Abstract
Chloroplast protein import is mediated by two hetero-oligomeric protein complexes, the Tic and Toc translocons, which are located in the inner and outer envelope membranes. At the inner membrane, many Tic components have been identified and characterized, but it remains unclear how these Tic proteins are organized to form a protein-conducting channel or whether a stable Tic core complex that binds translocating preproteins exists. Here, we report the identification of a 1-megadalton (MD) translocation complex as an intermediate during protein translocation across the inner membrane in Arabidopsis thaliana and pea (Pisum sativum). This complex can be detected by blue native PAGE using the mild detergent digitonin without any chemical cross-linkers. The preprotein arrested in the 1-MD complex can be chased into its fully translocated form after a subsequent incubation. While Tic20 and Tic21 appear to be involved in the 1-MD complex, Tic110, a well-characterized Tic component, exists as a distinct entity from the complex. Several lines of evidence suggest that the 1-MD complex functions in between the Toc and Tic110-containing complexes, most likely as a protein-conducting channel at the inner envelope.
INTRODUCTION
Nuclear-encoded chloroplast proteins are synthesized in the cytosol as preproteins with N-terminal targeting signals, called transit peptides. These proteins are then posttranslationally imported across the double envelope membranes of chloroplasts. The chloroplastic outer and inner envelope membranes contain multisubunit machinery for the import of preproteins, termed the Toc (translocon at the outer envelope membrane of chloroplasts) and the Tic (translocon at the inner envelope membrane of chloroplasts) complexes, respectively (for reviews, see Soll and Schleiff, 2004; Bédard and Jarvis, 2005; Jarvis, 2008; Kessler and Schnell, 2009). During import, the Toc and Tic complexes are thought to come together at contact sites where the outer and inner membranes are in proximity, allowing the preprotein to pass through both membranes simultaneously (Schnell and Blobel, 1993). During or shortly after import, the transit peptide is removed by a stromal processing peptidase, and the mature protein is then folded or targeted to one of the internal compartments.
Protein import into chloroplasts requires ATP hydrolysis. In the presence of low concentrations of ATP (<100 μM), irreversible binding of preproteins to the translocon components occurs. Although it is not clear where the ATP-utilizing component may reside, previous studies have shown that a stable association of preproteins with the translocon components, the so-called early import intermediate, was generated under low ATP concentrations (Waegemann and Soll, 1991; Perry and Keegstra, 1994; Schnell et al., 1994; Ma et al., 1996; Akita et al., 1997; Kouranov and Schnell, 1997; Nielsen et al., 1997; Young et al., 1999; Inoue and Akita, 2008). In the presence of higher concentrations of ATP (>1 mM), complete translocation of preproteins across the double envelope of chloroplasts occurs. This high ATP level is probably required by stromal molecular chaperones believed to provide the driving force for unidirectional translocation of preproteins.
At the outer membrane, Toc75, Toc159, and Toc34 form a stable complex and mediate the transfer of preproteins through the outer membrane (Schleiff et al., 2003; Kikuchi et al., 2006). At the inner membrane, eight proteins (Tic110, Tic40, Tic20, Tic21, Tic22, Tic55, Tic62, and Tic32) are reported to be involved in the import process (Soll and Schleiff, 2004; Bédard and Jarvis, 2005; Teng et al., 2006; Jarvis, 2008; Kessler and Schnell, 2009). Tic20 has been proposed to function as the protein-conducting channel of the inner membrane. Tic20 was identified by chemical cross-linking to translocating preproteins in pea (Pisum sativum) chloroplasts (Kouranov et al., 1998). Tic20 is an integral membrane protein and is predicted to have three or four transmembrane helices. The reduction of Tic20 levels in Arabidopsis thaliana antisense plants produced a specific defect in protein translocation across the inner membrane (Chen et al., 2002). Tic110 is predicted to have two transmembrane helices at its N terminus and a large hydrophilic C-terminal domain, which was shown to be exposed to the stromal compartment (Jackson et al., 1998). The stromal domain of Tic110 has been proposed to function as a molecular scaffold by binding the preprotein and recruiting the stromal chaperone Hsp93 with the assistance of the putative cochaperone Tic40 (Akita et al., 1997; Nielsen et al., 1997; Chou et al., 2003; Chou et al., 2006). These three proteins (Tic110, Tic40, and Hsp93) are thought to drive protein import into the stroma through repeated cycles of binding and release. Although an alternative model for the topology and function of Tic110 has also been proposed, in which Tic110 is a polytopic membrane protein that functions as a protein-conducting channel (Heins et al., 2002; Balsera et al., 2009), a truncated version of Tic110 lacking the N-terminal transmembrane helices was shown to exist as a soluble protein when expressed in Escherichia coli or in the stroma of transgenic Arabidopsis (Inaba et al., 2003).
However, the existence of a stable Tic complex containing a protein-conducting channel remains unclear. Here, we report the identification of a 1-MD translocation complex as an intermediate during protein translocation across the inner membrane. This complex can be detected by blue native PAGE (BN-PAGE) using the mild detergent digitonin without any chemical cross-linkers. The preprotein arrested in the 1-MD translocation complex can be chased into its fully translocated form after a subsequent incubation. Antibody-shift BN-PAGE, immunodepletion, and immunoprecipitation assays suggest that Tic20 and Tic21 are involved in the 1-MD translocation complex but that Tic110 is not involved in this complex.
RESULTS
A Translocation Intermediate Complex Was Observed by BN-PAGE
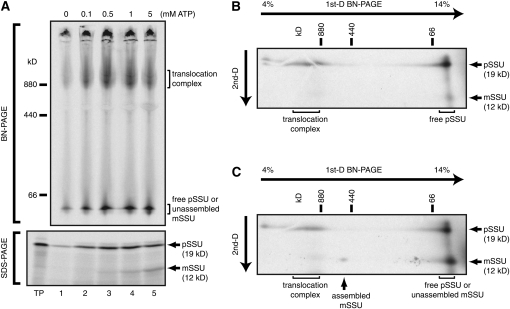
BN-PAGE allows the separation of membrane protein complexes under nondenaturing conditions (Schägger and von Jagow, 1991; Schägger et al., 1994). We examined whether BN-PAGE is applicable for the analysis of preproteins in the process of translocation across the double envelope membranes of chloroplasts. The precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (pSSU) was used as a model protein. pSSU was synthesized in vitro in the presence of [35S]Met. In vitro import reactions were performed in the presence of different concentrations of ATP using pea chloroplasts. Chloroplasts were reisolated, solubilized with 1% digitonin, and subjected to BN-PAGE and autoradiography (Figure 1A, top). Radioactive signals were found at, approximately, the 1-MD area and at a low molecular mass (<66 kD). By SDS-PAGE, the precursor form of SSU was observed at all tested concentrations of ATP, and the mature form of SSU (mSSU) was observed at relatively high concentrations of ATP (>1 mM) (Figure 1A, bottom).
Figure 1.
ATP-Dependent Formation of a Translocation Intermediate Complex.
(A) Energy-depleted pea chloroplasts were mixed with [35S]pSSU in HS buffer containing the indicated concentrations of ATP, 5 mM MgCl2, 5 mM DTT, 3 mM Met, 3 mM Cys, and 5 μL/mL protease inhibitor cocktail. The reactions were incubated for 10 min at 25°C in the dark. Reisolated chloroplasts were solubilized in BN-PAGE sample buffer (containing 1% digitonin) to a final concentration of 0.5 mg chlorophyll/mL for 10 min on ice. After ultracentrifugation, the supernatant was divided into two aliquots, one of which was mixed with Coomassie blue solution and subjected to 4 to 14% BN-PAGE (top). The other was mixed with 10% SDS and 2-mercaptoethanol to final concentrations of 3.3 and 5%, respectively, denatured by heating at 95°C for 2 min, and subjected to 15% SDS-PAGE (bottom).
(B) A gel strip corresponding to lane 3 (0.5 mM ATP) of (A) was subjected to SDS-PAGE as a second dimension (2D-BN/SDS-PAGE).
(C) A gel strip corresponding to lane 5 (5 mM ATP) of (A) was analyzed as in (B). Radioactive signals in dried gels were detected by digital autoradiography. Molecular mass markers are ferritin (880 and 440 kD) and BSA (66 kD). TP, 10% of the [35S]pSSU translation product added to each reaction. The positions of the 1-MD translocation intermediate complex, free pSSU, and unassembled mSSU migrating in the low molecular mass range are indicated by brackets.
Figure 1B shows two-dimensional (2D)-BN/SDS-PAGE analysis of lane 3 (0.5 mM ATP) from Figure 1A. pSSU was found in two peaks: one was in the 1-MD area, demonstrating that pSSU was arrested in the 1-MD translocation complex, and the other was in the low molecular mass range, probably representing pSSU dissociated from the translocation machinery in the presence of detergent. Figure 1C shows 2D-BN/SDS-PAGE analysis of lane 5 (5 mM ATP) from Figure 1A. pSSU was also found in this 1-MD area. In addition, mSSU was observed in the low molecular mass range. Formation of the 1-MD translocation intermediate complex was maximal at 0.5 mM ATP (Figure 1A, top). Therefore, we decided to use this concentration to generate the translocation intermediate complex for subsequent experiments.
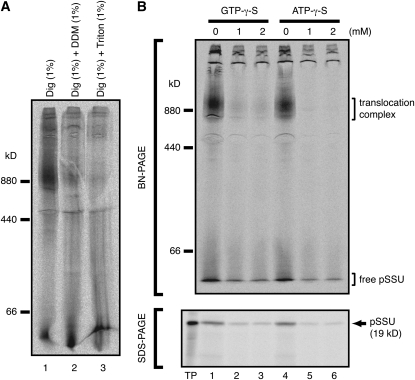
The 1-MD translocation complex was observed only when the mild detergent digitonin was used. All tested detergents except digitonin failed to preserve the 1-MD complex (i.e., 1% Triton X-100, 1% Nonidet P-40, 1% dodecyl maltoside, 1% decyl maltoside, or 1% octyl glucoside; data not shown). Moreover, addition of either dodecyl maltoside or Triton X-100 to the digitonin-solubilized BN-PAGE sample caused dissociation of pSSU from the 1-MD complex (Figure 2A). These observations suggest that pSSU is loosely associated with the 1-MD complex. The possibility that the association of pSSU occurred artificially after solubilization of the chloroplasts can be excluded because pSSU was efficiently associated with the 1-MD complex in the presence of ATP only when incubated with intact chloroplasts but not with solubilized chloroplast extract (see Supplemental Figure 1 online). A similar 1-MD translocation complex was also observed when the precursor of ferredoxin:NADP+ oxidoreductase was used (see Supplemental Figure 2 online).
