Abstract
The epithelial and non-epithelial cells of the intestinal wall experience a myriad of physical forces including strain, shear, and villous motility during normal gut function. Pathologic conditions alter these forces, leading to changes in the biology of these cells. The responses of intestinal epithelial cells to forces vary with both the applied force and the extracellular matrix proteins with which the cells interact, with differing effects on proliferation, differentiation, and motility, and the regulation of these effects involves similar but distinctly different signal transduction mechanisms. Although normal epithelial cells respond to mechanical forces, malignant gastrointestinal epithelial cells also respond to forces, most notably by increased cell adhesion, a critical step in tumor metastasis. This review will focus on the phenomenon of mechanical forces influencing cell biology and the mechanisms by which the gut responds these forces in both the normal as well as pathophysiologic states when forces are altered. Although more is known about epithelial responses to force, information regarding mechanosensitivity of vascular, neural, and endocrine cells within the gut wall will also be discussed, as will, the mechanism by which forces can regulate epithelial tumor cell adhesion.
Keywords: strain, pressure, gastrointestinal, signaling, tensegrity, integrins
Introduction
During normal gut function the intestinal mucosal layer is subjected to numerous forces. For instance, mucosal cells experience pressure and shear stress from interaction with relatively non-compressible endoluminal chyme, cyclic strain associated with rhythmic villous motility at the mucosal level, and further repetitive deformation engendered by peristaltic muscular contraction and relaxation deeper within the bowel wall. Although it is difficult to isolate the effects of mechanical forces on the gut in vivo from the other effects of the interventions required to manipulate such forces, increasing in vitro evidence suggests that such forces may substantially influence intestinal mucosal cell biology. This review will focus on the influence of mechanical forces, including strain, shear, and pressure, on intestinal mucosal cell physiology in both the normal state and in pathophysiologic states where mechanical forces or responses to such forces are altered such as ileus and malignancy.
Physical Forces in vivo
Macroscopic Forces
Numerous contractile activities occur in the bowel wall both in the circular and longitudinal muscle layer. This leads to deformation of the bowel wall in a irregularly irregular rhythm [1]. To ensure adequate and efficient digestion, absorption, and propulsion, a variety of deformation patterns exist [2]. Peristalsis contractions of circular and longitudinal muscle are highly synchronized and controlled to achieve the effects of the bowel [3]. Segmental contractions and ring contractions of the small bowel have been described that serve largely a propulsive function. These occur at frequencies of 7-20 per minute, with slower frequencies tending to be toward the distal ileum and more rapid frequencies toward the duodenum [1, 4]. Colonic frequencies tend to be lower, ranging from 2-13 per minute in humans [5]. Mathematical models have been proposed to simulate this complex process [6].
Peristaltic contractility of the intestinal tract induces deformation and pressure on the intestinal mucosa. Measurements of nocturnal jejunal pressures in healthy volunteers revealed an average pressure of 20 mmHg [7]. However, some pathologic conditions such as chronic inflammatory states, intraluminal pressures are often elevated and can adversely affect gut physiology and healing [8-10]. Luminal contents are generally non-compressible or minimally compressible so contraction of the muscular layers results in mucosal compression in this setting as the mucosa is squeezed between the contracting musculature and the non-compressible chyme [11]. In the colon, chronically increased luminal pressures can eventually deform the mucosa to the point of forming diverticulae [12]. The mucosa may come into physical contact with the opposing mucosa during contraction, generating shear stress, compression, and other forces. Even a large food bolus can aid in mucosal deformation as it passes through the intestines. While dramatic deformation obviously occurs at natural sphincters, radiographic evidence shows that it occurs throughout the bowel. Table 1 lists common forces seen by the intestinal mucosa during normal function as well as in malignancies (discussed later).
Table 1.
Forces experienced by the intestinal mucosa during normal function and in malignant states.
| Normal gut function | Malignancy |
|---|---|
| Peristalsis | Intraabdominal pressure |
| Shear from endoluminal chyme | Interstitial pressure |
| Interaction with luminal contents | Bowel wall edema |
| Villous motility | Shear from vascular/lymphatic transit |
| Remodeling activity | Pneumoperitoneum |
Microscopic Forces
On a microscopic level, villous motility is noted throughout the small intestine and is an entirely separate entity. Villous motility is a rhythmic extension and contraction of the intestinal villi within the mucosa that varies in magnitude and frequency depending upon the specific location within the bowel (duodenum to terminal ileum) and is regulated by many factors including temperature, pH, and protein and glucose concentrations [13]. Individual villi contract 0-15 times per minute, in a frequency distribution gradient that decreases progressively from duodenum to terminal ileum [13]. This motion is both rhythmic and regulated. Interestingly, villous motility is stimulated by increases in lymphatic flow but not altered by changes in arterial blood flow unless perfusion falls to levels at which tissue oxygenation drops [14]. Water absorption and vagal stimuli also correlate with increased villus motility [15].
Effects of Altered Physical Forces
Clearly, the regulation of mucosal deformation is very complex at both the macroscopic and microscopic levels and responds to both neurohumoral and luminal factors. Derangements in these complex processes result in a myriad of motility disorders [16] and pathophysiologic changes. Indeed, jejunal luminal pressures in irritable bowel syndrome can reach 50 mmHg [10]. Bowel wall edema after surgical procedures [17] or inflammation and injury from bowel diseases such as Crohn's disease [18] can further increase pressure within the bowel lumen and lead to motility derangements. Intestinal mucosa from patients with inflammatory bowel disease displays mucosal atrophy on histologic sections [19]. In states of ileus, prolonged fasting, or sepsis, the mucosa becomes atrophic as well. Normal gut forces are altered and aberrant, with peristaltic contractions sometimes absent completely [20], which could contribute to pathology. As the mucosa becomes atrophic, the villi shorten [21] while the luminal contents change in volume, concentration, and density. Ultimately, barrier function deteriorates [22, 23]. Eventually, albumin levels will fall resulting in bowel wall edema and increased interstitial pressure as well [24]. Chronic inflammatory bowel disease can increase colonic blood flow two to six-fold leading to increased capillary pressures [25] and subsequently increased interstitial pressures as well.
As the mucosa becomes atrophic, expression of brush border digestive enzymes tends to decrease [23]. Interestingly, during fasting states, some reports suggest a relative preservation of intestinal alkaline phosphatase while other brush border enzymes atrophy [26, 27]. It is interesting in this regard that intestinal epithelial cells subjected to repetitive mechanical strain show an increase in the specific activity of dipeptidyl dipeptidase (DPDD) while alkaline phosphatase specific activity is relatively reduced [28], consistent with the hypothesis that the increased repetitive deformation engendered by feeding may stimulate intestinal epithelial expression of DPDD and other brush border enzymes while affecting alkaline phosphatase expression differently [26]. It is clear that the motile intestinal epithelial cell is characterized by a distinct phenotype that is different from the static cell in terms of morphology, brush border enzyme expression, and cell signaling [29]. Malignant gut epithelial cells also respond to alterations in mechanical forces [30]; this will be discussed later in this review.
