Abstract
Aging has been reported to be accompanied by reduced mitochondrial function and insulin sensitivity. Whether these deleterious effects result from chronological age or lifestyle-related factors such as adiposity and physical inactivity remains debatable. The beneficial effects of exercise on mitochondrial function and insulin sensitivity are well documented; however, it is unclear whether exercise can effectively prevent, reverse, or delay the onset of these age-related dysfunctions. Other investigators and we have found that endurance exercise enhances mitochondrial function across the life span, highlighting the beneficial role of exercise in combating age-related mitochondrial dysfunction. The literature is mixed concerning the ability of endurance exercise to normalize age-related insulin resistance; however, emerging evidence points to adiposity rather than age per se as a primary determinant of age-related declines in insulin sensitivity. Recent data from our laboratory also shed some light on the controversial relation between mitochondrial function and insulin sensitivity. Although some investigators purport a causal role of mitochondrial dysfunction in the etiology of insulin resistance, we provide evidence that the reverse may be true based on the fact that insulin stimulates mitochondrial function in healthy control subjects but not in patients with type 2 diabetes. Furthermore, we find that these 2 variables are completely dissociated in some populations, such as Asian Indians, who exhibit elevated mitochondrial capacity despite marked insulin resistance compared with European Americans. Our data not only point to regular endurance exercise as a viable strategy to delay the onset of age-related dysfunctions but they suggest that mitochondrial function and insulin resistance may be linked by additional factors such as physical activity.
INTRODUCTION
The rapidly growing proportion of humans >60 y has spawned much interest in understanding the effects of old age on skeletal muscle, given its vital role in maintaining physical function, health, and quality of life across the life span. Although declines in muscle mass, strength, and maximal endurance with old age are well documented, the impact of aging per se on mitochondrial function is debatable and has received much attention at multiple levels. Substantial data support the view that mitochondrial function declines with old age. Indeed, oxidative capacity has been shown to be impaired with old age as evidenced by decreased mitochondrial enzyme activities (1, 2) and maximal ATP synthesis in permeabilized muscle fibers and isolated mitochondria (2, 3). Furthermore, underlying molecular and cellular disturbances, such as reduced mitochondrial protein synthesis rates (1), lower mitochondrial protein content (2), fewer gene transcripts for mitochondrial proteins (2, 4), decreased mitochondrial DNA (mtDNA) abundance (2, 5, 6), and increased DNA oxidative damage (2, 7), in older compared with young humans have been observed. This notion has been confirmed in vivo by phosphorous magnetic resonance spectroscopy (31P-MRS)-based measures of basal (8) and maximal (9) oxidative ATP production in skeletal muscle. However, both in vitro studies (10) and in vivo studies using 31P-MRS (11) have contradicted the notion of an age-related decline in muscle mitochondrial oxidative capacity. A unifying theme discussed in many of these works (10–13) is whether age-related changes in mitochondrial function are inevitable consequences of chronological age or are secondary to age-associated declines in physical activity, as observed in organisms such as nematodes (14), rodents (15), and humans (16). Spontaneous physical activity (defined as hypothalamic-regulated physical activity distinct from purposeful, voluntary activity) has been implicated in human obesity (17) and hypothesized to be reduced with senescence as a consequence of mitochondrial dysfunction (18). A vicious cycle has been proposed whereby a sedentary lifestyle leads to decreased mitochondrial function, decreased spontaneous physical activity, and, in turn, further decreases in mitochondrial function to the point where physical function is impaired.
EFFECTS OF ENDURANCE EXERCISE ON MITOCHONDRIAL FUNCTION
Since the publication of Holloszy's (19) pioneering work, an extensive body of literature has accumulated demonstrating that endurance exercise effectively stimulates mitochondrial biogenesis and increases muscle oxidative capacity. We recently investigated distinct mechanisms underlying exercise-induced improvements in mitochondrial function in a murine model of endurance training (20). Eight weeks of treadmill training (80% peak O2 uptake, 5 d/wk) augmented mitochondrial function, as reflected by increased mitochondrial enzyme activities (Figure 1A), maximal rate of ATP synthesis in isolated mitochondria (Figure 1B), and whole-body maximal O2 uptake (Figure 1C). Exercise-induced increases in mitochondrial function were accompanied by increased transcript levels of nuclear and mitochondrial genes encoding mitochondrial proteins, mitochondrial DNA abundance, and expression of peroxisome proliferator activated receptor γ coactivator 1α (PGC1α; protein) and mitochondrial transcription factor A (TFAM; mRNA). A key finding of this study was that exercise training also increased spontaneous physical activity (Figure 1D) as measured with infrared photocell sensors and was significantly correlated with mitochondrial ATP production capacity. This study has important implications for aging because it supports the notion of a relation between mitochondrial function and spontaneous physical activity; that is, endurance training increases mitochondrial function, stimulates spontaneous physical activity, and is a viable approach to interrupt the vicious cycle of aging.
