Abstract
The non-oncogene-bearing retrovirus SL3-3 murine leukemia virus induces strictly T-cell lymphomas with a mean latency of 2 to 4 months in mice of the NMRI-inbred (NMRI-i) strain. By high-throughput sequencing of retroviral tags, we have identified the genomic region carrying the transcriptional repressor and oncogene growth factor independence 1 (Gfi1) as a frequent target for SL3-3 in the NMRI-i mouse genome. Twenty-four SL3-3 insertions were identified within a 1-kb window of the 3′ untranslated region (3′UTR) of the Gfi1 gene, a clustering pattern unique for this lymphoma model. Expression analysis determined that the Gfi1 gene was transcriptionally activated by SL3-3 insertions, and an upregulation of Gfi1 protein expression was detected for tumors harboring insertions in the Gfi1 3′UTR. Here we provide data in support of a mechanism by which retroviral insertions in the Gfi1 3′UTR decouple microRNA-mediated posttranscriptional regulation.
The non-oncogene-bearing murine leukemia viruses (MLVs) induce leukemias and lymphomas when injected into newborn susceptible mice (1, 21, 75). The major determinant of MLV latency and disease specificity is the retroviral enhancer in the U3 region of the MLV long terminal repeat (LTR) (3, 5, 9, 17, 19, 24, 37, 38, 50, 52, 69, 70, 71). It comprises conserved areas which hold densely packed binding sites for several host transcription factors, including Runx, NF-1, Ets, c-Myb, the glucocorticoid response element, and basic helix-loop-helix factors. Small nucleotide alterations in the different binding sites influence latency, confer variations in cell-specific expression, and shift disease patterns from lymphoma to plasmacytoma, myeloid leukemia, megakaryoblastic leukemia, erythroleukemia, and mixed phenotype. The wild-type (wt) SL3-3 is a highly pathogenic ecotropic MLV that induces precursor T-cell lymphomas with a mean latency of 2 to 4 months and primary manifestations in thymus, spleen, and mesenteric lymph nodes when injected into mice of the NMRI-inbred (NMRI-i) strain (19, 43, 51). Tumor induction by SL3-3 and other MLVs is a complex process, where the most well defined step involves integration of the viral genome into the host genome and deregulation of nearby proto-oncogenes or tumor suppressors (6, 8, 10, 28, 53, 65, 66, 67). The effect of the provirus depends on its integration position relative to the target gene, where the most frequent mechanisms of insertional mutagenesis are enhancement and LTR promotion, both of which result in either upregulation of the wt gene and protein or generation of chimeric transcripts. Another way by which gene expression can be affected by retroviral insertions is by loss of regulatory regions. Early studies of insertional mutagenesis have demonstrated that retroviral integrations in the 3′ untranslated regions (3′UTRs) of genes may result in generation of prematurely terminated transcripts or transcripts with increased mRNA stability and elevated protein synthesis (6, 8, 10, 67). The 3′UTR may also harbor other regulatory sequences, namely, binding sites for microRNAs (miRNAs), which are noncoding 22-nucleotide RNAs encoded from introns or intergenic regions in the genome (36). They act by targeting primarily the 3′UTRs of mRNAs and mediate posttranscriptional downregulation of gene expression by complete complementarity or partial binding of their 5′-end nucleotides 2 to 7 (seed region) to mRNA targets (39). Theoretically, the short seed sequence permits a single miRNA to act on multiple target sites, and thereby each miRNA is able to recognize an average of 100 different mRNAs (2, 41).
The genomic locus on murine chromosome 5 encoding the transcriptional repressor and oncogene growth factor independence 1 (Gfi1) (25) and neuroblastoma 4S oncogene ecotropic viral integration site 5 (Evi5) (40) (hereafter also referred to as the gfi1 locus) is a frequent integration locus in T-cell lymphomas induced by Moloney MLV (MoMLV) (48, 62, 65) and in B-cell lymphomas induced by the Akv MLV (72, 73). Previous studies have demonstrated that retroviral insertions within the gfi1 locus lead to transcriptional activation of the Gfi1 gene (62, 65). Gfi1 is a key regulator of stem cell quiescence (29, 82) and plays a significant role in T-cell development (26, 54, 64, 81) and lineage commitment (80). It further influences maturation of myeloid precursors into granulocytes and monocytes and acts in limiting the inflammatory immune response (31). Gfi1 has a major oncogenic potential and has been associated with both murine and human cancers (15, 32, 59, 68).
In this study we have identified 130 retroviral insertions in the gfi1 locus and addressed their effect on Gfi1 mRNA and protein expression. Our results suggest that integrations in the Gfi1 3′UTR contribute to increased protein synthesis through a mechanism including loss of potential miRNA binding sites.
MATERIALS AND METHODS
Tumors and isolation of retroviral tags.
Tumors originated from previously published (17, 18, 19, 20, 27, 43, 45, 51, 69, 70) and unpublished pathogenicity studies of wt and enhancer mutated SL3-3, Akv, and Reilly-Finkel-Biskis (RFB) MLVs. Large-scale analysis of integrated retroviruses, performed by a splinklerette-based PCR method described previously (78), was able to identify 120 wt and enhancer-mutated SL3-3 integrations in the genomic region carrying Gfi1 from a total of 790 SL3-3 tags. Seven Akv integrations and three RFB integrations from 2,800 Akv tags and 85 RFB tags, respectively, were identified in the gfi1 locus.
PCR and sequencing.
Total RNA was extracted from snap-frozen tissue by use of TRIzol extraction reagent (Invitrogen). Full-genome cDNA was synthesized using the first-strand cDNA synthesis kit (GE Healthcare) according to the manufacturer's recommendations. PCR for identifying alternative transcripts was performed with a Gfi1 exon 2 forward primer (5′-CCGACTCTCAGCTTACCGAG-3′) and a Gfi1 exon 5 reverse primer (5′-CTGTGTGGATGAAGGTGTGTTT-3′) (DNA Technology). PCR for identifying retroviral insertions in the Gfi1 3′UTR was performed with a Gfi1 exon 6 forward primer (5′-CTCAGGAGGCACCGAGAGA-3′) and SL3-3 reverse primer (5′-CCCCAGAAATAGCTAAAACAACAACAGTTTCAA-3′) (DNA Technology). PCR fragments were purified on GFX columns (GE Healthcare) and sequenced by use of a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems).
Real-time PCR analysis.
Real-time PCR amplifications for gene mRNA quantification were performed using TaqMan expression assays for Gfi1 (Mm00515853_m1) and Ywhaz (Mm01158417_g1). For miRNA quantification, cDNA was synthesized according to the TaqMan MicroRNA assay protocol by use of a TaqMan MicroRNA reverse transcription kit and TaqMan MicroRNA assays for miR-155 (001806), miR-142-3p (001189), miR-330 (001062), miR-133a (002246), miR-34b-3p (002618), miR-879 (002473), miR-466l (002804), miR-10a (002288), and miR-467g (002811). Samples were set up in 20-μl reaction mixtures with 10 μl TaqMan universal PCR master mix, no AmpErase UNG, 0.5 μl TaqMan primer-probe, and 9 μl cDNA. All TaqMan reagents were purchased from Applied Biosystems. To obtain amplification efficiency, samples for gene quantification were run at four-point dilutions (1:10, 1:50, 1:100, and 1:500) and samples for miRNA quantification were run at three dilutions (1:10, 1:50, and 1:100). Each measurement was performed in duplicates. Controls without template and controls without reverse transcriptase for each tumor sample were included. Samples for Gfi1 quantification were normalized to Ywhaz (the housekeeping genes Ubc, Tfrc, B2m, and Gapdh were tested on 10 thymic, 10 splenic, and 10 mesenteric lymph node samples, where Ywhaz showed the most stable expression). miRNA expression was normalized to snoRNA420 (001239) (Applied Biosystems). Each tumor sample was further normalized to its own tissue control counterpart.
Western blot analysis.
