Abstract
Stem cells are undifferentiated cells that can self-renew and generate specialized (functional) cell types. The remarkable ability of stem cells to differentiate towards functional cells makes them suitable modalities in cellular therapy (which means treating diseases with the body's own cells). Potential targets for cellular therapy include diabetes and liver failure. However, in order for stem cells to be clinically useful, we must learn to identify them and to regulate their differentiation. We will use the intestine as a classical example of a stem cell compartment, and then examine the evidence for the existence of adult stem cells in two endodermally derived organs: pancreas and liver. We will review the characteristics of the putative stem cells in these tissues and the transcription factors controlling their differentiation towards functional cell types.
Keywords: cell differentiation, intestine, liver, pancreas, stem cell, transdifferentiation
Abbreviations: AP, anterior–posterior; BMP, bone morphogenetic protein; Cdx1, caudal-related; C/EBP, CCAAT/enhancer-binding protein; CK19, cytokeratin 19; DPPIV, dipeptidyl peptidase IV; E, embryonic day; ES cell, embryonic stem cell; FAH, fumarylacetoacetate hydrolase; FGF, fibroblast growth factor; Hox, homeobox; HSC, haematopoietic stem cell; IHH, Indian hedgehog; Ngn3, neurogenin 3; Pdx1, pancreatic and duodenal Hox 1; Prt, Prometheus; SCID, severe combined immunodeficiency; Tcf, T-cell factor
INTRODUCTION
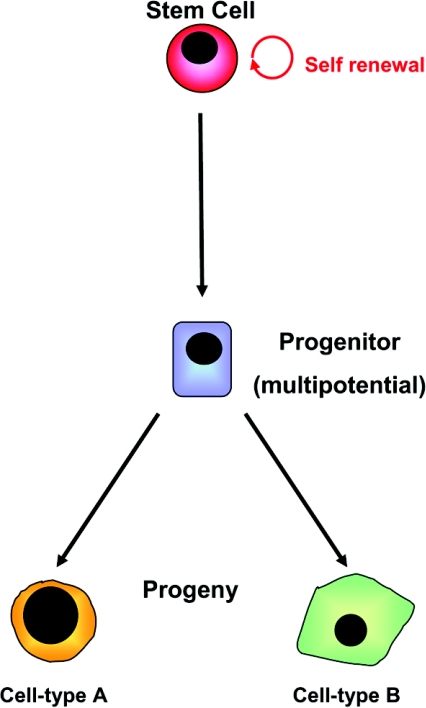
Stem cells are defined as cells that can divide without limit and whose progeny (or offspring) include both stem cells and cells destined to differentiate into specialized cell types (Figure 1; for an animated version of this Figure see http://www.BiochemJ.org/bj/404/0169/bj4040169add.htm). Many stem cells have the potential to generate more than one type of differentiated progeny (i.e. they are multipotent). Stem cells therefore hold great clinical promise in terms of their ability to provide replacements for tissues damaged by the processes of aging, injury and disease.
Figure 1. Scheme of stem cell self-renewal and differentiation.
Stem cells are capable of maintaining the stem cell compartment through self-renewal and can also give rise to progenitor cells that may undergo subsequent differentiation along more than one lineage to generate cell-type-specific derivatives. For an animated version of this Figure, see http://www.BiochemJ.org/bj/404/0169/bj4040169add.htm.
Stem cells are characterized by the fact they are undifferentiated. This presents a major obstacle to their identification, particularly for the more primitive stem cells which are normally in a quiescent state and do not express many antigens. It is, presumably, the overall combination of positive and negative antigen markers that are important in the identification of stem cells. Even though stem cells are undifferentiated, during development they are already committed to becoming one particular cell or tissue type. Therefore, an epidermal (skin) stem cell can only form keratinocytes, and an intestinal stem cell can only form intestinal cell types present in the villus. This demonstrates the phenomenon of ‘developmental hierarchy’, which means that cells of the early blastocyst can form any cell and then become restricted to a differentiated cell type. For example, the precursors of the intestinal stem cells are committed in the germ layer, which is known as the endoderm, and stem cells in the haematopoietic lineage are committed in the mesoderm. It is believed that once a cell is committed, it cannot cross the germ-layer boundary to form another cell type (for example, a mesodermally derived cell cannot form an endodermal cell; see below).
Stem cells are found in both the developing embryo [ES (embryonic stem) cells], as well as in adult tissues. ES cells are pluripotent, with the capacity to generate cells from all three germ layers (endoderm, mesoderm and ectoderm) [1]. The announcement that human stem cells could be derived from the inner cell mass of the blastocyst was met with much excitement, but the ethical issues surrounding the use of human ES cells in regenerative medicine [2] will not be dealt with in the present review.
Adult stem cells (sometimes referred to as tissue-specific or somatic stem cells) are undifferentiated cells found amongst differentiated cells that are present in certain organs of the body. The primary role of adult stem cells is to maintain and repair the tissue in which they are found. In adult tissues, stem cells exist in the gastrointestinal tract, bone marrow, the central nervous system, skin, muscle, cornea and retina (reviewed in [3]). Two tissues that exhibit a high cell-turnover rate, the intestine and skin, have relatively well-characterized stem cell compartments. However, in tissues such as the liver, where the true liver cells (or hepatocytes) have a low turnover rate [4], a stem cell capable of self-renewing and producing liver progeny has, to date, remained elusive [5]. In the present review, we will use the intestine as an example of a stem cell compartment, then examine the evidence for stem cell progenitors in the liver and pancreas, and also discuss briefly the process of transdifferentiation as a means of producing differentiated cells for therapeutic transplantation.
INTESTINAL STEM CELLS
The intestine is a constitutively developing tissue, undergoing constant renewal and differentiation from a stem cell compartment throughout the entire life of an organism. Interestingly, many of the molecular mechanisms underlying the maintenance and differentiation of adult intestinal stem cells are the same pathways regulating intestinal organogenesis. Therefore, understanding intestinal development will help identify the key extracellular (for example, morphogens and growth factors) and intracellular (signalling intermediates and transcription factors) components responsible for directing stem cells towards an intestinal phenotype.
The primitive gut tube is composed of three germ layers: the endoderm, which forms the epithelial lining of the intestine; the mesoderm, which forms the smooth muscle layers; and the ectoderm, from which the neural crest cells that will form the enteric nervous system are derived. The developing gut is subject to differentiation in several planes, the AP (anterior–posterior), DV (dorsal–ventral), LR (left–right) and the RAD (radial) axis, thus allowing the formation of varied and specialized structures along the entire length of the gut tube. Initial intestinal endoderm specification is regulated, in part, by epithelial–mesenchymal interactions along the AP axis. The specification is probably mediated by differential mesodermal expression of the Hox (homeobox)-containing transcription factors [6–8]. Cytodifferentiation of the intestinal epithelium in the developing rat begins at approx. E14 (embryonic day 14) when the endoderm undergoes a transformation from a pseudostratified epithelium to a columnar monolayer, a process resulting in the formation of numerous villi along the cranio–caudal axis (head-to-tail or AP axis) [9]. Intestinal villi are characteristically long and thin, and interspersed by areas of proliferating epithelium that will become the intervillus crypts (crypts of Lieberkühn). The crypt–villus structures are maintained into adulthood and form the basic functional units of the intestine. The crypt–villus axis is established in response to two signalling molecules: IHH (Indian hedgehog) and BMP (bone morphogenetic protein). IHH is expressed during development in the colonic epithelium and proliferating epithelial precursors of the small intestine. A dramatic reduction in villi size and the number of proliferating cells is observed in the intestine of Ihh−/− mice [10], supporting a role for IHH in promoting cell renewal. A possible role for IHH in controlling cell fate specification is also implicated by the observation that the colonic epithelium in Ihh−/− mice remains a multilayered epithelium of undifferentiated cells, rather than becoming a monolayer of cells organized into crypts and surface epithelium [11]. Maintenance of intestinal stem cell self-renewal is also mediated through the antagonistic effects of BMP on the Wnt signalling pathway [12] (see below). Suppression of BMP signalling leads to ectopic crypt formation in the villus and abnormal growth of the intestinal epithelium [13]. Cases of juvenile polyposis syndrome (an autosomal dominant disorder characterized by the development of polyps throughout the gastrointestinal tract in the first decade or two of life) have been shown to be due to mutations in SMAD4 and BMP receptor 1A, both components of the BMP signalling pathway [13].
