Abstract
Expression of multiple genes from the same target cell is required in several technological and therapeutic applications such as quantitative measurements of promoter activity or in vivo tracking of stem cells. In spite of such need, reaching independent and high level dual-gene expression cannot be reliably accomplished by current gene transfer vehicles. To address this issue, we designed a lentiviral vector carrying two transcriptional units separated by polyadenylation, terminator and insulator sequences. With this design, the expression level of both genes was as high as that yielded from lentiviral vectors containing only a single transcriptional unit. Similar results were observed with several promoters and cell types including epidermal keratinocytes, bone marrow mesenchymal stem cells and hair follicle stem cells. Notably, we demonstrated quantitative dynamic monitoring of gene expression in primary cells with no need for selection protocols suggesting that this optimized lentivirus may be useful in high-throughput gene expression profiling studies.
Keywords: lentivirus, gene expression, promoter interference, tissue-specific promoter, dynamic measurement
INTRODUCTION
Current bio-technologies necessitate co-expression of multiple genes in the same target cell. In gene therapy, certain applications may require expression of the protein of interest together with a marker gene to monitor or purify the modified cells. Other applications may require production of multiple subunits of a protein that is encoded by different genes; or production of several proteins that work synergistically as part of a network. Stem cell technologies may require co-expression of two marker genes, one driven by a lineage-specific promoter to monitor stem cell differentiation and the other driven by a constitutive promoter to monitor gene transfer or track the modified cells in vitro or in vivo. Despite the need for such vectors, independent and high level dual-gene expression cannot be reliably accomplished by current gene transfer technologies.
Although co-transfection of cells with two separate vectors has been used in the past, the copy number of each vector varies between target cells, resulting in heterogeneous population of cells expressing either one or two genes at different ratios. As a result some cells may express only one of the two proteins in detectable levels rendering quantitative measurements of gene expression or cell tracking difficult. Accordingly, several strategies have been developed and used extensively including use of internal ribosome entry site (IRES) 1,2, self-cleaving 2A-like peptides 3 or bidirectional synthetic promoters 4. However, as both genes are driven by the same promoter none of these strategies allows independent gene expression necessitating the development of vectors that allow expression of each gene from an independent promoter.
To this end, two transcriptional units in tandem cis-arrangement have been used but this approach has been hampered by low level and inconsistent - either one or the other gene is expressed but not both - gene expression mainly due to promoter interference or suppression 5-8, the extent of which may vary between cell types 9. Lentiviral vectors with two independent transcriptional units have been used with variable results. While some cells showed high gene expression level, for others e.g. CD34+ cells a significant fraction of the population expressed only one of the two genes 10,11. Others showed that expression of the second gene from dual-gene lentiviral vectors was reduced drastically possibly due to promoter interference 12.
In this study we designed and optimized a lentiviral vector carrying two independent transcriptional units that were spaced by a synthetic polyA, terminator and insulator sequences 13-15. A second insulator sequence was cloned downstream of the second gene polyA to alleviate potential suppression of gene expression from the viral enhancer or surrounding genomic sequence after lentivirus integration. All the internal transcription units were cloned in trans to avoid premature termination of viral mRNA by the inserted polyAs during virus production in packaging cells. With this design, flow cytometry and fluorescence microscopy showed that the vast majority of transduced cells co-expressed both genes to a similar extent as cells transduced with lentiviral vectors containing only a single transcriptional unit. Furthermore, the performance of the newly designed vector was independent of cell type and promoter used. Using this vector, we demonstrated tissue-specific gene expression in bone marrow mesenchymal stem cells, hair follicle stem cells and epidermal keratinocytes. Using different promoter response elements we also demonstrated quantitative dynamic monitoring of gene expression in response to cytokine stimulation suggesting that this lentiviral vector may be very useful in high-throughput gene expression profiling studies.
RESULTS
Polyadenylation signal increases the consistency and level of gene expression from two transcriptional units in tandem
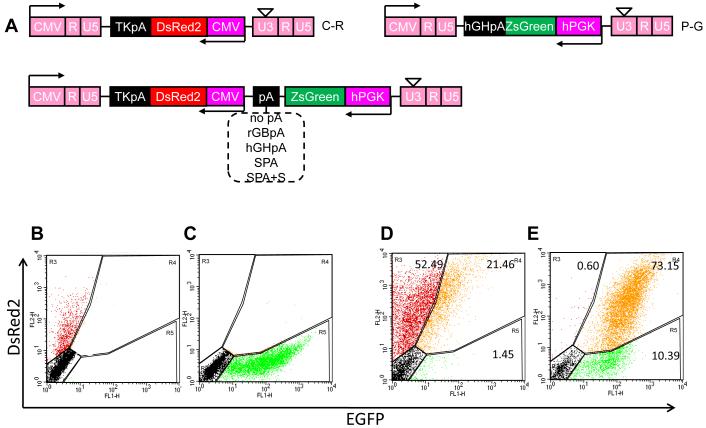
We employed a self-inactivating lentiviral vector with two promoters, PGK and CMV to achieve independent expression of two marker genes, ZsGreen and DsRed, respectively, in hair follicle derived stem cells (HF-SC) 16. Lentiviral vectors expressing a single gene, namely DsRed under the CMV promoter (C-R) or ZsGreen under the PGK promoter (P-G) served as controls (Fig. 1A). Note that to avoid disruption of viral mRNA production by poly(A) sequences in the middle of the vector, the promoters, genes and poly(A) sequences were cloned in antisense orientation relative to the viral LTR. As the mean fluorescence intensity depends on the number of integrated provirus copies per cell, all virus preparations were diluted to yield similar transduction efficiencies as indicated by the fraction of transduced cells and the mean fluorescent intensity, GFI and RFI.
Figure 1. Addition of polyA improves the consistency and level of dual-gene expression.
