Abstract
The regulatory proteins of polyomaviruses, including small and large T antigens, play important roles, not only in the viral life cycle but also in virus-induced cell transformation. Unlike many other tumor viruses, the transforming proteins of polyomaviruses have no cellular homologs but rather exert their effects mostly by interacting with cellular proteins that control fundamental processes in the regulation of cell proliferation and the cell cycle. Thus, they have proven to be valuable tools to identify specific signaling pathways involved in tumor progression. Elucidation of these pathways using polyomavirus transforming proteins as tools is critically important in understanding fundamental regulatory mechanisms and hence to develop effective therapeutic strategies against cancer. In this short review, we will focus on the structural and functional features of one polyomavirus transforming protein, that is, the small t-antigen of the human neurotropic JC virus (JCV) and the simian virus, SV40.
Polyomaviruses are comprised of a family of non-enveloped DNA viruses with icosahedral capsids. Their genome is small, circular and double stranded and they are isolated from a variety of species, including humans, monkeys, rodents, and birds (Cole, 1996). Simian vacuolating virus 40 (SV40) and mouse polyomavirus were the first in the family to be discovered (Stewart et al., 1958; Sweet and Hilleman, 1960), both of which have provided a simple model system to study many aspects of DNA structure for more complex eukaryotic genomes (White and Khalili, 2004; White et al., 2005). In addition, their oncogenic proteins were also used as tools to discover and understand many signaling and transduction pathways (Pipas, 1992; Brodsky and Pipas, 1998; Sullivan and Pipas, 2002). During the early 1970s, two new members from humans were isolated, JC virus (JCV) and BK virus (BKV). JCV was isolated from the brain tissue of a patient suffering from progressive multifocal encephalopathy (PML) (Padgett et al., 1971). BKV was isolated from the urine of a kidney transplant patient with advanced renal failure (Gardner et al., 1971). Recently two new human members of polyomavirus were also discovered, one isolated from patient with symptoms of acute respiratory tract infection and named WU virus (Gaynor et al., 2007), the other was isolated from human respiratory tract samples by PCR amplification and named KI polyomavirus (Allander et al., 2007).
JCV, SV40, and BKV are very closely related viruses because of their genomic organization and sequence similarity (White et al., 1992). Their genome is functionally divided into coding and noncoding regions (Cole, 1996). Coding regions can also be subdivided into early and late transcription units, early of which proceeds in a counter clockwise direction and encodes mainly regulatory proteins, including large T-Ag (LT-Ag), small t antigen (Sm t-Ag) (Major et al., 1992). The same region in JCV was also shown to encode alternatively spliced forms of LT-Ag, called T’ proteins (Bollag et al., 2006; Frisque et al., 2006). LT-Ag initiates viral DNA replication, transactivates late gene expression and plays a major role in cell transformation. The function of Sm t-Ag in these processes, however, is not clear although it was shown to play a major role in LT-Ag mediated cell transformation in SV40 (Bouck et al., 1978, 1984; Chang et al., 1984, 1985; Porras et al., 1996; Hahn et al., 2002; Zhao et al., 2003). T′ proteins was shown to differentially interact with pRb and play some roles in cell transformation (Bollag et al., 2006; Frisque et al., 2006). The late transcriptional unit encodes both regulatory (agnoprotein) and structural proteins (VP1, VP2, and VP3). Agnoprotein plays roles in viral replication, transcription (Safak et al., 2001, 2002) and virion biogenesis (Okada et al., 2005; Suzuki et al., 2005; Sariyer et al., 2006), whereas structural proteins are critical for viral attachment, penetration and delivery of viral DNA into the nucleus (Pho et al., 2000; Elphick et al., 2004; Daniels et al., 2006a,b). It was also recently discovered that SV40 late genome encodes a late protein, named VP4, whose synthesis initiates from a downstream start codon within the VP2 transcript. It appears that Vp4 mediates that the lysis of infected cells during the late phases of SV40 infection cycle (Daniels et al., 2007).
Polyomaviruses exhibit tissue and species-specific tropism and this is mostly due to, at least in the case of JCV, BKV, and SV40, the unique structural organization of the regulatory region of each virus. For example, JCV and BKV exhibits tropism for oligodendrocytes and kidney epithelial cells respectively (Major et al., 1992; Hirsch and Steiger, 2003; Hirsch, 2005; Hirsch et al., 2006). The replication of JCV in CNS results in PML, which is characterized by the multiple foci formation at the demyelinated areas caused by lytic infection of oligodendrocytes (Major et al., 1992; Safak and Khalili, 2003) and PML develops in immunocomprimized individuals including AIDS patients (6–9, 130), although occurrence of PML was also reported in normal individuals (Berger, 2003; Berger and Houff, 2006). Reactivation of BKV, on the other hand, may result in polyomavirus-associated nephropathy (PVN), which develops in renal transplant patients and characterized by the formation of the viral inclusion bodies in the nuclei of kidney epithelial cells and that of interstitial nephritis (Hirsch and Steiger, 2003; Hirsch et al., 2006). The natural host for SV40 is the rhesus monkey and not the human, however, inadvertent exposure of human population due to a SV40 contaminated polyomavirus vaccination between 1955 and 1963 put SV40 into a spotlight and raised some concerns for public health. However, it should be pointed out here that SV40 poorly replicates in human cells (Cole, 1996).
SV40, JCV, and BKV encode tumorogenic proteins including LT-Ag and Sm t-Ag. They have the potential to transform the cells in tissue culture and their tumorogenicity was demonstrated in experimental animals (London et al., 1978, 1983; Corallini et al., 1982; Miller et al., 1984; Butel, 1986; Small et al., 1986a,b; Butel et al., 1998, 1999; Butel and Lednicky, 1999; Gordon et al., 2000; Garcea, 2001). Their genomes were detected in a variety of human tumors (Corallini et al., 1987; Carbone et al., 1994, 1996; Krynska et al., 1999a) however it has yet to be conclusively determined whether they cause tumors in humans.
Structural Features of Sm t-Ag
Sm t-Ag was first identified by in vitro translation of SV40 mRNA (Prives and Beck, 1977) and by the characterization of several deletion mutants of the SV40 early genome. During the characterization of this mutant, it was initially thought that deletions were made only in the coding region of LT-Ag, but in fact they were also made in the coding region of Sm t-Ag (Shenk et al., 1976; Volckaert et al., 1979). This became clear after the phenomenon of mRNA splicing was discovered.
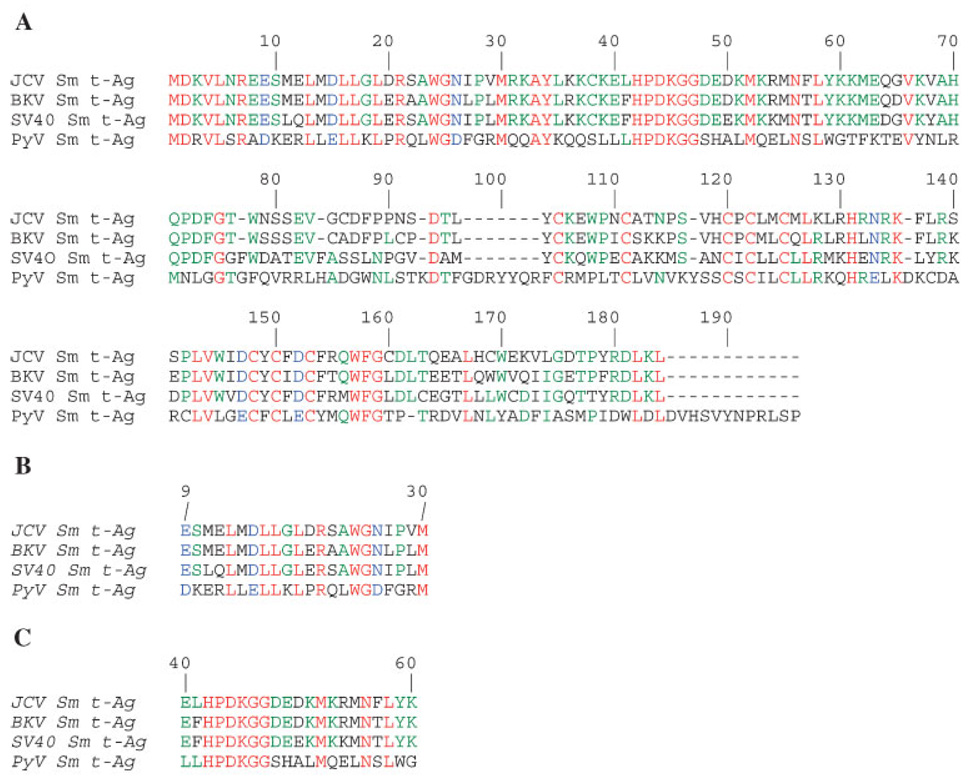
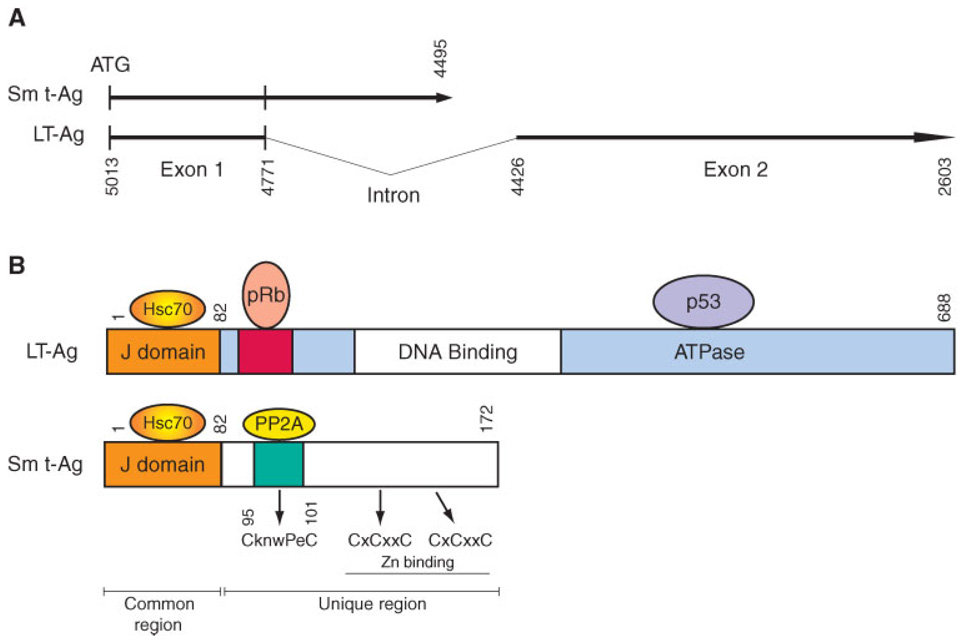
Amino acid alignment shows a high degree of sequence homology among JCV, SV40, BKV, and PyV Sm t-Ags. For example, JCV Sm t-Ag is a 172 amino acid long protein and shows 96% and 79% homology to both BKV and SV40 Sm t-Ag respectively. This alignment also reveals that the N-terminal sequences of each protein are highly conserved but those located in the middle portion are more divergent (Fig. 2), which may indicate a differential role for each protein during viral replication cycle and/or cell transformation. Sm t-Ags share a common region with LT-Ag and this corresponds to the N-terminus of each protein (Fig. 1). The unique region of each protein is located at the C-terminus. The common region contains a conserved domain (Cr1) (Fig. 2B) and heptapeptide region (HPDKGG) (Pipas, 1992) (Fig. 2C). Cr1 corresponds to amino acids 9 to 19 in SV40 LT/Sm t-Ags and is primarily involved in cell transformation (Peden et al., 1990; Yaciuk et al., 1991). The heptapeptide region is also conserved in all LT/sm t-Ags and corresponds to the residues 42–47 in Sm t-Ag (Fig. 2C). Amino acid substitution mutations within the heptapeptide region revealed that it is involved in viral replication rather than cell transformation (Peden and Pipas, 1992). The common region also contains a functional J domain (Fig. 1B), which functions as a chaperone. Chaperones are the proteins that promote the proper folding of proteins after translation and also prevent their aggregation during cellular stress (Hartl, 1996). There is structural and experimental evidence suggesting that the J domain of tumor antigens functions as a chaperone as follows: (i) The common region of tumor antigens and conserved residues of the type 3 DnaJ-like proteins show sequence similarity, (ii) Domain-swapping experiments showed that the J domain of the tumor antigens can functionally substitute for the J domain of E. coli DnaJ protein (Kelley and Georgopoulos, 1997), and (iii) Biochemical evidence suggests that the J domain of the tumor antigens functions as a J domain in multiple assays (Srinivasan et al., 1997).
