Abstract
Background
Knowledge of transmural Vm changes is important for understanding the mechanism of long-duration ventricular fibrillation (LDVF).
Methods
Vm was recorded optically at up to 8 transmural points separated by 1.5 mm in the left ventricle of Langendorff-perfused canine hearts (n=6) using a bundle of optical fibers (optrode) during 10 min of LDVF followed by 3 min of VF with reperfusion. Measurements were grouped into 4 layers: epicardium, sub-epicardium, midwall and sub-endocardium.
Results
Activation rates (ARs) and action potential durations (APDs) decreased while diastolic intervals (DIs) increased during LDVF in all transmural layers (p<0.05). After ~3 min of LDVF, ARs were faster and DIs shorter in the midwall and sub-endocardium than in the epicardium and sub-epicardium (p<0.05). Activations persisted at the sub-endocardium but disappeared from other layers after ~8 min of VF in the majority of hearts. There were no transmural differences in APD during LDVF or during pacing before and after LDVF (p>0.05). Restitution plots revealed no functional relationship between APD and DI in any layer at any stage of LDVF. Partial reperfusion during VF for 3 min restored transmural synchronicity of activation and eliminated gradients in activation parameters.
Conclusions
Vm dynamics evolve differently at different transmural layers. The sub-endocardium maintains persistent and the fastest activation during 10 min of LDVF suggesting it contains the source of VF wavefronts. There are no transmural APD gradients and no restitution relationship between APD and DI at any transmural layer indicating these are not the primary factors in the mechanism of LDVF.
Keywords: ventricular fibrillation, transmural activation, optrode, optical mapping
Introduction
Out-of-hospital sudden cardiac arrest (SCA) causes ~450,000 deaths each year in the US.1 For victims of SCA, the median survival rate is about 6.4%.2 One of the major causes of SCA is ventricular fibrillation (VF), which can be treated only by application of electrical shocks. The success of defibrillation and the patient survival rate strongly depend on the duration of VF, dropping 7–10% every min of VF without defibrillation.3 The first defibrillation shock is typically delivered 8–12 min after emergency call,4 i.e., during long-duration VF (LDVF, duration>1 min). However, the majority of studies on mechanisms of VF and defibrillation focused on short-duration VF lasting <1 min. Clinical studies indicate that optimal treatment may depend on VF duration. For instance, cardiopulmonary resuscitation (CPR) administered prior to defibrillation shocks increased survival rates as compared to immediate shocks for patients with VF lasting >4 min.5 The exact mechanism for this beneficial CPR effect is not clear and may be related to electrophysiological changes caused by partial reperfusion. Therefore, understanding the mechanism of LDVF and electrophysiological changes associated with LDVF and reperfusion may help to improve the treatment of SCA.
It is generally believed that VF is maintained by reentry, either by a single “mother rotor”,6 or by multiple reentry promoted by heterogeneous distribution of repolarization7 or steep action potential restitution.8 However, optical mapping of short-duration VF did not reveal reentrant circuits on the heart surface9 suggesting that any VF source may be intramural. Intramural reentry was often observed during VF in wedge preparations.10,11 It was also shown that intramural reentry could be caused by transmural heterogeneity of action potential duration (APD).11 Based on these data, it can be suggested that intramural Vm dynamics, particularly transmural APD heterogeneity and restitution, play an important role in LDVF. However, due to the lack of methods for intramural Vm mapping in whole hearts, these aspects of electrical activation remain unexplored.
Presently, the main approach for multi-site Vm measurements in the heart is the use of voltage-sensitive dyes and optical mapping techniques. Application of this method has provided a wealth of new information about VF mechanisms6,8–10 but so far these studies have been limited to the heart surface. To overcome this limitation, a new technique was recently developed which employs bundles of optical fibers (optrodes) to record Vm at multiple intramural sites.12,13 The purpose of the present study was to use this method to measure transmural Vm dynamics during LDVF and partial reperfusion in canine hearts.
Methods
Heart preparation
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85-23, revised 1996). Six male beagles weighing ~10 kg were anesthetized with Telazol (4.4 mg/Kg), Xylazine (4.4 mg/Kg) and Antropine (0.04 mg/Kg). Anesthesia was maintained with inhalation of Isoflurane (1.3–2.5%) in oxygen. The chest was opened via midline thoracotomy. Heparin (500 unit/kg) was given 10 min before heart removal. The pericardium was cut away and saved for later use. After removing the heart, the aorta was cannulated and retrogradely perfused at 37±0.5°C with Tyrode’s solution (in mmol/l, NaCl 128.5, Glucose 20, KCl 4.7, MgCl2 0.7, NaH2PO4 0.5, CaCl2 1.5, and NaHCO3 28) bubbled with 95% O2 and 5% CO2. The flow rate was maintained at ~1 mL/min per gram of heart weight. The heart was stained with Vm-sensitive dye RH-237 as previously described.13 A recently reported excitation-contraction uncoupler Blebbistatin (20 µmol/L) was used to stop heart contraction.14 To keep hearts warm during LDVF, the LV was covered with the pericardium and superfused with ischemia-mimicking solution (in mmol/L, NaCl 124, KCl 8, MgCl2 0.7, NaH2PO4 0.5, CaCl2 2.5, and NaHCO3 8) bubbled with 95% N2 and 5% CO2 at 37±0.5°C.