Evidence of mechanical forces influencing gut epithelium
The extracellular matrix with which intestinal epithelial cells may interact in vivo represents a complex gel of proteins and glycoproteins [31]. Normally, intestinal epithelial cells are anchored to the basement membrane which is predominantly composed of type IV collagen, laminin, some fibronectin, and heparin sulfate proteoglycans during normal function. The interstitial matrix, lying below the basement membrane, contains types I and III collagen and fibronectin but is only exposed to epithelial cells during periods of inflammation, wounding, or malignant invasion. The strain response to intestinal epithelial cells is matrix-dependent. While cells grown on collagen I, collagen IV, and laminin all behave similarly [32], the levels of fibronectin, which change in vivo with levels of inflammation, [33] lead to an entirely different response.
Cell Proliferation
Cell proliferation is stimulated by increasing stretch in vivo in pig jejunum [34]. In vitro strain stimulates cellular proliferation and differentiation in intestinal epithelial cells [28] and non-transformed primary human intestinal epithelial cells derived from surgical specimens [32]. Under normal circumstances, the small bowel derives most of its caloric needs from enteral glutamine, suggesting that glutamine could be at least a conditional nutritional requirement for the small bowel mucosa, and glutamine stimulates intestinal epithelial proliferation in vitro [35, 36]. However, glutamine supplementation into parenteral nutrition has on its own only been marginally successful at preventing gut mucosal atrophy in human [21]. Interestingly, the stimulation of intestinal epithelial proliferation by repetitive deformation is synergistic with that by glutamine in vitro, suggesting that parenteral glutamine may not be as effective in the absence of the increased intestinal epithelial deformation associated with enteral feeding [37].
The proliferative effects of strain are amplitude specific, with a greater effect seen as amplitude is increased, and frequency-dependent, responding optimally to 10-20 cycles per minute of strain. Interestingly, repetitive responses to strain in other cell types also appear to be frequency-specific. Vascular endothelial cells [38] and vascular smooth muscle cells [39] respond to frequencies of 60-90 cycles per minute, similar in frequency to an expected normal human heart rate. In contrast, osteoblasts respond best to significantly lower frequencies, on the order of 1-6 cycles per minute [40]. This is more similar in frequency to voluntary skeletal muscle movements. Pulmonary airway cells respond to a range of frequencies from 20-30 cycles per minute in some in vitro studies [41, 42] while 10 cycles per minute has been used to investigate lung parenchyma in vivo [43]. The physiologic respiratory rate varies with age and can be elevated in pathologic conditions or mechanical ventilation which may explain the response to a rather large range of frequencies. Clearly mechanical stimuli are specific in both magnitude and frequency.
Signaling in the proliferative pathway
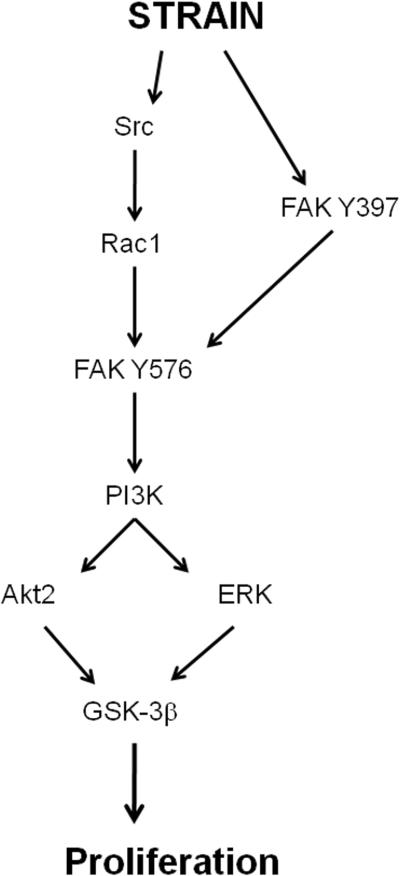
Recent studies have elucidated some of the signaling pathways that mediate the stimulation of intestinal epithelial proliferation by cyclic strain. Protein kinase C (PKC) and tyrosine kinase are activated in response to strain while blocking this kinases prevents the effect [44]. The cellular distribution of PKC involves the translocation of either the α or ζ isozymes [45]. Activation of tyrosine kinase under strain stimulation has been confirmed in vivo in rat intestine terminal ileum and colon [46]. The mitogen-activated protein kinase (MAPK) ERK is a key downstream mediator of the mitogenic effect of strain [47], and this molecule is stimulated by integrin-mediated cell adhesion [48]. In addition, the mitogenic effects require the activation of focal adhesion complex (FAK), which is in turn activated by integrin signaling when cell membrane integrins interact with extracellular matrix or other ligands [49]. Blocking FAK by a dominant negative construct prevents MAPK activation, so FAK is upstream of the MAPK. Although p38 and jnk are also activated by strain in intestinal epithelial cells, p38 blockade does not prevent proliferation [47], and the potential role of jnk in mediating the mitogenic effects of strain has not yet been evaluated in enterocytes. The pathway leading from FAK and ERK activation to the stimulation of proliferation involves Src, which modulates FAK, although a Src-independent FAK-dependent pathway also exists [50]. Blockade of α-actinin-1, which links the cytoskeletal structure to the focal adhesion proteins, prevents FAK and ERK activation but not that of Src [51] suggesting cytoskeletal mechanotransduction mediates some, although not all strain-induced intracellular signaling. Interestingly, cell stretch seems to trigger ongoing physical events in cell signaling via cytoskeletal rearrangements in both a mechanical and fluid manner [52]. More downstream, PI3K activation from upstream Src activation results in downstream involvement of not only ERK but also the specific Akt isoform Akt2 (but not Akt1). These molecules seem to coalesce on glycogen synthase kinase (GSK) to stimulate proliferation [53]. One potential pathway that explains these observations is summarized in figure 1.
Fig 1.
One possible pathway which regulates cell proliferation in response to strain on collagen. After PI3K is activated downstream in a Src-dependent fashion, the pathway diverges, and then re-converges on GSK. FAK, focal adhesion kinase; ERK, extracellular-related kinase; GSK, glycogen synthase kinase.
Matrix Interactions
Cells in the intestinal mucosa are attached to and, thus, interact with an extracellular matrix that is subject to change with various conditions. Indeed, the matrix varies from villous tip to the base of the crypts [54]. Cell-matrix interactions may profoundly affect the stimulation of intestinal cell proliferation by strain. Cyclic strain stimulates intestinal epithelial cell proliferation similarly whether they are plated on a matrix of collagen I, collagen IV, or laminin, or plated on a substrate precoated with antibody to the collagen-binding integrin subunits α2 and β1 or the laminin-binding α6 subunit [47], suggesting integrin involvement. However, intestinal epithelial cells plated on a fibronectin matrix or on a substrate of functional antibody to the α5 or αv integrin subunits, which are involved in cell-fibronectin interaction, display no mitogenic effect in response to cyclic strain. Furthermore, adding tissue fibronectin to a collagen matrix or adding plasma fibronectin to the media also inhibits the mitogenic effect of strain in such experiments. Interestingly, the MAPK activated by strain on collagen is not activated in subconfluent proliferating cell growing on a fibronectin matrix. These studies were done primarily on Caco-2 cells though primary non-malignant human intestinal epithelial cells display similar responses. Fibronectin-specific subunits α5 or αv seem to mediate the fibronectin interaction via integrin heterodimers which turns off the mitogenic response to mechanical forces [32].