Figure 2.
Characteristic Features of the 1-MD Translocation Complex.
(A) After pea chloroplasts carrying [35S]pSSU were solubilized with 1% digitonin (Dig), either dodecyl maltoside (DDM, lane 2) or Triton X-100 (lane 3) was added to give a final concentration of 1%. After incubation for 30 min on ice, samples were subjected to BN-PAGE.
(B) After pea chloroplasts were preincubated with the indicated concentrations of GTP-γ-S or ATP-γ-S for 10 min at 25°C in the dark, [35S]pSSU and ATP (final 0.5 mM) were added and incubated for another 10 min at 25°C in the dark. Reisolated chloroplasts were solubilized and subjected to BN-PAGE (top) and SDS-PAGE (bottom).
Formation of the 1-MD Translocation Complex Is Blocked by Slowly Hydrolyzable Analogs of GTP and ATP
It is well known that Toc34 and Toc159 have GTP binding motifs, and the binding of preproteins to the Toc complex is inhibited by non- or slowly hydrolyzable analogs of GTP (Young et al., 1999; Soll and Schleiff, 2004; Inoue and Akita, 2008; Jarvis, 2008; Kessler and Schnell, 2009). To test whether the 1-MD translocation intermediate complex is formed after translocation through the Toc complex via the general import pathway, we examined the effect of a slowly hydrolyzable analog of GTP (GTP-γ-S). Chloroplasts were preincubated with GTP-γ-S before addition of pSSU and ATP. GTP-γ-S drastically reduced the formation of the 1-MD complex (Figure 2B), suggesting that the 1-MD complex is formed after translocation through the well-defined Toc complex. In addition, by preincubating chloroplasts with ATP-γ-S, formation of the 1-MD complex was also drastically reduced (Figure 2B). This result shows that hydrolysis of ATP is also essential for the formation of the 1-MD complex.
The Preprotein Arrested in the 1-MD Translocation Complex Can Be Chased into Its Fully Translocated Mature Form
Chase experiments indicated that, in the absence of ATP, almost no import of pSSU, which had been arrested in the 1-MD translocation complex, was observed (Figure 3, top). In the presence of ATP (0.5 and 5 mM), the radiolabel in the 1-MD complex gradually decreased during the chase period, while radiolabeled ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco) holoenzyme found at the 520-kD position gradually increased (Figure 3, top). SDS-PAGE analysis also showed that pSSU gradually decreased, while fully imported mature SSU gradually increased (Figure 3, bottom). This means that pSSU arrested in the 1-MD complex was translocated, processed into its mature form, and further assembled into the 520-kD Rubisco holoenzyme in organello in the presence of higher concentrations of ATP. Since mature SSU contains only three of the six Met residues that are present in precursor SSU, the percentage of bound pSSU that was chased into mSSU under 5 mM ATP conditions for 30 min was estimated as 90%. The possibility that pSSU is associated with a stromal chaperonin can be excluded because addition of higher concentrations of ATP after solubilization of chloroplasts did not affect the signal intensity of the 1-MD complex (see Supplemental Figure 1 online). Therefore, we conclude that the radioactive 1-MD signal represents a chaseable genuine translocation intermediate complex.
Figure 3.
The Preprotein Arrested in the Translocation Intermediate Complex Can Be Chased into Its Fully Imported Mature Form under High ATP Concentrations.
The translocation intermediate was generated under 0.5-mM ATP as described in Methods. Reisolated pea chloroplasts carrying [35S]pSSU were resuspended in HS buffer containing the indicated concentrations of ATP, 5 mM MgCl2, 5 mM DTT, 3 mM Met, 3 mM Cys, and 5 μL/mL protease inhibitor cocktail on ice. Translocation (import) was resumed by transferring the reaction tubes to a water bath (25°C). The reactions were terminated by chilling and centrifuging the chloroplasts at the indicated time points. These samples were then solubilized and subjected to BN-PAGE (top) and SDS-PAGE (bottom). As a control, chloroplasts carrying preproteins were directly solubilized without the subsequent chase reaction (lane 1). The positions of the 1-MD translocation intermediate complex, the 520-kD Rubisco holoenzyme, free pSSU, and unassembled mSSU are indicated by brackets.
Protease Treatments Revealed That the Translocation Intermediate Complex Is Thermolysin Resistant and Partially Sensitive to Trypsin
To determine where the 1-MD translocation complex was accumulated in chloroplasts, we performed a selective proteolysis using exogenous thermolysin and trypsin. Thermolysin is an outer envelope–impermeable protease that selectively digests chloroplast surface-exposed proteins, whereas a certain concentration of trypsin is able to destroy the membrane integrity of the outer envelope and partially digest proteins within the intermembrane space while leaving the stromal proteins undigested (Kouranov et al., 1998; Hirohashi et al., 2001). Toc159, which has a large cytosolically exposed domain, was easily degraded by thermolysin and trypsin, as demonstrated by the resultant 52-kD protease-resistant fragment (Figure 4B). Toc34, which also has a cytosolically exposed domain, was resistant to up to 10 μg/mL of thermolysin (Chen et al., 2000) but was degraded by 100 μg/mL of thermolysin and trypsin. Toc75, an integral membrane protein, was largely resistant to thermolysin and trypsin. Tic22, which is known to reside in the intermembrane space (Kouranov et al., 1998), was resistant to thermolysin but sensitive to trypsin. Stromal Hsp70 and Tic110, an inner envelope membrane protein that is oriented toward the stroma (Jackson et al., 1998; Inaba et al., 2003), were completely resistant to both thermolysin and trypsin (Figure 4B).
Figure 4.
Protease Treatments of the Chloroplast Surface Reveal That the 1-MD Translocation Complex Is Resistant to Thermolysin but Partially Sensitive to Trypsin.
(A) to (E) The translocation intermediate was generated under 0.5-mM ATP as described in Methods. The pea chloroplast suspension was divided into six aliquots (lanes 1 to 6) and washed twice with HS buffer in the absence of protease inhibitor cocktail. Chloroplasts carrying [35S]pSSU were treated with the indicated concentrations of thermolysin (Thl) or trypsin (Trp) for 20 min on ice. After inactivation of the proteases, the chloroplast pellet was solubilized and subjected to BN-PAGE ([A], top) and SDS-PAGE ([A], bottom). When using trypsin, trypsin inhibitor was added to BN-PAGE sample buffer.
(B) Aliquots of the same samples in (A) were subjected to SDS-PAGE and immunoblotting with antibodies against indicated proteins. Arrows indicate intact Toc159, 86- and 52-kD fragments of Toc159, and Tic22. An asterisk denotes a nonspecific cross-reacting band.
(C) A gel strip corresponding to lane 1 (without protease) of (A) was subjected to 2D-BN/SDS-PAGE.
(D) A gel strip corresponding to lane 3 (with thermolysin) of (A) was analyzed as in (C).
(E) A gel strip corresponding to lane 6 (with trypsin) of (A) was analyzed as in (C). The three excised first-dimension BN-PAGE gel strips used in (C) to (E) were derived from the same first-dimension BN-PAGE gel. The positions of the 1-MD translocation intermediate complex, a partially degraded translocation intermediate complex, free pSSU, unassembled mSSU, and dissociated SSU-DP are indicated by brackets. mSSU assembled into the 520-kD Rubisco holoenzyme is indicated by arrows ([C] to [E]).
The 1-MD complex was largely resistant to exogenous thermolysin (Figure 4A, top, lanes 2 and 3). In SDS-PAGE, degradation products of SSU (SSU-DPs) were observed (Figure 4A, bottom, lanes 2 and 3), indicating that the C-terminal tail of pSSU, which was exposed to the surface of chloroplasts, was digested by exogenous thermolysin, while the N-terminal part of pSSU, which was probably deeply buried in a translocation channel, was resistant to proteolysis. Furthermore, 2D-BN/SDS-PAGE of lane 3 from Figure 4A confirmed that the radioactive signals in the 1-MD area remaining after thermolysin treatment were derived mostly from SSU-DP (Figure 4D). When chloroplasts carrying pSSU were treated with 100 μg/mL trypsin, the radiolabeled complex shifted slightly to a lower molecular mass on BN-PAGE (Figure 4A, top, lane 6), indicating partial degradation of the 1-MD complex. SSU-DPs were also found in the trypsin-treated samples by SDS-PAGE (Figure 4A, bottom, lanes 5 and 6). Figure 4E shows 2D-BN/SDS-PAGE of lane 6 from Figure 4A, confirming that the radioactive signals in the shifted complex were also mostly derived from SSU-DP.
As we have shown previously, the intact Toc complex is 800 to 1000 kD on BN-PAGE (Kikuchi et al., 2006). As a result, we initially suspected that the observed radiolabeled 1-MD complex corresponded to the Toc complex itself due to their almost identical sizes. Contrary to our expectations, the 1-MD complex shown here was resistant to thermolysin. This observation was inconsistent with the protease accessibilities of the Toc complex. When chloroplasts were isolated in the absence of protease inhibitor cocktail, the Toc complex became 350 to 500 kD in size because the cytosolically exposed A-domain of Toc159 was degraded by endogenous proteases during isolation of chloroplasts (Kikuchi et al., 2006; see Supplemental Figure 3 online, lane 5). Irrespective of the presence or absence of protease inhibitor cocktail during the isolation procedure, the radiolabeled 1-MD translocation complex was generated at nearly the same levels (see Supplemental Figure 3 online, top, lanes 1 and 3). Furthermore, as described above, while the 1-MD complex remained resistant by thermolysin treatment, the Toc complex was degraded to a 250- to 350-kD subcomplex in the same samples (lanes 6 and 8). Therefore, we conclude that the 1-MD complex does not correspond to the Toc complex itself. More importantly, the observed partial degradation of the translocation complex after trypsin treatment suggests that the 1-MD complex has an intermembrane space-facing domain.
The Translocation Intermediate Appears to Be Accumulated in the Inner Envelope Membrane
To determine where [35S]pSSU was accumulated in chloroplasts, we separated envelope membrane vesicles into fractions enriched in outer envelope membrane vesicles and inner envelope membrane vesicles using sucrose density gradient centrifugation (see Supplemental Figure 4 online). pSSU was found mainly in the mixed outer-inner membrane fraction (fractions 4 and 5). A very minor portion of pSSU found in the outer membrane fraction (fraction 8) may represent pSSU bound to Toc complex that was not associated with contact sites. Thermolysin-resistant SSU-DP was found exclusively in the mixed outer-inner membrane fraction but not in the outer membrane fraction. These observations suggest that the 1-MD complex in which the N-terminal region of pSSU is stably associated resides in the inner membrane rather than in the outer membrane.