Fig. 2.
A: Amino acid alignment of Sm t-Ags from JC virus (JCV), BK virus (BKV), Simian virus 40 (SV40) and mouse polyomavirus (PyV). B: Amino acid alignment of conserved region I (Cr I) of LT-Ag and Sm t-Ags of polyomaviruses (aa: 9–30). C: Amino acid alignment of HPDKGG box of polyomaviruses within the conserved region II (Cr II) (aa: 40–60). Amino acid alignments were performed according to “Match-box_server 1.3,” provided by University of Namur, Belgium.
Fig. 1.
A: Schematic representation of JCV Mad-1 early coding region. Numbering is according to JCV Mad-1 strain (GenBank # NC_001699, formerly J02226). B: Schematic representation of JCV LT-Ag and Sm t-Ag. pRB and p53 binding regions on LT-Ag is indicated. The J domains on both proteins are localized to the common region of both proteins. Two cystein clusters localized to the unique region of Sm t-Ag. An Amino acid cluster, CknwPeC, is also present in JCV, which may be important for PP2A binding.
The unique region of SV40 Sm t-Ag contains cysteine-rich sequences, grouped into two CxCxxC clusters, which appear to bind two Zn ions and are important for providing conformational stability to Sm t-Ag (Goswami et al., 1992; Turk et al., 1993) (Fig. 1). For instance, mutation within any of these cysteines results in a high turnover rate of Sm t-Ag (Jog et al., 1990). There is also another functionally important group of amino acids, CknwPeC, (amino acids 97–103) which are located within the unique region of Sm t-Ag. Of the sequences, cysteine and proline residues mediate the interaction between Sm t-Ag and its major downstream effector protein phosphatase-2A (PP2A), as described below (Mungre et al., 1994). These two cysteine clusters and CknwPeC sequences are also present within the unique region of JCV and BKV Sm t-Ag, which suggests that they function similarly as observed for SV40 Sm t-Ag (Fig. 1).
J Domain and Chaperone Function of Sm t-Ag
Living organisms protect themselves against environmental insults and invaders by inducing protective mechanisms of their own. These include chaperones that have unique activities in living organisms, which have been identified from bacteria to humans. Chaperone proteins are those that promote the proper folding of proteins and prevent their aggregation during the cellular stress (Hartl, 1996). One of the first chaperone molecules that was identified in bacteria belongs to heat shock class (Hsc) of proteins. These include Hsc60 family of proteins and the DnaK/DnaJ families of co-chaperones. DnaK is a member of the Hsc70 family of chaperones. Hsc70 family members contain a long amino terminal ATPase domain, a substrate binding domain and a carboxy-terminal variable domain. An intrinsic ATPase activity is associated with Hsc70 itself, but in the presence of co-chaperones and peptide substrates, the ATPase activity of Hsc70 increases dramatically (Karzai and McMacken, 1996; Misselwitz et al., 1998).
The J proteins and DnaJ are co-chaperone regulators. They promote the ATPase activity of Hsc70 and stimulate substrate interactions with Hsc70s (Silver and Way, 1993; Cyr et al., 1994; Kelley and Landry, 1994; Kelley, 1998). J proteins have also been implicated in the modulation of the phosphorylation state of their own substrates (Yaglom et al., 1996). A J protein contains an approximately 70 amino acid domain that binds directly to the Hsc70, which is known as the J domain (Fig. 1B). Crystal structure analyses and NMR studies of the J domains of the E. coli DnaJ, human HDJI, polyomavirus LT-Ag and SV40 LT-Ag revealed that the structure of a J domain is composed of the three or four alpha helices, where helices II and III are specifically linked by universally conserved amino acids, HPD. These three amino acids directly contact Hsc70 (Huang et al., 1999). J proteins regulate the function of their targets, including Hsc70 by recruiting and/or stabilizing the substrates into a complex, where the ATPase activity of Hsc70 is highly stimulated. The energy gained by ATP hydrolysis is used for the proper folding and transport of the target proteins. These activated targets in turn activate the specific protein signaling molecules.
The requirement of the J domain of LT-Ag in viral replication and cell transformation was investigated by mutational analysis as well as by use of chimeric constructs. The replacement of the J domain of the SV40 LT-Ag with the human HSJI J domain demonstrated an essential role for the J domain in viral DNA replication (Pipas et al., 1983; Campbell et al., 1997). Although the molecular mechanism by which the J domain exerts its role in viral DNA replication is unknown, it was suggested that this domain has either direct or indirect effects on the viral replication cycle. In a direct model, it is thought that the J domain is involved in the formation of the replication machinery that drives DNA replication in a manner analogous to the replication of bacteriophage lambda DNA. In an indirect model, it has been suggested that the J domain may be required to disrupt the chromatin complexes that are inhibitory to DNA replication through the recruitment of Hsc70. Alternatively, the J domain may be indirectly involved in viral replication through promoting the progression of the cell cycle into S-phase and hence allowing the induction of the cellular enzymes that are involved in DNA replication, including DNA polymerase alpha and thymidine kinase.
The role of J domain in transformation was also investigated by mutational analysis. Various mutants of this domain including a short deletion (d17–27) or amino acid substitution, D44N mutant, exhibited various degrees of penetration for transformation (Chen and Paucha, 1990; Marsilio et al., 1991; Sullivan et al., 2000, 2001). While the d17–27 mutant is 100% defective in transformation, the D44N mutant is capable of transformation but in a partially defective manner (Srinivasan et al., 1989, 1997; Zhu et al., 1992). A reason why a base substitution mutant (D44N) is less effective than the deletion (d17–27) mutant is that the D44N substitution may only partially disrupts the J domain function, while the deletion mutant may completely abolish its activity. Another possibility is that the deletion mutant causes a gross structural abnormality in LT-Ag protein, which could in turn affect the other transforming activities of LT-Ag that are associated with the N-terminal or C-terminal region of the protein.
Role of Sm t-Ag in the Viral Life Cycle
Small DNA viruses are absolutely dependent on the S phase of the cell cycle for their genomic replication and certain viral regulatory proteins are required for the S phase entry, through which they contribute to the viral replication cycle. For example, LT-Ag of JCV, BKV, and SV40 is required for the initiation of viral DNA replication (Lynch and Frisque, 1990, 1991; Frisque and White, 1992; Ranganathan and Khalili, 1993; Lynch et al., 1994) and without it, the viral genome cannot replicate. There is also experimental evidence supporting the involvement of Sm t-Ag in the viral life cycle. SV40 Sm t-Ag, for instance, has been shown to have a transregulatory activity on promoters transcribed by RNA polymerase II and III (Loeken et al., 1988). In this regard, mouse polyomavirus Sm t-Ag was also found to transactivate these two promoters (Cavender et al., 1999). These observations suggest the possibility that Sm t-Ag, like LT-Ag, may transactivate the promoters of the cellular genes that are involved in the successful completion of the viral replication cycle. SV40 Sm t-Ag was also shown to activate some other cellular promoters, including the cyclin A and cyclin D promoter (Yang et al., 1979; Watanabe et al., 1996). It appears that Sm t-Ag exerts this function indirectly, because it does not have DNA binding activity. The ability of Sm t-Ag to activate cellular promoters maps to its J domain, suggesting that a cluster of sequences that are important for the function of J domain also appears to be critical for its transactivation function (Porras et al., 1996).
