Abstract
HtrA1 belongs to a family of serine proteases found in organisms ranging from bacteria to humans. Bacterial HtrA1 (DegP) is a heat shock-induced protein that behaves as a chaperone at low temperature and as a protease at high temperature to help remove unfolded proteins during heat shock. In contrast to bacterial HtrA1, little is known about the function of human HtrA1. Here, we report the first evidence that human HtrA1 is a microtubule-associated protein and modulates microtubule stability and cell motility. Intracellular HtrA1 is localized to microtubules in a PDZ (PSD95, Dlg, ZO1) domain-dependent, nocodazole-sensitive manner. During microtubule assembly, intracellular HtrA associates with centrosomes and newly polymerized microtubules. In vitro, purified HtrA1 promotes microtubule assembly. Moreover, HtrA1 cosediments and copurifies with microtubules. Purified HtrA1 associates with purified α- and β-tubulins, and immunoprecipitation of endogenous HtrA1 results in coprecipitation of α-, β-, and γ-tubulins. Finally, downregulation of HtrA1 promotes cell motility, whereas enhanced expression of HtrA1 attenuates cell motility. These results offer an original identification of HtrA1 as a microtubule-associated protein and provide initial mechanistic insights into the role of HtrA1 in theregulation of cell motility by modulating microtubule stability.
HtrA1 (for high temperature requirement) belongs to a family of serine proteases and is so named because of its essential role in thermal tolerance in Escherichia coli, which requires HtrA (also known as DegP) for survival at elevated temperatures (14). This survival is attributed to the ability of HtrA proteins to switch from chaperones to proteases that reduce the amount of unfolded and aggregated protein upon heat stress (46). Human, as well as bacterial, HtrA proteins contain trypsin and PDZ (PSD95, Dlg, ZO1) domains that display a high degree of sequence conservation from bacteria to human (14). Of the four human HtrA proteins, HtrA1, HtrA3, and HtrA4 also contain a signal peptide, insulin-like growth factor binding protein (IGFBP), and Kazal-type trypsin inhibitor domains, while HtrA2 lacks these domains. Although HtrA1 contains signal peptide, an intracellular form of HtrA1 has been reported as well (15, 17). The mitochondrial protein HtrA2 is well characterized and has been shown to be involved in apoptosis (27, 37, 39, 47, 52, 53) and neurodegenerative disease (35). However, HtrA1 is the first in the family to be implicated as a tumor suppressor in ovarian cancer and melanoma (3, 5, 13). In addition, HtrA1 is implicated in various pathogenic and developmental processes, including osteoarthritis, Alzheimer's disease, neuronal maturation and development, age-related macular degeneration, and tumor progression (11, 23, 24, 33, 36, 50, 56). Specific to its role in tumor progression, HtrA1 is downregulated in various cancers, and its downregulation is associated with resistance to chemotherapy and a metastatic phenotype (4, 11, 19). Recently, we developed a mixture-based peptide library to determine the specificities of cleavage site motifs for HtrA1 serine protease. The results identified tubulins as potential substrates of HtrA1. Furthermore, we showed that exogenously expressed HtrA1 disrupts microtubules (MTs) and targets tubulins for degradation (data not shown). These results suggest a potential role for HtrA1 as an MT-associated protein (MAP) and its potential to regulate MT and tubulin stability and MT-associated cellular functions.
MTs are highly dynamic noncovalent polymers of α- and β-tubulins that undergo cyclical shrinking (catastrophe) and growing (rescue) (18, 31, 43). The dynamic instability of MTs is central to their diverse biological functions, including the coordination of cell division (40, 55), morphogenesis (25), cell polarity (42), and motility (48). MT instability is, in part, modulated by MAPs (2, 29). Many tumor suppressors, such as adenomatous polyposis coli (APC) (20), RASSF1A (45), and Dlg (6), associate with MTs and impose tumor suppressor activities by regulating their functions related to cell division, polarity, and motility. Deregulation of these processes, as a consequence of loss of function of these tumor suppressors, contributes to unchecked proliferation; cytoarchitecture disruption; and the ability to migrate, invade, and metastasize distant organs (6, 7, 26). Therefore, the regulation of MT stability and dynamics or the lack of it has dire consequences for normal cell functions.
Given the fact that HtrA1 is downregulated in various cancers, particularly in metastatic cancer, it is possible that HtrA1 may regulate certain aspects of cancer, namely, the motility of cancer cells, by modulating MT stability and dynamics. Therefore, to better characterize the interaction between HtrA1 and MTs and to gain mechanistic insights into the functional consequences of HtrA1 downregulation in cancer, we investigated the biochemical interaction between HtrA1 and tubulin, the domain within HtrA1 required for localization to MTs, and the effect on cell migration. Here, we describe the identification of HtrA1 as an MT-associated serine protease and a novel role of HtrA1 in the regulation of cell motility.
MATERIALS AND METHODS
Cell culture, transfection, and drug treatment.
SKOV3 cells were purchased from the ATCC and grown according to the provider's recommendation. The A2780 cell line was obtained from Thomas C. Hamilton, Fox Chase Cancer Center, and maintained in M199/MCDB 105 (Sigma-Aldrich, St. Louis, MO) with 5% fetal bovine serum from Invitrogen (Carlsbad, CA). The OV202 cell line was established at the Mayo Foundation and grown as previously described (16). Cells were transfected with plasmids using Lipofectamine Plus (Invitrogen) according to the manufacturer's recommendation. The cells were treated with 300 ng/ml nocodazole (Sigma-Aldrich) to destabilize MTs. The cells were also treated with 10 nM paclitaxel (Taxol) (Sigma-Aldrich) to stabilize MTs. GTP was purchased from Sigma-Aldrich. MitoTracker Red was purchased from Invitrogen, and a working concentration of 100 nM was used according to the protocol provided by the manufacturer.
Antibodies.
Polyclonal antibodies raised against a peptide corresponding to amino acids 161 to 480 of HtrA1 were affinity purified as previously described (33). Monoclonal antibodies against α-, β-, and γ-tubulins; β-actin; tau; and MAP2a/b were purchased from Sigma-Aldrich. Myc epitope tag antibodies were purchased from Cell Signaling (Danvers, MA), and glutathione S-transferase (GST) antibodies were purchased from Amersham (Piscataway, NJ). Polyclonal antibodies against HtrA2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Affinity-purified polyclonal antibodies against HtrA3 were generated using recombinant HtrA3 protein.
Transfection with siRNA and immunodetection of knocked-down cells.
Cells were transfected with small interfering RNA (siRNA) against HtrA1 as previously described (11). In some experiments, HtrA1 expression was rescued by RNA interference (RNAi)-resistant expression plasmids as previously described (12). Upon assay, coverslips were removed, fixed in prechilled methanol at −20°C, and immunostained with HtrA1 and tubulin antibodies. Whole-cell lysates (35 μg) from the same well were analyzed by Western blotting with HtrA1 and β-actin antibodies.
Immunostaining and fluorescence microscopy.
Immunostaining was performed as described previously (38). Slides were incubated for 1 h at room temperature in antibodies diluted as follows: anti-HtrA1, 1:100; anti-α-tubulin, 1:100. Some slides were stained with rhodamine-conjugated phalloidin during secondary-antibody incubation. Laser scanning confocal microscopy was performed on a Zeiss LSM510 with krypton-argon and helium-neon lasers.
Immunoblotting.
Cells were lysed in buffer (20 mM Tris-HCl at pH 7.6, 120 mM NaCl, 1 mM EDTA, 0.5% Nonidet P40, 1 mM dithiothreitol) supplemented with complete protease inhibitors (Roche, Indianapolis, IN). Whole-cell lysates and cytosolic extracts were analyzed by Western blotting with antibodies against HtrA1 (dilution, 1:500), HtrA2 (1:1,000), HtrA3 (1:1,000), β-actin (1:3,000), α-tubulin (1:1,000), β-tubulin (1:1,000), γ-tubulin (1:3,000), tau (1:1,000), MAP2 (1:1,000), and GST (1:1,000).
Recombinant GST fusion proteins.
GST-HtrA1PDZ (H1PDZ), GST-HtrA2PDZ (H2PDZ), and GST-HtrA3PDZ (H3PDZ) were purified from transformed BL21 cells carrying pGEX-5X-2 GST fusion constructs in MicroSpin GST columns (Amersham, Piscataway, NJ) according to the manufacturer's instructions.
GST pulldown assay.