FIGURE 1.
A: Training effects on mitochondrial function, V̇O2 peak, and spontaneous activity. Citrate synthase, l-3-hydroxyacyl coenzyme A dehydrogenase (BHAD), and cytochrome c oxidase activities increased with 8 wk of endurance exercise in Friend virus B-type (FVB) mice (n = 8 per group). B: Exercise also increased mitochondrial ATP production rates with the use of substrate combinations glutamate + malate (GM), palmitoyl-l-carnitine + malate (PCM), and succinate + rotenone (SR) (n = 8 per group). Maximal oxygen uptake expressed per unit of fat-free mass (FFM) (n = 8 per group) (C) and spontaneous physical activity (D) increased with exercise training (n = 12 untrained, 8 trained). Data are presented as means ± SEMs. Reprinted with permission from reference 20.
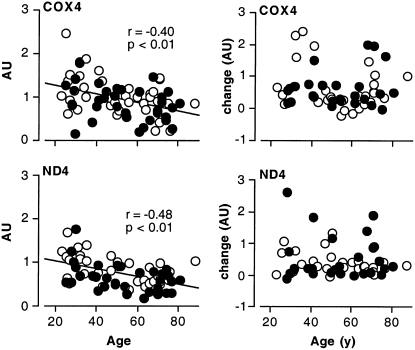
Indeed, there has been enormous interest in the potential utility of exercise as an intervention to prevent, reverse, or delay age-related mitochondrial and other metabolic dysfunctions in humans. An inclusive review of the numerous investigations into the adaptations of young and older individuals to exercise is beyond the scope of this short review. Briefly, older adults enrolled in exercise training programs respond with increased V̇O2 peak (21–23), mitochondrial content (5, 24), oxidative enzyme activities (5, 21, 23), muscle protein synthesis rates (25, 26), mitochondrial protein gene transcripts (23), and mitochondrial DNA copy number (5). For example, we investigated the effects of a 16-wk aerobic exercise program on several markers of mitochondrial function in previously sedentary individuals aged 21–87 y (23). Regression analyses revealed an age-related decline in citrate synthase (CS) and cytochrome c oxidase (COX) activities, 2 common markers of mitochondrial function. Furthermore, mRNA expression of COX4 and NADH dehydrogenase subunit 4 (ND4) also declined with age, as shown in Figure 2. In this study, we found that a 4-mo endurance-training program increased CS and COX activity as well as the mRNA expression of mitochondrial genes (COX4 and ND4) and transcription factors (PGC1α, NRF1, and TFAM). An important finding in this investigation was that the response to aerobic exercise training did not differ by age, as shown for COX and ND4 mRNA abundance in Figure 2. These data underscore the notion that endurance exercise confers significant improvements in mitochondrial function regardless of age.
FIGURE 2.
mRNA expression of mitochondrial proteins with age and exercise. mRNA transcripts of cytochrome c oxidase 4 (COX4) and NADH dehydrogenase 4 (ND4) declined significantly with age in men (open circles) and women (solid circles) combined (left panels). Exercise increased mRNA expression of COX4 and ND4, with no age differences in the absolute differences between pre- and posttraining measurements (right panels). Data are normalized to 28S rRNA; n = 78. AU, arbitrary units. Reprinted with permission from reference 23.