Cell Migration
From a teleological point of view, one might question why a system would evolve in which acute inflammation or illness turns off the mitogenic effect of repetitive strain on the intestinal epithelium? One might speculate that the cellular resources in such settings that normally support differentiation and proliferation may be required for epithelial cell motility and wound healing to maintain the gut mucosal barrier. The epithelial lining of the GI tract is constantly wounded during normal gut function and must be repaired regularly [55]. Cell migration is required for healing of wounds and requires the cell to interact with matrix proteins via integrins both at the trailing and leading edge of the cell [56]. Interestingly, fibronectin is an acute phase reactant and plasma fibronectin levels increase along with tissue deposition during times of inflammation such as occurs in IBD [57, 58] or severe illness such as sepsis [59]. When a mucosal injury occurs, gut epithelial cells are exposed to pre-existing fibronectin in the interstitial matrix, and tissue fibronectin deposition increases in larger or more chronic wounds. Fibronectin further is deposited into the tissues in chronic inflammatory states such as Crohn's disease [57] while septic states increase plasma fibronectin in vivo [60]. Failure of the gut mucosal barrier is a key element in the pathogenesis of such disease states as mucosal injury is increased with failure of restitution and bacterial translocation ensues [61, 62]. These states also will alter gut motility as alluded to earlier. Consistent with this hypothesis, repetitive deformation stimulates intestinal epithelial monolayer wound closure across fibronectin substrates, despite the slight inhibition of proliferation that such deformation induces. This contrasts with observations on collagen substrates where deformation stimulates proliferation but inhibits cell motility [33].
Signaling in the migratory pathway
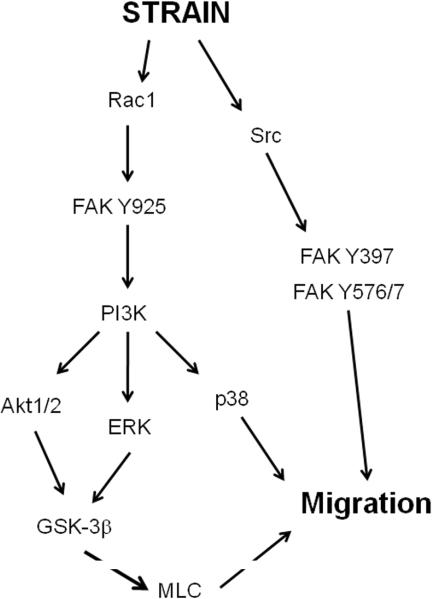
The pathway regulating strain-induced migration on fibronectin has some similarities to that regulating strain-induced cell proliferation on a collagen substrate. For instance, the MAPK ERK, FAK, and Src are all involved in both effects. However, several key differences are apparent. ERK is activated at the cell membrane in response to strain on a fibronectin matrix but not on a collagen matrix [33]. Interestingly, reducing FAK or inhibiting ERK may inhibit epithelial wound closure in vitro [33, 63] while the distribution of activated ERK immunoreactivity is each markedly abnormal at the edge of mucosal ulcers in Smad-3 knockout mice in which ulcer healing is also delayed [64]. Furthermore, a Src-independent FAK pathway involved Tyr-925 phosphorylation and eventual stimulation of ERK while Src and other FAK-binding site, including Tyr 397 and 576/577, control migration via another pathway [63]. The mitogenic pathway involves Src-independent FAK Tyr-397 activation and Src-dependent FAK Tyr-576 activation [50], though Tyr-925 has not been examined in proliferation. Interestingly, FAK Tyr-925 phosphorylation is generally viewed as a Src substrate though in response to strain it is Src-independent. This Src-independent arm of the pathway involves the eventual activation of PI3K and AKT. Although AKT is involved in both the mitogenic pathway on collagen and the motogenic pathway on fibronectin, the role of specific AKT isoforms differs in each pathway. While the mitogenic pathway stimulated by cyclic strain on non-fibronectin substrates involves the Akt2 but not the Akt1 isoform [53], there appears to be no Akt isoform specificity involved in the motogenic response on fibronectin, though GSK phosphorylation is required in both. Finally, p38 and GSK are downstream effectors in the motogenic pathway in preliminary studies, although they do not lie in parallel [65] while the mitogenic response does not require p38 activation at all. Myosin light-chain and its phosphorylating kinase myosin light chain kinase also appear involved in strain-induced cell migration as motility is more than simple cell spreading which does not result in net movement [66]. Although this has not been investigated, one would not expect a role for myosin light chain in the stimulation of proliferation by repetitive deformation. Figure 2 presents a hypothesized pathway that would be consistent with these published observations. It would appear then that repetitive deformation is an important trophic factor for intestinal epithelial cells that promote either a proliferative and differentiated phenotype or a migratory phenotype depending upon levels of plasma and tissue fibronectin. This divergent response pattern may be an important mechanism by which the intestinal epithelium perceives and adapts to healthy or pathophysiologic states.
Fig 2.
One possible pathway which regulates cell migration in response to strain on fibronectin. PI3K is phosphorylated in a Src-dependent manner that also requires Src-independnet phosphorylation of FAK at tyrosine-925. Although three arms of the pathway are depicted, all three are required for strain-induced migration. FAK, focal adhesion kinase; ERK, extracellular-related kinase; GSK, glycogen synthase kinase; MLC, myosin light-chain kinase.
Mechanical force effects on other gut cells
Non-epithelial Cells in the Mucosa
The intestinal mucosa includes cell types other than epithelial cells and these may also be important in how the gut responds to mechanical stimuli. Enterochromaffin (EC) cells, specialized endocrine cells of the GI tract, are sparsely dispersed in the intestinal epithelium and release 5-hydroxytryptamine (5-HT). 5-HT critically influences gut function by binding to a specific receptor on enteric afferent neurons and thus regulating intestinal motility, blood flow, and secretion [67]. In vivo, serotonin release from guinea pig ileum and other models have been stimulated by intestinal smooth muscle contraction and other mechanical [68, 69]. Serotonin then relays messages to the brain regarding sensory information about the bowel lumen. The mechanism of this phenomenon is not entirely elucidated though in vitro studies suggest the involvement of G-protein-coupled receptors and intracellular calcium mobilization in human carcinoid BON cells, used as a model for EC cells [70]. The details of the further intracellular signaling that mediate this effect await elucidation. Vascular endothelial and smooth muscle cells also exist in the bowel wall, lining the vessels that perfuse the tissue. Although these cells must also experience the forces engendered by bowel motility and peristalsis, it seems likely that their responses to such forces is more limited and dominated by their responses to hemodynamic forces since vascular endothelial and smooth muscle cells respond less to the relatively slow frequencies engendered by gut function than by the to much higher frequencies caused by cardiac pulsation as mentioned earlier [71, 72]. Inflammatory cells are also resident within the mucosa and further recruited during pathological states. In such states, pressure and shear stimulate monocyte adhesion to endovascular cells via activation of β1-integrins resulting in fibronectin and adhesion-molecule attachment [73]. Macrophages also respond to pressure by increased phagocytosis and modulation of cytokine release in a complex fashion [74]. In contrast to strain-mediated intestinal epithelial changes, which are mediated by FAK and ERK activation, the effects of pressure on macrophages seem due in part to FAK and ERK inhibition and p38 activation [75, 76]. However, similar to strain-induced mitogenesis, PI3K and Akt-2 but not other Akt isoforms, regulate this effect [77]. Table 2 lists some mechanosensitive cell types within the gut wall.