Two bipolar hook electrodes were placed on the anterior LV for pacing at 2x diastolic threshold, which was defined as the lowest stimulus current resulting in 1:1 rhythm capture. Two large mesh electrodes were sutured to the lateral LV and RV to deliver defibrillation shocks. To continuously monitor electrical activity, AC electrograms were recorded using two stainless steel hook electrodes placed on RV and LV and a ground electrode placed on aorta.
Transmural optrode recordings of Vm
The method for transmural optical recordings using optrodes has been described previously.13 In brief, an optrode consisted of 8 optical fibers (diameter=325 µm) spaced 1.5 mm apart. Fluorescence was excited at 530–585 nm using a 200-W Hg/Xe lamp and measured at >650 nm using a 16×16 photodiode array (C4675-102, Hamamatsu Corp.) with 2-kHz sampling rate and 12-bit resolution.
Study protocol
An optrode was inserted perpendicularly into the anterior LV wall (Figure 1A). Optical signals were recorded during pacing at a cycle length (CL) of 350 ms to verify dye staining and measure transmural APD distribution. VF was induced with a 9V battery. Coronary perfusion was stopped immediately after VF induction and epicardial superperfusion was started. Optical recordings were made at 10 s of VF and repeated at 1-min intervals from 1 to10 min. After 10 min, epicardial pacing was attempted to examine heart excitability using trains of 25 pulses with CL of 150 ms and strength of 6–10x diastolic threshold15, which was measured before LDVF. Then the heart was defibrillated using 10-ms rectangular shocks with ascending energy to measure the defibrillation threshold (data not reported in this manuscript).
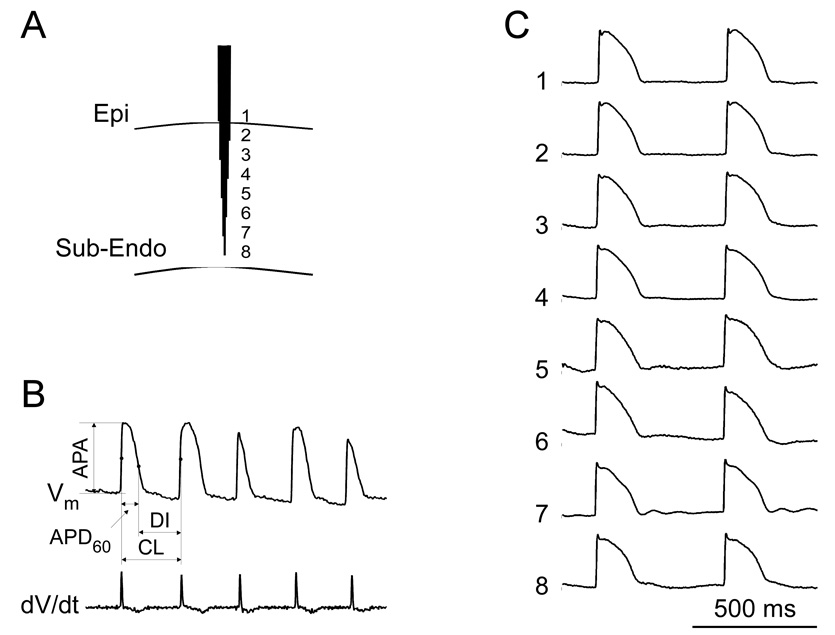
Figure 1.
A: Schematic diagram of optrode insertion in the anterior LV wall. Fibers are packed into hexagonal bundle with fiber ends separated by 1.5 mm. B: Parameters of AP measurements. The top trace is a Vm recording during VF; the bottom trace is its derivative. C: Transmural recordings during pacing at a CL of 500 ms. The mean APD60 at the 8 recording sites was 136±6 ms.
To assess the effects of reperfusion on VF dynamics, VF was re-induced immediately after defibrillation and perfusion was resumed at 20% of the normal flow rate. This flow rate is similar to coronary circulation during CPR.16 Optical recordings were made at 10 s, 1, 2, and 3 min of VF with reperfusion. The heart was defibrillated again as described before. The perfusion was changed to a normal rate and allowed to continue for 20–30 min. Then, the sinoatrial node was cut away and the heart was paced at CL varied between 0.5 and 4 s to measure transmural APD gradients.
Data analysis
Measured parameters of Vm signals are shown in Figure 1B. Action potential amplitude (APA) was measured as the difference between Vm before and after the AP upstroke; activation time (AT) - the time of the maximal upstroke slope (dV/dtmax); AP duration (APD60) - the time interval from AT to the 60% level of repolarization; CL - the interval between two adjacent ATs; diastolic interval (DI) - the difference between CL and APD60. Optical recordings were normalized assuming an APA during regular pacing of 100 mV.17 Positive Vm deflections during VF were classified as activations if they satisfied two requirements. First, Vm deflections should have a normalized amplitude >15 mV.18 Second, dV/dtmax of these deflections should be >5% of the normal value measured during regular pacing.19
Frequency content of VF signals may provide insight into VF mechanisms.9 To characterize spatial and temporal changes of the frequency content during LDVF, power spectra of optical recordings were calculated using Fast Fourier transform (Matlab) in the range of 2–30 Hz. To assess the transmural APD restitution, APD60 was plotted against the previous DI at different stages of LDVF. To simplify data presentation, intramural measurements were grouped into 4 sets. Measurements from the epicardium and from sites located at a depth of 0–33%, 34–66% and 67–100% of wall thickness were grouped into epicardial (Epi), sub-epicardial (Sub-Epi), midwall (Mid), and sub-endocardial (Sub-Endo) sets, respectively.