Tic21 Is Involved in the 1-MD Translocation Complex
To identify which components are involved in the 1-MD complex, we screened antisera against known Toc and Tic proteins by antibody-shift BN-PAGE. In antibody-shift BN-PAGE, chloroplasts carrying pSSU were first solubilized in digitonin-containing buffer, followed by the addition of antibodies. The specific binding of antibodies to a subunit(s) within the complex was predicted to result in a shift of the complex to a higher molecular mass on BN-PAGE. This assay was used in several reports (e.g., Johnston et al., 2002; Truscott et al., 2002). Arabidopsis chloroplasts were used in addition to pea chloroplasts for the assay because some of the antibodies used were raised against Arabidopsis antigens. We confirmed that incubation of Arabidopsis chloroplasts with pSSU and ATP resulted in a virtually identical translocation intermediate complex to that of the pea complex (see Supplemental Figure 5 online). By thorough screening of antisera, we found that an antibody against Arabidopsis Tic21 caused a significant shift in this assay when Arabidopsis chloroplasts were used (Figure 5A, lane 2). Tic21 was recently identified by Arabidopsis genetics (Teng et al., 2006). When antibodies against pea Tic110 and SPA-820 monoclonal antibody (StressGen), which recognizes an as yet unidentified intermembrane space Hsp70 (Schnell et al., 1994; Becker et al., 2004), were added, the mobility of the 1-MD complex on BN-PAGE was not affected (Figure 5A). All other antibodies, which we tested, against known translocon components failed to shift the 1-MD complex by antibody-shift BN-PAGE (i.e., antibodies against Toc159, Toc34, Tic40, Tic22, Cpn60α, Cpn60β, and stromal Hsp70) (see Supplemental Figure 6 online). These results indicate that at least Tic21 is involved in the 1-MD complex.
Figure 5.
Antibody-Shift BN-PAGE and Immunodepletion Experiments.
The translocation intermediate was generated using Arabidopsis chloroplasts by the same method described in Methods.
(A) The digitonin-solubilized chloroplastic extract containing [35S]translocation intermediate complex was mixed with purified anti-At Tic21 (8 μg), anti-Ps Tic110 (5 μg), or monoclonal SPA-820 (5 μg) antibodies and incubated for 30 min on ice. Samples were subjected to BN-PAGE and digital autoradiography. As a control, the solubilized chloroplastic extract without any antibody addition was applied (lane 1). The positions of a shifted complex, the 1-MD translocation intermediate complex, free pSSU, and unassembled mSSU are indicated by brackets.
(B) The digitonin-solubilized chloroplastic extract containing [35S]translocation intermediate complex was immunodepleted with anti-Ps Tic110 antibody that had been bound to rProtein A-Sepharose. The immunodepleted fractions were subjected to BN-PAGE and autoradiography (lanes 1 and 2). The same samples were subjected to BN-PAGE and immunoblotting with anti-Ps Tic110 antibody (lanes 3 and 4). The native Tic110 complex is indicated. An asterisk denotes a nonspecific cross-reacting band corresponding to the native Rubisco complex, which migrates in large quantities at this position.
The inability of anti-Tic110 antibody to shift the 1-MD translocation complex suggests that Tic110 is not involved in the 1-MD complex. To establish the absence of Tic110 in the 1-MD complex, we removed Tic110 proteins from the solubilized Arabidopsis chloroplast extract containing the translocation intermediate by immunodepletion with anti-Tic110 antibody bound to rProtein A-Sepharose. BN-PAGE followed by immunoblotting of the protein complexes remaining in the supernatant showed almost complete depletion of a 200- to 300-kD Tic110 complex (Figure 5B, lane 4). By contrast, the amount of radiolabeled 1-MD complex was not affected (Figure 5B, compare lanes 1 and 2). The size of the Tic110 complex that migrated in the 200- to 300-kD range is well consistent with previous reports (Caliebe et al., 1997; Küchler et al., 2002; Kikuchi et al., 2006). Based on observations of antibody-shift BN-PAGE and immunodepletion together with the substantial size difference between the 1-MD complex and the 200- to 300-kD Tic110 complex, we conclude that Tic110 is not involved in the 1-MD translocation complex.
We also performed immunodepletion with anti-Tic40, -Hsp93, and -Toc159 antibodies. Immunoblot analyses showed almost complete depletion of these proteins from digitonin-solubilized extract (see Supplemental Figure 7 online), demonstrating the binding ability of these antibodies to respective solubilized proteins. Nevertheless, these antibodies were unable to shift the 1-MD complex. Therefore, we think that not only Tic110 but also Tic40, Hsp93, and Toc159 are not involved in the 1-MD translocation complex. We were unable to conclude, at this stage, whether Tic20 is involved in the 1-MD complex either by antibody-shift BN-PAGE or by immunodepletion, since all available Tic20 antisera appear not to recognize solubilized native Tic20 proteins (see below).
Tic20 and a Minor Population of Tic21 Migrated at 1 MD on BN-PAGE
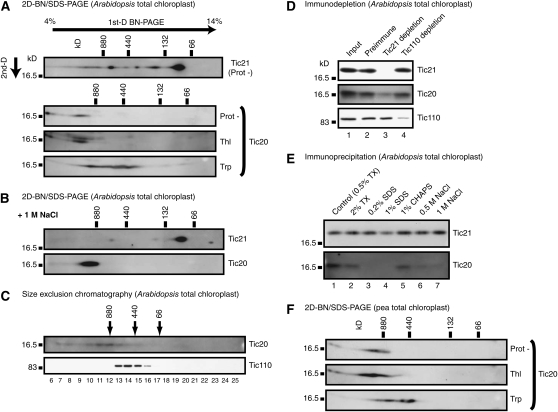
We analyzed the size of native Tic21-containing complex in Arabidopsis chloroplasts by BN-PAGE. Immunoblotting with anti-Tic21 antibody identified several populations of the Tic21 complexes (Figure 6A, top). The majority of Tic21 was found in the 100-kD area and thus is not present in the 1-MD complex. However, a minor population of Tic21 was found in the 1 MD area, which supports the hypothesis that Tic21 is involved in the 1-MD translocation complex.
Figure 6.
Two-Dimensional BN/SDS-PAGE Analyses and Size Exclusion Chromatography of Tic Proteins.
(A) Intact Arabidopsis chloroplasts were treated with 100 μg/mL thermolysin (Thl), 100 μg/mL trypsin (Trp), or without proteases (Prot-) for 20 min on ice. Reisolated chloroplasts were solubilized in BN-PAGE sample buffer (containing 1% digitonin) and subjected to 2D-BN/SDS-PAGE followed by immunoblotting with anti-At Tic21 or anti-At Tic20 ES antibodies.
(B) Arabidopsis chloroplasts were solubilized in 1 M NaCl-containing BN-PAGE sample buffer (1% [w/v] water-soluble digitonin, 50 mM BisTris-HCl, pH 7.0, 500 mM 6-amino-n-caproic acid, 1 M NaCl, 10% [w/v] glycerol, and 10 μL/mL protease inhibitor cocktail) and subjected to 2D-BN/SDS-PAGE. Immunoblotting was performed as in (A).
(C) Arabidopsis chloroplasts were solubilized with 1% digitonin and subjected to size exclusion chromatography on a Superose 6 column equilibrated with 0.1% digitonin, 50 mM HEPES-KOH, pH 7.5, and 150 mM NaCl. Immunoblotting was performed with anti-At Tic20 ES or anti-Ps Tic110 antibodies. Molecular mass markers are ferritin (880 and 440 kD) and BSA (132 and 66 kD).
(D) The digitonin (1%)-solubilized Arabidopsis chloroplastic extract was immunodepleted with anti-At Tic21 or anti-Ps Tic110 antibodies that had been bound to rProtein A-Sepharose. The immunodepleted fractions were subjected to SDS-PAGE and immunoblotting with anti-At Tic21, -At Tic20 ES, or -Ps Tic110 antibodies.
(E) The dodecyl maltoside (1%)-solubilized Arabidopsis chloroplastic extract was immunoprecipitated with anti-At Tic21 antibody that had been cross-linked to rProtein A-Sepharose by dimethyl pimelimidate. After the beads were washed with 0.5% Triton X-100 in TBS (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 10% [w/v] glycerol), the beads were further washed with various stringent conditions (2% Triton X-100 [TX], 0.2% SDS, 1% SDS, 1% CHAPS, 0.5 M NaCl, or 1 M NaCl; all reagents were in TBS). The proteins remained associated with the beads were eluted with 0.1 M glycine, pH 2.5, and 0.5% Triton X-100 and subjected to SDS-PAGE followed by immunoblotting with anti-At Tic21 or -At Tic20 ES antibodies.
(F) Experiments were performed as in (A), except that pea chloroplasts were used.
We next analyzed the size of native Tic20-containing complex since Tic20 is proposed to form the inner membrane translocation channel. Almost all Tic20 proteins were found in the 1-MD area (Figure 6A, second). Although the Tic20 protein itself was largely resistant to both thermolysin and trypsin, the Tic20 complex was resistant to thermolysin but partially sensitive to trypsin (Figure 6A, bottom two panels), suggesting that some other subunit(s) in the Tic20 complex has an intermembrane space-facing domain. The BN-PAGE profile of the 1-MD Tic20 complex and its protease accessibility were very similar to those of the 1-MD translocation complex shown in Figure 4. We also analyzed the pea Tic20 complex by the same procedure and obtained almost identical results (Figure 6F). In addition, by size exclusion chromatography on a Superose 6 column, Tic20 was found at, approximately, the 1-MD position, whereas Tic110 was not cofractionated with Tic20 (Figure 6C), consistent with the above observations using BN-PAGE.
Immunodepletion Experiments Suggest That Tic20 Is Associated with Tic21
We next investigated if there are direct interactions among Tic21, Tic20, and Tic110. In Tic21-depleted extract, the majority of Tic20 was depleted, while the amount of Tic110 was not affected (Figure 6D). By contrast, neither Tic21 nor Tic20 was reduced in Tic110-depleted extract. These results suggest that Tic21 and Tic20 are associated and form the 1-MD complex under steady state conditions but that Tic110 does not form any detectable complex with Tic20 or Tic21 after solubilization with digitonin.