Table 2.
Examples of cells in the gut wall that responds to mechanical force
| Mechanosensitive cells in the gut |
|---|
| Mucosa (epithelial cells) |
| Vascular endothelial cells |
| Vascular smooth muscle cells |
| Enterochromaffin (EC) cells |
| Interstitial cells of Cajal |
| Neurons - afferent, efferent, plexi (Auerbach's) |
Beyond the Gut Mucosa
Outside the mucosa, the muscular layers of the bowel wall are dominated by intestinal smooth muscle and nerve cells, which are exposed to similar mechanical forces. Although the intracellular signaling events that mediate the responses of these cells to mechanical forces have not been studied in as much detail, it is clear that they are also mechanosensitive. Colonic stretch results in an activation of polarized intrinsic neural reflexes in the colon by activating both ascending and descending interneurons, of which the descending inhibitory reflex dominates. This descending inhibitory reflex slows colonic emptying [78], at least in part due to nitric oxide release from descending neurons stimulated by colonic elongation [79]. Numerous classes of mechanosensory spinal afferents nerves have been described in mouse colon, each tuned to a distinct mechanical stimulus, leading some researchers to suggest that the diversity of mechanosensitive properties within the colon is similar to that innervating the skin [80]. Furthermore, the regulation seems to be specific enough to allow different signaling between the longitudinal and smooth muscle layer of the GI tract [81]. These effects become abnormal in some human disease processes. For instance, pathologic conditions with chronic ileal inflammation, as might occur in Crohn's disease, are characterized by increased thresholds for discomfort and greatly diminished systemic autonomic reflex responses [82]. Patients with inflammatory bowel disease and irritable bowel syndrome display altered rectal visceral perception which correlated with diminished perception thresholds [83]. Although the mechanisms for these aberrations are not fully understood, studies suggest that the primary abnormality may take place at the level of the mechanoreceptor, which may become sensitized by repeated inflammatory processes [84]. A more complete understanding of these complex interactions is required to eventually combat these pathologies.
Mechanical forces in gastrointestinal malignancy
Just as the normal mucosa experiences and responds to mechanical forces, so, too, does abnormal mucosa that has undergone malignant transformation. Colon cancer cells experience a variety of physical forces. Pressure can come not only from the bowel itself but also from external sources. During laparoscopic surgery, the abdomen is insufflated with gas to a pressure of approximately 15 mmHg above ambient, and some studies suggest that the pressure associated with pneumoperitoneum may play a role in tumor dissemination [85]. Whether via laparoscopic or more classical open surgical procedures, direct surgical manipulation of the tumor results in periods of increased pressure. Following an operation on the intestine, the bowel wall takes up fluid and becomes very edematous, a process known as “third-spacing” [24]. During this edematous phase, intraabdominal pressure increases as the bowel swells against the relatively non-distensible abdominal wall, yielding pressures as high as 15-40 mmHg above normal for a period of days [86, 87]. Although the primary tumor has been removed at this point, such pressures may affect residual unresected tumor or cancer cells that have been shed into the peritoneal cavity during the surgical procedure. Such shed cancer cells may correlate adversely with prognosis [88]. Cancer cells are subject to physical forces even outside the perioperative period. Rapidly growing tumors expand against a constraining stroma leading to increased interstitial pressure within the tumor itself, which may stimulate tumor proliferation depending upon the specific tumor type [89]. Increased proliferative states have also been observed in vivo [90]. In fact, some cancer treatments function by specifically decreasing interstitial pressure [91]. Metastasizing tumor cells also experience shear and pressure forces as they pass through lymphatics and the vasculature of the host. Thus, tumor cells are exposed to diverse forces in a variety of settings that may influence their phenotype.
Types of Cellular Responses to Mechanical Forces
Indeed, mechanical forces may influence a wide variety of epithelial cancers including colon cancers, stimulating a novel pathway by which malignant cells regulate their own adhesion [9]. Colorectal tumor cells from patients who have relapsed show increased cell adhesion to test monolayers in vitro versus cells from patients who were non-relapsing, suggesting a correlation between cancer invasion and adhesiveness [92]. Adhesion is a critical first step in the process of cancer metastasis, and increased adhesive ability or the expression of specific integrin adhesion receptors correlates with prognosis, malignant transformation, and dissemination [93, 94]. Indeed, pressure stimulates the adhesion of cancer cells in vitro from a variety of epithelial tumor types including colon cell lines, primary human colon tumor cells, head and neck squamous cancer cells, and breast adenocarcinoma [9, 95-97]. Laminar and non-laminar shear stress seems similar to activate similar intracellular signals and exert similar biologic effects as pressure [30, 98].
Cell Adhesion and Integrins
Cancer cell adhesion is a complex process. Most, although not all, epithelial cancer cell adhesion is mediated by integrins, heterodimeric cell surface proteins by which cells bind to matrix proteins [99]. The stimulation of adhesion by increased extracellular pressure appears mediated primarily by β1-integrin heterodimers since a functional antibody to the β1-integrin subunit blocks the effect [9]. Many integrins possess an extracellular divalent cation binding site which modulates integrin binding affinity. Both in vitro [100] and in vivo [101] data has shown that while magnesium stimulates colon tumor cell adhesion, calcium inhibits this effect. However, the stimulation of adhesion by extracellular pressure occurs independently of divalent cations [102], suggesting that while the cations modulate integrin binding affinity from outside the cell, pressure stimulates integrin-mediated adhesion by a different mechanism within the cell. Integrins clearly play a role in this phenomenon. β1-integrin subunit T788/9 phosphorylation is stimulated in response to increased extracellular pressure, causing a conformational change that exposes the extracellular matrix-binding epitope and increases adhesive potential [103]. Evidence that β1-integrin T788/9 phosphorylation is stimulated in suspended cells in the absence of ligand, in addition to data showing that intracellular signals translocate to the membrane and interact with β1-integrins to alter its binding affinity [95], supports the idea of “inside-out” signaling, in which intracellular signals modulate how the cell interacts with its environment.