(A). Schematic of third generation self-inactivated lentiviral vectors for single or dual-gene expression. TKpA, thymidine kinase polyA from herpes simplex virus; CMV, cytomegalovirus promoter; rGBpA, rabbit beta-globin polyA; hGHpA, human growth hormone polyA; SPA, synthetic polyA; S, a 400bp nonsense spacer sequence derived from the pEGFP-1 vector; PGK, human phosphoglycerate kinase promoter. (B-E) Flow cytometry analysis of HF-SC transduced with a lentivirus encoding for (B) CMV-driven DsRed (C-R); (C) PGK-driven ZsGreen (P-G); (D) both expression cassettes with no polyA after ZsGreen (P-G-C-R); (E) both expression cassettes with the synthetic polyA and spacer sequences (SPA+S) at the 3′ end of ZsGreen. (F-G) Flow cytometry analysis of HF-SC transduced with lentivirus encoding for both genes separated by the indicated polyA. Lentivirus preparations were diluted to achieve similar transduction efficiency (TE=53.5±2.6% ZsGreen+, DsRed+ or ZsGreen+/DsRed+ cells). To achieve this transduction efficiency each vector was used at the following MOI: 0.8±0.02 for control vector without poly(A) and 0.8±0.2 with SPA, 1.1±0.04 with rGBpA, 1.0±0.05 with hGHpA and 0.9±0.02 with SPA+S containing vectors). (F) Percentage of EGFP+/DsRed+ HF-SC. (G) Mean green fluorescence intensity (GFI, white bars) or DsRed fluorescence intensity (RFI, black bars). Data shown are representative of at least three experiments with similar results.
As expected, cells transduced with single gene vectors C-R or P-G expressed DsRed or ZsGreen, respectively (Fig. 1B, C). Surprisingly, transduction with the dual-promoter lentivirus (P-G-C-R) yielded only a modest fraction of cells (21.5% of total or 29% of transduced cells) expressing both genes despite use of a single vector. A large fraction of cells expressed DsRed (52.5%) but only a small fraction expressed ZsGreen (1.45%) (Fig. 1D). In agreement, fluorescence microscopy showed that the majority of cells expressed DsRed but only few cells expressed ZsGreen (Fig. S1), suggesting that the PGK promoter may be suppressed.
Interestingly, addition of the synthetic polyA (SPA) plus a spacer sequence (S) between the two transcriptional units increased the fraction of ZsGreen+/DsRed+ cells dramatically (Fig. 1E, S1). The spacer sequence - f1 origin for single-stranded DNA production, which is usually used as a probe or for DNA sequencing or site-directed mutagenesis - was derived from the pEGFP-1 vector (bases: 1067-1522; Clontech). Although other polyA sequences resulted in the same fraction of ZsGreen+/DsRed+ cells (Fig. 1F), the combination of SPA plus S resulted in the highest level of ZsGreen expression as quantified by the mean green fluorescence intensity (GFI) (Fig. 1G). Surprisingly, while all poly(A) sequences increased GFI significantly, no poly(A) increased red fluorescence intensity (RFI) and some of them (rGBpA and SPA) decreased RFI compared to control lentivirus (no polyA; P-G-C-R).
Pause and insulator sequences further enhances gene expression
Previous studies have shown that additional sequences downstream of polyA may play an important role in promoting transcriptional termination 17,18. These sequences include pause sites such as the G-rich MAZ4 19; CoTC, the sequence for transcriptional termination of human beta-globin gene 20,21; Tactb, a G-rich sequence from the extension of beta-actin gene; and Tmsa, the pause site for transcriptional termination of the mouse serum albumin gene 21.
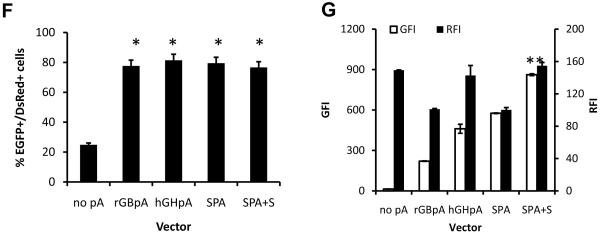
Based on these studies, we hypothesized that addition of pause sites between the two gene cassettes in the lentiviral vector might decrease promoter interference even further. To address this hypothesis, we cloned several pause sites downstream of SPA (Fig. 2A) and compared the expression level of both genes in HF-SC. We employed lentivirus preparations at concentrations that yielded similar transduction levels (42.3±3.6% of cells expressing at least one of the two genes) to ensure similar number of gene copies per cell.
Figure 2. Incorporation of a pause site improves the expression of both genes.
(A). Schematic of lentiviral vector for dual-gene expression in which different pause sites were cloned downstream of SPA. (B) Quantitative analysis of the expression level of both proteins at matched vector input (TE=42.4±3.6%; MOI=0.5±0.03). The symbol (*) denotes statistical significance of GFI (p<0.05) between the Tactb and SPA+S vectors. (C) Fluorescence image of HF cells transduced with lentivirus encoding for both genes separated by SPA plus the optimal terminator, Tactb. Data shown are representative of at least three experiments performed with similar results.
Using these vectors we found that the Tactb pause site increased ZsGreen expression significantly by 20.0±6.1% (n=3) compared to the vector containing the SPA plus S (Fig. 2B). MAZ4 containing lentivirus showed little improvement of GFI but decreased RFI. Surprisingly, both CoTC and Tmsa reduced ZsGreen expression compared to the nonsense spacer (S) sequence (Fig. 2B).
In naturally occurring genes that are spaced in close proximity, promoter interference is avoided by organization of genes in distinct domains separated by special DNA sequences termed “insulators” or “boundary elements” 13-15. Several insulators have been identified including the matrix attachment region from human beta-interferon gene (MAR) and the chicken hypersensitive site 4 (cHS4) 13,22,23. When cloned into expression vectors, both MAR and cHS4 were shown to improve the level and consistency of transgene expression through the reduction of position effects 12,24-26. In addition, these two insulators were also shown to alleviate promoter suppression 6,27.
