Abstract
The preimplantation embryo floats freely within the oviduct and is capable of developing into a blastocyst independently of the maternal reproductive tract. While establishment of the trophoblast lineage is dependent on expression of developmental regulatory genes, further differentiation leading to blastocyst implantation in the uterus requires external cues emanating from the microenvironment. Recent studies suggest that trophoblast differentiation requires intracellular signaling initiated by uterine-derived growth factors and integrin-binding components of the extracellular matrix. The progression of trophoblast development from the early blastocyst stage through the onset of implantation appears to be largely independent of new gene expression. Instead, extrinsic signals direct the sequential trafficking of cell surface receptors to orchestrate the developmental program that initiates blastocyst implantation. The dependence on external cues could coordinate embryonic activities with the developing uterine endometrium. Biochemical events that regulate trophoblast adhesion to fibronectin are presented to illustrate a developmental strategy employed by the peri-implantation blastocyst.
Keywords: Blastocyst implantation, Trophoblast, Integrins, Extracellular matrix, Cell adhesion, Cell invasion, Epigenetic regulation, Paracrine signaling, Intracellular calcium, Protein trafficking
Introduction and background
The trophoblast lineage is responsible for sustaining a mammalian conceptus throughout gestation. Beginning with their progenitors derived from the outer layer of the preimplantation embryo, the trophectoderm, trophoblast cells perform a multitude of functions essential for blastocyst formation, implantation, and placentation. At the late blastocyst stage, the trophoblast attaches to the endometrium and invades through the uterine epithelium to infiltrate the interstitium (Carson et al., 2000). Blastocyst implantation is the initial step of placentation and the trophoblast comprises a major component of the placenta. Trophoblast cells differentiate into several subtypes that serve a variety of functions, including invasive remodeling of the maternal vasculature and molecular transport at the maternal–fetal interface (Adamson et al., 2002; Pijnenborg et al., 1981). Thus, the trophoblast is ultimately responsible for ensuring adequate flow of maternal blood to the placenta, as well as shuttling nutrients, gases, and waste products between maternal and fetal blood.
While the complexity of trophoblast differentiation has been well described, the biochemical mechanisms that guide trophoblast development are still the subject of intense investigation. For example, it is clear that trophoblast stem cells are capable of converting to diverse phenotypes depending on where in the conceptus they are located. But how does cell position determine cell behavior? What is the molecular basis of the phenotypic plasticity of the trophoblast? Differentiation of the many trophoblast subtypes, as described below, is highly complex, making these questions difficult to answer. The major influence of extrinsic signals in directing trophoblast differentiation could account for their sensitivity to positional information. The molecular (Cross, 2000; Kunath et al., 2004; Red-Horse et al., 2004) and cellular (Denker, 1993; Sutherland, 2003) aspects of trophoblast differentiation based on studies in experimental species have been detailed in previous reviews. Here, we will examine trophoblast differentiation during blastocyst implantation in mice with respect to mechanisms that regulate their adhesion to the extracellular matrix (ECM) as they commence invasion of the endometrium. The biochemical process that establishes trophoblast adhesion provides a paradigm for understanding how the maternal and embryonic developmental programs operate in harmony. Trophoblast cells incorporate specific molecular cues obtained from their surroundings to advance stepwise toward an invasive phenotype. Through this directed process, trophoblast behavior can be intricately coordinated with changing conditions in the endometrium. Similar interactive strategies could guide other aspects of trophoblast development and conversion to trophoblast subtypes during placentation.
The maternal–embryonic dialogue
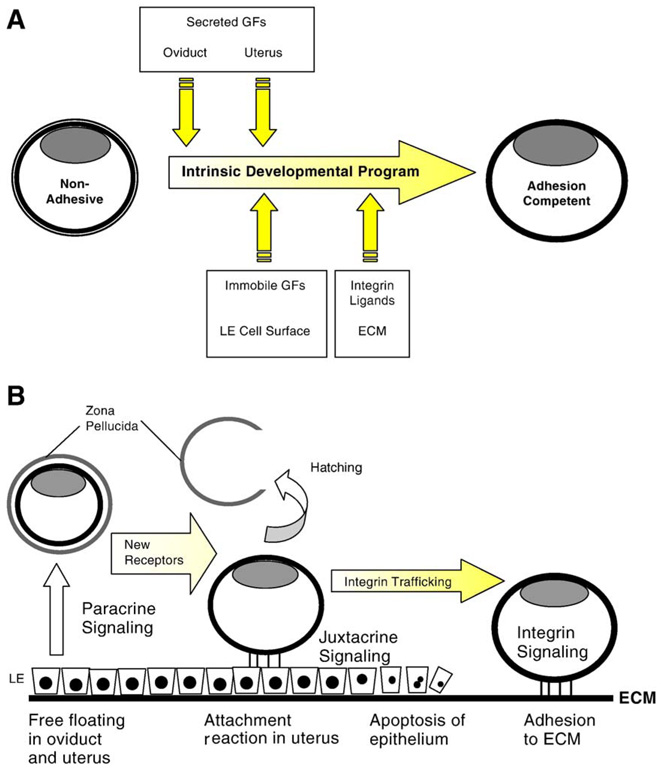
Blastocyst implantation is dependent on the intrinsic embryonic program, operating in conjunction with extrinsic signals emanating from the female reproductive tract (Fig. 1A). Uterine-derived signals maintain the pace of blastocyst development and mobilize embryonic receptors used for subsequent signaling (Armant et al., 2000). Decidualization of the endometrium is, in turn, regulated by the blastocyst (Psychoyos, 1973). The presence of a blastocyst is required for uterine expression of heparin-binding EGF-like growth factor (HB-EGF), followed by production of betacellulin, epiregulin, neuregulin-1, and cyclooxygenase-2 as the blastocyst attaches to the luminal epithelium (Dey et al., 2004). This molecular dialogue serves to synchronize embryonic and maternal tissues during the peri-implantation period. Like the mature oocyte, the blastocyst is primed to advance in development only after it receives an external signal. Rather than a sperm, the blastocyst awaits contact with the receptive endometrium. As in the developing zygote, blastocyst differentiation relies on transcripts synthesized at a prior stage (Armant et al., 2000; Schindler and Sherman, 1981). In this article, evidence will be reviewed illustrating that crucial gene products required for blastocyst implantation are activated only after the endometrium signals its readiness.
Fig. 1.
Maternal–embryonic interactions during peri-implantation development. (A) The intrinsic developmental program will direct blastocyst differentiation (yellow shading) to the adhesion competent stage, but extrinsic factors (intersecting yellow arrows) impact the outcome. As development progresses, the nature of the extrinsic factors is altered by embryo location and by physiological changes in both embryonic and uterine tissues. These signals accelerate the rate of trophoblast differentiation and induce cell competence for subsequent interactions with other extrinsic signals by mobilizing new receptors. (B) Growth factors (GFs) and other soluble bioactive molecules secreted by the oviduct and uterus provide paracrine signals to the embryo. These signals could trigger protein trafficking to the surface of trophoblast cells to activate receptors for new extrinsic signals. After hatching from the zona pellucida, juxtacrine signaling can occur at the interacting surfaces of trophoblast and luminal epithelial (LE) cells. Integrin trafficking induced by these interactions imparts competence for blastocyst adhesion to the ECM. As the blastocyst invades through the LE, contact with the basement membrane and decidual ECM activates integrin signaling required for strong trophoblast adhesion and subsequent invasion of the decidua. Later during placentation, extrinsic signaling could account for the differentiation of trophoblast cells along several divergent developmental pathways.
Mammals have evolved to sequentially deliver extrinsic signals to the preimplantation embryo by taking advantage of its location within the reproductive tract, temporal changes in the endometrium, and accessibility of embryonic cells to maternal compartments. Preimplantation embryos are initially surrounded by an ECM, the zona pellucida, that limits paracrine signaling; only soluble molecules present in fluids of the oviduct and uterus can penetrate the zona and reach the embryo (Fig. 1B). Once freed of the zona pellucida, the blastocyst can interact directly with cells and tissues. Growth factors tethered to cell surfaces become accessible for juxtacine signaling (Fig. 1B), widening the scope of interactions. For example, epithelial cells adjacent to the hatched blastocyst produce the transmembrane form of HB-EGF, which activates receptors on the surface of trophoblast cells (Raab et al., 1996). HB-EGF accelerates the rate of blastocyst differentiation in vitro, in part by stimulating α5β1 integrin trafficking to the apical surface of trophoblast cells (Wang et al., 2000). Later, the epithelial cells adjacent to the embryo are lost and ECM components of the underlying basement membrane become accessible to the trophoblast (Fig. 1B). We will discuss in this article how integrin signaling mediated by α5β1 mobilizes other integrins that strengthen trophoblast adhesion to fibronectin. An important second messenger integrating signals that reach the embryo from both growth factors and ECM is Ca2+, which plays a similar role in egg activation by initiating protein trafficking. The fine-tuning of trophoblast differentiation by extrinsic signals orchestrates the molecular interactions required for implantation in a precise and metabolically efficient sequence.
A brief overview of trophoblast differentiation
Trophectoderm is the first polarized epithelium to appear during mammalian development. It emerges through the assembly of tight cellular junctions and inward movement of water to generate the large cavity characteristic of a blastocyst (Collins and Fleming, 1995; Watson and Barcroft, 2001). The trophectoderm does not contribute any tissues to the fetus, but rather establishes the trophoblast lineage that produces the placenta and extraembryonic membranes. The fetus is derived solely from a cluster of non-polar, pluripotent cells situated eccentrically within the blastocyst, the inner cell mass (ICM).
At the implantation stage in mice, mural trophoblast cells (those not contacting the ICM) differentiate into primary trophoblast giant cells (TGCs) that undergo endoreduplication and initiate invasion of the endometrium (Dickson, 1963; Kirby et al., 1967). The primary TGCs anchor the embryo in the uterine wall and form the outermost layer of the parietal yolk sac (Cross et al., 1994; Gardner et al., 1973). The polar trophoblast cells remain diploid due to signals, including fibroblast growth factor-4 (FGF-4), they receive from the adjacent ICM (Chai et al., 1998; Tanaka et al., 1998). As polar trophoblast cells proliferate, those displaced away from the ICM are incorporated into the mural trophoblast and differentiate into primary TGCs (Gardner, 2000).
During mouse placentation, components of the ICM and polar trophoblast develop into the extraembryonic ectoderm and ectoplacental cone (EPC), respectively (Adamson et al., 2002; Gardner et al., 1973). Stem cells residing in the extraembryonic ectoderm provide a reservoir of trophoblast cells for the growing placenta (Tanaka et al., 1998). The core of the EPC contains diploid trophoblast cells that form the outer spongiotrophoblast and inner labyrinthine layers of the placenta. In the labyrinthine layer, the site of exchange between maternal and fetal blood, diploid trophoblast cells fuse to form syncytiotrophoblasts. At midgestation, a population of trophoblast glycogen cells appears transiently within the spongiotrophoblast layer and invade out to the distal regions of the decidua (Adamson et al., 2002; Redline and Lu, 1989). Trophoblast cells at the periphery of the placenta differentiate into secondary TGCs that closely resemble the primary TGCs. Secondary TGCs secrete hormones, including placental lactogens and proliferin, and invade the uterine arteries (Adamson et al., 2002; Faria et al., 1990). Using laboratory animal models and trophoblast stem cells induced to differentiate in vitro, genes have been described that determine each phenotype, ranging from undifferentiated trophoblast stem cells to syncytiotrophoblasts and TGCs (Rossant and Cross, 2001). These discoveries reveal the importance of intercellular signaling in placental morphogenesis.
Blastocyst implantation
Conversion of trophoblast cells to an adhesive phenotype during peri-implantation development proceeds in a stepwise fashion that is coordinately regulated with endometrial differentiation, as depicted in Fig. 1B. It is crucial that the blastocyst becomes competent for implantation during the brief “window” of uterine receptivity for implantation (Paria et al., 1993). The process of implantation may be subdivided into phases delineated by critical cellular interactions between the trophoblast and uterine endometrium (Schlafke and Enders, 1975). Initially, tight apposition occurs between the embryo and uterine epithelium as the blastocyst lodges in a crypt of the uterine folds and localized edema of the endometrium molds an implantation chamber around the embryo. During this period, the blastocyst hatches from the zona pellucida. The apical surface of the mural trophoblast then attaches to the opposing surface of the luminal epithelium. Blastocyst attachment initiates decidualization of fibroblast cells in the surrounding endometrial stroma. The attachment reaction is followed by apoptosis of those luminal epithelial cells contacting the blastocyst, providing access to the underlying basement membrane. In the final stage, trophoblast cells undergo changes in cell polarity and adhesiveness, allowing them to complete their differentiation to the invasive phenotype. Invading trophoblast cells breach the uterine basement membrane to infiltrate the decidua.
While trophoblast differentiation at implantation varies among species, the general processes of apposition, attachment, acquisition of apical adhesiveness, and invasion apply to human embryos, as well as mice. The basolateral surfaces of undifferentiated trophectoderm cells adhere to a basement membrane that surrounds the blastocyst cavity, but their apical surfaces are non-adhesive, typical of epithelial cells. After the blastocyst hatches from the zona pellucida, the apical surface of the mural trophoblast differentiates (Armant et al., 1986a; Sherman and Atienza-Samols, 1978), altering its ability to interact with other cells or ECM. Efforts in the past two decades have focused on understanding the ontogeny of adhesive mechanisms on the apical surface of trophoblast cells during this brief period of development. These changes are critical for the transition from an epithelial trophectoderm to the invasive TGC phenotype responsible for blastocyst implantation.
Experimental approaches for investigating implantation
The initial stages of implantation are largely unknown in humans due to ethical limitations on experimentation. Most of our understanding of blastocyst development and early trophoblast differentiation derives from work with animal models, including non-human primates, livestock, and rodents (Carson et al., 2000). Owing to its small diameter (approx. 100 µm) and location within a relatively large uterine lumen, the implanting mouse blastocyst is a rather intractable subject for in utero biochemical approaches. To explore the embryonic side of implantation, the in vitro culture of blastocysts has provided a useful experimental tool. Newly formed blastocysts are flushed from the uterus on embryonic day 3.5 (E3.5), initiating in vitro culture on gestation day 4 (GD4, where a copulatory plug is found on GD1). The two conventions for marking time are used here to distinguish in vivo (E) from in vitro (GD) development. Cultured blastocysts recapitulate many of the peri-implantation stages that occur in utero, but at a considerably slower rate (Table 1).