Although Sm t-Ag is not strictly required for the viral infection cycle in tissue culture, mutants of it are defective and grow slowly (Sugano et al., 1982). This suggests the possibility that Sm t-Ag may be only important during certain segments of the viral infection cycle perhaps when the level of LT-Ag is low in infected cells. The role of LT-Ag in regulation of SV40 and JCV promoters is clear in that it activates the viral late promoter but suppresses the early promoter (Jat et al., 1986; Matthews et al., 1987; DeCaprio et al., 1988; Chowdhury et al., 1990; Pipas, 1992; Zerrahn et al., 1993). In this respect, however, the function of Sm t-Ag was not properly analyzed. In an attempt to clarify this issue, Bikel and Loeken (1992) created different deletion mutants of Sm t-Ag and used them in transactivation studies. Full length Sm t-Ag was shown to increase the early promoter activity of SV40, but had no effect on the viral late promoter. Interestingly, Sm t-Ag was observed to have an additive effect on LT-Ag-mediated activation of the late promoter (Bikel and Loeken, 1992). Recently, in attempt to investigate the effect of SV40 Sm t-Ag on host cell gene expression, Moreno et al., performed a microarray analysis on human embryonic kidney cells expressing SV40 Sm t-Ag. It turns out that Sm t-Ag influences the expression of a quite number cells that are highly relevant to cell survival, motility, metastasis and cancer growth (Moreno et al., 2004) suggesting that Sm t-Ag promotes the expression of the host genes that are involved in survival while inhibiting those that are required for cell—cell adhesion pathways.
There is no published report regarding the analysis of the function of JCV Sm t-Ag in viral replication cycle. In an attempt to analyze its function, we introduced a stop codon at Ser 90 immediately after splice donor site of the JCV early gene so that the unique portion of Sm t-Ag is not produced. Subsequently we investigated the functional consequences of this mutation. The ability of this mutant virus with respect to replication and gene expression was substantially reduced compared to WT (Sariyer et al., unpublished work), which is consistent with the findings from SV40 Sm t-Ag studies. It should be mentioned here that the splicing of the viral early transcripts normally occurred after introduction of the stop codon, which indicates that the observed reduction in viral gene expression and replication cannot be attributed to the introduction of a stop codon, but rather to the functional ability of the C-terminal unique region of Sm t-Ag.
Sm t-Ag Promotes S Phase Entry
Evidence that Sm t-Ag promotes cell cycle progression prompted investigators to examine the known biochemical mediators in this process (Zaitsu and Kimura, 1988; Whalen et al., 1999). Recombinant adenovirus expression system that expresses the regulatory proteins of SV40 including Sm t-Ag and LT-Ag was used to study the growth promoting pathways of Sm t-Ag (Howe et al., 1998). Most of these studies were performed with human diploid fibroblasts, a cell type that exhibits tight growth arrest properties by contact inhibition. They do not re-enter the cell cycle even following the addition of fresh medium. As a result, these cells serve as a model system to examine SV40-induced cell transformation, because escape from density arrest is a key step in focus formation.
It is known that the growth of the cells is critically controlled by the cyclin-dependent kinase inhibitors (cdki). The Cdk inhibitor p27/Kip1 (Polyak et al., 1994; Hengst and Reed, 1996; Winston et al., 1996; Kato et al., 1997) and p53-induced p21/Waf1 (el-Deiry et al., 1993) were found to be differentially targeted by SV40 transforming proteins. It was observed that the expression levels of p27/Kip1 are reduced in confluent HDF cells expressing Sm t-Ag (Porras et al., 1999; Schuchner and Wintersberger, 1999; Whalen et al., 1999; Helmbrecht et al., 2000; Klucky et al., 2004). Similarly, p21/Waf1 was also found to be significantly lower in cells expressing LT-Ag (Porras et al., 1999). The mechanisms by which SV40 regulatory proteins (LT-Ag and Sm t-Ag) target cdkis are not fully understood. However, it is apparent that LT-Ag indirectly targets p21/Waf1 through the binding and inhibition of p53, which is a transcription factor that regulates p21/Waf1 levels in cells. To explain the effect of Sm t-Ag on p27/Kip1 levels, a theoretical mechanism was proposed, which is the possibility that Sm t-Ag exerts its inhibitory activity indirectly by inhibiting the dephosphorylation activity of PP2A. It is known that the level of p27/Kip1 is regulated by its phosphorylation by cyclinE/cdk2 complex. When inhibited, PP2A can no longer fulfill its dephosphorylation activity properly during cell cycle progression. This then leads to a rapid degradation of p27/Kip1 by ubiquitination and proteasome degradation (Sheaff et al., 1997) and cells undergo G/S phase transition more rapidly under the influence of Sm t-Ag.
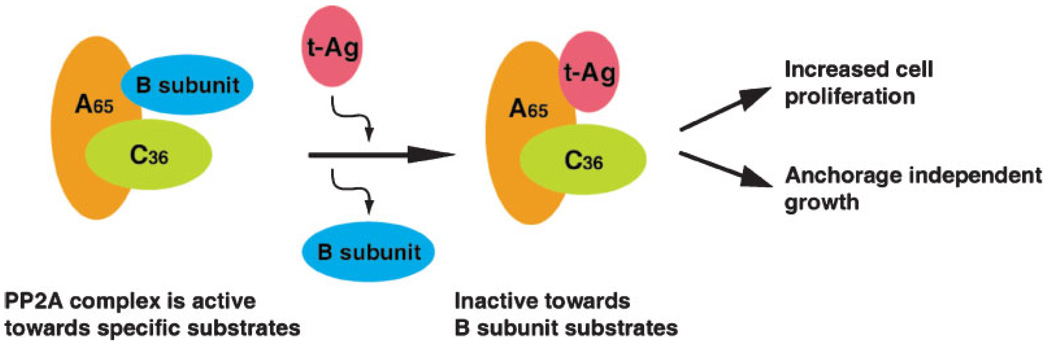
Studies showed that the J domain and the PP2A binding domain of Sm t-Ag are both required for its function in cell cycle progression, because mutations within these two regions differentially affect the Sm t-Ag mediated cell cycle progression (Ruediger et al., 1992). That is, a mutation at the cysteine 103 totally inactivates the effect of Sm t-Ag on cell cycle progression, but a mutation in J domain, although greatly reduced in its effect, does not totally inactivate the Sm t-Ag-mediated effect on cell cycle progression. It was also interesting to observe that cells can enter S phase more quickly when they are co-transfected with the above mutants (mut 43 and mut 103) (Ruediger et al., 1992). The influence of Sm t-Ag on cell cycle progression is linked to the interaction of this protein with one of the important cellular phosphatase in cells, PP2A. PP2A is one of the major serine/threonine phosphatases in mammalian cells and is critical for many biological processes, including development, differentiation and growth control (Janssens and Goris, 2001). This enzyme exists as a complex composed of a core subunit and a variable subunit. A cartoon of PP2A structure is shown in Figure 3. The core enzyme consists of a catalytic C subunit (~36 kDa) and a regulatory A subunit (~65 kDa). The variable unit exists as different isoforms but generally fall into three families designated B, B′, and B″ (Van Hoof and Goris, 2004; Janssens et al., 2005). This large number of variable subunits allows the formation of numerous but functionally distinct substrate-specific forms of PP2A.
Fig. 3.
Targeting of PP2A by Sm t-Ag. Sm t-Ag replaces B subunit on PP2A complex and inactivates it towards its substrates.
PP2A can be associated with and regulated by a number of cellular and viral proteins. For example, Tap42/a4, an important player in the TOR pathway, forms a complex with PP2Ac, independent of other two subunits and inhibits its function (Jacinto et al., 2001; Jacinto and Hall, 2003). Viruses have developed distinct strategies to deregulate cells via PP2A. Some viruses simply incorporate either PPA2 holoenzymes or downstream components of the PP2A signaling pathway. Human cytomegalovirus, for example, carries a cellular PP2A holoenzyme probably to shut-off the early cellular phosphorylation in infected cells (Michelson et al., 1996). The HIV regulatory protein, Vpr was also found to modulate PP2A activity when it complexes with the host cellular protein NCp7 and enhances the phosphatase activity of PP2A towards a model substrate (Goh et al., 1998). The tumor viruses, including polyomavirus middle T and SV40 small t antigen also target PP2A and modulate its activity (Pallas et al., 1990; Yang et al., 1991; Janssens et al., 2005).
The interaction domains of Sm t-Ag with PP2A was defined by truncated (Sontag et al., 1993) and point mutants in the CxxxPxC motif (Mungre et al., 1994). A mutation at the first cysteine severely reduced (~10-fold) the interaction of Sm t-Ag with PP2A compared to other single mutations at the proline or other cysteine residue. A combination of mutations was also further affected this interaction. Several cellular kinases are more active in cells expressing Sm t-Ag, including MAPK, ERK1, JNK and a key ion transporter, the Na/H antiporter (Sontag et al., 1993). It was also found that AP-1-driven promoter constructs are more active in Sm t-Ag expressing cells (Frost et al., 1994). These observations suggest that Sm t-Ag stimulates the intracellular kinases that are involved in growth promoting pathways.
