Abstract
Reduced glutathione (GSH) is critical for many cellular processes, and both its intracellular and extracellular concentrations are tightly regulated. Intracellular GSH levels are regulated by two main mechanisms: by adjusting the rates of synthesis and of export from cells. Some of the proteins responsible for GSH export from mammalian cells have recently been identified, and there is increasing evidence that these GSH exporters are multispecific and multifunctional, regulating a number of key biological processes. In particular, the multidrug resistance-associated proteins (Mrp/Abcc) appear to mediate GSH export and homeostasis. The Mrp proteins mediate not only GSH efflux, but they also export oxidized glutathione derivatives (e.g., glutathione disulfide (GSSG), S-nitrosoglutathione (GS-NO), and glutathione-metal complexes), as well as other glutathione S-conjugates. The ability to export both GSH and oxidized derivatives of GSH, endows these transporters with the capacity to directly regulate the cellular thiol-redox status, and therefore the ability to influence many key signaling and biochemical pathways. Among the many processes that are influenced by the GSH transporters are apoptosis, cell proliferation, and cell differentiation. This report summarizes the evidence that Mrps contribute to the regulation of cellular GSH levels and the thiol redox state, and thus to the many biochemical processes that are influenced by this tripeptide.
Keywords: Glutathione, glutathione transporters, multidrug resistance-associated proteins, thiol-redox status, apoptosis, xenobiotic export, signaling, cell proliferation and differentiation
Introduction
Reduced glutathione (GSH) plays an important role in cell metabolism, differentiation, proliferation, and apoptosis, and as a result, disturbances in its homeostasis are implicated in the etiology and/or progression of a number of human diseases, including cancer, diseases of aging, and cardiovascular, inflammatory, immune, and neurodegenerative diseases. GSH is synthesized in the cell cytosol, whereas its degradation occurs exclusively in the extracellular space, and thus export from the cell is required for normal GSH turnover from all mammalian cells. In the liver, a major site of GSH synthesis and export, GSH is released at high rates into both blood plasma and bile. GSH transport into bile functions as a driving force for bile secretion and plays an important role in the transport and hepatic detoxification of reactive compounds of both endogenous and exogenous origin. GSH is also released at high rates across the sinusoidal membrane into blood plasma, for delivery to other tissues. Although the molecular identity of GSH transporters has remained elusive, recent studies have implicated a major role for some Mrp/Abcc proteins in this process. The present overview will summarize the data demonstrating that the Mrp proteins are able to export GSH as well as various GSH derivatives, and in doing so may contribute to several processes, including the control of cellular redox status, delivery of cysteine to other tissues, export of signaling molecules, elimination of xenobiotics and reactive metabolic intermediates, as well as to cell differentiation, proliferation, and apoptosis.
Cellular GSH homeostasis
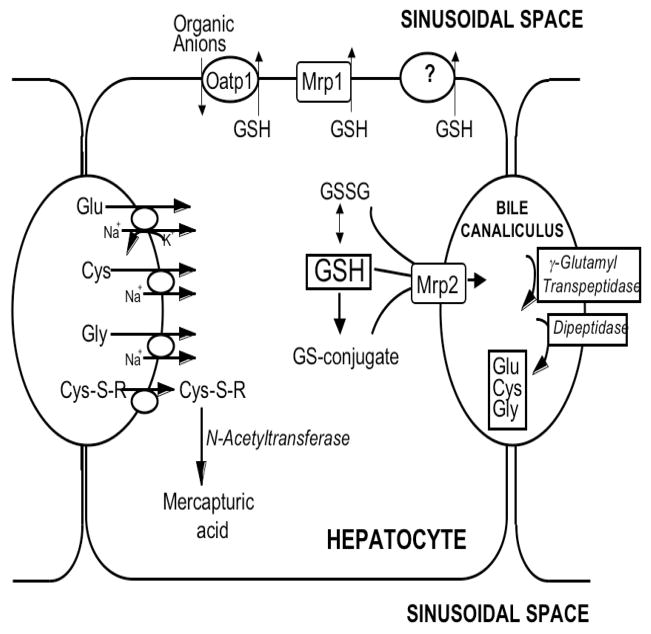
The synthesis and catabolism of GSH and GSH-adducts occurs by a regulated series of enzymatic and plasma membrane transport steps that are collectively referred to as the gamma-glutamyl cycle (Meister and Anderson, 1983; Meister and Tate, 1976;). Figure 1 depicts the steps of this cycle as they occur in hepatocytes, which are a major site of GSH biosynthesis and export. GSH is synthesized in the cell cytosol from its precursor amino acids. Within the cell, it exists mainly (>98%) in the thiol-reduced form (GSH), but some is also present in the thiol-oxidized (GSSG), thioether, mercaptide or other thioester forms (glutathione S-conjugates and complexes) (Fig. 1). After its synthesis, some of the GSH is delivered into specific intracellular compartments, including mitochondria and endoplasmic reticulum, but much of the GSH is delivered to extracellular spaces, including blood plasma, epithelial lining fluids, and exocrine secretions (e.g., bile; Fig. 1). In contrast to GSH synthesis, which occurs intracellularly, GSH degradation occurs exclusively in the extracellular space, and only on the surface of cells that express the ectoenzyme gamma-glutamyl transpeptidase (γGT; Fig. 1). Thus, export from the cell is required for normal GSH turnover, and for metabolism and disposition of GSH adducts.
Figure 1. Glutathione homeostasis in hepatocytes.
GSH is synthesized in the cell cytosol from its precursor amino acids, glutamate, cysteine and glycine. Within the cell, it exists mainly (>98%) in the thiol-reduced form (GSH), but some is also present in the thiol-oxidized (GSSG) and as glutathione S-conjugates. After its synthesis, some of the GSH is delivered into specific intracellular compartments, including mitochondria and endoplasmic reticulum, but much of the GSH is delivered to extracellular spaces, namely blood and bile. Transport of GSH and its conjugates into bile is mediated largely by Mrp2, whereas Mrp1 and Oatp1 may contribute to GSH efflux into blood, although this is still poorly defined. In contrast to GSH synthesis, which occurs intracellularly, GSH degradation occurs exclusively in the extracellular space, and only on the surface of cells that express the ectoenzyme gamma-glutamyl transpeptidase (γGT). In the liver this enzyme is most abundant on the canalicular membrane of hepatocytes and on the apical membrane of bile duct cells. Once GSH and GSH-containing compounds are released from liver cells there is an efficient intrahepatic cycle of glutathione degradation and utilization consisting of: (a) extensive catabolism within biliary spaces, as well as within sinusoidal compartments of some species; (b) direct hepatic reabsorption of some of the breakdown products; and (c) intracellular utilization of the amino acids, or conversion of cysteine S-conjugates to mercapturic acids, i.e., N-acetylcysteine S-conjugates.
The mechanisms of GSH transport into mitochondria and endoplasmic reticulum have not been established, although some possibilities have been suggested. Studies with kidney and liver mitochondria indicate that the dicarboxylate carrier (DIC, Slc25a10) and the oxoglutarate carrier (OGC, Slc25a11) contribute to the transport of GSH across the mitochondrial inner membrane (Lash 2006); however, differences between kidney and liver mitochondrial GSH transport suggest the existence of additional unidentified carriers (Fernandez-Checa and Kaplowitz 2005). For the endoplasmic reticulum, studies demonstrate that GSH accumulation by isolated membrane vesicles correlates with the abundance of ryanodine receptor type 1 (RyR1), is inhibited by RyR1 blockers, and the inhibitory effect is counteracted by RyR1 agonists, suggesting that RyR1 is associated with GSH transport in this organelle (Banhegyi et al. 2003; Csala et al. 2001). However, because ryanodine receptors contain many cysteine residues that are susceptible to S-glutathionylation (Aracena et al. 2003), it is not clear whether these effects on GSH accumulation by the vesicles are due to the actual transport of GSH across the membrane or to altered binding of GSH to the ryanodine receptors or to other proteins in the membrane vesicles (i.e., S-glutathionylation).
As noted above, although some of the GSH made within cells is delivered into intracellular compartments, much of it is exported across the plasma membrane into extracellular spaces. GSH turnover rates in most cells are relatively rapid, with half-lives of only 2–6 h (Meister and Anderson, 1983; Meister and Tate, 1976), indicating high rates of both GSH synthesis and export. Although large quantities of GSH are secreted into blood plasma, GSH concentrations in this compartment are relatively low, ~0.01 mM, owing to the rapid catabolism of the tripeptide in the circulation. Once GSH is released from cells it is rapidly degraded in the circulation by the plasma membrane-bound enzymes γGT and dipeptidases to release the free amino acids (i.e., Glu, Cys, and Gly), the dipeptide Cys-Gly, and other peptides (Meister and Anderson, 1983). The half-life of GSH in blood plasma is on the order of seconds to minutes (Meister and Tate, 1976; Meister and Anderson, 1983).
In rat liver, approximately one-half of the GSH is released into blood plasma, and one-half goes across the canalicular membrane into bile, with biliary GSH concentrations reaching 8–10 mM (Ballatori et al., 1986b, 1988, 1989; Ballatori and Truong, 1989, 1992). The GSH concentration within liver cells (~10 mM) is thus the result of a dynamic equilibrium between its synthesis and its efflux into blood plasma and bile. Once GSH is released from hepatocytes, there is an efficient intrahepatic cycle of glutathione degradation and utilization consisting of: (a) extensive catabolism within biliary spaces (Ballatori et al., 1986b, 1988, 1989), as well as within sinusoidal compartments of some species (Hinchman and Ballatori, 1990, 1994); (b) direct hepatic reabsorption of some of the breakdown products (Ballatori et al., 1986a, 1988; Simmons et al., 1991, 1992); and (c) intracellular utilization of the amino acids, or conversion of cysteine S-conjugates to mercapturic acids, i.e., N-acetylcysteine S-conjugates (Hinchman et al., 1991, 1993, 1998). Some of the functions of this cycle include regulation of GSH turnover, thiol-redox status of the cell, mercapturic acid biosynthesis, and metal transport and excretion. Another key function of GSH in bile is to serve as a primary osmotic driving force in bile formation (Ballatori and Truong, 1989, 1992).
As noted above, the catabolism of GSH is catalyzed by the ectoproteins γGT and dipeptidases, to release the free amino acids (i.e., Glu, Cys, and Gly), the dipeptide Cys-Gly, and other peptides via transpeptidation. Cellular reuptake of the amino acids is mediated by various amino acid transporters, whereas the reabsorption of Cys-Gly may be mediated by Pept2, a transporter of di- and tripeptides (Frey et al., 2007). Frey et al. (2007) demonstrated that dipeptides constitute a noticeable fraction of urinary amino acids in Pept2-deficient animals, and dipeptide-bound glycine and cystine are increased in their urine samples, suggesting that Pept2 is critical for the reabsorption of Cys-Gly originating from GSH breakdown, and that it contributes to reabsorptions of amino acids that may be used for GSH resynthesis.
Although some studies have suggested that GSH itself is transported from the extracellular space into mammalian cells (Lash 2005), this remains equivocal. The rapid catabolism of GSH in the circulation leads to low extracellular GSH levels (i.e., ~10 μM), whereas intracellular GSH concentrations are about three orders of magnitude higher, and thus any GSH uptake transporter would have to have both a relatively high affinity for GSH, and would have to overcome the large outwardly directed GSH electrochemical gradient in order to facilitate uptake. None of the putative GSH uptake transporters that have been identified to date exhibit the necessary kinetic properties that would enable them to mediate GSH uptake under physiological conditions.