Protein extraction was performed by homogenization of 60 to 120 ng snap-frozen tissue in 75 mM NaCl, 100 mM Tris-HCl (pH 8), 5 mM EDTA (pH 8), and 1 mM phenylmethylsulfonyl fluoride. Protein concentration was determined by use of a bicinchoninic acid assay kit (Pierce Biotechnology) according to the manufacturer's recommendations. Five micrograms of protein from each sample was loaded onto Criterion XT 12% bis-Tris precast gels (Bio-Rad) and run in 0.5× Criterion XT MOPS (morpholinepropanesulfonic acid) running buffer (Bio-Rad). Proteins were transferred onto a polyvinylidene fluoride membrane (Millipore Corporation), and blocking was performed overnight at 4°C in TBS-T (20 mM Tris-HCl [pH 7.6], 200 mM NaCl) containing 5% (wt/vol) fat-free milk and 0.05% Tween 20 (Sigma). Gfi1 primary antibody (ab21061) (Abcam) or β-actin primary antibody (sc-1616) (Santa Cruz Biotechnology) was diluted 1:1,000 in TBS-T-0.05% Tween 20 and incubated with the membranes for 1 h at room temperature. Secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (sc-2004) or rabbit anti-goat antibody (sc-2768) (Santa Cruz Biotechnology) was diluted 1:5,000 in blocking solution and incubated with the membranes for 30 min at room temperature. Membranes were washed in TBS-T. All samples were run simultaneously, and incubation of the membranes with antibodies was performed in the same solution to ensure sample comparability. The antigen-antibody complexes were visualized by use of an ECL Western blotting detection kit (GE Healthcare). The Western blot was repeated for 25 of the tumors with protein from a new round of purification to ensure reproducibility in observed expression patterns (data not shown).
Plasmid constructs and luciferase reporter assay.
The SL3-3 LTR, Gfi1 3′UTR, and Gfi1 3′UTR-SL3-3 constructs from tumor 2ML, 16T, and 25S (integration position are indicated in Table 1) were amplified using NotI and XhoI site-containing primers: Gfi1 3′UTR+XhoI forward primer (5′-CACTCGAGGTACCCTGGCAGCCCGCAA), Gfi1 3′UTR+NotI reverse primer (5′-CAGCGGCCGCGTAATAATCTTAATACTTTATTAAG-3′), SL3-3+XhoI forward primer (5′-CACTCGAGAATGAAAGACCCCTTCATAAGG-3′), and SL3-3+NotI reverse primer (5′-CAGCGGCCGCAATGAAAGACCCCCAGGCTGG-3′). Constructs were ligated into the PsiCheck-2 vector (Promega). 293T cells were cultured in 48-well plates with 2 × 104 cells/well in Dulbecco modified Eagle medium containing 10% fetal bovine serum (Invitrogen) and maintained at 37°C with 5% CO2 for 24 h prior to transfection. Cells were transfected by use of calcium phosphate in triplicates with 200 ng vector and 30 nM pre-miRNA precursor (PM13058 and PM10398) and anti-miRNA inhibitor (AM13058 and AM10398) (Ambion). Transfections were run in miRNA series so that all constructs were simultaneously cotransfected with a particular miRNA. Renilla/firefly activity was measured after 30 h by use of a dual-luciferase reporter assay (Promega) on a FLUOstar Optima luminometer. Renilla/firefly values for the construct with the wt Gfi1 3′UTR were on average 2.5-fold lower than those for the SL3-3 LTR, 2ML, 16T, and 25S constructs. Renilla/firefly values for the different constructs were normalized to values for the control transfection with no added miRNAs. The results presented here are representative of at least two independent transfection experiments for each miRNA, meaning that approximately the same downregulation patterns were observed in both experimental sets for each miRNA.
TABLE 1.
Retroviral integrations in the Gfi1 3′UTR
| Integrationa | Tissueb | Virusc | Provirus orientationd | Provirus positione | Reference(s) |
|---|---|---|---|---|---|
| 1 | S | SL3-3 UCR | + | 6641 | 45; unpublished data |
| 2* | ML | SL3-3 wt | + | 6646 | 51 |
| 3 | S | SL3-3 Ea/s | + | 6653 | 17 |
| 4* | ML | SL3-3 wt | + | 6654 | 51 |
| 5 | S | SL3-3 wt | + | 6654 | 51 |
| 6*** | S | SL3-3 UCR | + | 6656 | 45; unpublished data |
| 7 | S | SL3-3 wt | + | 6658 | 51 |
| 8 | T | SL3-3 (SL3-2Env) | + | 6694 | Unpublished data |
| 9 | S | Akv1-99 Egre+Ea/s | + | 6715 | 69 |
| 10 | T | SL3-3 wt | + | 6734 | 51 |
| 11 | ML | SL3-3 wt | + | 6734 | 51 |
| 12 | S | RFB wt | + | 6734 | Unpublished data |
| 13** | T | SL3-3 Turbo | + | 6787 | 20, 51 |
| 14 | S | SL3-3 GR+Ea/s | + | 6791 | 17 |
| 15** | T | SL3-3 Turbo | + | 6819 | 20, 51 |
| 16 | T | SL3-3 Turbo | + | 6819 | 20, 51 |
| 17 | T | SL3-3 wt | + | 6826 | 51 |
| 18 | T | SL3-3 wt | + | 6842 | 51 |
| 19 | T | SL3-3 wt | + | 6919 | 51 |
| 20*** | S | SL3-3 UCR | + | 6024 | 45; unpublished data |
| 21 | S | SL3-3 wt | + | 6932 | 51 |
| 22 | S | Akv1-99 Runx | + | 7024 | 19, 70 |
| 23 | T | SL3-3 Turbo | + | 7065 | 20, 51 |
| 24**** | S | SL3-3 GR+Ea/s | + | 7068 | 17 |
| 25 | S | SL3-3 wt | + | 7070 | 51 |
| 26**** | S | SL3-3 GR+Ea/s | + | 7086 | 17 |
| 27 | S | SL3-3 (AkvIN) | + | 7267 | Unpublished data |
Integrations that have been identified at more than one position in the Gfi1 3′UTRs from different purification rounds are here considered independent integration events and indicated by asterisks, where the same number of asterisks indicates that the integrations are derived from the same tumor.
T, thymus; S, spleen; ML, mesenteric lymph node.
NMRI-i mice were infected with wt SL3-3 (51), Akv (43, 69), and RFB (unpublished data) MLVs as well as several SL3-3 and Akv mutants with mutations in host transcription factor binding sites: Runx (19, 70), UCR (reference 45 and unpublished data), Egre and Ea/s(17, 69), Turbo (2Δ18-3) (20, 51), glucocorticoid response element (17, 70), SL3-2Env (SL3-3 envelope replaced with SL3-2 envelope) (unpublished data), AkvIN (SL3-3 integrase replaced with Akv integrase) (unpublished data), an Akv1-99 (single enhancer repeat variant of Akv) (43).
Integrated virus position in the same (+) transcriptional direction as Gfi1.
Retrovirus integration position from the Gfi1 transcriptional start site. The Gfi1 3′UTR is at positions 6606 to 7690 from the Gfi1 transcriptional start site.
RESULTS
The tumors assayed in this study originated from previously published and unpublished pathogenicity studies involving mainly wt SL3-3 (51) and Akv (43, 69), as well as SL3-3 and Akv mutated in the host transcription factor binding sites nuclear factor 1 (NF1) (18), Runx (19, 70), and glucocorticoid response element (17, 69) and the basic helix-loop-helix motifs Egre and Ea/s (17, 69). A panel of tumors originated from experimental studies of SL3-3 with replaced envelope and integrase sequences from SL3-2 and Akv, respectively (unpublished data). Furthermore, tumors induced by SL3-3 mutated in the upstream conserved region (UCR) (reference 45 and unpublished data) and the variant with two 18-bp deletions (SL3-3 Turbo) (20, 51) were included in this study. High-throughput sequencing of integrated retroviruses identified 2,800 and 790 tags in tumors induced by Akv and SL3-3, respectively. Additionally, 85 tags were obtained from tumors induced by the RFB MLV, which causes lymphomas, osteopetrosis, and osteomas when injected into NMRI-i mice (23, 63).
Frequent retroviral insertion in the gfi1 genomic locus.