Tic20 Is a Core Component of the 1-MD Complex, whereas Tic21 Appears to Be Loosely Associated with the Complex
Next, we looked for conditions in which Tic21 protein(s) are dissociated from the Tic20 complex. After the Tic21-containing Tic20 complex was immunoprecipitated with anti-At Tic21 antibody-immobilized (cross-linked) beads, the beads were incubated under various severe conditions to disrupt the interaction between Tic21 and the Tic20 complex. Incubation of this immunoprecipitated complex under high salt conditions resulted in a significant loss of Tic20 from the complex, while Tic21 remained associated with the beads (Figure 6E, lanes 6 and 7). Similar results were obtained when the immunoprecipitated complex was incubated with SDS (lanes 3 and 4). By contrast, in the presence of stringent detergent conditions of 2% Triton X-100 and 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), approximately half of the Tic20 subcomplex remained associated with the beads via Tic21 (lanes 2 and 5).
Then, we asked how the 1-MD complex containing Tic20 and Tic21 was influenced by treatment with high salt. To this end, we performed 2D-BN/SDS-PAGE analysis of Arabidopsis chloroplasts after solubilization with digitonin in the presence of 1 M NaCl. Almost all Tic21 was recovered in the 100-kD area, and a minor population of Tic21 found in the 1-MD area in the absence of NaCl disappeared (cf. Figures 6A with 6B, top panels). Correspondingly, the size of the Tic20 complex appeared to be slightly shifted to a lower molecular mass range (cf. Figures 6A, second panel, with 6B, bottom panel). Notably, Tic20 proteins that appeared as a smeared band around 1 MD and larger in the absence of NaCl migrated as an intense spot in the presence of 1 M NaCl. This observation suggests that Tic21 was dissociated from the Tic20 complex under high salt conditions, and the size of the Tic20 complex was decreased slightly because of the removal of Tic21 protein(s) and other potential unidentified protein(s). More importantly, this observation suggests that Tic20 exists as a core component of the 1-MD complex, whereas Tic21 is loosely associated with the 1-MD complex.
Both the tic20 and tic21 Mutants Were Defective in Plastid Protein Import
There are two conflicting reports about the function of Tic21. Teng et al. (2006) identified Tic21 as a preprotein translocon at the inner envelope membrane, whereas Duy et al. (2007) independently identified the same protein as an iron transporter (PERMEASE IN CHLOROPLASTS1 [PIC1]). To clarify these controversial findings, we employed two independent experimental approaches. First, we performed transient expression and targeting of preproteins using Arabidopsis knockout mutants. Because both homozygous tic21/pic1 and tic20 mutants show severe albino phenotypes (Teng et al., 2006), standard import experiments using isolated plastids are difficult. Therefore, we used transient expression system in protoplasts (Jin et al., 2001; Lee et al., 2008). In this system, plasmids encoding a fusion protein consisting of the N-terminal transit peptide (79 amino acids) of the small subunit of Rubisco and green fluorescent protein (RbcS-nt:GFP) or a fusion protein consisting of the N-terminal transit peptide (80 amino acids) of the pyruvate dehydrogenase E1α subunit and GFP (E1α-nt:GFP) were introduced into protoplasts by polyethylene glycol–mediated transformation (Jin et al., 2001). RbcS-nt:GFP was used as a model for photosynthetic proteins, whereas E1α-nt:GFP was used as a model for nonphotosynthetic proteins (Smith et al., 2004). Protein import into plastids was assessed by immunoblotting with anti-GFP antibody using protein extracts prepared from transformed protoplasts (Lee et al., 2008).
In the wild-type protoplasts, the majority of RbcS-nt:GFP and E1α-nt:GFP were found in processed forms (Figure 7, lanes 1 and 2), indicating that the transiently expressed preproteins were efficiently imported into wild-type plastids. By contrast, approximately half of RbcS-nt:GFP remained unprocessed in tic21/pic1 and tic20 mutants (lanes 3 and 5), indicating the significant impairments in the protein import of photosynthetic proteins in these mutants. On the other hand, the majority of E1α-nt:GFP was imported into plastids in the tic21/pic1 and tic20 mutants, although slightly less efficient than the wild type (lanes 4 and 6). This observation suggests that some nonphotosynthetic preproteins are normally imported into plastids in spite of the lack of Tic21/PIC1 and Tic20. Indeed, stromal Hsp70 and thylakoidal Albino3, which are nonphotosynthetic and housekeeping proteins, were accumulated normally in the tic21/pic1 and tic20 mutants, while accumulation of photosynthetic proteins were severely affected (Teng et al., 2006; Duy et al., 2007; see Supplemental Figure 8 online). As a control experiment, another albino mutant, albino3, was used for the transient expression assay. Albino3 is a thylakoidal membrane protein and unrelated to protein transport across the envelope (Sundberg et al., 1997; Asakura et al., 2008). In the albino3 protoplasts, both RbcS-nt:GFP and E1α-nt:GFP were found in their processed form (see Supplemental Figure 9 online, lanes 3 and 4), suggesting that the observed impairments of the import of RbcS-nt:GFP are specific to the tic21/pic1 and tic20 mutants but not common in other albino mutants. From these results, we propose that both Tic21/PIC1 and Tic20 play critical roles in import of photosynthetic proteins into chloroplasts (see Discussion for details).
Figure 7.
Protein Import into Plastids Analyzed by Transient Expression in Protoplasts.
Protoplasts isolated from Arabidopsis wild-type, homozygous tic21/pic1-1 (SALK_104852), and tic20 (SALK_039676) mutants were transformed with RbcS-nt:GFP or E1α-nt:GFP. After 8 h of incubation at 22°C, proteins were extracted from transformed protoplasts and subjected to SDS-PAGE followed by immunoblotting with anti-GFP antibody. RbcS-nt, the N-terminal transit peptide of the small subunit of Rubisco; E1α-nt, the N-terminal transit peptide of the pyruvate dehydrogenase E1α subunit.
Upregulation of Iron Homeostasis Proteins Are Common in Some Albino Mutants
In a second approach, we examined expression levels of iron homeostasis proteins in the tic21/pic1 mutant and other albino mutants. One reason that Duy et al. (2007) termed Tic21/PIC1 as an iron transporter is based on the observation that genes related to iron homeostasis are upregulated in the tic21/pic1 mutant. Supplemental Figure 8 online shows immunoblot analyses of several plastid-localized proteins in the tic21/pic1, tic20, and albino3 mutants, all of which show albino phenotypes. As reported by Duy et al. (2007), in the tic21/pic1 mutant, upregulation of ferritin and copper superoxide dismutase 1 (CSD1) and CSD2, which are related to iron homeostasis in plastids, was reproduced. However, this upregulation was also observed in other albino mutants, tic20 and albino3. This means that the upregulation of these proteins is not specific to the tic21/pic1 mutant but is seen in other albino mutants.
Chemical Cross-Linking of the Preprotein to Tic20
To obtain direct evidence that Tic20 associates with the preprotein in the translocation intermediate, chemical cross-linking of the preprotein to translocon components was performed. In this experiment, a fusion preprotein pSSU-DHFR, which consists of full-length pSSU and dihydrofolate reductase (DHFR), was used because it produced a much higher amount of cross-linked products than pSSU. We then selected m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS) as a noncleavable cross-linker after screening of more than a dozen reagents. Arabidopsis chloroplasts were incubated with pSSU-DHFR under either low or high ATP conditions. After reisolation, chloroplasts were cross-linked with MBS followed by SDS-PAGE and autoradiography. Several cross-linked products were observed, and the relative amounts of these products varied between low and high ATP conditions (e.g., a 48-kD and a 100-kD band were prominent under low ATP conditions, whereas a 140-kD band was prominent under high ATP conditions) (Figure 8A, compare lanes 2 and 3). This indicates dynamic changes of association partners in the translocation event.
Figure 8.
Chemical Cross-Linking of a Preprotein to Toc/Tic Proteins.
(A) Energy-depleted Arabidopsis chloroplasts were incubated with [35S]pSSU-DHFR under either low ATP concentrations (100 μM) or high ATP concentrations (4 mM) in HS buffer containing 5 mM MgCl2, 5 mM DTT, 3 mM Met, 3 mM Cys, 5 μL/mL protease inhibitor cocktail, and 20 μM methotrexate (MTX) for 10 min at 25°C in the dark. The DHFR fusion protein, pSSU-DHFR, was used in this experiment instead of pSSU. Reisolated chloroplasts carrying [35S]pSSU-DHFR, at 0.24 mg chlorophyll/mL in HS buffer in the presence of 20 μM MTX, were treated with (lanes 2 and 3) or without (lane 1) 0.2 mM MBS. Noncleavable cross-linked products were subjected to 7.5% SDS-PAGE and autoradiography. Single asterisks indicate cross-linked products of pSSU-DHFR with Toc75 or Tic20. The double asterisk indicates a cross-linked product with unidentified protein.
(B) The translocation intermediate was generated under low ATP concentrations and cross-linked with 0.2 mM MBS as in (A). Chloroplasts carrying cross-linked products were solubilized under denaturing conditions containing 2% SDS. The resulting extract was diluted 18.5-fold with 0.5% Triton X-100 in TBS followed by immunoprecipitation with anti-At Tic20 FL (lane 3) or anti-Ps Toc75 (lane 4) antibodies or with preimmune serum (lane 2). The eluates were subjected to 7.5% SDS-PAGE and autoradiography. Input represents 30% of the material used for immunoprecipitation (lane 1). The region marked with a diamond probably represents cross-linked products containing at least one Toc75 molecule.
(C) The translocation intermediate was generated under either low ATP concentrations (lanes 1 to 5) or high ATP concentrations (lanes 6 to 9) as in (A). Cross-linking was performed with 0.2 mM DSP, which is cleavable with reducing agents. Chloroplasts carrying cross-linked products were solubilized under denaturing conditions containing 2% SDS. The resulting extract was diluted 18.5-fold with 0.5% Triton X-100 in TBS followed by immunoprecipitation with anti-At Tic20 FL (lanes 3 and 7), anti-Ps Toc75 (lanes 4 and 8), or anti-Ps Tic110 (lanes 5 and 9) antibodies or with preimmune serum (lane 2). Bound proteins were eluted by boiling in 2× Laemmli sample buffer containing 10% 2-mercaptoethanol and subjected to 12.5% SDS-PAGE and autoradiography. Input represents 30% of the material used for immunoprecipitation (lanes 1 and 6).
To examine whether Tic20 is cross-linked with the preprotein, immunoprecipitation was performed after denaturation with SDS. All four anti-At Tic20 antisera which we prepared and raised in different rabbits can recognize denatured At Tic20 antigens, but are unable to immunoprecipitate or immunodeplete native Tic20 proteins. The most probable explanation for this is that Tic20 is deeply embedded within its large complex and does not have surface-exposed epitopes. Therefore, cross-linked products were denatured with 2% SDS to dissociate the 1-MD Tic20 complexes and allow Tic20 to be immunoprecipitated. Anti-At Tic20 antibody immunoprecipitated the single 48-kD cross-linked band (Figure 8B, lane 3), demonstrating a direct interaction between the preprotein and Tic20. Please note that a slight shift of this 48-kD band to a lower molecular mass was caused by an overlap of IgG that migrated in large quantities at this position (cf. lanes 1 and 3).