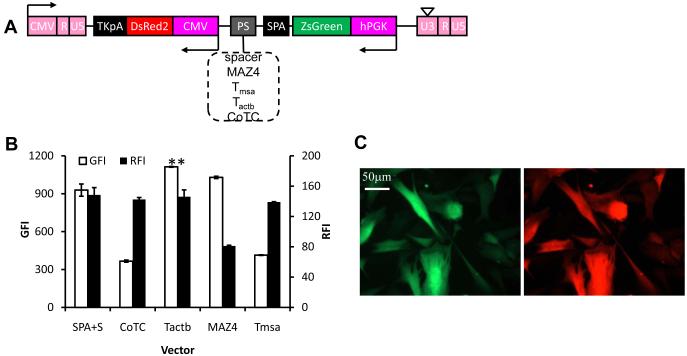
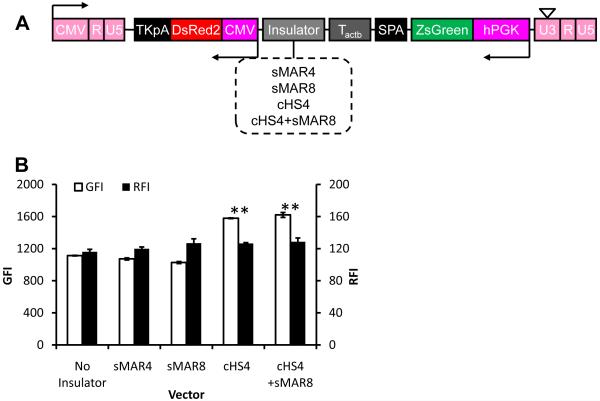
To examine whether addition of insulator elements might improve gene expression further we cloned the following insulators downstream of the optimum terminator, Tactb: synthetic MAR sequence (sMAR4, sMAR8); the extended core sequence of cHS4 26 or the combination of cHS4 and sMAR8 (Fig. 3A). Interestingly, cHS4 improved expression of both ZsGreen and DsRed by 41.8±0.8% and 9.0±3.6% (n=3), respectively (Fig. 3B). On the other hand, sMAR4 or sMAR8 had no effect on gene expression when added between the two units but addition of sMAR8 downstream of TKpA further improved expression of ZsGreen and DsRed by 14.7±3.2% and 30.9±6.9% (n=3), respectively (Fig. 3C, D). Interestingly, other insulator elements at that site decreased RFI and to a lesser extent GFI levels.
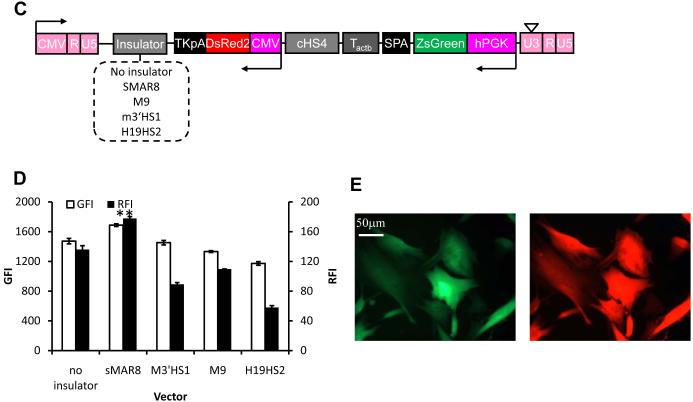
Figure 3. Insulator sequences reduced promoter interference and enhanced gene expression.
(A) Schematic of lentiviral vector for dual-gene expression in which different insulators were cloned downstream of Tactb. (B) Quantitative analysis of the expression level of both proteins in cells modified at matched vector input (TE=41.1±2.9%; MOI=0.6±0.04). (C) Schematic of lentiviral vector for dual-gene expression in which different insulators were cloned downstream of the second polyA sequence, TKpA. (D) Quantitative analysis of the expression level of both proteins in cells modified at matched vector input (TE=28.7±1.7%; MOI=0.4±0.05). (E) Fluorescence image of HF cells transduced by the optimum vector, LVDP, containing sMAR8 downstream of TKpA and cHS4, Tactb, SPA downstream of EGFP. Data shown are representative of at least three experiments performed with similar results.
Overall, compared to P-G-C-R the optimized lentiviral dual-promoter (LVDP) vector containing SPA, Tactb and cHS4 between the two transcriptional units as well as sMAR8 after TKpA (Fig. 4A) improved DsRed expression by approximately 40% (RFI increased from 128.8±1.7 to 177.8±2.6; n=3) and ZsGreen expression more than 120-fold (GFI increased from 13.7±0.4 to 1688.3±17.3; n=3).
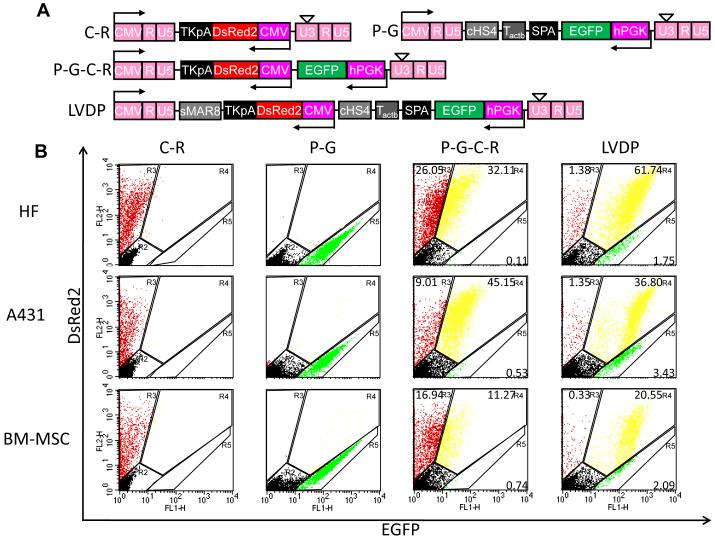
Figure 4. LVDP exhibits consistent and high level dual-gene expression in different cell types.