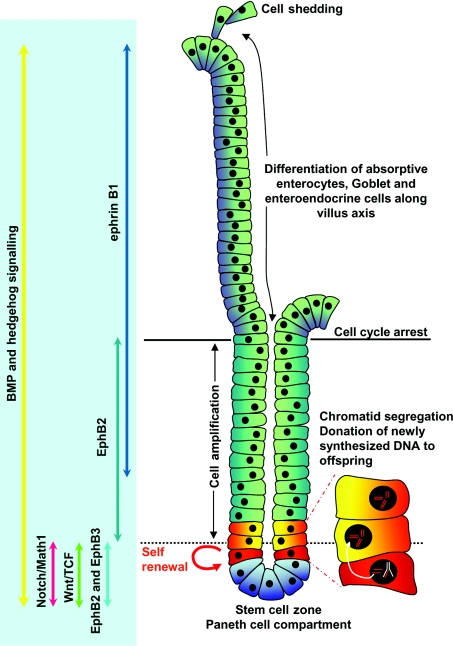
Intestinal stem cells located in the base of the crypts of Lieberkühn give rise to progenitors that will differentiate into absorptive enterocytes or secretory cells, such as enteroendocrine, goblet and Paneth cells [14]. In 1981 Bjerknes and Cheng [15] put forward the stem cell zone model that proposed the stem cell state is supported by the microenvironment (or niche) around cell positions 1–4. However, cells moving up to position 5 are induced to begin a programme of differentiation into one of the intestinal cell lineages. Each crypt is estimated to contain between one and six slowly dividing stem cells that give rise to a transient population of rapidly dividing progenitor cells, the majority of which migrate out of the crypt into the villi where they will differentiate and eventually be shed and replaced by new cells. The Paneth cells, in contrast, differentiate and migrate back into the stem cell zone at the base of the crypt [15,16]. A model to explain the ability of intestinal stem cells to maintain and protect their genetic integrity was first described by Cairns and colleagues over 25 years ago [17]. In this study [17], it was proposed that stem cells could selectively donate newly synthesized DNA strands to their descendants while retaining old DNA strands (Figure 2). Although controversial at first, the Cairns model has now been confirmed by the demonstration of asymmetric chromatid segregation using a technique to label template strands of DNA during development with tritiated thymidine and newly synthesized strands with bromodeoxyuridine [18].
Figure 2. Representation of the cellular components of the intestinal crypt and the signalling pathways involved in differentiation and maintenance of the crypt–villus axis.
The intestinal crypt–villus axis can be divided into three zones: the lower stem cell and Paneth cell compartment, the cell amplification zone, both located in the crypt; and the cell differentiation zone that constitutes the villus epithelium. Stem cells located at approx. position 5 maintain genomic integrity through chromatid segregation by donating newly synthesized DNA to their offspring while retaining their original genomic content. Progenitors arising in the stem cell compartment may differentiate into Paneth cells that migrate into the bottom of the crypt, directed by interactions between EphB2/3 and ephrin, where they will reside for up to 20 days before being replaced by new Paneth cells. Alternatively, stem cell progenitors enter the amplification zone in the crypt. When the stem cell progenitors reach the base of the villus they will undergo cell-cycle arrest and differentiate along an absorptive enterocyte, goblet or enteroendocrine cell lineage. Cells shed at the tip of the villus are replaced by constant generation of new progenitors in the crypt. Wnt and Notch signalling components are restricted to the base of the crypt where they play an important role in maintaining the stem cell compartment. BMP and hedgehog signalling along the crypt–villus axis have been shown to regulate cell renewal and lineage specification.
Maintenance of the gut stem cell compartment is dependent, in part, on signalling through the Wnt–β-catenin–Tcf-4 (T-cell factor 4) pathway [19,20]. Wnt genes encode secreted proteins that act via membrane-bound Wnt receptors, which in turn, lead to the nuclear translocation of β-catenin and Tcf complexes where they mediate the transcriptional activation of numerous genes. Expression of Wnt genes during mouse development suggested a role for Wnt signalling in determining AP boundaries in the gut [21]. Whereas β-catenin is localized to the membrane of all cells in the villi and crypts in adult intestine, nuclear β-catenin is localized only in the epithelial cells within the crypt. The Wnt effector Tcf-4 is also expressed at high levels in the cells of the crypt, and Tcf-4-null mice exhibit loss of the intestinal stem cell compartment and die at birth, prior to crypt formation [22,23]. Furthermore, expression of the Wnt repressor Dickopf, under the control of the villin promoter in the intestine of transgenic mice, leads to a dramatic reduction in proliferative activity, the absence of crypts and perturbation of villi formation [24].
Cellular differentiation in the intestine is also regulated by the Notch–Delta–Hes1 signalling pathway (reviewed in [25]). Math1, a downstream target of Hes1, has been localized to the progenitor cell compartment of the developing and adult crypt. Math1 homozygous mutant mice show a complete loss of goblet, Paneth and enteroendocrine cells, indicating a requirement for Math1 in lineage specification. Interestingly, enterocytes arise from the Math1-negative progenitors, suggesting that two progenitor cell types normally reside in the crypt [26]. Math1-positive cells are directed towards an endocrine phenotype through the activity of Ngn3 (neurogenin 3), whereas the expression of the transcriptional repressor Gfi1 (growth factor independence 1) directs the cells toward a secretory cell lineage (Paneth/goblet cells). Differentiation of intestinal progenitors is also dependent on their ability to migrate out of the crypt, a process regulated by the expression of ephrin receptors and their ligands. Differentiating cells, migrating away from the crypt, express the ligand ephrin B1, whereas the receptors EphB2 and EphB3 are expressed in the progenitor cell compartment [27]. Paneth cells normally reside in the base of the crypt, a region to which the EphB3 receptor is restricted in the adult intestine. In EphB3−/− mice, Paneth cells are seen scattered throughout the base of the villus and the crypt, further supporting a role for ephrin signalling in maintaining the correct cellular patterning of the intestinal crypt [27].