Although numerous studies demonstrated a robust adaptability of older skeletal muscle to training, the function of older muscle after training often remained at or below the level of young untrained subjects and well below the level of young subjects who engaged in similar training (5, 21, 23, 25). A lingering question is whether older muscle is less adaptable to exercise training than young muscle or whether the volume and duration of most training regimes are insufficient to reveal the full potential for adaptation in both age groups. Indeed, practical considerations limit most prospective training studies to weeks or months in duration, whereas the chronic effects of aging manifest over the bulk of the entire life span. Long-term training studies in previously sedentary individuals or longitudinal studies of habitual exercisers are needed to determine the extent to which regular endurance exercise is able to prevent or delay the onset of age-related mitochondrial dysfunction.
DETERMINANTS OF AGE-RELATED DECLINES IN INSULIN SENSITIVITY AND THE EFFECTS OF EXERCISE
In addition to age-related mitochondrial defects, numerous investigators have shown impaired glucose tolerance and insulin sensitivity in older adults (8, 23, 27, 28). Once again, however, the literature is divided concerning whether age per se is an independent predictor of insulin resistance. Numerous groups have found that body composition and physical fitness are primary determinants of age-related insulin resistance (23, 29–31). For example, Basu et al (29) measured insulin action in 67 elderly and 21 young individuals by means of mixed meal and intravenous glucose-tolerance test models. Both models indicated decreased insulin action in the elderly but not after adjustments for percentage body fat and visceral fat were made. Similarly, we previously reported an age-related decline in insulin sensitivity that was more strongly correlated to abdominal adiposity than to the chronological age of the participants (Figure 3, A and B) (23). This appears to be a recurrent finding in the literature. Although some investigators report age-related declines in insulin sensitivity despite matching age groups for body mass index (BMI) (8, 32), there are many reports demonstrating that body composition and central adiposity, but not age, are powerful predictors of whole-body insulin action (23, 28–31).
FIGURE 3.
Relation between insulin sensitivity and age, abdominal fat content, and postexercise change in insulin sensitivity. The insulin sensitivity index (SI) was more closely related to abdominal adiposity (B) than age (A) in men (open circles) and women (solid circles) combined. The difference between pre- and posttraining SI (C) and the percentage change in SI (D) were inversely related to age, indicating blunted increases in insulin sensitivity with training in older individuals; n = 78. Reprinted with permission from reference 23.
Regardless of the exact determinants, insulin resistance is prevalent in the elderly and is implicated in the development of type 2 diabetes and metabolic syndrome with age. The onus is on clinicians and researchers to investigate and use effective strategies to prevent or delay the onset of age-related insulin resistance. One such strategy is exercise, which has well-documented effects on increasing insulin action (33). These beneficial effects of exercise on insulin action have been observed in the elderly as well (34–36). Similar to the effects of exercise on mitochondrial function, there remains some question as to whether the insulin-sensitizing effects of exercise are lessened with old age. Data from our laboratory showed that 16 wk of moderately intense cycling increased GLUT4 mRNA and protein expression to similar levels in young and older adults, although the increment in whole-body insulin sensitivity with training was blunted in the older group (Figure 3, C and D). This study suggests that the beneficial effects of exercise on insulin sensitivity may be blunted, slower to occur, or more rapidly lost after exercise; however, additional longer-term prospective training studies or cross-sectional comparisons of chronically endurance-trained young and older individuals are required to more completely address this issue. Nevertheless, endurance exercise training is a promising tool for combating the insulin resistance that would likely otherwise occur in sedentary, overweight individuals as they age.
CONTROVERSIAL RELATION BETWEEN MITOCHONDRIAL FUNCTION AND INSULIN SENSITIVITY
Concurrent impairments in mitochondrial function and insulin sensitivity are often observed not only with old age (2, 8, 23) but also in people with type 2 diabetes (37) and obese individuals (38). The associations between insulin resistance and mitochondrial dysfunction have prompted 3 distinct hypotheses concerning this relation: 1) insulin resistance may be a consequence of mitochondrial dysfunction, 2) mitochondrial dysfunction may result from insulin resistance, and 3) mitochondrial dysfunction and insulin resistance may be coincidental. Some recent data from our laboratory point to impaired insulin sensitivity as a determinant of mitochondrial dysfunction rather than the reverse. A report by Stump et al (39) demonstrates that an 8-h insulin infusion (1.5 mU/kg fat-free mass/min) in humans increased the capacity for skeletal muscle mitochondrial ATP production in parallel with increased mitochondrial enzyme activities, mRNA expression of mitochondrial proteins, and fractional synthesis rates of mitochondrial proteins. The effect of insulin on stimulating mitochondrial ATP production was absent in people with type 2 diabetes, which was later confirmed in a larger cohort of type 2 diabetic and nondiabetic subjects (40). Moreover, in contrast to aging populations in which mtDNA copy number and maximal ATP production are reduced (2, 5, 6), individuals with type 2 diabetes have no reduction in baseline mtDNA copy number or ATP production despite blunted increments in ATP production with increasing insulin levels (40). These studies have not only established a role for insulin as a key regulator of mitochondrial function, they also support the hypothesis that mitochondrial dysfunction in insulin-resistant individuals may be a consequence of impaired insulin signaling and possibly reduced fuel metabolism.