(A) Schematic of lentiviral vectors encoding DsRed only driven by CMV promoter (C-R) or EGFP only driven by PGK promoter (P-G); or both DsRed and EGFP with no intermediate sequences (P-G-C-R); or with the optimum combination of regulatory sequences (LVDP) separating the two transcriptional units. (B) Flow cytometry analysis of transduced HF cells, A431 cells and BM-MSC by vectors described in (A). (C) Quantitative analysis of the expression level of both proteins in cells modified by vectors described in (A) at MOI: 1.2±0.04 for P-G and C-R; 1.4±0.05 for P-G-C-R and 2.6±0.15 for LVDP. TE=62.2±2.3% for HF cells; 51.3±3.2% for A431 cells or 28.9±3.1% for BM-MSC, respectively. Data shown are representative of at least three experiments performed with similar results.
The optimized lentiviral vector confers high level dual-gene expression independent of cell type
Next we examined whether LVDP (Fig. 4A) could yield high level and independent expression of both genes in multiple cell types including HF-SC, A431 epidermoid carcinoma cells and bone marrow derived mesenchymal stem cells (BM-MSC). As controls we use P-G-C-R as well as single-gene lentiviral vectors, P-G and C-R (Fig. 4A). Since the optimized vector yielded GFI that was about 10 times higher than RFI, the rest of the experiments were conducted with EGFP rather than ZsGreen in order to measure GFI that was comparable to RFI and avoid high compensation during flow cytometry.
Cells transduced with the P-G-C-R lentivirus exhibited a significant population of DsRed+ cells that were not EGFP+ (Fig. 4B). The percentage of DsRed+/EGFP- cells varied for different cell types and was the smallest for BM-MSC (Fig. 4B). In contrast, transduction with the LVDP lentivirus resulted in the vast majority of all cell types expressing both genes to a higher level. Indeed, all cells transduced with the LVDP virus exhibited GFI and RFI as high as cells transduced with lentiviral vectors encoding only for a single reporter, C-R or P-G (Fig. 4C). In contrast, all cells transduced with the non-optimized P-G-C-R exhibited lower RFI and very low - if any - GFI (Fig. 4C). Fluorescence microscopy confirmed these results showing bright green and red cells transduced with the LVDP virus (Fig. S2) as opposed to the P-G-C-R virus (Fig. S1).
LVDP confers independent and high level dual-gene expression from constitutive and tissue-specific promoters
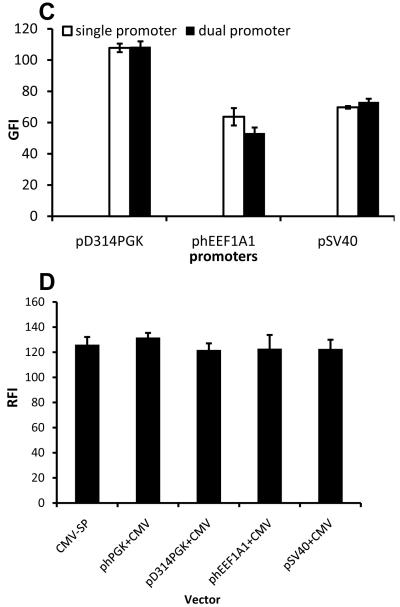
Next we examined whether independent expression of EGFP - the gene that was mostly suppressed by the second expression cassette (CMV-DsRed) - depended on the choice of promoter. To this end, in addition to PGK we employed the following promoters to drive expression of EGFP: pD314PGK, a truncated PGK with reduced strength 28, pSV40 or phEEF1A1 (Fig. 5A; also see Table S1). DsRed was expressed by CMV and gene expression was investigated in HF-SC.
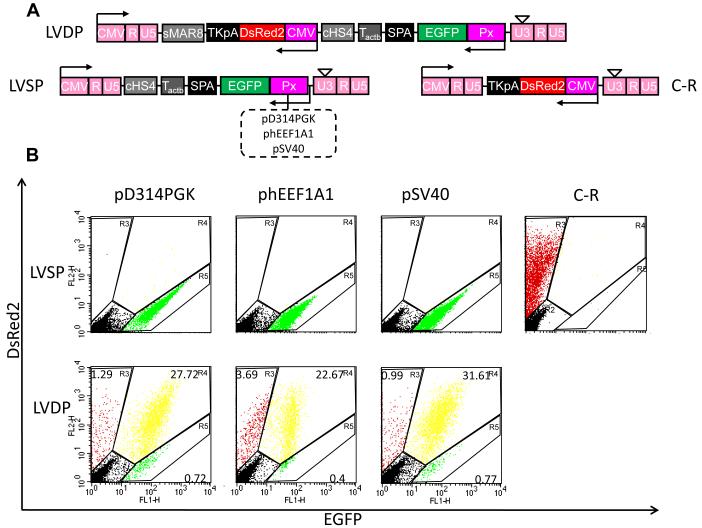
Figure 5. LVDP-transduced cells exhibit consistent and high level dual-gene expression independent of internal promoters used.
(A) Schematic of lentiviral vector (LVDP) in which the PGK promoter is replaced by promoter Px or control single-gene encoding vectors (LVSP) in which promoter Px drives expression of EGFP or CMV drives expression of DsRed. Px=pD314PGK, truncated PGK promoter; phEEF1A1, human translation elongation factor 1 alpha 1 promoter; pSV40, simian virus promoter. (B) Flow cytometry analysis of HF cells transduced by vectors described in (A). (C-D) Quantitative analysis of the expression level of both proteins in cells modified by vectors described in (A) at TE=28.1±2.9% (MOI: 0.7±0.07 for phEEF1A1 and 0.4±0.05 for hPGK, D314PGK, or pSV40 dual-promoter vectors). Data shown are representative of at least three experiments performed with similar results.
Interestingly, flow cytometry (Fig. 5B) and fluorescence microscopy (Fig. S3) showed that close to 100% of transduced cells expressed both proteins (EGFP+/DsRed+), indicating consistent expression from all promoters. Although each promoter exhibited different activity, the level of gene expression (GFI) from LVDP transduced cells was similar to that of cells modified with lentivirus encoding for a single gene i.e. EGFP under the same promoter (Fig. 5C). In addition, the DsRed expression level (RFI) remained unaffected regardless of the promoter driving EGFP (Fig. 5D), indicating that expression of each transgene was expressed independent of the other.