The percentages of earliest activations in each of the 4 intramural layers were quantified during different stages of VF. Similar to a method used previously,20 activations were considered to be from the same beat if their upstrokes were within 50-ms time interval.
Statistical differences in AP parameters among the 4 layers were analyzed by Duncan’s multivariance analysis (ANOVA) with animal, intramural layers and time as factors. All data are expressed as mean±standard deviation. Results were considered statistically significant if p<0.05.
Results
Transmural APD distribution during regular rhythm
As shown in Figure 1C and Table 1, APD60 did not show any significant transmural differences during pacing at a 350 ms CL before LDVF induction. It was reported previously that repolarization gradients may be revealed at long CL.21 Therefore, after completion of LDVF measurements, hearts were allowed to recover with a normal perfusion rate, and the sinoatrial node was cut away, which allowed pacing at long CLs. As shown in Table 1, APD60 measurements at CLs ranging from 0.5 to 4 s also revealed no significant differences among all intramural layers (p>0.05). This is consistent with our previous study in porcine hearts where no transmural APD gradients were detected and where possible reasons for the discrepancies with other studies were discussed.13
Table 1.
Transmural APD Distribution during regular rhythm
| CL (ms) | APD60 (ms) | |||
|---|---|---|---|---|
| Epi | Sub-Epi | Mid | Sub-Endo | |
| 350* | 122±11 | 127±14 | 137±16 | 135±12 |
| 500 | 156±20 | 155±21 | 156±20 | 147±20 |
| 1000 | 178±20 | 177±27 | 179±27 | 168±28 |
| 2000 | 193±22 | 197±28 | 203±28 | 190±22 |
| 4000 | 207±21 | 211±23 | 218±20 | 215±22 |
CL, pacing cycle length;
measurements made before LDVF; other measurements were made after LDVF. N=6 for each layer.
Vm dynamics during LDVF
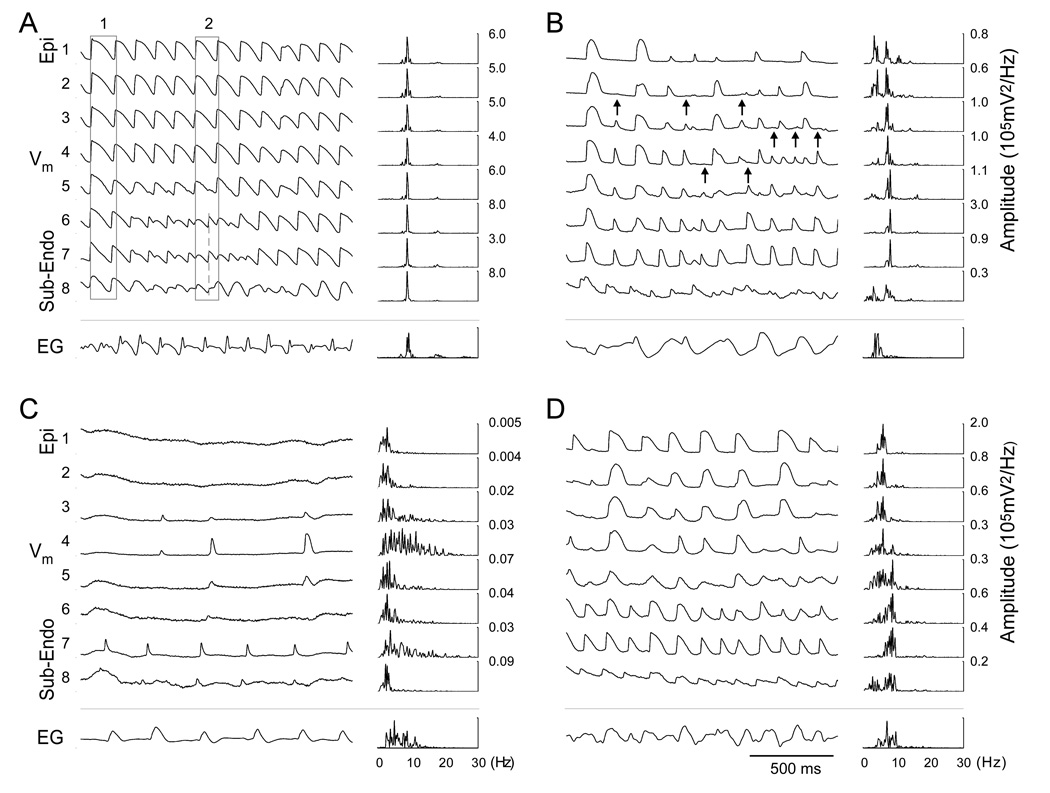
Figure 2A illustrates an example of transmural Vm recordings during early VF (10 s after VF induction). At this stage, the majority of intramural sites were activated nearly synchronously, as exemplified by AP upstrokes within rectangle 1. However, some intramural sites (traces 6–8) exhibited transient periods of irregular activations (rectangle 2) and double potentials, which is typical for sites of the reentry core.22 The average CL from all 6 hearts was 113±15 ms. Activation rate (AR), the inverse of the CL, was 9.1±1.1 Hz. Action potentials (APs) followed each other closely with mean APD60 and DI of 75±10 ms and 38±6 ms, respectively (n=6). Despite transient irregularities of local activation, there were no significant differences in average CL, APD60 or DI (p>0.05) between all intramural layers. This high order of activation periodicity was also observed in the power spectra of Vm recordings. Spectra from all transmural sites had a single narrow high-amplitude peak at an average frequency of 8.6±0.9 Hz (n=6).