We and other investigators recently reported a dissociation between mitochondrial function and insulin sensitivity. As discussed above, we showed that 16 wk of aerobic exercise training increases mitochondrial oxidative capacity but not insulin sensitivity in older adults (23). More recently, we further demonstrated this apparent dissociation in Asian Indian adults who, despite stark insulin resistance, exhibited higher oxidative capacity and mtDNA abundance compared with people of northern European descent (41) (Figure 4). Other examples of this dissociation can be seen in recently published work from other groups. For example, Toledo et al (42) showed that weight loss by diet without exercise significantly improves insulin sensitivity but not mitochondrial oxidative capacity. Others have found that mitochondrial function is normal in skeletal muscle of patients with type 2 diabetes (43). Pospisilik et al (44) demonstrated that apoptosis-inducing factor (AIF) knockout mice exhibit reduced mitochondrial capacity but are more insulin-sensitive than control animals. These data underscore the fact that, although mitochondrial dysfunction and insulin resistance often occur commensurately in some populations, an accumulating body of evidence suggests that this relation may be coincidental or linked by some common factor such as physical inactivity.
FIGURE 4.
Insulin sensitivity and mitochondrial ATP production in nondiabetic European Americans and diabetic and nondiabetic Asian Indians. Glucose infusion rates per unit of fat-free mass (FFM) during an 8-h euglycemic hyperinsulinemic clamp were significantly lower in Asian Indians compared with European Americans (A). ATP production rates with substrates succinate + rotenone (SR), pyruvate + malate (PM), glutamate + malate (GM), palmitoyl-l-carnitine + α-ketoglutarate + malate (PPKM), α-ketoglutarate (KG), and palmitoyl-l-carnitine + malate (PCM) were significantly higher in Asian Indians than in European Americans despite lower insulin sensitivity. Data presented as means ± SEMs; n = 13 per group. *Significantly (P < 0.05) different from European Americans. Reprinted with permission from reference 41.
CONCLUSIONS
In conclusion, there is emerging evidence that although mitochondrial dysfunction and insulin resistance are often considered hallmarks of the aging process, many of these detrimental effects appear to be secondary to physical inactivity and adiposity rather than chronological age. Numerous investigations by our group and others have demonstrated clear beneficial effects of endurance-exercise training on mitochondrial function and insulin sensitivity in young and older adults. The extent to which regular endurance exercise is able to prevent, delay, or reverse such detrimental effects of aging will be revealed by longitudinal studies of habitual exercisers, longer-term training studies, or cross-sectional comparisons of sedentary and chronically endurance-trained young and older adults. It remains to be shown whether decreased activity levels are an obligatory or preventable consequence of aging in humans and other species. Evidence suggests that deliberate maintenance of motor activities may slow the decline in spontaneous activity, mitochondrial function, and insulin action that occurs with old age. (Other articles in this supplement to the Journal include references 45 and 46.)
Acknowledgments
The authors' responsibilities were as follows—IRL: contributed to writing of the manuscript; KSN: conceived and designed the studies, interpreted the findings, and contributed to writing of the manuscript. Both authors read and edited the draft of the manuscript. Neither author had any financial or personal conflicts of interest.
