Abstract
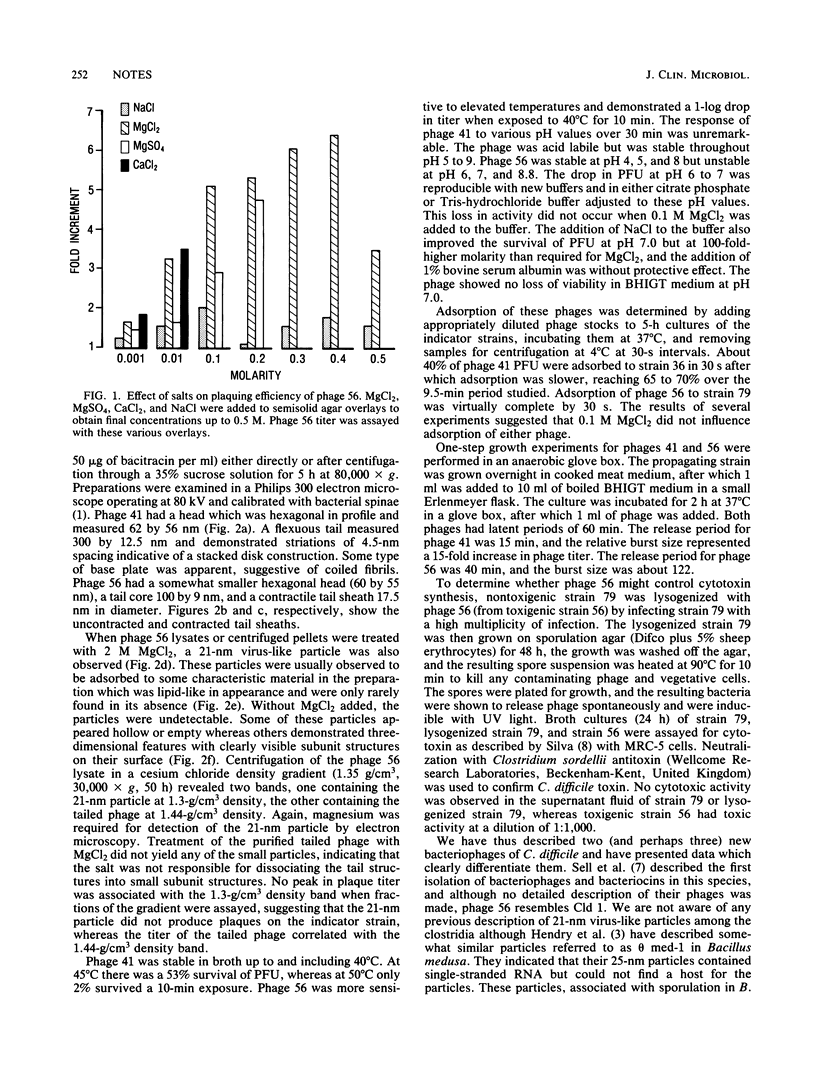
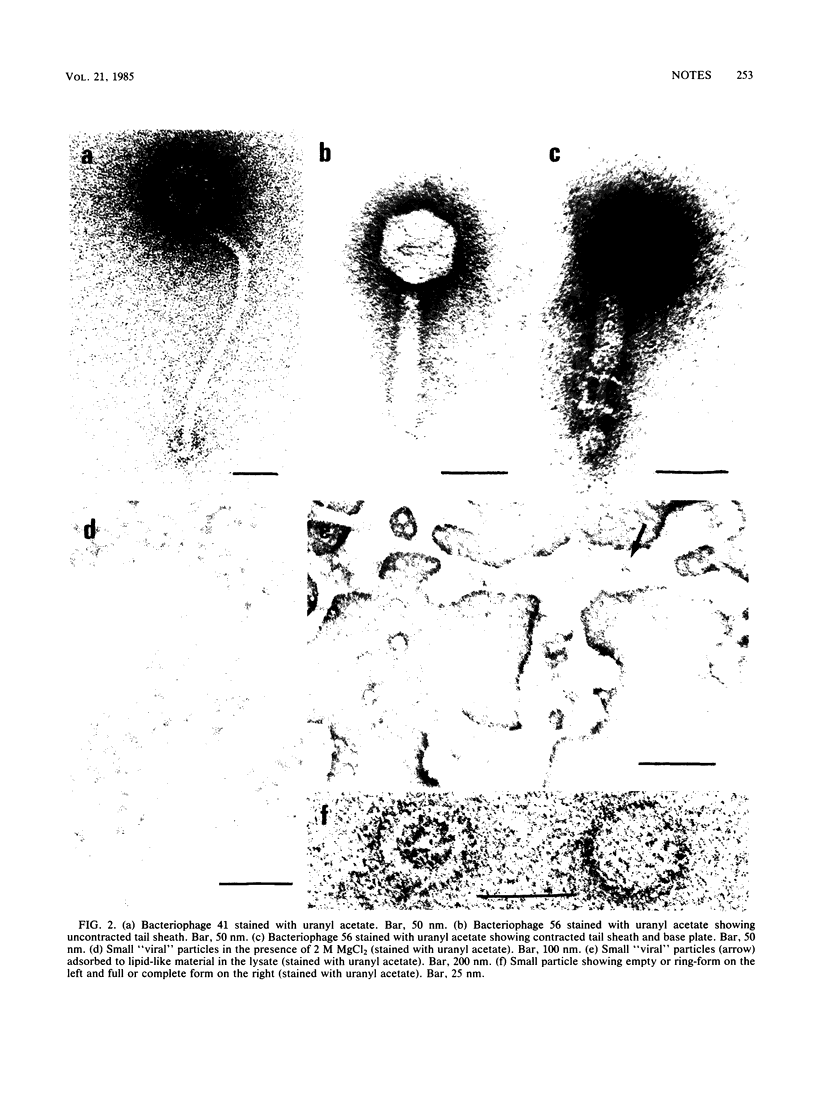
Two temperate bacteriophages of differing morphology and host range were isolated by screening 94 isolates of Clostridium difficile. Phage 41 had a 300-nm flexible tail, whereas phage 56 had a shorter tail with a contractile sheath. Electron microscopy of phage 56 lysates exposed to elevated magnesium concentrations showed small virus-like particles which were 21 nm in diameter. The addition of MgCl2 to semisolid agar overlays enhanced both the titer and plaque size of phage 56. Phage 56 was more temperature labile than phage 41 and demonstrated unusual lability in buffer at pH 7.0. One-step growth and adsorption experiments revealed that both phages had latent periods of about 60 min, but phage 56 adsorbed to its indicator strain more efficiently. Phage 56, which was obtained from a toxigenic strain of C. difficile, was used to lysogenize its nontoxigenic indicator strain, but no conversion to toxigenicity was observed in this strain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Easterbrook K. B., Willison J. H., Coombs R. W. Arrangement of morphological subunits in bacterial spinae. Can J Microbiol. 1976 May;22(5):619–629. doi: 10.1139/m76-092. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Buggy B. P., Fekety R., Schaberg D. R. Epidemiology of colitis induced by Clostridium difficile in hamsters: application of a bacteriophage and bacteriocin typing system. J Infect Dis. 1984 May;149(5):775–780. doi: 10.1093/infdis/149.5.775. [DOI] [PubMed] [Google Scholar]
- Hendry G. S., Gillespie J. B., Fitz-James P. C. Bacteriophage and bacteriophage-like structures carried by Bacillus medusa and their effect on sporulation. J Virol. 1976 Jun;18(3):1051–1062. doi: 10.1128/jvi.18.3.1051-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar J. J., Silva J. Pseudomembranous enterocolitis and the aetiological role of Clostridium difficile. An overview of the recent literature. S Afr Med J. 1981 Oct 17;60(16):623–625. [PubMed] [Google Scholar]
- Riley T. V., Mee B. J. Simple method for detecting Bacteroides spp. bacteriocin production. J Clin Microbiol. 1981 Mar;13(3):594–595. doi: 10.1128/jcm.13.3.594-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell T. L., Schaberg D. R., Fekety F. R. Bacteriophage and bacteriocin typing scheme for Clostridium difficile. J Clin Microbiol. 1983 Jun;17(6):1148–1152. doi: 10.1128/jcm.17.6.1148-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]