Although the high rate of self-renewal required for general maintenance of the intestine has facilitated the location of stem cell and progenitor cell compartments, rapid cell turnover may contribute to intestinal stem cell susceptibility to genetic alteration under conditions of inflammation or infection, the consequence of which is the development of metaplastic lesions. For example, the Drosophila Hox transcription factor Cdx1 (caudal-related 1), a direct target of Tcf-4, is expressed in the developing intestinal endoderm and the proliferating cells of the adult crypt [28,29]. Cdx1 and another member of the caudal-type Hox gene family, Cdx2, have been localized to intestinal metaplastic tissue of the human stomach, suggesting that ectopic expression of this gene may contribute to the mechanism underlying intestinal metaplasia [30]. Animal models in which Cdx2 is misexpressed in the gastric mucosa also develop intestinal metaplasia that are characterized by the presence of intestinal cell types [31]. Together, these data indicate the importance of strict regional expression of Cdx1 for the maintenance of the intestinal stem cell compartment and normal boundaries within the gut.
Despite the apparent wealth of information regarding the maintenance and differentiation of intestinal progenitor cells, lack of a stem-cell-specific marker continues to hamper progress. A study [32] suggests that Mushasi-1, a marker for neural stem cells expressed in the lower crypt, may be a marker for intestinal stem and early progenitor cells.
LIVER STEM CELLS
The embryonic liver arises as an outgrowth of the ventral foregut endoderm. Hepatic specification occurs at E8.0, when the foregut endoderm is brought into close proximity with the cardiac mesenchyme, a process dependent on both BMP and FGF (fibroblast growth factor) signalling. In the absence of FGF and BMP signals in the mouse, the anteroventral endoderm assumes a pancreatic fate [33]. The transcription factors Foxa1 (forkhead box A1) and Foxa2 are also required for initiation of liver development [34]. Following specification, the hepatic endoderm proliferates to form the liver bud (E9.5), and by E10.5 the fundamental sinusoidal structure of the liver becomes apparent as the early hepatoblasts migrate into the mesenchyme of the surrounding septum transversum. In addition to interactions with the developing vascular system [35] and the effects of factors such as HGF (hepatocyte growth factor) [36], liver outgrowth is regulated by the transcription factors Prox1 and Hex. The lack of hepatocytes observed in Hex−/− mice indicate an essential role for Hex in differentiation of the hepatic endoderm [37], whereas in Prox1−/− animals the differentiating hepatocytes are encapsulated by the presence of a basement membrane abnormally rich in collagen IV and laminin, suggesting a role for Prox1 in hepatocyte migration [38].
A role for Wnt signalling during liver development has also been implicated [39]. A recent report by Ober and co-workers [39] has demonstrated the requirement for a mesodermally derived Wnt signal to direct liver specification in the zebrafish embryo. The Prt (Prometheus) gene, identified as a novel homologue of Wnt2b (and closely resembles the mouse orthologue Wnt13), is expressed in the lateral plate mesoderm in the time frame during which liver specification occurs (reviewed in [40]). Prt mutants exhibit an absent or severely reduced liver due to defects in hepatic fate specification. In addition, a role for Wnt signalling in promoting the specification and survival of the biliary cell lineage in mouse has been suggested [41]. In the absence of serum, cultured embryonic liver exhibits extensive loss of parenchymal and biliary architecture, reduction in proliferation and increased apoptosis. When supplemented with Wnt3a-conditioned medium, however, duct-like structures comprising proliferating cells, positive for the biliary marker CK19 (cytokeratin 19), were observed in the embryonic livers. These data suggest that Wnt signalling may play a role not only in the early phase of liver specification, but also in the determination of specific hepatic cell lineages.
The epithelial cells of the foetal liver, the hepatoblasts, are bipotential progenitor cells. Both the hepatocytes (i.e. the liver parenchymal cells) and biliary epithelial cells are derived from the hepatoblast [42]. Sandhu et al. [43] demonstrated the ability of E14 rat hepatoblasts to replicate and differentiate when transplanted into the liver of DPPIV (dipeptidyl peptidase IV)-deficient rats (DPPIV is an exopeptidase located at the apical membrane of hepatocytes). The transplanted cells generated clusters of DPPIV+ hepatocytes and comprised 5–10% of the liver population 6 months post-transplantation. The hepatoblasts also generated bile duct structures that became integrated into the host biliary system. Similar replicative and differentiation capacities have been shown in isolated human foetal hepatoblasts transplanted into mice with SCID (severe combined immunodeficiency) [44]. As an alternative to using primary hepatoblasts, some research groups have now generated embryonic progenitor cell precursor lines from mouse and rat, although such a cell line has not yet been described in human liver [45,46]. The BMEL (bipotential mouse embryonic liver) cell line described by Strick-Marchand and Weiss [45] has been shown to differentiate into both bile duct cells and hepatocytes during liver regeneration in Alb-uPA (albumin-urokinase plasminogen activator)/SCID transgenic mice. These data reinforce the importance of such cells for the development of cell-based therapies for liver disease, as they provide a pool of cells that can be readily expanded, genetically manipulated and potentially used in transplantation.
The fate and differentiation of wild-type foetal liver cells was determined recently by Oertel et al. [47], following transplantation into DPPIV− rats. Foetal liver cells showed continuous proliferation potential for 6 months following transplantation, and subsequent differentiation into hepatocytes re-populating 23.5% of the total liver mass. Interestingly, the transplanted cells exhibited reduced apoptosis and a greater proliferative activity than host hepatocytes. Increased apoptosis of host hepatocytes adjacent to the transplanted cells led Oertel and colleagues [47] to propose that a process termed cell–cell competition (described previously in Drosophila [48]) may operate to replace functional hepatic tissue in the transplanted animals.
Considerable controversy continues to surround the existence of an adult liver stem cell. Under conditions of severe parenchymal loss, the hepatocytes themselves may be considered as functional stem cells due to their remarkable ability to self-replicate to restore liver mass during regeneration [49,50]. Furthermore, animal models of liver disease have demonstrated that transplanted hepatocytes undergo substantial clonal expansion and are sufficient to rescue mutant phenotypes. For example, the FAH (fumarylacetoacetate hydrolase)-deficient mouse strain is a model for tyrosinaemia type 1. This mutant mouse can normally survive only by liver transplantation or treatment with the drug NTBC [2-(2-nitro-4-trifluro-methylbenozyl)-1,3-cyclohexanedione]. NTBC treatment prevents tyrosinaemia-induced liver failure by inhibiting the enzyme 4-hydroxyphenylpyruvate dioxygenase, which functions upstream of FAH. However, in FAH-deficient mice, transplanted wild-type mature hepatocytes readily proliferate within the diseased liver and alleviate symptoms [51]. What is more interesting is the remarkable re-population potential of hepatocytes. Using the FAH-knockout mouse model, Grompe and co-workers [51] demonstrated that normal adult hepatocytes serially transplanted are capable of at least 80 doublings.