Figure 2.
Transmural Vm recordings and power spectra during different stages of VF: 10 s (A), 5 min (B), 10 min (C) and reperfusion (D). Trace numbers on the left are the same as in Figure 1 (1-Epi, 8-Sub-Endo). Arrows indicate sites of conduction block. Numbers on the right designate peak amplitudes of corresponding power spectra. EG, epicardial electrogram recorded between LV and RV.
Analysis of the earliest activation sites in all experiments (n=6) demonstrated that beats originated with approximately equal frequency in Sub-Endo, Mid and Sub-Epi layers where percentages of EAs were 38%, 29% and 26%, respectively. In the Epi layer, EAs were observed in 7% of beats.
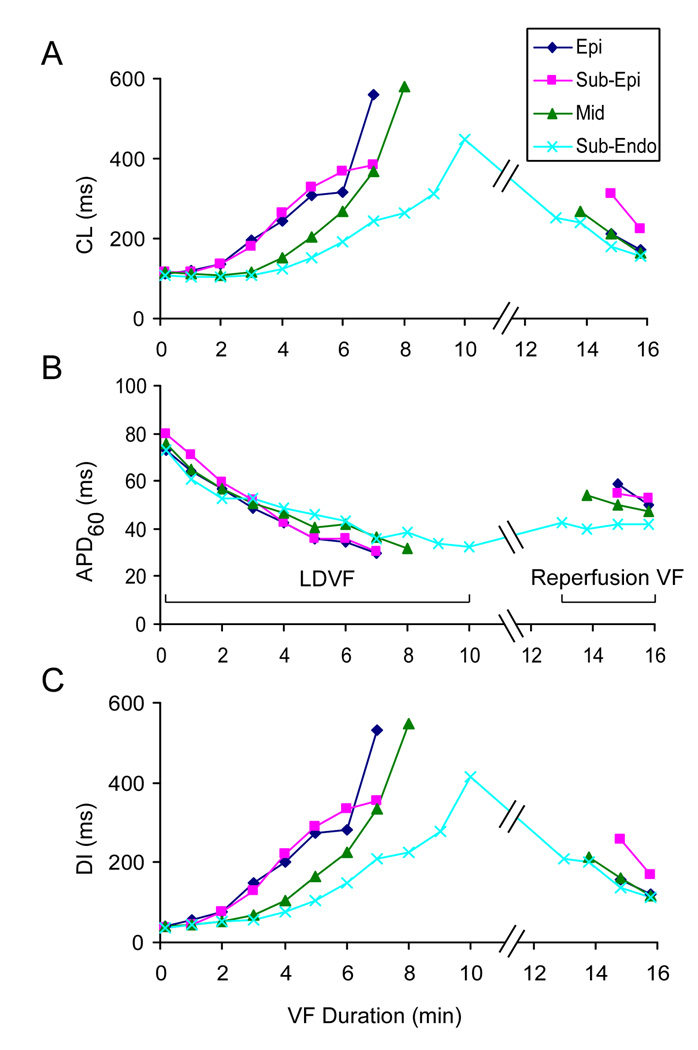
At 5 min of LDVF (Figure 2B), Vm recordings revealed significant changes of activation characteristics from early VF. The average CL increased to 235±48 ms (n=6, Figure 3A), which was approximately 108% larger than at the beginning of VF (p<0.05). This increase of CL was paralleled with ~45% decrease of average APD60 to 42±8 ms (Figure 3B, p<0.05) whereas average DI increased by ~414% to 194±51 ms (Figure 3C, p<0.05).
Figure 3.
Changes of intramural activation parameters CL (A), APD60 (B), and DI (C) during LDVF and VF with partial reperfusion. Blue, red, green and cyan plots represent data from Epi, Sub-Epi, Mid, and Sub-Endo layers, respectively. Mean data for all 6 hearts are shown. At the end of LDVF, data are shown only when at least 3 hearts exhibited activations at an intramural level.