Role of Sm t-Ag in Cell Transformation
It is known that SV40 induces cell transformation in tissue culture and in experimental animals. Without Sm t-Ag, the ability of SV40 to transform cells is significantly reduced (Bouck et al., 1978; Sleigh et al., 1978). For example, SV40 cannot form foci in soft agar without Sm t-Ag, although it can do so in adherent cultures. In other cell types, including rat 3Y1 and human diploid fibroblasts, Sm t-Ag is required for focus formation as well (Sugano et al., 1982; Chang et al., 1985; Porras et al., 1996). It appears that Sm t-Ag is critical for the initial stages of transformation process, during which it optimizes the ability of LT-Ag to carry out the transforming activities that are required for transformed phenotype. In some cases, the need for Sm t-Ag is less apparent, if the expression level of LT-Ag is high. In such cases, however, LT-Ag may direct the cell transformation itself by influencing the Rb/E2F pathways (Jat et al., 1986; Bikel et al., 1987).
It was demonstrated that Sm t-Ag enhances the transforming ability of SV40 LT-Ag (Sontag et al., 1993; Porras et al., 1996, 1999; Ali and DeCaprio, 2001; Gaillard et al., 2001; Rundell and Parakati, 2001; Chen and Hahn, 2003; Nunbhakdi-Craig et al., 2003; Zhao et al., 2003; Cho et al., 2007). It is also believed that Sm t-Ag antagonizes LT-Ag-induced cellular apoptosis and thereby contributes to more efficient transformation of rat embryo fibroblasts (Kolzau et al., 1999). In addition, transgenic animals created with a Sm t-Ag deletion mutant of SV40 genome developed tumors almost exclusively in highly mitotic tissues such as lymphoid organs, suggesting that Sm t-Ag may play an important role in tumor induction in nonproliferative tissues (Choi et al., 1988; Carbone et al., 1989). In particular, Hahn et al. demonstrated that SV40 Sm t-Ag is absolutely required for the full transformation of human cells (Hahn et al., 2002). In this study, it was demonstrated that an oncogenic allele of the H-Ras gene, telomerase catalytic subunit (hTERT) and SV40 large T antigen were shown to be not sufficient to transform normal human fibroblast, kidney epithelial and mammary epithelial cells. However, expression of Sm t-Ag in this system let these cells undergo to a tumorigenic state. These findings clearly suggest that Sm t-Ag is required for transformation of the human cells (Hahn and Meyerson, 2001; Hahn, 2002; Hahn et al., 2002). The precise mechanism by which Sm t-Ag contributes to the tumorigenic state is not known. However, the targeting and inhibition of PP2A by Sm t-Ag were suggested to be, at least in part, one of the mechanisms that would account for this event (Sontag et al., 1993; Ali and DeCaprio, 2001; Rundell and Parakati, 2001; Cho et al., 2007). Concomitant crystallization of PP2A and Sm t-Ag appears to support this notion in that the J domain and unique domain of Smt-Ag were both recently found to be in contact with PP2A, strongly arguing in favor of inhibition of PP2A by Sm t-Ag (Chen et al., 2007; Cho et al., 2007). Moreover, a recent report indicates that Sm t-Ag may also function as a transfer factor to specify PP2A targeting in cells and thereby modulate the transcriptional activity of cellular genes including the androgen receptor (Yang et al., 2005), which might be also one of the ways that Sm t-Ag may contribute to the cell transformation.
JCV LT-Ag was also shown to have an oncogenic potential and can induce tumors of neuronal origin in experimental animals (Varakis et al., 1978; Small et al., 1986a; Krynska et al., 1999b). The function of JCV Sm t-Ag in these processes is currently unknown.
Sm t-Ag and mTOR Pathway
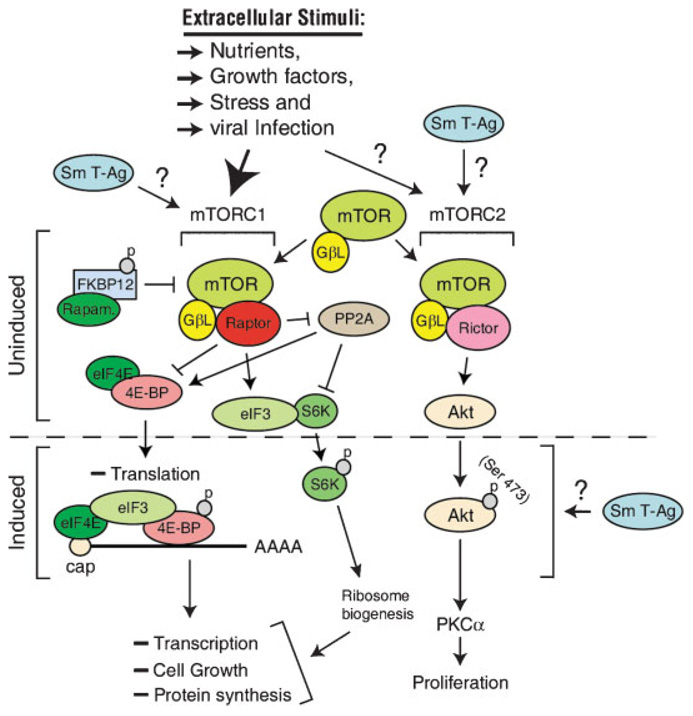
Mammalian target of rapamycin (mTOR) is a Ser/Thr-specific protein kinase that controls many aspects of cellular physiology, including transcription, translation, cell size, and autophagy (Sarbassov et al., 2004; Reiling and Sabatini, 2006). mTOR exists in two heteromeric complexes mTORC1 and mTORC2 (Fig. 4). The activity of mTORC1 is regulated by a diverse array of upstream signals, including growth factors, nutrients, cellular stress and viral infection; and is inhibited by Rapamycin (Corradetti et al., 2004; Corradetti and Guan, 2006). In contrast, the physiologic regulators of mTORC2 have yet to be defined and it is insensitive to rapamycin inhibition. The best-described function of mTOR is its regulation of translation. One mechanism by which mTOR regulates translation is the following. Upon activation by stimuli, it phosphorylates the downstream effector translational control proteins, including a p70 ribosomal protein S6 kinase (S6K) and initiation factor 4E binding protein 1 (4E-BP1). Both effectors inhibit translation by binding initiation factor 3 (eIF3) and initiation factor 4 (eIF4E) respectively (Fig. 4). Upon phosphorylation by mTOR, these two inhibitors (S6K and 4E-BP1) are released from eIF3 and eIF4E and translation proceeds (Mamane et al., 2006). Although the stimulators of mTORC2 are not known, it was recently reported that it phosphorylates and activates Akt at Ser473 (Sarbassov et al., 2004). PP2A is also a player in this pathway. It is inhibited by mTORC1 but activates 4E-BP. It also inhibits S6K as indicated in Figure 4.
Fig. 4.
mTOR signaling pathway. This pathway is stimulated by a number of extracellular stimuli, including nutrients, growth factors, stress and viral infection. Signaling generated by these stimuli is relayed to downstream effectors by the mTORC1 complex. The mTORC1 can be inhibited by rapamycin treatment. The upstream activators of the mTORC2 complex however are currently unknown and mTORC2 is insensitive to rapamycin treatment. Upon activation, the mTORC1 complex phosphorylates 4E-BPI and S6K resulting in their dissociation from the cap-binding protein, eIF4E, and initiation factor 3(eIF3)respectively. This signals to impact downstream events including ribosome biogenesis, translation and cell growth. The effector molecules of mTOR pathway are targeted and modified by many viruses to preferentially translate their own mRNA as explained in the text. The Sm t-Ag of polyomaviruses also targets this pathway at different levels as indicated.
Many viruses have developed diverse and elaborate mechanisms to target this signaling pathway and to suppress cellular mRNA translation through which they exploit the host translational machinery for their own advantage. There are many examples for such strategies developed by viruses. Picornaviruses, for example, including rhinovirus, coxsackie virus and foot-and-mouth disease virus inhibit cellular protein synthesis while simultaneously translating their own mRNAs (Schneider and Mohr, 2003) by targeting and cleaving the host cap-binding protein, eIF4E, through their own encoded protease known as 2A-pro. eIF4 then can no longer bind to mRNA and regulate translation but they allow initiation of the translation of their own mRNA in a 5′CAP-independent manner (Ali et al., 2001). Similarly, adenovirus was also shown to inhibit translation of host-cell mRNAs but efficiently promote translation of their own mRNAs during the late phase of infection through alterations in the host cap-initiation complex and through redirecting the initiation of their own mRNA by a mechanism known as ribosome shunting (Yueh and Schneider, 1996, 2000). Herpes viruses was also shown to induce alterations in translation initiation complexes in quiescent cells by phosphorylation of eIF4E by mink-1 and by promoting assembly of an active translation initiation complex of its own (Yueh and Schneider, 1996, 2000). Recently, SV40 was also reported to induce dephosphorylation of 4E-BP1 late during the infection cycle through which it modifies the translation machinery for its own use (Yu et al., 2005) through its Sm t-Ag. It is also possible that JCV Sm t-Ag may affect the mTOR signaling pathways and its effector molecules at different levels as indicated in the Figure 4, through which it may influence the tumorigenic pathways.