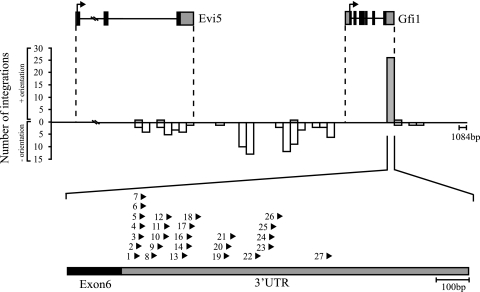
By comparison of isolated tags within publicly available databases, 130 retroviral integrations from 95 tumors were mapped to a 150-kb genomic region on the murine chromosome 5 carrying Gfi1 and Evi5 (the gfi1 locus) (Fig. 1). The majority of the integrations were mapped to the intergenic region between Gfi1 and Evi5, with the provirus oriented mainly in the opposite transcriptional direction of the genes. In a small number of tumors, provirus was positioned downstream of the Gfi1 gene in the same transcriptional direction and in the 3′ end of the Evi5 gene in the opposite transcriptional direction. Notably, 27 retroviral insertions (24 SL3-3 insertions) were tightly clustered within a 1-kb window in the 3′UTR of Gfi1, all oriented in the same transcriptional direction as the Gfi1 gene (Fig. 1 and Table 1). Integrations in the gfi1 locus which were not positioned in the Gfi1 3′UTR will be referred to as “integrations outside the Gfi1 3′UTR.”
FIG. 1.
MLV integrations identified in the genomic region carrying Gfi1 and Evi5. Gfi1 and Evi5 gene structures are shown at the top with coding sequences (black) and UTRs (gray). The transcriptional direction of the genes is indicated by arrows. The number of integrations is indicated by bars. Gray bars represent retroviral integrations in the same transcriptional orientation; white bars represent integrations in the opposite transcriptional direction. Each bar represents 1,084 nucleotides, corresponding to the size of the Gfi1 3′UTR. Integrations in the Gfi1 3′UTR are shown below the graph. The position and transcriptional orientation of the provirus are indicated by arrowheads.
The vast majority of the tumors with 3′UTR insertions were induced by the wt or enhancer-mutated SL3-3. Akv insertion was identified in only two tumors, and one tumor was found to possess an integration of the RFB MLV. Each integration in the Gfi1 3′UTR was validated by PCR using specific primers positioned in the sixth exon of Gfi1 and in the SL3-3 LTR. In all cases, sequencing revealed the presence of chimeric transcripts containing both Gfi1 and SL3-3 LTR sequences (data not shown). The exact position of the provirus with respect to the Gfi1 gene is indicated in Table 1.
This region was the most frequently targeted locus in the NMRI-i mouse genome and contained retroviral insertions in 15% (120 of 790) of all SL3-3-induced tumors, where 90% (120 of 130) were integrations of wt SL3-3 or SL3-3 enhancer mutants (data not shown). There was no significant correlation between integration patterns and virus mutants. Our observations demonstrated that the genomic locus carrying Gfi1, and the Gfi1 3′UTR in particular, are hot spots for retroviral insertions in the SL3-3/NMRI-i lymphoma model.
Retroviral insertions in the gfi1 genomic locus generate truncated forms of Gfi1 mRNA.
To study Gfi1 mRNA expression, 40 tumors were selected for splicing analysis based on accessibility and integration relative to the Gfi1 gene. Samples included tumor material from thymus, spleen, and mesenteric lymph node. PCR was performed using gene-specific primers complementary to sequences in the second and fifth exons in the murine Gfi1 gene. Sequencing revealed the presence of three alternative Gfi1 transcripts, none of which have been previously identified (Fig. 2). The transcripts were characterized by exon 4 skipping (alternative transcript 1) and use of alternative 5′ and 3′ splice sites in exons 3 and 4, respectively (alternative transcripts 2 and 3, respectively). Moreover, alternative transcripts 1 and 2 had maintained their open reading frames. In the panel “integrations in the Gfi1 3′UTR,” transcripts 1 and 3 were detected in SL3-3 (7 of 14)- and Akv (1 of 2)-induced tumors, while transcript 2 was detected in all tumors from this tumor group. Five of the tumors with insertions in the Gfi1 3′UTR had all three alternative transcripts. In tumors with insertions outside the Gfi1 3′UTR, transcripts 1 and 3 were detected only in SL3-3-induced tumors (in 1 of 19 and 7 of 19 cases, respectively), while transcript 2 was identified in both SL3-3 (16 of 19)- and Akv (1 of 4)-induced tumors. Due to a lack of tumor material, it was not possible to include more Akv- or RFB-induced tumors. Table 2 summarizes the frequency of alternative splicing within these two tumor groups. The alternative transcripts showed relatively equal distribution among the thymus, spleen, and mesenteric lymph node tumors, with no apparent correlation to either integration position, virus variant, or provirus orientation. Alternative splicing was also detected in MLV-induced tumors without known integration on chromosome 5 but not in uninfected tissue or in either of the control cell lines, L691, MPC11, or NIH 3T3, indicating that the aberrant splicing of the Gfi1 gene observed in our study is a general phenomenon of MLV-induced lymphomas. We have not determined the relative abundances of the transcripts, and the alternative splicing was not investigated further in this study.
FIG. 2.
Gfi1 alternative transcripts identified by sequencing. The wt Gfi1 is shown at the top. Coding exons are in black, UTRs are in gray. Three alternative transcripts were detected in SL3-3- and Akv-induced tumors, here referred to as alternative transcripts 1, 2, and 3. Sequencing revealed exon 4 skipping (alternative transcript 1) and use of alternative 5′ and 3′ splice sites in exons 3 and 4, respectively (alternative transcripts 2 and 3).
TABLE 2.
Frequency of Gfi1 alternative splicing
| Alternative transcript | No. of tumors with alternative transcript/no. of tumors analyzeda
|
|||||
|---|---|---|---|---|---|---|
| Integrations in Gfi1 3′UTR
|
Integrations outside Gfi1 3′UTR
|
|||||
| SL3-3 | Akv | RFB | SL3-3 | Akv | RFB | |
| 1 | 7/14 | 1/2 | 0/1 | 1/19 | 0/4 | 0/0 |
| 2 | 14/14 | 2/2 | 1/1 | 16/19 | 1/4 | 0/0 |
| 3 | 7/14 | 1/2 | 0/1 | 7/19 | 0/4 | 0/0 |
From a total of 40 tumors analyzed.
Gfi1 is transcriptionally activated by the SL3-3 MLV.
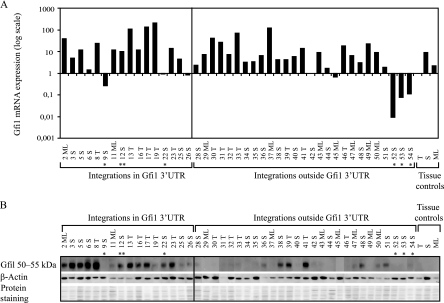
Previous small-scale studies have demonstrated that all MoMLV insertions in the genomic region carrying Gfi1 and Evi5 activate the Gfi1 gene, leading to a three- to sixfold transcriptional upregulation (62, 65). To evaluate the effect of retrovirus integration in the gfi1 genomic locus on Gfi1 mRNA expression, 43 tumors were screened by TaqMan real-time PCR (Fig. 3A). Our data confirmed a general upregulation of Gfi1 mRNA regardless of the position of the provirus in this 150-kb region. Notably, an up-to-200-fold upregulation in tumor 19T and a 10- to 100-fold mRNA increase in 16 other tumors were observed. The transcription level of Gfi1 was found to be significantly elevated in nearly all tumors analyzed, regardless of tissue type or provirus orientation. The upregulation was most prominent in SL3-3-induced tumors but was not observed in Akv-induced tumors, possibly indicating that Gfi1 upregulation takes place primarily in development of T-cell lymphomas. In normal tissue, Gfi1 was most abundant in spleen, with a somewhat lower expression in mesenteric lymph node and thymus.
FIG. 3.
(A) Gfi1 expression in MLV-induced lymphomas. TaqMan real-time PCR was performed on 43 tumors harboring integrations in the Gfi1 3′UTR and elsewhere in the gfi1 locus as well as on the three control tissues, i.e., thymus (T), spleen (S), and mesenteric lymph node (ML). Tumors are indicated by numbers. *, Akv integrations; **, RFB integrations. Gfi1 mRNA expression was normalized to the expression of the tyrosine 3-monooxygenase housekeeping gene, Ywhaz. Thymic, splenic, and mesenteric lymph node tumors were further normalized to thymus, spleen, and mesenteric lymph node tissue controls, respectively. All control tissue was normalized to the thymus control. (B) Gfi1 protein expression in tumors with integrations in the Gfi1 3′UTR and outside of the Gfi1 gene. Tumors are indicated by numbers. Gfi1 was detected at 50 to 55kDa. β-Actin and amido black protein stainings were used as a loading controls.