Table 1.
Pre- and peri-implantation mouse embryonic developmental rates in vivo and in vitro
| Embryonic stage | Time in vivo |
Time in vitro |
|---|---|---|
| Fertilization | E0 | |
| 2-Cell embryo | E1.0 | |
| Compacted 8-cell embryo | E2.5 | |
| Early blastocyst | E3.5 | GD4 |
| Expanded blastocyst and hatching | E3.8 | GD5 |
| Attachment reaction/attachment competent | E4.0 | GD6 |
| At basement membrane/adhesion competent | E5.2 | GD7 |
| Invasion of stroma/trophoblast outgrowth | E6.2 | GD7–10 |
For in vitro culture, blastocysts are removed from the uterus on embryonic day (E) 3.5 and then cultured on a surface treated with fibronectin in serum-free medium, beginning on gestation day (GD) 4. Note that E3.5 is noon of GD4.
The central event of peri-implantation embryonic development is the conversion of the apical domain of the trophoblast plasma membrane to an adhesive surface. Initially, trophoblast cells are not reactive with maternal cells or ECM. As the trophoblast differentiates, the apical surface first becomes attachment competent, acquiring receptors that mediate interactions with the uterine luminal epithelium. The attachment reaction appears to be governed by a broad variety of molecules that inhibit or promote the attachment of trophoblast to uterine epithelial cells (Dey et al., 2004; Kimber, 2000; Kimber and Spanswick, 2000). In the next step, the blastocyst becomes adhesion competent by activating receptors for ECM on the apical surface of primary TGCs. Trophoblast adhesion to the uterine basement membrane and interstitial ECM has been particularly amenable to study in vitro. Blastocysts become adhesion competent in serum-free medium on GD7 (Table 1) and quickly undergo trophoblast dispersion and migration to form an outgrowth on surfaces coated with adhesive proteins (Armant et al., 2000).
Using the in vitro experimental approach, a great deal of biochemical information has accumulated, indicating the nature and molecular basis of embryo-endometrial interactions during implantation, as outlined in Fig. 1. Adhesive mechanisms can be analyzed by perturbing trophoblast outgrowth with specific reagents to yield information about the processes that directly mediate trophoblast adhesion (Wang and Armant, 2002) and the developmental program that converts trophoblast cells from an epithelial phenotype to invasive cells (Armant et al., 2000). Combined with data on the in utero localization of key proteins, experimental studies of blastocysts in culture have revealed novel mechanisms that orchestrate adhesive interactions of primary TGCs with molecular components of the uterine endometrium during implantation.
Blastocyst adhesion to ECM
The culture of blastocysts in vitro and formation of trophoblast outgrowths was first developed in the 1960s as an in vitro model of implantation (Gwatkin, 1966; Gwatkin and Meckley, 1966). Trophoblast outgrowth is easily observed during blastocyst culture as a spreading monolayer of cells around the base of the embryo concomitant with disappearance of the spherical blastocyst morphology. This experimental approach was used by several laboratories to examine morphological and biochemical aspects of peri-implantation blastocyst development (Enders et al., 1981; Sherman and Atienza-Samols, 1978; Spindle and Pedersen, 1973; Surani, 1979). Although there was much interest in understanding the molecular basis of trophoblast adhesion in vitro, the original media used for blastocyst culture was supplemented with serum, which contains adhesive ECM components, including fibronectin and vitronectin. For this approach to be viable, it was necessary to develop more defined culture conditions. Toward that end, successful trophoblast outgrowth was reported in serum-free medium when culture plates were coated with collagen (Jenkinson and Wilson, 1973; Wordinger and McGrath, 1979), establishing that a major function of serum was the provision of a biologically active substrate for cell adhesion. Later, fibronectin, laminin, vitronectin, entactin/nidogen, and other ECM components were used as surface coatings to generate trophoblast outgrowths in defined, serum free-medium for the purpose of understanding the biochemical basis of trophoblast adhesion to specific ECM proteins (Armant et al., 1986a,c; Yelian et al., 1993).
Trophoblast cells adhere to complex ECM and basement membranes (Cammarata et al., 1987; Wordinger et al., 1991), and are capable of differentiating on and migrating through ECM purified from mouse endometrium (Armant and Kameda, 1994). Ultrastructural evidence has established that mouse trophoblast cells adhere to the uterine basement membrane around E5.2 in vivo (Blankenship and Given, 1992), after the juxtaembryonic uterine epithelium is lost to apoptosis (Schlafke et al., 1985; Parr et al., 1987; Welsh and Enders, 1991). During blastocyst implantation, the newly formed decidua secretes a rich ECM composed of collagens, proteoglycans, fibronectin, laminin, and entactin/nidogen (Kisalus et al., 1987; Leivo et al., 1980; Rider et al., 1992; Wartiovaara et al., 1979; Wewer et al., 1986; Wu et al., 1983), which are available to support trophoblast adhesion during the invasive phase. Therefore, the interactions observed between trophoblast cells and ECM components in vitro most likely correspond to adhesive interactions that occur in utero as the primary TGCs initially adhere to the basement membrane and commence their invasion of the underlying decidua. A challenge to investigators is to understand how trophoblast cells efficiently adhere to the complex mixture of ECM proteins encountered in vivo. Studies of adhesion to fibronectin, described below, provide some insight into how that could be accomplished.
Trophoblast integrins
While many classes of proteins participate in trophoblast–epithelial interactions during the attachment reaction (Dey et al., 2004; Kimber, 2000), trophoblast adhesion to ECM appears to be mediated predominantly by integrins (Sutherland, 2003; Wang and Armant, 2002). Integrins are a family of heterodimeric (α/β) transmembrane glycoproteins that bind extracellularly to the ECM and intracellularly to adapter proteins that link them to cytoskeletal and signaling proteins (Giancotti and Ruoslahti, 1999; Hynes, 2002). A role for integrins in trophoblast adhesion was initially implicated by the inhibitory activity of an antibody (GP140) recognizing a complex of integrins (Richa et al., 1985) and a small peptide (GRGDSP) representing a sequence within the tenth type III homology repeat of fibronectin (FIII-10) recognized by integrins (Armant et al., 1986c). Conversely, trophoblast outgrowth on fibronectin is specifically inhibited by a peptide corresponding to the region of the β1 integrin subunit that recognizes the RGD sequence (Shiokawa et al., 1999). The RGD sequence appears in other ECM proteins and mediates their binding to integrins during cell adhesion cooperatively with other sequence motifs (Plow et al., 2000; Ruoslahti, 1996). The peptide GRGDSP specifically inhibits trophoblast outgrowth on fibronectin, vitronectin, type II collagen, and entactin/nidogen (Armant et al., 1986c; Carson et al., 1988; Yelian et al., 1993). However, outgrowth on laminin 1 is not blocked by this peptide (Armant et al., 1986c). The E8 domain of laminin 1, which is devoid of an RGD sequence, is responsible for that outgrowth activity (Armant, 1991). Evidence suggests that mouse trophoblast cells adhere to laminin 1 through the α7β1 integrin (Klaffky et al., 2001). The diverse array of adhesive ECM ligands and integrin receptors provides functional redundancy to ensure that trophoblast cells are able to adhere to and invade the interstitial components of the endometrium. A challenge for investigators is to now decipher individual roles of the ECM components in directing the movements and organization of trophoblast cells during placentation. Studies of knockout mice lacking specific laminin subunits suggest that laminin isoforms have different functions in directing trophoblast morphogenesis (Miner et al., 2004). Transgenic models like these could be useful for revealing the physiological impact of interactions between specific integrin–ligand partners.
Based on experiments using proteolytic fragments of fibronectin and peptide inhibitors, the specific target of adhering primary TGCs has been defined as the RGD site in a 120-kDa fragment of fibronectin (FN-120) containing the FIII-10 repeat (Yelian et al., 1995). Adhesion is unsupported by another fragment containing the IIICS segment, which serves as an alternate (non-RGD) cell recognition site. These findings are consistent with the observed expression in mouse preimplantation embryos of RGD-recognizing integrins (α5β1, αVβ3, and αIIbβ3), but not α4β1 or α4β7 that recognize the IIICS repeat of fibronectin (Schultz et al., 1997; Sutherland et al., 1993; Yelian et al., 1995). However, α4 is expressed on E8.0 in the chorion, and α4 integrin-mediated adhesion is indispensable for chorio-allantoic union (Downs, 2002; Yang et al., 1995). Many integrins are expressed as early as the oocyte stage, including the subunits α5, α6, αV, β1, and β3 (Evans et al., 1995; Fusi et al., 1993; Hierck et al., 1993; Sutherland et al., 1993; Tarone et al., 1993), demonstrating that the preimplantation embryo accumulates integrins capable of binding to fibronectin and other ECM proteins long before the implantation stage. Although integrin genes are highly expressed during preimplantation development, we will see that the presence of integrin proteins at the outer surface of the embryo is restricted. Integrin trafficking appears to be a rate-limiting step in establishing blastocyst adhesion to the ECM.
As adherent cells spread and migrate, integrins are first clustered in the plasma membrane and then organized into focal adhesions that anchor the actin cytoskeleton to the ECM to regulate cell shape and migration (Sastry and Burridge, 2000). α5β1, αVβ3, and αIIbβ3 all appear in focal adhesions formed by trophoblast cells during fibronectin-mediated outgrowth, and blocking antibodies against the β1 or β3 subunits delay outgrowth (Rout et al., 2004; Sutherland et al., 1993; Yelian et al., 1995). Although gene knockout studies suggest that none of these integrins are essential for implantation (Hynes, 1996), the three integrins appear to operate synergistically in trophoblast cells adhering to fibronectin (Rout et al., 2004). Details of the latter study will be discussed below.
Determining the onset of blastocyst adhesion
A more refined approach than the blastocyst outgrowth model is required to investigate the adhesive activity of integrins at the apical surface of mural trophoblast cells before they dissociate and migrate outward. To address this dilemma, a fibronectin-binding assay was devised using fluorescent polystyrene microspheres coated with FN-120 that are incubated for 30 min at 4°C with intact blastocysts (Schultz and Armant, 1995). The fibronectin-binding assay is ideal to investigate the onset of adhesion at the apical surface of trophoblast cells, since it is performed rapidly and detection does not require any cellular activity beyond ligand binding. Image analysis is used to quantify the intensity of the fluorescence imparted by the bound microspheres. Binding is restricted to the mural trophoblast where TGCs first differentiate. The specificity of fibronectin-binding activity is demonstrated by competitive inhibition with FN-120 or the GRGDSP peptide, but not other proteins or fragments of fibronectin lacking FIII-10. Importantly, binding activity is specifically blocked by antibodies against the integrin subunits α5, αV, αIIb, β1, and β3, which comprise the RGD-recognizing integrins α5β1, αVβ3, and αIIbβ3 (Rout et al., 2004; Schultz and Armant, 1995). The fibronectin-binding assay provides an important tool for assessing the adhesive activity of integrins on the embryo surface during peri-implantation development. The assay is useful for comparative analysis of adhesion throughout development if embryos are first treated with heparinase to eliminate microsphere binding to heparan sulfate proteoglycans. As would be expected, fibronectin-binding activity parallels trophoblast outgrowth by first appearing on GD7 (Schultz et al., 1997). Agonists capable of accelerating the rate of blastocyst outgrowth concomitantly shift the onset of fibronectin-binding activity from GD7 to GD6, substantiating the physiological relationship between this activity and the adhesion competence of blastocysts (Wang et al., 1998, 1999, 2000).
Molecular interactions between the trophoblast and fibronectin
Fibronectin is abundant in the mouse endometrial basement membrane and stromal ECM on E4.5–E5.5, when the trophoblast breaches the luminal epithelium and commences invasion (Wartiovaara et al., 1979). As peri-implantation development proceeds, fibronectin accumulates in the ECM subjacent to the basement membrane and becomes remodeled in the stroma (Rider et al., 1992; Slater and Murphy, 1999). In vitro studies of mouse E7.0 decidua explants reveal constitutive levels of fibronectin synthesis and deposition (Babiarz et al., 1996), indicative of its dynamic role during blastocyst implantation. Other ECM components, including laminins and collagens, also undergo remodeling (Farrar and Carson, 1992; Glasser et al., 1987; Wewer et al., 1986) and are deposited in isoform-specific patterns that could influence trophoblast morphology as placentation commences (Miner et al., 2004). Fibronectin colocalizes with invasive extravillous trophoblast cells of the early human implantation site (Earl et al., 1990; Feinberg et al., 1991) and it promotes anchorage of placental villous explants (Aplin et al., 1999). Some in vitro experiments suggest that fibronectin promotes trophoblast adhesion, which may restrict migration, while other studies indicate that it facilitates motility (Burrows et al., 1993; Damsky et al., 1994; Irving et al., 1995; Stephens et al., 1995; Yelian et al., 1995). Fibronectin has the ability to modulate both integrin-mediated adhesion (detailed below) and protease activity (Zhang et al., 1996) of trophoblast cells. In vivo, it could have diverse functions at different locations within the implantation site, depending on integrin expression and proximal levels of matrix-degrading proteases. Therefore, fibronectin is a relevant and instructive model ligand for studies of mouse primary TGC adhesion to ECM.
A key question addressed in studies of trophoblast interactions with fibronectin is how the non-adhesive surface of the early blastocyst acquires its adhesiveness during peri-implantation development. Since some fibronectin-binding integrins are expressed on the apical surface earlier during development, it is important to understand exactly how their activities are regulated during conversion an adhesive trophoblast surface. The evidence to be presented here suggests that trophoblast differentiation is controlled at three levels: (1) the intrinsic developmental program of the blastocyst, (2) responses to extrinsic developmental triggers, and (3) subsequent autocrine signaling by the trophoblast to reinforce extrinsic input.
Regulation of adhesion by the intrinsic developmental program
It is first important to establish the nature of the intrinsic program that advances development during the blastocyst stage in the context of trophoblast adhesiveness to fibronectin. What cellular processes are required to unfold the entire program? Is progression through the peri-implantation period dependent on new gene expression? Assessments of outgrowth and fibronectin-binding activity have been used in conjunction with metabolic inhibitors to examine the role of gene transcription, translation, and protein trafficking in regulating trophoblast development.