REFERENCES
- 1.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 1996;93:15364–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 2005;102:5618–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonkonogi M, Fernstrom M, Walsh B, et al. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch 2003;446:261–9 [DOI] [PubMed] [Google Scholar]
- 4.Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: comparison between young and old. J Appl Physiol 2000;89:297–304 [DOI] [PubMed] [Google Scholar]
- 5.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 2006;61:534–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 2000;275:3343–7 [DOI] [PubMed] [Google Scholar]
- 7.Melov S, Shoffner JM, Kaufman A, Wallace DC. Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle. Nucleic Acids Res 1995;23:4122–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 2000;526:203–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol 2003;38:877–86 [DOI] [PubMed] [Google Scholar]
- 11.Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol 2000;89:1072–8 [DOI] [PubMed] [Google Scholar]
- 12.Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med 2004;34:221–9 [DOI] [PubMed] [Google Scholar]
- 13.Brierley EJ, Johnson MA, James OF, Turnbull DM. Effects of physical activity and age on mitochondrial function. QJM 1996;89:251–8 [DOI] [PubMed] [Google Scholar]
- 14.Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002;419:808–14 [DOI] [PubMed] [Google Scholar]
- 15.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol 1997;82:399–403 [DOI] [PubMed] [Google Scholar]
- 16.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr 1996;50:72–92 [PubMed] [Google Scholar]
- 17.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science 2005;307:584–6 [DOI] [PubMed] [Google Scholar]
- 18.Nair KS. Aging muscle. Am J Clin Nutr 2005;81:953–63 [DOI] [PubMed] [Google Scholar]
- 19.Holloszy JO. Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 1967;242:2278–82 [PubMed] [Google Scholar]
- 20.Chow LS, Greenlund LJ, Asmann YW, et al. Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function. J Appl Physiol 2007;102:1078–89 [DOI] [PubMed] [Google Scholar]
- 21.Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 1992;72:1780–6 [DOI] [PubMed] [Google Scholar]
- 22.Kohrt WM, Malley MT, Coggan AR, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J Appl Physiol 1991;71:2004–11 [DOI] [PubMed] [Google Scholar]
- 23.Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52:1888–96 [DOI] [PubMed] [Google Scholar]
- 24.Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 2001;90:1663–70 [DOI] [PubMed] [Google Scholar]
- 25.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab 2001;280:E203–8 [DOI] [PubMed] [Google Scholar]
- 26.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 2000;278:E620–6 [DOI] [PubMed] [Google Scholar]
- 27.DeFronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes 1979;28:1095–101 [DOI] [PubMed] [Google Scholar]
- 28.Houmard JA, Weidner MD, Dolan PL, et al. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 1995;44:555–60 [DOI] [PubMed] [Google Scholar]
- 29.Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003;52:1738–48 [DOI] [PubMed] [Google Scholar]
- 30.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes 1993;42:273–81 [PubMed] [Google Scholar]
- 31.Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care 2008;31:539–43 [DOI] [PubMed] [Google Scholar]
- 32.Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest 1983;71:1523–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes VA, Fiatarone MA, Fielding RA, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol 1993;264:E855–62 [DOI] [PubMed] [Google Scholar]
- 34.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. JAMA 1984;252:645–9 [PubMed] [Google Scholar]
- 35.Cox JH, Cortright RN, Dohm GL, Houmard JA. Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol 1999;86:2019–25 [DOI] [PubMed] [Google Scholar]
- 36.Kahn SE, Larson VG, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol 1990;258:E937–43 [DOI] [PubMed] [Google Scholar]
- 37.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–50 [DOI] [PubMed] [Google Scholar]
- 38.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 1997;83:166–71 [DOI] [PubMed] [Google Scholar]
- 39.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 2003;100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asmann YW, Stump CS, Short KR, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006;55:3309–19 [DOI] [PubMed] [Google Scholar]
- 41.Nair KS, Bigelow ML, Asmann YW, et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin-resistance. Diabetes 2008;57:1166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 2008;57:987–94 [DOI] [PubMed] [Google Scholar]
- 43.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007;50:790–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pospisilik JA, Knauf C, Joza N, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007;131:476–91 [DOI] [PubMed] [Google Scholar]
- 45.Holloway GP, Bonen A, Spriet LL. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr 2009;89(suppl):455S–62S [DOI] [PubMed] [Google Scholar]
- 46.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 2009;89(suppl):463S–6S [DOI] [PubMed] [Google Scholar]