H1PDZ, GST, H2PDZ, and H3PDZ were purified as indicated above. After the columns were equilibrated with B-PER (Thermo Fisher Scientific, Rockford, IL), A2780 lysates were added to the columns and incubated for 1 h at 4°C. The columns were washed four times with B-PER and eluted with Laemmli buffer (50 mM Tris, pH 6.8, 2% sodium dodecyl sulfate [SDS], 0.1% bromophenol blue, 10% glycerol). The eluted proteins were resolved on SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted with anti-α-, -β-, and -γ-tubulin, as well as anti-HtrA1, -HtrA2 (1:200), -HtrA3, and -GST antibodies.
MT sedimentation assays.
For cosedimentation assay, proteins were extracted in MT stabilizing buffer {80 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 6.8, 138 mM KCl, 3 mM MgCl, 4 mM EGTA} containing 0.5% NP-40. Postnuclear supernatants were centrifuged at 100,000 × g for 1 h. Tubulin polymerization was performed in supernatants by incubating them with 10 μM paclitaxel and 2 mg/ml GTP for 1 h at 37°C, and tubulin polymers were pelleted by centrifugation at 100,000 × g for 30 min. The pellet and supernatant were designated P1 and S1, respectively, and 10 μg of sample from P1 and S1 were analyzed by Western blotting using anti-α-tubulin and anti-HtrA1 antibodies.
Purified tubulins and MAP fraction.
Tubulin protein, purified from bovine brain by an adaptation of the method of Shelanski et al. (44), was purchased from Cytoskeleton (Denver, CO). Further purification to >99% purity was achieved by cation-exchange chromatography. A MAP fraction, isolated from bovine brain by temperature-induced tubulin polymerization followed by ionic-exchange chromatography over a phosphocellulose matrix and salt elution, was purchased from Cytoskeleton.
In vitro binding assay.
C-terminal His-tagged HtrA1 was purified as previously described (23). Purified tubulins (10 μg in wash buffer with complete protease inhibitor from Roche [50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate]) were incubated with or without HtrA1 (2 μg) in a His SpinTrap column (Amersham) for 30 min at 4°C. The columns were washed five times with wash buffer supplemented with protease inhibitors and two times with wash buffer and eluted successively with increasing concentrations of NaCl in wash buffer. The final elution was carried out in Laemmli buffer. Eluted samples were resolved by 10% SDS-PAGE and immunoblotted with various antibodies.
In vitro tubulin polymerization assay.
An in vitro tubulin polymerization assay was carried out according to the manufacturer's instructions (Cytoskeleton, Denver, CO). In brief, a master mixture with purified MAP-rich tubulin monomers (2 mg/ml) was prepared on ice in G-PEM buffer (80 mM PIPES, pH 6.9, 2 mM MgCl2, 0.5 mM EGTA, 1 mM GTP). Using a multichannel pipetter, the master mixture was added to wells containing various concentrations of HtrA1 diluted in G-PEM buffer. Paclitaxel (3 μM) was used as a positive control. Tubulin polymerization was monitored by measuring absorbance at 340 nm kinetically for 45 min at 25°C.
Immunoprecipitation assay.
SKOV3 cells with endogenous HtrA1 expression were lysed in wash buffer supplemented with complete protease inhibitors from Roche, centrifuged at 10,000 × g for 5 min to obtain postnuclear supernatant, and immunoprecipitated using control immunoglobulin G or polyclonal HtrA1 antibodies. Immunocomplexes were precipitated by protein A-agarose (Thermo Fisher Scientific, Rockford, IL) and washed four times with wash buffer. Immunoprecipitated protein samples were eluted with Laemmli buffer with 100 mM dithiothreitol, resolved by 10% SDS-PAGE, and immunoblotted with various antibodies.
Migration assay.
SKOV3 cells (20,000 cells/well in a 24-well plate) were transfected with control and HtrA1-specific siRNA using Oligofectamine (Invitrogen) as previously described (11). Forty-eight hours after transfection, scratch wounds were created with 200-μl pipette tips. The medium was replaced with fresh medium to remove cells that became suspended as a result of the scratch wound. Two fiduciary lines perpendicular to the scratch wounds were drawn on the bottom of each well using a black marker pen. Photomicrographs were taken at the intersection between a black fiduciary line and the scratch wound at time zero (immediately following the scratch wound) and at 24 h. The wound gaps at time zero and at 24 h were measured using the SPOT program (Diagnostic Instruments, Sterling Heights, MI). The percent migration was determined by considering no gap as 100% migration. Since the growth rate was not affected by siRNA transfection, the filling of the gap represented cell migration.
Video microscopy.
A stable pool of SKOV3 cell lines were generated by infecting the cells with lentiviruses containing nontargeting short hairpin RNA (shRNA) or shRNA targeted against HtrA1 (Sigma-Aldrich Mission shRNA clones in pLKO.1 vector). Cells resistant to 1 μg/ml puromycin were used as batch-stable cells. The cells (105) were plated in 35-mm wells as confluent cultures. Following the scratch wound, motility was studied over a 20-h period under a Nikon Eclipse TE 300 microscope with a 40× phase-contrast objective in an attached, hermetically sealed Plexiglas Nikon NP-2 incubator at 37°C. Cell motility was recorded using a Cohu High Performance charge-coupled device camera. Image analysis was performed with a MicroImage analysis system (Lucia G; Laboratory Imaging s.r.o., Prague, Czech Republic) and an IBM-compatible system equipped with a video card (S3 Virge-DX/GXPCI 375/385) by digitally saving images at 30-min intervals. Migration tracks were generated by marking the positions of the nuclei of individual cells on each image. The net migratory speed (velocity straight line) was calculated by the MicroImage software based on the straight-line distance between the starting and ending points divided by the time of observation. The migrations of at least 30 cells were analyzed for each experimental condition. Values are given as mean ± standard deviation. All migration assays were performed in triplicate. Cells did not begin to divide to any significant degree during the experiments.
Proliferation assay by CyQuant.
SKOV3 cells, transfected with siRNA (Sigma-Aldrich) as previously described (11), were plated at a density of 2 × 103 cells per well in 96-well plates in M199/MCDB105 with 5% fetal bovine serum and were harvested after 24 h. Proliferation was determined by CyQuant assay (Invitrogen) according to the manufacturer's procedure.
RESULTS
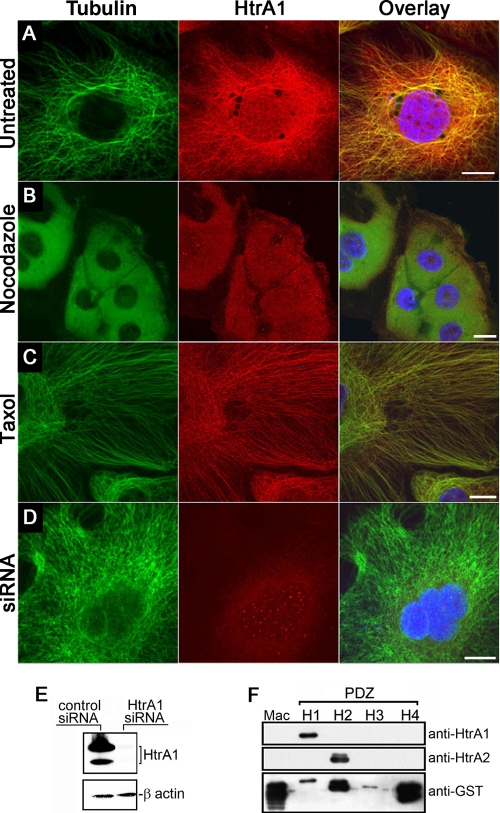
To confirm the exact intracellular localization of HtrA1, we first examined the subcellular localization of endogenous HtrA1 by immunofluorescence analysis in the ovarian cancer cell line SKOV3 with affinity-purified HtrA1 antibodies. Figure 1A shows cytoplasmic staining of endogenous HtrA1 that coincides with MTs. To test whether the localization of HtrA1 to MTs is dependent on intact MTs, cells were pretreated with the MT-destabilizing agent nocodazole. Nocodazole treatment resulted in diffuse distribution of HtrA1 and tubulin (Fig. 1B), indicating that localization of HtrA1 to MTs is dependent on intact MTs. Alternatively, when MTs were stabilized with paclitaxel, enhanced localization of HtrA1 to MTs was observed (Fig. 1C). To demonstrate the specificity of the affinity-purified polyclonal HtrA1 antibody, immunofluorescence analysis was performed on cells made HtrA1 deficient by siRNA or antisense transfection. No MT-like immunoreactivity was detected with the HtrA1 antibody in these cells, although intact MTs were observed with antitubulin antibody (Fig. 1D), indicating the specificity of HtrA1 antibody, as well as the specificity of immunofluorescence analysis. Immunoblot analyses confirmed the efficient downregulation of HtrA1 expression in these cells made deficient by siRNA (Fig. 1E). Since the antibody was generated toward the PDZ domain of HtrA1, to demonstrate the specificity of the antibody toward the HtrA1 PDZ domain, immunoblot analysis was performed on a blot containing GST fusion proteins with the Mac25 domain of HtrA1 and the PDZ domains of HtrA1, HtrA2, HtrA3, and HtrA4. HtrA1 antibodies specifically detected the PDZ domain of HtrA1 (Fig. 1F). No cross-reactivity with the PDZ domain of HtrA2, HtrA3, or HtrA4 was observed. Similarly, HtrA2 antibodies showed no cross-reactivity to the PDZ domains of other HtrA proteins. These results indicate the specificity of HtrA1 antibodies and support our conclusion that HtrA1 colocalizes with MTs.