Liver regeneration is also mediated through the activation of a potential stem cell compartment localized to the periductular junction or canals of Hering [52,53]. In response to injury by carcinogenic agents, small cells containing oval nuclei and limited cytoplasm (termed oval cells in rodents or hepatic progenitor cells in humans) proliferate within the smallest branches of the intrahepatic biliary tract [49,54]. The oval cell is considered to be the progeny of the elusive hepatic stem cell and is bipotential in nature, capable of giving rise to both hepatocytes and bile duct cells. It was speculated at one stage that oval cells were derived from bone-marrow precursor cells. This was based on the observation that oval cells express many of the markers typically associated with haematopoietic cells, for example, CD34, Thy-1, c-Kit and Flt-3 (Fms-related tyrosine kinase 1) [55]. However, a study carried out by Dabeva et al. [56] now forms the basis of the widely accepted idea that oval cells do arise from the stem cell niche within the canals of Hering. In order to determine the origin of oval cells, Dabeva et al. [56] transplanted 5×107 wild-type male bone-marrow cells into lethally irradiated DPPIV− female rats, and subsequently subjected the rats to three different protocols to promote activation and expansion of the oval cell compartment. Although in each model a marked accumulation and increase in CK19-expressing oval cells were observed, less than 1% of the oval cells showed dual CK19/DPPIV+ labelling, indicating that they probably do not arise from bone-marrow stem cells. Interestingly, oval cells have been identified in the ductal system of the pancreas in copper-depleted rats [57]. The copper-depleted rat is significant, because the copper-deficiency regime followed by re-feeding with a normal copper-containing diet leads to the appearance of hepatocyte-like cells in the pancreas of treated animals. The ectopic hepatocytes were thought to be derived from pancreatic oval cells. When oval cells from copper-depleted rats were transplanted into rats they did, indeed, begin to express hepatic markers, such as albumin [56].
Although the in vivo models for the transdifferentiation of pancreatic cells into hepatocytes have been extremely useful and demonstrate the potential for pancreas-to-liver transdifferentiation [58], it is more difficult to determine the molecular or cellular mechanisms from these studies. The generation of an in vitro model for transdifferentiation (discussed below) has provided some of the molecular details for the switch in phenotype from a pancreatic to a hepatic cell type.
PANCREATIC STEM CELLS
The pancreas comprises two distinct cell types that are referred to as exocrine and endocrine cells. The exocrine region of the pancreas produces and secretes digestive enzymes (for example, amylase) in response to hormones from the gastrointestinal tract. The endocrine cells of the pancreas are arranged in compact spheroidal clusters called the islets of Langerhans which are dispersed throughout the acinar matrix. There are five endocrine cell types and each secretes a distinct pancreatic hormone. The cell types are: α-cells (glucagon), β-cells (insulin), δ-cells (somatostatin), F- or PP-cells (pancreatic polypeptide) and ϵ-cells (ghrelin). In Type 1 diabetes, the insulin-producing β-cells of the endocrine pancreas are inappropriately destroyed by autoimmune attack, resulting in diabetes. An ideal therapy for diabetes would achieve normoglycaemia through β-cell replacement. Pancreatic stem cells have been proposed as a potentially feasible source of novel β-cells for transplantation. However, there has been considerable controversy as to the existence and exact nature of any stem cells in the adult pancreas. As shown by early studies, the pancreas has a very limited regenerative capacity. The exocrine tissue in hamsters can be selectively damaged by the replacement of methionine in the diet with ethionine, and regeneration of the damaged tissue can be provoked by the restoration of methionine [59]. Local inflammation and fibrosis caused by the cellophane wrapping of the pancreas in hamsters can also induce islet neogenesis from hyperplastic ducts [60]. Such results have led to the proposal of potential candidates for adult pancreatic stem cells. They include pancreatic ductal cells, exocrine cells, Ngn3-positive cells, nestin-positive cells and oval cells, all of which are discussed below. Some of these cells have the potential for lineage-restricted differentiation into pancreatic phenotypes.
During normal embryogenesis, the pancreas, similar to the liver and the intestine, is derived from the definitive endoderm [61]. However, commitment to a pancreatic fate is set up by an activin-βB- and FGF2-mediated repression of sonic hedgehog expression in pre-pancreatic dorsal endoderm [62,63]. This induces the expression of the master regulator of pancreatic development, Pdx1 (pancreatic and duodenal homeobox 1), and marks the precursor cell population for pancreatic development. A hierarchical cascade of pancreatic developmental transcription factor expression in these progenitor cells then gives rise to the endocrine and exocrine cell populations and the distinct cell types within each of the populations [64,65]. For example, transcription factors such as Ngn3 and NeuroD1 are essential for early endocrine lineage determination, whereas Hes1, p48 and Mist1 are expressed in the cells which contribute to the exocrine lineage [65–67]. Furthermore, sequential expression of Nkx2.2, Pax4, Nkx6.1, MafA, Pax6 and Pdx1 determine insulin-producing β-cell fate, whereas Brn4, Arx1, Nkx6.2 and MafB expression promote the glucagon-producing α-cell fate [65,66,68]. The understanding of the transcription factor hierarchy and the various growth factors involved in pancreatic development and maturation has proved useful in reprogramming other cell types into pancreatic cells. Recently, a five-stage protocol that mimics pancreatic development, taking human ES cells through different identifiable endodermal intermediates, has made it possible to eventually produce endocrine-hormone-expressing cells in vitro, simply by the application of different combinations of growth factors, such as activins, Wnts, BMPs and FGFs when ES cells are in culture [69].
In vivo, endocrine cells may arise by sequential differentiation of ductal epithelial stem cells [70,71]. Islet-like structures then bud off and migrate into the acinar tissue. Immunohistochemical localization of single β-cells near and within ductal tissue, following pancreatic injury, was originally presented as evidence that new β-cells in the adult could similarly originate from stem cells within the ductal tissue [72]. Furthermore, it is possible to generate islets containing α-, β- and δ-cells from pluripotent stem cells isolated from adult pancreatic ductal epithelial cells in long-term culture [73]. However, rapid proliferation of ductal cells could make them lose their ductal phenotype and revert to multipotent cells that then differentiate into other cell types [74]. Ductal cells can also transdifferentiate into other cell types, including squamous, mucinous and pyloric cells, and undergo malignant transformations into pancreatic adenocarcinomas [75]. Given these two lines of evidence, some controversy exists as to whether the cells within the ducts that give rise to β-cells are true ductal stem cells or differentiated ductal cells with some transdifferentiation capacity.
The exocrine and endocrine pancreas arises from a common Pdx-1-expressing domain of the foregut endoderm [61,76]. This observation revoked the idea that the adult pancreatic stem cells, which can generate both endocrine and exocrine cell types, might reside among the exocrine cell population [77]. The non-endocrine pancreatic epithelial cells that remain after islet isolation when co-transplanted with foetal pancreatic cells under the kidney capsule of immunodeficient mice are capable of differentiating into endocrine cells [77]. However, lineage-tracing studies have demonstrated that acinar–islet transitions, which occur both in vivo and in vitro, could primarily be due to transdifferentiation and not as a result of the existence of endocrine stem or progenitor cells within the pancreatic exocrine tissue [78,79].
Nestin-positive cells within the pancreatic islets and ducts were also postulated to be putative multipotent stem cells that provide precursors for neogenesis of endocrine cells [80,81]. Nestin is an intermediate filament protein that is a marker of neural stem cells [82]. The nestin-positive cells within the islets and ducts are negative for endocrine hormones and ductal markers respectively, but, when isolated and expanded in vitro, they can differentiate into insulin- and glucagon-producing cells [80,81]. However, later studies characterized the nestin-positive cells of the pancreas as being either reactive stellate cells or angiogenic endothelial cells [83]. Immunohistochemical analyses of nestin-positive cells in vivo showed that the cells were mesenchymal in nature, and nestin was not co-expressed with the epithelial marker E-cadherin at any stage during development of the mouse pancreas [82]. Furthermore, although nestin demarcates a cell population that differentiates along neuronal and muscle lineages, these cells do not contribute to insulin-producing cells during normal human development [84].