When exposed to oxidant stress or electrophilic chemicals, GSSG and glutathione S-conjugates are generated within the cell, and the GSH-adducts are exported from cells mainly by the Mrp/Abcc proteins. A total of nine functional Mrp genes have been identified (Mrp1 to Mrp9), although the physiological functions of many remain poorly defined (Borst et al., 2007; Borst and Oude Elferink, 2002; Deeley et al., 2006; Kruh et al., 2007a;). In general, the Mrps function as organic anion export pumps, and they appear to have broad and partially overlapping substrate specificity (Table 1). Nearly all of the Mrps accept glutathione S-conjugates as substrates (Table 1: Borst et al., 2007; Deeley et al., 2006; Ilias et al., 2002; Konig et al. 1999; Kruh et al., 2007a; Lai and Tan, 2002).
Table 1.
Endogenous compounds that are substrates for human MRP1–MRP9 and CFTR.
| Endogenous substrates | MRP1 | MRP2 | MRP3 | MRP4 | MRP5 | MRP6 | MRP7 | MRP8 | MRP9 | CFTR |
|---|---|---|---|---|---|---|---|---|---|---|
| GSH conjugates | X | X | X | X | X | X | X | X | ||
| Cysteinyl leukotrienes (e.g., LTC4) | X | X | X | X | X | X | X | |||
| Glucuronic acid conjugates | X | X | X | X | X | X | X | |||
| Bile salts | X | X | ||||||||
| Bilirubin | X | X | X | |||||||
| Estradiol, 17B- | X | X | X | X | X | X | ||||
| Sulfate conjugates | X | X | X | X | X | |||||
| Bile Salts | X | X | X | |||||||
| Sulfated Steroids | X | X | X | X | ||||||
| Cyclic nucleotides | X | X | X | |||||||
| GSH (+/− other substrates) | X | X | X | X | X | |||||
| GSSG | X | X | X |
Endogenous substrates for these proteins are indicated by (X); however, the absence of an (X) does not eliminate the possibility of transport for the given substrate, as this may have not yet been examined. Data were obtained from Borst et al., 2000, 2007; Ilias et al., 2002;Konig et al., 1999, Kruh et al., 2007a; Leslie et al., 2001b; Lindsell and Hanrahan, 1998; Rius et al., 2006, 2008; Wijnholds et al., 2000; Zelcer et al., 2003.
Mechanisms of GSH export from cells
In contrast to the transport of glutathione S-conjugates, the functional characterization and the molecular identity of GSH transporters remain poorly defined. As discussed in a recent review article (Ballatori et al., 2005), several factors have contributed to the slow progress in identifying and characterizing GSH transport proteins, including: (a) The relative difficulty of studying efflux transporters. (b) The low catalytic efficiency of GSH transport (Ballatori and Dutczak, 1994; Paulusma et al., 1999; Rebbeor et al., 1998a, 1998b, 2000b, 2002). (c) The fact that all cells have endogenous GSH export mechanisms (Meister and Anderson, 1983). This high background rate of GSH transport is a major confounding variable in GSH transport measurements, and severely limits the choice of cells with which this process can be studied. (d) GSH is chemically reactive and is present in several chemical forms (glutathione-thioethers, –thioesters, and –mercaptides). GSH participates in nucleophilic displacement reactions, in thiol-redox reactions, and forms coordinate-covalent adducts with several transition metals (Ballatori 1994, 2002). These adducts can form non-enzymatically, and many of them are chemically unstable, present in low concentrations, and difficult to detect and quantify. (e) In addition to the sulfhydryl-dependent reactions, GSH and GSH-containing molecules are also subject to degradation by the ectoenzyme γGT. (f) GSH transporters are present both on the plasma membrane and on intracellular membranes. Thus, in studies with subcellular membrane fractions, these intracellular GSH transporters may contribute to the overall transport rate. (g) The lack of specific GSH transport inhibitors, and (h) In addition, the search for GSH transporters was significantly hampered by the publication of two papers that claimed to have identified cDNA clones for canalicular (RcGshT) and sinusoidal (RsGshT) GSH transporters (Yi et al. 1994, 1995). Subsequent experiments demonstrated that these putative gene products not only have no GSH transport activity, but that they are cloning artifacts (Li et al., 1997).
Despite these difficulties in studying mechanisms of GSH transport, two plasma membrane GSH export mechanisms have now been identified (Fig. 1). First, a role for the MRP proteins in GSH transport was indicated by studies in the yeast Saccharomyces cerevisiae (Rebbeor et al., 1998a, 1998b, 2002), and in membrane vesicles isolated from the liver of the little skate, Leucoraja erinacea (Rebbeor et al., 2000) or from yeast and from rat liver (Rebbeor et al., 2002). These studies in yeast provided the first direct evidence for ATP-dependent, low-affinity transport of GSH in any cell type (Rebbeor et al., 1998a), and demonstrated that this ATP-dependent GSH transport in yeast is mediated by Ycf1p, the yeast orthologue of mammalian MRP1 and MRP2 (Rebbeor et al., 1998b). Additionally, the yeast transporter Bpt1p, which is a homologue of Ycf1p, also transports GSH and glutathione conjugates (Klein et al., 2002; Sharma et al., 2002). Because Ycf1p and Bpt1p are structurally and functionally homologous to the MRP proteins, these data suggest that GSH efflux from mammalian cells is mediated in part by MRP1 and MRP2. Second, rat Oatp1, the sinusoidal organic solute uptake transporter, was shown to function as a GSH/organic solute exchanger (Li et al., 1998), and thus could potentially contribute to GSH release from cells. However, more recent studies with two human OATPs demonstrated no role of GSH in their transport mechanism (Mahagita et al., 2007).
Studies in rat liver canalicular membrane vesicles by Rebbeor et al. (2002) provided the first direct evidence for GSH transport on Mrp2. This study demonstrated that the inability to detect ATP-dependent GSH transport in previous studies with mammalian plasma membrane vesicles was due in part to the inhibitory effect of dithiothreitol (DTT) and some other reducing agents on Mrp2-mediated transport. DTT is an effective reducing agent that is normally added to prevent GSH oxidation in membrane vesicle studies. Because all previous studies of GSH transport utilized high concentrations of reducing agents, they probably underestimated GSH transport rates. Thus, these studies demonstrated that both rat Mrp2 and yeast Ycf1p are able to transport GSH by an ATP-dependent, low-affinity mechanism (Rebbeor et al., 1998a, 1998b, 2002). Additional evidence in support of a role for the Mrps in GSH transport is summarized in reviews by Ballatori et al. (2005) and Cole and Deeley (2006), and is discussed further below.
Evidence for GSH transport on Mrp1, Mrp2, Mrp4, Mrp5, and Cftr, and proposed transport mechanisms
MRP1 was cloned in 1992 from the human small cell lung cancer cell line H69 that had been repeatedly exposed to doxorubicin, an anthracyclin (Cole et al., 1992). LTC4 and other glutathione S-conjugates were identified as substrates for MRP1 (Jedlitschky et al., 1994; Leier et al., 1994) (Table 1). Further analysis revealed that in addition to glutathione S-conjugates, MRP1 is capable of transporting glucuronide and sulfate conjugates, antineoplastics, antivirals, antibiotics, non-steroidal anti-inflammatory and antiepileptic drugs, flavonoids, natural folates, fluorescent probes, and some peptides (Conseil et al., 2005) (Table 1).
As noted above, there is now strong evidence that GSH is a substrate for MRP1. One of the first indications that GSH may be exported by the Mrps came from experiments conducted in an MRP1-overexpressing lung carcinoma cell line, which had lower intracellular GSH levels and higher extracellular GSH levels (Zaman et al., 1995). Subsequent studies confirmed that MRP1 overexpressing cells have lower intracellular GSH (Laberge et al., 2007; Lautier et al., 1996; Mao et al., 2000; Marchan et al., 2008). Furthermore, MDCKII cells overexpressing MRP1 have increased amounts of GSH to the basal compartment (Paulusma et al., 1999). Subsequent studies in membrane vesicles described GSH as a low affinity substrate for MRP1 (Km > 1mM: Leslie et al., 2001a; Paulusma et al., 1999; Qian et al., 2001).
Support for a role of both Mrp1 and Mrp2 in GSH export is provided by studies in knockout mouse models. Measurements of GSH levels in tissues of mice deficient in Mrp1, Mrp2, or Cftr, reveal that these mice have altered tissue GSH levels (Chu et al., 2006; Kruh et al., 2007b; Lorico et al., 1997; Velsor et al., 2001). Lorico et al. (1997) reported that GSH levels are about 20–40% higher in tissues of Mrp1−/− mice that normally express relatively high levels of this protein. In Mrp2-deficient rats and mice, hepatic GSH levels are increased about 2-fold (e.g., Ballatori et al., 1995; Chu et al., 2006). In Cftr−/− mice, the epithelial lining fluid GSH concentration is slightly lower than that of wild type mice, but the GSH concentration in the lung tissue is not affected (Velsor et al., 2001). Because Mrp1 is expressed in all tissues, it may play a ubiquitous role in GSH export from cells, whereas Mrp2 expression is more restricted (Table 2), and thus can only contribute to GSH export in those cells.
Table 2.
Tissue mRNA expression of human MRP1–MRP9 and CFTR.
| Tissues | MRP1 | MRP2 | MRP3 | MRP4 | MRP5 | MRP6 | MRP7 | MRP8 | MRP9 | CFTR |
|---|---|---|---|---|---|---|---|---|---|---|
| Adrenal Glands | X | X | X | X | X | |||||
| Bladder | X | X | X | |||||||
| Brain | X | X | X | X | X | X | X | X | ||
| Breast | X | X | X | X | X | X | ||||
| Colon | X | X | X | X | X | X | X | |||
| Gallbladder | X | X | X | |||||||
| Heart | X | X | X | X | X | |||||
| Kidney | X | X | X | X | X | X | X | X | ||
| Liver | X(Low) | X | X | X(Low) | X | X | X | X | X | |
| Lung | X | X | X | X | X | X | X(Low) | |||
| Ovary | X | X | X | |||||||
| Pancreas | X | X | X | X | X | X | ||||
| Placenta | X | X | X | X | X | X | X | |||
| Prostate | X | X | X | X | X | X | ||||
| Skeletal Muscle | X | X | X | X | ||||||
| Skin | X | X | X | X | X | X | ||||
| Small Intestine | X | X | X | X | X | X | X | |||
| Spleen | X | X | X | X | ||||||
| Stomach | X | X | X | |||||||
| Sweat gland | X | |||||||||
| Testis | X | X | X | X | X | X | X | X | ||
| Thymus | X | X |
mRNA detection is indicated by (X); however, please note that the absence of an (X) does not exclude gene expression, as this may have not yet been examined. Data were obtained from Beck et al., 2005; Deeley et al., 2006; Dreuw et al., 2005; Duan et al., 2005; Englund et al., 2006; Hihnala et al., 2006; Kruh et al., 2007a; Lacueva et al., 2005; Leslie et al., 2005; McCarthy et al., 2005; Morales et al., 1996; Mylona et al., 1996; Patrizio et al., 1998; Scheffer et al., 2002; Serrano et al., 2007; Strong et al., 1994; Taipalensuu et al., 2001; Torky et al., 2005; Zimmermann et al., 2005.