Sm t-Ag and ARF-p53 Pathway
In order to facilitate their own replication, small DNA tumor viruses subvert many of the pivotal growth regulatory pathways within the cells by targeting specific pathways. The p53 tumor suppressor pathway, for example, is a critical cellular defense against neoplastic transformation. Genotoxic stress or viral infection can activate p53, which then induces the transcription of downstream effectors that prevent cell cycle progression or induce apoptosis. Triggering of a p53 response in viral-infected cells, therefore, could terminate the viral life cycle. In order to overcome such an obstacle, DNA tumor viruses have developed different strategies to inactivate this pathway. Indeed, p53 was first identified as a cellular protein that formed a complex with SV40 LT-Ag (Lane and Crawford, 1979; Linzer and Levine, 1979). p53 can be inactivated either through a mutation in the genome or through loss of ARF, a critical upstream regulator (Sherr, 2004; Sherr and McCormick, 2002). ARF is designated as the Alternative Reading Frame of the p16INKA gene in the INK4a locus (Quelle et al., 1995) and is not induced in normal cellular proliferation. Activated oncogenes or unscheduled cell proliferation induce the ARF expression, which in turn prevents the MDM2-mediated degradation of p53 (Zindy et al., 2003). Recent developments in PyV infection cycle suggest that both PyV middle t antigen (PyMT t-Ag) and PyV small t antigen (PyMT t-Ag) have differential effects on the ARF-p53 pathway. That is, while PyMT t-Ag inhibits ARF-mediated up-regulation of p53, PyMT-Ag activates the ARF-p53 tumor suppressor pathway (Lomax and Fried, 2001).
Although the mechanism whereby the small DNA virus infections activate the p53 pathways is not precisely known, several aspects of the viral infection cycle can be influential in this regard. For instance, p53 can be activated by a DNA damage response triggered by the extensive DNA replication or unusual DNA structure/intermediates of viral genomes. This subsequently can trigger the DNA damage repair machinery. Concurrently, potential DNA breaks may also occur in infected cells causing the activation of ATM, which in turn results in the phosphorylation and induction of p53 (Motoyama and Naka, 2004). Aberrant ARF proliferative signals that drive viral DNA replication can also activate p53 pathways.
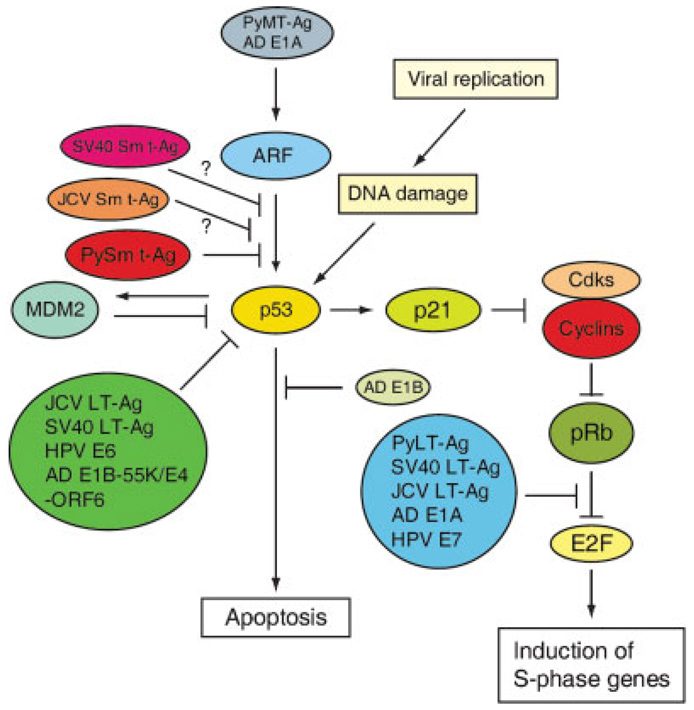
Unlike the large DNA viruses, small DNA tumor viruses have limited genetic capacity and do not specify the DNA replication enzymes required for their propagation. As such, small DNA viruses have evolved common strategies to induce an unscheduled S phase entry by which they replicate their own DNA while the host DNA is also being replicated. They induce an unscheduled S phase entry, in part by targeting the Rb/E2F complex. This complex is critically regulated by cyclin-dependent kinase complexes in normal cell proliferation (Trimarchi and Lees, 2002). In small DNA virus-infected cells however, virus-encoded proteins, including PYLT-Ag, SV40 LT-Ag, JCV LT-Ag, HPE7, and AdeE1, target this complex and inactivate it, irrespective of Cdk activation. E2F is then released from Rb and activates S phase specific genes that are critical for DNA replication (Chellappan et al., 1992). This unscheduled S phase entry can then also induce the activation of p53 (Fig. 5).
Fig. 5.
Effect of small DNA viruses on the ARF-p53 pathway. Different viral proteins from DNA tumor viruses affect the ARF-p53 pathway both negatively and positively. Genotoxic and oncogenic stress created by small DNA virus infections can induce p53 activation, which in turn results in the activation of downstream effectors including p21/Waf1 and MDM2. If the DNA damage created by the stress is extensive and irreparable, cells undergo apoptosis. If the DNA damage is mild, the p53 pathway induces a cell cycle arrest through p21/Waf1 until the DNA damage is repaired. Middle t antigen (PyMT-Ag) from murine polyomavirus, and immediate early protein (E1A) protein from adenovirus can induce the ARF-p53 pathway, which results in cell cycle arrest/apoptosis. Therefore, different viruses have evolved a number of strategies to inactivate the p53 pathway. JCV LT-Ag, SV40 LT-Ag HPV E6, and AD E1A-55K/E4-ORF6 can directly bind to p53 and inactivate it. AD E1B-19K inhibits p53-induced apoptosis. JCV LT-Ag, SV40 LT-Ag HPV E7, and AD E1A bind and inactivate pRB and thus bypass the p21/Waf1-induced cell cycle arrest. PySm t-Ag inhibits ARF-mediated activation of p53 by targeting PP2A. Sm t-Ag from SV40 and JCV may also inactivate the same pathway by targeting PP2A.
ARF is itself a direct target of E1A and PyMT-Ag. These proteins directly bind to ARF and this binding consequently leads to the activation of p53 (Fig. 5). In addition, E2F targets ARF but in normal cellular proliferation, this protein is not induced. In the case of aberrant proliferative signals and activated oncogenes, this protein is significantly induced (Aslanian et al., 2004; Zindy et al., 2003). ARF is activated by viral oncoprotein PyMT-Ag, although this protein does not have an intrinsic enzymatic activity. It binds and modulates a number of cellular proteins that are involved in growth promoting signaling, including non-receptor tyrosine kinases (e.g., c-src), PI3 kinase, PLC-γ, and the Ras-Raf-MAP kinase pathway. Inappropriate activation of one or a combination of these growth stimulatory pathways by PyMT-Ag causes the activation of AFR and consequently induces the p53 checkpoint.
A viral infection can trigger p53 activation at multiple levels. This event could halt the viral life cycle unless delicately regulated. That is, p53 activation could inhibit DNA replication or induce the premature death of infected cells, resulting in an abortive infection. The small tumor DNA viruses appear to have developed interesting strategies to inactivate p53. How do these small DNA viruses prevent this negative effect coming from p53 signaling? The short answer to this question is by directly targeting p53. There are a number of viral-encoded proteins from different viruses, which directly bind and inactivate p53 (Fig. 5). These include JCV LT-Ag (Bollag et al., 1989), BKV LT-Ag (Shivakumar and Das, 1996), SV40 LT-Ag (Lane and Crawford, 1979; Linzer and Levine, 1979) Adenovirus E1B-55K and E4-ORF6 (Harada and Berk, 1999; Querido et al., 1997, 2001) and HPV E7 protein (Huibregtse et al., 1991; Scheffner et al., 1990, 1992).
Mouse polyomavirus, although related to SV40, JCV, and BKV, is an unusual virus among the small DNA tumor viruses in that none of its tumor antigen proteins (PyLT, PyMT, and PyST) directly binds to p53 (Dilworth, 1990). This was a very surprising finding. Then the question became what are the mechanisms by which PyV is able to prevent the harmful effect of p53 activation? It appears that PyV accomplishes this primarily by inhibiting ARF-mediated activation of p53 by its own tumor antigen, PySm t-Ag (Moule et al., 2004), suggesting that PySm t-Ag plays a critical role in protecting cells from apoptosis initiated by activated p53. In the case of other small DNA virus infections, including JCV and SV40, although p53 is directly targeted by their own LT-Ag, their Sm t-Ags, as is the case of PySm t-Ag, may also play a protective role by preventing the harmful effect of p53 activation through a direct inhibition of ARF.
Conclusion
In this short review, we touched upon the structural and functional characteristics of SV40 Sm t-Ag, JCV Sm t-Ag, and PyVSm t-Ag. Over the years, we have learned much with respect to the roles of SV40 and PyV Sm t-Ag in cell transformation. However, we need to find new targets of these tumorigenic proteins in the cell and study their function to further understand the process of cell transformation. In addition, there is virtually no published research on the function of JCV Sm t-Ag with respect to viral life cycle and tumor progression. Since JCV Sm t-Ag shows a high sequence homology to both SV40 and PyV Sm t-Ags at the amino acid level, it is likely that JCV Sm t-Ag functions in a similar fashion as indicated by the data obtained for these two tumorigenic proteins. However, it is still important to establish the functions of JCV Sm t-Ag experimentally. As such, there is a need for a new research for understanding of the function of JCV Sm t-Ag at the level of viral life cycle as well as that of in cell transformation. Such an effect will shed on light on the biology of JCV and address many questions surrounding whether JCV is involved in tumor induction in humans. Understanding the functions of JCV Sm t-Ag may also be important in the development of new therapeutic strategies to inhibit JCV replication which is the proven cause of the fatal demyelinating disease, progressive multifocal leukoencephalopathy.
Acknowledgments
We would like to thank the members of the Center for Neurovirology for sharing of ideas and reagents, particularly to Dr. M. White for insightful discussion. This work was made possible by grants awarded by NIH to KK and MS.