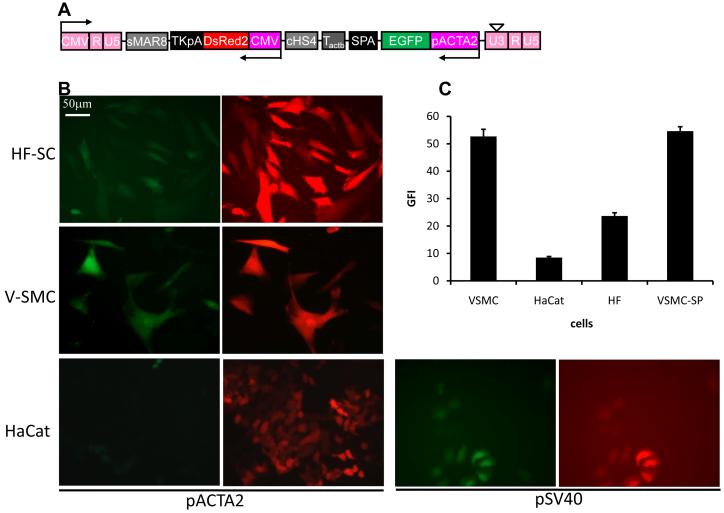
To test whether LVDP could be used for studies that require tissue-specific gene expression, we replaced the PGK with the human smooth muscle alpha-actin (pACTA2) promoter (Fig. 6A; LVDP-ACTA2) and transduced three cell types: vascular smooth muscle cells (V-SMC); HF-SC, which were previously shown to contain a significant fraction of alpha-actin expressing cells 16; and the epidermal cell line, HaCaT, which served as negative control. Almost 100% of V-SMC and about 77.1±2.1% (n=3) of HF-SC expressed EGFP (Fig. 6B). As expected, HaCaT cells did not express EGFP but expressed high level of DsRed indicating successful gene delivery. Substitution of pACTA2 by the constitutive pSV40 promoter restored the high level of EGFP expression, suggesting that the weak activity of pACTA2 may reflect the physiology of HaCaT cells rather than pACTA2 suppression by the downstream CMV promoter. Indeed, the EGFP fluorescence intensity of LVDP-ACTA2-modified V-SMC was as high as that of V-SMC transduced by single promoter lentivirus (LVSP-ACTA2; Fig. 6C), further supporting the conclusion that pACTA2 was not suppressed by CMV.
Figure 6. Tissue-specific gene expression using LVDP.
(A) Schematic of lentiviral LVDP vector in which PGK promoter is replaced by smooth muscle alpha-actin promoter or pSV40. (B) Fluorescence imaging of vascular smooth muscle cells (VSMC), HF cells or HaCaT cells transduced by vectors described in (A). (C) Quantitative analysis of EGFP expression level in cells modified by vectors described in (A) at TE=12.7±2.0% (MOI: 0.3±0.02). VSMC-SP denotes V-SMC transduced with a single-gene encoding lentiviral (LVSP) in which EGFP is driven by the ACTA2 promoter. Data shown are representative of at least three experiments performed with similar results.
Dynamic gene expression profiling independent of transduction efficiency
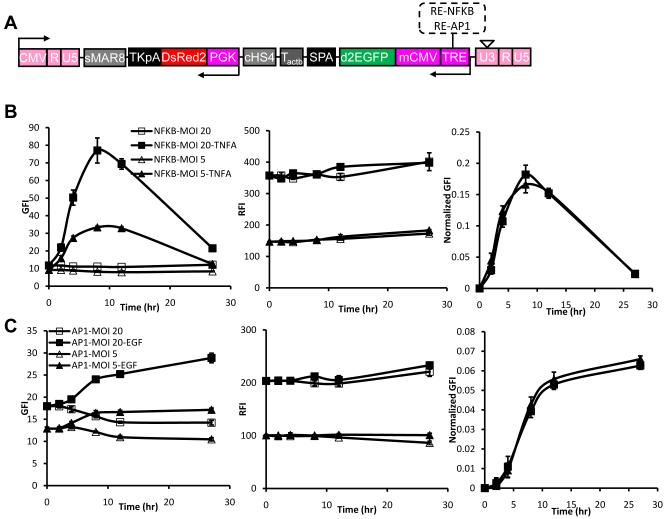
Next we employed two response elements, NFκB or AP1 along with a minimum CMV promoter 29,30 to drive expression of destabilized d2EGFP (t1/2=2 hr) in order to monitor the dynamics of gene expression in response to exogenous signals. Since these response elements are accompanied by a minimum CMV promoter and the presence of recurring sequences in lentiviral vectors has been shown to result in gene deletions and vector instability 31,32, we employed the PGK promoter - instead of CMV - to drive DsRed2 expression in these experiments (Fig. 7A). Primary human keratinocytes were transduced with lentivirus at two different dilutions, expanded and treated with TNF-α (25 ng/ml) or EGF (25 ng/ml) to activate the NFκB or AP1 response elements, respectively. Transduced but untreated keratinocytes were used as negative control.
Figure 7. Using LVDP for dynamic gene expression profiling.
(A) Schematic of lentiviral LVDP vector in which PGK promoter is replaced by response element NFκB (RE-NFκB) or AP1 (RE-AP1) along with the CMV minimal promoter and EGFP is replaced by destabilized EGFP with t1/2=2hr (d2EGFP). (B) Kinetics of EGFP and DsRed expression in response to TNF-α (25 ng/ml). Primary keratinocytes were transduced with two dilutions: 1/5 (MOI=20) or 1/20 (MOI=5) of lentivirus encoding for EGFP under RE-NFκB and DsRed under the PGK promoter, respectively. (C) Kinetics of EGFP and DsRed expression in response to EGF (25 ng/ml). Primary keratinocytes were transduced with lentivirus encoding for EGFP under RE-AP1 and DsRed under the PGK promoter at MOI=5 or 20 as indicated. Data shown are representative of at least three experiments performed with similar results.