Further expression analysis of Evi5 mRNA (data not shown) revealed significant Evi5 upregulation in Akv-induced tumors without known integrations on chromosome 5, suggesting an oncogenic potential for Evi5 in B-cell lymphomagenesis. Evi5 was also activated in the RFB-induced tumor 12S harboring integration in the Gfi1 3′UTR but not in the Akv-induced tumor 22S. Expression for tumor 9S was not investigated, and no other Akv tumors with insertions in the gfi1 locus were included in the study due to a lack of tumor material. In the SL3-3-induced tumors from both tumor groups, Evi5 expression varied, with no unambiguous expression pattern.
Decoupling of Gfi1 mRNA and protein expression in tumors harboring retroviral insertions in the Gfi1 3′UTR.
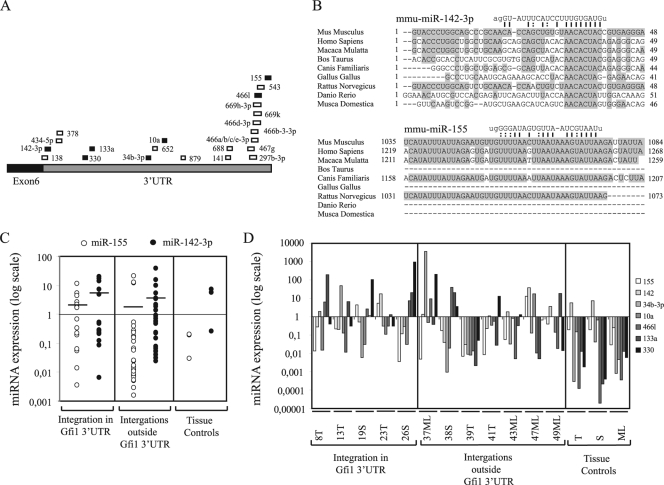
To investigate the correlation between Gfi1 mRNA and protein expression, Western blot analysis was performed with a polyclonal antibody detecting Gfi1 at 50 to 55 kDa (Fig. 3B). Surprisingly, our results demonstrated major differences in Gfi1 protein expression, which appeared to be most abundant in tumors possessing insertions in the Gfi1 3′UTR. Gfi1 protein was identified at a relative high level in all tumors with Gfi1 3′UTR insertions except 9S, 11ML, 19T, 25S, and 26S, which showed vague or no protein expression. In tumors with integrations elsewhere in the gfi1 locus, only 38S, 39T, 41T, 48S, and 51S expressed the Gfi1 protein at equally high levels. No (or vague) protein expression was observed in the remaining tumors from this panel, and no Gfi1 protein was detected in normal-tissue controls. Based on the decoupled mRNA and protein expression patterns, we hypothesized that integrations in the Gfi1 3′UTR might disrupt a potential posttranscriptional miRNA regulation of Gfi1. Previous studies have indicated that Gfi1 might be targeted by several miRNAs (7), and numerous predicted miRNA target sites in the Gfi1 3′UTR (Fig. 4A) can also be found in the miRNA registries (http://microrna.sanger.ac.uk and http://microrna.org). However, experimental validation of whether Gfi1 is subjected to miRNA regulation has not to our knowledge been presented yet. From alignment of predicted potential target sites for different miRNAs in the Gfi1 3′UTRs of various species, miR-142-3p and miR-155 showed the most conservation in the seed binding region (Fig. 4B). Moreover, miR-142-3p displayed perfect base pairing of nucleotides 2 to 8, while a single wobble at position 6 was present in the miR-155 seed sequence. Due to the conservation between species and to the already established expression of miR-142-3p and miR-155 in T and B lymphocytes (47), these were selected as main candidates for further analysis, and real-time quantitative PCR was performed on all 46 samples. Of the remaining miRNAs, which showed no major conservation between species in the region binding the miRNA seed sequence (alignment not shown), miR-330, miR-133a, miR-34b-3p, miR-10a, miR-879, miR-466l, and miR-467g were selected for expression analysis. Thymus, spleen, mesenteric lymph node, and 12 tumors from the two tumor groups with and without Gfi1 protein expression were assayed for miRNA expression. All data were calculated by the ΔCT method, and the values were normalized to snoRNA420 and tissue controls (Fig. 4C). miR-879 and miR-467g were not detected in any of the samples. miR-142-3p and miR-155 expression patterns varied, while expression of miR-330, miR-133a, miR-34b-3p, miR-10a, and miR-466l was mainly downregulated in comparison to control tissue. There was no significant difference in expression between the two tumor groups for any of the miRNAs. The expression data indicate that the increase in Gfi1 protein in tumors with integrations in the Gfi1 3′UTR was not due to a decrease in miRNA levels.
FIG. 4.
(A) Predicted miRNA binding sites in the Gfi1 3′UTR from miRNA registries (http://microrna.sanger.ac.uk and http://microrna.org, April 2009). Positions of the different miRNA binding sites in the Gfi1 3′UTR are indicated by boxes, and miRNAs are indicated by numbers. miRNAs indicated by black boxes were selected for expression analysis. (B) Alignment of miR-142-3p and miR-155 binding sites in different species. Conserved nucleotides are boxed. Presumed binding sites and nucleotide sequences for miR-142-3p and miR-155 are shown, and complementary nucleotides are connected by lines. G·U base pairing is indicated by dashed lines. (C) miR-142-3p and miR-155 expression in 43 tumors with integrations in the Gfi1 3′UTR and outside the Gfi1 gene and three control tissue, i.e., thymus (T), spleen (S), and mesenteric lymph node (ML), assayed by TaqMan real-time PCR. The data were calculated by the ΔCT method, and the values were normalized to snoRNA420. Thymic, splenic, and mesenteric lymph node tumors were further normalized to thymus, spleen, and mesenteric lymph node tissue controls, respectively. Expression for tissue controls is shown as normalized to snoRNA420. Lines indicate mean values for miR-142-3p and miR-155 expression. (D) Real-time PCR expression analysis for miR-155, miR-142, miR-34b-3p, miR-10a, miR-466l, miR-133a, and miR-330 on 12 tumors with integrations in the Gfi1 3′UTR and elsewhere in the gfi1 locus and three control tissues, i.e., thymus (T), spleen (S), and mesenteric lymph node (ML). The data were processed as described above.
Downregulation of the Gfi1 3′UTR by miR-142-3p, miR-155, miR-10a, and miR-133a.
To determine if any of the selected miRNAs were able to recognize the 3′UTR and mediate translational regulation of the Gfi1 transcript, we made a Renilla/luciferase reporter system with different constructs containing the Gfi1 3′UTR, SL3-3 LTR, and 3′UTR-SL3-3 LTR sequences representing retrovirus integration in the tumors 2ML, 16T, and 25S (Fig. 5A). The 2ML and 16T constructs contained only the miR-142-3p binding site, while 25S also contained the miR-330 and miR-133a binding sites. The Gfi1 3′UTR contained all miRNA binding sites.
FIG. 5.
Downregulation of the Gfi1 3′UTR determined by Renilla luciferase assay. (A) Constructs containing the SL3-3 LTR, Gfi1 3′UTR, and Gfi1 3′UTR-SL3-3 chimeric sequences representing integrations of tumor 2ML, 16T, and 25S were ligated into the psiCheck-2 vector. miR-142-3p, miR-155, miR-10a, and miR-133a binding sites in the Gfi1 3′UTR and in the chimeric constructs are indicated. (B) Constructs were cotransfected into 293T cells with miRNA precursors and their respective anti-miRNA inhibitors. Single transfections with the different constructs (− miRNA) and the empty psiCheck-2 vector were used as controls. Renilla/firefly activity for each cotransfection was normalized to the activity for the control transfections (minus miRNA control). The results presented here are representative of at least two independent transfection experiments for each miRNA, meaning that approximately the same downregulation patterns were observed in both experimental sets for each miRNA. Error bars indicate standard deviations.