Contract grant sponsor: National Institute of Neurological Disorders and Stroke (NINDS);
Contract grant number: RO1 NS43108.
Contract grant sponsor: NIH.
Literature Cited
- Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: Interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- Ali IK, McKendrick L, Morley SJ, Jackson RJ. Truncated initiation factor eIF4G lacking an eIF4E binding site can support capped mRNA translation. EMBO J. 2001;20:4233–4242. doi: 10.1093/emboj/20.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanian A, Iaquinta PJ, Verona R, Lees JA. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 2004;18:1413–1422. doi: 10.1101/gad.1196704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR. Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: Explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions. J Neurovirol. 2003;9:38–41. doi: 10.1080/13550280390195261. [DOI] [PubMed] [Google Scholar]
- Berger JR, Houff S. Progressive multifocal leukoencephalopathy: Lessons from AIDS and natalizumab. Neurol Res. 2006;28:299–305. doi: 10.1179/016164106X98198. [DOI] [PubMed] [Google Scholar]
- Bikel I, Loeken MR. Involvement of simian virus 40 (SV40) small t antigen in trans activation of SV40 early and late promoters. J Virol. 1992;66:1489–1494. doi: 10.1128/jvi.66.3.1489-1494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I, Montano X, Agha ME, Brown M, McCormack M, Boltax J, Livingston DM. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987;48:321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Bollag B, Chuke WF, Frisque RJ. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: Identification of sequences important for efficient transformation. J Virol. 1989;63:863–872. doi: 10.1128/jvi.63.2.863-872.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag B, Kilpatrick LH, Tyagarajan SK, Tevethia MJ, Frisque RJ. JC virus T′ 135, T′ 136 and T′ 165 proteins interact with cellular p107 and p130 in vivo and influence viral transformation potential. J Neurovirol. 2006;12:428–442. doi: 10.1080/13550280601009553. [DOI] [PubMed] [Google Scholar]
- Bouck N, Beales N, Shenk T, Berg P, di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci USA. 1978;75:2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N, Fikes J, Rundell MK. Large-T-antigen-p53 complex formation is not cold sensitive in a cold-sensitive transformant induced by simian virus 40 mutant ts A1499. J Virol. 1984;49:997–1001. doi: 10.1128/jvi.49.3.997-1001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Pipas JM. Polyomavirus T antigens: Molecular chaperones for multiprotein complexes. J Virol. 1998;72:5329–5334. doi: 10.1128/jvi.72.7.5329-5334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel JS. SV40 large T-antigen: Dual oncogene. Cancer Surv. 1986;5:343–365. [PubMed] [Google Scholar]
- Butel JS, Lednicky JA. Cell and molecular biology of simian virus 40: Implications for human infections and disease. J Natl Cancer Inst. 1999;91:119–134. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- Butel JS, Jafar S, Stewart AR, Lednicky JA. Detection of authentic SV40 DNA sequences in human brain and bone tumours. Dev Biol Stand. 1998;94:23–32. [PubMed] [Google Scholar]
- Butel JS, Arrington AS, Wong C, Lednicky JA, Finegold MJ. Molecular evidence of simian virus 40 infections in children. J Infect Dis. 1999;180:884–887. doi: 10.1086/314915. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Mullane KP, Aksoy IA, Stubdal H, Zalvide J, Pipas JM, Silver PA, Roberts TM, Schaffhausen BS, DeCaprio JA. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- Carbone M, Lewis AM, Jr, Matthews BJ, Levine AS, Dixon K. Characterization of hamster tumors induced by simian virus 40 small t deletion mutants as true histiocytic lymphomas. Cancer Res. 1989;49:1565–1571. [PubMed] [Google Scholar]
- Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, Mew DJ, Levine AS, Procopio A. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- Carbone M, Rizzo P, Procopio A, Giuliano M, Pass HI, Gebhardt MC, Mangham C, Hansen M, Malkin DF, Bushart G, Pompetti F, Picci P, Levine AS, Bergsagel JD, Garcea RL. SV40-like sequences in human bone tumors. Oncogene. 1996;13:527–535. [PubMed] [Google Scholar]
- Cavender JF, Mummert C, Tevethia MJ. Transactivation of a ribosomal gene by simian virus 40 large-T antigen requires at least three activities of the protein. J Virol. 1999;73:214–224. doi: 10.1128/jvi.73.1.214-224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LS, Pater MM, Hutchinson NI, di Mayorca G. Transformation by purified early genes of simian virus 40. Virology. 1984;133:341–353. doi: 10.1016/0042-6822(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Chang LS, Pan S, Pater MM, Di Mayorca G. Differential requirement for SV40 early genes in immortalization and transformation of primary rat and human embryonic cells. Virology. 1985;146:246–261. doi: 10.1016/0042-6822(85)90008-x. [DOI] [PubMed] [Google Scholar]
- Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hahn WC. SV40 early region oncoproteins and human cell transformation. Histol Histopathol. 2003;18:541–550. doi: 10.14670/HH-18.541. [DOI] [PubMed] [Google Scholar]
- Chen S, Paucha E. Identification of a region of simian virus 40 large T antigen required for cell transformation. J Virol. 1990;64:3350–3357. doi: 10.1128/jvi.64.7.3350-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xu Y, Bao Q, Xing Y, Li Z, Lin Z, Stock JB, Jeffrey PD, Shi Y. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nature Struct Mol Biol. 2007;14:527–534. doi: 10.1038/nsmb1254. [DOI] [PubMed] [Google Scholar]
- Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Structural basis of PP2A inhibition by small t antigen. BLos Biol. 2007;5:1–10. doi: 10.1371/journal.pbio.0050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YW, Lee IC, Ross SR. Requirement for the simian virus 40 small tumor antigen in tumorigenesis in transgenic mice. Mol Cell Biol. 1988;8:3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M, Taylor JP, Tada H, Rappaport J, Wong-Staal F, Amini S, Khalili K. Regulation of the human neurotropic virus promoter by JCV-T antigen and HIV-1 tat protein. Oncogene. 1990;5:1737–1742. [PubMed] [Google Scholar]
- Cole CN. Polyomaviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fundemental virology. Philadelphia: Lippincott, Williams and Wilkins; 1996. pp. 917–946. [Google Scholar]
- Corallini A, Altavilla G, Carra L, Grossi MP, Federspil G, Caputo A, Negrini M, Barbanti-Brodano G. Oncogenity of BK virus for immunosuppressed hamsters. Arch Virol. 1982;73:243–253. doi: 10.1007/BF01318078. [DOI] [PubMed] [Google Scholar]
- Corallini A, Pagnani M, Viadana P, Silini E, Mottes M, Milanesi G, Gerna G, Vettor R, Trapella G, Silvani V, et al. Association of BK virus with human brain tumors and tumors of pancreatic islets. Int J Cancer. 1987;39:60–67. doi: 10.1002/ijc.2910390111. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: Do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LK B1: Evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DM, Langer T, Douglas MG. DnaJ-like proteins: Molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Daniels R, Rusan NM, Wadsworth P, Hebert DN. SV40 VP2 and VP3 insertion into ER membranes is controlled by the capsid protein V P1: Implications for DNA translocation out of the ER. Mol Cell. 2006a;24:955–966. doi: 10.1016/j.molcel.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Daniels R, Rusan NM, Wilbuer AK, Norkin LC, Wadsworth P, Hebert DN. Simian virus 40 late proteins possess lytic properties that render them capable of permeabilizing cellular membranes. J Virol. 2006b;80:6575–6587. doi: 10.1128/JVI.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R, Sadowicz D, Daniel NH. A very late viral protein trigers the lytic release fo SV40. PLoS Pathogens. 2007;3:928–938. doi: 10.1371/journal.ppat.0030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, Marsilio E, Paucha E, Livingston DM. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dilworth SM. Cell alterations induced by the large T-antigens of SV40 and polyoma virus. Semin Cancer Biol. 1990;1:407–414. [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, Roth BL, Atwood WJ. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- Frisque RJ, White FA. The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. In: Roos R, editor. Molecular Neurovirology. Totowa, NJ: Humana Press, Inc.; 1992. pp. 25–158. [Google Scholar]
- Frisque RJ, Hofstetter C, Tyagarajan SK. Transforming activities of JC virus early proteins. Adv Exp Med Biol. 2006;577:288–309. doi: 10.1007/0-387-32957-9_21. [DOI] [PubMed] [Google Scholar]
- Frost JA, Alberts AS, Sontag E, Guan K, Mumby MC, Feramisco JR. Simian virus 40 small t antigen cooperates with mitogen-activated kinases to stimulate AP-1 activity. Mol Cell Biol. 1994;14:6244–6252. doi: 10.1128/mcb.14.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard S, Fahrbach KM, Parkati R, Rundell K. Overexpression of simian virus 40 small-T antigen blocks centrosome function and mitotic progression in human fibroblasts. J Virol. 2001;75:9799–9807. doi: 10.1128/JVI.75.20.9799-9807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea RL. SV40: A human pathogen? Dis Markers. 2001;17:149–151. doi: 10.1155/2001/515039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SD, Feild AM, Colleman DV, Hulme B. New human papovavirus (BK) isolated from urine after renal transplantation. Lancet. 1971;i:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gaynor AMN, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Guang W, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infection. Plos Pathogens. 2007;3:1–10. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: A mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Gordon J, Del Valle L, Otte J, Khalili K. Pituitary neoplasia induced by expression of human neurotropic polyomavirus, JCV, early genome in transgenic mice. Oncogene. 2000;19:4840–4846. doi: 10.1038/sj.onc.1203849. [DOI] [PubMed] [Google Scholar]