Molecular pathways involved
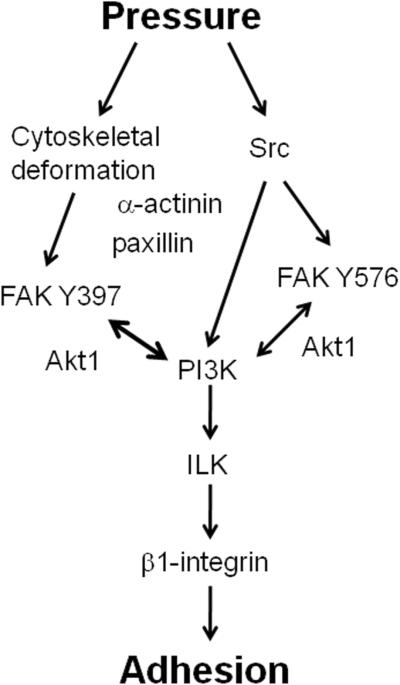
This phenomenon of integrin-mediated adhesion is regulated in part via FAK and Src, similar to strain stimuli within non-malignant gut epithelial cells. However, unlike the gut mucosal response to strain, PKC and ERK are not involved in the stimulation of malignant cell adhesion by pressure [9, 102]. Tumor cells activated by pressure prior to experimentation adhere more readily to surgical wounds in mice, and in a Src-dependent and cytoskeletal-dependent manner [104]. Other forces such as shear and turbulence also activate tumor cells similarly in a Src-dependent fashion [30, 105]. The activation of FAK via shear similarly stimulates the adhesion of tumor cells within sinusoids [98]. PI3K and Akt are other key molecules involved in pressure-stimulated adhesion. While Src interacts with FAK at the tyrosine 576/577 residue, PI3K interacts with FAK at its tyrosine 397 residue, leading to Akt-1 activation and translocation of FAK and AKT to the membrane, ultimately activating integrins as described above [95]. Furthermore, the phosphorylation of β1-integrin at T788/9 discussed earlier requires activation of α-actinin, FAK, Src, and PI3K [103]. Pharmacologic manipulation of the cytoskeleton prevents pressure stimulation of adhesion [106], and both pressure-stimulated signaling in cancer cells and strain-stimulated signaling in non-malignant epithelial cells are ablated by cytoskeletal manipulation [51] suggesting that the cytoskeleton plays a large role in this phenomenon, perhaps as a primary mechanosensor. Ingber has suggested that the cytoskeleton functions as a tensegrity structure that may sense and respond to physical forces by altering its physical conformation, perhaps thereby also altering the conformation of more conventional signaling molecules to which the cytoskeleton attaches within focal adhesions [107]. The adapter protein paxillin, which facilitates focal adhesion assembly and linkage to the cytoskeleton, is phosphorylated in response to pressure, in a cytoskeletally dependent fashion [96, 108]. Colchicine, which disrupts microtubule dynamics by irreversibly binding to tubulin dimmers and preventing microtubule polymerization [109], blocks this effect. Figure 3 summarizes what is currently known about the pathway involved in cell adhesion stimulated by pressure.
Fig 3.
One possible pathway in which pressure regulates cellular adhesion. The cytoskeleton plays a key role in transducing many of the relevant signals, but Src is activated independently of the cytoskeleton. Akt1 seems to aid in keeping FAK phosphorylated at the focal adhesion, though its role is complex and not completely understood. FAK, focal adhesion kinase; ILK, integrin-linked kinase.
Mitogenic Effects on Cancer Cells
In addition to stimulating cancer cell adhesion, pressure also stimulates the proliferation of colon cancer cells in vitro [110]. Interestingly, although cancer cell adhesion may be stimulated by an exposure to increased pressure as short as one minute [103], the mitogenic effects of pressure require exposure to durations of pressure exceeding four hours. The mitogenic effects of pressure in malignant epithelial cells also differ from the mitogenic effects of strain in non-malignant intestinal epithelial cells. For instance, although the strain effect is inhibited by fibronectin [32], the stimulation of cancer cell proliferation by pressure is matrix-independent. Finally, although ERK, p38, Src, and PKC are each activated by pressure, just as they are in response to strain, specifically preventing Src or MAPK activitiy does not prevent the pressure effect, which is inhibited only by PKC or global tyrosine kinase blockade [110]. This difference in pathways between proliferation and adhesion may offer targets to intervene in cancer progression on multiple levels.
In vivo evidence for intestinal responsiveness to physical forces
Strain and GI Mucosa
In vitro data clearly suggests the involvement of physical forces on cell biology in both the normal and pathologic states, but does experimental in vivo work validate this concept? It is obviously difficult to manipulate physical forces within the gut mucosa in vivo without affecting a host of other neurohumoral factors. However, studies performed so far support the paradigm. Non-malignant rat intestine subjected to rhythmic distension with isotonic balanced polyethylene glycol and electrolyte solution displays a time-dependent increase in mucosal tyrosine kinase activity within ten minutes, both in the small bowel and colon [46]. When pig jejunum is stretched over a period of days, epithelial growth and proliferation are induced, while the mucosal barrier and absorptive functions are maintained. The architecture of the epithelium does change, but this occurs proportionally with a decrease in villus height offset by an increase in crypt depth [34]. Partially obstructed Roux-en-Y anastomoses have been created in mice, leading to a proximal partially obstructed limb in continuity with gut peristalsis and nutrient flow, a partially obstructed defunctional bowel limb disconnected from the proximal peristaltic pacemaker and without luminal flow, and a distal collapsed bowel limb. Luminal pressure is increased in the proximal and defunctionalized limbs and decreased distally. In these studies, mucosal proliferation was increased similarly in both the proximal and defunctionalized limbs while mucosal wounds healed more slowly in both of these limbs in comparison to the distal low pressure low strain bowel, suggesting that strain and pressure can together influence cell responses [111]. Although this model is complex and it is difficult to dissect the relative contributions of strain versus pressure here, such observations again suggest that physical forces can modulate non-malignant intestinal epithelial biology in vivo.
Pressure and GI Cancer
The in vitro observation that cancer cell adhesion is stimulated by pressure has been validated in vivo as well. When murine transplantable tumor cells are preincubated under increased pressure conditions, cell adhesion to murine wounds is increased [104]. However, when α-actinin is blocked in pressure-exposed cancer cells, subsequent adhesion to murine surgical wounds is impaired. Even more importantly, pressure activation of tumor cells allowed to adhere to murine surgical wounds substantially impairs subsequent tumor-free survival, and this effect is eliminated if α-actinin (which participates in cytoskeletal-focal adhesion linkage) is blocked in pressure-treated cells, suggesting that α-actinin may be a reasonable target to reduce the activation of tumor cell adhesion by physical forces [112]. Pressure-stimulated adhesion is also decreased and tumor-free survival is increased if either the treating cancer cells or the donor mice are treated with colchicines to disrupt microtubule organization [113]. Increased pressure also potentiates peritoneal metastasis and this effect is also prevented by colchicine [113]. Thus, pressure stimulation seems to have a real effect on tumor biology in vivo, and modulation of the cytoskeleton is a critical upstream mechanosensor in these effects.
Summary
Intestinal cells are subjected to a wide variety of mechanical forces in both normal function and pathophysiologic states. Such forces play a major role in cell biology and, interacting with other stimuli including neurohumoral and matrix input, markedly influence cell phenotype. These forces are often altered in pathologic conditions that adversely impact cell biology, sometimes to the detriment of the organism. A more complete understanding of the pathways governing these complex processes is required in order to attempt therapeutic manipulation and potentially improve such pathologic conditions. The cytoskeleton is likely to play an important role in all of these effects, by serving as a primary mechanosensor, becoming distorted as external forces compress the cell. These pathways offer a possible series of reasonable targets for pharmacologic intervention into non-malignant and malignant disease states.