Our data (Fig. 5B) demonstrated that miR-142-3p was capable of downregulating all constructs, including the empty psiCheck-2 vector, indicating that the effect was not specific for the Gfi1 3′UTR only. In all cases, downregulation by miR-142-3p was rescued by cotransfection with miR-142-3p inhibitor, establishing a specific effect of miR-142-3p on all constructs. Screening of the psiCheck-2 vector sequence detected a perfect seed match between miR-142-3p and Renilla (positions 982 to 987 and 1075 to 1080 [data not shown]). Likewise, screening of the SL3-3 LTR sequence identified weak mir-142-3p complementarity (nucleotides 2 to 6 with one G·U base pairing and nucleotides 3 to 9 with two G·U base pairings) (not shown in Fig. 5).
In contrast, both miR-155 and miR-10a were able to downregulate the Gfi1 3′UTR significantly in comparison to the SL3-3 LTR, and a full rescue was observed in both cases. miR-133a downregulated the Gfi1 3′UTR and 25S constructs, and a small knockdown was also observed on the SL3-3 LTR. A region complementary to the miR-133a seed sequence was found in the SL3-3 LTR (nucleotides 2 to 7 with one G·U base-pairing), however, downregulation of the 2ML and 16T constructs was not observed. The miRNAs 34b-3b, 330, and 466l did not have a specific effect on any of the constructs (data not shown). Our data suggested that miR-155, miR-10a, and miR-133a were able to recognize and bind to sequences present in the 3′UTR of Gfi1 and that the main silencing effect of miR-142-3p was due to recognition of additional binding to complementarity sequences in Renilla and possibly also the SL3-3 LTR.
DISCUSSION
The mechanism of insertional mutagenesis in murine models and identification of retroviral insertion sites by high-throughput screening of the mouse genome have been widely used in identification of genes contributing to murine lymphomagenesis (34, 48, 73).
By large-scale analysis of integrated retroviruses in MLV-infected NMRI-i mice, we have identified the genomic region carrying Gfi1 as the most frequently targeted locus and have addressed the effect of these insertions on expression of the Gfi1 gene. Gfi1 has previously been identified as a common integration site for several retroviruses, including MoMLV (22, 30, 62, 65), Akv (72, 73), and MCF (40). Accumulating retroviral insertions identified in various mouse strains have made Gfi1 a highly targeted gene in MLV-induced lymphomas, with 82 integrations available from the Retrovirus Tagged Cancer Gene Database (http://rtcgd.abcc.ncifcrf.gov/) and many more which have been identified in recent studies (4, 76, 78). We here report on further identification of 130 MLV insertions in and adjacent to Gfi1. The majority of the integrations were of wt or enhancer-mutated SL3-3. In most of the tumors the provirus was found in the intergenic region between Gfi1 and Evi5 in the opposite transcriptional direction, displaying integration patterns similar to those described previously (http://rtcgd.abcc.ncifcrf.gov/). Additionally, a tight cluster of 24 SL3-3 insertions was mapped to a 1-kb region in the Gfi1 3′UTR. Such clustering in the Gfi1 gene has not been documented in other virus/host models and appears to be unique for SL3-3 in the NMRI-i mouse strain.
The differences in integration patterns between studies are often a result of different combinations of mouse genetic background and virus strain. For instance, both Gfi1 and Myc are frequently targeted in by MoMLV in p27kip (30)- and Cdkn2a (44)-deficient mice of the C57Bl6/129 strain but are rarely targeted in BHX2 mice. Likewise, the SL3-3 Turbo enhancer variant has distinct integration hot spots in the c-Myc promoter compared to the wt SL3-3 (51), and the wt SL3-3 has different integration patterns in the Fos/Jdp2/Batf locus in comparison to other SL3-3 enhancer variants (55). The variation of targets in different model systems may reflect different but overlapping pathways to lymphoma development (55).
To determine the effect of the provirus on Gfi1 expression, Gfi1 mRNA and protein levels were determined in 43 tumors. In agreement with earlier studies (62, 65), we found that MLV integration activated Gfi1 expression regardless of provirus position. Gfi1 mRNA upregulation was most profound in SL3-3-induced tumors, supporting the involvement of Gfi1 in T-cell lymphomas. In Akv-induced lymphomas Gfi1 was downregulated compared to in control tissue, strongly indicating that Gfi1 does not have an oncogenic effect in B-cell lymphomagenesis. Thus, we failed to support previous reports of frequent Akv integration in this locus (72, 73). However, evidence from several studies points toward a role for Gfi1 in development of B-cell tumors, a notion supported by findings of plasmacytosis in Gfi1-deficient mice (56) and increased Gfi1 levels in a subset of murine B-cell lymphomas in the marginal zone (68). Furthermore, Gfi1 expression has been detected in early B-cell progenitors (81), and it has been suggested that Gfi1 controls cytokine-dependent B-cell differentiation (57). Taken together, these observations point toward a definite role for Gfi1 in both T-cell and B-cell development and in lymphomagenesis.
Here, we have demonstrated that retroviral insertions in the Gfi1 3′UTR result in elevated Gfi1 mRNA levels in nearly all tumors. However, Gfi1 protein was detected primarily in tumors with retroviral integrations in the 3′UTR and in only a few tumors from the panel “integrations outside the Gfi1 3′UTR.” The observation that integrations outside the Gfi1 3′UTR activate Gfi1 on the mRNA level but do not have an effect on Gfi1 protein expression may indicate that Gfi1 is posttranscriptionally downregulated.
A stabilizing function of retroviral insertions in the 3′UTR in the same transcriptional orientation as the gene has previously been proposed for several genes, including Pim-1 (8, 66), Myc (6), and Int-2 (10). Thereby, 3′noncoding sequences that negatively affect the mRNA stability are removed, rendering the normal mRNA unstable and leading to accumulation of abnormal mRNA and protein levels. Retroviral insertions are further able to facilitate the use of cryptic promoters (6) or destroy important regulatory elements such as A/U-rich regions implicated in mRNA destabilization (79). Based on the preferred integration clustering in the 5′ end of the Gfi1 3′UTR and the Gfi1 protein expression patterns observed here, we speculated on whether retroviral integrations decoupled miRNA binding to the Gfi1 3′UTR, resulting in an increase in protein synthesis. In this case, it would be possible that the high Gfi1 protein expression observed in some of the tumors from the panel “integrations outside of the Gfi1 3′UTR” (38S, 39T, 41T, 48S, and 51S) could reflect deregulation of other proteins important for miRNA processing, although we did not succeed in identifying such integrations.
The Gfi1 3′UTR holds predicted binding sites for several miRNAs, including miR-142-3p and miR-155, which are found with relatively high abundance in most hematopoietic cells (47) and show highly conserved binding sites in the Gfi1 3′UTRs of various species. Real-time PCR expression analysis for the miRNAs 155 and 142-3p showed varying expression patterns, while miRNAs 34b-3p, 10a, 466l, 133a, and 330 demonstrated a general downregulation in most of the tumors in comparison to control tissue. Our data indicated that the increase in Gfi1 protein in tumors with integrations in the Gfi1 3′UTR was not due to a decrease in miRNA levels for any of the miRNAs investigated here.
In order to determine if any of these miRNAs could be potential downregulators of Gfi1, Renilla luciferase reporter assays with different constructs were performed. Our results demonstrated that miR-142-3p was able to downregulate all constructs, suggesting that this downregulation results from an interaction with competing target sequences in the psiCheck-2 vector and the SL3-3 LTR. Previous studies have demonstrated that multiple target sites can potentially increase the degree of translational suppression (13), possibly explaining the higher silencing observed here for miR-142-3p on the 3′UTR 16T and 25S constructs. Only a minor downregulation by miR-142-3p was observed on the 2ML construct, although this contained the full binding site for miR-142-3p. The integration in tumor 2ML is positioned just three nucleotides downstream of the miRNA binding region, possibly influencing the structure of the small Gfi1 3′UTR fragment. Several studies have addressed the role of mRNA structure in miRNA target recognition and suggest that the affinity of binding of a miRNA to its mRNA target is determined by both the sequence and structure of the mRNA (11, 12, 14, 33, 42). A possible explanation for the variability that we observed in our experiments may simply arise from differences in accessibility imposed by the sequence surrounding the target.