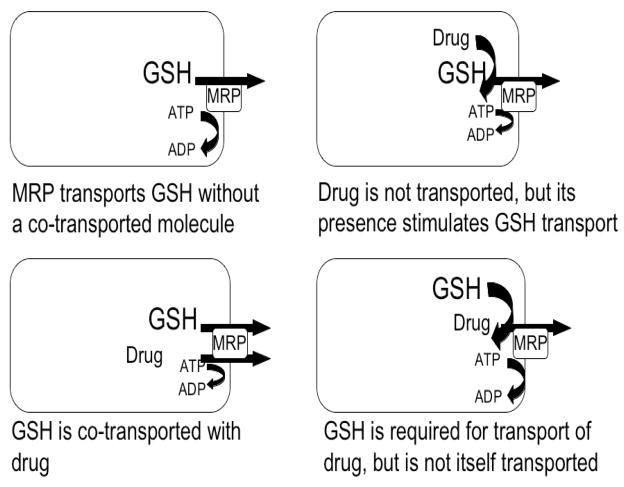
The mechanism of GSH transport by the Mrp proteins is still not understood. There are at least four potential mechanisms by which GSH interacts with the Mrps (Fig. 2). The first proposed mechanism is that GSH itself is a substrate for the Mrps, and preliminary studies in MRP1-containing proteoliposomes support this hypothesis; however, the observed GSH transport was quite low in the absence of other substrates (Mao et al., 2000). Co-transport of GSH with another substrate is a second possible mechanism of GSH transport on the Mrp proteins. Studies utilizing membrane vesicles isolated from MRP1 overexpressing cells suggest that transport of certain drugs is increased in the presence of GSH (Loe et al., 1996, 1997; Mao et al., 2000; Morrow et al., 2006; Renes et al., 1999). Additionally, depleting GSH with L-buthionine sulfoximine (BSO) inhibited transport of these compounds (Gekeler et al., 1995; Loe et al., 1998; Rappa et al., 1997;Salerno and Garnier-Suillerot, 2001; Schneider et al., 1995; Vanhoefer et al., 1996; Versantvoort et al., 1995). In support of a co-transport mechanism, vincristine, etoposide, and vinblastine have been shown to stimulate GSH transport (Loe et al., 1998; Mao et al., 2000; Rappa et al., 1997). A third possible interaction between the Mrps and GSH is that transport of some substrates is stimulated by or is dependent on GSH, but GSH itself is not transported. It is still unknown what specific characteristics determine whether a substrate requires GSH for transport (Conseil et al., 2005; Leslie et al., 2001a; Loe et al., 1998; Qian et al., 2001). The fourth possible mechanism by which the Mrps mediates GSH transport is that GSH transport is stimulated by the presence of drugs that are not themselves transported by the Mrps. Compounds in this category include verapamil and some bioflavonoids, such as the dietary flavone apigenin (Leslie et al., 2001a, 2003; Loe et al., 2000).
Figure 2. Four possible mechanisms by which GSH interacts with the Mrp proteins.
(1) GSH itself is a substrate for the Mrp protein; (2) GSH is co-transport with another substrate; (3) Transport of some substrates is stimulated by or is dependent on GSH, but GSH itself is not transported; and (4) GSH transport is stimulated by the presence of drugs that are not themselves transported by the Mrp protein.
Approximately four years after the cloning of MRP1, the second member of the ABCC family, Mrp2 was identified in normal rat liver using probes against evolutionarily conserved regions of MRP1 (Buchler et al., 1996). Many functional characteristics of Mrp2 were already known prior to its molecular identification because it was discovered that Mrp2 is the protein responsible for transporting organic anions into the bile, a functional entity previously identified as the canalicular multispecific organic anion transporter (cMoat) (Jansen et al., 1985; Oude Elferink et al., 1989). The characterization of cMoat, including its substrate specificity, was performed in vesicles isolated from wild type rats and rats deficient in biliary transport, namely the Wistar transport deficient (TR-), the Groningen Yellow (GY), and the Sprague-Dawley Eisai hyperbilirubinuric rat (EHBR) (Ballatori et al., 1995; Dijkstra et al., 1990; Fernandez-Checa et al., 1992; Jansen et al., 1985; Kurisu et al., 1991; Oude Elferink et al., 1989). The observed phenotypes in these rats, including the defect in transport of endogenous and exogenous conjugated anions from hepatocytes to bile leading to hyperbilirubinemia, were similar to those observed in patients diagnosed with Dubin-Johnson syndrome. Mutations in the human MRP2 gene were eventually recognized as the underlying cause of Dubin-Johnson syndrome (Paulusma et al., 1999; Toh et al., 1999; Wada et al., 1998).
Unlike MRP1, which is expressed in all tissues, MRP2 has a more limited tissue distribution and is primarily found on the apical membrane of epithelial cells (Table 2). MRP2 is found in relatively high levels on the canalicular membrane of hepatocytes, and on the apical membrane of the kidney proximal tubule, small intestine, colon, gallbladder, bronchi, and placenta (Jedlitschky et al., 2006; Nies and Keppler, 2007). The substrate specificity of MRP2 is similar to MRP1 (Table 1), having the highest affinity for glucuronide conjugates, and glutathione S-conjugates of lipophilic compounds, including LTC4, LTD4, and LTE4 (Oude Elferink et al., 1989; Paulusma et al., 1999). Other endogenous compounds that are substrates for these proteins include conjugated steroids and bile salts. MRP2 also transports many exogenous compounds including the anticancer drugs doxorubicin, etoposide, methotrexate, cisplatin, vinblastine, and vincristine as well as several HIV drugs, antibiotics, environmental toxicants, and metal complexes (Jedlitschky et al., 2006; Nies and Keppler, 2007).
MRP2 also appears to mediate transport of GSH itself as well as cotransport of GSH with other compounds (Paulusma et al., 1999; Rebbeor et al., 2002). For many MRP2 substrates shared by MRP1, the mechanism of transport appears similar to MRP1. Transport of compounds such as vinblastine, vincristine, and etoposide all require GSH for their transport (Cui et al., 1999; Evers et al., 2000; Kawabe et al., 1999; van Aubel et al., 1999). Similar to MRP1, vinblastine and GSH are co-transported by MRP2 (Evers et al., 2000) and GSH appears to stimulate ATPase activity of MRP2 (Bakos et al., 2000). Further evidence that MRP2 transports GSH is observed in MRP2-overexpressing cells that have higher rates of GSH transport to the apical compartment (Evers et al., 2000; Paulusma et al., 1999). Conversely, hepatocytes transfected with antisense MRP2 have higher levels of intracellular GSH (Koike et al., 1997).
MRP4 (ABCC4) and MRP5 (ABCC5) were both identified in 1997 by searching databases of human expressed sequence tags (Kool et al., 1997). Both MRP4 and MRP5 lack the additional N-terminal spanning domain present in MRP1 and MRP2 (MSD0) (Belinsky et al., 1998; Lee et al., 1998), indicating that their functions might be distinct from the other members of the MRP family. Indeed, the substrates identified for these proteins appear to be somewhat unique; however, a common substrate may be GSH. MRP4 mRNA is found at low levels in lung, kidney, bladder, tonsil, liver, prostate, testes, ovary, and brain (Table 2: Kool et al., 1997; Lee et al., 1998, 2000; Schuetz et al., 2001). Although levels in the liver appear to be low across species, a recent study localizes MRP4/Mrp4 to the basolateral membrane in human, mouse, and rat hepatocytes, and in HepG2 cells, providing an alternative pathway for these cells to transport GSH and monoanionic bile salts across the sinusoidal membrane into the blood (Rius et al., 2003). Another recent study indicates a potential function of this protein by showing that upregulation of Mrp4 is associated with increased export of cAMP into plasma and urine in mice that had undergone bile duct ligation (Denk et al., 2004). MRP5 appears to be ubiquitously expressed, with high levels of mRNA found in brain, skeletal muscle, lung, and heart (Table 2: Belinsky et al., 1998; Kool et al., 1997; McAleer et al., 1999).
GSH has been suggested to be a substrate for both MRP4 and MRP5 (Lai and Tan, 2002; Wijnholds et al., 2000). Overexpression of MRP4 in HepG2 cells is associated with a marked increase in GSH efflux (Lai and Tan, 2002), and MDCKII cells transfected with MRP5 exhibit higher GSH transport across the basolateral membrane with a concurrent decrease in intracellular GSH levels (Wijnholds et al., 2000). Similar to MRP1 and MRP2 that co-transport compounds out of cells with GSH, fibroblasts transfected with MRP4 appear to co-transport bile salts (glycocholate, taurocholate and cholate) with GSH or S-methylglutathione (Rius et al., 2003, 2006). However, additional studies are needed to establish this possibility and to determine whether co-transport is necessary for all MRP4 substrates.
Interestingly, there is now significant evidence that Cftr/Abcc7 may also be able to export GSH across the plasma membrane, although this remains controversial. As noted above, the GSH concentration in epithelial lining fluid of Cftr−/− mice is slightly lower than that of wild type mice, but the GSH concentration in the lung tissue is not affected (Velsor et al., 2001). Likewise, the GSH content of epithelial lining fluid from cystic fibrosis patients is approximately one-third of normal humans (Roum et al., 1993), and this deficiency may render the lung epithelia more susceptible to oxidative damage from chronic infection and inflammation (Gao et al., 1999). CFTR was originally shown to allow passage of GSH using the patch clamp technique, but whether this is physiologically relevant is debatable (Linsdell and Hanrahan, 1998). Cells from cystic fibrosis patients lacking functional CFTR still transport GSH, albeit at a significantly slower rate (Gao et al., 1999). When chloride transport is restored to these cells by an artificial peptide channel that cannot transport or conduct GSH itself, GSH transport increased to a normal rate (Gao et al., 2001). Thus, CFTR may not actually conduct GSH, but may regulate its transport indirectly through chloride transport. As mice have more chloride channels than humans in their lungs, it is possible that the cystic fibrosis mouse does not have as much of a decrease in GSH because of this.
More recently, direct transport of GSH on CFTR was observed in membrane vesicle and proteoliposome experiments (Kogan et al., 2003). ATP binding and hydrolysis in CFTR regulates channel gating from open and closed conductance states, suggesting that ATP may alter substrate affinity on CFTR (Ikuma and Welsh, 2000; Li et al., 1996;). Interestingly, GSH appears to inhibit CFTR ATPase activity (Kogan et al., 2001), and this inhibition may alter the properties of CFTR such that it now favors GSH flux over chloride flux (Kogan et al., 2003). In addition, the MRP1 substrates taurolithocholate-3-sulphate and 17β-estradiol 17-(β-D-glucuronide) are able to block the CFTR channel, suggesting that MRP1 and CFTR share some binding properties (Linsdell and Hanrahan, 1999). Because CFTR is the only MRP family member that is in the apical membrane of lung epithelial cells, it could play a key role in GSH delivery into the apical compartment of this tissue.
Mrp and cellular thiol redox control
Cellular oxidant stresses almost invariably lead to increased intracellular concentrations of GSSG and of other oxidized glutathione derivatives. Although GSSG may be reduced back to GSH, excess GSSG is also eliminated from the cell by export into the extracellular space. For example, in the liver, oxidants lead to a marked increase in biliary GSSG excretion, with GSSG concentrations in bile usually exceeding those in hepatocytes, indicating an active canalicular membrane transport process (Keppler et al., 1997; Sies et al., 1980; Suzuki and Sugiyama, 1998). Strong evidence that this export step is mediated by Mrp2 was provided by studies of GSSG transport with canalicular membrane-enriched vesicles derived from normal and EHBR rats (Fernandez-Checa et al. 1992). This study demonstrated ATP-dependent GSSG transport in control, but not in EHBR-derived vesicles, indicating that the defect in the EHBR rats is associated with GSSG transport (Fernandez-Checa et al. 1992).