Acknowledgments
Supported in part by: a VA Merit Award, NIH RO1 DK067257, NIH RO1DK060771, (all MDB) and NIH 2 T32 GM008420 (MDB and CG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Otterson MF, Sarr MG. Surg Clin North Am. 1993;73(6):1173–1192. doi: 10.1016/s0039-6109(16)46186-4. [DOI] [PubMed] [Google Scholar]
- [2].Sarna SK, Otterson MF. Gastroenterol Clin North Am. 1989;18(2):375–404. [PubMed] [Google Scholar]
- [3].Hennig GW, Costa M, Chen BN, Brookes SJ. J Physiol. 1999;517(Pt 2):575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grivel ML, Ruckebusch Y. J Physiol. 1972;227(2):611–625. doi: 10.1113/jphysiol.1972.sp010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Karaus M, Wienbeck M. Baillieres Clin Gastroenterol. 1991;5(2):453–478. doi: 10.1016/0950-3528(91)90037-2. [DOI] [PubMed] [Google Scholar]
- [6].Miftakhov RN, Wingate DL. Med Eng Phys. 1994;16(5):406–415. doi: 10.1016/1350-4533(90)90007-u. [DOI] [PubMed] [Google Scholar]
- [7].Scott SM, Knowles CH, Wang D, Yazaki E, Picon L, Wingate DL, Lindberg G. Neurogastroenterol Motil. 2006;18(10):927–935. doi: 10.1111/j.1365-2982.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- [8].Brodribb AJ, Condon RE, Cowles V, DeCosse JJ. Gastroenterology. 1979;77(1):70–74. [PubMed] [Google Scholar]
- [9].Basson MD, Yu CF, Herden-Kirchoff O, Ellermeier M, Sanders MA, Merrell RC, Sumpio BE. J Cell Biochem. 2000;78(1):47–61. doi: 10.1002/(sici)1097-4644(20000701)78:1<47::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [10].Kellow JE, Phillips SF. Gastroenterology. 1987;92(6):1885–1893. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- [11].Alizadeh H, Weems WA, Castro GA. Gastroenterology. 1989;97(6):1461–1468. doi: 10.1016/0016-5085(89)90390-9. [DOI] [PubMed] [Google Scholar]
- [12].Mimura T, Emanuel A, Kamm MA. Best Pract Res Clin Gastroenterol. 2002;16(4):563–576. doi: 10.1053/bega.2002.0298. [DOI] [PubMed] [Google Scholar]
- [13].Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Am J Physiol. 1987;252(2 Pt 1):G250–256. doi: 10.1152/ajpgi.1987.252.2.G250. [DOI] [PubMed] [Google Scholar]
- [14].Womack WA, Tygart PK, Mailman D, Kvietys PR, Granger DN. Gastroenterology. 1988;94(4):977–983. doi: 10.1016/0016-5085(88)90556-2. [DOI] [PubMed] [Google Scholar]
- [15].Womack WA, Mailman D, Kvietys PR, Granger DN. Am J Physiol. 1988;255(2 Pt 1):G162–167. doi: 10.1152/ajpgi.1988.255.2.G162. [DOI] [PubMed] [Google Scholar]
- [16].Kuemmerle JF. J Clin Gastroenterol. 2000;31(4):276–281. doi: 10.1097/00004836-200012000-00003. [DOI] [PubMed] [Google Scholar]
- [17].Granger DN, Barrowman JA. Gastroenterology. 1983;84(4):846–868. [PubMed] [Google Scholar]
- [18].Allan A, Wyke J, Allan RN, Morel P, Robinson M, Scott DL, Alexander-Williams J. Gut. 1989;30(5):627–633. doi: 10.1136/gut.30.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Gastroenterology. 1994;107(3):755–763. doi: 10.1016/0016-5085(94)90124-4. [DOI] [PubMed] [Google Scholar]
- [20].Kehlet H, Holte K. Am J Surg. 2001;182(5A Suppl):3S–10S. doi: 10.1016/s0002-9610(01)00781-4. [DOI] [PubMed] [Google Scholar]
- [21].Inoue Y, Grant JP, Snyder PJ. JPEN J Parenter Enteral Nutr. 1993;17(2):165–170. doi: 10.1177/0148607193017002165. [DOI] [PubMed] [Google Scholar]
- [22].Fink MP. Curr Opin Crit Care. 2003;9(2):143–151. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- [23].Boza J, Jimenez J, Baro L, Martinez O, Suarez MD, Gil A. J Pediatr Gastroenterol Nutr. 1996;22(2):186–193. doi: 10.1097/00005176-199602000-00010. [DOI] [PubMed] [Google Scholar]
- [24].Granger DN, Barrowman JA. Gastroenterology. 1983;84(5 Pt 1):1035–1049. [PubMed] [Google Scholar]
- [25].Hulten L, Lindhagen J, Lundgren O, Fasth S, Ahren C. Gastroenterology. 1977;72(3):388–396. [PubMed] [Google Scholar]
- [26].Ecknauer R, Raffler H. Digestion. 1978;18(132):45–55. doi: 10.1159/000198183. [DOI] [PubMed] [Google Scholar]
- [27].Hodin RA, Chamberlain SM, Meng S. Am J Physiol. 1995;269(2 Pt 1):C385–391. doi: 10.1152/ajpcell.1995.269.2.C385. [DOI] [PubMed] [Google Scholar]
- [28].Basson MD, Li GD, Hong F, Han O, Sumpio BE. J Cell Physiol. 1996;168(2):476–488. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [29].Basson MD, Rashid Z, Turowski GA, West AB, Emenaker NJ, Sgambati SA, Hong F, Perdikis DM, Datta S, Madri JA. Yale J Biol Med. 1996;69(2):119–129. [PMC free article] [PubMed] [Google Scholar]
- [30].Thamilselvan V, Patel A, van der Voort van Zyp J, Basson MD. J Cell Biochem. 2004;92(2):361–371. doi: 10.1002/jcb.20072. [DOI] [PubMed] [Google Scholar]
- [31].Aumailley M, Gayraud B. J Mol Med. 1998;76(334):253–265. doi: 10.1007/s001090050215. [DOI] [PubMed] [Google Scholar]
- [32].Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. FASEB J. 2003;17(8):926–928. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]
- [33].Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. Gastroenterology. 2006;131(4):1179–1189. doi: 10.1053/j.gastro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [34].Spencer AU, Sun X, El-Sawaf M, Haxhija EQ, Brei D, Luntz J, Yang H, Teitelbaum DH. Surgery. 2006;140(2):212–220. doi: 10.1016/j.surg.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Turowski GA, Rashid Z, Hong F, Madri JA, Basson MD. Cancer Res. 1994;54(22):5974–5980. [PubMed] [Google Scholar]
- [36].Larson SD, Li J, Chung DH, Evers BM. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1262–1271. doi: 10.1152/ajpgi.00254.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murnin M, Kumar A, Li GD, Brown M, Sumpio BE, Basson MD. J Gastrointest Surg. 2000;4(4):435–442. doi: 10.1016/s1091-255x(00)80025-6. [DOI] [PubMed] [Google Scholar]