In contrast, miR-155, miR-10a, and miR-133a all had a downregulating effect on the Gfi1 3′UTR construct, possibly suggesting a role for these miRNAs in posttranscriptional regulation of the Gfi1 gene. Furthermore, the 25S construct, which was the only chimeric construct containing the miR133a binding site, was also downregulated. Together, our results support the hypothesis that Gfi1 may be downregulated by one or more miRNAs. However, we have assessed the function of only a small number of potential miRNAs. Other miRNAs may also have an effect on Gfi1 regulation. To further validate whether any of the miRNAs investigated here targets the Gfi1 gene, additional experiments, including miRNA knockdown in different cell lines and subsequent analysis of Gfi1 expression, need to be performed.
Our results suggest that retroviral integrations in the Gfi1 3′UTR contribute to Gfi1 activation and possibly T-cell lymphomagenesis through loss of miRNA binding sites. In the majority of the tumors with insertions elsewhere in the gfi1 locus, no Gfi1 protein expression was observed. It is unclear how these integrations contribute to the development of these tumors. Ccnd3, Myc/Pvt1, Ras2, and RasGrp1, which were previously identified as possible Gfi1 cooperative partners in lymphoma development (76), were found as recurring integrations in several of the tumors with integrations in the gfi1 locus. Development of T-cell lymphomas in tumors with integrations outside the Gfi1 3′UTR that do not express the Gfi1 protein could be a result of activation of these or other oncogenes. Of the 130 insertions, Ccnd3 was a cotarget in 10 tumors and was also found as a target in tumor 42S, 45ML, 49ML, and 50ML; Myc/Pvt1was targeted four times, including in 8T and 48S; Ras2 was cotargeted in 9 tumors, including 42S; and RasGRP1 was cotargeted in 5 tumors including 37 M and 43ML. We do not know how Ccnd3, Myc/Pvt1, Ras2, and RasGrp1 are expressed in the tumors investigated in this study, and the techniques used to identify retroviral tags do not necessarily identify all integrations. Overall, more extensive analyses need to be performed in order to obtain a clearer impression of how these tumors were initiated.
In comparison to tumor development through insertional mutagenesis of proto-oncogenes or tumor suppressors, a recently discovered mode of tumor induction includes retroviral targeting of miRNA loci and deregulation of single miRNAs or miRNA cistrons. The SL3-3 retrovirus has been shown to activate the 17-92 miRNA cistron (78), while the avian leukosis virus targets the BIC gene (the chromosomal region encoding miR-155) (16, 74) and the Radiation MLV frequently integrates into a locus encoding a group of five differentially spliced noncoding RNAs known as Kis2 (35). Retroviral integration in all these regions caused significant upregulation of the miRNA clusters, demonstrating a role for these miRNAs in oncogenesis.
In this study, we have introduced an SL3-3/NMRI-i model with high retroviral integration frequency in the gfi1 locus and deregulated Gfi1 mRNA and protein expression patterns. Our data indicate that retroviral insertions in the Gfi1 3′UTR contribute to activation of Gfi1 by loss of regulatory regions important for miRNA posttranscriptional downregulation of the gene. It is possible that such loss of regulatory regions in the 3′UTR of the human GFI1 gene might likewise contribute to human lymphomagenesis. The human GFI1 gene is carried in the chromosomal region 1p22 (58), a locus commonly affected in several cancers, including mantle cell lymphoma (60, 61), mucosa-associated lymphoid tissue lymphoma (77), and neuroblastoma (46). Although there has been no direct correlation between translocations in this region and the effect on the GFI1 gene, our studies support the importance of this genomic region in tumor development. In humans, precursor T-cell lymphoma is a rare disease with a poor prognosis but with a clear diagnostic parallel to the same type of tumors observed in murine models (49). In time, the results presented here may contribute to understanding of the oncogenic mechanisms by which Gfi1 is involved in development of both murine and human T-cell lymphomas.
Acknowledgments
The technical assistance of Astrid van der Aa Kühle is gratefully acknowledged.
This project was supported by grants from The Karen Elise Jensen Foundation (M.J.D. and K.D.), the Danish Cancer Society (H.E.J. and F.S.P.), and The Danish Agency for Science Technology and Innovation (F.S.P.) and by NIH grant R01AI41570 (M.W.).
Footnotes
Published ahead of print on 27 May 2009.
REFERENCES
- 1.Ben-David, Y., E. B. Giddens, K. Letwin, and A. Bernstein. 1991. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 6908-918. [DOI] [PubMed] [Google Scholar]
- 2.Brennecke, J., A. Stark, R. B. Russell, and S. M. Cohen. 2005. Principles of microRNA-target recognition. PLoS Biol. 3e85-e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celander, D., and W. A. Haseltine. 1987. Glucocorticoid regulation of murine leukemia virus transcription elements is specified by determinants within the viral enhancer region. J. Virol. 61269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborty, J., H. Okonta, H. Bagalb, S. J. Lee, B. Fink, R. Changanamkandat, and J. Duggan. 2008. Retroviral gene insertion in breast milk mediated lymphomagenesis. Virology 377100-109. [DOI] [PubMed] [Google Scholar]
- 5.Chatis, P. A., C. A. Holland, J. W. Hartley, W. P. Rowe, and N. Hopkins. 1983. Role for the 3′ end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc. Natl. Acad. Sci. USA 804408-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcoran, L. M., J. M. Adams, A. R. Dunn, and S. Cory. 1984. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell 37113-122. [DOI] [PubMed] [Google Scholar]
- 7.Costa, I. G., S. Roepcke, and A. Schliep. 2007. Gene expression trees in lymphoid development. BMC Immunol. 825-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuypers, H. T., G. Selten, W. Quint, M. Zijlstra, E. R. Maandag, W. Boelens, P. van Wezenbeek, C. Melief, and A. Berns. 1984. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell 37141-150. [DOI] [PubMed] [Google Scholar]
- 9.DesGroseillers, L., and P. Jolicoeur. 1984. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are primary determinant of their leukemogenic potential. J. Virol. 52945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson, C., R. Smith, S. Brookes, and G. Peters. 1990. Proviral insertions within the int-2 gene can generate multiple anomalous transcripts but leave the protein-coding domain intact. J. Virol. 64784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didiano, D., and O. Hobert. 2006. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nat. Struct. Mol. Biol. 13849-851. [DOI] [PubMed] [Google Scholar]
- 12.Didiano, D., and O. Hobert. 2008. Molecular architecture of a miRNA-regulated 3′UTR. RNA 141297-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doench, J. G., C. P. Petersen, and P. A. Sharp. 2003. siRNAs can function as miRNAs. Genes Dev. 17438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doench, J. G., and P. A. Sharp. 2004. Specificity of microRNA target selection in translational repression. Genes Dev. 18504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwivedi, P. P., P. H. Anderson, J. L. Omdahl, H. L. Grimes, H. A. Morris, and B. K. May. 2005. Identification of growth factor independent-1 (GFI1) as a repressor of 25-hydroxyvitamin D 1-alpha hydroxylase (CYP27B1) gene expression in human prostate cancer cells. Endocrinol. Relat. Cancer 12351-365. [DOI] [PubMed] [Google Scholar]
- 16.Eis, P. S., W. Tam, L. Sun, A. Chadburn, Z. Li, M. F. Gomez, E. Lund, and J. E. Dahlberg. 2005. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA 1023627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ejegod, D., K. D. Sørensen, I. Mossbrugger, L. Quintanilla-Martinez, J. Schmidt, and F. S. Pedersen. 2009. Control of pathogenicity and disease specificity of a T-cell lymphomagenic gammaretrovirus by E-box motifs but not by an overlapping glucocorticoid response element. J. Virol. 83336-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ethelberg, S., B. Hallberg, J. Lovmand, J. Schmidt, A. Luz, T. Grundström, and F. S. Pedersen. 1997. Second-site proviral enhancer alterations in lymphomas induced by enhancer mutants of SL3-3 murine leukemia virus: negative effects of nuclear factor 1 binding site. J. Virol. 711196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ethelberg, S., J. Lovmand, J. Schmidt, A. Luz, and F. S. Pedersen. 1997. Increased lymphomagenicity and restored disease specificity of AML1 site (core) mutant SL3-3 murine leukemia virus by a second-site enhancer variant evolved in vivo. J. Virol. 717273-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ethelberg, S., A. B. Sørensen, J. Schmidt, A. Luz, and F. S. Pedersen. 1997. An SL3-3 murine leukemia virus enhancer variant more pathogenic than the wild type obtained by assisted molecular evolution in vivo. J. Virol. 719796-9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan, H., B. K. Brightman, B. R. Davis, and Q. X. Li. 1991. Leukemogenesis by Moloney murine leukemia virus, p. 155-174. In H. Y. Fan et al. (ed.), Viruses that affect the immune system. American Society for Microbiology, Washington, DC.