Likewise, MRP1 has been implicated in the GSSG export that is observed in various cell types challenged with oxidant stresses, including human endothelial cells (Mueller et al., 2005; Widder et al., 2007), rat cardiac myocytes (Krause et al., 2007), and primary cultures of rat astrocytes (Ronaldson and Bendayan, 2008) and mouse astrocytes (Minich et al., 2006).
In addition to GSSG export, the Mrp proteins may also mediate the export of other oxidized GSH derivatives, including nitric oxide (NO) the various oxidized metabolites of arachidonic acid. For example, Kolberg et al. (2006) reported that the low expression of Mrp1 in lymphocytes of Walker 256 tumour-bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Watts et al. (2006) demonstrated that cells overexpressing MRP1 exhibited a 3- to 4-fold increase in NO-mediated Fe and GSH efflux compared with wild type cells. Similar results were found for other MRP1-overexpressing cell types but not those expressing another drug efflux pump, MDR1. NO-mediated Fe and GSH efflux were temperature- and energy-dependent and were significantly decreased by MRP1 transport inhibitors and by GSH-depletion, suggesting that MRP1 may mediate the export of the GS-Fe-NO complex, but this was not measured directly.
Trauner et al. (1997) reported that the NO donors sodium nitroprusside (SNP) and S-nitroso-acetyl-penicillamine stimulate bile flow and increase both bicarbonate and total glutathione excretion in perfused rat liver. Increases in bile flow were linearly related to increases in biliary glutathione concentration and output (P < .0001), which were almost entirely caused by increases in GSSG excretion, whereas GSH excretion remained unchanged. In contrast to the NO donors, dibutyryl cGMP did not increase glutathione excretion. Furthermore, the NO donors failed to stimulate bile flow in the Mrp2-deficient TR- rats. These findings indicate that exogenous sources of NO increase bile acid-independent bile flow by stimulating Mrp2-mediated GSSG and GS-NO excretion.
In addition to the many organic chemicals that can disrupt the cellular thiol-redox status, a number of metals can also disrupt this balance. As discussed below, GSH and its transporters play important roles in preventing these reactions and in transporting toxic metals across cell membranes.
GSH transport and the disposition of endo- and xenobiotics
As illustrated in figures 1 and 2, GSH facilitates the plasma membrane transport of endo- and xenobiotics by at least four different mechanisms. Perhaps the best-known and most important role of GSH is in the formation of glutathione S-conjugates. Conjugation may occur either with the parent compound, its metabolites, and/or reactive metabolic intermediates that may be formed as a result of the processing of the compound. Because GSH adducts are typically excellent substrates for the Mrps, GSH conjugation is critical for the membrane transport and eventual elimination of many toxic chemicals of both endogenous and exogenous origin, including metals (Ballatori, 1994). In terms of metal binding, GSH contains six potential coordination sites for metals: the cysteinyl thiolate, the glutamyl amino, and the glycyl and glutamyl carboxyl groups, and the peptide linkages. Of these, the thiolate residue exhibits high affinity for a number of metals, including Hg, Cd, Cu, Zn, Ag, As and Pb (Ballatori, 1994). GSH modulates the disposition and toxicity of metals by at least four mechanisms: 1) it functions in the mobilization and delivery of metals between ligands; 2) it functions to transport metals across cell membranes as GSH-complexes; 3) serves as a source of cysteine, an amino acid that plays a central role in metal homeostasis; and 4) serves as a cofactor for redox reactions, yielding metal compounds with different speciation or biochemical forms (Ballatori, 1994).
Second, GSH appears to serve as a driving force for uptake of organic molecules into cells via the rat Oatp1 transporter (Fig. 1; Li et al., 1998). That is, the large outwardly-directed GSH electrochemical gradient serves to energize the uptake of drugs and other xenobiotics into the cells by an exchange mechanism (Li et al., 1998). However, more recent studies with two human OATPs demonstrated no role of GSH in their transport mechanism (Mahagita et al., 2007).
Third, MRP-mediated transport of many compounds occurs by a GSH-cotransport mechanism. As noted earlier, transport of certain compounds by MRP1-containing plasma membrane vesicles is increased in the presence of GSH (Loe et al., 1996, 1997; Mao et al., 2000; Morrow et al., 2006; Renes et al., 1999), and conversely, GSH depletion decreases transport of these compounds (Gekeler et al., 1995; Loe et al., 1998; Rappa et al., 1997;Salerno and Garnier-Suillerot, 2001; Schneider et al., 1995; Vanhoefer et al., 1996; Versantvoort et al., 1995). In support of a co-transport mechanism, vincristine, etoposide, and vinblastine have been shown to stimulate GSH transport (Loe et al., 1998; Mao et al., 2000; Rappa et al., 1997).
The fourth mechanism by which GSH facilitates membrane transport of endo- and xenobiotics is by stimulating MRP-mediated transport, but the tripeptide itself is not transported. It is still unknown what specific characteristics determine whether a substrate requires GSH for transport or cotransport (Conseil et al., 2005; Leslie et al., 2001a; Loe et al., 1998; Qian et al., 2001).
GSH transport during apoptosis
Apoptosis is critical throughout life, from embryonic development through adulthood in mammals. Dysregulation within apoptotic signaling pathways can result in too much or insufficient apoptosis, and this has been linked to several diseases, including cancer, neurodegenerative diseases, inflammation, and autoimmune diseases (Fadeel and Orrenius, 2005; Reed, 2002).
Intracellular GSH levels appear to regulate the ability of cells to undergo apoptosis. Experimentally increasing intracellular GSH decreases apoptosis (Chiba et al., 1996; Devadas et al., 2003; Wang, 2001), and alternatively, cells with less GSH and hence, less antioxidant capacity, are more susceptible to apoptotic stimuli (Anderson et al., 1999; Beaver and Waring, 1995; Chiba et al., 1996; Devadas et al., 2003; Haouzi et al., 2001). Furthermore, several studies demonstrate that depleting GSH is enough to induce apoptosis in cells sensitive to oxidative stress (Anderson et al., 1999; Celli et al., 1998; Merad-Boudia et al., 1998), and can accelerate apoptosis in apoptotic resistant cell lines (Chiba et al., 1996; Filomeni et al., 2005; Friesen et al., 2004). Overall, the data support a protective role for GSH against cellular demise. Although the exact mechanism is unknown, high GSH levels are likely protecting cells against reactive oxygen species and are facilitating the conjugation and elimination of reactive intermediates or signaling molecules (Vlachaki and Meyn, 1998).
Cells undergoing apoptosis also appear to rapidly and selectively release GSH into the extracellular space (Ghibelli et al., 1995; Ghibelli et al., 1998; Hammond et al., 2004 and 2007; He et al., 2003; van den Dobbelsteen et al., 1996). Most of the GSH can be recovered in the extracellular media within hours after apoptosis induction. Organic anion transport inhibitors decrease apoptotic GSH release and plasma membrane integrity is not compromised during this time interval, indicating that a specific transport mechanism is involved.
Both the MRP and OATP families of transporters have been implicated in apoptotic GSH export. Franco and colleagues (2006, 2007) suggested that the OATP transporters are responsible for GSH export from apoptotic cells; however, only indirect evidence was provided for this conclusion. In contrast, Hammond et al. (2007) demonstrated a direct role for the MRPs in apoptotic GSH export. Basal and apoptotic GSH release were decreased after RNAi reduction of MRP1 expression in Jurkat cells, indicating that MRP1 is a major player in both of these processes (Hammond et al., 2007). In addition, a comparison of two cell lines, one of which releases GSH during apoptosis (Jurkat cells) and one that does not (Raji cells), showed that differences exist in MRP1 localization and function. In Jurkat cells, MRP1 is largely localized to the plasma membrane and this MRP is functional, as evidenced by the export of calcein. Calcein export was further enhanced during apoptosis. In contrast, Raji cells have little MRP1 at the plasma membrane and did not export calcein under basal or apoptotic conditions, indicating that these cells lack functional MRPs at the plasma membrane (Hammond et al., 2007).
Additional evidence that MRP1 is directly involved in GSH release was provided by measuring basal and apoptotic GSH efflux in HEK293 cells overexpressing human MRP1 (Marchan et al., 2008). MRP1-overexpressing cells have lower intracellular GSH levels and higher levels of GSH release, under both basal conditions and after apoptosis induction. Despite the enhanced GSH efflux in MRP1-overexpressing cells, intracellular GSH levels are not further depleted after apoptotic induction, suggesting that there is an increase in GSH synthesis. The higher GSH levels may have protective effects as these cells are more resistant to apoptosis. Overall, these results indicate that MRP1 is a major mediator of both basal and apoptotic glutathione release. The enhanced GSH release, with a concurrent decrease of intracellular GSH, appears to be necessary for the progression of apoptosis (Marchan et al., 2008). Other studies have also associated increases in cytotoxicity for cells overexpressing MRP1 due to the loss of intracellular GSH (Laberge et al., 2007; Trompier et al., 2004).
Interestingly, there is also a direct connection between the Bcl-2 familiy of apoptotic proteins and GSH export. Cells overexpressing the antiapoptotic proteins Bcl-2 and Bcl-XL have higher levels of intracellular GSH and conversely, cells with low levels of Bcl-2 have lower intracellular GSH (Benlloch et al., 2005; Bojes et al., 1997; Ellerby et al., 1996; Ortega et al., 2003; Voehringer, 1999). The higher levels of intracellular GSH in cells with elevated Bcl-2 are achieved by a decrease in export rather than an increase in synthesis (Benlloch et al., 2005; Meredith et al., 1998; Ortega et al., 2003). The mechanism in which Bcl-2 prevents GSH export is not yet understood, although a recent paper suggests that there is an interaction between the BH3 domain of Bcl-2 and GSH (Zimmermann et al., 2007).
GSH and glutathione S-conjugate transport, and cell signaling
As summarized by Wang and Ballatori (1998), in addition to the multitude of foreign chemicals that require GSH for their detoxification and elimination, GSH is also required for the formation of specific biological mediators and for the disposition of many endogenous signaling molecules, including certain hormones, second messengers, and neurotransmitters. For example, GSH forms thioether conjugates with leukotrienes, prostaglandins, hepoxilin, nitric oxide, hydroxyalkenals, ascorbic acid, dopa, dopamine, and maleic acid, and it forms thioesters with cysteine, coenzyme A, proteins, and other cellular thiols. The glycine carboxyl group of GSH binds to the amino group of spermidine to produce glutathione-amides, in reactions catalyzed by glutathionylspermidine synthetase and trypanothione synthetase in bacterial systems. GSH also binds endogenous metals such as Cu, Se, Cr and Zn via nonenzymatic reactions (Ballatori, 1994).
Binding of GSH to these endogenous compounds serves several important roles: it serves to limit and regulate the reactivity of the chemicals, it facilitates their membrane transport and elimination from the cell and organism, and in some cases it leads to the formation of essential biological mediators. The cysteinyl leukotrienes, for example, are involved in inflammatory and anaphylactic reactions. The GSH conjugate of 9-deoxy-▴9,▴12-prostaglandin D2 may modulate the antiproliferative activity of the parent compound (Atsmon et al., 1990), whereas S-nitrosoglutathione, a relatively stable intermediate derived from the nonenzymatic reaction of nitric oxide with intracellular GSH, appears to have the same biological functions as nitric oxide itself (Ignarro, 1990). Recent studies by Alexander and coworkers (2006), demonstrate that GSH conjugation of nitrolinoleic acid and the subsequent MRP1-mediated transport of the conjugate can attenuate nitrolinoleic acid bioactivity and thereby play important roles in the regulation of cellular signaling. Nitrolinoleic acid is an electrophilic derivative of linoleic acid that has several important bioactivities including anti-inflammatory, anti-platelet, and vasorelaxation activities, and is a ligand of PPARgamma.
