Summary
Both acute and chronic stress can impair maternal behavior and increase rates of infant abuse in several species. The mechanisms inducing these effects are unknown, but experimental manipulation of circulating corticosterone levels alters maternal behavior in rats, and circulating or excreted cortisol concentrations have been found to correlate either positively or negatively with maternal behavior in humans and nonhuman primates. In this study, therefore, we experimentally tested the hypothesis that both acute and chronic treatment with exogenous glucocorticoids would alter maternal behavior in a primate, the common marmoset (Callithrix jacchus). Multiparous females, approximately 3−5 weeks postpartum, received daily injections of either cortisol (hydrocortisone sodium succinate and hydrocortisone acetate; N = 7) or vehicle (N = 7) for 8 days, and maternal behavior was characterized under baseline conditions as well as during exposure to a noise stressor. Cortisol treatment successfully elevated both morning and afternoon plasma cortisol concentrations and suppressed circulating levels of adrenocorticotropic hormone. In home-cage observations, cortisol-treated females carried their infants significantly less than control mothers, and in noise-stressor tests, several hours after the first cortisol or vehicle treatment, cortisol-treated mothers inspected their infants significantly more often than controls. Aggression towards infants was infrequent and mild, and did not differ between treatment groups. These findings provide the first experimental evidence that cortisol elevations can alter maternal behavior in primates. As these effects were limited in scope, however, they suggest that other stress-responsive hormones or neuropeptides may additionally play a role in mediating the effects of stress on maternal behavior.
Keywords: common marmoset, stress, maternal behavior, glucocorticoids, infant abuse, infant neglect
1. Introduction
Stress can impair maternal behavior and increase the likelihood of child abuse. In humans, it has long been appreciated that a variety of acute and chronic psychosocial and environmental stressors, such as poverty, domestic violence, sexual assault, natural disasters, and lack of social support, may contribute to deficient maternal behavior and increased abusive behavior (e.g., Kotch et al., 1995; Brockington, 1996; Banyard et al., 2003; Tolan et al., 2005). Stress also contributes to the onset of several psychopathological conditions, including major depressive disorder, postpartum depression, and post-traumatic stress disorder, which in turn are associated with deficient maternal behavior, increased child abuse, and impaired mother-child relationships (e.g., Kotch et al., 1995; Lovejoy et al., 2000; Oyserman et al., 2000; Nonacs, 2002; Bailham and Joseph, 2003; Banyard et al., 2003; Brockington, 2004; Windham et al., 2004; Cohen et al., 2008). Compared to mothers without obvious signs or symptoms of depression, for example, mothers with clinically diagnosed depression engage in lower rates of positive behaviors and higher rates of negative and disengaged behaviors towards their children, and may be more likely to commit severe physical abuse (Lovejoy et al., 2000; Dawson et al., 2003; Windham et al., 2004).
Both acute and chronic stress have also been found to impair maternal behavior and increase infant abuse in animal models. In nonhuman primates, as in humans, anxiety and psychosocial or environmental stressors, such as lack of social support, crowding, and receipt of aggression, increase rates of infant abuse (Reite and Caine, 1983; Troisi and D'Amato, 1994; Maestripieri and Carroll, 1998a,b). In a study of group-housed pigtail macaques (Macaca nemestrina), for example, acute stress was the most common context for the spontaneous occurrence of infant abuse (Maestripieri and Carroll, 1998a). Similarly, mother rats (Rattus norvegicus) exhibited diminished maternal behavior and/or increased abusive behavior immediately following acute restraint stress (Yamada et al., 2002), during acute confinement in a novel chamber with limited bedding (Roth and Sullivan, 2005), and during exposure to chronic stressors involving wet bedding and forced foraging (Léonhardt et al., 2007) or limited nesting material (Ivy et al., 2008). Conversely, the spontaneous onset of maternal behavior in new rat mothers is associated with, and may in fact depend upon, a general reduction in fearfulness and anxiety as well as reduced hormonal and behavioral responses to stress (Neumann, 2003; Numan and Insel, 2003; Tu et al., 2005).