Besides changes in average parameters, optical recordings revealed significant transmural heterogeneities of activation at 5 min of LDVF. APs became much slower and irregular in the Epi and Sub-Epi layers in comparison to the Sub-Endo layer (Figure 2B). At the Epi layer, the mean AR decreased by 63% to 3.4±1.1 Hz (p<0.05, n=6 hearts) from early VF, whereas at the Sub-Endo layer, AR decreased by only 29% to 6.7±1.3 Hz (p<0.05). Similar transmural differences were observed in DI values whereas APD showed a nearly uniform transmural distribution. Thus, DI in the Epi and Sub-Endo layers increased to 273±112 and 105±26 ms (p<0.05, n=6), respectively (585% and 187% changes from the start of VF). APD60 in their respective layers decreased to 36±3 and 46±10 ms (NS).
Transmural differences in activation were also reflected in changes of the Vm power spectra (Figure 2B). Whereas the majority of Sub-Endo recordings retained a single narrow spectrum peak reflecting more organized Vm dynamics, the Epi and Sub-Epi layers exhibited widened spectra with double peaks suggesting a lower degree of organization.
Analysis of AT sequences indicated that activations became partially de-synchronized across the LV wall. In contrast to early VF, a much larger portion of EAs was observed at the Sub-Endo than in the Sub-Epi layer (53% vs. 6% of all beats). Activation appeared to spread from Sub-Endo to Epi with frequent conduction blocks between the Mid and the Sub-Epi regions (arrows in Figure 2B). Transition from the Sub-Endo to Mid layers was characterized by shift of dominant spectral peak from 7.4±1.5 Hz to 3.8±0.8 Hz (n=6), which is typical for sites of conduction block.9
Figure 2C shows transmural recordings and power spectra at 10 min of LDVF. Activations ceased at the majority of sites in the Epi and Sub-Epi layers but irregular activations with long CL and DI were still present in the Mid and Sub-Endo layers. Cessation of Epi and Sub-Epi activation was observed starting from 8th min of LDVF in 4 out of 6 hearts. At sites with no activation, power spectra exhibited broad low amplitude peaks reflecting noise. At sites with slow activation and small AP amplitude, the spectra had small but detectable peaks. Earliest activation was observed more frequently in the Sub-Endo layer than in the Mid layer (76% vs. 24% of beats).
To examine whether the disappearance of activation at the end of LDVF was due to loss of tissue excitability or due to transmural conduction block, epicardial pacing was attempted after 10 min of LDVF. In all 6 hearts, no APs were detected indicating complete loss of tissue excitability.
Effect of reperfusion on LDVF
Figure 2D shows examples of transmural recordings at 3 min of VF with reperfusion. Activations were restored at all transmural sites in 5 out of 6 hearts. In the remaining heart, Vm deflections during reperfusion VF did not meet activation criteria. In the 5 hearts with improved activation, reperfusion increased the average APD60 to 49±8 ms while it decreased CL and DI to 196±76 ms and 147±76 ms, respectively (Figure 3). ARs were similar across the wall, ranging from 5.8 to 7.1 Hz. Toward the end of the 3-min reperfusion interval, mean activation parameters returned to levels similar to those observed at 3–5 min of LDVF. Transmural heterogeneities in AR and DI observed during late LDVF were abolished (p>0.05).
APD restitution during LDVF
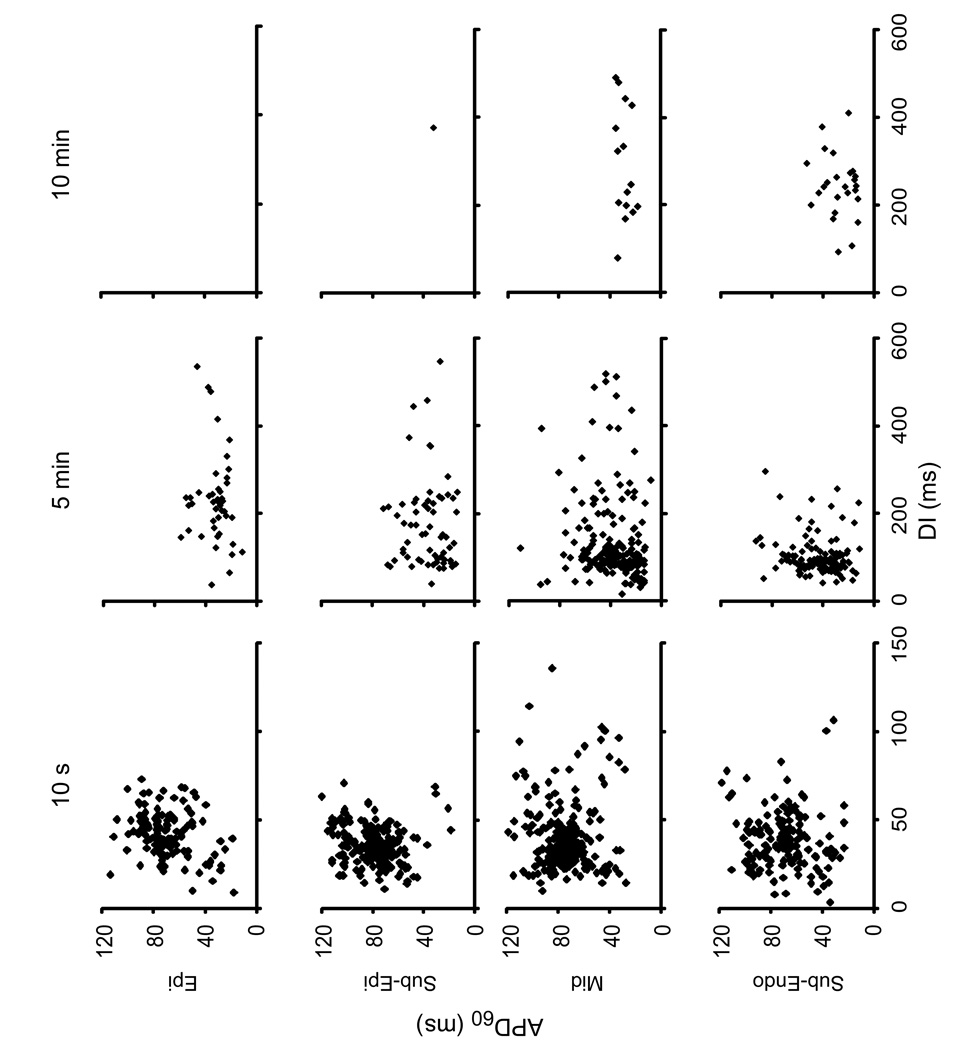
Figure 4 shows plots of APD60 vs. the preceding DI in different intramural layers at 10 s, 5 min and 10 min of LDVF from all 6 hearts. There were no clear functional relationships between APD60 and DI in any layer. At 10 s of VF, restitution plots for all sites showed relatively tight clusters of points elongated along the APD60 axis. Standard deviations of APD60 and DI were 17–20 and 10–18 ms, respectively. With progression of LDVF, APD(DI) clusters became progressively looser in all tissue layers with standard deviations of DI in Sub-Endo and Mid layers increasing 16–19 fold at 10 min compared to the start of VF.
Figure 4.
Plots of APD restitution for different intramural layers and different LDVF stages for all animals. Epi and Sub-Epi layers exhibited almost no activations at 10 min of LDVF.
Discussion