Regulation of gene expression
The RNA polymerase II inhibitor, α-amanitin, was originally found to have no effect on blastocyst outgrowth when present during any 24-h period between GD4 and GD7 (Schindler and Sherman, 1981). A less specific inhibitor of transcription, actinomycin D, is also a poor inhibitor of trophoblast outgrowth (Glass et al., 1976). Conditions of α-amanitin treatment have been established that completely inhibit poly(A+) RNA synthesis within 3 h in mouse blastocysts without affecting total RNA synthesis (Khidir et al., 1995). Culture of blastocysts with α-amanitin between GD4 and GD7 has no effect on the appearance of fibronectin-binding activity early on GD7 (Schultz et al., 1997), suggesting that genes regulating trophoblast adhesion are expressed prior to the early blastocyst stage, over 72 h before the event occurs. De novo gene expression during the blastocyst stage could impact ICM development, TGC endoreduplication, or postimplantation events, but appears to have little effect on the progression of trophoblast cells through adhesion and outgrowth.
The congruent findings of Schindler and Sherman (1981) and Schultz et al. (1997) stand in stark contrast to transcriptional inhibition studies at an earlier developmental stage. Treatment of 16-cell morulae with α-amanitin within 2 h of the onset of cavitation inhibits blastocyst formation (Khidir et al., 1995; Kidder and McLachlin, 1985), demonstrating temporal regulation by de novo gene expression. The effectiveness of α-amanitin at the 16-cell morula stage diminishes concern that the drug failed to inhibit transcription of critical genes at the blastocyst stage, and strengthens the view that adhesive differentiation of the trophoblast relies on genes expressed earlier during development.
So when are the genes that direct this aspect of trophoblast development transcribed? This question can be addressed by collecting embryos 24 h earlier, at the morula stage, for treatment with α-amanitin. Embryos exposed for 8 h to α-amanitin late on GD3, but not earlier in the day, exhibit significantly reduced fibronectin-binding activity after culture to GD7 (Schultz et al., 1997). This experiment defines a brief period during blastocyst formation when genes required several days later for trophoblast adhesion to fibronectin are transcribed. Based on similar experiments using cycloheximide, it has been determined that the period of development when protein synthetic activity is required for trophoblast adhesion occurs late (between 16 and 24 h) on GD4 (Schultz et al., 1997), which is in agreement with an earlier investigation that measured outgrowth rates (Blake et al., 1982). Results of studies using α-amanitin and cycloheximide suggest that genes required for the adhesive and migratory activity of primary TGCs are translated about 24 h after they are transcribed (Table 2). Whether the transcripts are sequestered as ribonucleoprotein complexes for later translation is not known.
Table 2.
Effect of metabolic inhibitors on trophoblast differentiation
| Stage of development | Effective inhibitors |
Required metabolic processes |
|---|---|---|
| Late cavitation (GD3) | α-Amanitin | mRNA transcription |
| Early blastocyst (GD4) | Cycloheximide | Protein synthesis |
| Mid-blastocyst (GD5–6) | Tunicamycin | N-glycosylation |
| Late blastocyst (GD6–7) | Brefeldin A | Protein trafficking |
| Adhesion competent (GD7) [exposed to fibronectin] |
Brefeldin A | Protein trafficking |
Morulae or blastocysts cultured to GD7 were inhibited in their ability to express strong fibronectin-binding activity or form trophoblast outgrowths when treated during the indicated stages of embryonic development with the metabolic inhibitors shown. Cycloheximide and α-amanitin were both ineffective when used before or after the indicated stages.
Global gene expression analysis of preimplantation development using microarrays indicates that relatively few mRNAs increase in abundance after the 8-cell stage (Hamatani et al., 2004a) with the possible exception of a burst of transcript accumulation between the 16-cell and blastocyst stages (Wang et al., 2004), the period of development when a-amanitin impacts trophoblast differentiation. The 16-cell morula to 32-cell blastocyst transition encompasses the period of outer cell commitment to the trophoblast lineage, since blastomeres at the 16-cell stage retain totipotency (Ziomek et al., 1982). Therefore, it is not surprising that transcription at that time is critical for trophoblast function. Discerned patterns of global gene expression during the preimplantation period support a “wave of activation” hypothesis in which transcription of gene clusters occurs well before execution of their developmental function (Hamatani et al., 2004a). Trophoblast adhesion at implantation appears to be regulated in this manner.
Two genes indispensable for trophoblast differentiation are the caudal-related homeodomain transcription factor, Cdx2 (Chawengsaksophak et al., 1997), and the mouse homologue of a T-box transcription factor, eomesodermin (mEomes) (Russ et al., 2000), which are both expressed exclusively in the trophectoderm on E3.5 (Beck et al., 1995; Hancock et al., 1999). Blastocysts lacking either of these genes fail to outgrow in vitro. Later, the genes are expressed in the extraembryonic ectoderm in response to FGF-4 signaling and are downregulated as trophoblast stem cells differentiate (Cross, 2000; Tanaka et al., 1998). The regulation of Cdx2 and mEomes and the identity of their target genes are not well understood. Cdx2 is involved in mesodermal pattern formation and activates expression of certain Hox genes (Lohnes, 2003). mEomes is associated with mesoderm differentiation from the epiblast at gastrulation and induction of genes regulating mesoderm migration (Showell et al., 2004). Sutherland (2003) points out that both trophoblast and pre-mesodermal epiblast cells express mEomes and subsequently undergo an epithelial to mesenchymal transition. Expression patterns obtained by microarray-based gene transcription profiling show a sudden accumulation of Cdx2 and mEomes between the 16-cell and blastocyst stages (Wang et al., 2004), suggesting that these regulatory genes are activated during blastocyst formation. It is thought that their expression in trophectoderm cells confers the ability to differentiate into trophoblast. Implantation rescue of Cdx2 null embryos by aggregation with wild type tetraploid blastomeres demonstrates that trophoblast failure is directly responsible for the implantation defect in mutant blastocysts (Chawengsaksophak et al., 2004). The putative functions of Cdx2 and mEomes and their patterns of expression support the prediction of α-amanitin experiments that transcription during blastocyst formation is requisite for trophoblast adhesive and migratory activities (Schindler and Sherman, 1981; Schultz et al., 1997).
Post-translational regulation
Presumably, genes activated during blastocyst formation, together with other embryonic gene products, perform the work of transforming trophoblast cells to the invasive phenotype. Since the embryonic program operates without further need for transcription or protein synthesis, post-translational mechanisms must temporally regulate peri-implantation development. During this period of 48–72 h, the apical surface of the trophoblast cell undergoes sequential modifications that direct blastocyst hatching from the zona pellucida, attachment competence, adhesion to ECM, and invasive activity. Protein turnover, glycosylation, and trafficking are among the metabolic processes that could direct trophoblast differentiation during this period in the absence of further genetic instruction.
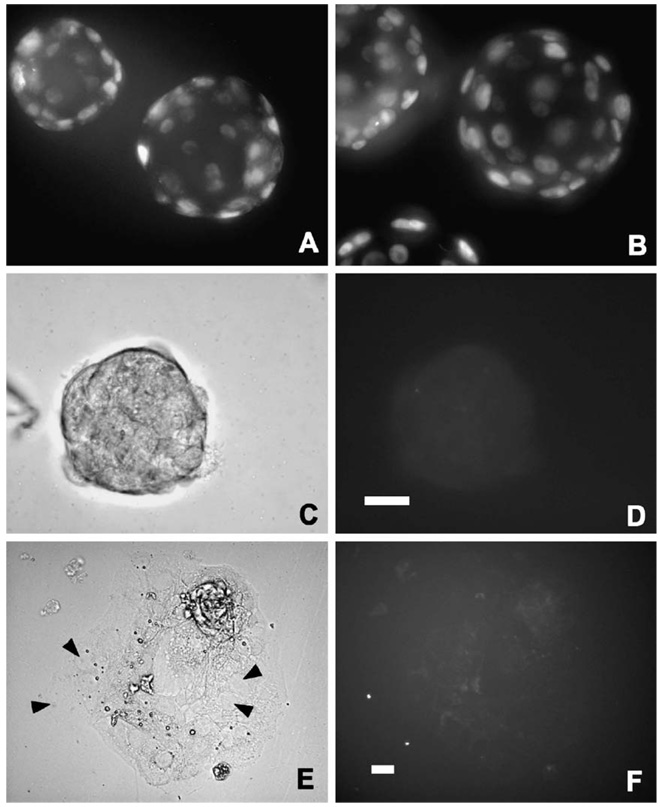
Trophoblast differentiation could be regulated in the absence of new gene induction through the degradation of RNA or proteins that maintain the non-adhesive phenotype. Targeted protein turnover is accomplished through the ubiquitination pathway, which directs specific proteins to the proteosome for degradation (Jackson et al., 2000). A component of the ubiquitination pathway, the E2 (ubiquitin conjugating) enzyme, is among genes induced at the early blastocyst stage by 0.1% ethanol (Rout et al., 1997), a treatment that activates intracellular Ca2+ signaling to accelerate blastocyst outgrowth in vitro and increase implantation rates in vivo (Stachecki et al., 1994). Ubiquitinated proteins accumulate in mouse blastocysts specifically within the trophoblast (Sutovsky et al., 2001), indicative of an increase in protein turnover as the trophoblast develops. Expression of Cdx2 and mEomes, which are abundant in trophoblast stem cells of the extraembyronic ectoderm, is downregulated during differentiation to other phenotypes, including the invasive secondary TGCs (Tanaka et al., 1998). A similar mechanism operates during primary TGC differentiation in blastocysts. As blastocysts become adhesion competent and initiate outgrowth, strong nuclear expression of Cdx2 observed at earlier stages is downregulated (Fig. 2). The half-life of proteins like Cdx2 that are transcribed during blastocyst formation could regulate trophoblast differentiation. Cdx2 and mEomes have been ascribed roles in maintaining the undifferentiated trophoblast phenotype, although no experimental evidence is available to support this hypothesis. Without more information that specifically addresses the role of protein turnover in the ontogeny of trophoblast adhesion competence, this concept remains speculative.
Fig. 2.
Downregulation of the Cdx2 transcription factor during primary TGC differentiation. Immunofluorescence was conducted according to published procedures (Liu et al., 2004) using 10 µg/ml of a mouse monoclonal antibody against the Cdx2 protein (BioGenex, San Ramon, CA) in fixed, permeablilized blastocysts (A–D) or outgrowing trophoblast cells (E–F). For blastocysts, 1-µm optical sections were obtained by fluorescence microscopy and deconvolution, as previously described (Liu et al., 2004). The deconvolved images were then recombined to produce a montage of the entire embryo. Mouse embryos were obtained at the early blastocyst stage on E3.5 (A), cultured to the mid-blastocyst stage approximately 24 h before attaining adhesion competence (B), cultured to the adhesion competent stage (C,D) or cultured on fibronectin-coated plates for 24 h after becoming adhesion competent (E,F). Nuclear localization of Cdx2 is evident in trophoblast cells before blastocysts become adhesion competent (A,B). However, the protein was dramatically downregulated in blastocysts capable of adhesion and outgrowth. A collapsed, adhesion competent blastocyst shown in bright field (C) is devoid of labeled Cdx2 (D). Giant nuclei (arrowheads in E) are abundant in outgrowing trophoblast cells, but none are labeled by the antibody (F). Scale bars, 30 µm, shown for blastocysts in D and for outgrowth in F.
The oligosaccharide moieties of glycoproteins and proteoglycans have a vital function in blastocyst attachment to the uterine epithelium (Carson, 2002; Kimber, 2000), and could impact adhesion to the ECM. Genes expressed during blastocyst formation may require post-translational modification by asparagine (N)- or serine (O)-linked oligosaccharides before they are fully functional. Integrins that mediate trophoblast adhesion to the ECM are among the proteins that require N-glycosylation (Bellis, 2004). Indeed, inhibition of N-glycosylation by treatment with tunicamycin at the mid-blastocyst stage (see Table 2) inhibits trophoblast outgrowth in vitro (Armant et al., 1986b; Surani, 1979). During the blastocyst stage, there is a sharp increase in the activity of UDP-GlcNAc: dolichol phosphate N-acetylglucosamine-1-phosphate transferase (GPT), a rate-limiting enzyme in the assembly of N-linked oligosaccharide precursors bound to dolichol (Armant et al., 1986b). Elevation of GPT enzymatic activity at the blastocyst stage is unimpeded by α-amanitin, consistent with the ability of trophoblast cells to differentiate independently of de novo mRNA synthesis. Targeted deletion of the GPT gene results in peri-implantation lethality and generates trophoblast cells incapable of survival in culture (Marek et al., 1999), establishing a requirement for N-glycosylation during implantation. The time required for protein processing by glycosylation in the endoplasmic reticulum and Golgi apparatus could influence the rate of development to the adhesive blastocyst stage.
Gene products required for cell adhesion must be processed, transported to the plasma membrane, and assembled into functional multiprotein complexes. Therefore, protein trafficking constitutes another level of intrinsic regulation during trophoblast differentiation. Brefeldin A interferes in vivo with intracellular protein trafficking to block secretion and membrane transfer between intracellular organelles (Klausner et al., 1992). Treatment with brefeldin A between GD6 and GD7 delays blastocyst differentiation, reducing fibronectin-binding activity on GD7 (Schultz et al., 1997). The effectiveness of the drug during the period that immediately precedes the onset of trophoblast adhesion (Table 2) indicates that protein trafficking directly regulates blastocyst adhesion competence. Moreover, the fibronectin-binding integrin, α5β1, translocates to the apical plasma membrane of trophoblast cells on GD7 coincident with the appearance of adhesion competence (Schultz et al., 1997). Linkage between insertion of α5β1 into the apical domain of the plasma membrane and trophoblast adhesiveness is supported by experiments that jointly shift α5β1 trafficking and the onset of fibronectin-binding activity from GD7 to GD6 through treatments that accelerate in vitro blastocyst development (Wang et al., 1998, 1999, 2000). mEomes knockout blastocysts, which are incompetent to outgrow, express α5β1 (Russ et al., 2000), but it is not known whether the protein trafficks normally to the apical surface of trophoblast cells. As discussed below, the trafficking of additional integrins may be required for trophoblast adhesion to fibronectin.
The intrinsic developmental program of the primary trophoblast utilizes mRNAs synthesized during cavitation and proteins that are translated shortly after blastocyst formation, but probably not fully functional until they are processed and transported to their sites of action. Outside of the uterus, completion of the entire developmental process from the time of gene activation to attaining adhesion competence takes 3–4 days (summarized in Table 1). However, the coordinate regulation of blastocyst and uterine development to achieve strong adhesion between trophoblast cells and the endometrium requires the intervention of extrinsic factors that modify and accelerate the embryonic developmental program.