Some of the most convincing evidence points to Ngn3-positive cells as pancreatic progenitors, both during embryogenesis and in the adult mouse. Using an inducible Cre-ER™-LoxP system to follow the progeny of Ngn3- and Pdx1-expressing cells, Ngn3-positive cells specifically give rise to endocrine cells, whereas Pdx1-positive cells contribute to exocrine, endocrine and ductal tissue of the pancreas [85]. Although these Ngn3-positive cells behave as endocrine progenitors, their self-renewal capabilities and the possibility that a subpopulation of these cells might be true pluripotent stem cells remain unresolved.
The anatomical proximity of the developing liver and pancreas has also led to the suggestion of the existence of a common hepatopancreatic stem cell [86]. These endodermal cells, exhibiting bipotentiality, follow a default pancreatic programme to start initially expressing Pdx1, and subsequently markers specific to endocrine (Ngn3, Isl1 and NeuroD) or exocrine (p48) lineages. It is only the FGF signalling programme from the cardiac mesoderm that diverts the bipotential cells to commit to a hepatic lineage [86].
An intrinsic pancreas-derived multipotent precursor population from the adult pancreas which is capable of differentiating in vitro into pancreatic α-, β- and δ-cells, pancreatic exocrine cells and stellate cells, neurons and glial cells has also been reported [87]. However, controversy still remains as to whether these precursor or stem cells are predominantly responsible for the adult pancreatic regeneration observed in vivo. In the case of β-cells, in vivo genetic lineage tracing showed that normal β-cell replenishment and generation of novel β-cells following pancreatectomy occur by the replication of pre-existing β-cells, rather than via the postulated pluripotent stem cell population [88]. This study [88] questions the significance of the in vivo roles of any pancreatic stem cell, but it does not rule out the possibility of their existence. Although the use of adult pancreatic stem cells would prove to be highly revolutionary in developing an innovative diabetes therapy, to date, a fully defined and clinically applicable true pluripotent pancreatic stem cell still awaits to be identified, isolated and characterized.
METAPLASIA AND TRANSDIFFERENTIATION AS A MEANS OF GENERATING DIFFERENTIATED CELL TYPES
Metaplasia is defined as the conversion, in postnatal life, of one cell type into another, and can include conversion between stem cells of different tissues. Transdifferentiation belongs to a subset of metaplasia and involves conversion of one terminally differentiated cell type into another [89]. Both metaplasia and transdifferentiation are largely associated with tissue damage.
There is now some evidence to suggest that, through a process of cell fusion, tissue-specific stem cells can give rise to cells characteristic of a completely different tissue. One of the best-documented examples of this type of cell conversion is seen in adult bone-marrow-derived stem cells. Bone marrow contains mesenchymal stem cells and HSCs (haematopoietic stem cells), which can generate neuronal cells, pneumocytes, skeletal and cardiac muscle, as well as hepatocytes (reviewed in [90]). The derivation of hepatocytes from circulating HSCs was first documented in the rat following a complex protocol designed to prevent normal liver regeneration and cause oval cell activation. Lethally irradiated female rats were subjected to treatment with 2-acetylaminofluorene and carbon tetrachloride to block hepatocyte regeneration and cause hepatocyte necrosis respectively. The fate of transplanted Y-chromosome-positive oval cells was then monitored in the recipient females [91]. Hepatic engraftment of bone-marrow-derived cells was observed by the appearance of Y-chromosome-positive hepatocytes approx. 13 days after liver injury. In subsequent studies, examination of the liver of female recipients of male bone marrow revealed that hepatocytes may be derived from HSCs in humans as well [92,93]. Lagasse and colleagues [94] also reported that HSCs differentiated into hepatocytes to alleviate the symptoms in FAH knockout mice; however, closer examination of the data revealed that regeneration in their model arises through cell fusion, rather than by a true metaplastic change [95]. The reverse conversion from a hepatic progenitor cell line into cells of a pancreatic lineage has also been demonstrated in an in vitro model. WB cells represent a liver progenitor cell line and can be reprogrammed into insulin-producing cells by the lentivirus-mediated transduction of the pancreatic transcription factor Pdx1 [96]. Pdx1 was modified to include the Vp16 activation domain from herpes simplex virus. The β-cells produced from WB cells were transplanted into a mouse model of diabetes and hyperglycaemia was reversed.
In addition to stem-cell-derived hepatocytes and pancreatic cells, several laboratories have now demonstrated the generation of these cell types by transdifferentiation. Transdifferentiation of pancreatic cells into hepatocytes has been observed in a number of model systems which include: the copper-depleted rat described by Rao et al. [57,97], following transplantation of a cell population enriched for pancreatic epithelial progenitors into the rat liver [56], and in transgenic mice overexpressing keratinocyte growth factor in the pancreas [98] (Figure 3). Hepatic transdifferentiation has also been described as a naturally occurring phenomenon in the vervet monkey (Cercopithecus aethiops) and in human pancreatic tumours [99,100].
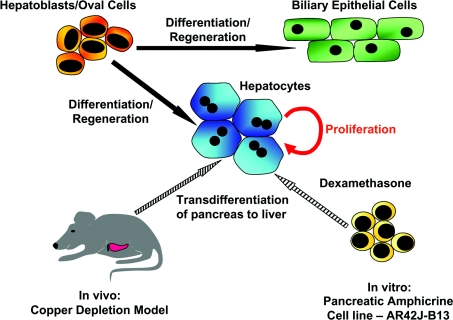
Figure 3. Diagram showing the generation of hepatocytes from embryonic and adult cells through normal development, regeneration and transdifferentiation.
During normal liver development, hepatocytes and biliary epithelial cells are generated by differentiation of embryonic hepatoblasts. Under conditions of regeneration, both hepatocyte and bile duct lineages arise from oval cells, the progeny of the putative hepatic stem cell. Hepatocytes can also be generated from pancreatic cell types: either through the treatment of rats with a copper-deficient diet in vivo or through the treatment of the pancreatic cell line AR42J-B13 with 1 μM dexamethasone in vitro.
Studies carried out in our laboratory have described the conversion of the rat pancreatic tumour cell line AR42J-B13 into hepatocyte-like cells. The transdifferentiated hepatocytes possess many of the functional characteristics of normal hepatocytes [101–104]. These observations have been confirmed by two independent groups [105,106]. Bouwens and colleagues [107] have also shown that addition of dexamethasone can provoke a hepatic phenotype in cultured adult rat exocrine cells. Furthermore, the molecular basis of the switch from pancreatic to hepatic phenotype in vitro is mediated, at least in part, by the transcription factor C/EBPβ (CCAAT/enhancer-binding protein β) [101]. Embryonic expression of C/EBPβ (and C/EBPα) is confined to the region of the developing liver, supporting the idea that C/EBPs are important in liver development [108].
The conversion from liver cells into pancreatic cells has also been achieved [109–114]. The ability to interconvert between liver and pancreas reflects the close developmental relationship between these two tissues and supports the theory that transdifferentiation is driven by one or two so-called ‘masterswitch’ genes that distinguish the two tissues during development [115]. Identification of such masterswitch genes will be useful in the future for directing stem cell differentiation towards liver, pancreatic, intestinal or other phenotypes.