- [38].Frangos SG, Knox R, Yano Y, Chen E, Di Luozzo G, Chen AH, Sumpio BE. Endothelium. 2001;8(1):1–10. doi: 10.3109/10623320109063153. [DOI] [PubMed] [Google Scholar]
- [39].Li C, Hu Y, Mayr M, Xu Q. J Biol Chem. 1999;274(36):25273–25280. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- [40].Matsuda N, Morita N, Matsuda K, Watanabe M. Biochem Biophys Res Commun. 1998;249(2):350–354. doi: 10.1006/bbrc.1998.9151. [DOI] [PubMed] [Google Scholar]
- [41].Chaturvedi LS, Marsh HM, Basson MD. Am J Physiol Cell Physiol. 2007;292(5):C1701–1713. doi: 10.1152/ajpcell.00529.2006. [DOI] [PubMed] [Google Scholar]
- [42].Waters CM, Ridge KM, Sunio G, Venetsanou K, Sznajder JI. J Appl Physiol. 1999;87(2):715–721. doi: 10.1152/jappl.1999.87.2.715. [DOI] [PubMed] [Google Scholar]
- [43].Kumar A, Lnu S, Malya R, Barron D, Moore J, Corry DB, Boriek AM. FASEB J. 2003;17(13):1800–1811. doi: 10.1096/fj.02-1148com. [DOI] [PubMed] [Google Scholar]
- [44].Han O, Li GD, Sumpio BE, Basson MD. Am J Physiol. 1998;275(3 Pt 1):G534–541. doi: 10.1152/ajpgi.1998.275.3.G534. [DOI] [PubMed] [Google Scholar]
- [45].Han O, Sumpio BE, Basson MD. Biochem Biophys Res Commun. 1998;250(3):668–673. doi: 10.1006/bbrc.1998.9372. [DOI] [PubMed] [Google Scholar]
- [46].Basson MD, Coppola CP. Metabolism. 2002;51(12):1525–1527. doi: 10.1053/meta.2002.36303. [DOI] [PubMed] [Google Scholar]
- [47].Li W, Duzgun A, Sumpio BE, Basson MD. Am J Physiol Gastrointest Liver Physiol. 2001;280(1):G75–87. doi: 10.1152/ajpgi.2001.280.1.G75. [DOI] [PubMed] [Google Scholar]
- [48].Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. J Biol Chem. 1994;269(43):26602–26605. [PubMed] [Google Scholar]
- [49].Schlaepfer DD, Hauck CR, Sieg DJ. Prog Biophys Mol Biol. 1999;71(334):435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- [50].Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. J Biol Chem. 2007;282(1):14–28. doi: 10.1074/jbc.M605817200. [DOI] [PubMed] [Google Scholar]
- [51].Craig DH, Zhang J, Basson MD. Am J Surg. 2007;194(5):618–622. doi: 10.1016/j.amjsurg.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Nature. 2007;447(7144):592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. J Biol Chem. 2008 doi: 10.1074/jbc.M804576200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Microsc Res Tech. 2000;51(2):169–178. doi: 10.1002/1097-0029(20001015)51:2<169::AID-JEMT8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [55].McNeil PL, Ito S. Gastroenterology. 1989;96(5 Pt 1):1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- [56].Dunn GA, Zicha D, Fraylich PE. J Cell Sci. 1997;110(Pt 24):3091–3098. doi: 10.1242/jcs.110.24.3091. [DOI] [PubMed] [Google Scholar]
- [57].Verspaget HW, Biemond I, Allaart CF, van Weede H, Weterman IT, Gooszen HG, Pena AS, Lamers CB. Hepatogastroenterology. 1991;38(3):231–234. [PubMed] [Google Scholar]
- [58].Ito M, Hirata S, Arai S, Takahashi T. J Gastroenterol. 1995;30(Suppl 8):70–72. [PubMed] [Google Scholar]
- [59].Velky TS, Yang JC, Greenburg AG. J Trauma. 1984;24(9):824–829. doi: 10.1097/00005373-198409000-00008. [DOI] [PubMed] [Google Scholar]
- [60].Kiener JL, Saba TM, Cho E, Blumenstock FA. Am J Physiol. 1986;251(4 Pt 2):R724–734. doi: 10.1152/ajpregu.1986.251.4.R724. [DOI] [PubMed] [Google Scholar]
- [61].Yu P, Martin CM. Crit Care Med. 2000;28(7):2573–2577. doi: 10.1097/00003246-200007000-00065. [DOI] [PubMed] [Google Scholar]
- [62].De Hertogh G, Aerssens J, Geboes KP, Geboes K. World J Gastroenterol. 2008;14(6):845–852. doi: 10.3748/wjg.14.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chaturvedi LS, Gayer CP, Marsh HM, Basson MD. Am J Physiol Cell Physiol. 2008;294(6):C1350–1361. doi: 10.1152/ajpcell.00027.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Owen CR, Yuan L, Basson MD. Lab Invest. 2008;88(10):1101–1109. doi: 10.1038/labinvest.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gayer CP, Chaturvedi LS, Basson MD. J Am Coll Surg. 2008;207(3 Supplemental):S13. [Google Scholar]
- [66].Cunningham CC, Vegners R, Bucki R, Funaki M, Korde N, Hartwig JH, Stossel TP, Janmey PA. J Biol Chem. 2001;276(46):43390–43399. doi: 10.1074/jbc.M105289200. [DOI] [PubMed] [Google Scholar]
- [67].Read NW, Gwee KA. Pharmacol Ther. 1994;62(132):159–173. doi: 10.1016/0163-7258(94)90009-4. [DOI] [PubMed] [Google Scholar]
- [68].Bertrand PP. J Physiol. 2006;577(Pt 2):689–704. doi: 10.1113/jphysiol.2006.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kirchgessner AL, Liu MT, Raymond JR, Gershon MD. J Comp Neurol. 1996;364(3):439–455. doi: 10.1002/(SICI)1096-9861(19960115)364:3<439::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [70].Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ. J Clin Invest. 2001;108(7):1051–1059. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chien S. Am J Physiol Heart Circ Physiol. 2007;292(3):H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- [72].Li YS, Haga JH, Chien S. J Biomech. 2005;38(10):1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- [73].Ashida N, Takechi H, Kita T, Arai H. J Biol Chem. 2003;278(11):9327–9331. doi: 10.1074/jbc.M212316200. [DOI] [PubMed] [Google Scholar]
- [74].Shiratsuch H, Basson MD. Am J Surg. 2005;190(5):757–762. doi: 10.1016/j.amjsurg.2005.07.016. [DOI] [PubMed] [Google Scholar]
- [75].Shiratsuchi H, Basson MD. Am J Physiol Cell Physiol. 2004;286(6):C1358–1366. doi: 10.1152/ajpcell.00553.2003. [DOI] [PubMed] [Google Scholar]
- [76].Shiratsuchi H, Basson MD. Am J Physiol Cell Physiol. 2005;288(5):C1083–1093. doi: 10.1152/ajpcell.00543.2004. [DOI] [PubMed] [Google Scholar]
- [77].Shiratsuchi H, Basson MD. J Cell Biochem. 2007;102(2):353–367. doi: 10.1002/jcb.21295. [DOI] [PubMed] [Google Scholar]