Extrinsic factors that regulate trophoblast adhesion
Advanced blastocyst development is dependent on extrinsic factors provided by the maternal reproductive tract. Uterine growth factors alter the rate of blastocyst development to synchronize the embryo with the “window” of uterine receptivity for implantation. Several endogenous agonists that accelerate blastocyst development have been identified and their impact on intracellular signaling to regulate trophoblast differentiation has been described (reviewed in Armant et al., 2000). As embryonic and uterine tissues interact more intimately, additional synchronization is achieved between trophoblast cells and the molecular milieu of the endometrium. For example, the local ECM composition may vary as trophoblast cells begin to implant and invade, requiring adjustments in cell surface receptors. Evidence of the fine tuning that occurs on the surface of the trophoblast during adhesion to fibronectin (reviewed in Wang and Armant, 2002) suggests a mechanism that mobilizes receptors with ligand specificity appropriate for the composition of the ECM engaged by the cells.
Uterine products that accelerate the intrinsic program
Blastocysts cultured in serum-free medium beginning on GD4 are adhesion competent by GD7 (Armant et al., 1986a; Schultz et al., 1997; Yelian et al., 1995), about 24 h later than when they would have adhered to the endometrial ECM had they been allowed to develop in vivo (Blankenship and Given, 1992). With prolonged exposure to the maternal environment prior to in vitro culture (harvesting blastocysts on E4.5/GD5), α5β1 trafficking to the apical surface of trophoblast cells and blastocyst adhesion to fibronectin are achieved by GD6 (Wang et al., 2000). This experiment demonstrates that the uterus provides an optimal environment for development of the free-floating blastocyst. A similar acceleration of trophoblast differentiation is achieved when early blastocysts (E3.5) are cultured in medium supplemented with hormones (Wang et al., 1998), endocannabinoids (Wang et al., 1999), phospholipids (Liu and Armant, 2004), or growth factors (Wang et al., 2000) produced by the uterus during the peri-implantation period. A large number of molecules produced within the uterus are capable of accelerating preimplantation development (Armant et al., 2000; Cavagna and Mantese, 2003; Dey et al., 2004), but discussion here will be limited to a few examples representing the above listed categories. These bioactive agonists account for at least some of the benefit derived from the uterine environment.
Signaling pathways induced by bioactive factors produced in the receptive uterus activate downstream biochemical steps in the intrinsic developmental program of the blastocyst. Paracrine and autocrine signaling during the pre-and peri-implantation periods supports embryogenesis, in part, through the generation of intracellular Ca2+ transients that operate downstream of at least three important agonists: calcitonin, lysophosphatidic acid (LPA), and HB-EGF (Liu and Armant, 2004; Wang et al., 1998, 2000). Receptors for each protein are expressed by the mouse blastocyst. Calcitonin and LPA bind to G protein-coupled receptors that mobilize Ca2+ from intracellular stores through the inositol 1,4,5-trisphosphate (IP3) pathway (Contos et al., 2000; Goldring et al., 1993). HB-EGF also increases intracellular Ca2+ levels, but through influx mediated by N-type voltage-gated Ca2+ channels in the plasma membrane (Wang et al., 2000). Downstream of Ca2+, both calmodulin and protein kinase C play important roles in the acquisition of adhesion competence by mouse blastocysts (Armant et al., 2000). The cortical reaction in oocytes (Abbott and Ducibella, 2001) and exocytosis in a number of cell types follows the activation of calmodulin (Chamberlain et al., 1995; Peters and Mayer, 1998) and protein kinase C (Chen et al., 1999; Lan et al., 2001; Quetglas et al., 2000) downstream of intracellular Ca2+ transients (Guo et al., 1996; Kiraly-Borri et al., 1996). In blastocysts treated with calcitonin or HB-EGF, the induction of a Ca2+ transient on GD4 or GD5 leads to precocious trafficking of α5 to the apical surface of the trophoblast on GD6, implying that reactions downstream of cytosolic free Ca2+ control protein trafficking required for blastocyst adhesion to fibronectin (Wang et al., 1998, 2000). Indeed, direct pharmacological induction of a Ca2+ transient at the early blastocyst stage using ethanol accelerates the rate of trophoblast outgrowth and doubles implantation rates after transferring embryos back to the uterus (Stachecki et al., 1994).
In humans, autocrine and paracrine roles for calcitonin and HB-EGF are prominent during implantation and placentation. Both are expressed by the uterine epithelium during the “window” of implantation (Kumar et al., 1998; Leach et al., 1999a; Yoo et al., 1997) and HB-EGF stimulates human blastocyst development in vitro (Chobotova et al., 2002; Martin et al., 1998). In the placenta, invasive extravillous trophoblast cells express high levels of HB-EGF (Leach et al., 1999a) that can alter integrin expression in cultured cytotrophoblast cells to increase their invasive motility (Leach et al., 2004). Additionally, HB-EGF is a survival factor (Iwamoto and Mekada, 2000) that could moderate assaults by the maternal immune system and other stresses encountered in the reproductive tract. It is notable that aberrantly low levels of HB-EGF are found in placentas of women with pre-eclampsia, a disorder associated with poor trophoblast invasion and survival (Leach et al., 2002).
The endocannabinoid, anandamide, accelerates blastocyst development in a bimodal fashion, becoming inhibitory at higher concentrations (Wang et al., 1999). Anandamide is present at implantation sites, but its levels are much higher in the interimplantation region (Schmid et al., 1997), suggesting a role in embryo spacing within the rodent uterus. Interestingly, at low, stimulatory levels, anandamide has no effect on intracellular Ca2+ levels, but at high concentrations that are detrimental to embryonic development anandamide prevents influx of Ca2+ through voltage-gated channels (Wang et al., 2003). Furthermore, low, stimulatory concentrations of anandamide activate mitogen-activated protein kinase (MAPK). Whether the Ca2+-dependent acceleration of blastocyst development involves MAPK is not presently known. However, pharmacological elevation of intracellular Ca2+ levels in blastocysts increases expression of c-Myc (Leach et al., 1999b), a MAPK substrate that is required for preimplantation development (Paria et al., 1992). Invasiveness of cultured human cytotrophoblast cells stimulated by the urokinase plasminogen activator receptor is dependent on MAPK activation downstream of an intracellular Ca2+ transient (Liu et al., 2003). Therefore, MAPK could be a target of anandamide, as well as agonists that stimulate trophoblast differentiation through intracellular Ca2+ signaling.
Integration of multiple uterine signals
Temporal and spatial availability within the uterus varies among paracrine-acting factors that influence blastocyst development, as indicated in Fig. 1. LPA, calcitonin, histamine, and several other bioactive agents are secreted into the uterine fluid as the blastocyst exits the oviduct on E3.5 (Armant et al., 2000). However, HB-EGF is produced on E4.5 by murine uterine epithelial cells solely at the site of blastocyst apposition (Das et al., 1994). This transmembrane peptide can operate locally through juxtacrine signaling to receptors on adjacent trophoblast cells (Raab et al., 1996). The ectodomain of HB-EGF is secreted through metalloproteinase-mediated cleavage (Iwamoto and Mekada, 2000), but may function as a proximal signal due to its strong binding to heparan sulfate proteoglycans in the ECM. Juxtacrine signaling to the trophoblast by HB-EGF during the attachment reaction stimulates integrin trafficking required in the final preparation for adhesion to the ECM and invasion of the endometrium.
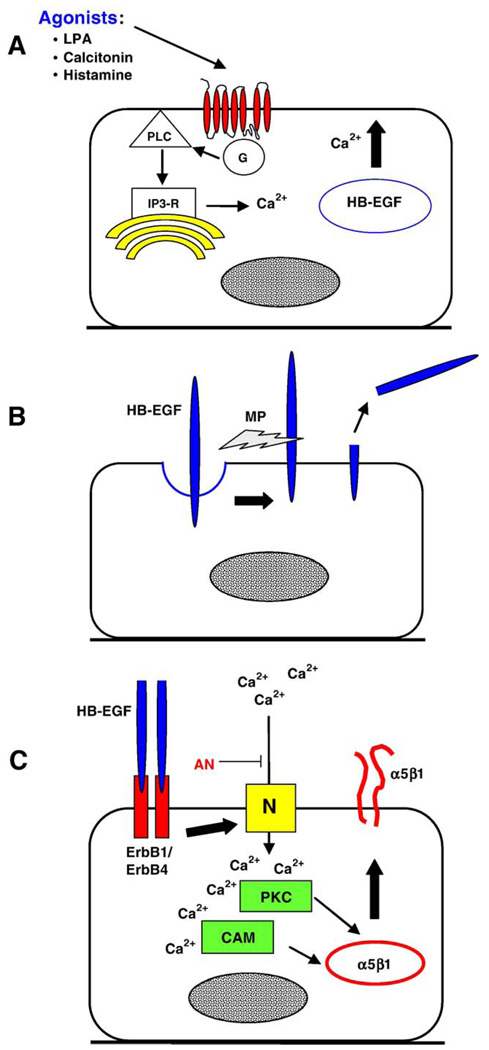
In addition to its expression in the uterus, HB-EGF is induced in embryonic tissues during activation of dormant blastocysts (Hamatani et al., 2004b). Embryonic HB-EGF can potentially stimulate uterine differentiation to the receptive state (Paria et al., 2001). We now have evidence that autocrine signaling by HB-EGF in trophoblast cells, as depicted in Fig. 3, could integrate diverse paracrine signals arising from the endometrium. For example, the Ca2+-dependent acceleration of blastocyst development by LPA requires HB-EGF, based on the inhibitory effects of antibodies against HB-EGF or its receptors, ErbB1 and ErbB4 (Liu and Armant, 2004). HB-EGF trafficks from the cytoplasm to the surface of trophoblast cells in cultured blastocysts treated on GD5 with LPA (Fig. 3A). G protein-coupled receptors can transactivate ErbB1 or ErbB4 through ectodomain shedding of HB-EGF (Umata et al., 2001). Transactivation occurs downstream to either intracellular Ca2+ or protein kinase C activation (Dethlefsen et al., 1998; Dong and Wiley, 2000). The LPA-induced trafficking of HB-EGF to the apical surface of trophoblast cells is blocked by chelation of intracellular Ca2+ with BAPTA-AM or by interrupting the IP3 pathway (Liu and Armant, 2004), supporting our hypothesis that LPA initiates Ca2+-dependent trafficking of HB-EGF to the cell surface where it is shed by metalloproteinases (Fig. 3B) and binds its receptors in an autocrine loop (Fig. 3C). Intracellular Ca2+ signaling is also required downstream of HB-EGF/ErbB binding (Fig. 3C) for integrin trafficking and precocious blastocyst differentiation (Wang et al., 2000). Blastocyst exposure to high, detrimental concentrations of anandamide interferes with Ca2+ entry through voltage-gated channels (Wang et al., 2003), perhaps by interrupting autocrine HB-EGF signaling (Fig. 3C). It is plausible that intracellular Ca2+ mobilization by other endogenous agonists of G protein-coupled receptors, including calcitonin and histamine (Goldring et al., 1993; Hill et al., 1997; Paria et al., 1998), operate similarly through the transactivation of ErbB receptors by HB-EGF, accounting for their similar biological activities.
Fig. 3.
Transactivation of ErbB receptors by G protein-coupled receptors in trophoblast cells. (A) LPA, and other agonists of G protein-coupled receptors, mobilizes intracellular Ca2+ through the IP3 receptor (IP3-R) after activating phospholipase C (PLC). (B) The increased Ca2+ concentration induces trafficking of HB-EGF to the plasma membrane, where it is cleaved by metalloproteinases (MP). (C) HB-EGF is then able to bind its receptor in an autocrine loop. Experimental evidence points to Erb4 as the primary receptor for HB-EGF (Paria et al., 1999; Wang et al., 2000). Intracellular Ca2+ levels are again raised, this time by influx through N-type Ca2+ channels (N). A recent report by Wang et al. (2003) suggests that voltage-gated channels might be inhibited in the presence of high concentrations of anandamide (AN), blocking this autocrine pathway. Protein kinase C (PKC) and calmodulin (CAM) operate downstream of Ca2+ to accelerate α5β1 trafficking to the plasma membrane. As a result, blastocysts are adhesion competent approximately 1 day earlier than in unstimulated embryos (Wang et al., 2000).
Fibronectin engagement alters blastocyst adhesion to fibronectin
The positioning of fibronectin receptors on the surface of the blastocyst is requisite, but not necessarily sufficient, for strong adhesion to fibronectin. The integrin αVβ3 is available on the apical surface of trophoblast cells by GD4 (Sutherland et al., 1993), but FN-120-coated microspheres will not adhere to intact blastocysts until GD7 (Schultz and Armant, 1995). Another fibronectin-binding integrin, α5 β1, translocates to the apical plasma membrane of trophoblast cells between GD6 and GD7 (Schultz et al., 1997). Although the timing of α5β1 insertion suggests that this is required for adhesion to fibronectin, the fibronectin-binding activity of blastocysts remains extremely low until they are exposed to fibronectin for at least 1 h (Schultz and Armant, 1995). Therefore, trafficking of αVβ3 and α5β1 to the surface of trophoblast cells is, in itself, insufficient for strong adhesion to fibronectin. However, after ligand-mediated upregulation by fibronectin, fibronectin-binding activity is inhibited by antibodies that recognize αVβ3 or α5β1; evidence that they contribute an essential element of the adhesion mechanism.
In the presence of brefeldin A, fibronectin fails to upregulate fibronectin-binding activity of adhesion competent blastocysts (Table 2), revealing a requirement for additional protein trafficking after α5β1 insertion in the apical cell surface (Schultz and Armant, 1995). Competitive inhibition experiments indicate no increase in receptor affinity (IC50) upon exposure to fibronectin, suggesting that some fully active receptors are present before exposure, but the majority of receptors are inactive. Hypotheses that might account for the increased adhesive activity include, (1) the number of fibronectin receptors on the apical cell surface is increased, (2) additional integrin subtypes are recruited to the apical surface, and (3) fibronectin receptors must be reorganized with other proteins into adhesion complexes. Blastocysts treated with fibronectin show no readily apparent change in the expression of α5β1 or αVβ3 on their surface (Schultz et al., 1997), suggesting that the first hypothesis does not fully account for the altered adhesiveness. However, the second hypothesis is supported by the discovery of a third fibronectin-binding integrin that trafficks to the apical surface of the trophoblast following exposure to fibronectin on GD7 (Rout et al., 2004). The third hypothesis could prove valid, as well, since the cytoplasmic domains of integrins must interact with cytoskeletal and signaling components to promote adhesion (Sastry and Burridge, 2000). Indeed, disruption of the actin cytoskeleton during exposure to fibronectin prevents the upregulation of fibronectin activity (Schultz and Armant, 1995).