SUMMARY AND CONCLUSIONS
In recent years, there has been a considerable amount of interest in using stem cells to repair or regenerate damaged tissues. Stem cells can be used as a source for cell transplantation due to their ability to differentiate into a variety of cell types. This strategy is one branch of ‘regenerative medicine’ which refers to the stimulation of regeneration of damaged organs and constitutes one of the fundamental challenges to tissue engineers and biologists.
Several problems still remain to be resolved before pancreatic and hepatic stem cells can be therapeutically useful. The first is to determine whether the studies performed on animal cells can be applied to human cells. At present, it is not clear if the markers identified on rodent liver and pancreas progenitor stem cells are also expressed in the equivalent human cells. Therefore the first hurdle will involve the identification of suitable stem cell markers. One approach to this problem might be through large-scale genomic or proteomic screening of putative stem cells. Once reliable markers have been found this should make isolation and expansion easier. The second problem to be resolved will be to elucidate the rules for differentiation of stem cells towards functional cell types. Identification of the extracellular cues in stem cell differentiation and the key regulatory transcription factors will require the development of new in vitro culture models. Identifying the individual steps in the differentiation of stem cells to functional cells is important for two distinct reasons. First, the bioassay provided by stem cell differentiation might enable a shortcut to the molecular understanding of the development of individual tissues, since the steps involved in stem cell differentiation are probably similar to what happens during embryonic development (the same logic can also be applied to the steps in transdifferentiation). The information obtained from stem cell studies may also be complementary to that obtained from the direct investigation of embryonic development by knockout studies and transgenic approaches. Secondly, understanding the molecular basis of stem cell differentiation may have a practical value in the design of novel therapies for treating degenerative diseases. For example, differentiation of pancreatic stem cells to glucose-responsive insulin-expressing β-cells is an important therapeutic goal in diabetes research. Ultimately, identifying tissue-specific adult stem cells and the mechanisms involved in their differentiation to specific cell lineages will pave the way to developing novel therapeutic strategies for treating liver failure and diabetes.
Animation of Figure 1 Scheme of stem cell self-renewal and differentiation
Acknowledgments
We thank the Wellcome Trust and the Overseas Research Studentship committee for financial support.
References
- 1.Odorico J. S., Kaufman D. S., Thomson J. A. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 2.de Wert G., Mummery C. Human embryonic stem cells: research, ethics and policy. Hum. Reprod. 2003;18:672–682. doi: 10.1093/humrep/deg143. [DOI] [PubMed] [Google Scholar]
- 3.Preston S. L., Alison M. R., Forbes S. J., Direkze N. C., Poulsom R., Wright N. A. The new stem cell biology: something for everyone. Mol. Pathol. 2003;56:86–96. doi: 10.1136/mp.56.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magami Y., Azuma T., Inokuchi H., Kokuno S., Moriyasu F., Kawai K., Hattori T. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver. 2002;22:419–425. doi: 10.1034/j.1600-0676.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- 5.Tosh D., Strain A. Liver stem cells – prospects for clinical use. J. Hepatol. 2005;42(Suppl.):S75–S84. doi: 10.1016/j.jhep.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Roberts D. J., Smith D. M., Goff D. J., Tabin C. J. Epithelial–mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- 7.Beck F., Tata F., Chawengsaksophak K. Homeobox genes and gut development. Bioessays. 2000;22:431–441. doi: 10.1002/(SICI)1521-1878(200005)22:5<431::AID-BIES5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.Sekimoto T., Yoshinobu K., Yoshida M., Kuratani S., Fujimoto S., Araki M., Tajima N., Araki K., Yamamura K. Region-specific expression of murine Hox genes implies the Hox code-mediated patterning of the digestive tract. Genes Cells. 1998;3:51–64. doi: 10.1046/j.1365-2443.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- 9.Mathan M., Moxey P. C., Trier J. S. Morphogenesis of fetal rat duodenal villi. Am. J. Anat. 1976;146:73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- 10.Ramalho-Santos M., Melton D. A., McMahon A. P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 11.van den Brink G. R., Bleuming S. A., Hardwick J. C., Schepman B. L., Offerhaus G. J., Keller J. J., Nielsen C., Gaffield W., van Deventer S. J., Roberts D. J., Peppelenbosch M. P. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 12.He X. C., Zhang J., Tong W. G., Tawfik O., Ross J., Scoville D. H., Tian Q., Zeng X., He X., Wiedemann L. M., et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nat. Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 13.Haramis A. P., Begthel H., van den Born M., van Es J., Jonkheer S., Offerhaus G. J., Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 14.Stappenbeck T. S., Mills J. C., Gordon J. I. Molecular features of adult mouse small intestinal epithelial progenitors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1004–1009. doi: 10.1073/pnas.242735899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjerknes M., Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am. J. Anat. 1981;160:51–63. doi: 10.1002/aja.1001600105. [DOI] [PubMed] [Google Scholar]
- 16.Bjerknes M., Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 17.Potten C. S., Hume W. J., Reid P., Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- 18.Potten C. S., Owen G., Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 19.Huelsken J., Behrens J. The Wnt signalling pathway. J. Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 20.Pinto D., Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Lickert H., Kispert A., Kutsch S., Kemler R. Expression patterns of Wnt genes in mouse gut development. Mech. Dev. 2001;105:181–184. doi: 10.1016/s0925-4773(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 22.Barker N., Huls G., Korinek V., Clevers H. Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am. J. Pathol. 1999;154:29–35. doi: 10.1016/S0002-9440(10)65247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P. J., Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 24.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson E. M., Lendahl U., Chapman G. Notch signaling in development and disease. Semin. Cancer Biol. 2004;14:320–328. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q., Bermingham N. A., Finegold M. J., Zoghbi H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 27.Batlle E., Henderson J. T., Beghtel H., van den Born M. M., Sancho E., Huls G., Meeldijk J., Robertson J., van de Wetering M., Pawson T., Clevers H. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 28.Duprey P., Chowdhury K., Dressler G. R., Balling R., Simon D., Guenet J. L., Gruss P. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 1988;2:1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian V., Meyer B., Evans G. S. The murine Cdx1 gene product localises to the proliferative compartment in the developing and regenerating intestinal epithelium. Differentiation. 1998;64:11–18. doi: 10.1046/j.1432-0436.1998.6410011.x. [DOI] [PubMed] [Google Scholar]
- 30.Mizoshita T., Inada K., Tsukamoto T., Kodera Y., Yamamura Y., Hirai T., Kato T., Joh T., Itoh M., Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa – with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer. 2001;4:185–191. doi: 10.1007/pl00011741. [DOI] [PubMed] [Google Scholar]