FIG. 1.
HtrA1 is localized to MTs. (A) Localization of endogenous HtrA1 to MTs in SKOV3 cells. (B) Localization of endogenous HtrA1 to MTs is dependent on intact MTs in SKOV3 cells. (C) Localization of endogenous HtrA1 to paclitaxel (Taxol)-stabilized MTs. (D) MT localization of HtrA1 disappears when endogenous HtrA1 expression is downregulated by siRNA (bar = 10 μm). (E) Endogenous HtrA1 is efficiently downregulated by siRNA expression. (F) HtrA1 antibodies recognize the PDZ domain of HtrA1 but do not recognize the Mac25 domain of HtrA1 (Mac) and the PDZ domains of HtrA2 (H2), HtrA3 (H3), and HtrA4 (H4).
The PDZ domain of HtrA1 is required for its localization to MTs.
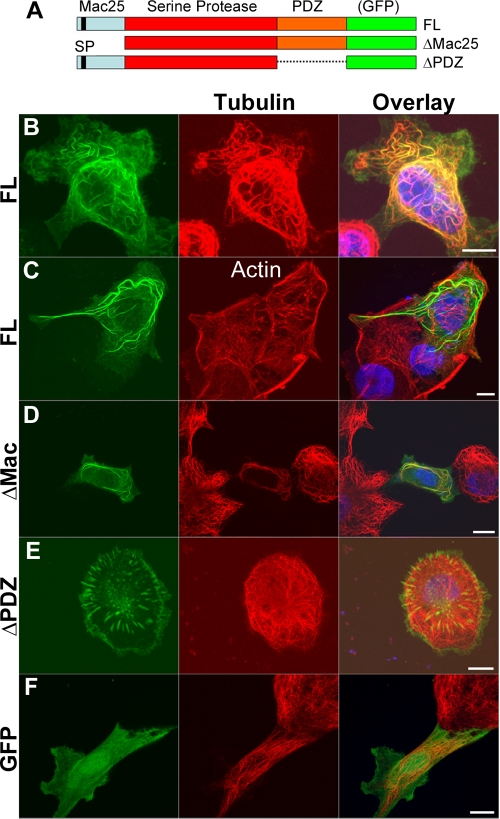
HtrA1 contains three distinct domains, namely, Mac25, trypsin-like protease, and PDZ domains (Fig. 2A). To determine the domain responsible for MT association, full-length HtrA1 constructs with Mac25 or PDZ deleted were subcloned into pcDNA3.1/CT-GFP-TOPO plasmids (Fig. 2A). The ovarian cell line OV202 was selected for its high efficiency of transfection and relatively low levels of endogenous HtrA1 (11, 13). Upon transfection of these green fluorescent protein (GFP)-tagged constructs into OV202 cells, full-length HtrA1 colocalized with MTs (Fig. 2B), but not with actin filaments (Fig. 2C). Deletion of the Mac25 domain (ΔMac25) did not disrupt HtrA1-MT association (Fig. 2D), while deletion of the PDZ domain (ΔPDZ) did (Fig. 2E). The interaction was independent of GFP, since free GFP produced a diffuse staining pattern throughout the cells (Fig. 2F). These results indicate that the PDZ domain of HtrA1 is required for its localization to MTs.
FIG. 2.
The PDZ domain is required for MT association. (A) Domain organization and deletion constructs of GFP-tagged HtrA1. FL, full-length. (B and C) HtrA1-GFP fusion protein is localized to MTs (B), but not to actin filaments (C). (D and E) Deletion of the N-terminal Mac25 domain (ΔMAC) does not disrupt MT association (D), but deletion of the PDZ domain (ΔPDZ) disrupts the association (E). (F) Control GFP is not localized to MTs. Bars, 10 μm.
HtrA3 and HtrA2 do not localize to MTs.
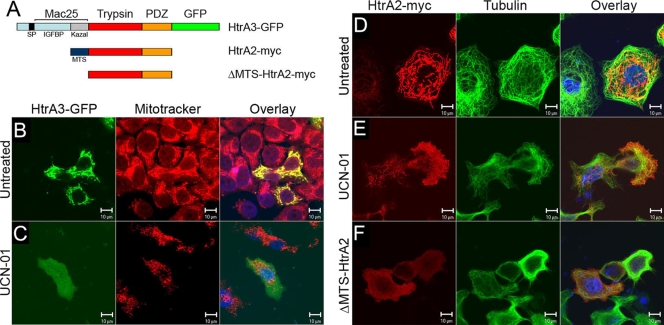
To test whether association with MTs is unique to HtrA1, GFP-tagged HtrA3 and myc epitope-tagged HtrA2 were transfected into OV202 cells (Fig. 3A). HtrA3 was localized to mitochondria, as evidenced by colocalization of HtrA3 with MitoTracker-Red in HtrA3-GFP-transfected cells (Fig. 3B). In addition, myc epitope-tagged HtrA3 was not colocalized with MTs (data not shown). These results indicate that both HtrA2 and HtrA3 are not MAPs (data not shown). Mitochondrial localization of HtrA2 was previously reported by various groups (27, 37, 39, 47, 52, 53), and therefore, our observation is consistent with these previous studies. However, mitochondrial localization of HtrA3 was unexpected, since it contains signal peptide and would be expected to be secreted. These results suggest the possibility of posttranslational modification resulting in retargeting of HtrA3 to mitochondria. To test whether HtrA3 or HtrA2 might associate with MTs if they were displaced from mitochondria during apoptosis, cells were treated with a broad kinase inhibitor, UCN-01, resulting in the release of HtrA3 and HtrA2 into the cytoplasm in cells undergoing apoptosis. No MT-like staining pattern was observed with these proteins (Fig. 3C and E, respectively). Extramitochondrial expression of HtrA2 lacking a mitochondrial targeting sequence, ΔMTS-HtrA2 (47), also did not produce MT-like staining (Fig. 3F). These results suggest that localization to MTs is unique to HtrA1.
FIG. 3.
HtrA3 and HtrA2 do not localize to MTs. (A) Domain organization and deletion constructs of tagged HtrA3 and HtrA2. (B) GFP-tagged HtrA3 is localized to mitochondria and can be colocalized with a mitochondrial dye, MitoTracker Red. (C) When HtrA3 is released into the cytosol upon induction of apoptosis induced by a broad kinase inhibitor, UCN-01, diffuse staining of HtrA3 is observed. No MT-like staining of HtrA3 is observed. (D) myc-tagged HtrA2 is localized to mitochondria, and the immunofluorescence of HtrA2 is not consistent with MT localization. (E) When HtrA2 is released into the cytosol upon induction of apoptosis by UCN-01, the diffused staining pattern of HtrA2 is not consistent with MT localization. (F) ΔMTS-HtrA2 is localized to the cytosol, and the diffused staining of HtrA2 in the cytosol is not consistent with MT localization. Bars = 10 μm.
The PDZ domain of HtrA1 associates with tubulins.