We also performed immunoprecipitations using several Toc and Tic antibodies. Immunoprecipitation with anti-Toc75 antibody gave a strong band at 100 kD, which most likely represents a 1:1 cross-linked product with Toc75, and smeared bands of >120 kD, which probably represent products including at least one molecule of Toc75 (lane 4). Immunoprecipitation with anti-At Tic21 antibody did not detect any specific band (data not shown), probably because Tic21 is not positioned close to the preprotein-translocating channel. Also, immunoprecipitation with anti-Tic110 antibody after MBS cross-linking did not detect any specific band (see Supplemental Figure 10 online). Almost all major cross-linked bands except a 55-kD band observed under low ATP conditions were immunoprecipitated with either anti-Toc75 or -Tic20 antibodies, strongly suggesting that Toc75 and Tic20 are the proteins in the closest contact with the translocating preprotein.
Moreover, we used the cleavable cross-linker dithiobis(succinimidyl propionate) (DSP), which has been successfully used to cross-link preproteins to Toc and Tic proteins (Akita et al., 1997; Chou et al., 2003). Consistent with the results of MBS cross-linking, anti-Toc75 and -Tic20 antibodies immunoprecipitated pSSU-DHFR more efficiently under low ATP conditions than under high ATP conditions (Figure 8C, lanes 3, 4, 7, and 8). In addition, immunoprecipitation with anti-Tic110 antibody was able to capture a small amount of pSSU-DHFR under low ATP conditions (lane 5) and both pSSU-DHFR and mSSU-DHFR under high ATP conditions (lane 9), which are consistent with the results of Chou et al. (2003).
DISCUSSION
We report the identification of a 1-MD translocation complex as an intermediate during preprotein import into chloroplasts. Characteristic features of the 1-MD translocation complex are summarized as follows. (1) This complex is formed under limited ATP conditions and can be detected by BN-PAGE with the mild detergent digitonin. (2) Preproteins arrested in this complex can be chased under high ATP conditions. (3) Protease accessibility assays and sucrose density gradient centrifugation revealed that this complex resides in the inner membrane. (4) Tic20 forms a 1-MD complex with a minor population of Tic21 at the inner membrane under steady state conditions, and this complex most likely corresponds to the 1-MD translocation complex.
All tested detergents except digitonin failed to preserve the 1-MD translocation complex (Figure 2A; data not shown), suggesting a loose association between the preprotein and the translocation complex. This feature may have impeded previous identification of the 1-MD translocation complex.
Although the 1-MD translocation complex described in this study was derived from the inner membrane, protease treatments shown in Figure 4 revealed that the C-terminal tail of the preprotein was exposed to the surface of chloroplasts, suggesting that the arrested preprotein spans both Toc and Tic complexes. Indeed, in the presence of cross-linkers, the preprotein was cross-linked with Toc75 and Tic20 (Figure 8). On BN-PAGE, high concentrations of Coomassie blue (0.125%), which has an anionic feature, was added to the detergent-solubilized protein complexes, potentially causing dissociation of loosely associated proteins (Schägger and Pfeiffer, 2000; Gavin et al., 2003; Wittig and Schägger, 2005). It is highly probable that the Toc complex was dissociated from the preprotein under the conditions of BN-PAGE, whereas the Tic complex remained associated with the preprotein during BN-PAGE and was observable as the 1-MD complex. Considering unidirectional protein transport from the outer membrane to the inner membrane, it seems reasonable that the translocating preprotein at this stage binds more tightly to the Tic complex than to the Toc complex. When chemical cross-linking was performed to stabilize the associations of Toc and Tic proteins prior to solubilization, the 1-MD translocation complex was shifted to a higher molecular mass range around 1.4 to 1.8 MD on BN-PAGE, which most likely corresponds to a Toc-Tic supercomplex (see Supplemental Figure 11 online).
The 1-MD translocation complex characterized in this study is neither the Toc complex, to which preproteins initially bind, nor the Tic110-containing complex, which should mediate a later translocation step on the stromal side. We propose that the 1-MD translocation complex functions in between the Toc- and Tic110-containing complexes. Tic110 and Hsp93 migrated in the range of 200 to 300 kD on BN-PAGE (see Supplemental Figure 7 online; Caliebe et al., 1997; Küchler et al., 2002), and Tic22 migrated at a low molecular mass (<66 kD) (data not shown), supporting the idea that these Tic components are not involved in the 1-MD translocation complex. Figure 8C shows that Toc75 is preferentially associated with the precursor form of the preprotein, whereas Tic110 is associated with both the precursor and mature forms of the preprotein, consistent with the results of Chou et al. (2003). Meanwhile, Tic20 is preferentially associated with the precursor form of the preprotein, supporting the idea that the Tic20-containing 1-MD complex functions in between the Toc- and Tic110-containing complexes. The preprotein arrested in the 1-MD complex would be transferred to the Tic110/Hsp93 complex in a subsequent step, where the transit peptide would be processed.
By analogy with mitochondrial import machinery, it is often stated that Tic110 is an analogous component to Tim44 (Bédard and Jarvis, 2005), which serves as a binding site for matrix Hsp70. The situation that the 1-MD complex containing Tic20 and Tic21 holds preproteins in the absence of Tic110 is very similar to that of the mitochondrial import since a Tim core complex containing Tim23 and Tim17 can hold preproteins in the absence of Tim44 in digitonin extracts (Dekker et al., 1997). It should be noted that, despite the absence of any significant sequence similarities, Tic20 and Tic21, and mitochondrial Tim23 and Tim17, all have similar molecular weights and contain three or four predicted transmembrane domains, suggesting functional similarity.
We have observed the protease-resistant fragment SSU-DPs arrested in the 1-MD complex (Figure 4). Earlier studies have depicted similar protease-resistant fragments (Friedman and Keegstra, 1989; Waegemann and Soll, 1991, 1993; Chigri et al., 2005; Inoue and Akita, 2008). Protease-resistant fragments are called deg and classified into deg1, deg2, deg3, or deg4 based on different molecular sizes, from the largest (deg1) to the smallest (deg4). deg1 and deg2 were shown to cofractionate with the outer membrane, whereas deg3 and deg4, which would correspond to SSU-DPs in this study, were shown to cofractionate with the inner membrane (Waegemann and Soll, 1993; Soll and Tien, 1998), supporting our observations.
Akita et al. (1997) have shown that a translocation intermediate complex of ∼600 kD could be isolated using chemical cross-linkers. They generated the intermediate under incubation conditions on ice for 20 min in the presence of 75 μM ATP, whereas we incubated at 25°C for 10 min in the presence of 0.5 mM ATP. Under their conditions, an early intermediate is predicted to be formed; therefore, the 600-kD complex most likely corresponds to the Toc subcomplex (II) in our previous study (Kikuchi et al., 2006). In addition, Chen and Li (2007) have recently shown that two intermediate complexes of ∼880 and 1320 kD, which were referred to as C1 and C2, respectively, were observed by BN-PAGE. They concluded that both C1 and C2 contained the Toc complex, while C2 additionally contained Tic110, Hsp93, and the intermembrane space Hsp70. There are significant experimental differences between their study and ours. After the import reaction at 20°C for 20 min, they first performed chemical cross-linking to preserve the translocation intermediate complexes. Then, isolated total membranes were solubilized with the more stringent detergent decyl maltoside and fractionated by sucrose density gradient centrifugation. C1 most likely corresponds to the intact Toc complex characterized in our previous study (Kikuchi et al., 2006). C2 likely corresponds to the Toc-Tic supercomplex shown in Supplemental Figure 11 online. However, the involvement of Tic21 and/or Tic20 in formation of C2 was not analyzed. Moreover, since there are significant experimental differences, some Tic proteins may be added to or removed from the C2 complex.
Kouranov and Schnell (1997) have shown that the association of the preprotein with Tic20 was increased in the presence of high ATP (2 mM) compared with low ATP (0.1 mM). By contrast, the cross-linked product between the preprotein and Tic20 was more prominent in low ATP (0.1 mM) than in high ATP (4 mM) in this study (Figure 8A). This difference can be explained by the following reasons. In the study by Kouranov and Schnell (1997), they used a urea-denatured preprotein that had been overexpressed in E. coli and purified from inclusion bodies. By contrast, we used in vitro–translated soluble preproteins. In addition, the preprotein used by Kouranov and Schnell contained an IgG binding domain of Protein A at the C terminus. Perhaps this are why the preprotein they used seems to require more ATP to be unfolded and reach the Tic20-containing complex than that required for the in vitro–synthesized preprotein used in this study.
There are two conflicting reports about the function of Tic21, which has been reported to be a component of the protein import machinery at the inner envelope membrane (Teng et al., 2006) or an iron transporter (Duy et al., 2007). To clarify the function of Tic21, we performed transient expression and targeting of preproteins in mutant protoplasts and compared expression levels of metal homeostasis proteins in mutants. Figure 7 shows a defect in protein import of photosynthetic proteins in the tic21/pic1 mutant, which is comparable to that in the tic20 mutant. Supplemental Figure 8 online shows that upregulation of ferritin, CSD1, and CSD2, all of which are iron homeostasis-related proteins, is not specific to the tic21/pic1 mutant but is seen in other albino mutants. These observations support the proposal by Teng et al. (2006) that Tic21/PIC1 functions in chloroplast protein import.
The results shown in Figures 6A and 6B suggest that Tic21 is not a central component of the 1-MD translocation complex but loosely associated component of the complex. Nevertheless, the lack of Tic21 causes severe defects in chloroplast protein import, similar to those observed in the tic20 mutant. Preliminary analysis of the Tic20 complex in the tic21 mutant by 2D-BN/SDS-PAGE showed that the Tic20 complex of the tic21 mutant did not migrate at the same position as that of the wild type but accumulated at the top of the separation gel in BN-PAGE (S. Kikuchi and M. Nakai, unpublished data), suggesting that an improper assembly or an aggregation of the Tic20 complex probably occurs in the tic21 mutant. Tic21 may function in the proper assembly of the Tic20 complex. This hypothesis can explain similar severe albino phenotypes of the tic20 and tic21 mutants (Teng et al., 2006).