- 31.Silberg D. G., Sullivan J., Kang E., Swain G. P., Moffett J., Sund N. J., Sackett S. D., Kaestner K. H. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 32.Potten C. S., Booth C., Tudor G. L., Booth D., Brady G., Hurley P., Ashton G., Clarke R., Sakakibara S., Okano H. Identification of a putative intestinal stem cell and early lineage marker: musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 33.Rossi J. M., Dunn N. R., Hogan B. L., Zaret K. S. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C. S., Friedman J. R., Fulmer J. T., Kaestner K. H. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto K., Yoshitomi H., Rossant J., Zaret K. S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., Gherardi E., Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 37.Keng V. W., Yagi H., Ikawa M., Nagano T., Myint Z., Yamada K., Tanaka T., Sato A., Muramatsu I., Okabe M., et al. Homeobox gene Hex is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem. Biophys. Res. Commun. 2000;276:1155–1161. doi: 10.1006/bbrc.2000.3548. [DOI] [PubMed] [Google Scholar]
- 38.Sosa-Pineda B., Wigle J. T., Oliver G. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 39.Ober E. A., Verkade H., Field H. A., Stainier D. Y. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 40.Burke Z. D., Thowfeequ S., Tosh D. Liver specification: a new role for Wnts in liver development. Curr. Biol. 2006;16:R688–R690. doi: 10.1016/j.cub.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Hussain S. Z., Sneddon T., Tan X., Micsenyi A., Michalopoulos G. K., Monga S. P. Wnt impacts growth and differentiation in ex vivo liver development. Exp. Cell Res. 2004;292:157–169. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Notenboom R. G., van den Bergh Weerman M. A., Dingemans K. P., Vermeulen J. L., van den Eijnde S., Reutelingsperger C. P., Hut H., Willemsen R., Offerhaus G. J., Lamers W. H. Timing and sequence of differentiation of embryonic rat hepatocytes along the biliary epithelial lineage. Hepatology. 2003;38:683–691. doi: 10.1053/jhep.2003.50365. [DOI] [PubMed] [Google Scholar]
- 43.Sandhu J. S., Petkov P. M., Dabeva M. D., Shafritz D. A. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am. J. Pathol. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhi H., Irani A. N., Gagandeep S., Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J. Cell Sci. 2002;115:2679–2688. doi: 10.1242/jcs.115.13.2679. [DOI] [PubMed] [Google Scholar]
- 45.Strick-Marchand H., Morosan S., Charneau P., Kremsdorf D., Weiss M. C. Bipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8360–8365. doi: 10.1073/pnas.0401092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allain J. E., Dagher I., Mahieu-Caputo D., Loux N., Andreoletti M., Westerman K., Briand P., Franco D., Leboulch P., Weber A. Immortalization of a primate bipotent epithelial liver stem cell. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3639–3644. doi: 10.1073/pnas.062038599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oertel M., Menthena A., Dabeva M. D., Shafritz D. A. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 48.Moreno E., Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 49.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 50.Michalopoulos G. K., DeFrances M. C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 51.Overturf K., al-Dhalimy M., Ou C. N., Finegold M., Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am. J. Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 52.Theise N. D., Saxena R., Portmann B. C., Thung S. N., Yee H., Chiriboga L., Kumar A., Crawford J. M. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 53.Roskams T. A., Theise N. D., Balabaud C., Bhagat G., Bhathal P. S., Bioulac-Sage P., Brunt E. M., Crawford J. M., Crosby H. A., Desmet V., et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 54.Alison M. R., Golding M., Sarraf C. E., Edwards R. J., Lalani E. N. Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology. 1996;110:1182–1190. doi: 10.1053/gast.1996.v110.pm8613008. [DOI] [PubMed] [Google Scholar]
- 55.Crosby H. A., Kelly D. A., Strain A. J. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534–544. doi: 10.1053/gast.2001.21175. [DOI] [PubMed] [Google Scholar]
- 56.Dabeva M. D., Hwang S. G., Vasa S. R., Hurston E., Novikoff P. M., Hixson D. C., Gupta S., Shafritz D. A. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7356–7361. doi: 10.1073/pnas.94.14.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao M. S., Subbarao V., Reddy J. K. Induction of hepatocytes in the pancreas of copper-depleted rats following copper repletion. Cell Differ. 1986;18:109–117. doi: 10.1016/0045-6039(86)90005-9. [DOI] [PubMed] [Google Scholar]
- 58.Rao M. S., Reddy J. K. Hepatic transdifferentiation in the pancreas. Semin. Cell Biol. 1995;6:151–156. doi: 10.1006/scel.1995.0021. [DOI] [PubMed] [Google Scholar]
- 59.Scarpelli D. G., Rao M. S. Differentiation of regenerating pancreatic cells into hepatocyte-like cells. Proc. Natl. Acad. Sci. U.S.A. 1981;78:2577–2581. doi: 10.1073/pnas.78.4.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberg L., Duguid W. P., Vinik A. I. The effect of cellophane wrapping of the pancreas in the Syrian golden hamster: autoradiographic observations. Pancreas. 1989;4:31–37. doi: 10.1097/00006676-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Slack J. M. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 62.Hebrok M., Kim S. K., St. Jacques B., McMahon A. P., Melton D. A. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- 63.Kim S. K., Hebrok M., Melton D. A. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 64.Wilson M. E., Scheel D., German M. S. Gene expression cascades in pancreatic development. Mech. Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 65.Jensen J. Gene regulatory factors in pancreatic development. Dev. Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- 66.Chakrabarti S. K., Mirmira R. G. Transcription factors direct the development and function of pancreatic β-cells. Trends Endocrinol. Metab. 2003;14:78–84. doi: 10.1016/s1043-2760(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 67.Watada H. Neurogenin 3 is a key transcription factor for differentiation of the endocrine pancreas. Endocr. J. 2004;51:255–264. doi: 10.1507/endocrj.51.255. [DOI] [PubMed] [Google Scholar]
- 68.Samson S. L., Chan L. Gene therapy for diabetes: reinventing the islet. Trends Endocrinol. Metab. 2006;17:92–100. doi: 10.1016/j.tem.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 69.D'Amour K. A., Bang A. G., Eliazer S., Kelly O. G., Agulnick A. D., Smart N. G., Moorman M. A., Kroon E., Carpenter M. K., Baetge E. E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 70.Rosenberg L. In vivo cell transformation: neogenesis of beta cells from pancreatic ductal cells. Cell Transplant. 1995;4:371–383. doi: 10.1177/096368979500400408. [DOI] [PubMed] [Google Scholar]
- 71.Wang R. N., Kloppel G., Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 72.Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 73.Ramiya V. K., Maraist M., Arfors K. E., Schatz D. A., Peck A. B., Cornelius J. G. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 74.Bonner-Weir S., Taneja M., Weir G. C., Tatarkiewicz K., Song K. H., Sharma A., O'Neil J. J. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pour P. M., Pandey K. K., Batra S. K. What is the origin of pancreatic adenocarcinoma? Mol. Cancer. 2003;2:13. doi: 10.1186/1476-4598-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fishman M. P., Melton D. A. Pancreatic lineage analysis using a retroviral vector in embryonic mice demonstrates a common progenitor for endocrine and exocrine cells. Int. J. Dev. Biol. 2002;46:201–207. doi: 10.1387/ijdb.011552. [DOI] [PubMed] [Google Scholar]