Many proteins are activated or inhibited in vitro by disulfide exchange between the protein and GSH or GSSG (Dalle-Donne et al., 2007; Ghezzi and Disimplicio, 2007; Lillig et al., 2008; Shelton Mieyal, 2008; Townsend, 2007; Ziegler, 1985). For example, GSSG can activate enzymes such as glucose-6-phosphatase, acid phosphatase, γ-aminolaevulinate synthetase, and fructose 1,6-bisphosphatase, whereas it inhibits glycogen synthetase, pyruvate kinase, adenylate cyclase, phosphorylase/phosphatase, ribonucleotide reductase, phosphofructokinase, glycogen debranching enzyme, and fatty acid synthase.
GSH and Cell Proliferation and Cell Differentiation
The ratio of GSH to GSSG reflects the intracellular thiol redox environment. Thiol redox environment is a critical determinant of cell function, and is thought to modulate major cell processes, including cell differentiation (Anselmo and Cobb, 2004; Ardite et al., 2004; Henmi et al., 2001; Huh et al., 2005; Kim et al., 2004; Chenais et al., 2000; Smith et al., 2000), proliferation (Kang et al., 1994; Shaw and Chou, 1986; Suthanthiran et al., 1990), and apoptosis (Ghibelli et al., 1995; Hall, 1999; Voehringer, 1999). It does so through affecting signal transduction, (Blackburn et al., 1999; Staal et al., 1990; Suzuki et al., 1997), gene expression (Arrigo, 1999; Hammond et al., 2001), and modulating protein function (Barrett et al., 1999; Dinkova-Kostova et al., 2002; Staal et al., 1994).
It has become clear in recent years that redox modification of intracellular proteins plays a significant role in cell signaling, not only in periods of oxidative stress, but under physiological conditions as well (Chiarugi and Cirri, 2003; Filomeni et al., 2005; Forman et al., 2004; Salmeen and Barford, 2005; Sauer et al., 2001; Sen, 2000). The sulfhydryl group in cysteine residues appears to be a primary target for redox regulation, and many enzymes and transcription factors contain cysteine in their active sites or in their binding domains. These cysteine residues exist in the thiolate anion form at physiological pH, making them excellent targets for specific and reversible redox reactions (Dalle-Donne et al., 2007).
The ability of redox signaling events to take place depends largely on the availability of redox-active thiol groups. Although a few proteins have been identified as thiol-sensitive, the specific mechanisms as to how thiols affect protein function remain unclear. Some possible mechanisms through which GSH affects cell signaling include the scavenging of free radicals, maintenance of protein thiol status, and the covalent binding to cysteine moieties of cytosolic proteins (S-glutathionylation). Although all of these mechanisms are likely contributing to thiol-associated effects on cell processes, S-glutathionylation has come to the forefront as a viable redox regulatory mechanism (Anselmo and Cobb, 2004; Fratelli et al., 2005; Ghezzi, 2005; Ghezzi et al., 2005; Giustarini et al., 2004).
In particular, S-glutathionylation has been identified as a regulator of signaling proteins, transcription factors, ras proteins, ion channels and pumps, mitochondrial proteins, as well as cytoskeletal proteins, some of which play roles in cell differentiation (Dalle-Donne et al., 2007; Ghezzi and Disimplicio, 2007; Lillig et al., 2008; Shelton Mieyal, 2008; Townsend, 2007; Ziegler, 1985). The targets of these reactions are proteins that contain cysteine residues in their catalytic, activation, and/or binding domains. Recently, S-glutathionylation has been implicated in the expression of a number of genes involved in NFkB activation, transcription (AP-1, JNK), DNA methylation, and immune function (cytokines and cytokine receptors) (Fratelli et al., 2005). S-Glutathionylation occurs under both basal conditions and during periods of oxidative stress (Chai et al., 2003; Lind et al., 2002; Reynaert et al., 2006).
The intracellular redox environment also influences cellular differentiation. However, the effects of thiols on differentiation are quite varied and in many aspects contradictory. Whereas some reports suggest that thiol supplementation maintains a cell’s proliferative capacity or inhibits differentiation (Chenais et al., 2000; Debbas et al., 2007; Fidelus et al., 1987; Iemata et al., 2007; Mayer and Noble, 1994), other reports demonstrate that increasing thiol levels promotes differentiation or decreases proliferative capacity (Jun et al., 2008; Kim et al., 2001; Paranjpe et al., 2007; Parasassi et al., 2005). Likewise, some studies report that thiol depletion enhances differentiation (Benard and Balasubramanian, 1997; Davison et al., 2003; Dietrich et al., 2006; Smith et al., 2000), whereas others suggest that it inhibits the process (Esposito et al., 1994; Hansen et al., 2001; Huh et al., 2005; Kim et al., 2004). Many of these discrepancies are undoubtedly due to differences in cell type. Careful consideration must be paid not only to the cell type, but normal versus immortal status, the background activity of cell signaling pathways, endogenous GSH levels, as well as the extent and timing of thiol supplementation or depletion. A spectrum of thiol redox conditions can exist within a cell, with either extreme (i.e., too reduced or too oxidized) adversely affecting cell physiology and perhaps creating similar effects. Although data interpretation remains complicated and no coalescing theory has been established, it is clear that thiol status affects the differentiation program. Additional research is needed to elucidate how thiol status affects differentiation and proliferation and to identify the cellular factors that mediate these effects.
Summary
Recent studies provide strong evidence that GSH is a substrate for the Mrp proteins, and thus indicate that these proteins play an important role in cellular GSH homeostasis. Because the Mrp proteins also mediate export of GSSG and glutathione S-conjugates and complexes, they likely contribute to the control of cellular redox status, delivery of cysteine to other tissues, export of signaling molecules, elimination of xenobiotics and reactive metabolic intermediates, as well as to cell differentiation, proliferation, and apoptosis.
Acknowledgments
Preparation of this overview was supported in part by National Institute of Health Grants DK48823 and DK67214, NIEHS Center Grants ES01247 and ES03828, and Environmental Toxicology Training Grant ES07026.
Abbreviations
- ABC
ATP-binding cassette
- BSO
L-buthionine (S,R)-sulfoximine
- CFTR
cystic fibrosis transmembrane conductance regulator
- EHBR
Sprague Dawley Eisai hyperbilirubinuric rat, mutant rat strain that lacks Mrp2 activity
- GY
Groningen Yellow rat, Wistar rat strain that lacks Mrp2 activity
- GSH
reduced glutathione
- GS-NO
S-nitrosoglutathione
- GSSG
glutathione disulfide
- MDR1
multidrug resistance protein 1
- MRP and Mrp
multidrug resistance-associated protein
- OATP and Oatp
organic anion transporting polypeptide
- TR-
transport deficient rat, Wistar rat strain that lacks Mrp2 activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander RL, Bates DJ, Wright MW, King SB, Morrow CS. Modulation of nitrated lipid signaling by multidrug resistance protein 1 (MRP1): glutathione conjugation and MRP1-mediated efflux inhibit nitrolinoleic acid-induced, PPARgamma-dependent transcription activation. Biochemistry. 2006;45:7889–7896. doi: 10.1021/bi0605639. [DOI] [PubMed] [Google Scholar]
- Anderson CP, Tsai JM, Meek WE, Liu RM, Tang Y, Forman HJ, Reynolds CP. Depletion of glutathione by buthionine sulfoxine is cytotoxic for human neuroblastoma cell lines via apoptosis. Exp Cell Res. 1999;246 (1):183–192. doi: 10.1006/excr.1998.4303. [DOI] [PubMed] [Google Scholar]
- Anselmo AN, Cobb MH. Protein kinase function and glutathionylation. Biochem J. 2004;381:e1–2. doi: 10.1042/BJ20040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracena P, Sánchez G, Donoso P, Hamilton SL, Hidalgo C. S-Glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J Biol Chem. 2003;278(44):42927–42935. doi: 10.1074/jbc.M306969200. [DOI] [PubMed] [Google Scholar]
- Ardite E, Barbera JA, Roca J, Fernandez-Checa JC. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am J Pathol. 2004;165:719–728. doi: 10.1016/s0002-9440(10)63335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med. 1999;27:936–944. doi: 10.1016/s0891-5849(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Atsmon J, Freeman ML, Meredith MJ, Sweetman BJ, Roberts LJ. Conjugation of 9-deoxy-▴9, ▴ 12(E)-prostaglandin D2 with intracellular glutathione and enhancement of its antiproliferative activity by glutathione depletion. Cancer Res. 1990;50:1879–1885. [PubMed] [Google Scholar]
- Bakos E, Evers R, Sinko E, Varadi A, Borst P, Sarkadi B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol. 2000;57 (4):760–768. doi: 10.1124/mol.57.4.760. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Glutathione mercaptides as transport forms of metals. Adv Pharmacol. 1994;27:271–298. doi: 10.1016/s1054-3589(08)61036-4. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect. 2002;110 (Suppl 5):689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N, Dutczak WJ. Identification and characterization of high and low affinity transport systems for reduced glutathione in liver cell canalicular membranes. J Biol Chem. 1994;269 (31):19731–19737. [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Relation between biliary glutathione excretion and bile acid- independent bile flow. Am J Physiol. 1989;256(1 Pt 1):G22–G30. doi: 10.1152/ajpgi.1989.256.1.G22. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992;263(5 Pt 1):G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: Role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204 (3):238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Moseley RH, Boyer JL. Sodium gradient-dependent L-glutamate transport is localized to the canalicular domain of liver plasma membranes. Studies in rat liver sinusoidal and canalicular membrane vesicles. J Biol Chem. 1986a;261:6216–6221. [PubMed] [Google Scholar]
- Ballatori N, Jacob R, Boyer JL. Intrabiliary glutathione hydrolysis - a source of glutamate in bile. J Biol Chem. 1986b;261:7860–7865. [PubMed] [Google Scholar]
- Ballatori N, Jacob R, Barrett C, Boyer JL. Biliary catabolism of glutathione and differential reabsorption of its amino acid constituents. Am J Physiol. 1988;254:G1–G7. doi: 10.1152/ajpgi.1988.254.1.G1. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT, Ma AK, Boyer JL. Determinants of glutathione efflux and biliary GSH/GSSG ratio in perfused rat liver. Am J Physiol. 1989;256:G482–G490. doi: 10.1152/ajpgi.1989.256.3.G482. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Gatmaitan Z, Truong AT. Impaired biliary excretion and whole body elimination of methylmercury in rats with a congenital defect in biliary glutathione excretion. Hepatology. 1995;22:1469–1473. [PubMed] [Google Scholar]
- Bánhegyi G, Csala M, Nagy G, Sorrentino V, Fulceri R, Benedetti A. Evidence for the transport of glutathione through ryanodine receptor channel type 1. Biochem J. 2003;376(Pt 3):807–812. doi: 10.1042/BJ20031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- Beaver JP, Waring P. A decrease in intracellular glutathione concentration precedes the onset of apoptosis in murine thymocytes. Eur J Cell Biol. 1995;68 (1):47–54. [PubMed] [Google Scholar]
- Beck K, Hayashi K, Dang K, Hayashi M, Boyd CD. Analysis of ABCC6 (MRP6) in normal human tissues. Histochem Cell Biol. 2005;123 (4–5):517–528. doi: 10.1007/s00418-004-0744-3. [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD. Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst. 1998;90:1735–1741. doi: 10.1093/jnci/90.22.1735. [DOI] [PubMed] [Google Scholar]