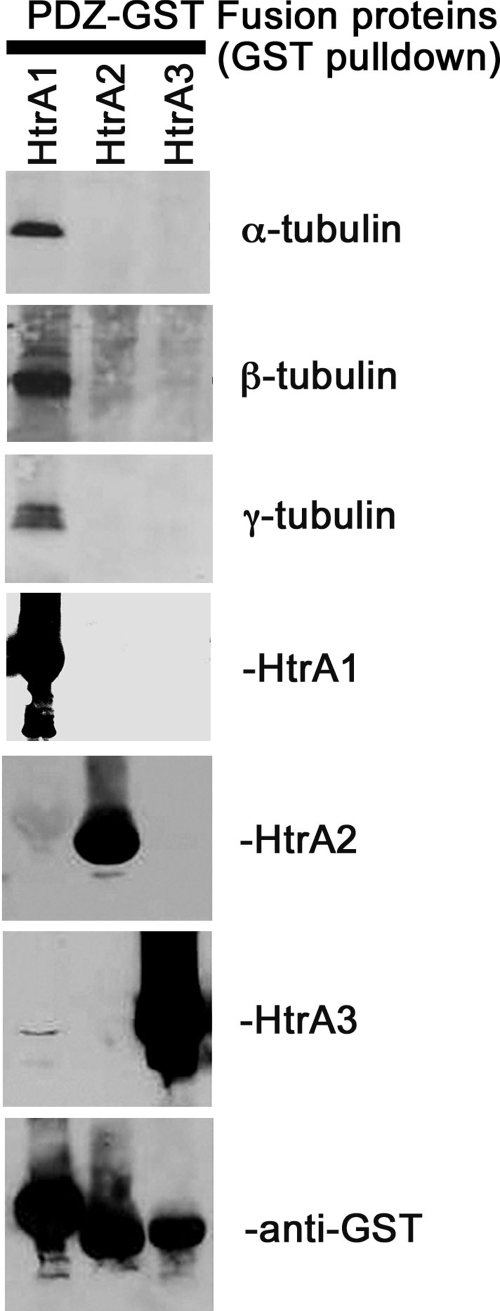
To determine whether the Y domain of HtrA1 associates with tubulin in vitro, a PDZ pulldown assay was performed with postnuclear lysates from the ovarian cancer cell line A2780. This cell line was selected because it expresses low levels of HtrA1 (11), to minimize endogenous interaction between HtrA1 and tubulins. Recombinant PDZ domains of human HtrA1, HtrA2, and HtrA3, purified as GST fusion proteins, were allowed to bind to a glutathione-agarose minicolumn and were incubated with lysates from A2780 cells. The eluted proteins were resolved by SDS-PAGE and immunoblotted with anti-α-, -β-, and -γ-tubulin antibodies. The results shown in Fig. 4 indicate that all three isoforms of tubulin were present in the eluents from the column containing HtrA1 PDZ domains, but not in eluents from columns containing the PDZ domain of HtrA2 or HtrA3. The PDZ domain of HtrA4, in similar experiments, did not associate with tubulins (data not shown). These data suggest that the PDZ domain of HtrA1 is sufficient for its association with tubulins in vitro.
FIG. 4.
The PDZ domain of HtrA1 interacts with tubulins. The PDZ domains of HtrA1, HtrA2, and HtrA3 were expressed as GST fusion proteins and purified with a glutathione column. The purified PDZs were incubated with postnuclear supernatant of A2780 cells, and unbound proteins were washed off the columns with Tris-buffered saline containing 1% NP-40 and 0.5% sodium deoxycholate. Bound proteins were eluted with 2× Laemmli buffer and resolved by SDS-PAGE. Eluted proteins were immunoblotted with α-, β-, and γ-tubulin antibodies. The PDZ domain of HtrA1 interacts with all three forms of tubulins (top three blots). The PDZ domains of HtrA2 and HtrA3 do not interact with tubulins. The lower four blots show loading controls for purified HtrA1, HtrA2, and HtrA3 and GST-fusion proteins, respectively.
HtrA1 associates with the MTOC and MTs during polymerization.
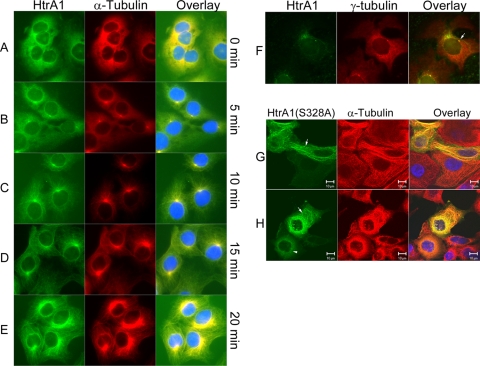
To determine whether HtrA1 associates with MTs during polymerization and to establish the dynamic nature of HtrA1 association with MTs, we incubated SKOV3 cells on ice for 20 min to depolymerize MTs into soluble tubulins. This treatment resulted in depolymerization of MTs, as was evident from diffuse staining of α-tubulin (Fig. 5A). This treatment also resulted in diffuse staining of HtrA1 (Fig. 5A). Within 5 min of reincubation at 37°C, we observed repolymerization of MTs beginning at an MT-organizing center (MTOC) (Fig. 5B). Similarly, we observed endogenous HtrA1 staining at the MTOC and growing MTs (Fig. 5B). At 10 min post-warm up, we observed tubulin staining and HtrA1 staining colocalizing with growing MTs (Fig. 5C). At 15 and 20 min post-warm up, we continued to observe colocalization of tubulins and HtrA1 along the entire length of MTs (Fig. 5D and E). Consistent with it localization to the MTOC (Fig. 5B) and its interaction with γ-tubulins (Fig. 4), we observed endogenous colocalization of HtrA1 and γ-tubulins in nocodazole-pretreated SKOV3 cells (Fig. 5F). Similar results were obtained with exogenously expressed HtrA1 in HeLa and OV202 cells (data not shown). In these experiments, we elected to use the protease mutant HtrA1 (S328A) because overexpression of wild-type proteases severely affected MT assembly, as previously reported (12). Exogenous expression of the protease mutant HtrA1 resulted in bundling of some of the MTs (Fig. 5G). Furthermore, these bundled MTs were resistant to cold-induced depolymerization (Fig. 5H). Nonetheless, exogenously expressed HtrA1 associates with the MTOC and polymerizing MTs in a manner similar to that of endogenous HtrA1. These results suggest a possible role of HtrA1 in MT assembly and stabilization.
FIG. 5.
Endogenous HtrA1 associates with polymerized MTs. SKOV3 cells were incubated at 4°C for 20 min and allowed to recover at 37°C for 20 min. (A) MTs are destabilized when cells are incubated at 4°C for 20 min. The end of the 4°C incubation was considered 0 min of recovery. (B) At 5 min of recovery from cold, MTs begin to assemble at the MTOC and HtrA1 is found colocalizing with the assembled MTs. (C) At 10 min, HtrA1 is colocalized with MTs as they continue to grow outward from the MTOC. (D and E) At 15 and 20 min of recovery, HtrA1 is colocalized with MTs throughout the cell (bar = 10 μm). (F) Endogenous HtrA1 colocalizes with γ-tubulins in nocodazole-pretreated SKOV3 cells (indicated by the arrow). (G) Exogenous expression of protease mutant HtrA1 (S328A) causes bundling of MTs (indicated by the arrow). (H) The MT bundles are resistant to cold-induced depolymerization. The arrowhead indicates a cell with depolymerized tubulins. The arrow indicates a cell with cold-resistant MT bundles.
HtrA1 promotes MT polymerization.
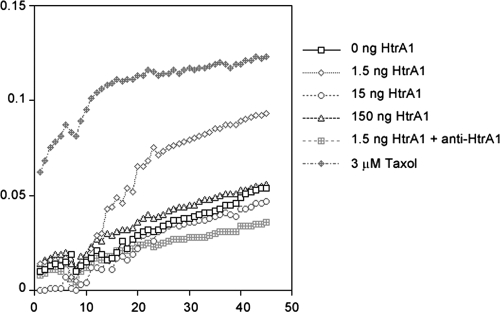
To test whether HtrA1 could affect MT assembly and stability, we investigated whether HtrA1 promotes MT polymerization. A recombinant protein corresponding to 35-kDa HtrA1 was purified from bacteria and used in the MT polymerization assay. Polymerization-competent MAP-rich bovine brain tubulin was purchased from Cytoskeleton (Denver, CO) and used in the assay. When 100 μg of MAP-rich tubulin was incubated in assay buffer containing 2 mM GTP at 25°C, we observed a steady increase in polymerization as assessed by absorbance at 340 nm (Fig. 6). It reached a polymerization plateau within 30 min. The highest steady-state levels of tubulin polymerization were achieved in the presence of paclitaxel. Steady-state levels of tubulin polymerization achieved in the presence of 1.5 ng of HtrA1 were higher than those without HtrA1. In addition, when neutralizing antibody targeted against the PDZ domain of HtrA1 was preincubated with recombinant HtrA1, it no longer promoted tubulin polymerization. Interestingly, higher levels of HtrA1 did not promote tubulin polymerization. Immunoblot analysis with antitubulin antibody to the postreaction lysates did indicate partial degradation of tubulins at higher HtrA1 concentrations (data not shown). These results further support the role of the PDZ domain in association with tubulins and the critical role of HtrA levels in regulating tubulin polymerization.
FIG. 6.