The platelet-associated integrin αIIbβ3 strengthens trophoblast adhesion to fibronectin
Preimplantation embryos express mRNA encoding the alpha subunit of the integrin, αIIb β3 (Schultz et al., 1997), which is associated primarily with cells of the megakaryocytic lineage (Ginsberg et al., 1993; Phillips et al., 1988). In platelets, αIIbβ3 binds fibrinogen during thrombus formation, although its recognition of fibronectin and several other RGD-containing proteins, including vitronectin, von Willebrand factor, and thrombospondin, is probably also physiologically relevant for hemostasis (Savage et al., 1996). Semi-quantitative assessment of αIIb mRNA during preimplantation development reveals an apparent fivefold increase in gene expression during blastocyst formation that is blocked by α-amanitin (Rout et al., 2004), suggesting that αIIb is among the gene products transcribed during cavitation that later direct trophoblast outgrowth (Table 2). Immunofluorescence microscopy reveals αIIb in focal adhesions of trophoblast cells outgrowing on fibronectin (Rout et al., 2004; Yelian et al., 1995), as expected of an integrin that mediates adhesion to fibronectin. Most interesting was the observation that αIIb remains intracellular after αV and α5 have translocated to the apical surface of trophoblast cells on GD7; however, upon exposure to fibronectin, there is a significant increase in αIIb at the blastocyst surface (Rout et al., 2004). In support of its role in trophoblast adhesion, a function-blocking antibody against the αIIb subunit inhibits fibronectin-binding activity (Rout et al., 2004).
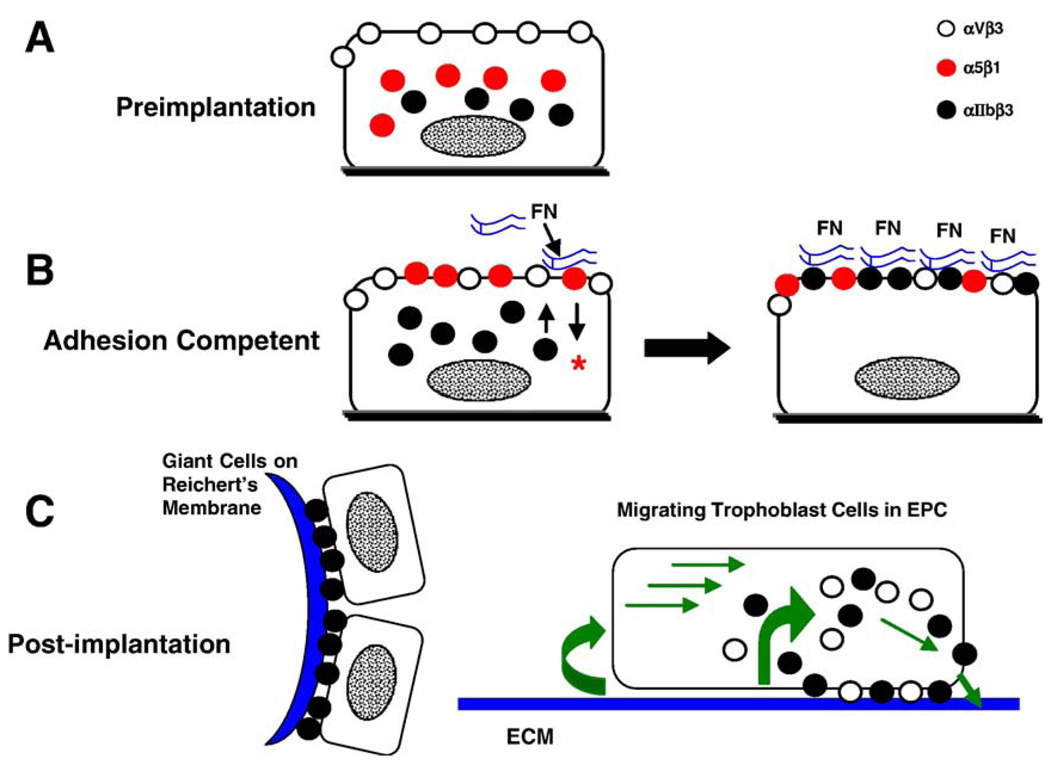
The trafficking of αIIb to the apical surface of the trophoblast coincides with the second period of sensitivity to brefeldin A (Table 2). As the blastocyst becomes adhesion competent, intracellular vesicles accumulate in mural trophoblast cells; however, they are absent after exposure to fibronectin (Wang et al., 2002). Brefeldin A inhibits vesicle depletion induced by fibronectin, consistent with a mechanism involving their insertion into the plasma membrane. Thus, a strong correlation exists between vesicle translocation, αIIb trafficking, and the upregulation of fibronectin-binding activity. The content of the intracellular vesicles is unknown, but it is tempting to speculate that they deliver to the apical plasma membrane αIIb and other proteins required for strong adhesion. The studies by Rout et al. (2004) and Wang et al. (2002) suggest that αIIb trafficking is a key step in establishing strong adhesion between trophoblast cells and fibronectin (Figs. 4A,B). Examination of embryo implantation sites reveals αIIb localized in primary TGCs of the parietal yolk sac adhering to the Reichert’s membrane on E5.5 (Rout et al., 2004). By E6.5, αIIb also appears in migrating secondary TGCs as they differentiate near the margins of the EPC. During implantation, αIIbβ3-mediated adhesion could initially anchor mural trophoblast cells to the uterine wall, and may later function in secondary TGC migratory activity (Fig. 4C).
Fig. 4.
Integrin trafficking during trophoblast development. (A) In the early blastocyst, αVβ3 (white circles) is present at the apical surface of non-adhesive trophoblast cells, whereas α5β1 (red circles) and αIIb subunits (black circles) are localized either intracellularly or at the basal cell surface. (B) In adhesion competent blastocysts, α5β1 has been inserted into the apical plasma membrane of trophoblast cells. Integrin ligation by fibronectin (FN), particularly its binding to α5β1, initiates intracellular signaling (*) required for trafficking of αIIb subunits to the apical surface. Once αIIbβ3 is available, fibronectin-binding activity is strengthened. (C) During early postimplantation development, αIIbβ3 is the predominant fibronectin-binding integrin of trophoblast cells. It is highly expressed in primary TGCs adhering to the Reichert’s membrane (left) and in secondary TGCs migrating at the periphery of the EPC. In vitro analysis of migrating trophoblast cells suggests a model (right) in which the β3 integrins are primarily responsible for motility. They appear in focal adhesions near the leading edge of cells, where they presumably attach the plasma membrane to ECM. Focal adhesions are disassembled in the middle and at the trailing edge of cells to allow forward movement.
Cooperative activities of fibronectin receptors
As mural trophoblast cells acquire adhesive activity, some division of labor can be discerned among the fibronectin-binding integrins. According to antibody inhibition studies (Rout et al., 2004; Schultz and Armant, 1995), contributions from three fibronectin-binding integrins, αVβ3, αIIbβ3, and α5β1, are required to achieve strong adhesion to fibronectin and promote cell migration. The contribution of additional integrins that recognize fibronectin, in particular α8β1 and other αV integrins, has yet to be examined. Culturing blastocysts on fibrinogen, which is a poor substrate for α5β1, in the presence of function-blocking antibodies against β3 to eliminate αVβ3 and αIIbβ3 activity completely abolishes trophoblast outgrowth (Rout et al., 2004), suggesting that these three integrins function as the principal receptors for fibronectin.
Trophoblast outgrowth on fibronectin is inhibited almostcompletely by an antibody against β3 or a combination of antibodies against αV and αIIb, whereas inhibition of α5β1 has no significant effect on migration. From this result, it appears that the β3 integrins are primarily responsible for migratory activity. Anti-αIIb antibody disrupts focal adhesions of trophoblast cells, displacing them from the migrating edge of the cell. The redistribution of focal adhesions to the center of cells could convert them from migratory to stationary. While there has been much attention paid to the promotion of cell invasion by αVβ3 (Chatterjee and Chatterjee, 2001; Huang et al., 2000; Leavesley et al., 1992; Marshall and Hart, 1996), more studies are needed to understand the role of αIIbβ3 in trophoblast migration. It has come to light in recent years that αIIb expression is not restricted to the megakaryocyte lineage, and that αIIbβ3 serves a critical function in the motility of highly invasive tumors (Chen et al., 1997; Trikha et al., 2002). The documented activity of αVβ3 and αIIbβ3 in other invasive cell types is in keeping with their essential function in trophoblast migration.
Ligation of α5β1 influences the initiation of trophoblast outgrowth (Rout et al., 2004). Fibrinogen, which binds only β3 integrins, supports trophoblast outgrowth after a substantial delay and upregulates the fibronectin-binding activity of intact blastocysts at a rate three times slower than fibronectin. Mice lacking the β1 gene produce primary TGCs that adhere to and outgrow on fibronectin (Stephens et al., 1995), consistent with the ability of fibrinogen to support outgrowth. While the β3 integrins can operate in the absence of α5β1, antibody inhibition demonstrates that α5β1 most efficiently initiates the pathway that establishes strong adhesion (Rout et al., 2004).
Although none of these fibronectin-binding integrins are indispensable for trophoblast outgrowth or implantation (Hynes, 1996), the accumulated evidence indicates that the default pathway in wild type trophoblast cells relies on α5β1 signaling to upregulate adhesion, αIIbβ3 to mediate strong adhesion (Figs. 4A,B), and the β3 integrins for cell motility (Fig. 4C). The survival and invasive integrity of primary TGCs in mutant mice lacking fibronectin or its integrin receptors suggests that functional redundancies in the developmental program ensure the success of this critical step. It is interesting that trophoblast cells can manage a “work around” when an integrin gene is deleted, but they are considerably less agile when inhibitory antibodies are introduced immediately prior to blastocyst outgrowth. Perhaps there is a point at which the embryo commits to an adhesive mechanism incorporating the available integrin genes. The use of RNA interference (Svoboda et al., 2001) to knock down integrin transcripts might have advantages over gene knockouts as an approach to understanding how integrins are recruited for specific functions, although these experiments are complicated somewhat by the disparity between the periods of integrin synthesis and function in peri-implantation embryos.
Cellular recognition of ECM components
Trophoblast adhesion to the ECM in vivo is complex, considering that the cells initially adhere to the epithelial basement membrane, migrate throughout the decidua and finally invade uterine blood vessels. Invasion would be facilitated by an efficient mechanism to recruit adhesive receptors as trophoblast cells encounter various ECM components. The ligand-mediated upregulation of trophoblast adhesion to fibronectin could operate as a fibronectin sensor that activates its cognate receptors. Together with sensors for other ECM components, invading trophoblast cells could rapidly adapt within a heterogeneous uterine ECM.
The ECM-dependent selectivity of integrin activation can be demonstrated by measuring fibronectin-binding activity after exposing blastocysts to either fibronectin or laminin. Only fibronectin upregulates fibronectin-binding activity (Wang et al., 2002). Conversely, laminin, but not fibronectin, can upregulate laminin binding activity (Schultz and Armant, unpublished). These results support the idea that contact with different components of the ECM activates the appropriate receptors at the surface of trophoblast cells. This example of physiological fine-tuning during trophoblast differentiation may reflect an increased reliance after blastocyst formation on environmental cues over a rigid developmental program. Recent studies of trophoblast adhesion to fibronectin have begun to shed light on the biochemical basis of ECM recognition, which incorporates an autocrine signaling mechanism.
Autocrine signaling in trophoblast cells contacting fibronectin
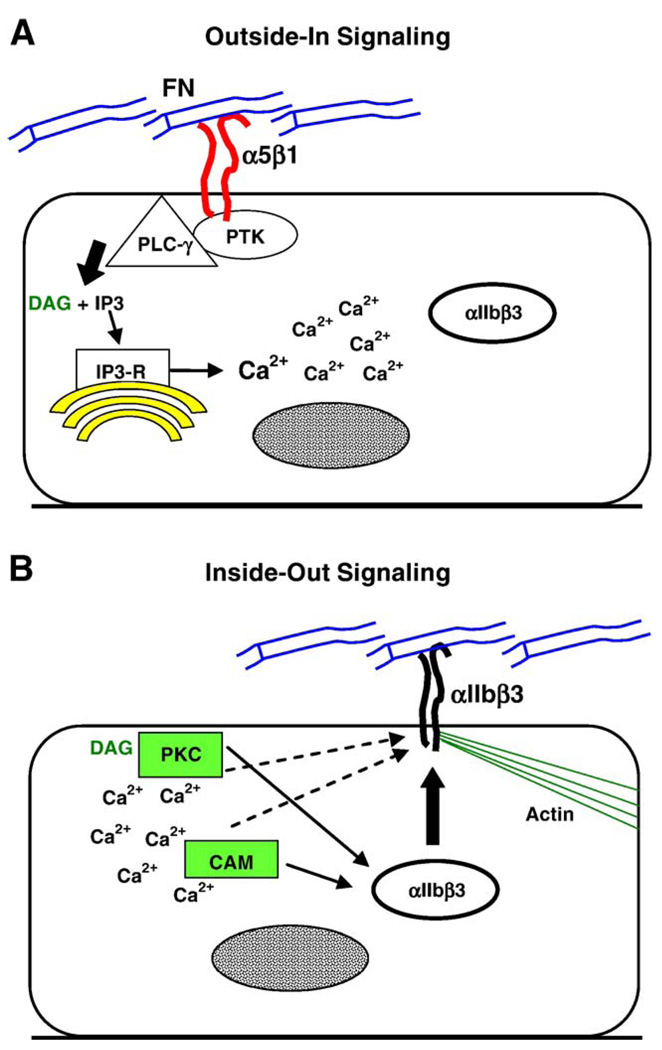
The ability of integrins to mediate intracellular signaling upon ligation by ECM components (Kornberg et al., 1991) has been known for over a decade to regulate cell growth, adhesion, motility, and differentiation (Aplin et al., 1998; Burridge and Chrzanowska-Wodnicka, 1996; Hynes, 1992). Autocrine signaling involving integrins includes both the initiation and transduction of intracellular signals. Integrin ligation produces “outside-in signaling” through integrin clustering and association with cytoplasmic proteins that initiate intracellular biochemical cascades (Fig. 5A). Signals originating within cells can activate cytoplasmic proteins that associate with integrins to modulate their adhesive function, a process termed “inside-out signaling” (Fig. 5B). Ligand-mediated upregulation of fibronectin-binding activity in trophoblast cells is an example of integrin autocrine signaling.