- 22.Gilks, C. B., S. E. Bear, H. L. Grimes, and P. N. Tsichlis. 1993. Progression of interleukin-2 (IL-2)-dependent rat T-cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol. Cell. Biol. 131759-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimbel, W., J. Schmidt, J. Barack-Werner, A. Luz, P. G. Strauss, V. Erfle, and T. Werner. 1996. Molecular and pathogenic characterization of the RFB osteoma virus: lack of oncogene and induction of osteoma, osteopetrosis and lymphoma. Virology 224533-538. [DOI] [PubMed] [Google Scholar]
- 24.Golemis, E. A., N. A. Speck, and N. Hopkins. 1990. Alignment of U3 region sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J. Virol. 64534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimes, H. L., T. O. Chan, P. A. Zweidler-McKay, B. Tong, and P. N. Tsichlis. 1996. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol. Cell. Biol. 166263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimes, H. L., C. B. Gilks, T. O. Chan, S. Porter, and P. N. Tsichlis. 1996. The Gfi-1 protooncoprotein represses Bax expression and inhibits T-cell death. Proc. Natl. Acad. Sci. USA 9314569-14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallberg, B., J. Schmidt, A. Luz, F. S. Pedersen, and T. Grundström. 1991. SL3-3 enhancer factor I transcriptional activators are required for tumor formation by SL3-3 murine leukemia virus. J. Virol. 654177-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayward, W. S., B. G. Neel, and S. M. Astrin. 1981. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature 290475-480. [DOI] [PubMed] [Google Scholar]
- 29.Hock, H., M. J. Hamblen, H. M. Rooke, J. W. Schindler, S. Saleque, Y. Fujiwara, and S. H. Orkin. 2004. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 4311002-1007. [DOI] [PubMed] [Google Scholar]
- 30.Hwang, H. C., C. P. Martins, Y. Bronkhorst, E. Randel, A. Berns, M. Fero, and B. E. Clurman. 2002. Identification of oncogenes collaborating with p27kip1 loss by insertional mutagenesis and high-throughput insertion site analysis. Proc. Natl. Acad. Sci. USA 9911293-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karsunky, H., H. Zeng, T. Schmidt, B. Zevnik, R. Kluge, K. W. Schmid, U. Dührsen, and T. Möröy. 2002. Inflamatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat. Genet. 30295-300. [DOI] [PubMed] [Google Scholar]
- 32.Kazanjian, A., D. Wallis, N. Au, R. Nigam, K. J. T. Venken, P. T. Cagle, B. F. Dickey, H. J. Bellen, C. B. Gilks, and H. L. Grimes. 2004. Growth factor independence-1 is expressed in primary human neuroendocrine lung carcinomas and mediates the differentiation of murine pulmonary neuroendocrine cells. Cancer Res. 646874-6882. [DOI] [PubMed] [Google Scholar]
- 33.Kertesz, M., N. Iovino, U. Unnerstall, U. Gaul, and E. Segal. 2007. The role of site accessibility in miRNA target recognition. Nat. Genet. 391278-1284. [DOI] [PubMed] [Google Scholar]
- 34.Kim, R., A. Trubetskoy, T. Suzuki, N. A. Jenkins, N. G. Copeland, and J. Lenz. 2003. Genome-based identification of cancer genes by proviral tagging in mouse retrovirus-induced T-cell lymphomas. J. Virol. 772056-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landais, S., S. Landry, P. Legault, and E. Rassart. 2007. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res. 675699-5707. [DOI] [PubMed] [Google Scholar]
- 36.Lee, R. C., R. L. Feinbaum, and V. Ambros. 1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75843-854. [DOI] [PubMed] [Google Scholar]
- 37.Lenz, J., D. Celander, R. L. Crowther, R. Patarca, D. W. Perkins, and W. A. Haseltine. 1984. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. Nature 308467-470. [DOI] [PubMed] [Google Scholar]
- 38.Lewis, A. F., T. Stacy, W. R. Green, L. Taddesse-Heath, J. W. Hartley, and N. A. Speck. 1999. Core-binding factor influences the disease specificity of Moloney murine leukemia virus. J. Virol. 735535-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis, B. P., C. B. Burge, and D. P. Bartel. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 12015-20. [DOI] [PubMed] [Google Scholar]
- 40.Liao, X., A. M. Buchberg, N. A. Jenkins, and N. G. Copeland. 1995. Evi-5, a common site of retroviral integration in AKXD T-cell lymphomas, maps near Gfi1 on mouse chromosome 5. J. Virol. 697132-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim, L. P., N. C. Lau, P. Garrett-Engele, A. Grimson, J. M. Schelter, J. Castle, D. P. Bartel, P. S. Linsley, and J. M. Johnson. 2005. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433769-773. [DOI] [PubMed] [Google Scholar]
- 42.Long, D., R. Lee, P. Williams, C. Y. Chan, V. Ambros, and Y. Ding. 2007. Potent effect of target structure on microRNA function. Nat. Struct. Mol. Biol. 14287-294. [DOI] [PubMed] [Google Scholar]
- 43.Lovmand, J., A. B. Sørensen, J. Schmidt, M. Østergaard, A. Luz, and F. S. Pedersen. 1998. B-cell lymphoma induction by Akv murine leukemia viruses harboring one or both copies of the tandem repeat in the U3 enhancer. J. Virol. 725745-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund, A. H., G. Turner, A. Trubetskoy, E. Verhoeven, E. Wientjens, D. Hulsman, R. Russell, R. A. DePinho, J. Lenz, and M. van Lohuizen. 2002. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat. Genet. 32160-165. [DOI] [PubMed] [Google Scholar]
- 45.Ma, S. L., J. Lovmand, A. B. Sørensen, A. Luz, J. Schmidt, and F. S. Pedersen. 2003. Triple basepair changes within and adjacent to the conserved YY1 motif upstream of the U3 enhancer repeats of SL3-3 murine leukemia virus cause a small but significant shortening of latency of T-lymphoma induction. Virology 313638-644. [DOI] [PubMed] [Google Scholar]
- 46.Mead, R. S., and J. K. Cowell. 1995. Molecular characterization of a (1;10)(p22;q21) constitutional translocation from a patient with neuroblastoma. Cancer Genet. Cytogenet. 81151-157. [DOI] [PubMed] [Google Scholar]
- 47.Merkerova, M., M. Belickova, and H. Bruchova. 2008. Differential expression of microRNA in hematopoietic cell lineages. Eur. J. Haematol. 81304-310. [DOI] [PubMed] [Google Scholar]
- 48.Mikkers, H., J. Allen, P. Knipscheer, L. Romeijn, A. Hart, E. Vink, and A. Berns. 2002. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat. Genet. 32153-159. [DOI] [PubMed] [Google Scholar]
- 49.Morse, H. C., III, M. R. Anver, T. N. Fredrickson, D. C. Haines, A. W. Harris, N. L. Harris, E. S. Jaffe, S. C. Kogan, I. C. MacLennan, P. K. Pattengale, and J. M. Ward. 2002. Hematopathology subcommittee of the Mouse Models of Human Cancers Consortium. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood 100246-258. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen, A. L., P. L. Nørby, F. S. Pedersen, and P. J.ørgensen. 1996. Various models of basic helix-loop-helix protein-mediated regulation of murine leukemia virus transcription in lymphoid cells. J. Virol. 705893-5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen, A. A., A. B. Sørensen, J. Schmidt, and F. S. Pedersen. 2005. Analysis of wild-type and mutant SL3-3 murine leukemia virus insertions in the c-myc promoter during lymphomagenesis reveals target site hot spots, virus-dependent patterns, and frequent error-prone gap repair. J. Virol. 7967-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nieves, A., L. S. Levy, and J. Lenz. 1997. Importance of a c-Myb binding site for lymphomagenesis by the retrovirus SL3-3. J. Virol. 711213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nusse, R., and H. E. Varmus. 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 3199-109. [DOI] [PubMed] [Google Scholar]