After stimulation with TNF-α or EGF, the EGFP and DsRed fluorescence intensity - GFI and RFI - were monitored using flow cytometry. As shown in Fig. 7B&S4A NFκB-driven GFI increased with time after TNF-α stimulation, peaked at about 8 hr and decreased to background level 27 hr later. On the other hand, AP1-driven GFI increased with time after EGF treatment and reached a plateau at 8-12 hr later (Fig. 7C, S4B). Decreasing the concentration of lentivirus decreased NFκB- and AP1-driven GFI without altering the kinetic profiles. As expected, RFI also depended strongly on lentivirus concentration but was not affected by TNF-α or EGF. Notably, in both cases the kinetic profile of the normalized signal (GFI/RFI) was independent of virus concentration, indicating that the signal accurately depicts the intrinsic response of cells to the stimulus and is unaffected by variability in transduction efficiency between individual cells or cell populations.
DISCUSSION
In this study, we seek to improve the level and consistency of gene expression from self-inactivating dual-gene lentiviral vectors by systematically incorporating polyadenylation, terminator and insulator sequences between the two expression cassettes. Several investigators reported efficient dual-gene expression lentiviral vectors from two different promoters with one in reverse orientation and the other in forward 33,34 or with both promoters in forward orientation 31. With two genes in tandem, the two transcriptional units elongate in the same direction and share the same polyadenylation signal in the “R” region of the 3′ LTR. Therefore, two transcripts are made, which overlap in the downstream gene sequence 10. In contrast, placing both promoters in opposite orientation to the viral LTRs enabled insertion of poly(A) sequences between the two gene cassettes without disrupting lentiviral production due to premature termination of the viral mRNA transcript in packaging cells (data not shown and 12). In addition, this orientation allowed insertion of another poly(A) and insulator sequence downstream of the second gene as well. These sequences further enhanced DsRed expression and may also provide enough insulation to avoid interference from flanking genomic elements after lentiviral integration.
With the two transcriptional units in trans, we observed that addition of polyadenylation sequence between the two units increased the efficiency and consistency of EGFP expression, albeit to an extent that depended on the specific sequence. The combination of SPA+S yielded the highest EGFP expression while maintaining the level of DsRed expression. The spacer sequence (S) increased RFI and GFI compared to SPA alone, possibly by providing additional space between the two transcription units 18. Surprisingly, two of the polyA sequences, rGBpA and SPA decreased expression of the downstream gene, DsRed. Although the mechanism is not clear, one possible explanation may be reduction of read-through transcription by the PGK promoter 10. Finally, some polyA sequences e.g. SV40 reduced viral titer significantly even when positioned in trans to the viral LTR (data not shown), possibly because they are able to terminate transcription in a bi-directional manner 35.
Compared to the spacer, S, addition of terminator sequence Tactb further improved EGFP expression while maintaining expression of DsRed. Surprisingly, MAZ4 decreased only DsRed expression, while CoTC and Tmsa decreased EGFP expression but maintained DsRed levels. Interestingly, MAZ4 is only 200bp in length while CoTC and Tmsa are larger than 400bp, suggesting that the length of the terminator sequence may determine which of the two promoters is suppressed.
In agreement with a recent report using non-viral vectors 27, the cHS4 insulator improved lentivirus-mediated EGFP expression over and above the enhancement afforded by the polyA and pause sequences. cHS4 also improved DsRed gene expression albeit to a lesser extent. Notably, the expression level of each gene from the optimized LVDP vector was similar to that of vectors carrying a single expression cassette, suggesting that promoter interference was eliminated. Taken collectively, these results suggest that terminator and insulator sequences must be carefully optimized to ensure efficient and consistent gene expression.
Promoter activity measurement is one area where consistency of gene expression is very important because the level of expression must reflect the true biological activity of the promoter and be independent of strong adjacent promoters or the efficiency of gene transfer. Current state of the art assays rely on two plasmid co-transfection, where one plasmid encodes for a marker gene e.g. luc under a tissue specific promoter and the other employs a constitutive promoter to drive expression of a second marker e.g. Renilla luc. As a result, it is not possible to ensure the same transfection efficiency for both plasmids thereby affecting the accuracy of signal normalization. In addition, low transfection efficiency restricts application of this assay to a small group of easily transfectable cell lines such as 293T cells. Application to other cell types may require long antibiotic selection protocols often followed by selection of single cell clones that exhibit the highest promoter activity in response to a particular treatment. Concerns then arise regarding the duration and physiological significance of such studies. More importantly, following experiments with one cell type, application of the same approach to a second cell line requires tedious re-engineering of a new cell line, a process that can take several more weeks.
Our data showed that the optimized LVDP dual-gene lentivirus overcomes these problems and may be ideal for dynamic promoter activity assays especially in the more physiologically relevant context of primary cells and stem cells. Using the NF-κB or AP-1 response elements and a destabilized EGFP marker gene we determined the kinetics of gene expression in response to cytokine treatment, TNF-α or EGF, respectively. As expected the level of EGFP and DsRed expression depended on lentivirus concentration but only EGFP expression changed dynamically in response to TNF-α or EGF. Notably, normalizing the EGFP signal by the intensity of DsRed (GFI/RFI) maintained the shape of the kinetic profile and rendered it independent of transduction efficiency or the site of lentivirus integration. This data clearly demonstrates that the two promoters acted independent of each other and yielded consistent gene expression data. Therefore, LVDP may be useful in high-throughput gene expression profiling studies.
Our results showed that the combination of polyA, insulator and terminator sequences eliminated promoter interference yielding high-level gene expression that was similar to that obtained by single-gene encoding vectors. These elements may also shield these two transcriptional units from the influence of cellular chromatin and reduce potential activation of adjacent cellular genes by internal promoters after provirus integration into the cellular genome 13,36,37. Indeed, shielding from surrounding genomic elements may account for the increased DsRed expression upon addition of sMAR8 insulator downstream of TKpA. If true, this hypothesis may also suggest that the insulator and/or terminator sequences may reduce the oncogenic potential of lentiviral vectors ultimately improving their safety and therapeutic potential.