Fig. 5.
Hypothetical model of autocrine signaling in adhesion competent trophoblast cells exposed to fibronectin. (A) With ligation of α5β1 by fibronectin (FN), cytoplasmic proteins associate with integrin aggregates at the plasma membrane. Protein tyrosine kinases (PTK) phosphorylate PLC-γ within this complex, leading to the production of IP3 and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3-R), releasing stored Ca2+ into the cytoplasm. During development to the adhesion competent stage, intracellular vesicles accumulate in mural trophoblast cells. It is hypothesized that the vesicles contain αIIbβ3 and other proteins that promote cell adhesion. (B) With the elevation of intracellular Ca2+, calmodulin (CAM) is activated. Depending on the isoform, PKC may be activated by DAG or Ca2+. Signaling by both proteins is required for the upregulation of fibronectin-binding activity. Ca2+ signaling is needed for the depletion (apparent trafficking, solid arrows from PKC and CAM) of the intracellular vesicles. CAM and PKC could also activate αIIbβ3 after its trafficking to the apical plasma membrane (dashed arrows). Presumably, αIIbβ3 is integrated into a nascent adhesion complex linked to elements of the actin cytoskeleton, which strengthens adhesion to fibronectin.
The participation of integrins in the upregulation of fibronectin-binding activity can be demonstrated by directly ligating α5β1 or αVβ3 with appropriate antibodies, which increases binding activity to the same extent as fibronectin (Wang et al., 2002). Integrins become clustered in the plasma membrane upon ligation by fibronectin, which recruits protein tyrosine kinases, their substrates and other signaling proteins to associate in focal adhesion complexes with cytoskeletal elements of the cortical cytoplasm (Miyamoto et al., 1995). Focal adhesion complexes mediate adhesion at the basal surface of cells in culture. Occupied integrins can also aggregate with signaling and cytoskeletal proteins at the apical cell surface, as demonstrated by Miyamoto et al. (1995). The same process could occur at the apical surface of trophoblast cells in blastocysts exposed to fibronectin in vitro or contacting fibronectin in the endometrial ECM during implantation (see Fig. 5).
In addition to protein tyrosine phosphorylation (Kornberg et al., 1991), early reports demonstrated that integrin ligation could elevate intracellular levels of Ca2+ in lymphocytes (Pardi et al., 1989), neutrophils (Ng-Sikorski et al., 1991), platelets (Smith et al., 1991), and endothelial cells (Schwartz, 1993). Ligation of fibronectin receptors on the surface of mouse blastocysts induces an immediate increase in intracellular Ca2+ that is sustained over a 30- to 60-min period (Wang et al., 2002). Suppression of Ca2+ signaling by pretreating embryos with the intracellular Ca2+ chelator BAPTA-AM inhibits the upregulation of fibronectin-binding activity by FN-120. Moreover, pharmacological elevation of intracellular Ca2+ upregulates fibronectin-binding activity and induces vesicle depletion in the mural trophoblast. These experiments show that Ca2+ signaling induced by integrin ligation is both necessary and sufficient to strengthen trophoblast adhesion to fibronectin.
Integrins elevate intracellular Ca2+ levels either through influx or release from intracellular Ca2+ stores (McNamee et al., 1993; Sjaastad et al., 1996; Somogyi et al., 1994; Wu et al., 1998). In adrenal glomerulosa cells, fibronectin ligation of α5β1 or αVβ3 elevates intracellular Ca2+ by a mechanism involving release from intracellular stores through the IP3 receptor (Campbell et al., 2003). Phospholipase C-γ (PLC-γ) is among the cytoplasmic proteins that colocalize with clustered integrins (Miyamoto et al., 1995). Its phosphorylation by protein tyrosine kinases that also associate with aggregated integrins (e.g., Src, FAK) could activate PLC-γ to produce IP3 (discussed in Wang and Armant, 2002). In mouse blastocysts, the ligand-mediated upregulation of fibronectin-binding activity and associated Ca2+ transients are blocked by antagonists of protein tyrosine kinases, PLC and the IP3 receptor, but not by inhibition of Ca2+ influx or voltage-gated Ca2+ channels (Wang, Mayernik, Kilburn, and Armant, in submission). This information suggests a mechanism of outside-in integrin signaling (Fig. 5A). In this scheme, integrin ligation by fibronectin activates associated protein tyrosine kinases and PLC-γ to generate IP3, which activates IP3 receptors that release stored Ca2+ into the cytoplasm.
Integrin downstream signaling leads to inside-out signaling, as depicted in Fig. 5B. As in the Ca2+-dependent acceleration of blastocyst differentiation by growth factors, fibronectin-induced upregulation of adhesion requires both calmodulin and protein kinase C activities (Wang et al., 2002). Calmodulin is directly activated by Ca2+ (Lu and Means, 1993) and calmodulin-dependent proteins can alter integrin binding activity (Pomies et al., 1995). Protein kinase C is recruited to integrins clustered by fibronectin (Miyamoto et al., 1995) where it could be activated by diacylglycerol or Ca2+ mobilized through the IP3 receptor. Once active, protein kinase C could upregulate integrin binding activity. Protein kinase C-α activates αIIbβ3 during platelet aggregation (Tabuchi et al., 2003), suggesting the intriguing idea that a similar process occurs in the trophoblast. Additionally, both calmodulin and protein kinase C regulate exocytosis and protein trafficking (Chen et al., 1999). Calmodulin is required for essential steps in the fusion of secretory vesicles with the plasma membrane (Quetglas et al., 2000; Peters and Mayer, 1998). Delivery of proteins to the plasma membrane is stimulated by the activity of protein kinase C (Lan et al., 2001), particularly the β isoform (Long et al., 2001; Marinari et al., 2003; Nechushtan et al., 2000). The protein kinase C isoform that regulates fibronectin-binding activity in trophoblast cells has not yet been identified, nor have the critical proteins downstream of it or calmodulin. Several isoforms of protein kinase C are expressed by the early blastocyst stage and most appear to accumulate in the apical or junctional plasma membranes of the trophectoderm (Eckert et al., 2004; Pauken and Capco, 2000). These or other protein kinase C isoforms that are expressed by the late blastocyst stage could mediate the inside-out signaling pathway. During peri-implantation development, it appears that the second messenger Ca2+, operating through established downstream pathways, orchestrates trophoblast adhesive differentiation by initiating the sequential trafficking of critical proteins into the plasma membrane (Fig. 3 and Fig. 5). Whether other aspects of trophoblast phenotype are impacted by this process remains to be determined.
Concluding remarks
The developing blastocyst has proven to be an informative model for investigating the ontogeny of cell adhesion during mammalian embryogenesis. Reminiscent of the early zygote, the intrinsic developmental program of primary trophoblast cells is directed by transcripts or proteins synthesized at an earlier stage (Schultz et al., 1997). Post-translational processing and trafficking of gene products, rather than biosynthesis, determines the onset of trophoblast adhesiveness in preparation for blastocyst implantation. One caveat of this paradigm is a lack of information about the integrity of other embryonic functions, such as proliferation and endoredupication, in embryos blocked for transcription or translation. Ca2+ signaling plays a major role in advancing blastocyst development to the adhesive stage. Transit through key steps in differentiation is initiated by the activity of Ca2+-dependent proteins, including calmodulin and protein kinase C, that trigger the delivery of proteins needed for cell adhesion to the apical cell domain. The regulation of trophoblast adhesion independently of gene activation enables cells to rapidly adapt to variations in the local environment. As trophoblast cells migrate through a heterogeneous ECM, adhesion is maintained by switching integrin receptors. The integrin switching hypothesis (Damsky et al., 1994) suggests that trophoblast cells recognize the molecular composition of the local ECM and modify their cell surfaces to optimally interact with it for purposes of both embryo anchorage and cell invasion.
Evidence discussed here suggests that trophoblast adhesion is orchestrated through a series of trafficking events that position receptors at the embryo surface for subsequent signaling, which initiates further trafficking (see Fig. 1B). Paracrine signaling by secreted uterine agonists (e.g., LPA, calcitonin) advance blastocyst development, perhaps in part through protein trafficking. Trafficking of ErbB4 to the surface of early blastocysts (E4.0) is required before HB-EGF becomes an effective agonist (Wang et al., 2000). Ca2+ influx downstream of HB-EGF binding to ErbB4 drives the insertion of α5β1 into the apical plasma membrane, enabling integrin signaling (Wang et al., 2000). Once the embryo contacts fibronectin within the endometrial ECM, integrin signaling initiates the Ca2+-dependent events that translocate αIIb to the apical surface and strengthen adhesion. Signaling at each of these steps is dependent on a previous trafficking event, providing a glimpse of the developmental program that guides the conversion of primary trophoblast cells to an adhesion competent phenotype.
Many unanswered questions remain regarding the appropriateness of generalizing processes observed during the upregulation of fibronectin-binding activity to other ECM components or to later stages of trophoblast development. Functions unrelated to trophoblast adhesion and outgrowth could depend on de novo gene expression. It would be interesting to know whether intracellular signaling initiated by integrin ligation differentially regulates embryonic gene expression. While the emergence of trophoblast subtypes during placentation is certainly regulated by specific genes (Cross, 2000; Rossant and Cross, 2001), regulation by extrinsic factors operating at the post-transcriptional level remains an open and intriguing possibility. The paradigm represented by fibronectin-mediated adhesion in the implanting blastocyst suggests how positional information could be translated to direct the rapid emergence of trophoblast subtype populations during pattern formation within the developing placenta. The innovation of new technologies for investigating trophoblast stem cells in vitro (Gerami-Naini et al., 2004; Tanaka et al., 1998; Xu et al., 2002) presents an opportunity to examine the role of extrinsic factors on trophoblast differentiation into its various subtypes.
Acknowledgments
Studies by the author that were discussed in this review were supported by National Institutes of Health grants HD36764 and AA12057. The author thanks Drs. Stephen Krawetz and Daniel Rappolee for helpful discussions and Brian A. Kilburn for technical assistance with mouse embryo culture and Cdx2 protein immunofluorescence microscopy.