Effect of HtrA1 on tubulin polymerization. Bovine brain tubulin (2 mg/ml) was polymerized at 25°C in the presence of various concentrations of HtrA1 or 3 μM paclitaxel (Taxol). Tubulin polymerization was kinetically monitored by absorbance measurements at 340 nm (y axis) for 45 min. Low levels of HtrA1 (1.5 ng in a 100-μl reaction volume) promote higher steady-state levels of in vitro tubulin polymerization. Higher concentrations of HtrA1, however, do not promote tubulin polymerization. Antibody-neutralized HtrA1 does not promote tubulin polymerization. Paclitaxel-stabilized tubulins displayed the highest steady-state level of tubulin polymerization.
HtrA1 is a MAP.
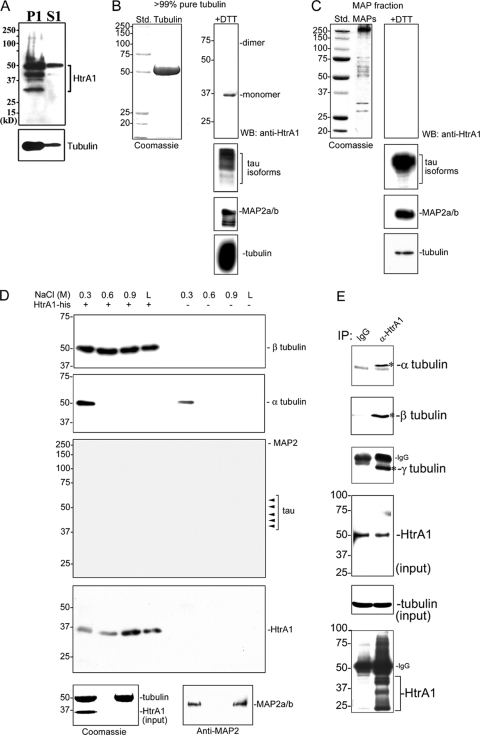
To further characterize the interaction between HtrA1 and tubulins, MTs from SKOV3 cells were purified by ultracentrifugation, and MT pellets (P1) and cytosolic proteins (S1) were resolved by 12% SDS-PAGE and immunoblotted sequentially with antibodies against α-tubulin and HtrA1. The results shown in Fig. 7A indicate that several proteolytic products of HtrA1 copelleted with MTs. It should be noted that autodegradation is a common feature of most HtrA proteases. Similarly, HtrA1 was also detected in the fraction containing purified brain tubulins (Fig. 7B, right), but not in the fraction enriched for MAPs (Fig. 7C, right). These results demonstrate that HtrA1 coprecipitated with MT pellets and copurified with tubulins, suggesting that HtrA1 is a MAP, but unlike MAPs, HtrA1 is not detected in MAP-rich fractions.
FIG. 7.
HtrA1 is a MAP. (A) Precipitation of tubulins by ultracentrifugation (100,000 × g) of MTs results in coprecipitation of endogenous HtrA1. P1 is the MT pellet; S1 is the soluble fraction. (B) Purification of tubulin from bovine brain also results in copurification of HtrA1. The 35-kDa HtrA1 monomer under reducing conditions is detected in the purified tubulin. Std., molecular weight standard; WB, Western blot. (C) HtrA1 is not detected in the MAP fraction. The three blots at the bottom, immunoblotted with anti-MAP2 or anti-tau antibodies, also indicate copurification of MAPs with tubulin purification. (D) In vitro interaction between tubulin and HtrA1. Ten micrograms of bovine brain tubulin was incubated in an Ni affinity column with or without 2 μg of HtrA1. Unbound proteins were washed away, and bound proteins were eluted sequentially with increasing concentrations of NaCl elution buffer. The eluents were resolved by SDS-PAGE and immunoblotted with the various antibodies indicated. Sequential elutions with 0.3 M, 0.6 M, and 0.9 M NaCl resulted in the elution of small fractions of HtrA1 and β-tubulin from the column (top blot, left three lanes). Final elution with Laemmli (L) buffer resulted in the elution of the remaining HtrA1 and β-tubulin. β-Tubulin was not detectable in the eluents from the Ni affinity column without HtrA1, demonstrating the absence of nonspecific interaction of β-tubulin with the Ni affinity column (top blot, right four lanes). Unlike β-tubulin, all of the α-tubulin was eluted from the column with 0.3 M NaCl elution (second blot from top, left lane). α-Tubulin was not detected in subsequent elution fractions (second blot from top, second, third, and fourth lanes from left). A small amount of α-tubulin was also detected in the first eluent from the Ni affinity column without HtrA1 (second blot from top, fifth lane from left), indicating a weak nonspecific interaction of α-tubulin with resin in the Ni affinity column. Isoforms of tau and MAP2 were not detectable in any of the eluents (third blot from top). The expected isoforms of tau and MAP2 are indicated on the right. Immunoblotting with anti-HtrA1 indicated the presence of HtrA1 in the eluents from the Ni affinity column with HtrA1 (fourth blot from top, left four lanes) but the absence of HtrA1 in the eluents from the Ni affinity column without HtrA1 (fourth blot from top, right four lanes). (Bottom left) Coomassie-stained gel showing purified proteins in the input fraction used in the corresponding studies. (Bottom right) Immunoblot with anti-MAP2 antibody indicating the presence of MAP2 in the input fraction. Units of numbers to the left (A to D) are kDa. (E) Immunoprecipitation (IP) of endogenous HtrA1 in SKOV3 cells also resulted in precipitation of α-, β-, and γ-tubulins. Specific signals are indicated by asterisks. IgG, immunoglobulin G.
The MAP-rich fraction was generally isolated from a tubulin pellet by salt extraction (0.35 M NaCl) (51). Therefore, our results indicating that HtrA1 is not detected in the MAP fraction suggest that the interaction between HtrA1 and tubulins is resistant to high-salt extraction. To demonstrate this interaction, purified tubulins were incubated with purified his-tagged HtrA1 and subjected to Ni chromatography. Bound proteins were sequentially eluted from the column with increasing concentrations of NaCl in wash buffer. A small fraction of β-tubulin and all of the α-tubulin were eluted with 0.3 M NaCl elution (Fig. 7D, top two blots, left lane). No β-tubulin and a small amount of α-tubulin (in the first elution) were eluted from the column without HtrA1 (Fig. 7D, top two blots, four right lanes), demonstrating the negligible nonspecific binding of tubulins to the His SpinTrap column. In subsequent elutions, small amounts of β-tubulins and HtrA1 were eluted (Fig. 7D, top blot, second and third lanes from left). The final elution with Laemmli buffer also contained additional β-tubulins and HtrA1 (Fig. 7D, top blot, lane L). No other MAPs (tau and MAP2) were retained by HtrA1 or tubulins (Fig. 7D, third blot from the top), although purified tubulins contain detectable amounts of tau and MAP2 proteins (Fig. 7B). These results demonstrate an interaction between β-tubulin and HtrA1 stronger than the interaction between α-tubulin and HtrA1 or the interaction between MAPs and tubulins. In the reciprocal experiment, immunoprecipitation of endogenous HtrA1 in SKOV3 cells also resulted in coimmunoprecipitation of α-, β-, and γ-tubulins (Fig. 7E, top three blots). These results therefore suggest that HtrA1 is a MAP and that it associates with all three types of tubulins.
Endogenous HtrA1 modulates cell migration.
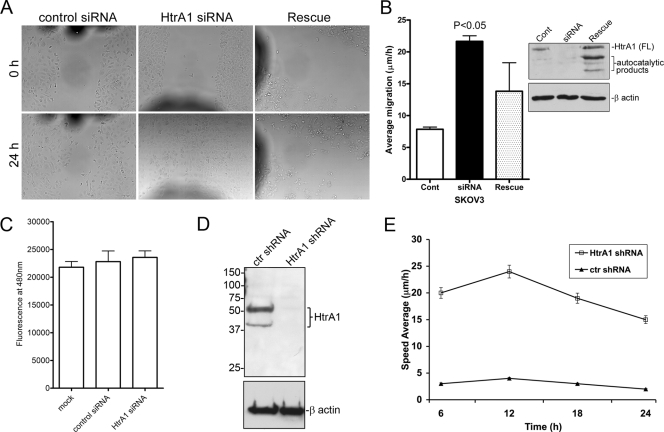
Since MTs affect cell motility (34), our observations raised the possibility that HtrA1 might affect cell motility by regulating MTs. We therefore determined whether downregulation of HtrA1 by RNAi in SKOV3 cells affected cell migration. SKOV3 cells were transfected with control or HtrA1-specific siRNAs for 48 h, and scratch wounds were made with 200-μl pipette tips. We selected this time point because preliminary data indicated efficient downregulation of HtrA1 (Fig. 1F) and confluent culture were achieved then (data not shown). Cell migration was assessed by determining how quickly denuded areas were filled up by migrating cells at the wound edges. Downregulation of endogenous HtrA1 by siRNA resulted in increased cell migration (Fig. 8A and B). Enhanced cell migration following HtrA1 knockdown was attenuated when cells were transfected with RNAi-resistant HtrA1 expression plasmid (Fig. 8A and B, Rescue). Since gap filling of the wound area is also dependent on cell proliferation, we determined the rate of cell proliferation at 72 h after siRNA transfection. The results from CyQuant cell proliferation assays indicated that there was no significant difference in cell proliferation in SKOV3 cells transfected with control siRNA or HtrA1-specific siRNA (Fig. 8C). In addition, we generated a batch-stable cell line expressing shRNA against HtrA1 in SKOV3 cells. These cells expressed reduced levels of HtrA1 compared to cells transfected with nontargeting shRNA (Fig. 8D). Time-lapse video microscopy of scratch wound migration assays performed in these cell lines indicated that SKOV3 cells with reduced levels of HtrA1 migrated significantly faster than SKOV3 cells with endogenous levels of HtrA1 (Fig. 8E). Collectively, these results suggest that endogenous HtrA1 negatively regulates cell migration.