MATERIALS & METHODS
Cell culture
293T/17 cells (ATCC, Manassas, VA), A431 cells (ATCC, VA), HaCaT cells, vascular smooth muscle cells (V-SMC), bone marrow mesenchymal stem cells (BM-MSC) and hair follicle stem cells (HF-SC) were cultured in DMEM (GIBCO BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (GIBCO, NY). Isolation and culture of primary human keratinocytes was performed as described previously 38,39. For experiments involving cytokine treatment, keratinocytes were cultured in serum-free medium (KC-SFM) without supplements (EGF and BPE) but with addition of 1% BSA for 24 hr before stimulation. Keratinocytes were treated with human recombinant TNF-α (25 ng/ml, Sigma, MO) or EGF (25ng/ml, GIBCO, NY) and harvested for flow cytometry at 0, 2, 4, 8, 12 and 27 hr post treatment. At each time point untreated cells served as negative controls.
Cloning and lentivirus production
The third generation of lentiviral system was described elsewhere 40. The cloning sites, primers and templates (genomic DNA or other plasmids) are listed in Table S1. All PCR reactions were carried out with either High-Fidelity-Plus polymerase (Roche, Indianapolis, IN), Accuzyme™ DNA polymerase (Bioline, Taunton, MA) or Phusion™ High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA) following manufacturer’s protocols. For oligonucleotide annealing, sense and antisense oligonucleotides were mixed at 1:1 ratio to a final concentration of 50 μM and the mixture was incubated at 95°C for 30 sec, followed by stepwise cooling at 72°C for 2 min, 37°C for 2 min and 25°C for 2 min. All cloning products were confirmed by sequencing with ABI PRISM 3130XL Genetic Analyzers (Applied Biosystems, Foster City, CA).
For lentivirus production 293T/17 cells (ATCC) were transiently co-transfected with four plasmids using the standard calcium phosphate precipitation method 41 when they reached 70-80% confluence. The following plasmids were used: 15 μg lentiviral vector, 5μg pMDL-g/p, 3μg pSRV-rev and 1.5μg pMDG-VSVG. After 24 hr, transfected cells were washed with PBS and the culture medium was replaced with medium containing 5 mM sodium butyrate (Aldrich, St. Louis, MO). Virus was harvested twice at 24 and 48 hr post transfection, filtered through 0.45μm filter (Millipore, Bedford, MA), pelleted by ultracentrifugation (50,000g at 4°C for 2 hr) and resuspended in fresh medium. The titer of lentiviral preparation was determined on HF-SC and lentiviral mRNA was measured using the Lenti-X qRT-PCR titration kit (Clontech Laboratories Inc., Mountain View, CA) for the indicated lentivirus stocks (Fig. S5).
Lentivirus transduction
Non-tissue culture treated 24-well plates were incubated overnight at 4°C with 400μl of recombinant fibronectin (rFN) fragment CH296 (10 μg/mL; Takara Mirus Bio Corporation, Madison, WI). Lentivirus (200 μL/well) at the indicated dilution was preloaded in rFN-coated wells and incubated at 37°C for 2 hr. At the end of virus incubation, 30,000 cells in 300 μl of culture medium were added in each well to initiate gene transfer. The culture medium was replenished the next day and cells were processed for flow cytometry at 72 hr post-transduction (usually >90% confluence).
Statistical analysis
Statistical analysis of the data was performed using a two-tailed Student’s t-test (α = 0.05) using Microsoft Excel (Microsoft, Redwood, CA).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (R01 EB000876-01, R01 HL086582) and the New York Stem Cell Science Funding Program (NYSTEM) to S.T.A.
The authors are thankful to Dr. Arul Jayaraman (Texas A&M University) for providing the plasmids containing the NF-κB and AP-1 response elements.
REFERENCES
- 1.Martinez-Salas E. Internal ribosome entry site biology and its use in expression vectors. Curr Opin Biotechnol. 1999;10:458–464. doi: 10.1016/s0958-1669(99)00010-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Feuer G, Day SL, Wrzesinski S, Planelles V. Multigene lentiviral vectors based on differential splicing and translational control. Mol Ther. 2001;4:375–382. doi: 10.1006/mthe.2001.0469. [DOI] [PubMed] [Google Scholar]
- 3.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 4.Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. Epub 2004 Dec 2026. [DOI] [PubMed] [Google Scholar]
- 5.Wilson C, Bellen HJ, Gehring WJ. Position effects on eukaryotic gene expression. Annu Rev Cell Biol. 1990;6:679–714. doi: 10.1146/annurev.cb.06.110190.003335. [DOI] [PubMed] [Google Scholar]
- 6.Villemure JF, Savard N, Belmaaza A. Promoter suppression in cultured mammalian cells can be blocked by the chicken beta-globin chromatin insulator 5′HS4 and matrix/scaffold attachment regions. J Mol Biol. 2001;312:963–974. doi: 10.1006/jmbi.2001.5015. [DOI] [PubMed] [Google Scholar]
- 7.Milot E, Fraser P, Grosveld F. Position effects and genetic disease. Trends Genet. 1996;12:123–126. doi: 10.1016/0168-9525(96)30019-x. [DOI] [PubMed] [Google Scholar]
- 8.Shearwin KE, Callen BP, Egan JB. Transcriptional interference--a crash course. Trends Genet. 2005;21:339–345. doi: 10.1016/j.tig.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eszterhas SK, Bouhassira EE, Martin DI, Fiering S. Transcriptional interference by independently regulated genes occurs in any relative arrangement of the genes and is influenced by chromosomal integration position. Mol Cell Biol. 2002;22:469–479. doi: 10.1128/MCB.22.2.469-479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Zhan X, D’Costa J, Tanavde VM, Ye Z, Peng T, et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 11.Pan H, Mostoslavsky G, Eruslanov E, Kotton DN, Kramnik I. Dual-promoter lentiviral system allows inducible expression of noxious proteins in macrophages. J Immunol Methods. 2008;329:31–44. doi: 10.1016/j.jim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osti D, Marras E, Ceriani I, Grassini G, Rubino T, Vigano D, et al. Comparative analysis of molecular strategies attenuating positional effects in lentiviral vectors carrying multiple genes. J Virol Methods. 2006;136:93–101. doi: 10.1016/j.jviromet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Bell AC, West AG, Felsenfeld G. Insulators and boundaries: versatile regulatory elements in the eukaryotic genome. Science. 2001;291:447–450. doi: 10.1126/science.291.5503.447. [DOI] [PubMed] [Google Scholar]