Although the tic20 and tic21 mutants show severe albino phenotypes and are seedling lethal, they are able to produce albino leaves and occasionally inflorescence tissues on synthetic media supplemented with sucrose. This suggests residual import capacities in the tic20 and tic21 mutants. As shown in Figure 7, import defects in the tic20 and tic21 mutants were clearly observed using photosynthetic preprotein (RbcS-nt:GFP). However, interestingly, less severe import defects were observed using nonphotosynthetic preprotein (E1α-nt:GFP). Indeed, stromal Hsp70, ferritin, and thylakoidal Albino3, which are nonphotosynthetic and housekeeping proteins, accumulated normally in the plastids of the tic20 and tic21 mutants (see Supplemental Figure 8 online). From these observations, we propose that Tic20 and Tic21 have substrate specificity for photosynthetic preproteins. The phenotypes of the tic20 and tic21 mutants are very similar to that of an Arabidopsis ppi2 mutant in which the Toc159 gene is disrupted (Bauer et al., 2000; Teng et al., 2006). Many photosynthetic proteins are deficient in the ppi2 mutant, whereas nonphotosynthetic proteins seem to accumulate normally. In the ppi2 mutant, Toc132 and Toc120, which are homologs of Toc159, compensate for the absence of Toc159, at least to some extent. The Tic20 family consists of four genes in Arabidopsis: Tic20-I, Tic20-IV, Tic20-II, and Tic20-V (Jarvis, 2008). Tic20-I characterized in this study is the most abundant isoform among four proteins and is the closest homolog to the biochemically identified pea Tic20 (Kouranov et al., 1998). The other three Tic20 isoforms in Arabidopsis may be responsible for the import of nonphotosynthetic and housekeeping proteins. Preliminary experiments indicated that certain double knockout mutations introduced into the four Arabidopsis Tic20 genes resulted in more severe embryo lethal phenotype (S. Kikuchi, Y. Hirabayashi, and M. Nakai, unpublished data), suggesting that the residual import capacities observed in the tic20 and tic21 mutants may be due to the presence of another Tic20 isoform-containing channel that probably has different substrate specificity. These issues are currently under investigation.
To date, the Tic110/Tic40/Hsp93 complex, which mediates stromal side events, has received considerable attention with regard to translocation across the inner membrane. However, a Tic core complex that functions in between the Toc complex and the Tic110/Tic40/Hsp93 complex has not yet been reported. We believe that the 1-MD translocation complex characterized in this study corresponds to this Tic core complex, which should contain a protein-conducting channel. While Tic20 and Tic21 would play a crucial role in the 1-MD complex, we can easily speculate that most constituents of the 1-MD complex have not yet been identified. Therefore, further work will be required to identify new components.
METHODS
Plant Material and Growth Conditions
Pea (Pisum sativum var Alaska) seedlings were grown on vermiculite in a growth chamber under 14 h light at 25°C/10 h dark at 23°C cycles for 12 to 13 d. The Arabidopsis thaliana mutants tic20 (SALK_039676) and tic21/pic1-1 (SALK_104852) carrying T-DNA insertion(s) were kindly provided by the Salk Institute Genomic Analysis Laboratory (Alonso et al., 2003). Arabidopsis ecotype Columbia was used as the wild type. Arabidopsis (wild type and mutants) were grown on MS plates (1× Murashige and Skoog salts [Sigma-Aldrich], 1× Gamborg's B5 vitamin [Sigma-Aldrich], 2% sucrose, pH 5.8, and 0.3% phytagel [Sigma-Aldrich]) in a growth chamber under 16 h light at 23°C /8 h dark at 21°C cycles for 18 to 21 d (Yabe et al., 2004; Asakura et al., 2008).
Isolation of Chloroplasts
Chloroplast isolation from pea leaves was described in our previous report (Kikuchi et al., 2006). Arabidopsis chloroplasts were isolated by direct homogenization method as described (Bruce et al., 1994; Aronsson and Jarvis, 2002; Schulz et al., 2004) with the following modifications. Approximately 20 g of aerial parts were homogenized in 400 mL of blending buffer (50 mM HEPES-KOH, pH 7.8, 330 mM sorbitol, 2 mM EDTA, 1 mM MnCl2, 1 mM MgCl2, and 50 mM sodium ascorbate [freshly added in powder]) with or without 5 μL/mL protease inhibitor cocktail (for plant extracts, P-9599; Sigma-Aldrich), in five 2-s pulses in a kitchen blender equipped with disposable razor blades. The homogenate was filtered through four layers of Miracloth (Calbiochem) and then centrifuged at 4000g for 3 min. The crude chloroplast pellet was resuspended in HS buffer (50 mM HEPES-KOH, pH 7.8, and 330 mM sorbitol) and overlaid onto 30% (v/v) Percoll in HS buffer. After centrifugation at 1350g for 15 min in a swinging bucket rotor, the pellet was washed twice with HS buffer. Purified intact chloroplasts were kept on ice in the dark and used within 3 h.
In Vitro Transcription and Translation of Preproteins
Plasmid pGEM-4Z-pSSU for the in vitro expression of a precursor protein of SSU was constructed by inserting the coding sequence derived from its cDNA prepared from pea into pGEM-4Z vector (Promega) at a XbaI/SalI site. The mRNA of pSSU was synthesized from linearized pGEM-4Z-pSSU construct using RiboMAX in vitro transcription system (Promega) with SP6 RNA polymerase. The resulting mRNA was translated in a wheat germ extract (Promega) at 25°C for 2 h in the presence of [35S]Met. A plasmid for the in vitro expression of pSSU-DHFR, which consists of the full-length precursor to SSU fused to the entire mouse dihydrofolate reductase, was constructed by inserting the pSSU coding sequence lacking the stop codon into pPC-DHFR/SP (Endo et al., 1994) at a PstI/BamHI site. The fusion protein pSSU-DHFR was synthesized using TNT SP6-coupled reticulocyte lysate system (Promega) at 30°C for 90 min in the presence of [35S]Met. The translation mixtures were kept on ice and used within 3 h.
Formation of a Translocation Intermediate, Protease Treatments, and Gel Electrophoresis
Isolated intact chloroplasts in HS buffer containing 5 μL/mL protease inhibitor cocktail were preincubated for 10 min at 25°C in the dark to deplete endogenous ATP. Energy-depleted chloroplasts were mixed with in vitro–translated preproteins in HS buffer containing 0.5 mM Mg-ATP, 5 mM MgCl2, 5 mM DTT, 3 mM Met, and 3 mM Cys. The reactions were incubated for 10 min at 25°C in the dark to form a translocation intermediate. Each reaction contained chloroplasts equivalent to 100 μg chlorophyll in a reaction volume of 400 μL. The reactions were terminated by reisolation of chloroplasts by centrifugation at 1500g for 1 min at 4°C, and chloroplasts were washed once with HS buffer containing 5 μL/mL protease inhibitor cocktail. When chase experiments or protease treatments were performed, the above reactions were performed as a batch reaction corresponding to the number of various conditions. Before protease accessibility assays, chloroplasts carrying preproteins were washed twice with HS buffer in the absence of protease inhibitor cocktail. For thermolysin treatment, chloroplasts in HS buffer were incubated with 10 to 100 μg/mL of thermolysin (Sigma-Aldrich) and 1 mM CaCl2 for 20 min on ice. Thermolysin was inactivated by the addition of 10 mM EDTA. For trypsin treatment, chloroplasts in HS buffer were incubated with 10 to 100 μg/mL of trypsin (Sigma-Aldrich) for 20 min on ice. Trypsin was inactivated by the addition of a fivefold excess of soybean trypsin inhibitor (0.5 mg/mL; Sigma-Aldrich). Proteolyzed chloroplasts were pelleted and washed twice with HS buffer containing either 5 μL/mL protease inhibitor cocktail or 0.5 mg/mL trypsin inhibitor.
Samples prepared by the above methods were analyzed by two different electrophoresis methods: BN-PAGE and SDS-PAGE. The BN-PAGE method was described in detail in our previous report (Kikuchi et al., 2006). The chloroplast pellet was solubilized in freshly prepared BN-PAGE sample buffer (1% [w/v] water-soluble digitonin, 50 mM BisTris-HCl, pH 7.0, 500 mM 6-amino-n-caproic acid, and 10% [w/v] glycerol) containing 10 μL/mL protease inhibitor cocktail to a final concentration of 0.5 mg chlorophyll/mL for 10 min on ice. Water-soluble digitonin was prepared as described (Mori et al., 1999). When using trypsin as a protease, trypsin inhibitor was added to a final concentration of 0.5 mg/mL to BN-PAGE sample buffer. Insoluble material was removed by ultracentrifugation at 100,000g for 10 min at 4°C. The supernatant was divided into two aliquots. One of which (40 μL) was mixed with Coomassie Brilliant Blue G 250 solution (5% [w/v] Serva blue G, 50 mM BisTris-HCl, pH 7.0, and 500 mM 6-amino-n-caproic acid) to give a detergent:Coomassie ratio of 8:1 (w:w) and subjected to 4 to 14% BN-PAGE. The other was mixed with 10% SDS and 2-mercaptoethanol to final concentrations of 3.3 and 5%, respectively. The SDS-denatured sample was heated at 95°C for 2 min and subjected to SDS-PAGE. The radioactive signals in dried gels were detected using a BAS-2000II image analysis system (FujiFilm) and quantified using Image Gauge version 3.45 software (FujiFilm).
Antibody Preparation, Purification, and Immunoblotting
Two overlapping but different antigens of Arabidopsis Tic20 were overexpressed as N-terminal hexahistidine-tagged fusion proteins in Escherichia coli using recently developed cold shock–inducible system (Qing et al., 2004). An antigen of Arabidopsis Tic21 was overexpressed as a fusion protein with N-terminal 260 amino acids of T7 gene10 protein in E. coli. An antigen of pea Tic22 was overexpressed as a C-terminal hexahistidine-tagged fusion protein in E. coli. Regions of antigens used were as follows: At Tic20 FL (full-length sequence not including transit peptide), At Tic20 ES (C-terminal half part, amino acids 157 to 274), At Tic21 (C-terminal part, amino acids 210 to 296), and Ps Tic22 (full-length sequence including transit peptide). Recombinant At Tic20 FL, At Tic20 ES, and Ps Tic22 proteins were purified from inclusion bodies with nickel chelate chromatography under denaturing conditions. Recombinant At Tic21 protein was purified from inclusion bodies using SDS-PAGE and subsequent electroelution. Purified recombinant At Tic20 FL, At Tic20 ES, At Tic21, and Ps Tic22 proteins were injected into rabbits using Freund's complete adjuvant and (for subsequent boosts) Freund's incomplete adjuvant (Harlow and Lane, 1988). Preparation of antibodies against Ps Toc75, Ps Toc159, Ps Toc34, Ps Tic110, So cpHsp70 (Spinacia oleracea stromal Hsp70), So Cpn60α, and So Cpn60β was described in our previous reports (Nishio et al., 1999; Asakura et al., 2004; Kikuchi et al., 2006). Anti-At Tic21, -Ps Toc75, -Ps Toc159, and -Ps Toc34 antibodies were affinity purified using the purified antigens as described previously (Kikuchi et al., 2006). Anti-Ps Tic110 antibody was purified using rProtein A-Sepharose (GE Healthcare). Anti-At Tic20 ES antibody was purified by blot affinity purification (Tang, 1993).