- 77.Hao E., Tyrberg B., Itkin-Ansari P., Lakey J. R., Geron I., Monosov E. Z., Barcova M., Mercola M., Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat. Med. 2006;12:310–316. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 78.Baeyens L., De Breuck S., Lardon J., Mfopou J. K., Rooman I., Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- 79.Minami K., Okuno M., Miyawaki K., Okumachi A., Ishizaki K., Oyama K., Kawaguchi M., Ishizuka N., Iwanaga T., Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zulewski H., Abraham E. J., Gerlach M. J., Daniel P. B., Moritz W., Muller B., Vallejo M., Thomas M. K., Habener J. F. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 81.Hunziker E., Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem. Biophys. Res. Commun. 2000;271:116–119. doi: 10.1006/bbrc.2000.2611. [DOI] [PubMed] [Google Scholar]
- 82.Selander L., Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech. Dev. 2002;113:189–192. doi: 10.1016/s0925-4773(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 83.Lardon J., Rooman I., Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem. Cell Biol. 2002;117:535–540. doi: 10.1007/s00418-002-0412-4. [DOI] [PubMed] [Google Scholar]
- 84.Piper K., Ball S. G., Turnpenny L. W., Brickwood S., Wilson D. I., Hanley N. A. Beta-cell differentiation during human development does not rely on nestin-positive precursors: implications for stem cell-derived replacement therapy. Diabetologia. 2002;45:1045–1047. doi: 10.1007/s00125-002-0864-z. [DOI] [PubMed] [Google Scholar]
- 85.Gu G., Dubauskaite J., Melton D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 86.Deutsch G., Jung J., Zheng M., Lora J., Zaret K. S. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 87.Seaberg R. M., Smukler S. R., Kieffer T. J., Enikolopov G., Asghar Z., Wheeler M. B., Korbutt G., van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 88.Dor Y., Brown J., Martinez O. I., Melton D. A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 89.Okada T. Oxford: Calderon Press; 1991. Transdifferentiation: Flexibility in Cell Differentiation. [Google Scholar]
- 90.Pomerantz J., Blau H. M. Nuclear reprogramming: a key to stem cell function in regenerative medicine. Nat. Cell Biol. 2004;6:810–816. doi: 10.1038/ncb0904-810. [DOI] [PubMed] [Google Scholar]
- 91.Petersen B. E., Bowen W. C., Patrene K. D., Mars W. M., Sullivan A. K., Murase N., Boggs S. S., Greenberger J. S., Goff J. P. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 92.Alison M. R., Poulsom R., Jeffery R., Dhillon A. P., Quaglia A., Jacob J., Novelli M., Prentice G., Williamson J., Wright N. A. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 93.Theise N. D., Nimmakayalu M., Gardner R., Illei P. B., Morgan G., Teperman L., Henegariu O., Krause D. S. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 94.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 95.Wang X., Willenbring H., Akkari Y., Torimaru Y., Foster M., Al-Dhalimy M., Lagasse E., Finegold M., Olson S., Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 96.Tang D. Q., Lu S., Sun Y. P., Rodrigues E., Chou W., Yang C., Cao L. Z., Chang L. J., Yang L. J. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab. Invest. 2006;86:83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rao M. S., Dwivedi R. S., Subbarao V., Usman M. I., Scarpelli D. G., Nemali M. R., Yeldandi A., Thangada S., Kumar S., Reddy J. K. Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem. Biophys. Res. Commun. 1988;156:131–136. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- 98.Krakowski M. L., Kritzik M. R., Jones E. M., Krahl T., Lee J., Arnush M., Gu D., Sarvetnick N. Pancreatic expression of keratinocyte growth factor leads to differentiation of islet hepatocytes and proliferation of duct cells. Am. J. Pathol. 1999;154:683–691. doi: 10.1016/S0002-9440(10)65315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolfe-Coote S., Louw J., Woodroof C., Du Toit D. F. The non-human primate endocrine pancreas: development, regeneration potential and metaplasia. Cell Biol. Int. 1996;20:95–101. doi: 10.1006/cbir.1996.0013. [DOI] [PubMed] [Google Scholar]
- 100.Paner G. P., Thompson K. S., Reyes C. V. Hepatoid carcinoma of the pancreas. Cancer. 2000;88:1582–1589. doi: 10.1002/(sici)1097-0142(20000401)88:7<1582::aid-cncr12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 101.Shen C. N., Slack J. M., Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- 102.Tosh D., Shen C. N., Slack J. M. Differentiated properties of hepatocytes induced from pancreatic cells. Hepatology. 2002;36:534–543. doi: 10.1053/jhep.2002.35060. [DOI] [PubMed] [Google Scholar]
- 103.Kurash J. K., Shen C. N., Tosh D. Induction and regulation of acute phase proteins in transdifferentiated hepatocytes. Exp. Cell Res. 2004;292:342–358. doi: 10.1016/j.yexcr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 104.Burke Z. D., Shen C. N., Ralphs K. L., Tosh D. Characterization of liver function in transdifferentiated hepatocytes. J. Cell. Physiol. 2006;206:147–159. doi: 10.1002/jcp.20438. [DOI] [PubMed] [Google Scholar]
- 105.Marek C. J., Cameron G. A., Elrick L. J., Hawksworth G. M., Wright M. C. Generation of hepatocytes expressing functional cytochromes P450 from a pancreatic progenitor cell line in vitro. Biochem. J. 2003;370:763–769. doi: 10.1042/BJ20021545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mashima H., Ueda N., Ohno H., Suzuki J., Ohnishi H., Yasuda H., Tsuchida T., Kanamaru C., Makita N., Iiri T., et al. A novel mitochondrial Ca2+-dependent solute carrier in the liver identified by mRNA differential display. J. Biol. Chem. 2003;278:9520–9527. doi: 10.1074/jbc.m208398200. [DOI] [PubMed] [Google Scholar]
- 107.Lardon J., De Breuck S., Rooman I., Van Lommel L., Kruhoffer M., Orntoft T., Schuit F., Bouwens L. Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte-like cells in primary culture. Hepatology. 2004;39:1499–1507. doi: 10.1002/hep.20213. [DOI] [PubMed] [Google Scholar]
- 108.Westmacott A., Burke Z. D., Oliver G., Slack J. M., Tosh D. C/EBPα and C/EBPβ are markers of early liver development. Int. J. Dev. Biol. 2006;50:653–657. doi: 10.1387/ijdb.062146aw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horb M. E., Shen C. N., Tosh D., Slack J. M. Experimental conversion of liver to pancreas. Curr. Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 110.Li W. C., Horb M. E., Tosh D., Slack J. M. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech. Dev. 2005;122:835–847. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Kaneto H., Nakatani Y., Miyatsuka T., Matsuoka T. A., Matsuhisa M., Hori M., Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- 112.Kojima H., Fujimiya M., Matsumura K., Younan P., Imaeda H., Maeda M., Chan L. NeuroD–β-cellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 113.Imai J., Katagiri H., Yamada T., Ishigaki Y., Ogihara T., Uno K., Hasegawa Y., Gao J., Ishihara H., Sasano H., et al. Constitutively active PDX1 induced efficient insulin production in adult murine liver. Biochem. Biophys. Res. Commun. 2005;326:402–409. doi: 10.1016/j.bbrc.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 114.Fodor A., Harel C., Fodor L., Armoni M., Salmon P., Trono D., Karnieli E. Adult rat liver cells transdifferentiated with lentiviral IPF1 vectors reverse diabetes in mice: an ex vivo gene therapy approach. Diabetologia. 2007;50:121–130. doi: 10.1007/s00125-006-0509-8. [DOI] [PubMed] [Google Scholar]
- 115.Slack J. M., Tosh D. Transdifferentiation and metaplasia-switching cell types. Curr. Opin. Genet. Dev. 2001;11:581–586. doi: 10.1016/s0959-437x(00)00236-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.