- 14.Gaszner M, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 15.Razin SV, Iarovaia OV, Sjakste N, Sjakste T, Bagdoniene L, Rynditch AV, et al. Chromatin domains and regulation of transcription. J Mol Biol. 2007;369:597–607. doi: 10.1016/j.jmb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu JY, Peng HF, Andreadis ST. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot NJ, Furger A, Dye MJ. Integrating mRNA processing with transcription. Cell. 2002;108:501–512. doi: 10.1016/s0092-8674(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 18.Orozco IJ, Kim SJ, Martinson HG. The poly(A) signal, without the assistance of any downstream element, directs RNA polymerase II to pause in vivo and then to release stochastically from the template. J Biol Chem. 2002;277:42899–42911. doi: 10.1074/jbc.M207415200. [DOI] [PubMed] [Google Scholar]
- 19.Yonaha M, Proudfoot NJ. Specific transcriptional pausing activates polyadenylation in a coupled in vitro system. Mol Cell. 1999;3:593–600. doi: 10.1016/s1097-2765(00)80352-4. [DOI] [PubMed] [Google Scholar]
- 20.Dye MJ, Proudfoot NJ. Multiple transcript cleavage precedes polymerase release in termination by RNA polymerase II. Cell. 2001;105:669–681. doi: 10.1016/s0092-8674(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 21.Gromak N, West S, Proudfoot NJ. Pause sites promote transcriptional termination of mammalian RNA polymerase II. Mol Cell Biol. 2006;26:3986–3996. doi: 10.1128/MCB.26.10.3986-3996.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon YS, Jeong S, Rong Q, Park KY, Chung JH, Pfeifer K. Analysis of the H19ICR insulator. Mol Cell Biol. 2007;27:3499–3510. doi: 10.1128/MCB.02170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girod PA, Nguyen DQ, Calabrese D, Puttini S, Grandjean M, Martinet D, et al. Genome-wide prediction of matrix attachment regions that increase gene expression in mammalian cells. Nat Methods. 2007;4:747–753. doi: 10.1038/nmeth1076. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- 25.Girod PA, Zahn-Zabal M, Mermod N. Use of the chicken lysozyme 5′ matrix attachment region to generate high producer CHO cell lines. Biotechnol Bioeng. 2005;91:1–11. doi: 10.1002/bit.20563. [DOI] [PubMed] [Google Scholar]
- 26.Aker M, Tubb J, Groth AC, Bukovsky AA, Bell AC, Felsenfeld G, et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- 27.Yahata K, Maeshima K, Sone T, Ando T, Okabe M, Imamoto N, et al. cHS4 insulator-mediated alleviation of promoter interference during cell-based expression of tandemly associated transgenes. J Mol Biol. 2007;374:580–590. doi: 10.1016/j.jmb.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Dor I, Itsykson P, Goldenberg D, Galun E, Reubinoff BE. Lentiviral vectors harboring a dual-gene system allow high and homogeneous transgene expression in selected polyclonal human embryonic stem cells. Mol Ther. 2006;14:255–267. doi: 10.1016/j.ymthe.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Thompson DM, King KR, Wieder KJ, Toner M, Yarmush ML, Jayaraman A. Dynamic gene expression profiling using a microfabricated living cell array. Anal Chem. 2004;76:4098–4103. doi: 10.1021/ac0354241. [DOI] [PubMed] [Google Scholar]
- 30.King KR, Wang S, Irimia D, Jayaraman A, Toner M, Yarmush ML. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7:77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert-Richard E, Richard E, Malik P, Ged C, de Verneuil H, Moreau-Gaudry F. Murine retroviral but not human cellular promoters induce in vivo erythroid-specific deregulation that can be partially prevented by insulators. Mol Ther. 2007;15:173–182. doi: 10.1038/sj.mt.6300030. [DOI] [PubMed] [Google Scholar]
- 32.ter Brake O, t Hooft K, Liu YP, Centlivre M, von Eije KJ, Berkhout B. Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol Ther. 2008;16:557–564. doi: 10.1038/sj.mt.6300382. [DOI] [PubMed] [Google Scholar]
- 33.Persons DA, Hargrove PW, Allay ER, Hanawa H, Nienhuis AW. The degree of phenotypic correction of murine beta -thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- 34.Chang AH, Stephan MT, Lisowski L, Sadelain M. Erythroid-specific human factor IX delivery from in vivo selected hematopoietic stem cells following nonmyeloablative conditioning in hemophilia B mice. Mol Ther. 2008;16:1745–1752. doi: 10.1038/mt.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng XH, Hughes SH. An avian sarcoma/leukosis virus-based gene trap vector for mammalian cells. J Virol. 1999;73:6946–6952. doi: 10.1128/jvi.73.8.6946-6952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emery DW, Yannaki E, Tubb J, Stamatoyannopoulos G. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc Natl Acad Sci U S A. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arumugam PI, Scholes J, Perelman N, Xia P, Yee JK, Malik P. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- 38.Geer DJ, Swartz DD, Andreadis ST. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am J Pathol. 2005;167:1575–1586. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajaj BG, Lei P, Andreadis ST. Efficient gene transfer to human epidermal keratinocytes on fibronectin: in vitro evidence for transduction of epidermal stem cells. Mol Ther. 2005;11:969–979. doi: 10.1016/j.ymthe.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.