FIG. 8.
Endogenous HtrA1 decreases the motility of cancer cells. (A and B) Downregulation of HtrA1 in SKOV3 cells by RNAi enhanced cell migration in a scratch assay, whereas expression of RNAi-resistant HtrA1 in rescue experiments attenuated cell migration. Photomicrographs of migrated cells at time zero and at 24 h following the initial scratch in the cell monolayer are shown. The dark shadows on the photographs represent fiduciary marks. Each experiment contained five replicates, with two measurements for each replicate. Analysis of the migration rate showed a significant increase in cell migration in cells transfected with HtrA1 siRNA compared to scrambled siRNA (control [Cont]). The error bars represent standard errors of the mean. Immunoblot analysis indicated efficient downregulation of HtrA1 by transient transfection with siRNA and rescue of HtrA1 expression by an RNAi-resistant expression plasmid. (C) CyQuant cell proliferation analysis indicated no significant differences in cell proliferation. (D) Pooled stable cells infected with lentiviral HtrA1 shRNA had reduced levels of HtrA1 (HtrA1 shRNA) compared to cells infected with lentiviruses containing ctr shRNA. Numbers are kDa. (E) Pooled stable cells (HtrA1 shRNA and ctr shRNA) were plated on 35- by 10-mm tissue culture dishes in RPMI medium, and cell motility was monitored with a time-lapse cinematography system as described in Materials and Methods. Each experiment contained three replicates. The values are the averages of 10 measurements for each replicate ± standard deviation (P < 0.0001).
DISCUSSION
In this report, we have provided several lines of evidence to show that HtrA1 is a MAP. Our data revealed that HtrA1 localizes to the MT network and regulates MT functions related to cell motility. While our immunolocalization data clearly indicate that HtrA1 associates with MTs, such interaction could be direct or indirect, requiring additional proteins. However, results from in vitro binding assays using purified HtrA1 and tubulins strongly suggest the probability that HtrA1 may directly associate with tubulin subunits, since other MAPs were not detected in the eluents. These results strongly support our conclusion that HtrA1 is a MAP. Several tumor suppressor gene products, such as APC (57), von Hippel Lindau tumor suppressor protein (pVHL) (28), BRCA1 (32), and RASSF1A (45), have been reported to associate with the MT network. Like APC, which has been implicated in the regulation of cell motility, HtrA1 associates with growing MTs and regulates cell motility. Similar to RASSF1A, which has a strong cytoprotective effect against cold treatment in vivo, protease mutant HtrA1 causes MT bundles and protects them against cold-induced depolymerization. Like pVHL, which carries specific mutations that disrupt MT association, leading to a specific cell phenotype in VHL disease (28), HtrA1 is a target of deletion in ovarian cancer (13), and loss of HtrA1 expression is associated with a metastatic phenotype (3). Thus, HtrA1 joins the growing list of tumor suppressors that associate with MTs. Loss of these genes may have implications in tumorigenesis specifically related to MT function.
MT association is unique to HtrA1, since neither HtrA2 nor HtrA3 associates with MTs under physiological conditions or upon release from the mitochondria following apoptotic stress. Additionally, we have demonstrated that the PDZ domain of HtrA1 is required for MT association. Several PDZ-containing proteins have been shown to regulate cell polarization during directed migration. For example, the Par6-PKCζ complex promotes the association of APC with MT plus ends and the assembly of Dlg-containing puncta in the plasma membrane at the leading edges of migrating cells (21). Recent studies have shown that the PDZ-containing protein Dlg1 interacts with APC protein and regulates epithelial migration by providing a link between MTs and the plasma membrane through interactions with PDZ proteins (41). Therefore, it will be of great interest to determine whether the PDZ domain of HtrA1 also provides such functional and structural links between MTs and the plasma membrane so that the migration processes can be properly regulated. The PDZ domain of HtrA1 interacts with all three types of tubulins, whereas the PDZ domains of HtrA2 and HtrA3 do not interact with any tubulin. Consistent with its ability to interact with all three forms of tubulins, HtrA1 associates with the MTOC and growing MTs during MT repolymerization following cold-induced depolymerization. These results also suggest that HtrA1 does not preferentially associate with the plus or minus ends of MTs. Rather, HtrA1 associates with tubulins and MTs as they are polymerized. It is tempting to speculate that HtrA1 may play a role in regulating the stability of tubulin polymer. This speculation is consistent with the observation that HtrA1 at low concentrations promotes higher steady-state levels of tubulin polymerization.
However, it should be noted that higher levels of HtrA1 result in the disruption of MTs and partially degrade tubulins (data not shown). It should also be noted that the purified HtrA1 used in the tubulin polymerase assay is an active protease that does not contain N-terminal Kazal-type trypsin inhibitor. Several attempts were made to purify the full-length HtrA1 that contains N-terminal Kazal-type trypsin inhibitor. However, full-length HtrA1 is inherently unstable and produces inclusion bodies. Therefore, the role of full-length HtrA1 in tubulin polymerization could not be ascertained in this study. Full-length HtrA1 contains an N-terminal Kazal-type trypsin-inhibitory domain that is likely to act as an intramolecular inhibitory domain against serine protease activity. Consequently, full-length HtrA1 is suspected to act as a zymogen, and its protease activity may be masked by the Kazal-type inhibitory domain. In addition, under physiological conditions in intact cells, endogenous serine protease inhibitors, known as serpins, are expected to regulate the protease activity of HtrA1. Therefore, higher levels of HtrA1 under physiological conditions in intact cells may have the ability to associate with MTs without resulting in the proteolysis of tubulin. Under physiological conditions, where protease activity is held in check by several mechanisms, such as intramolecular inhibition by the Kazal domain or intermolecular inhibition by serpins, it is conceivable that full-length HtrA1 may associate with MTs and regulate tubulin polymerization or MT stability. Consistent with its ability to regulate MT polymerization, exogenous expression of protease-inactive HtrA1 resulted in bundled MTs that were resistant to cold-induced depolymerization. These results further support a role of HtrA1 in regulating MT stability and dynamics.
Our observation that HtrA1 is an MT-associated serine protease also have several implications for the pathobiology of HtrA1 in several diseases. For example, expression of HtrA1 is upregulated by cisplatin and paclitaxel, and such upregulation often results in activation of HtrA1 by N-terminal removal of Kazal-type trypsin-inhibitory domains. Active HtrA serine proteases contribute to cell death through caspase-dependent, as well as caspase-independent, mechanisms. Moreover, we have recently identified tubulins as potential substrates of HtrA1. Therefore, our observation that HtrA1 is an MT-associated serine protease suggests a potential role of this protease in the cytoskeletal disruption that accompanies cell death. In this regard, the role of this serine protease is analogous to that of granzyme B, which also targets the cytoskeleton and perturbs MT polymerization dynamics (1). Like granzyme B, which enhances the MT polymerization rate (1), HtrA1 also promotes higher steady-state levels of tubulin polymerization. However, unlike with granzyme B, higher levels of HtrA1 are not associated with higher steady-state levels of tubulin polymerization.
Our results also indicate that although HtrA1 associates with MTs and therefore could be considered a MAP, it does not copurify with the MAP-rich fraction. Additional results in follow-up experiments suggest that HtrA1 associates with tubulins even under high-ionic-buffer conditions. This higher affinity may explain why HtrA1 does not copurify with MAP fractions. However, it is surprising that HtrA1 possesses such strong affinity to tubulin, and the physiological relevance of such high affinity is not currently known.