In this work, transmural Vm was measured optically during LDVF in the LV wall of intact canine hearts. The main findings are as follows: 1) LDVF caused shortening of APD, transmurally heterogeneous prolongation of CL and DI and heterogeneous loss of tissue excitability. Vm dynamics deteriorated faster at Epi and Sub-Epi tissue layers than at Mid and Sub-Endo layers resulting in significant transmural de-synchronization of activation. The Sub-Endo level was the most viable exhibiting persistent activations at 10 min of VF, suggesting that it contains the source of activation in late LDVF; 2) There was no transmural dispersion of APD during pacing and LDVF; 3) Partial reperfusion for 3 min restored transmural synchronicity of activation and eliminated gradients in activation parameters caused by LDVF; 4) There was no clear functional relationship between APD and the preceding DI at any transmural site during all VF stages.
Dynamics of transmural Vm during LDVF
Previously, Vm changes during LDVF were measured from the epicardial surface in several studies using optical mapping18,23,24 or monophasic AP recordings.25 Optical mapping in rabbits showed that as VF progressed, the dominant frequency in Fourier power spectra decreased23,24 indicating slowing of activation rate. Monophasic AP recordings showed a significant decrease in APD accompanied by increases in CL and DI during the first 5 min of VF.25 Qualitatively similar Vm changes were observed on epicardium in the present study. However, optrode measurements demonstrated that epicardial Vm recordings were representative of intramural activation only at the early VF stage when transmural activations were highly synchronous.
Starting from ~3 min of VF, significant differences in Vm dynamics appeared between different intramural layers. The main difference was that AR decreased and DI increased much faster in Epi/Sub-Epi layers in comparison to Mid/Sub-Endo layers between 3 and 10 min of LDVF. The reason for the development of transmural AR gradient is likely to be tissue ischemia, since AR gradients did not develop during VF in hearts with cardiopulmonary bypass.26 It is less clear why Sub-Endo myocardium was more resistant to ischemia than Sub-Epi tissue. It could be due to transmural gradients in expression of ion channels27 or due to the endocardial resistance to ischemia.28 The latter explanation is supported by the results of experiments with chemical ablation of the endocardial regions which caused early termination of LDVF and elimination of transmural AR gradients.29
In contrast to the other electrophysiological parameters, APD changes during LDVF progression were nearly uniform across the LV wall. This is surprising taking into account significant transmural differences in activation rates during late LDVF. The reasons for such uniform APD changes are unclear. Transmural APD gradients were also absent during regular rhythm. Altogether, these data indicate that APD gradients did not play a significant role in VF maintenance. It should be noted, however, that APD measurements during late VF are limited in predicting the occurrence of conduction block since ischemia at this VF stage causes post-repolarization refractoriness.30
An important question is about the origin of beats during VF and whether it changes with the LDVF progression. Intramural optical recordings demonstrated that the incidence of earliest activations at the Sub-Endo layer gradually increased with LDVF progression. In addition, activation frequency in Epi and Sub-Epi levels became significantly slower than in the Sub-Endo after ~3 min of LDVF (until disappearance of Epi/Sub-Epi activation at ~8 min). Although VF source couldn’t be localized with the limited number of intramural recordings, these data indicate that VF source at this LDVF stage was likely to be located in the Sub-Endo tissue layers. This is consistent with recent studies utilizing plunge needle recordings showing that earliest activations at 2–10 min of LDVF appeared more frequently at endocardial sites.20,29
Transmural differences became exacerbated towards the end of LDVF when almost no activations were detected in Epi and Sub-Epi tissue layers whereas slow activations persisted in the Sub-Endo regions. This lack of activation could be either due to loss of tissue excitability or due to transmural conduction block between the deeper tissue layers and sub-epicardium. The fact that heart epicardium could not be paced with stimuli up to 10x diastolic threshold in strength indicates complete loss of tissue excitability on epicardium and sub-epicardium at this LDVF stage.