Immunoblotting was performed as described previously (Kikuchi et al., 2006). Toc75, Toc159, Toc34, Tic110, Tic22, and cpHsp70 were detected by an enhanced chemiluminescence system (GE Healthcare). Tic20 and Tic21 were detected by the ECL plus system (GE Healthcare). We found that At Tic21 protein band was lost in SDS-PAGE/immunoblotting after standard denaturation by heating at 95°C in Laemmli sample buffer; therefore, when detecting Tic21 and distantly related Tic20, samples were incubated at 37°C for 30 min.
Antibody-Shift BN-PAGE
Antibody-shift BN-PAGE is used in several reports (e.g., Johnston et al., 2002; Truscott et al., 2002). Chloroplasts carrying [35S]translocation intermediate were solubilized in BN-PAGE sample buffer containing 10 μL/mL protease inhibitor as described above. After ultracentrifugation at 100,000g for 10 min at 4°C, 35 μL of the supernatant was mixed with each purified antibody of known concentrations (0 to 10 μg) and incubated for 30 min on ice with occasional mixing. After a clarifying spin, the supernatant was mixed with Coomassie Brilliant Blue G 250 solution as described above and subjected to BN-PAGE and autoradiography.
Immunodepletion
Chloroplasts carrying [35S]translocation intermediate were solubilized in BN-PAGE sample buffer containing 10 μL/mL protease inhibitor as described above. Insoluble material was removed by ultracentrifugation at 100,000g for 10 min at 4°C. Fifty to 100 μL of the supernatant was incubated twice with 10 μL packed volume of rProtein A-Sepharose that had been coupled with IgG for 1 h in cold room (6°C) with rotational mixing. The unbound fraction (supernatant) was recovered by centrifugation and was filtered through a 0.2-μm membrane filter by centrifugation to remove residual beads.
Cross-Linking and Immunoprecipitation under Denaturing Conditions
Chloroplasts carrying [35S]pSSU-DHFR, at 0.24 mg chlorophyll/mL in HS buffer in the presence of 20 μM MTX and in the absence of protease inhibitor cocktail, were treated with 0.2 mM MBS (Pierce) or 0.2 mM DSP (Pierce) for 15 min on ice. The MBS cross-linking was quenched by adding glycine and 2-mercaptoethanol to final concentrations of 20 mM and 2%, respectively, and incubating on ice for another 15 min. The DSP cross-linking was quenched by adding glycine to a final concentrations of 20 mM and incubating on ice for another 15 min. Chloroplasts were recovered by centrifugation, washed with HS buffer containing 20 μM MTX and 5 μL/mL protease inhibitor cocktail. Immunoprecipitation under denaturing conditions was performed as described (Cline and Mori, 2001) with the following modifications. Chloroplasts carrying cross-linked preproteins were solubilized under denaturing conditions (2% SDS, 0.5% Triton X-100, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 10 μL/mL protease inhibitor cocktail) at 25°C for 5 min at 1 mg chlorophyll/mL. Insoluble material was removed by centrifugation, and the supernatant was diluted 18.5-fold with 0.5% Triton X-100 in TBS (50 mM Tris-HCl, pH 7.5, and 150 mM NaCl) to enable immunoprecipitations. The diluted sample (370 μL) was mixed with 10 μL packed volume of rProtein A-Sepharose that had been coupled with IgG and incubated for 2 h at 25°C with rotational mixing. After the beads were washed twice with 0.5% Triton X-100 in TBS, bound proteins were eluted by boiling 2 min in 2× Laemmli sample buffer. When using DSP as the cross-linker, bound proteins were eluted by boiling 5 min in 2× Laemmli sample buffer containing 10% 2-mercaptoethanol to cleave the cross-linker. The eluates were subjected to SDS-PAGE and autoradiography.
Transient Expression in Protoplasts
Arabidopsis (wild type and mutants) were grown on MS plates. Homozygous tic20 and tic21 mutants were selected from pools of plants based on the visible albino phenotypes. Protoplasts were prepared as described previously (Jin et al., 2001). The GFP fusion constructs were introduced into protoplasts by polyethylene glycol–mediated transformation (Jin et al., 2001). Protein import into wild-type and mutant plastids was analyzed by immunoblotting with anti-GFP antibody using protein extracts from transformed protoplasts as described previously (Lee et al., 2008).
Miscellaneous
2D-BN/SDS-PAGE was performed as described previously (Kikuchi et al., 2006) except when detecting At Tic21, At Tic20, and Ps Tic20, where excised first-dimension native gels were incubated at 37°C for 30 min. Size exclusion chromatography was also performed as described previously (Kikuchi et al., 2006) except that 1% water-soluble digitonin was used for solubilization and 0.1% water-soluble digitonin was used in equilibration buffer instead of dodecyl maltoside. Water-soluble digitonin was prepared as described (Mori et al., 1999). Monoclonal antibody SPA-820 against Hsp70/Hsc70 was purchased from StressGen.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: Tic20 (At1g04940), Tic21 (At2g15290), pea Tic22 (AF095284), and pea pSSU (X00806).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The 1-MD Translocation Complex Is Not a Stromal Chaperonin Complex.
Supplemental Figure 2. The 1-MD Translocation Complex Was Generated Using Ferredoxin:NADP+ Oxidoreductase as a Preprotein.
Supplemental Figure 3. Partial Degradation of the Toc Complex, Which Had Occurred during the Isolation of Chloroplasts, Did Not Affect the Formation of the 1-MD Translocation Complex.
Supplemental Figure 4. Sucrose Density Gradient Separation of Outer and Inner Envelope Membrane Vesicles.
Supplemental Figure 5. The Arabidopsis 1-MD Translocation Complex Has Identical Protease Accessibility Properties to That of the Pea Complex.
Supplemental Figure 6. Antibody-Shift BN-PAGE.
Supplemental Figure 7. Tests for the Ability of Anti-Tic and -Toc Antibodies to Native Proteins by Immunodepletion.
Supplemental Figure 8. Immunoblot Analyses of Several Proteins in Albino Mutants.
Supplemental Figure 9. Protein Import into Plastids in a Transient Expression System Using Protoplasts from the albino3 Mutant.
Supplemental Figure 10. Immunoprecipitation after Chemical Cross-Linking with MBS.
Supplemental Figure 11. Chemical Cross-Linking of the Translocation Intermediate.
Supplementary Material
Acknowledgments
We thank Toshiharu Hase, Yoko Kimata-Ariga, Guy T. Hanke, Yukari Asakura, Toshiki Yabe, Jocelyn Bédard, and coworkers of our laboratory for valuable discussions, Tazu Uchida for technical assistance, and Rieko Nakanishi for secretarial assistance. We also thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants tic20 and tic21/pic1, Hsou-min Li for the gift of anti-At Tic40 antibody, Brigitte Touraine for the gift of anti-ferritin antibody, Marinus Pilon for the gift of anti-CSD2 antibody, Toshiya Hirohashi for the preparation of anti-Ps Tic22 antibody, Kazuaki Nishio for the preparation of anti-stromal So Hsp70, So Cpn60α, and So Cpn60β antibodies, and Tetsuya Nohara for the gift of pea first-strand cDNA. This work was supported by Grants-in-Aid for Scientific Research on Priority Areas (17028034 and 17051020) from MEXT of Japan to M.N. This work was also supported in part by Korea Science and Engineering Foundation through the Creative Research Initiatives Program (20090063529) to I.H. S.K. was supported by a research fellowship from the Japan Society for the Promotion of Science.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Masato Nakai (nakai@protein.osaka-u.ac.jp).
Online version contains Web-only data.
References
- Akita, M., Nielsen, E., and Keegstra, K. (1997). Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking. J. Cell Biol. 136 983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J.M., Stepanova, A.N., Leisse, T.J., Kim, C.J., Chen, H., Shinn, P., Stevenson, D.K., Zimmerman, J., Barajas, P., Cheuk, R., Gadrinab, C., and Heller, C. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Aronsson, H., and Jarvis, P. (2002). A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 529 215–220. [DOI] [PubMed] [Google Scholar]
- Asakura, Y., Hirohashi, T., Kikuchi, S., Belcher, S., Osborne, E., Yano, S., Terashima, I., Barkan, A., and Nakai, M. (2004). Maize mutants lacking chloroplast FtsY exhibit pleiotropic defects in the biogenesis of thylakoid membranes. Plant Cell 16 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura, Y., Kikuchi, S., and Nakai, M. (2008). Non-identical contributions of two membrane-bound cpSRP components, cpFtsY and Alb3, to thylakoid biogenesis. Plant J. 56 1007–1017. [DOI] [PubMed] [Google Scholar]
- Balsera, M., Goetze, T.A., Kovács-Bogdán, E., Schürmann, P., Wagner, R., Buchanan, B.B., Soll, J., and Bölter, B. (2009). Characterization of Tic110, a channel-forming protein at the inner envelope membrane of chloroplasts, unveils a response to Ca2+ and a stromal regulatory disulfide bridge. J. Biol. Chem. 284 2603–2616. [DOI] [PubMed] [Google Scholar]
- Bauer, J., Chen, K., Hiltbunner, A., Wehrli, E., Eugster, M., Schnell, D.J., and Kessler, F. (2000). The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403 203–207. [DOI] [PubMed] [Google Scholar]
- Becker, T., Hritz, J., Vogel, M., Caliebe, A., Bukau, B., Soll, J., and Schleiff, E. (2004). Toc12, a novel subunit of the intermembrane space preprotein translocon of chloroplasts. Mol. Biol. Cell 15 5130–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard, J., and Jarvis, P. (2005). Recognition and envelope translocation of chloroplast preproteins. J. Exp. Bot. 56 2287–2320. [DOI] [PubMed] [Google Scholar]
- Bruce, B.D., Perry, S., Froehlich, J., and Keegstra, K. (1994). In vitro import of protein into chloroplasts. In Plant Molecular Biology Manual, S.B. Gelvin and R.A. Schilperoot, eds (Belgium: Kluwer Academic Publishers), pp. 1–15.