- Benlloch M, Ortega A, Ferrer P, Segarra R, Obrador E, Asensi M, Carretero J, Estrela JM. Acceleration of glutathione efflux and inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells to endothelium-induced cytotoxicity. J Biol Chem. 2005;280 (8):6950–6959. doi: 10.1074/jbc.M408531200. [DOI] [PubMed] [Google Scholar]
- Benard O, Balasubramanian KA. Modulation of glutathione level during butyrate-induced differentiation in human colon derived HT-29 cells. Mol Cell Biochem. 1997;170:109–114. doi: 10.1023/a:1006892929652. [DOI] [PubMed] [Google Scholar]
- Blackburn RV, Spitz DR, Liu X, Galoforo SS, Sim JE, Ridnour LA, Chen JC, Davis BH, Corry PM, Lee YJ. Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells. Free Radic Biol Med. 1999;26:419–430. doi: 10.1016/s0891-5849(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Bojes HK, Datta K, Xu J, Chin A, Simonian P, Nunez G, Kehrer JP. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem J. 1997;325 (Pt 2):315–319. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, Oude Elferink RPJ. Mammalian ABC transporters in health and disease. Ann Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Eur J Physiol. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wijnholds J. A Family of Drug Transporters: the Multidrug Resistance-Associated Proteins. J Natl Cancer Inst. 2000;92(16):1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Buchler M, Konig J, Brom M, Kartenbeck J, Spring H, Horie T, Keppler D. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271 (25):15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol. 1998;275 (4 Pt 1):G749–G757. doi: 10.1152/ajpgi.1998.275.4.G749. [DOI] [PubMed] [Google Scholar]
- Chai YC, Hoppe G, Sears J. Reversal of protein S-glutathiolation by glutaredoxin in the retinal pigment epithelium. Exp Eye Res. 2003;76:155–159. doi: 10.1016/s0014-4835(02)00309-3. [DOI] [PubMed] [Google Scholar]
- Chenais B, Andriollo M, Guiraud P, Belhoussine R, Jeannesson P. Oxidative stress involvement in chemically induced differentiation of K562 cells. Free Radic Biol Med. 2000;28:18–27. doi: 10.1016/s0891-5849(99)00195-1. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- Chiba T, Takahashi S, Sato N, Ishii S, Kikuchi K. Fas-mediated apoptosis is modulated by intracellular glutathione in human T cells. Eur J Immunol. 1996;26 (5):1164–1169. doi: 10.1002/eji.1830260530. [DOI] [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, Evers R. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2) J Pharmacol Exp Ther. 2006;317 (2):579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci. 2006;27:438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258 (5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Conseil G, Deeley RG, Cole SP. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet Genomics. 2005;15 (8):523–533. doi: 10.1097/01.fpc.0000167333.38528.ec. [DOI] [PubMed] [Google Scholar]
- Csala M, Fulceri R, Mandl J, Benedetti A, Bánhegyi G. Ryanodine receptor channel-dependent glutathione transport in the sarcoplasmic reticulum of skeletal muscle. Biochem Biophys Res Commun. 2001;287(3):696–700. doi: 10.1006/bbrc.2001.5648. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55 (5):929–937. [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–940. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- Debbas V, Arai RJ, Ferderbar S, Schindler F, Stern A, Monteiro HP. Regulation of p21Waf1 expression and TNFalpha biosynthesis by glutathione modulators in PMA induced-THP1 differentiation: involvement of JNK and ERK pathways. Biochem Biophys Res Commun. 2007;363:965–970. doi: 10.1016/j.bbrc.2007.09.091. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Devadas S, Hinshaw JA, Zaritskaya L, Williams MS. Fas-stimulated generation of reactive oxygen species or exogenous oxidative stress sensitize cells to Fas-mediated apoptosis. Free Radic Biol Med. 2003;35 (6):648–661. doi: 10.1016/s0891-5849(03)00391-5. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra M, Kuipers F, Havinga R, Smit EP, Vonk RJ. Bile secretion of trace elements in rats with a congenital defect in hepatobiliary transport of glutathione. Pediatr Res. 1990;28 (4):339–343. doi: 10.1203/00006450-199010000-00008. [DOI] [PubMed] [Google Scholar]
- Dreuw A, Hermanns HM, Heise R, Joussen S, Rodríguez F, Marquardt Y, Jugert F, Merk HF, Heinrich PC, Baron JM. Interleukin-6-type cytokines upregulate expression of multidrug resistance-associated proteins in NHEK and dermal fibroblasts. J Invest Dermatol. 2005;124(1):28–37. doi: 10.1111/j.0022-202X.2004.23499.x. [DOI] [PubMed] [Google Scholar]
- Duan DY, Liu LL, Bozeat N, Huang ZM, Xiang SY, Wang GL, Ye L, Hume JR. Functional role of anion channels in cardiac diseases. Acta Pharmacol Sin. 2005;26 (3):265–278. doi: 10.1111/j.1745-7254.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. J Neurochem. 1996;67 (3):1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- Englund G, Rorsman F, Rönnblom A, Karlbom U, Lazorova L, Gråsjö J, Kindmark A, Artursson P. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29 (3–4):269–277. doi: 10.1016/j.ejps.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Esposito F, Agosti V, Morrone G, Morra F, Cuomo C, Russo T, Venuta S, Cimino F. Inhibition of the differentiation of human myeloid cell lines by redox changes induced through glutathione depletion. Biochem J. 1994;301 (Pt 3):649–653. doi: 10.1042/bj3010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers R, de Haas M, Sparidans R, Beijnen J, Wielinga PR, Lankelma J, Borst P. Vinblastine and sulfinpyrazone export by the multidrug resistance protein MRP2 is associated with glutathione export. Br J Cancer. 2000;83 (3):375–383. doi: 10.1054/bjoc.2000.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258 (6):479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204 (3):263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Takikawa H, Horie T, Ookhtens M, Kaplowitz N. Canalicular transport of reduced glutathione in normal and mutant Eisai hyperbilirubinemic rats. J Biol Chem. 1992;267 (3):1667–1673. [PubMed] [Google Scholar]
- Fidelus RK, Ginouves P, Lawrence D, Tsan MF. Modulation of intracellular glutathione concentrations alters lymphocyte activation and proliferation. Exp Cell Res. 1987;170 (2):269–275. doi: 10.1016/0014-4827(87)90305-3. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR. Disulfide relays and phosphorylative cascades: partners in redox-mediated signaling pathways. Cell Death Differ. 2005;12:1555–1563. doi: 10.1038/sj.cdd.4401754. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- Fratelli M, Goodwin LO, Orom UA, Lombardi S, Tonelli R, Mengozzi M, Ghezzi P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci USA. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281 (40):29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- Franco R, Panayiotidis MI, Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem. 2007;282 (42):30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey IM, Rubio-Aliaga I, Siewert A, Sailer D, Drobyshev A, Beckers J, de Angelis MH, Aubert J, Bar Hen A, Fiehn O, Eichinger HM, Daniel H. Profiling at mRNA, protein, and metabolite levels reveals alterations in renal amino acid handling and glutathione metabolism in kidney tissue of Pept2−/− mice. Physiol Genomics. 2007;28 (3):301–310. doi: 10.1152/physiolgenomics.00193.2006. [DOI] [PubMed] [Google Scholar]
- Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ Suppl. 2004;1:S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol. 1999;277:L113–L118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- Gao L, Broughman JR, Iwamoto T, Tomich JM, Venglarik CJ, Forman HJ. Synthetic chloride channel restores glutathione secretion in cystic fibrosis airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L24–L30. doi: 10.1152/ajplung.2001.281.1.L24. [DOI] [PubMed] [Google Scholar]
- Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208 (1):345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- Ghezzi P. Regulation of protein function by glutathionylation. Free Radic Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Di Simplicio P. Glutathionylation pathways in drug response. Curr Opin Pharmacol. 2007;7 (4):398–403. doi: 10.1016/j.coph.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Ghibelli L, Coppola S, Rotilio G, Lafavia E, Maresca V, Ciriolo MR. Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem Biophys Res Commun. 1995;216:313–320. doi: 10.1006/bbrc.1995.2626. [DOI] [PubMed] [Google Scholar]
- Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C, Civitareale P, Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. Faseb J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med. 2004;8:201–212. doi: 10.1111/j.1582-4934.2004.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AG. Review: The role of glutathione in the regulation of apoptosis. Eur J Clin Invest. 1999;29:238–245. doi: 10.1046/j.1365-2362.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Lee TK, Ballatori N. Novel roles for glutathione in gene expression, cell death, and membrane transport of organic solutes. J Hepatol. 2001;34 (6):946–954. doi: 10.1016/s0168-8278(01)00037-x. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Madejczyk MS, Ballatori N. Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol. 2004;195 (1):12–22. doi: 10.1016/j.taap.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282:14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Carney EW, Harris C. Altered differentiation in rat and rabbit limb bud micromass cultures by glutathione modulating agents. Free Radic Biol Med. 2001;31:1582–1592. doi: 10.1016/s0891-5849(01)00751-1. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Lekehal M, Tinel M, Vadrot N, Caussanel L, Lettéron P, Moreau A, Feldmann G, Fau D, Pessayre D. Prolonged, but not acute, glutathione depletion promotes Fas-mediated mitochondrial permeability transition and apoptosis in mice. Hepatology. 2001;33:1181–1188. doi: 10.1053/jhep.2001.24235. [DOI] [PubMed] [Google Scholar]
- He YY, Huang JL, Ramirez DC, Chignell CF. Role of reduced glutathione efflux in apoptosis of immortalized human keratinocytes induced by UVA. J Biol Chem. 2003;278 (10):8058–8064. doi: 10.1074/jbc.M207781200. [DOI] [PubMed] [Google Scholar]
- Henmi K, Tsuboi S, Demura T, Fukuda H, Iwabuchi M, Ogawa KI. A possible role of glutathione and glutathione disulfide in tracheary element differentiation in the cultured mesophyll cells of Zinnia elegans. Plant Cell Physiol. 2001;42:673–676. doi: 10.1093/pcp/pce072. [DOI] [PubMed] [Google Scholar]
- Hihnala S, Kujala M, Toppari J, Kere J, Holmberg C, Höglund P. Expression of SLC26A3, CFTR and NHE3 in the human male reproductive tract: role in male subfertility caused by congenital chloride diarrhoea. Mol Hum Reprod. 2006;12 (2):107–111. doi: 10.1093/molehr/gal009. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol. 1990;40 (5):1131–1135. doi: 10.1016/0006-2952(90)90503-d. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J Toxicol Environ Health. 1994;41:387–409. doi: 10.1080/15287399409531852. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Matsumoto H, Simmons TW, Ballatori N. Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. J Biol Chem. 1991;266:22179–22185. [PubMed] [Google Scholar]
- Hinchman CA, Truong AT, Ballatori N. Hepatic uptake of intact glutathione S-conjugate, inhibition by organic anions, and sinusoidal catabolism. Am J Physiol. 1993;265:G547–G554. doi: 10.1152/ajpgi.1993.265.3.G547. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Rebbeor JF, Ballatori N. Efficient hepatic uptake and concentrative biliary excretion of a mercapturic acid. Am J Physiol. 1998;275:G612–G619. doi: 10.1152/ajpgi.1998.275.4.G612. [DOI] [PubMed] [Google Scholar]
- Huh YJ, Kim JM, Kim H, Song H, So H, Lee SY, Kwon SB, Kim HJ, Kim HH, Lee SH, Choi Y, Chung SC, Jeong DW, Min BM. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2005;13 (7):1138–1146. doi: 10.1038/sj.cdd.4401793. [DOI] [PubMed] [Google Scholar]