- [78].Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NJ, Smith TK. J Physiol. 2008;586(Pt 17):4225–4240. doi: 10.1113/jphysiol.2008.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. Gastroenterology. 2007;132(5):1912–1924. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- [80].Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Gastroenterology. 2004;127(1):166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [81].Miller SM, Szurszewski JH. Am J Physiol Gastrointest Liver Physiol. 2003;285(6):G1129–1138. doi: 10.1152/ajpgi.00292.2003. [DOI] [PubMed] [Google Scholar]
- [82].Bernstein CN, Niazi N, Robert M, Mertz H, Kodner A, Munakata J, Naliboff B, Mayer EA. Pain. 1996;66(233):151–161. doi: 10.1016/0304-3959(96)03062-x. [DOI] [PubMed] [Google Scholar]
- [83].Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Gastroenterology. 1995;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- [84].Delvaux M. Gut. 2002;51(Suppl 1):i67–71. doi: 10.1136/gut.51.suppl_1.i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shen MY, Huang IP, Chen WS, Chang JT, Lin JK. Hepatogastroenterology. 2008;55(84):947–951. [PubMed] [Google Scholar]
- [86].Williams M, Simms HH. Am Surg. 1997;63(6):555–558. [PubMed] [Google Scholar]
- [87].Madl C, Druml W. Best Pract Res Clin Gastroenterol. 2003;17(3):445–456. doi: 10.1016/s1521-6918(03)00022-2. [DOI] [PubMed] [Google Scholar]
- [88].Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, Harder F, Heberer M, Marti WR. Ann Surg. 2002;236(6):768–775. doi: 10.1097/00000658-200212000-00009. discussion 775-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Diresta GR, Nathan SS, Manoso MW, Casas-Ganem J, Wyatt C, Kubo T, Boland PJ, Athanasian EA, Miodownik J, Gorlick R, Healey JH. Ann Biomed Eng. 2005;33(9):1270–1280. doi: 10.1007/s10439-005-5732-9. [DOI] [PubMed] [Google Scholar]
- [90].Nathan SS, DiResta GR, Casas-Ganem JE, Hoang BH, Sowers R, Yang R, Huvos AG, Gorlick R, Healey JH. Clin Cancer Res. 2005;11(6):2389–2397. doi: 10.1158/1078-0432.CCR-04-2048. [DOI] [PubMed] [Google Scholar]
- [91].Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E, Molokhia P, Attia K, Sullivan T, Kuter I, Boucher Y, Powell SN. J Clin Oncol. 2005;23(9):1951–1961. doi: 10.1200/JCO.2005.08.119. [DOI] [PubMed] [Google Scholar]
- [92].Benoliel AM, Pirro N, Marin V, Consentino B, Pierres A, Vitte J, Bongrand P, Sielezneff I, Sastre B. Anticancer Res. 2003;23(6C):4891–4896. [PubMed] [Google Scholar]
- [93].Streit M, Schmidt R, Hilgenfeld RU, Thiel E, Kreuser ED. Recent Results Cancer Res. 1996;142:19–50. doi: 10.1007/978-3-642-80035-1_3. [DOI] [PubMed] [Google Scholar]
- [94].Haier J, Nasralla M, Nicolson GL. Ann Surg. 2000;231(1):11–24. doi: 10.1097/00000658-200001000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Thamilselvan V, Craig DH, Basson MD. FASEB J. 2007;21(8):1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- [96].Conway WC, Van der Voort van Zyp J, Thamilselvan V, Walsh MF, Crowe DL, Basson MD. J Cell Biochem. 2006;98(6):1507–1516. doi: 10.1002/jcb.20819. [DOI] [PubMed] [Google Scholar]
- [97].Downey C, Alwan K, Thamilselvan V, Zhang L, Jiang Y, Rishi AK, Basson MD. Am J Surg. 2006;192(5):631–635. doi: 10.1016/j.amjsurg.2006.08.006. [DOI] [PubMed] [Google Scholar]
- [98].von Sengbusch A, Gassmann P, Fisch KM, Enns A, Nicolson GL, Haier J. Am J Pathol. 2005;166(2):585–596. doi: 10.1016/S0002-9440(10)62280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Stupack DG. Oncology (Williston Park) 2007;21(9 Suppl 3):6–12. [PubMed] [Google Scholar]
- [100].Thamilselvan V, Fomby M, Walsh M, Basson MD. J Surg Res. 2003;110(1):255–265. doi: 10.1016/s0022-4804(03)00028-3. [DOI] [PubMed] [Google Scholar]
- [101].van der Voort van Zyp J, Conway WC, Thamilselvan V, Polin L, Basson MD. Am J Surg. 2005;190(5):701–707. doi: 10.1016/j.amjsurg.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [102].Thamilselvan V, Basson MD. Gastroenterology. 2004;126(1):8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- [103].Craig DH, Gayer CP, Schaubert KL, Wei Y, Li J, Laouar Y, Basson MD. Am J Physiol Cell Physiol. 2008 doi: 10.1152/ajpcell.00355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].van der Voort van Zyp J, Thamilselvan V, Walsh M, Polin L, Basson MD. Am J Surg. 2004;188(5):467–473. doi: 10.1016/j.amjsurg.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [105].Haier J, Gallick GE, Nicolson GL. J Exp Ther Oncol. 2002;2(4):237–245. doi: 10.1046/j.1359-4117.2002.01051.x. [DOI] [PubMed] [Google Scholar]
- [106].Thamilselvan V, Basson MD. Carcinogenesis. 2005;26(10):1687–1697. doi: 10.1093/carcin/bgi135. [DOI] [PubMed] [Google Scholar]
- [107].Ingber DE. J Cell Sci. 2003;116(Pt 7):1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- [108].Downey C, Craig DH, Basson MD. Cell Mol Life Sci. 2008;65(9):1446–1457. doi: 10.1007/s00018-008-8038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Downing KH. Annu Rev Cell Dev Biol. 2000;16:89–111. doi: 10.1146/annurev.cellbio.16.1.89. [DOI] [PubMed] [Google Scholar]
- [110].Walsh MF, Woo RK, Gomez R, Basson MD. Cell Prolif. 2004;37(6):427–441. doi: 10.1111/j.1365-2184.2004.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Flanigan TL, Owen CR, Gayer C, Basson MD. Am J Surg. 2008;196(5):683–689. doi: 10.1016/j.amjsurg.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Craig DH, Downey C, Basson MD. Neoplasia. 2008;10(3):217–222. doi: 10.1593/neo.07945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Craig DH, Owen CR, Conway WC, Walsh MF, Downey C, Basson MD. J Clin Invest. 2008;118(9):3170–3180. doi: 10.1172/JCI34279. [DOI] [PMC free article] [PubMed] [Google Scholar]