Effects of Reperfusion on VF
Although assessing the effects of partial reperfusion on LDVF may be important for understanding of the effect of CPR on defibrillation efficacy, data in this regard are rare. It was shown previously in rabbit hearts that after 10 min of VF with full reperfusion, dominant frequencies measured on the epicardium returned to levels similar to those at the beginning of VF.23,24 However, the effect of long full perfusion may not be the same as the effect of partial reperfusion during relatively short periods of CPR. Also, intramural electrophysiological changes may be different from that on the epicardium. Optrode recordings of Vm during VF in the present study showed that after 3 min of partial reperfusion epicardial excitability recovered and transmural differences in activation parameters produced by LDVF were abolished. These parameters returned to levels similar to the first 3–5 min of LDVF. The likely reason for the rapid recovery of excitability is the removal of hypoxia and ischemic metabolites from the extracellular environment by partial reperfusion.28
The role of APD restitution in VF
Experimental data regarding the role of APD restitution in VF mechanisms are still controversial. Some optical mapping studies found that there was a functional relationship between epicardial APD and DI during VF and that drugs flattening the restitution curve converted VF to VT.8 However, other studies did not find any restitution relationship during VF.18 This discrepancy could be possibly explained by non-uniform distribution of restitution properties and intramural localization of VF source. However, data obtained in the present study do not support this explanation. Consistent with reports showing no restitution in epicardial recordings,18 we found no clear relationship between APD60 and preceding DI in intramural tissue layers during LDVF. A previous study in porcine hearts demonstrated that, in addition to restitution, cardiac memory played an important role in APD variability during short-duration VF, but even their combination did not have a decisive predictive value.31 Also, it was shown in a computer model that restitution of conduction velocity may play a role in activation irregularities leading to VF.32 It is likely that multiple factors are involved in APD variability and VF mechanism.
Limitations
The isolated and perfused heart model used in this study lacks neurohumoral factors raising the possibility that the development of LDVF in this model may be different from that in whole animals or in patients. Only one optrode with 8 recording sites was used, which precluded mapping of the activation pathways and VF source. Intramural recordings were made only from one region on anterior LV, which does not exclude the possibility for existence of transmural APD gradients during pacing or LDVF in other heart regions. These issues could be resolved by using multiple optrodes in future studies. Due to dye photobleaching, recordings were limited to 3-s duration every min of LDVF. Certain fast physiological changes during the 1-min interval could be missed but this limitation is not likely to affect the conclusions of this study.
Acknowledgements
This work was supported by NIH grants HL28429 and HL85370. We would like to thank Southern Research Institute for providing resources for this study; Dr. Sholuan Ding for help with statistical analysis; Reuben Collins and Frank Vance for technical support.
Lists of Abbreviations
- AP
action potential
- APD
AP duration
- APD
AP duration
- APD60
APD at the 60% level of repolarization
- AT
activation time
- AR
activation rate
- CL
cycle length
- CPR
cardiopulmonary resuscitation
- DI
diastolic interval
- Epi
epicardium
- LDVF
long-duration ventricular fibrillation
- Mid
mid-myocardium
- Sub-Endo
sub-endocardium
- Sub-Epi
sub-epicardium
- SCA
sudden cardiac arrest
- VF
ventricular fibrillation
- Vm
transmembrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Stiell IG, Laupacis A, et al. A cumulative meta-analysis of the effectiveness of defibrillator-capable emergency medical services for victims of out-of-hospital cardiac arrest. Ann Emerg Med. 1999;34:517–525. [PubMed] [Google Scholar]
- 3.Cummins RO. From concept to standard-of-care? Review of the clinical experience with automated external defibrillators. Ann Emerg Med. 1989;18:1269–1275. doi: 10.1016/s0196-0644(89)80257-4. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JP, Kroshus KS, Lindholm DJ, et al. Measuring the call-receipt-to-defibrillation interval: evaluation of prehospital methods. Ann Emerg Med. 1995;26:697–701. doi: 10.1016/s0196-0644(95)70040-4. [DOI] [PubMed] [Google Scholar]
- 5.Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 6.Samie FH, Berenfeld O, Anumonwo J, et al. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 7.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 8.Garfinkel A, Kim YH, Voroshilovsky O, et al. Preventing ventricular fibrillation by flattening cardiac restitution. PNAS. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaitsev AV, Berenfeld O, Mironov SF, et al. Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circ Res. 2000;86:408–417. doi: 10.1161/01.res.86.4.408. [DOI] [PubMed] [Google Scholar]