References
- Abbott AL, Ducibella T. Calcium and the control of mammalian cortical granule exocytosis. Front Biosci. 2001;6:D792–D806. doi: 10.2741/abbott. [DOI] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin–cell adhesion molecules, and selectins. Pharm. Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Aplin JD, Haigh T, Jones CJ, Church HJ, Vicovac L. Development of cytotrophoblast columns from explanted first-trimester human placental villi: role of fibronectin and integrin alpha 5 beta 1. Biol. Reprod. 1999;60:828–838. doi: 10.1095/biolreprod60.4.828. [DOI] [PubMed] [Google Scholar]
- Armant DR. Cell interactions with laminin and its proteolytic fragments during outgrowth of mouse primary trophoblast cells. Biol. Reprod. 1991;45:664–672. doi: 10.1095/biolreprod45.5.664. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kameda S. Mouse trophoblast cell invasion of extracellular matrix purified from endometrial tissue: a model for peri-implantation development. J. Exp. Zool. 1994;269:146–156. doi: 10.1002/jez.1402690208. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kaplan HA, Lennarz WJ. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev. Biol. 1986a;116:519–523. doi: 10.1016/0012-1606(86)90152-1. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kaplan HA, Lennarz WJ. N-linked glycoprotein biosynthesis in the developing mouse embryo. Dev. Biol. 1986b;113:228–237. doi: 10.1016/0012-1606(86)90125-9. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kaplan HA, Mover H, Lennarz WJ. The effect of hexapeptides on attachment and outgrowth of mouse blastocysts cultured in vitro: evidence for the involvement of the cell recognition tripeptide Arg-Gly-Asp. Proc. Natl. Acad. Sci. U. S. A. 1986c;83:6751–6755. doi: 10.1073/pnas.83.18.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant DR, Wang J, Liu Z. Intracellular signaling in the developing blastocyst as a consequence of the maternal–embryonic dialogue. Semin. Reprod. Med. 2000;18:273–287. doi: 10.1055/s-2000-12565. [DOI] [PubMed] [Google Scholar]
- Babiarz B, Romagnano L, Afonso S, Kurila G. Localization and expression of fibronectin during mouse decidualization in vitro: mechanisms of cell:matrix interactions. Dev. Dyn. 1996;206:330–342. doi: 10.1002/(SICI)1097-0177(199607)206:3<330::AID-AJA10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extraembryonic membranes. Dev. Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Bellis SL. Variant glycosylation: an underappreciated regulatory mechanism for beta1 integrins. Biochim. Biophys. Acta. 2004;1663:52–60. doi: 10.1016/j.bbamem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Blake EJ, Schindler J, Sherman MI. Protein synthetic requirements for the outgrowth of trophoblast cells from mouse blastocysts. J. Exp. Zool. 1982;224:401–408. doi: 10.1002/jez.1402240313. [DOI] [PubMed] [Google Scholar]
- Blankenship TN, Given RL. Penetration of the uterine epithelial basement membrane during blastocyst implantation in the mouse. Anat. Rec. 1992;233:196–204. doi: 10.1002/ar.1092330204. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burrows TD, King A, Loke YW. Expression of integrins by human trophoblast and differential adhesion to laminin or fibronectin. Hum. Reprod. 1993;8:475–484. doi: 10.1093/oxfordjournals.humrep.a138075. [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Oakford L, Cantu-Crouch D, Wordinger R. Attachment of blastocysts to lens capsule: a model system for trophoblast–epithelial cell interaction on a natural basement membrane. Cell Tissue Res. 1987;250:633–640. doi: 10.1007/BF00218957. [DOI] [PubMed] [Google Scholar]
- Campbell S, Otis M, Otis M, Cote M, Gallo-Payet N, Payet MD. Connection between integrins and cell activation in rat adrenal glomerulosa cells: a role for Arg-Gly-Asp peptide in the activation of the p42/p44(mapk) pathway and intracellular calcium. Endocrinology. 2003;144:1486–1495. doi: 10.1210/en.2002-220903. [DOI] [PubMed] [Google Scholar]
- Carson DD. The glycobiology of implantation. Front Biosci. 2002;7:d1535–d1544. doi: 10.2741/A858. [DOI] [PubMed] [Google Scholar]
- Carson DD, Tang JP, Gay S. Collagens support embryo attachment and outgrowth in vitro: effects of the Arg-Gly-Asp sequence. Dev. Biol. 1988;127:368–375. doi: 10.1016/0012-1606(88)90323-5. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev. Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Cavagna M, Mantese JC. Biomarkers of endometrial receptivity—A review. Placenta. 2003;24 Suppl B:S39–S47. doi: 10.1016/s0143-4004(03)00184-x. [DOI] [PubMed] [Google Scholar]
- Chai N, Patel Y, Jacobson K, McMahon J, McMahon A, Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev. Biol. 1998;198:105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Roth D, Morgan A, Burgoyne RD. Distinct effects of alpha-SNAP, 14-3-3 proteins, and calmodulin on priming and triggering of regulated exocytosis. J. Cell Biol. 1995;130:1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Chatterjee A. Role of alphavbeta3 integrin receptor in the invasive potential of human cervical cancer (SiHa) cells. J. Environ. Pathol., Toxicol. Oncol. 2001;20:211–221. [PubMed] [Google Scholar]
- Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Trikha M, Gao X, Bazaz R, Porter AT, Timar J, Honn KV. Ectopic expression of platelet integrin alphaIIb beta3 in tumor cells from various species and histological origin. Int. J. Cancer. 1997;72:642–648. doi: 10.1002/(sici)1097-0215(19970807)72:4<642::aid-ijc16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Chen YA, Duvvuri V, Schulman H, Scheller RH. Calmodulin and protein kinase C increase Ca(2+)-stimulated secretion by modulating membrane-attached exocytic machinery. J. Biol. Chem. 1999;274:26469–26476. doi: 10.1074/jbc.274.37.26469. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ, Barlow DH, Sargent IL, Mardon HJ. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech. Dev. 2002;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Collins JE, Fleming TP. Epithelial differentiation in the mouse preimplantation embryo: making adhesive cell contacts for the first time. Trends Biochem. Sci. 1995;20:307–312. doi: 10.1016/s0968-0004(00)89057-x. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol. Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin. Cell Dev. Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Denker HW. Implantation: a cell biological paradox. J. Exp. Zool. 1993;266:541–558. doi: 10.1002/jez.1402660606. [DOI] [PubMed] [Google Scholar]
- Dethlefsen SM, Raab G, Moses MA, Adam RM, Klagsbrun M, Freeman MR. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J. Cell Biochem. 1998;69:143–153. doi: 10.1002/(sici)1097-4644(19980501)69:2<143::aid-jcb5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr. Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Dickson AD. Trophoblastic giant cell transformation of mouse blastocysts. J. Reprod. Fertil. 1963;6:465–466. doi: 10.1530/jrf.0.0060465. [DOI] [PubMed] [Google Scholar]
- Dong J, Wiley HS. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains. J. Biol. Chem. 2000;275:557–564. doi: 10.1074/jbc.275.1.557. [DOI] [PubMed] [Google Scholar]
- Downs KM. Early placental ontogeny in the mouse. Placenta. 2002;23:116–131. doi: 10.1053/plac.2001.0763. [DOI] [PubMed] [Google Scholar]
- Earl U, Estlin C, Bulmer JN. Fibronectin and laminin in the early human placenta. Placenta. 1990;11:223–231. doi: 10.1016/s0143-4004(05)80268-1. [DOI] [PubMed] [Google Scholar]
- Eckert JJ, McCallum A, Mears A, Rumsby MG, Cameron IT, Fleming TP. Specific PKC isoforms regulate blastocoel formation during mouse preimplantation development. Dev. Biol. 2004;274:384–401. doi: 10.1016/j.ydbio.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Enders AC, Chavez DJ, Schlafke S. Comparison of implantation in utero and in vitro. In: Glasser SR, Bullock DW, editors. Cellular and Molecular Aspects of Implantation. New York, NY: Plenum Press; 1981. pp. 365–382. [Google Scholar]
- Evans JP, Schultz RM, Kopf GS. Identification and localization of integrin subunits in oocytes and eggs of the mouse. Mol. Reprod. Dev. 1995;40:211–220. doi: 10.1002/mrd.1080400210. [DOI] [PubMed] [Google Scholar]
- Faria TN, Deb S, Kwok SC, Talamantes F, Soares MJ. Ontogeny of placental lactogen-I and placental lactogen-II expression in the developing rat placenta. Dev. Biol. 1990;141:279–291. doi: 10.1016/0012-1606(90)90384-u. [DOI] [PubMed] [Google Scholar]
- Farrar JD, Carson DD. Differential temporal and spatial expression of mRNA encoding extracellular matrix components in decidua during the peri-implantation period. Biol. Reprod. 1992;46:1095–1108. doi: 10.1095/biolreprod46.6.1095. [DOI] [PubMed] [Google Scholar]
- Feinberg RF, Kliman HJ, Lockwood CJ. Is oncofetal fibronectin a trophoblast glue for human implantation? Am. J. Pathol. 1991;138:537–543. [PMC free article] [PubMed] [Google Scholar]
- Fusi FM, Vignali M, Gailit J, Bronson RA. Mammalian oocytes exhibit specific recognition of the RGD (Arg-Gly-Asp) tripeptide and express oolemmal integrins. Mol. Reprod. Dev. 1993;36:212–219. doi: 10.1002/mrd.1080360212. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Flow of cells from polar to mural trophectoderm is polarized in the mouse blastocyst. Hum. Reprod. 2000;15:694–701. doi: 10.1093/humrep/15.3.694. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Papaioannou VE, Barton SC. Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. J. Embryol. Exp. Morphol. 1973;30:561–572. [PubMed] [Google Scholar]
- Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Xiaoping D, O’Toole TE, Loftus JC, Plow EF. Platelet integrins. Thromb. Haemostasis. 1993;70:87–93. [PubMed] [Google Scholar]
- Glass RH, Spindle AI, Pedersen RA. Differential inhibition of trophoblast outgrowth and inner cell mass growth by actinomycin D in cultured mouse embryos. J. Reprod. Fertil. 1976;48:443–445. doi: 10.1530/jrf.0.0480443. [DOI] [PubMed] [Google Scholar]
- Glasser SR, Lampelo S, Munir MI, Julian J. Expression of desmin, laminin and fibronectin during in situ differentiation (decidualization)of rat uterine stromal cells. Differentiation. 1987;35:132–142. doi: 10.1111/j.1432-0436.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Goldring SR, Gorn AH, Yamin M, Krane SM, Wang JT. Characterization of the structural and functional properties of cloned calcitonin receptor cDNAs. Horm. Metab. Res. 1993;25:477–480. doi: 10.1055/s-2007-1002153. [DOI] [PubMed] [Google Scholar]
- Guo X, Przywara DA, Wakade TD, Wakade AR. Exocytosis coupled to mobilization of intracellular calcium by muscarine and caffeine in rat chromaffin cells. J. Neurochem. 1996;67:155–162. doi: 10.1046/j.1471-4159.1996.67010155.x. [DOI] [PubMed] [Google Scholar]
- Gwatkin RB. Defined media and development of mammalian eggs in vitro. Ann. N. Y. Acad. Sci. 1966;139:79–90. doi: 10.1111/j.1749-6632.1966.tb41186.x. [DOI] [PubMed] [Google Scholar]
- Gwatkin RB, Meckley PE. Chromosomes of the mouse blastocyst following its attachment and outgrowth in vitro. Ann. Med. Exp. Biol. Fenn. 1966;44:125–127. [PubMed] [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell. 2004a;6:117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc. Natl. Acad. Sci. U. S. A. 2004b;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock SN, Agulnik SI, Silver LM, Papaioannou VE. Mapping and expression analysis of the mouse ortholog of Xenopus Eomesodermin. Mech. Dev. 1999;81:205–208. doi: 10.1016/s0925-4773(98)00244-5. [DOI] [PubMed] [Google Scholar]
- Hierck BP, Thorsteinsdottir S, Niessen CM, Freund E, Iperen LV, Feyen A, Hogervorst F, Poelmann RE, Mummery CL, Sonnenberg A. Variants of the alpha-6 beta-1 laminin receptor in early murine development: distribution, molecular cloning and chromosomal localization of the mouse integrin alpha-6 subunit. Cell Adhes. Commun. 1993;1:33–53. doi: 10.3109/15419069309095680. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. International union of pharmacology: XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- Huang S, Stupack D, Liu A, Cheresh D, Nemerow GR. Cell growth and matrix invasion of EBV-immortalized human B lymphocytes is regulated by expression of alpha(v) integrins. Oncogene. 2000;19:1915–1923. doi: 10.1038/sj.onc.1203509. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? Dev. Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Irving JA, Lysiak JJ, Graham CH, Hearn S, Han VK, Lala PK. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta. 1995;16:413–433. doi: 10.1016/0143-4004(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Mekada E. Heparin-binding EGF-like growth factor: a juxtacrine growth factor. Cytokine Growth Factor Rev. 2000;11:335–344. doi: 10.1016/s1359-6101(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK, Reimann JD. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 2000;10:429–439. doi: 10.1016/s0962-8924(00)01834-1. [DOI] [PubMed] [Google Scholar]
- Jenkinson EJ, Wilson IB. In vitro studies on the control of trophoblast outgrowth in the mouse. J. Embryol. Exp. Morphol. 1973;30:21–30. [PubMed] [Google Scholar]
- Khidir MA, Stachecki JJ, Krawetz SA, Armant DR. Rapid inhibition of mRNA synthesis during preimplantation embryo development: vital permeabilization by lysolecithin potentiates the action of α-amanitin. Exp. Cell. 1995;219:619–623. doi: 10.1006/excr.1995.1272. [DOI] [PubMed] [Google Scholar]
- Kidder GM, McLachlin JR. Timing of transcription and protein synthesis underlying morphogenesis in preimplantation mouse embryos. Dev. Biol. 1985;112:265–275. doi: 10.1016/0012-1606(85)90397-5. [DOI] [PubMed] [Google Scholar]
- Kimber SJ. Molecular interactions at the maternal–embryonic interface during the early phase of implantation. Semin. Reprod Med. 2000;18:237–253. doi: 10.1055/s-2000-12562. [DOI] [PubMed] [Google Scholar]
- Kimber SJ, Spanswick C. Blastocyst implantation: the adhesion cascade. Semin. Cell Dev. Biol. 2000;11:77–92. doi: 10.1006/scdb.2000.0154. [DOI] [PubMed] [Google Scholar]
- Kiraly-Bor CE, Morgan A, Burgoyne RD, Weller U, Wollheim CB, Lang J. Soluble N-ethylmaleimide-sensitive-factor attachment protein and N-ethylmaleimide-insensitive factors are required for Ca2+-stimulated exocytosis of insulin. Biochem. J. 1996;314:199–203. doi: 10.1042/bj3140199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DR, Potts DM, Wilson IB. On the orientation of the implanting blastocyst. J. Embryol. Exp. Morphol. 1967;17:527–532. [PubMed] [Google Scholar]
- Kisalus LL, Herr JC, Little CD. Immunolocalization of extracellular matrix proteins and collagen synthesis in first-trimester human decidua. Anat. Rec. 1987;218:402–415. doi: 10.1002/ar.1092180408. [DOI] [PubMed] [Google Scholar]
- Klaffky E, Williams R, Yao CC, Ziober B, Kramer R, Sutherland A. Trophoblast-specific expression and function of the integrin alpha 7 subunit in the peri-implantation mouse embryo. Dev. Biol. 2001;239:161–175. doi: 10.1006/dbio.2001.0404. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Zhu LJ, Polihronis M, Cameron ST, Baird DT, Schatz F, Dua A, Ying YK, Bagchi MK, Bagchi IC. Progesterone induces calcitonin gene expression in human endometrium within the putative window of implantation. J. Clin. Endocrinol. Metab. 1998;83:4443–4450. doi: 10.1210/jcem.83.12.5328. [DOI] [PubMed] [Google Scholar]
- Kunath T, Strumpf D, Rossant J. Early trophoblast determination and stem cell maintenance in the mouse—A review. Placenta. 2004;25 Suppl A:S32–S38. doi: 10.1016/j.placenta.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell specific expression during the human endometrial cycle and early placentation. J. Clin. Endocrinol. Metab. 1999a;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- Leach RE, Rout UK, Schultz JF, Saunders DE, Armant DR. Ethanol elevates c-Myc levels in cultured mouse preimplantation embryos. Alcohol. Clin. Exp. Res. 1999b;23:778–784. [PubMed] [Google Scholar]