Sedimentation of MT pellets by ultracentrifugation also precipitated HtrA1. Lower-molecular-weight immunoreactive bands were also observed in MT pellets. Previous studies had shown that HtrA1 at higher concentrations underwent proteolytic removal of the N-terminal Mac25 domain during apoptosis, resulting in low-molecular-weight proteolytic products with intact PDZ domains (11). Therefore, the lower-molecular-weight products observed in tubulin pellets during cosedimentation experiments likely represent partial proteolytic products of HtrA1 with intact PDZ domains. These results are consistent with our observation that the PDZ domain is required for association with MTs.
Finally, our results show that endogenous HtrA1 attenuates cancer cell migration. Cancer cell migration is significantly increased when endogenous HtrA1 is transiently knocked down by siRNA, but cell migration is attenuated when HtrA1 expression is rescued by an RNAi-resistant expression plasmid, thus excluding the possibility of off-target effects of siRNA. Cell proliferation is not affected by HtrA1 knockdown, and therefore, increased gap filling following HtrA1 knockdown is not due to increased cell proliferation. This conclusion is further supported by the results from real-time imaging of migrating cells in which HtrA1 expression is stably knocked down by stable expression of shRNA against HtrA1. These cell lines are generated as batch-stable lines following lentiviral transduction of nontargeting shRNA (ctr shRNA) or HtrA1 shRNA. After being wounded, migrating cells were individually tracked, and the average migratory speeds were calculated. As a result of this real-time tracking of migrating cells, we were able to observe not only the migratory speed, but also the status of cell division. We observed no significant differences in cell division between the two isogenic cell lines. These results indicate that cells with endogenous HtrA1 have lower migratory rates than cells without HtrA1, further supporting the role of endogenous HtrA1 in regulating cancer cell migration.
Cell migration is regulated at various levels (30, 54). At the leading edge, several proteins associate with actin and tubulin filaments and regulate the dynamic remodeling of cytoskeletal structure and focal adhesion complexes, thereby contributing to the generation of physical forces required to propel the cell forward. At the trailing edge, proteases, like calpains and other cytoskeleton-associated proteins, interact with actin filaments and MTs to regulate the adhesion turnover and cytoskeleton-remodeling processes that are required for physical transformation and detachment from a substratum. Inside the cell, away from the leading and trailing edges, processes such as MTOC reorientation and nuclear movement are also coordinated with the migratory processes. Cytoskeletal reorganization is an essential attribute of these processes. Several proteases, such as calpains and granzymes, are reported to regulate cytoskeletal reorganization, FAK turnover, membrane protrusion, and cell migration (>8-10, 22, 49). Therefore, our observation that the serine protease HtrA1 associates with MTs and regulates MT stability and cell migration is consistent with previous studies indicating the roles of several proteases in the regulation of cytoskeletal organization and cell migration, and it provides initial biological insight into the potential role of HtrA1 in regulating MT organization associated with cell migration. Accordingly, it will be important to investigate the exact mechanism(s) by which HtrA1 regulates cell migration. In summary, the identification of HtrA1 as a serine protease that associates with MT is novel and significant, as it provides initial insight into the mechanistic framework for the tumor-suppressive activity of HtrA1, whereby HtrA1 may affect the cytoskeletal remodeling associated with malignant-cell biology related to invasion and motility.
Acknowledgments
We declare that we have no competing financial interests.
We thank Jeffrey L. Salibury and Wilma L. Lingle, for their discussions and critical review of the paper, James Tarara for his assistance with confocal microscopy, and Ryosuke Takahashi for the HtrA2-myc and HtrA2ΔN(MVPS) constructs.
This work was funded by grants from the National Cancer Institute (1R01CA123249 to V.S. and J.C.), the Mayo Clinic Bernard and Edith Waterman Center for Cancer Genetics and the Mayo Foundation (to V.S.), Ovarian Cancer Research Fund Liz Tilberis Scholars (to J.C.), and FUTURA-onlus and Second University of Naples (to A.B.).
Footnotes
Published ahead of print on 26 May 2009.
REFERENCES
- 1.Adrain, C., P. J. Duriez, G. Brumatti, P. Delivani, and S. J. Martin. 2006. The cytotoxic lymphocyte protease, granzyme B, targets the cytoskeleton and perturbs microtubule polymerization dynamics. J. Biol. Chem. 2818118-8125. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, S. S. 2000. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 10261-267. [DOI] [PubMed] [Google Scholar]
- 3.Baldi, A., A. De Luca, M. Morini, T. Battista, A. Felsani, F. Baldi, C. Catricala, A. Amantea, D. M. Noonan, A. Albini, P. G. Natali, D. Lombardi, and M. G. Paggi. 2002. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene 216684-6688. [DOI] [PubMed] [Google Scholar]
- 4.Baldi, A., M. Mottolese, B. Vincenzi, M. Campioni, P. Mellone, M. Di Marino, V. G. di Crescenzo, P. Visca, S. Menegozzo, E. P. Spugnini, G. Citro, A. Ceribelli, A. Mirri, J. Chien, V. Shridhar, M. Ehrmann, M. Santini, and F. Facciolo. 2008. The serine protease HtrA1 is a novel prognostic factor for human mesothelioma. Pharmacogenomics 91069-1077. [DOI] [PubMed] [Google Scholar]
- 5.Baldi, A., D. Santini, P. Russo, C. Catricala, A. Amantea, M. Picardo, F. Tatangelo, G. Botti, E. Dragonetti, R. Murace, G. Tonini, P. G. Natali, F. Baldi, and M. G. Paggi. 2004. Analysis of APAF-1 expression in human cutaneous melanoma progression. Exp. Dermatol. 1393-97. [DOI] [PubMed] [Google Scholar]
- 6.Bilder, D., M. Li, and N. Perrimon. 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289113-116. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4915-925. [DOI] [PubMed] [Google Scholar]
- 8.Bovenschen, N., P. J. de Koning, R. Quadir, R. Broekhuizen, J. M. Damen, C. J. Froelich, M. Slijper, and J. A. Kummer. 2008. NK cell protease granzyme M targets alpha-tubulin and disorganizes the microtubule network. J. Immunol. 1808184-8191. [DOI] [PubMed] [Google Scholar]
- 9.Carragher, N. O., S. M. Walker, L. A. Scott Carragher, F. Harris, T. K. Sawyer, V. G. Brunton, B. W. Ozanne, and M. C. Frame. 2006. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene 255726-5740. [DOI] [PubMed] [Google Scholar]
- 10.Carragher, N. O., M. A. Westhoff, V. J. Fincham, M. D. Schaller, and M. C. Frame. 2003. A novel role for FAK as a protease-targeting adaptor protein: regulation by p42 ERK and Src. Curr. Biol. 131442-1450. [DOI] [PubMed] [Google Scholar]
- 11.Chien, J., G. Aletti, A. Baldi, V. Catalano, P. Muretto, G. L. Keeney, K. R. Kalli, J. Staub, M. Ehrmann, W. A. Cliby, Y. K. Lee, K. C. Bible, L. C. Hartmann, S. H. Kaufmann, and V. Shridhar. 2006. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J. Clin. Investig. 1161994-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien, J., X. He, and V. Shridhar. 2009. Identification of tubulins as substrates of serine protease HtrA1 by mixture-based oriented peptide library screening. J. Cell Biochem. 107253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien, J., J. Staub, S. I. Hu, M. R. Erickson-Johnson, F. J. Couch, D. I. Smith, R. M. Crowl, S. H. Kaufmann, and V. Shridhar. 2004. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene 231636-1644. [DOI] [PubMed] [Google Scholar]
- 14.Clausen, T., C. Southan, and M. Ehrmann. 2002. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10443-455. [DOI] [PubMed] [Google Scholar]
- 15.Clawson, G. A., V. Bui, P. Xin, N. Wang, and W. Pan. 2008. Intracellular localization of the tumor suppressor HtrA1/Prss11 and its association with HPV16 E6 and E7 proteins. J. Cell Biochem. 10581-88. [DOI] [PubMed] [Google Scholar]