- Iemata M, Takarada T, Hinoi E, Taniura H, Yoneda Y. Suppression by glutamate of proliferative activity through glutathione depletion mediated by the cystine/glutamate antiporter in mesenchymal C3H10T1/2 stem cells. J Cell Physiol. 2007;213:721–729. doi: 10.1002/jcp.21145. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Ikuma M, Welsh MJ. Regulation of CFTR Cl- channel gating by ATP binding and hydrolysis. Proc Natl Acad Sci USA. 2000;97:8675–8680. doi: 10.1073/pnas.140220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, Sarkadi B, Varadi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- Jansen PL, Peters WH, Lamers WH. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985;5 (4):573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Hoffmann U, Kroemer HK. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin Drug Metab Toxicol. 2006;2 (3):351–366. doi: 10.1517/17425255.2.3.351. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Leier I, Buchholz U, Center M, Keppler D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994;54 (18):4833–4836. [PubMed] [Google Scholar]
- Jun JH, Lee SH, Kwak HB, Lee ZH, Seo SB, Woo KM, Ryoo HM, Kim GS, Baek JH. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J Cell Biochem. 2008;103 (4):1246–1255. doi: 10.1002/jcb.21508. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Feng Y, Hatcher EL. Glutathione stimulates A549 cell proliferation in glutamine-deficient culture: the effect of glutamate supplementation. J Cell Physiol. 1994;161:589–596. doi: 10.1002/jcp.1041610323. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chen ZS, Wada M, Uchiumi T, Ono M, Akiyama S, Kuwano M. Enhanced transport of anticancer agents and leukotriene C4 by the human canalicular multispecific organic anion transporter (cMOAT/MRP2) FEBS Lett. 1999;456 (2):327–331. doi: 10.1016/s0014-5793(99)00979-5. [DOI] [PubMed] [Google Scholar]
- Keppler D, Leier I, Jedlitschky G. Transport of glutathione conjugates and glucuronides by the multidrug resistance proteins MRP1 and MRP2. Biol Chem. 1997;378:787–791. [PubMed] [Google Scholar]
- Kim JM, Kim H, Kwon SB, Lee SY, Chung SC, Jeong DW, Min BM. Intracellular glutathione status regulates mouse bone marrow monocyte-derived macrophage differentiation and phagocytic activity. Biochem Biophys Res Commun. 2004;325:101–108. doi: 10.1016/j.bbrc.2004.09.220. [DOI] [PubMed] [Google Scholar]
- Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- Klein M, Mamnun YM, Eggmann T, Schuller C, Wolfger H, Martinoia E, Kuchler K. The ATP-binding cassette (ABC) transporter Bpt1p mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett. 2002;520:63–67. doi: 10.1016/s0014-5793(02)02767-9. [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Huan LJ, Wang Y, Bear CE. Perturbation of the pore of the cystic fibrosis transmembrane conductance regulator (CFTR) inhibits its ATPase activity. J Biol Chem. 2001;276:11575–11581. doi: 10.1074/jbc.M010403200. [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. Embo J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Kawabe T, Tanaka T, Toh S, Uchiumi T, Wada M, Akiyama S, Ono M, Kuwano M. A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res. 1997;57 (24):5475–5479. [PubMed] [Google Scholar]
- Kolberg A, Rosa TG, Puhl MT, Scola G, da Rocha Janner D, Maslinkiewicz A, Lagranha DJ, Heck TG, Curi R, de Bittencourt PI., Jr Low expression of MRP1/GS-X pump ATPase in lymphocytes of Walker 256 tumour-bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Cell Biochem Funct. 2006;24 (1):23–39. doi: 10.1002/cbf.1290. [DOI] [PubMed] [Google Scholar]
- König J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461 (2):377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- Krause MS, Oliveira LP, Jr, Silveira EM, Vianna DR, Rossato JS, Almeida BS, Rodrigues MF, Fernandes AJ, Costa JA, Curi R, de Bittencourt PI., Jr MRP1/GS-X pump ATPase expression: is this the explanation for the cytoprotection of the heart against oxidative stress-induced redox imbalance in comparison to skeletal muscle cells? Cell Biochem Funct. 2007;25:23–32. doi: 10.1002/cbf.1343. [DOI] [PubMed] [Google Scholar]
- Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen Z. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007a;453:675–684. doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG, Gallo JM, Lee K. Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice. Cancer Metastasis Rev. 2007b;26 (1):5–14. doi: 10.1007/s10555-007-9039-1. [DOI] [PubMed] [Google Scholar]
- Kurisu H, Kamisaka K, Koyo T, Yamasuge S, Igarashi H, Maezawa H, Uesugi T, Tagaya O. Organic anion transport study in mutant rats with autosomal recessive conjugated hyperbilirubinemia. Life Sci. 1991;49 (14):1003–1011. doi: 10.1016/0024-3205(91)90301-q. [DOI] [PubMed] [Google Scholar]
- Laberge RM, Karwatsky J, Lincoln MC, Leimanis ML, Georges E. Modulation of GSH levels in ABCC1 expressing tumor cells triggers apoptosis through oxidative stress. Biochem Pharmacol. 2007;73 (11):1727–1737. doi: 10.1016/j.bcp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lacueva J, Perez-Ramos M, Soto JL, Oliver I, Andrada E, Medrano J, Perez-Vazquez T, Arroyo A, Carrato A, Ferragut JA, Calpena R. Multidrug resistance-associated protein (MRP1) gene is strongly expressed in gastric carcinomas. Analysis by immunohistochemistry and real-time quantitative RT-PCR. Histopathology. 2005;46 (4):389–395. doi: 10.1111/j.1365-2559.2005.02100.x. [DOI] [PubMed] [Google Scholar]
- Lai L, Tan TM. Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem J. 2002;361:497–503. doi: 10.1042/0264-6021:3610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH. Role of glutathione transport processes in kidney function. Toxicol Appl Pharmacol. 2005;204(3):329–342. doi: 10.1016/j.taap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2007;163 (12):54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautier D, Canitrot Y, Deeley RG, Cole SP. Multidrug resistance mediated by the multidrug resistance protein (MRP) gene. Biochem Pharmacol. 1996;52 (7):967–977. doi: 10.1016/0006-2952(96)00450-9. [DOI] [PubMed] [Google Scholar]
- Lee K, Belinsky MG, Bell DW, Testa JR, Kruh GD. Isolation of MOAT-B, a widely expressed multidrug resistance-associated protein/canalicular multispecific organic anion transporter-related transporter. Cancer Res. 1998;58:2741–2747. [PubMed] [Google Scholar]
- Lee K, Klein-Szanto AJ, Kruh GD. Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst. 2000;92:1934–1940. doi: 10.1093/jnci/92.23.1934. [DOI] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994;269 (45):27807–27810. [PubMed] [Google Scholar]
- Leslie EM, Ito K, Upadhyaya P, Hecht SS, Deeley RG, Cole SP. Transport of the beta -O-glucuronide conjugate of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) by the multidrug resistance protein 1 (MRP1). Requirement for glutathione or a non-sulfur-containing analog. J Biol Chem. 2001a;276 (30):27846–27854. doi: 10.1074/jbc.M102453200. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001b;167 (1):3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Bowers RJ, Deeley RG, Cole SP. Structural requirements for functional interaction of glutathione tripeptide analogs with the human multidrug resistance protein 1 (MRP1) J Pharmacol Exp Ther. 2003;304 (2):643–653. doi: 10.1124/jpet.102.044073. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: a role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem. 1998;273 (26):16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Ballatori N. Functional re-evaluation of the putative glutathione transporters, RcGshT and RsGshT. Yale J Biol Med. 1997;70:301–310. [PMC free article] [PubMed] [Google Scholar]
- Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, von Lowenhielm HB, Holmgren A, Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am J Physiol. 1998;275:C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Substrates of multidrug resistance-associated proteins block the cystic fibrosis transmembrane conductance regulator chloride channel. Br J Pharmacol. 1999;126:1471–1477. doi: 10.1038/sj.bjp.0702458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem. 1996;271 (16):9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- Loe DW, Deeley RG, Cole SP. Characterization of vincristine transport by the M(r) 190,000 multidrug resistance protein (MRP): evidence for cotransport with reduced glutathione. Cancer Res. 1998;58 (22):5130–5136. [PubMed] [Google Scholar]
- Loe DW, Deeley RG, Cole SP. Verapamil stimulates glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1) J Pharmacol Exp Ther. 2000;293 (2):530–538. [PubMed] [Google Scholar]
- Loe DW, Stewart RK, Massey TE, Deeley RG, Cole SP. ATP-dependent transport of aflatoxin B1 and its glutathione conjugates by the product of the multidrug resistance protein (MRP) gene. Mol Pharmacol. 1997;51 (6):1034–1041. doi: 10.1124/mol.51.6.1034. [DOI] [PubMed] [Google Scholar]
- Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57:5238–5242. [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol. 2007;293 (1):G271–G278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- Mao Q, Deeley RG, Cole SP. Functional reconstitution of substrate transport by purified multidrug resistance protein MRP1 (ABCC1) in phospholipid vesicles. J Biol Chem. 2000;275 (44):34166–34172. doi: 10.1074/jbc.M004584200. [DOI] [PubMed] [Google Scholar]
- Marchan R, Hammond CL, Ballatori N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.06.011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Noble M. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer MA, Breen MA, White NL, Matthews N. pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem. 1999;274 (33):23541–23548. doi: 10.1074/jbc.274.33.23541. [DOI] [PubMed] [Google Scholar]
- McCarthy VA, Harris A. The CFTR gene and regulation of its expression. Pediatr Pulmonol. 2005;40 (1):1–8. doi: 10.1002/ppul.20199. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A, Tate SS. Glutathione and related γ-glutamyl compounds: biosynthesis and utilization. Ann Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Merad-Boudia M, Nicole A, Santiard-Baron D, Saille C, Ceballos-Picot I. Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease. Biochem Pharmacol. 1998;56 (5):645–655. doi: 10.1016/s0006-2952(97)00647-3. [DOI] [PubMed] [Google Scholar]
- Meredith MJ, Cusick CL, Soltaninassab S, Sekhar KS, Lu S, Freeman ML. Expression of Bcl-2 increases intracellular glutathione by inhibiting methionine-dependent GSH efflux. Biochem Biophys Res Commun. 1998;248 (3):458–463. doi: 10.1006/bbrc.1998.8998. [DOI] [PubMed] [Google Scholar]
- Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2006;97 (2):373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- Morales MM, Carroll TP, Morita T, Schwiebert EM, Devuyst O, Wilson PD, Lopes AG, Stanton BA, Dietz HC, Cutting GR, Guggino WB. Both the wild type and a functional isoform of CFTR are expressed in kidney. Am J Physiol. 1996;270 (6 Pt 2):F1038–F1048. doi: 10.1152/ajprenal.1996.270.6.F1038. [DOI] [PubMed] [Google Scholar]
- Morrow CS, Peklak-Scott C, Bishwokarma B, Kute TE, Smitherman PK, Townsend AJ. Multidrug resistance protein 1 (MRP1, ABCC1) mediates resistance to mitoxantrone via glutathione-dependent drug efflux. Mol Pharmacol. 2006;69 (4):1499–1505. doi: 10.1124/mol.105.017988. [DOI] [PubMed] [Google Scholar]
- Mueller CF, Widder JD, McNally JS, McCann L, Jones DP, Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ Res. 2005;97 (7):637–644. doi: 10.1161/01.RES.0000183734.21112.b7. [DOI] [PubMed] [Google Scholar]
- Mylona P, Glazier JD, Greenwood SL, Sides MK, Sibley CP. Expression of the cystic fibrosis (CF) and multidrug resistance (MDR1) genes during development and differentiation in the human placenta. Mol Hum Reprod. 1996;2 (9):693–698. doi: 10.1093/molehr/2.9.693. [DOI] [PubMed] [Google Scholar]
- Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453 (5):643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Ortega A, Ferrer P, Carretero J, Obrador E, Asensi M, Pellicer JA, Estrela JM. Down-regulation of glutathione and Bcl-2 synthesis in mouse B16 melanoma cells avoids their survival during interaction with the vascular endothelium. J Biol Chem. 2003;278 (41):39591–39599. doi: 10.1074/jbc.M303753200. [DOI] [PubMed] [Google Scholar]
- Oude Elferink RP, de Haan J, Lambert KJ, Hagey LR, Hofmann AF, Jansen PL. Selective hepatobiliary transport of nordeoxycholate side chain conjugates in mutant rats with a canalicular transport defect. Hepatology. 1989;9 (6):861–865. doi: 10.1002/hep.1840090612. [DOI] [PubMed] [Google Scholar]
- Paranjpe A, Cac–lano NA, Hume WR, Jewett A. N-acetylcysteine protects dental pulp stromal cells from HEMA-induced apoptosis by inducing differentiation of the cells. Free Radic Biol Med. 2007;43:1394–1408. doi: 10.1016/j.freeradbiomed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T, Brunelli R, Bracci-Laudiero L, Greco G, Gustafsson AC, Krasnowska EK, Lundeberg J, Lundeberg T, Pittaluga E, Romano MC, Serafino A. Differentiation of normal and cancer cells induced by sulfhydryl reduction: biochemical and molecular mechanisms. Cell Death Differ. 2005;12:1285–1296. doi: 10.1038/sj.cdd.4401663. [DOI] [PubMed] [Google Scholar]
- Patrizio P, Salameh WA. Expression of the cystic fibrosis transmembrane conductance regulator (CFTR) mRNA in normal and pathological adult human epididymis. J Reprod Fertil Suppl. 1998;53:261–270. [PubMed] [Google Scholar]
- Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, Oude Elferink RP. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J. 1999;338 (Pt 2):393–401. [PMC free article] [PubMed] [Google Scholar]
- Qian YM, Song WC, Cui H, Cole SP, Deeley RG. Glutathione stimulates sulfated estrogen transport by multidrug resistance protein 1. J Biol Chem. 2001;276 (9):6404–6411. doi: 10.1074/jbc.M008251200. [DOI] [PubMed] [Google Scholar]
- Rappa G, Lorico A, Flavell RA, Sartorelli AC. Evidence that the multidrug resistance protein (MRP) functions as a co-transporter of glutathione and natural product toxins. Cancer Res. 1997;57 (23):5232–5237. [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Dumont ME, Ballatori N. ATP-Dependent transport of reduced glutathione in yeast secretory vesicles. Biochem J. 1998a;334:723–729. doi: 10.1042/bj3340723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Dumont ME, Ballatori N. ATP-Dependent transport of reduced glutathione on YCF1, the yeast orthologue of mammalian multidrug resistance associated proteins. J Biol Chem. 1998b;273:33449–33454. doi: 10.1074/jbc.273.50.33449. [DOI] [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Henson JH, Boyer JL, Ballatori N. ATP-dependent GSH and glutathione S-conjugate transport in skate liver: role of an Mrp functional homologue. Am J Physiol. 2000;279:G417–G425. doi: 10.1152/ajpgi.2000.279.2.G417. [DOI] [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Ballatori N. Inhibition of Mrp2- and Ycf1p-mediated transport by reducing agents: evidence for GSH transport on rat Mrp2. Biochim Biophys Acta. 2002;1559 (2):171–178. doi: 10.1016/s0005-2736(01)00454-0. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1 (2):111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- Renes J, de Vries EG, Nienhuis EF, Jansen PL, Muller M. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126 (3):681–688. doi: 10.1038/sj.bjp.0702360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL, Ckless K, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta. 2006;1760:380–7. doi: 10.1016/j.bbagen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rius M, Nies AT, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38 (2):374–384. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am J Phys. 2006;290:G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Keppler D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4) J Pharmacol Exp Ther. 2008;324:86–94. doi: 10.1124/jpet.107.131342. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05479.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82 (2):193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- Schneider E, Yamazaki H, Sinha BK, Cowan KH. Buthionine sulphoximine-mediated sensitisation of etoposide-resistant human breast cancer MCF7 cells overexpressing the multidrug resistance-associated protein involves increased drug accumulation. Br J Cancer. 1995;71 (4):738–743. doi: 10.1038/bjc.1995.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ, Venkataramanan R, Cai H, Sinal CJ, Gonzalez FJ, Schuetz JD. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- Sen CK. Cellular thiols and redox-regulated signal transduction. Curr Top Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- Serrano MA, Macias RI, Briz O, Monte MJ, Blazquez AG, Williamson C, Kubitz R, Marin JJ. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta. 2007;28 (2–3):107–117. doi: 10.1016/j.placenta.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Sharma KG, Mason DL, Liu G, Rea PA, Bachhawat AK, Michaelis S. Localization, regulation, and substrate transport properties of Bpt1p, a Saccharomyces cerevisiae MRP-type ABC transporter. Eukaryot Cell. 2002;1:391–400. doi: 10.1128/EC.1.3.391-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Chou IN. Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J Cell Physiol. 1986;129:193–198. doi: 10.1002/jcp.1041290210. [DOI] [PubMed] [Google Scholar]
- Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25 (3):332–346. [PMC free article] [PubMed] [Google Scholar]
- Sies H, Wahlländer A, Waydhas C, Soboll S, Höberle D. Functions of intracellular glutathione in hepatic hydroperoxide and drug metabolism and the role of extracellular glutathione. Adv Enzyme Regul. 1980;18:303–320. doi: 10.1016/0065-2571(80)90022-9. [DOI] [PubMed] [Google Scholar]
- Simmons TW, Hinchman CA, Ballatori N. Polarity of hepatic glutathione and glutathione S-conjugate efflux, and intraorgan mercapturic acid formation in the skate. Biochem Pharmacol. 1991;42:2221–2228. doi: 10.1016/0006-2952(91)90359-d. [DOI] [PubMed] [Google Scholar]
- Simmons TW, Anders MW, Ballatori N. Cysteine and S-(1,2-dichlorovinyl)-L-cysteine transport in rat liver canalicular membrane vesicles: Potential reabsorption mechanisms for biliary metabolites of glutathione and its S-conjugates. J Pharmacol Exp Ther. 1992;262:1182–1188. [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Anderson MT, Staal GE, Herzenberg LA, Gitler C. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx. Proc Natl Acad Sci USA. 1994;91:3619–3622. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Roederer M, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994;93 (1):347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci USA. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cMOAT/MRP2. Semin Liver Dis. 1998;18:359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction: Free Radic. Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Törnblom H, Lindberg G, Einarsson C, Sjöqvist F, Melhus H, Garberg P, Sjöström B, Lundgren B, Artursson P. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 2001;299 (1):164–170. [PubMed] [Google Scholar]
- Toh S, Wada M, Uchiumi T, Inokuchi A, Makino Y, Horie Y, Adachi Y, Sakisaka S, Kuwano M. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. Am J Hum Genet. 1999;64 (3):739–746. doi: 10.1086/302292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torky AR, Stehfest E, Viehweger K, Taege C, Foth H. Immuno-histochemical detection of MRPs in human lung cells in culture. Toxicology. 2005;207 (3):437–450. doi: 10.1016/j.tox.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7 (6):313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Nathanson MH, Mennone A, Rydberg SA, Boyer JL. Nitric oxide donors stimulate bile flow and glutathione disulfide excretion independent of guanosine 3′,5′-cyclic [corrected] monophosphate in the isolated perfused rat liver. Hepatology. 1997;25 (2):263–269. doi: 10.1002/hep.510250202. [DOI] [PubMed] [Google Scholar]
- Trompier D, Chang XB, Barattin R, du Moulinet D’Hardemare A, Di Pietro A, Baubichon-Cortay H. Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res. 2004;64:4950–4956. doi: 10.1158/0008-5472.CAN-04-0143. [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Koenderink JB, Peters JG, Van Os CH, Russel FG. Mechanisms and interaction of vinblastine and reduced glutathione transport in membrane vesicles by the rabbit multidrug resistance protein Mrp2 expressed in insect cells. Mol Pharmacol. 1999;56 (4):714–719. [PubMed] [Google Scholar]
- van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cotgreave IA, Orrenius S, Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem. 1996;271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- Vanhoefer U, Cao S, Minderman H, Toth K, Skenderis BS, 2nd, Slovak ML, Rustum YM. d,l-buthionine-(S,R)-sulfoximine potentiates in vivo the therapeutic efficacy of doxorubicin against multidrug resistance protein-expressing tumors. Clin Cancer Res. 1996;2 (12):1961–1968. [PubMed] [Google Scholar]
- Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L31–L38. doi: 10.1152/ajplung.2001.281.1.L31. [DOI] [PubMed] [Google Scholar]
- Versantvoort CH, Bagrij T, Wright KA, Twentyman PR. On the relationship between the probenecid-sensitive transport of daunorubicin or calcein and the glutathione status of cells overexpressing the multidrug resistance-associated protein (MRP) Int J Cancer. 1995;63 (6):855–862. doi: 10.1002/ijc.2910630617. [DOI] [PubMed] [Google Scholar]
- Voehringer DW. BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic Biol Med. 1999;27:945–950. doi: 10.1016/s0891-5849(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, Yoshida I, Kimura A, Sakisaka S, Adachi Y, Kuwano M. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum Mol Genet. 1998;7 (2):203–207. doi: 10.1093/hmg/7.2.203. [DOI] [PubMed] [Google Scholar]
- Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50 (3):335–356. [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15 (22):2922–2933. [PubMed] [Google Scholar]
- Watts RN, Hawkins C, Ponka P, Richardson DR. Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc Natl Acad Sci USA. 2006;103 (20):7670–7675. doi: 10.1073/pnas.0602515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci USA. 2000;97 (13):7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JR, Lu S, Fernandez-Checa J, Kaplowitz N. Expression cloning of a rat hepatic reduced glutathione transporter with canalicular characteristics. J Clin Invest. 1994;93:1841–1845. doi: 10.1172/JCI117170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J-R, Lu S, Fernandez-Checa J, Kaplowitz N. Expression cloning of the cDNA for a polypeptide associated with rat hepatic sinusoidal reduced glutathione transport: characteristics and comparison with the canalicular transporter. Proc Natl Acad Sci USA. 1995;92:1495–1499. doi: 10.1073/pnas.92.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman GJ, Lankelma J, van Tellingen O, Beijnen J, Dekker H, Paulusma C, Oude Elferink RP, Baas F, Borst P. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci USA. 1995;92 (17):7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371 (Pt 2):361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Gutmann H, Hruz P, Gutzwiller JP, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos. 2005;33 (2):219–224. doi: 10.1124/dmd.104.001354. [DOI] [PubMed] [Google Scholar]
- Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: A molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282 (40):29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]