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn BA, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, Armant DR. Pre-eclampsia and expression of heparin-binding EGF-likegrowth factor. Lancet. 2002;360:1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev. Biol. 2004;266:223–237. doi: 10.1016/j.ydbio.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J. Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I, Vaheri A, Timpl R, Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev. Biol. 1980;76:100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp. Cell Res. 2004;296:317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Liu J, Chakraborty C, Graham CH, Barbin YP, Dixon SJ, Lala PK. Noncatalytic domain of uPA stimulates human extravillous trophoblast migration by using phospholipase C, phosphatidylinositol 3-kinase and mitogen-activated protein kinase. Exp. Cell Res. 2003;286:138–151. doi: 10.1016/s0014-4827(03)00089-2. [DOI] [PubMed] [Google Scholar]
- Lohnes D. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. BioEssays. 2003;25:971–980. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- Long A, Kelleher D, Lynch S, Volkov Y. Cutting edge: protein kinase C beta expression is critical for export of Il-2 from T cells. J. Immunol. 2001;167:636–640. doi: 10.4049/jimmunol.167.2.636. [DOI] [PubMed] [Google Scholar]
- Lu KP, Means AR. Regulation of the cell cycle by calcium and calmodulin. Endocr. Rev. 1993;14:40–58. doi: 10.1210/edrv-14-1-40. [DOI] [PubMed] [Google Scholar]
- Marek KW, Vijay IK, Marth JD. A recessive deletion in the GlcNAc-1-phosphotransferase gene results in peri-implantation embryonic lethality. Glycobiology. 1999;9:1263–1271. doi: 10.1093/glycob/9.11.1263. [DOI] [PubMed] [Google Scholar]
- Marinari UM, Nitti M, Pronzato MA, Domenicotti C. Role of PKC-dependent pathways in HNE-induced cell protein transport and secretion. Mol. Aspects Med. 2003;24:205–211. doi: 10.1016/s0098-2997(03)00015-3. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Hart IR. The role of alpha v-integrins in tumour progression and metastasis. Semin. Cancer Biol. 1996;7:129–138. doi: 10.1006/scbi.1996.0018. [DOI] [PubMed] [Google Scholar]
- Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum. Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- McNamee HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J. Cell Biol. 1993;121:673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase Cbeta. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- Ng-Sikorski J, Andersson R, Patarroyo M, Andersson T. Calcium signaling capacity of the CD11b/CD18 integrin on human neutrophils. Exp. Cell Res. 1991;195:504–508. doi: 10.1016/0014-4827(91)90402-g. [DOI] [PubMed] [Google Scholar]
- Pardi R, Bender JR, Dettori C, Giannazza E, Engleman EG. Heterogeneous distribution and transmembrane signaling properties of lymphocyte function-associated antigen (LFA-1) in human lymphocyte subsets. J. Immunol. 1989;143:3157–3166. [PubMed] [Google Scholar]
- Paria BC, Dey SK, Andrews GK. Antisense c-myc effects on preimplantation mouse embryo development. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10051–10055. doi: 10.1073/pnas.89.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Das N, Das SK, Zhao X, Dileepan KN, Dey SK. Histidine decarboxylase gene in the mouse uterus is regulated by progesterone and correlates with uterine differentiation for blastocyst implantation. Endocrinology. 1998;139:3958–3966. doi: 10.1210/endo.139.9.6173. [DOI] [PubMed] [Google Scholar]
- Paria BC, Elenius K, Klagsburn M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for herparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol. Reprod. 1987;36:211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- Pauken CM, Capco DG. The expression and stage-specific localization of protein kinase C isotypes during mouse preimplantation development. Dev. Biol. 2000;223:411–421. doi: 10.1006/dbio.2000.9763. [DOI] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Phillips DR, Charo IF, Parise LV, Fitzgerald LA. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988;71:831–843. [PubMed] [Google Scholar]
- Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2:71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J. Biol. Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Pomies P, Frachet P, Block MR. Control of the alpha 5 beta 1 integrin/fibronectin interaction in vitro by the serine/threonine protein phosphatase calcineurin. Biochemistry. 1995;34:5104–5112. doi: 10.1021/bi00015a022. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astood EG, Geiger SR, editors. Handbook of Physiology. Washington, DC: American Physiology Society; 1973. pp. 187–215. [Google Scholar]
- Quetglas S, Leveque C, Miquelis R, Sato K, Seagar M. Ca2+-dependent regulation of synaptic SNARE complex assembly via a calmodulin-and phospholipid-binding domain of synaptobrevin. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9695–9700. doi: 10.1073/pnas.97.17.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal–fetal interface. J. Clin. Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW, Lu CY. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta, Implications for maternal–fetal immunological relationship. Lab. Invest. 1989;61:27–36. [PubMed] [Google Scholar]
- Richa J, Damsky CH, Buck CA, Knowles BB, Solter D. Cell surface glycoproteins mediate compaction, trophoblast attachment, and endoderm formation during early mouse development. Dev. Biol. 1985;108:513–521. doi: 10.1016/0012-1606(85)90054-5. [DOI] [PubMed] [Google Scholar]
- Rider V, Carlone DL, Witrock D, Cai C, Oliver N. Uterine fibronectin mRNA content and localization are modulated during implantation. Dev. Dyn. 1992;195:1–14. doi: 10.1002/aja.1001950102. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat. Rev., Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Rout UK, Krawetz SA, Armant DR. Ethanol-induced intracellular calcium mobilization rapidly alters gene expression in the mouse blastocyst. Cell Calcium. 1997;22:463–474. doi: 10.1016/s0143-4160(97)90074-9. [DOI] [PubMed] [Google Scholar]
- Rout UK, Wang J, Paria BC, Armant DR. alpha5beta1, alphaVbeta3 and the platelet-associated integrin alphaIIbbeta3 coordinately regulate adhesion and migration of differentiating mouse trophoblast cells. Dev. Biol. 2004;268:135–151. doi: 10.1016/j.ydbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Schindler J, Sherman MI. Effect of α-amanatin on programming of mouse blastocyst development. Dev. Biol. 1981;84:332–340. doi: 10.1016/0012-1606(81)90401-2. [DOI] [PubMed] [Google Scholar]
- Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol. Reprod. 1975;12:41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- Schlafke S, Welsh AO, Enders AC. Penetration of the basal lamina of the uterine luminal epithelium during implantation in the rat. Anat. Rec. 1985;212:47–56. doi: 10.1002/ar.1092120107. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JF, Armant DR. Beta1-and beta3-class integrins mediate fibronectin binding activity at the surface of developing mouse peri-implantation blastocysts. Regulation by ligand-induced mobilization of stored receptor. J. Biol. Chem. 1995;270:11522–11531. doi: 10.1074/jbc.270.19.11522. [DOI] [PubMed] [Google Scholar]
- Schultz JF, Mayernik L, Rout UK, Armant DR. Integrin trafficking regulates adhesion to fibronectin during differentiation of mouse peri-implantation blastocysts. Dev. Genet. 1997;21:31–43. doi: 10.1002/(SICI)1520-6408(1997)21:1<31::AID-DVG4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J. Cell Biol. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MI, Atienza-Samols SB. In vitro studies on the surface adhesiveness of mouse blastocysts. In: Ludwig H, Tauber PF, editors. Human fertilization. Stuttgart, Germany: Georg Thieme Publishers; 1978. pp. 179–183. [Google Scholar]
- Shiokawa S, Yoshimura Y, Sawa H, Nagamatsu S, Hanashi H, Sakai K, Ando M, Nakamura Y. Functional role of arg-gly-asp (RGD)-binding sites on beta1 integrin in embryo implantation using mouse blastocysts and human decidua. Biol. Reprod. 1999;60:1468–1474. doi: 10.1095/biolreprod60.6.1468. [DOI] [PubMed] [Google Scholar]
- Showell C, Binder O, Conlon FL. T-box genes in early embryogenesis. Dev. Dyn. 2004;229:201–218. doi: 10.1002/dvdy.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaastad MD, Lewis RS, Nelson WJ. Mechanisms of integrin-mediated calcium signaling in MDCK cells: regulation of adhesion by IP3- and store-independent calcium influx. Mol. Biol. Cell. 1996;7:1025–1041. doi: 10.1091/mbc.7.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M, Murphy CR. Temporal changes in the expression of platelet-derived growth factor and fibronectin in the uterine epithelium during early pregnancy. Anat. Rec. 1999;255:1–6. doi: 10.1002/(SICI)1097-0185(19990501)255:1<1::AID-AR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Smith JB, Dangelmaier C, Selak MA, Daniel JL. Facile platelet adhesion to collagen requires metabolic energy and actin polymerization and evokes intracellular free calcium mobilization. J. Cell. Biochem. 1991;47:54–61. doi: 10.1002/jcb.240470108. [DOI] [PubMed] [Google Scholar]
- Somogyi L, Lasic Z, Vukicevic S, Banfic H. Collagen type IV stimulates an increase in intracellular Ca2+ in pancreatic acinar cells via activation of phospholipase C. Biochem. J. 1994;299:603–611. doi: 10.1042/bj2990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle AI, Pedersen RA. Hatching, attachment, and outgrowth of mouse blastocysts in vitro: fixed nitrogen requirements. J. Exp. Zool. 1973;186:305–318. doi: 10.1002/jez.1401860308. [DOI] [PubMed] [Google Scholar]
- Stachecki JJ, Yelian FD, Leach RE, Armant DR. Mouse blastocyst outgrowth and implantation rates following exposure to ethanol or A23187 during culture in vitro. J. Reprod. Fertil. 1994;101:611–617. doi: 10.1530/jrf.0.1010611. [DOI] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Surani MA. Glycoprotein synthesis and inhibition of glycosylation by tunicamycin in preimplantation mouse embryos: compaction and trophoblast adhesion. Cell. 1979;18:217–227. doi: 10.1016/0092-8674(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Sutherland A. Mechanisms of implantation in the mouse: differentiation and functional importance of trophoblast giant cell behavior. Dev. Biol. 2003;258:241–251. doi: 10.1016/s0012-1606(03)00130-1. [DOI] [PubMed] [Google Scholar]
- Sutherland AE, Calarco PG, Damsky CH. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development. 1993;119:1175–1186. doi: 10.1242/dev.119.4.1175. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Motlik J, Neuber E, Pavlok A, Schatten G, Palecek J, Hyttel P, Adebayo OT, Adwan K, Alberio R, Bagis H, Bataineh Z, Bjerregaard B, Bodo S, Bryja V, Carrington M, Couf M, de la FR, Diblik J, Esner M, Forejt J, Fulka Jr, J, Geussova G, Gjorret JO, Libik M, Hampl A, Hassane MS, Houshmand M, Hozak P, Jezova M, Kania G, Kanka J, Kandil OM, Kishimoto T, Klima J, Kohoutek J, Kopska T, Kubelka M, Lapathitis G, Laurincik J, Lefevre B, Mihalik J, Novakova M, Oko R, Omelka R, Owiny D, Pachernik J, Pacholikova J, Peknicova J, Pesty A, Ponya Z, Preclikova H, Sloskova A, Svoboda P, Strejcek F, Toth S, Tepla O, Valdivia M, Vodicka P, Zudova D. Accumulation of the proteolytic marker peptide ubiquitin in the trophoblast of mammalian blastocysts. Cloning Stem Cells. 2001;3:157–161. doi: 10.1089/153623001753205115. [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Schultz RM. RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem. Biophys. Res. Commun. 2001;287:1099–1104. doi: 10.1006/bbrc.2001.5707. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Yoshioka A, Higashi T, Shirakawa R, Nishioka H, Kita T, Horiuchi H. Direct demonstration of involvement of protein kinase Calpha in the Ca2+-induced platelet aggregation. J. Biol. Chem. 2003;278:26374–26379. doi: 10.1074/jbc.M212407200. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Tarone G, Russo MA, Hirsch E, Odorisio T, Altruda F, Silengo L, Siracusa G. Expression of beta 1 integrin complexes on the surface of unfertilized mouse oocyte. Development. 1993;117:1369–1375. doi: 10.1242/dev.117.4.1369. [DOI] [PubMed] [Google Scholar]
- Trikha M, Timar J, Zacharek A, Nemeth JA, Cai Y, Dome B, Somlai B, Raso E, Ladanyi A, Honn KV. Role for beta3 integrins in human melanoma growth and survival. Int. J. Cancer. 2002;101:156–167. doi: 10.1002/ijc.10521. [DOI] [PubMed] [Google Scholar]
- Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, Tsuneoka M, Miura Y, Masuda M, Horiguchi Y, Mekada E. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J. Biol. Chem. 2001;276:30475–30482. doi: 10.1074/jbc.M103673200. [DOI] [PubMed] [Google Scholar]
- Wang J, Armant DR. Integrin-mediated adhesion and signaling during blastocyst implantation. Cells Tissues Organs. 2002;172:190–201. doi: 10.1159/000066970. [DOI] [PubMed] [Google Scholar]
- Wang J, Rout UK, Bagchi IC, Armant DR. Expression of calcitonin receptors in mouse preimplantation embryos and their function in the regulation of blastocyst differentiation by calcitonin. Development. 1998;125:4293–4302. doi: 10.1242/dev.125.21.4293. [DOI] [PubMed] [Google Scholar]
- Wang J, Paria BC, Dey SK, Armant DR. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol. Reprod. 1999;60:839–844. doi: 10.1095/biolreprod60.4.839. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Armant DR. Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: intracellular calcium transients and vesicle trafficking in primary trophoblast cells. Dev. Biol. 2002;245:270–279. doi: 10.1006/dbio.2002.0644. [DOI] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev. Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J, Leivo I, Vaheri A. Expression of the cell surface-associated glycoprotein, fibronectin, in the early mouse embryo. Dev. Biol. 1979;69:247–257. doi: 10.1016/0012-1606(79)90289-6. [DOI] [PubMed] [Google Scholar]
- Watson AJ, Barcroft LC. Regulation of blastocyst formation. Front. Biosci. 2001;6:D708–D730. doi: 10.2741/watson. [DOI] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Chorioallantoic placenta formation in the rat: I. Luminal epithelial cell death and extracellular matrix modifications in the mesometrial region of implantation chambers. Am. J. Anat. 1991;192:215–231. doi: 10.1002/aja.1001920302. [DOI] [PubMed] [Google Scholar]
- Wewer UM, Damjanov A, Weiss J, Liotta LA, Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation. 1986;32:49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, McGrath M. The collagen substratum influences in vitro hatching and attachment of the mouse blastocyst in a serumless medium. Experientia. 1979;35:1683–1684. doi: 10.1007/BF01953272. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Brun-Zinkernagel AM, Jackson T. An ultrastructural study of in-vitro interaction of guinea-pig and mouse blastocysts with extracellular matrices. J. Reprod. Fertil. 1991;93:585–597. doi: 10.1530/jrf.0.0930585. [DOI] [PubMed] [Google Scholar]
- Wu TC, Wan YJ, Chung AE, Damjanov I. Immunohistochemical localization of entactin and laminin in mouse embryos and fetuses. Dev. Biol. 1983;100:496–505. doi: 10.1016/0012-1606(83)90242-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J. Cell Biol. 1998;143:241–252. doi: 10.1083/jcb.143.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Yelian FD, Edgeworth NA, Dong LJ, Chung AE, Armant DR. Recombinant entactin promotes mouse primary trophoblast cell adhesion and migration through the Arg-Gly-Asp (RGD) recognition sequence. J. Cell Biol. 1993;121:923–929. doi: 10.1083/jcb.121.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelian FD, Yang Y, Hirata JD, Schultz JF, Armant DR. Molecular interactions between fibronectin and integrins during mouse blastocyst outgrowth. Mol. Reprod. Dev. 1995;41:435–448. doi: 10.1002/mrd.1080410406. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev. Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shu MA, Harvey MB, Schultz GA. Regulation of urokinase plasminogen activator production in implanting mouse embryo: effect of embryo interaction with extracellular matrix. Biol. Reprod. 1996;54:1052–1058. doi: 10.1095/biolreprod54.5.1052. [DOI] [PubMed] [Google Scholar]
- Ziomek CA, Johnson MH, Handyside AH. The developmental potential of mouse 16-cell blastomeres. J. Exp. Zool. 1982;221:345–355. doi: 10.1002/jez.1402210310. [DOI] [PubMed] [Google Scholar]