- 16.Conover, C. A., L. C. Hartmann, S. Bradley, P. Stalboerger, G. G. Klee, K. R. Kalli, and R. B. Jenkins. 1998. Biological characterization of human epithelial ovarian carcinoma cells in primary culture: the insulin-like growth factor system. Exp. Cell Res. 238439-449. [DOI] [PubMed] [Google Scholar]
- 17.De Luca, A., M. De Falco, V. Fedele, L. Cobellis, A. Mastrogiacomo, V. Laforgia, I. L. Tuduce, M. Campioni, D. Giraldi, M. G. Paggi, and A. Baldi. 2004. The serine protease HtrA1 is upregulated in the human placenta during pregnancy. J. Histochem. Cytochem. 52885-892. [DOI] [PubMed] [Google Scholar]
- 18.Desai, A., and T. J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1383-117. [DOI] [PubMed] [Google Scholar]
- 19.Esposito, V., M. Campioni, A. De Luca, E. P. Spugnini, F. Baldi, R. Cassandro, A. Mancini, B. Vincenzi, A. Groeger, M. Caputi, and A. Baldi. 2006. Analysis of HtrA1 serine protease expression in human lung cancer. Anticancer Res. 263455-3459. [PubMed] [Google Scholar]
- 20.Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421753-756. [DOI] [PubMed] [Google Scholar]
- 21.Etienne-Manneville, S., J. B. Manneville, S. Nicholls, M. A. Ferenczi, and A. Hall. 2005. Cdc42 and Par6-PKCζ regulate the spatially localized association of Dlg1 and APC to control cell polarization. J. Cell Biol. 170895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco, S., B. Perrin, and A. Huttenlocher. 2004. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp. Cell Res. 299179-187. [DOI] [PubMed] [Google Scholar]
- 23.Grau, S., A. Baldi, R. Bussani, X. Tian, R. Stefanescu, M. Przybylski, P. Richards, S. A. Jones, V. Shridhar, T. Clausen, and M. Ehrmann. 2005. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA 1026021-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grau, S., P. J. Richards, B. Kerr, C. Hughes, B. Caterson, A. S. Williams, U. Junker, S. A. Jones, T. Clausen, and M. Ehrmann. 2006. The role of human HtrA1 in arthritic disease. J. Biol. Chem. 2816124-6129. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen, G. G. 2002. Evolutionary conservation of microtubule-capture mechanisms. Nat. Rev. Mol. Cell Biol. 3296-304. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 10057-70. [DOI] [PubMed] [Google Scholar]
- 27.Hegde, R., S. M. Srinivasula, Z. Zhang, R. Wassell, R. Mukattash, L. Cilenti, G. DuBois, Y. Lazebnik, A. S. Zervos, T. Fernandes-Alnemri, and E. S. Alnemri. 2002. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 277432-438. [DOI] [PubMed] [Google Scholar]
- 28.Hergovich, A., J. Lisztwan, R. Barry, P. Ballschmieter, and W. Krek. 2003. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 564-70. [DOI] [PubMed] [Google Scholar]
- 29.Hirokawa, N. 1994. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol. 674-81. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz, A. R., and J. T. Parsons. 1999. Cell migration—movin’ on. Science 2861102-1103. [DOI] [PubMed] [Google Scholar]
- 31.Howard, J., and A. A. Hyman. 2003. Dynamics and mechanics of the microtubule plus end. Nature 422753-758. [DOI] [PubMed] [Google Scholar]
- 32.Hsu, L. C., and R. L. White. 1998. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA 9512983-12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu, S. I., M. Carozza, M. Klein, P. Nantermet, D. Luk, and R. M. Crowl. 1998. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J. Biol. Chem. 27334406-34412. [DOI] [PubMed] [Google Scholar]
- 34.Hubbert, C., A. Guardiola, R. Shao, Y. Kawaguchi, A. Ito, A. Nixon, M. Yoshida, X. F. Wang, and T. P. Yao. 2002. HDAC6 is a microtubule-associated deacetylase. Nature 417455-458. [DOI] [PubMed] [Google Scholar]
- 35.Jones, J. M., P. Datta, S. M. Srinivasula, W. Ji, S. Gupta, Z. Zhang, E. Davies, G. Hajnoczky, T. L. Saunders, M. L. Van Keuren, T. Fernandes-Alnemri, M. H. Meisler, and E. S. Alnemri. 2003. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 425721-727. [DOI] [PubMed] [Google Scholar]
- 36.Launay, S., E. Maubert, N. Lebeurrier, A. Tennstaedt, M. Campioni, F. Docagne, C. Gabriel, L. Dauphinot, M. C. Potier, M. Ehrmann, A. Baldi, and D. Vivien. 2008. HtrA1-dependent proteolysis of TGF-beta controls both neuronal maturation and developmental survival. Cell Death Differ. 151408-1416. [DOI] [PubMed] [Google Scholar]
- 37.Li, W., S. M. Srinivasula, J. Chai, P. Li, J. W. Wu, Z. Zhang, E. S. Alnemri, and Y. Shi. 2002. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nat. Struct. Biol. 9436-441. [DOI] [PubMed] [Google Scholar]
- 38.Lingle, W. L., and J. L. Salisbury. 2001. Methods for the analysis of centrosome reproduction in cancer cells. Methods Cell Biol. 67325-336. [DOI] [PubMed] [Google Scholar]
- 39.Martins, L. M. 2002. The serine protease Omi/HtrA2: a second mammalian protein with a Reaper-like function. Cell Death Differ. 9699-701. [DOI] [PubMed] [Google Scholar]
- 40.McIntosh, J. R., and M. P. Koonce. 1989. Mitosis. Science 246622-628. [DOI] [PubMed] [Google Scholar]
- 41.Mimori-Kiyosue, Y., C. Matsui, H. Sasaki, and S. Tsukita. 2007. Adenomatous polyposis coli (APC) protein regulates epithelial cell migration and morphogenesis via PDZ domain-based interactions with plasma membranes. Genes Cells. 12219-233. [DOI] [PubMed] [Google Scholar]
- 42.Nabi, I. R. 1999. The polarization of the motile cell. J. Cell Sci. 1121803-1811. [DOI] [PubMed] [Google Scholar]
- 43.Nogales, E. 2000. Structural insights into microtubule function. Annu. Rev. Biochem. 69277-302. [DOI] [PubMed] [Google Scholar]
- 44.Shelanski, M. L., F. Gaskin, and C. R. Cantor. 1973. Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA 70765-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, M. S., S. J. Song, N. G. Ayad, J. S. Chang, J. H. Lee, H. K. Hong, H. Lee, N. Choi, J. Kim, H. Kim, J. W. Kim, E. J. Choi, M. W. Kirschner, and D. S. Lim. 2004. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat. Cell Biol. 6129-137. [DOI] [PubMed] [Google Scholar]
- 46.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97339-347. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, Y., Y. Imai, H. Nakayama, K. Takahashi, K. Takio, and R. Takahashi. 2001. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8613-621. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, E., T. Ho, and M. W. Kirschner. 1995. The role of microtubule dynamics in growth cone motility and axonal growth. J. Cell Biol. 128139-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tonami, K., Y. Kurihara, H. Aburatani, Y. Uchijima, T. Asano, and H. Kurihara. 2007. Calpain 6 is involved in microtubule stabilization and cytoskeletal organization. Mol. Cell. Biol. 272548-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuchiya, A., M. Yano, J. Tocharus, H. Kojima, M. Fukumoto, M. Kawaichi, and C. Oka. 2005. Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone 37323-336. [DOI] [PubMed] [Google Scholar]
- 51.Vallee, R. B. 1982. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J. Cell Biol. 92435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Loo, G., M. van Gurp, B. Depuydt, S. M. Srinivasula, I. Rodriguez, E. S. Alnemri, K. Gevaert, J. Vandekerckhove, W. Declercq, and P. Vandenabeele. 2002. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 920-26. [DOI] [PubMed] [Google Scholar]
- 53.Verhagen, A. M., J. Silke, P. G. Ekert, M. Pakusch, H. Kaufmann, L. M. Connolly, C. L. Day, A. Tikoo, R. Burke, C. Wrobel, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2002. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J. Biol. Chem. 277445-454. [DOI] [PubMed] [Google Scholar]
- 54.Vicente-Manzanares, M., D. J. Webb, and A. R. Horwitz. 2005. Cell migration at a glance. J. Cell Sci. 1184917-4919. [DOI] [PubMed] [Google Scholar]
- 55.Wittmann, T., A. Hyman, and A. Desai. 2001. The spindle: a dynamic assembly of microtubules and motors. Nat. Cell Biol. 3E28-E34. [DOI] [PubMed] [Google Scholar]
- 56.Yang, Z., N. J. Camp, H. Sun, Z. Tong, D. Gibbs, D. J. Cameron, H. Chen, Y. Zhao, E. Pearson, X. Li, J. Chien, A. Dewan, J. Harmon, P. S. Bernstein, V. Shridhar, N. A. Zabriskie, J. Hoh, K. Howes, and K. Zhang. 2006. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 314992-993. [DOI] [PubMed] [Google Scholar]
- 57.Zumbrunn, J., K. Kinoshita, A. A. Hyman, and I. S. Nathke. 2001. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 1144-49. [DOI] [PubMed] [Google Scholar]