- 10.Valderrabano M, Lee MH, Ohara T, et al. Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res. 2001;88:839–848. doi: 10.1161/hh0801.089259. [DOI] [PubMed] [Google Scholar]
- 11.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–645. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell BJ, Legrice IJ, Hooks DA, et al. Intramural measurement of transmembrane potential in the isolated pig heart: validation of a novel technique. J Cardiovasc Electrophysiol. 2005;16:1001–1010. doi: 10.1111/j.1540-8167.2005.40558.x. [DOI] [PubMed] [Google Scholar]
- 13.Kong W, Fakhari N, Sharifov OF, et al. Optical measurements of intramural action potentials in isolated porcine hearts using optrodes. Heart Rhythm. 2007;4:1430–1436. doi: 10.1016/j.hrthm.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedorov VV, Lozinsky IT, Sosunov EA, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 15.Newton JC, Huang J, Rogers JM, et al. Pacing during ventricular fibrillation: factors influencing the ability to capture. J Cardiovasc Electrophysiol. 2001;12:76–84. doi: 10.1046/j.1540-8167.2001.00076.x. [DOI] [PubMed] [Google Scholar]
- 16.Maier GW, Tyson GS, Jr, Olsen CO, et al. The physiology of external cardiac massage: high-impulse cardiopulmonary resuscitation. Circulation. 1984;70:86–101. doi: 10.1161/01.cir.70.1.86. [DOI] [PubMed] [Google Scholar]
- 17.Gray RA, Jalife J. Effects of atrial defibrillation shocks on the ventricles in isolated sheep hearts. Circulation. 1998;97:1613–1622. doi: 10.1161/01.cir.97.16.1613. [DOI] [PubMed] [Google Scholar]
- 18.Huizar JF, Warren MD, Shvedko AG, et al. Three distinct phases of VF during global ischemia in the isolated blood-perfused pig heart. Am J Physiol. 2007;293:H1617–H1628. doi: 10.1152/ajpheart.00130.2007. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi H, Miyauchi Y, Chou CC, et al. Effects of cytochalasin D on electrical restitution and the dynamics of ventricular fibrillation in isolated rabbit heart. J Cardiovasc Electrophysiol. 2003;14:1077–1084. doi: 10.1046/j.1540-8167.2003.03234.x. [DOI] [PubMed] [Google Scholar]
- 20.Allison JS, Qin H, Dosdall DJ, et al. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol. 2007;18:1306–1312. doi: 10.1111/j.1540-8167.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 21.Sicouri S, Quist M, Antzelevitch C. Evidence for the presence of M cells in the guinea pig ventricle. J Cardiovasc Electrophysiol. 1996;7:503–511. doi: 10.1111/j.1540-8167.1996.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 22.Valderrabano M, Chen PS, Lin SF. Spatial distribution of phase singularities in ventricular fibrillation. Circulation. 2003;108:354–359. doi: 10.1161/01.CIR.0000080322.67408.B4. [DOI] [PubMed] [Google Scholar]
- 23.Wu TJ, Lin SF, Hsieh YC, et al. Ventricular fibrillation during no-flow global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2006;17:1112–1120. doi: 10.1111/j.1540-8167.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 24.Mandapati R, Asano Y, Baxter WT, et al. Quantification of effects of global ischemia on dynamics of ventricular fibrillation in isolated rabbit heart. Circulation. 1998;98:1688–1696. doi: 10.1161/01.cir.98.16.1688. [DOI] [PubMed] [Google Scholar]
- 25.Tovar OH, Jones JL. Electrophysiological deterioration during long-duration ventricular fibrillation. Circulation. 2000;102:2886–2891. doi: 10.1161/01.cir.102.23.2886. [DOI] [PubMed] [Google Scholar]
- 26.Worley SJ, Swain JL, Colavita PG, et al. Development of an endocardial-epicardial gradient of activation rate during electrically induced, sustained ventricular fibrillation in dogs. Am J Cardiol. 1985;55:813–820. doi: 10.1016/0002-9149(85)90162-6. [DOI] [PubMed] [Google Scholar]
- 27.Cordeiro JM, Greene L, Heilmann C, et al. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol. 2004;286:H1471–H1479. doi: 10.1152/ajpheart.00748.2003. [DOI] [PubMed] [Google Scholar]
- 28.Gilmour RF, Jr, Zipes DP. Different electrophysiological responses of canine endocardium and epicardium to combined hyperkalemia, hypoxia, and acidosis. Circ Res. 1980;46:814–825. doi: 10.1161/01.res.46.6.814. [DOI] [PubMed] [Google Scholar]
- 29.Dosdall DJ, Tabereaux PB, Kim JJ, et al. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am J Physiol. 2008;295:H883–H889. doi: 10.1152/ajpheart.00466.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downar E, Janse MJ, Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation. 1977;56:217–224. doi: 10.1161/01.cir.56.2.217. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Zhou X, Smith WM, et al. Restitution properties during ventricular fibrillation in the in situ swine heart. Circulation. 2004;110:3161–3167. doi: 10.1161/01.CIR.0000147618.93579.56. [DOI] [PubMed] [Google Scholar]
- 32.Cao JM, Qu Z, Kim YH, et al. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: importance of cardiac restitution properties. Circ Res. 1999;84:1318–1331. doi: 10.1161/01.res.84.11.1318. [DOI] [PubMed] [Google Scholar]