- Caliebe, A., Grimm, R., Kaiser, G., Lübeck, J., Soll, J., and Heins, L. (1997). The chloroplastic protein import machinery contains a Rieske-type iron-sulfur cluster and a mononuclear iron-binding protein. EMBO J. 16 7342–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K., Chen, X., and Schnell, D.J. (2000). Initial binding of preproteins involving the Toc159 receptor can be bypassed during protein import into chloroplasts. Plant Physiol. 122 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K.-Y., and Li, H.-m. (2007). Precursor binding to an 880-kDa Toc complex as an early step during active import of protein into chloroplasts. Plant J. 49 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Smith, M.D., Fitzpatrick, L., and Schnell, D.J. (2002). In vivo analysis of the role of atTic20 in protein import into chloroplasts. Plant Cell 14 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigri, F., Soll, J., and Vothknecht, U.C. (2005). Calcium regulation of chloroplast protein import. Plant J. 42 821–831. [DOI] [PubMed] [Google Scholar]
- Chou, M.-L., Fitzpatrick, L.M., Tu, S.-L., Budziszewski, G., Potter-Lewis, S., Akita, M., Levin, J.Z., Keegstra, K., and Li, H.-m. (2003). Tic40, a membrane-anchored co-chaperone homolog in the chloroplast protein translocon. EMBO J. 22 2970–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, M.-L., Chu, C.-C., Chen, L.-J., Akira, M., and Li, H.-M. (2006). Stimulation of transit-peptide release and ATP hydrolysis by a cochaperone during protein import into chloroplasts. J. Cell Biol. 175 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., and Mori, H. (2001). Thylakoid ΔpH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J. Cell Biol. 154 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker, P.J.T., Martin, F., Maarse, A.C., Bömer, U., Müller, H., Guiard, B., Meijer, M., Rassow, J., and Pfanner, N. (1997). The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16 5408–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy, D., Wanner, G., Meda, A.R., von Wirén, N., Soll, J., and Philippar, K. (2007). PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell 19 986–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, T., Kawakami, M., Goto, A., America, T., Weisbeek, P., and Nakai, M. (1994). Chloroplast protein import. Chloroplast envelopes and thylakoids have different abilities to unfold proteins. Eur. J. Biochem. 225 403–409. [DOI] [PubMed] [Google Scholar]
- Friedman, A.L., and Keegstra, K. (1989). Chloroplast protein import quantitative analysis of precursor binding. Plant Physiol. 89 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, P.D., Devenish, R.J., and Prescott, M. (2003). FRET reveals changes in the F1-stator stalk interaction during activity of F1F0-ATP synthase. Biochim. Biophys. Acta 1607 167–179. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Heins, L., Mehrle, A., Hemmler, R., Wagner, R., Küchler, M., Hörmann, F., Sveshnikov, D., and Soll, J. (2002). The preprotein conducting channel at the inner envelope membrane of plastids. EMBO J. 21 2616–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi, T., Hase, T., and Nakai, M. (2001). Maize non-photosynthetic ferredoxin precursor is mis-sorted to the intermembrane space of chloroplasts in the presence of light. Plant Physiol. 125 2154–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba, T., Li, M., Alvarez-Huerta, M., Kessler, F., and Schnell, D.J. (2003). atTic110 functions as a scaffold for coordinating the stromal events of protein import into chloroplasts. J. Biol. Chem. 278 38617–38627. [DOI] [PubMed] [Google Scholar]
- Inoue, H., and Akita, M. (2008). Three sets of translocation intermediates are formed during the early stage of protein import into chloroplasts. J. Biol. Chem. 283 7491–7502. [DOI] [PubMed] [Google Scholar]
- Jackson, D.T., Froehlich, J.E., and Keegstra, K. (1998). The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment. J. Biol. Chem. 273 16583–16588. [DOI] [PubMed] [Google Scholar]
- Jarvis, P. (2008). Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol. 179 257–285. [DOI] [PubMed] [Google Scholar]
- Jin, J.B., Kim, Y.A., Kim, S.J., Lee, S.H., Kim, D.H., Cheong, G.-W., and Hwang, I. (2001). A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13 1511–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, A.J., Hoogenraad, J., Dougan, D.A., Truscott, K.N., Yano, M., Mori, M., Hoogenraad, N.J., and Ryan, M.T. (2002). Insertion and assembly of human Tom7 into the preprotein translocase complex of the outer mitochondrial membrane. J. Biol. Chem. 277 42197–42204. [DOI] [PubMed] [Google Scholar]
- Kessler, F., and Schnell, D. (2009). Chloroplast biogenesis: Diversity and regulation of the protein import apparatus. Curr. Opin. Cell Biol. 21: in press. [DOI] [PubMed]
- Kikuchi, S., Hirohashi, T., and Nakai, M. (2006). Characterization of the preprotein translocon at the outer envelope membrane of chloroplasts by blue native PAGE. Plant Cell Physiol. 47 363–371. [DOI] [PubMed] [Google Scholar]
- Kouranov, A., Chen, X., Fuks, B., and Schnell, D.J. (1998). Tic20 and Tic22 are new components of the protein import apparatus at the chloroplast inner envelope membrane. J. Cell Biol. 143 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranov, A., and Schnell, D.J. (1997). Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts. J. Cell Biol. 139 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küchler, M., Decker, S., Hörmann, F., Soll, J., and Heins, L. (2002). Protein import into chloroplasts involves redox-regulated proteins. EMBO J. 21 6136–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.W., Kim, J.K., Lee, S., Choi, S., Kim, S., and Hwang, I. (2008). Arabidopsis nuclear-encoded plastid transit peptides contain multiple sequence subgroups with distinctive chloroplast-targeting sequence motifs. Plant Cell 20 1603–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., Kouranov, A., LaSala, S.E., and Schnell, D.J. (1996). Two components of the chloroplast protein import apparatus, IAP86 and IAP75, interact with the transit sequence during the recognition and translocation of precursor proteins at the outer envelope. J. Cell Biol. 134 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., Summer, E.J., Ma, X., and Cline, K. (1999). Component specificity for the thylakoidal sec and delta pH-dependent protein transport pathways. J. Cell Biol. 146 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, E., Akita, M., Davila-Aponte, J., and Keegstra, K. (1997). Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 16 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio, K., Hirohashi, T., and Nakai, M. (1999). Chloroplast chaperonins: Evidence for heterogeneous assembly of alpha and beta Cpn60 polypeptides into a chaperonin oligomer. Biochem. Biophys. Res. Commun. 266 584–587. [DOI] [PubMed] [Google Scholar]
- Perry, S.E., and Keegstra, K. (1994). Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell 6 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing, G., et al. (2004). Cold-shock induced high-yield protein production in Escherichia coli. Nat. Biotechnol. 22 877–882. [DOI] [PubMed] [Google Scholar]
- Schägger, H., Cramer, W.A., and von Jagow, G. (1994). Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217 220–230. [DOI] [PubMed] [Google Scholar]
- Schägger, H., and Pfeiffer, K. (2000). Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger, H., and von Jagow, G. (1991). Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199 223–231. [DOI] [PubMed] [Google Scholar]
- Schleiff, E., Soll, J., Küchler, M., Kühlbrandt, W., and Harrer, R. (2003). Characterization of the translocon of the outer envelope of chloroplasts. J. Cell Biol. 160 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell, D.J., and Blobel, G. (1993). Identification of intermediates in the pathway of protein import into chloroplasts and their localization to envelope contact sites. J. Cell Biol. 120 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell, D.J., Kessler, F., and Blobel, G. (1994). Isolation of components of the chloroplast protein import machinery. Science 266 1007–1012. [DOI] [PubMed] [Google Scholar]
- Schulz, A., Knoetzel, J., Scheller, H.V., and Mant, A. (2004). Uptake of a fluorescent dye as a swift and simple indicator of organelle intactness: Import-competent chloroplasts from soil-grown Arabidopsis. J. Histochem. Cytochem. 52 701–704. [DOI] [PubMed] [Google Scholar]
- Smith, M.D., Rounds, C.M., Wang, F., Chen, K., Afitlhile, M., and Schnell, D.J. (2004). atToc159 is a selective transit peptide receptor for the import of nucleus-encoded chloroplast proteins. J. Cell Biol. 165 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll, J., and Schleiff, E. (2004). Protein import into chloroplasts. Nat. Rev. Mol. Cell Biol. 5 198–208. [DOI] [PubMed] [Google Scholar]
- Soll, J., and Tien, R. (1998). Protein translocation into and across the chloroplastic envelope membranes. Plant Mol. Biol. 38 191–207. [PubMed] [Google Scholar]
- Sundberg, E., Slagter, J.G., Fridborg, I., Cleary, S.P., Robinson, C., and Coupland, G. (1997). ALBINO3, an Arabidopsis nuclear gene essential for chloroplast differentiation, encodes a chloroplast protein that shows homology to proteins present in bacterial membranes and yeast mitochondria. Plant Cell 9 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W.-J.Y. (1993). Blot-affinity purification of antibodies. Methods Cell Biol. 37 95–104. [DOI] [PubMed] [Google Scholar]
- Teng, Y.-S., Su, Y.-s., Chen, L.-J., Lee, Y.J., Hwang, I., and Li, H.-m. (2006). Tic21 is an essential translocon component for protein translocation across the chloroplast inner envelope membrane. Plant Cell 18 2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott, K.N., Wiedemann, N., Rehling, P., Müller, H., Meisinger, C., Pfanner, N., and Guiard, B. (2002). Mitochondrial import of the ADP/ATP carrier: the essential TIM complex of the intermembrane space is required for precursor release from the TOM complex. Mol. Cell. Biol. 22 7780–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waegemann, K., and Soll, J. (1991). Characterization of the protein import apparatus in isolated outer envelopes of chloroplasts. Plant J. 1 149–158. [Google Scholar]
- Waegemann, K., and Soll, J. (1993). Isolation and characterization of a functionally active protein translocation apparatus from chloroplast envelopes. In Molecular Mechanisms of Membrane Traffic, NATO ASI Ser. H74, D.J. Morré, K.E. Howell, and J.J.M. Bergeron, eds (Berlin/Heidelberg: Springer-Verlag), pp. 101–104.
- Wittig, I., and Schägger, H. (2005). Advantages and limitations of clear-native PAGE. Proteomics 5 4338–4346. [DOI] [PubMed] [Google Scholar]
- Yabe, T., Morimoto, K., Kikuchi, S., Nishio, K., Terashima, I., and Nakai, M. (2004). The Arabidopsis chloroplastic NifU-like protein CnfU, which can act as an iron-sulfur cluster scaffold protein, is required for biogenesis of ferredoxin and photosystem I. Plant Cell 16 993–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M.E., Keegstra, K., and Froehlich, J.E. (1999). GTP promotes the formation of early-import intermediates but is not required during the translocation step of protein import into chloroplasts. Plant Physiol. 121 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.