- 54.Pargmann, D., R. Yücel, C. Kosan, I. Saba, L. Klein-Hitpass, S. Schimmer, F. Heyd, U. Dittmer, and T. Möröy. 2007. Differential impact of the transcriptional repressor Gfi1 on mature CD4+ and CD8+ T lymphocyte function. Eur. J. Immunol. 373551-3563. [DOI] [PubMed] [Google Scholar]
- 55.Rasmussen, M. H., A. B. Sørensen, D. W. Morris, J. C. Dutra, E. K. Engelhard, C. L. Wang, J. Schmidt, and F. S. Pedersen. 2005. Tumor model-specific proviral insertional mutagenesis of the Fos/Jdp2/Batf locus. Virology 337353-364. [DOI] [PubMed] [Google Scholar]
- 56.Rathinam, C., H. Lassmann, M. Mengel, and C. Klein. 2008. Transcription factor Gfi1 restricts B-cell mediated autoimmunity. J. Immunol. 1816222-6229. [DOI] [PubMed] [Google Scholar]
- 57.Rathinam, C., and C. Klein. 2007. Transcriptional repressor Gfi1 integrates cytokine-receptor signals controlling B-cell differentiation. PLoS ONE 2e306-e317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Roberts, T., and J. K. Cowell. 1997. Cloning of the human Gfi-1 gene and its mapping to chromosome region 1p22. Oncogene 141003-1005. [DOI] [PubMed] [Google Scholar]
- 59.Sakai, I., H. Yamauchi, M. Yasukawa, H. Kohno, and S. Fujita. 2001. Expression of the Gfi-1 gene in HTLV-I-transformed T cells. Int. J. Hematol. 73507-516. [DOI] [PubMed] [Google Scholar]
- 60.Salaverria, I., B. Espinet, A. Carrió, D, Costa, L, Astier, J. Slotta-Huspenina, L, Quintanilla-Martinez, F. Fend, F. Solé, D. Colomer, S. Serrano, R. Miró, S. Beà, and E. Campo. 2008. Multiple recurrent chromosomal breakpoints in mantle cell lymphoma revealed by a combination of molecular cytogenetic techniques. Genes Chromosomes Cancer 471086-1097. [DOI] [PubMed] [Google Scholar]
- 61.Sander, S., L. Bullinger, E. Leupolt, A. Benner, D. Kienle, T. Katzenberger, J. Kalla, G. Ott, H. K. Müller-Hermelink, T. F. Barth, P. Möller, P. Lichter, H. Döhner, and S. Stilgenbauer. 2008. Genomic aberrations in mantle cell lymphoma detected by interphase fluorescence in situ hybridization. Incidence and clinicopathological correlations. Haematologica 93680-687. [DOI] [PubMed] [Google Scholar]
- 62.Scheijen, B., J. Jonkers, D. Acton, and A. Berns. 1997. Characterization of pal-1, a common proviral insertion site in murine leukemia virus-induced lymphomas of c-myc and pim-1 transgenic mice. J. Virol. 719-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt, J., K. Lumniczky, B. D. Tzschaschel, H. L. Guenther, A. Luz, S. Riemann, W. Gimbel, V. Erfle, and R. G. Erben. 1999. Onset and dynamics of osteosclerosis in mice induced by Reilly-Finkel-Biskis (RFB) murine leukemia virus. Am. J. Pathol. 155557-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt, T., H. Karsunky, B. Rödel, B. Zevnik, H-P. Elsässer, and T. Möröy. 1998. Evidence implicating Gfi-1 and Pim-1 in pre-T-cell differentiation steps associated with β-selection. EMBO J. 175349-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt, T., M. Zörnig, R. Benke, and T. Möröy. 1996. MoMuLV proviral integrations identified by Sup-F selection in tumors from infected myc/pim bitransgenic mice correlate with activation of the gfi-1 gene. Nucleic Acids Res. 242528-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selten, G. H., H. T. Cuypers, and A. Berns. 1985. Proviral activation of the putative oncogene Pim-1 in MuLV induced T-cell lymphomas. EMBO J. 41793-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selten, G. H., H. T. Cuypers, M. Zijlstra, C. Melief, and A. Berns. 1984. Involvement of c-Myc in T cell lymphomas in mice: frequency and mechanism of activation. EMBO J. 33215-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin, M. S., T. N. Fredrickson, J. W. Hartley, T. Suzuki, K. Akagi, and H. C. Morse III. 2004. High-throughput retroviral tagging for identification of genes involved in initiation and progression of mouse splenic marginal zone lymphomas. Cancer Res. 644419-4427. [DOI] [PubMed] [Google Scholar]
- 69.Sørensen, K. D., S. Kunder, L. Quintanilla-Martinez, J. Sørensen, J. Schmidt, and F. S. Pedersen. 2007. Enhancer mutations of Akv murine leukemia virus inhibit the induction of mature B-cell lymphomas and shift disease specificity towards the more differentiated plasma cell stage. Virology 362179-191. [DOI] [PubMed] [Google Scholar]
- 70.Sørensen, K. D., L. Quintanilla-Martinez, S. Kunder, J. Schmidt, and F. S. Pedersen. 2004. Mutation of all Runx (AML1/core) sites in the enhancer of T-lymphomagenic SL3-3 murine leukemia virus unmasks a significant potential for myeloid leukemia induction and favors enhancer evolution toward induction of other disease patterns. J. Virol. 7813216-13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Speck, N. A., B. Renjifo, E. Golemis, T. N. Fredrickson, J. W. Hartley, and N. Hopkins. 1990. Mutation of the core or adjacent LVb elements of the Moloney murine leukemia virus enhancer alters disease specificity. Genes Dev. 4233-242. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki, T., K. Minehata, K. Agaki, N. A. Jenkins, and N. G. Copeland. 2006. Tumor suppressor gene identification using retroviral insertional mutagenesis in Blm-deficient mice. EMBO J. 253422-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki, T., H. Shen, K. Akagi, H. C. Morse, J. D. Malley, D. Q. Naiman, N. A. Jenkins, and N. G. Copeland. 2002. New genes involved in cancer identified by retroviral tagging. Nat. Genet. 32166-174. [DOI] [PubMed] [Google Scholar]
- 74.Tam, W., D. Ben-Yehuda, and W. S. Hayward. 1997. bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA. Mol. Cell. Biol. 171490-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsichlis, P. N., and P. A. Lazo. 1991. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr. Top. Microbiol. Immunol. 17195-173. [DOI] [PubMed] [Google Scholar]
- 76.Uren, A. G., J. Kool, K. Matentzoglu, J. de Ridder, J. Mattison, M. van Uitert, W. Lagcher, D. Sie, E. Tanger, T. Cox, M. Reinders, T. J. Hubbard, J. Rogers, J. Jonkers, L. Wessels, D. J. Adams, M. van Lohuizen, and A. Berns. 1998. Large-scale mutagenesis in p19ARF- and p53-deficient mice identifies cancer genes and their collaborative networks. Cell 133727-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vega, F., and L. J. Medeiros. 2001. Marginal-zone B-cell lymphoma of extranodal mucosa-associated lymphoid tissue type: molecular genetics provides new insights into pathogenesis. Adv. Anat. Pathol. 8313-326. [DOI] [PubMed] [Google Scholar]
- 78.Wang, C. L., B. B. Wang, G. Bartha, L. Li, N. Channa, M. Klinger, N. Killeen, and M. Wabl. 2006. Activation of an oncogenic microRNA cistron by provirus integration. Proc. Natl. Acad. Sci. USA 10318680-18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wingett, D., R. Reeves, and N. S. Magnuson. 1992. Characterization of the testes-specific pim-1 transcript in rat. Nucleic Acids Res. 203183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yücel, R., H. Karsunky, L. Klein-Hitpass, and T. Möröy. 2003. The transcriptional repressor Gfi1 affects development of early uncommitted c-kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J. Exp. Med. 197831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yücel, R., C. Kosan, F. Heyd, and T. Möröy. 2004. Gfi1:Green fluorescent protein knock-in mutant reveals different expression and autoregulation of the growth factor independence 1 (Gfi1) gene during lymphocyte development. J. Biol. Chem. 27940906-40917. [DOI] [PubMed] [Google Scholar]
- 82.Zeng, H., R. Yücel, C. Kosan, L. Klein-Hitpass, and T. Möröy. 2004. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J. 234116-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]