- Goswami R, Turk B, Enderle K, Howe A, Rundell K. Effect of zinc ions on the biochemical behavior of simian virus 40 small-t antigen expressed in bacteria. J Virol. 1992;66:1746–1751. doi: 10.1128/jvi.66.3.1746-1751.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC. Immortalization and transformation of human cells. Mol Cells. 2002;13:351–361. [PubMed] [Google Scholar]
- Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Ann Med. 2001;33:123–129. doi: 10.3109/07853890109002067. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, DeCaprio JA, Weinberg RA. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada JN, Berk AJ. p53-Independent and-dependent requirements for E1B-55K in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: A review. Cell Prolif. 2000;33:341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Hirsch HH. BK virus: Opportunity makes a pathogen. Clin Infect Dis. 2005;41:354–360. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- Hirsch HH, Drachenberg CB, Steiger J, Ramos E. Polyomavirus-associated nephropathy in renal transplantation: Critical issues of screening and management. Adv Exp Med Biol. 2006;577:160–173. doi: 10.1007/0-387-32957-9_11. [DOI] [PubMed] [Google Scholar]
- Howe AK, Gaillard S, Bennett JS, Rundell K. Cell cycle progression in monkey cells expressing simian virus 40 small t antigen from adenovirus vectors. J Virol. 1998;72:9637–9644. doi: 10.1128/jvi.72.12.9637-9644.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Flanagan JM, Prestegard JH. The influence of C-terminal extension on the structure of the “J-domain” in E. coli DnaJ. Protein Sci. 1999;8:203–214. doi: 10.1110/ps.8.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Guo B, Arndt KT, Schmelzle T, Hall MN. TIP41 interacts with TAP42 and negatively regulates the TOR signaling pathway. Mol Cell. 2001;8:1017–1026. doi: 10.1016/s1097-2765(01)00386-0. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J, Van Hoof C. PP2A: The expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Jat PS, Cepko CL, Mulligan RC, Sharp PA. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986;6:1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog P, Joshi B, Dhamankar V, Imperiale MJ, Rutila J, Rundell K. Mutational analysis of simian virus 40 small-t antigen. J Virol. 1990;64:2895–2900. doi: 10.1128/jvi.64.6.2895-2900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzai AW, McMacken R. A bipartite signaling mechanism involved in DnaJ-mediated activation of the Escherichia coli DnaK protein. J Biol Chem. 1996;271:11236–11246. doi: 10.1074/jbc.271.19.11236. [DOI] [PubMed] [Google Scholar]
- Kato A, Takahashi H, Takahashi Y, Matsushime H. Inactivation of the cyclin D-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J Biol Chem. 1997;272:8065–8070. doi: 10.1074/jbc.272.12.8065. [DOI] [PubMed] [Google Scholar]
- Kelley WL. The J-domain family and the recruitment of chaperone power. Trends Biochem Sci. 1998;23:222–227. doi: 10.1016/s0968-0004(98)01215-8. [DOI] [PubMed] [Google Scholar]
- Kelley WL, Georgopoulos C. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc Natl Acad Sci USA. 1997;94:3679–3684. doi: 10.1073/pnas.94.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WL, Landry SJ. Chaperone power in a virus? Trends Biochem Sci. 1994;19:277–278. doi: 10.1016/0968-0004(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Klucky B, Koch B, Radolf M, Steinlein P, Wintersberger E. Polyomavirus tumor antigens have a profound effect on gene expression in mouse fibroblasts. Oncogene. 2004;23:4707–4721. doi: 10.1038/sj.onc.1207640. [DOI] [PubMed] [Google Scholar]
- Kolzau T, Hansen RS, Zahra D, Reddel RR, Braithwaite AW. Inhibition of SV40 large T antigen induced apoptosis by small T antigen. Oncogene. 1999;18:5598–5603. doi: 10.1038/sj.onc.1202942. [DOI] [PubMed] [Google Scholar]
- Krynska B, Del Valle L, Croul S, Gordon J, Katsetos CD, Carbone M, Giordano A, Khalili K. Detection of human neurotropic JC virus DNA sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc Natl Acad Sci USA. 1999a;96:11519–11524. doi: 10.1073/pnas.96.20.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV(CY) T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999b;18:39–46. doi: 10.1038/sj.onc.1202278. [DOI] [PubMed] [Google Scholar]
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17:43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Loeken M, Bikel I, Livingston DM, Brady J. Trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988;55:1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Lomax M, Fried M. Polyoma virus disrupts ARF signaling to p53. Oncogene. 2001;20:4951–4960. doi: 10.1038/sj.onc.1204717. [DOI] [PubMed] [Google Scholar]
- London WT, Houff SA, Madden DL, Fuccillo DA, Gravell M, Wallen WC, Palmer AE, Sever JL, Padgett BL, Walker DL, ZuRhein GM, Ohashi T. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus) Science. 1978;201:1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- London WT, Houff SA, McKeever PE, Wallen WC, Sever JL, Padgett BL, Walker DL. Viral-induced astrocytomas in squirrel monkeys. Prog Clin Biol Res. 1983;105:227–237. [PubMed] [Google Scholar]
- Lynch KJ, Frisque RJ. Identification of critical elements within the JC virus DNA replication origin. J Virol. 1990;64:5812–5822. doi: 10.1128/jvi.64.12.5812-5822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KJ, Frisque RJ. Factors contributing to the restricted DNA replicating activity of JC virus. Virology. 1991;180:306–317. doi: 10.1016/0042-6822(91)90035-a. [DOI] [PubMed] [Google Scholar]
- Lynch KJ, Haggerty S, Frisque RJ. DNA replication of chimeric JC virus-simian virus 40 genomes. Virology. 1994;204:819–822. doi: 10.1006/viro.1994.1600. [DOI] [PubMed] [Google Scholar]
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- Marsilio E, Cheng SH, Schaffhausen B, Paucha E, Livingston DM. The T/t common region of simian virus 40 large T antigen contains a distinct transformation-governing sequence. J Virol. 1991;65:5647–5652. doi: 10.1128/jvi.65.10.5647-5652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Levine AS, Dixon K. Deletion mutations in the small t antigen gene alter the tissue specificity of tumors induced by simian virus 40. J Virol. 1987;61:1282–1285. doi: 10.1128/jvi.61.4.1282-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S, Turowski P, Picard L, Goris J, Landini MP, Topilko A, Hemmings B, Bessia C, Garcia A, Virelizier JL. Human cytomegalovirus carries serine/threonine protein phosphatases PP1 and a host-cell derived PP2A. J Virol. 1996;70:1415–1423. doi: 10.1128/jvi.70.3.1415-1423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, McKeever PE, London W, Padgett BL, Walker DL, Wallen WC. Brain tumors of owl monkeys inoculated with JC virus contain the JC virus genome. J Virol. 1984;49:848–856. doi: 10.1128/jvi.49.3.848-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misselwitz B, Staeck O, Rapoport TA. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol Cell. 1998;2:593–603. doi: 10.1016/s1097-2765(00)80158-6. [DOI] [PubMed] [Google Scholar]
- Moreno CS, Ramachandran S, Ashby DG, Laycock N, Plattner CA, Chen W, Hahn WC, Pallas DC. Signaling and transcriptional changes critical for transformation of human cells by simian virus 40 small tumor antigen or protein phosphatase 2A B56gamma knockdown. Cancer Res. 2004;64:6978–6988. doi: 10.1158/0008-5472.CAN-04-1150. [DOI] [PubMed] [Google Scholar]
- Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev. 2004;14:11–16. doi: 10.1016/j.gde.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Moule MG, Collins CH, McCormick F, Fried M. Role for PP2A in ARF signaling to p53. Proc Natl Acad Sci USA. 2004;101:14063–14066. doi: 10.1073/pnas.0405533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungre S, Enderle K, Turk B, Porras A, Wu YQ, Mumby MC, Rundell K. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–1681. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Craig L, Machleidt T, Sontag E. Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J Virol. 2003;77:2807–2818. doi: 10.1128/JVI.77.5.2807-2818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Suzuki T, Sunden Y, Orba Y, Kose S, Imamoto N, Takahashi H, Tanaka S, Hall WW, Nagashima K, Sawa H. Dissociation of heterochromatin protein 1 from lamin B receptor induced by human polyomavirus agnoprotein: Role in nuclear egress of viral particles. EMBO Rep. 2005;6:452–457. doi: 10.1038/sj.embor.7400406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett BL, Zu Rhein GM, Walker DL, Echroade R, Dessel B. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;i:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, Roberts TM. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Peden KW, Pipas JM. Simian virus 40 mutants with amino-acid substitutions near the amino terminus of large T antigen. Virus Genes. 1992;6:107–118. doi: 10.1007/BF01703060. [DOI] [PubMed] [Google Scholar]
- Peden KW, Spence SL, Tack LC, Cartwright CA, Srinivasan A, Pipas JM. A DNA replication-positive mutant of simian virus 40 that is defective for transformation and the production of infectious virions. J Virol. 1990;64:2912–2921. doi: 10.1128/jvi.64.6.2912-2921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pho MT, Ashok A, Atwood WJ. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74:2288–2292. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas JM. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas JM, Peden KW, Nathans D. Mutational analysis of simian virus 40 T antigen: Isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983;3:203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras A, Gaillard S, Rundell K. The simian virus 40 small-t and large-T antigens jointly regulate cell cycle reentry in human fibroblasts. J Virol. 1999;73:3102–3107. doi: 10.1128/jvi.73.4.3102-3107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C, Beck Y. characterization of SV40 T antigen polypeptides synthesized in vivo an in vitro. Inserm EMBO. 1977;69:175–188. [Google Scholar]
- Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Querido E, Marcellus RC, Lai A, Charbonneau R, Teodoro JG, Ketner G, Branton PE. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido E, Blanchette P, Yan Q, Kamura T, Morrison M, Boivin D, Kaelin WG, Conaway RC, Conaway JW, Branton PE. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 2001;15:3104–3117. doi: 10.1101/gad.926401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan PN, Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993;21:1959–1964. doi: 10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- Ruediger R, Roeckel D, Fait J, Bergqvist A, Magnusson G, Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992;12:4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K, Parakati R. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin Cancer Biol. 2001;11:5–13. doi: 10.1006/scbi.2000.0341. [DOI] [PubMed] [Google Scholar]
- Safak M, Khalili K. An overview: Human polyomavirus JC virus and its associated disorders. J Neurovirol. 2003;9:3–9. doi: 10.1080/13550280390195360. [DOI] [PubMed] [Google Scholar]
- Safak M, Barrucco R, Darbinyan A, Okada Y, Nagashima K, Khalili K. Interaction of JC virus agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J Virol. 2001;75:1476–1486. doi: 10.1128/JVI.75.3.1476-1486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safak M, Sadowska B, Barrucco R, Khalili K. Functional interaction between JC virus late regulatory agnoprotein and cellular Y-box binding transcription factor, YB-1. J Virol. 2002;76:3828–3838. doi: 10.1128/JVI.76.8.3828-3838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sariyer IK, Akan I, Palermo V, Gordon J, Khalili K, Safak M. Phosphorylation mutants of JC virus agnoprotein are unable to sustain the viral infection cycle. J Virol. 2006;80:3893–3903. doi: 10.1128/JVI.80.8.3893-3903.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Munger K, Huibregtse JM, Howley PM. Targeted degradation of the retinoblastoma protein by human papillomavirus E7-E6 fusion proteins. EMBO J. 1992;11:2425–2431. doi: 10.1002/j.1460-2075.1992.tb05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RJ, Mohr I. Translation initiation and viral tricks. Trends Biochem Sci. 2003;28:130–136. doi: 10.1016/S0968-0004(03)00029-X. [DOI] [PubMed] [Google Scholar]
- Schuchner S, Wintersberger E. Binding of polyomavirus small T antigen to protein phosphatase 2A is required for elimination of p27 and support of S-phase induction in concert with large T antigen. J Virol. 1999;73:9266–9273. doi: 10.1128/jvi.73.11.9266-9273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Shenk T, Carbon J, Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976;18:664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Shivakumar CV, Das GC. Interaction of human polyomavirus BK with the tumor-suppressor protein p53. Oncogene. 1996;13:323–332. [PubMed] [Google Scholar]
- Silver PA, Way JC. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993;74:5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- Sleigh MJ, Topp WC, Hanich R, Sambrook JF. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978;14:79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Small JA, Khoury G, Jay G, Howley PM, Scangos GA. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc Natl Acad Sci USA. 1986a;83:8288–8292. doi: 10.1073/pnas.83.21.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JA, Scangos GA, Cork L, Jay G, Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986b;46:13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Peden KW, Pipas JM. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989;63:5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, McClellan AJ, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky JL, Pipas JM. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SE, Eddy BE, Borgese N. Neoplasms in mice inoculated with a tumor agent carried in tissue culture. J Natl Cancer Inst. 1958;20:1223–1243. doi: 10.1093/jnci/20.6.1223. [DOI] [PubMed] [Google Scholar]
- Sugano S, Yamaguchi N, Shimojo H. Small t protein of simian virus 40 is required for dense focus formation in a rat cell line. J Virol. 1982;41:1073–1075. doi: 10.1128/jvi.41.3.1073-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CS, Pipas JM. T antigens of simian virus 40: Molecular chaperones for viral replication and tumorigenesis. Microbiol Mol Biol Rev. 2002;66:179–202. doi: 10.1128/MMBR.66.2.179-202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CS, Cantalupo P, Pipas JM. The molecular chaperone activity of simian virus 40 large T antigen is required to disrupt Rb-E2F family complexes by an ATP-dependent mechanism. Mol Cell Biol. 2000;20:6233–6243. doi: 10.1128/mcb.20.17.6233-6243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CS, Gilbert SP, Pipas JM. ATP-dependent simian virus 40 T-antigen-Hsc70 complex formation. J Virol. 2001;75:1601–1610. doi: 10.1128/JVI.75.4.1601-1610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Okada Y, Semba S, Orba Y, Yamanouchi S, Endo S, Tanaka S, Fujita T, Kuroda S, Nagashima K, Sawa H. Identification of FEZ1 as a protein that interacts with JC virus agnoprotein and microtubules: Role of agnoprotein-induced dissociation of FEZ1 from microtubules in viral propagation. J Biol Chem. 2005;280:24948–24956. doi: 10.1074/jbc.M411499200. [DOI] [PubMed] [Google Scholar]
- Sweet BH, Hilleman MR. The vacuolating virus, S.V.40. Proc Soc Exp Biol Med. 1960;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Turk B, Porras A, Mumby MC, Rundell K. Simian virus 40 small-t antigen binds two zinc ions. J Virol. 1993;67:3671–3673. doi: 10.1128/jvi.67.6.3671-3673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof C, Goris J. PP2A fulfills its promises as tumor suppressor: Which subunits are important? Cancer cell. 2004;5:105–106. doi: 10.1016/s1535-6108(04)00027-3. [DOI] [PubMed] [Google Scholar]
- Varakis J, ZuRhein GM, Padgett BL, Walker DL. Induction of peripheral neuroblastomas in Syrian hamsters after injection as neonates with JC virus, a human polyoma virus. Cancer Res. 1978;38:1718–1722. [PubMed] [Google Scholar]
- Volckaert G, Feunteun J, Crawford LV, Berg P, Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol. 1979;30:674–682. doi: 10.1128/jvi.30.3.674-682.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Howe A, Lee RJ, Albanese C, Shu IW, Karnezis AN, Zon L, Kyriakis J, Rundell K, Pestell RG. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc Natl Acad Sci USA. 1996;93:12861–12866. doi: 10.1073/pnas.93.23.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen B, Laffin J, Friedrich TD, Lehman JM. SV40 small T antigen enhances progression to >G2 during lytic infection. Exp Cell Res. 1999;251:121–127. doi: 10.1006/excr.1999.4572. [DOI] [PubMed] [Google Scholar]
- White MK, Khalili K. Polyomaviruses and human cancer: Molecular mechanisms underlying patterns of tumorigenesis. Virology. 2004;324:1–16. doi: 10.1016/j.virol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- White FA, III, Ishaq M, Stoner GL, Frisque RJ. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Gordon J, Reiss K, Del Valle L, Croul S, Giordano A, Darbinyan A, Khalili K. Human polyomaviruses and brain tumors. Brain Res Brain Res Rev. 2005;50:69–85. doi: 10.1016/j.brainresrev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Winston J, Dong F, Pledger WJ. Differential modulation of G1 cyclins and the Cdk inhibitor p27kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J Biol Chem. 1996;271:11253–11260. doi: 10.1074/jbc.271.19.11253. [DOI] [PubMed] [Google Scholar]
- Yaciuk P, Carter MC, Pipas JM, Moran E. Simian virus 40 large-T antigen expresses a biological activity complementary to the p300-associated transforming function of the adenovirus E1A gene products. Mol Cell Biol. 1991;11:2116–2124. doi: 10.1128/mcb.11.4.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, Goldberg AL, Finley D, Sherman MY. The molecular chaperone Ydj1 is required for the p34CDC28-dependent phosphorylation of the cyclin Cln3 that signals its degradation. Mol Cell Biol. 1996;16:3679–3684. doi: 10.1128/mcb.16.7.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Hearing P, Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979;32:147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SI, Lickteig RL, Estes R, Rundell K, Walter G, Mumby MC. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Vitto MJ, Busby SA, Garcia BA, Kesler CT, Gioeli D, Shabanowitz J, Hunt DF, Rundell K, Brautigan DL, Paschal BM. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol Cell Biol. 2005;25:1298–1308. doi: 10.1128/MCB.25.4.1298-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Kudchodkar SB, Alwine JC. Effects of simian virus 40 large and small tumor antigens on mammalian target of rapamycin signaling: Small tumor antigen mediates hypophosphorylation of eIF4E-binding protein 1 late in infection. J Virol. 2005;79:6882–6889. doi: 10.1128/JVI.79.11.6882-6889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 1996;10:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 2000;14:414–421. [PMC free article] [PubMed] [Google Scholar]
- Zaitsu H, Kimura G. Simian virus 40 compensates a cellular mutational defect of a serum-dependent function controlling cell cycle progression in the G2 phase. Virology. 1988;164:165–170. doi: 10.1016/0042-6822(88)90632-0. [DOI] [PubMed] [Google Scholar]
- Zerrahn J, Knippschild U, Winkler T, Deppert W. Independent expression of the transforming amino-terminal domain of SV40 large I antigen from an alternatively spliced third SV40 early mRNA. EMBO J. 1993;12:4739–4746. doi: 10.1002/j.1460-2075.1993.tb06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Gjoerup OV, Subramanian RR, Cheng Y, Chen W, Roberts TM, Hahn WC. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Rice PW, Gorsch L, Abate M, Cole CN. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992;66:2780–2791. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Williams RT, Baudino TA, Rehg JE, Skapek SX, Cleveland JL, Roussel MF, Sherr CJ. Arf tumor suppressor promoter monitors latent oncogenic signals in vivo. Proc Natl Acad Sci USA. 2003;100:15930–15935. doi: 10.1073/pnas.2536808100. [DOI] [PMC free article] [PubMed] [Google Scholar]