The mechanisms by which stress impairs maternal behavior are not known. One possibility is that such effects are mediated by elevated concentrations of glucocorticoid hormones (i.e., cortisol and corticosterone) (Wingfield and Sapolsky, 2003). Few studies, however, have experimentally investigated the effects of glucocorticoids on maternal behavior. A recent series of experiments by Rees and colleagues (Rees et al., 2004, 2006; Graham et al., 2006) has indicated that glucocorticoids can either facilitate or inhibit aspects of maternal behavior in rats, depending on the reproductive history of the individual animal. In postpartum females, adrenalectomy decreased and corticosterone replacement increased licking of pups, time spent in the nest, and maternal memory (Rees et al., 2004; Graham et al., 2006). Conversely, in sensitized virgin female rats, adrenalectomy increased licking of pups and time spent crouching over pups, whereas corticosterone replacement had opposite effects (Rees et al., 2006).
Correlational studies in primates have suggested a similar dichotomy of associations between cortisol and maternal behavior. In women, salivary or plasma cortisol concentrations during the first few days postpartum were positively correlated with attraction to infant-related odors and affectionate behavior toward the infant in first-time mothers (Fleming et al., 1987, 1997). At roughly 6 weeks postpartum, higher salivary cortisol levels were similarly associated with more affectionate behavior towards infants in primiparous mothers aged 19−25 years old but were associated with less instrumental care-taking behavior in younger primiparous mothers (Krpan et al., 2005). Circulating or excreted cortisol concentrations have also been found to correlate negatively with specific aspects of maternal behavior in Western lowland gorillas (Gorilla gorilla gorilla; Bahr et al., 1998), Japanese macaques (M. fuscata; Bardi et al., 2003), and baboons (Papio hamadryas anubis sp.; Bardi et al., 2004; Ramirez et al., 2004). To our knowledge, however, effects of glucocorticoids on maternal behavior have not been tested experimentally in any primate species.
In the present study, therefore, we experimentally manipulated circulating cortisol concentrations in female common marmosets (Callithrix jacchus) to determine whether elevated cortisol levels alter maternal behavior. These small-bodied (∼350 g) New World monkeys live in small groups (∼3−16 individuals) in which the behaviorally dominant female gives birth, usually to fraternal twins or triplets, at roughly 6-month intervals (Digby et al., 2007). Infants are weaned at approximately 8−10 weeks of age (Tardif et al., 2003), and infant care is shared by all members of the social group, including the father and older siblings; however, mothers spend substantial amounts of time carrying their infants (e.g., approximately 30−40% of observation time during the first month postpartum and approximately 10−20% during the second month postpartum; Tardif et al., 1986; Ximenes and Sousa, 1996). This biparental and cooperative care of infants is unusual among primates and makes marmosets a particularly suitable model for human parental behavior. Moreover, stress has been reported to increase rates of infant abuse and infanticide by marmoset parents and to markedly reduce infant survival rates (Johnson et al., 1991).
We treated multiparous females with exogenous cortisol for 8 days during the midlactational period and examined both the acute and chronic effects on maternal behavior. Because glucocorticoid hormones are important regulators of fear and anxiety and may be especially likely to affect behavior under stressful, anxiogenic, or frightening circumstances (Korte, 2001; Schulkin et al., 2005), we characterized maternal behavior both under baseline conditions and during exposure to an auditory stressor.
2. Materials and Methods
2.1. Animals
Subjects were 14 multiparous female common marmosets housed at the National Primate Research Center at the University of Wisconsin (UW) – Madison (WNPRC). Seven females were assigned to the cortisol treatment condition, and the remaining seven served as vehicle-treated controls. Cortisol-treated and control animals did not differ in age (60.7 ± 8.5 vs. 59.5 ± 7.9 months, respectively, mean ± SEM; T = −0.110, df = 12, p = 0.915), body mass (446 ± 16 vs. 436 ± 13 g, T = −0.467, df = 12, p = 0.6489), or parity (5 ± 1 vs. 5 ± 1 litters; T = −0.402, df = 12, p = 0.695) at the outset of data collection. Each female was housed with her mate and up to 8 offspring, including 1−2 infants and 1−7 older offspring.
Marmosets were housed indoors in aluminum and wire mesh cages (61 × 91 × 183 cm or 122 × 61 × 183 cm) that permitted visual, auditory, and olfactory contact between animals in different groups. The animals were fed Mazuri Hi-Fiber Callitrichid Diet (Mazuri, Richmond, IN) supplemented with vitamin D, at 1230−1330h; however, food was typically available in the cages at all times. Water was available ad libitum. Lights were on from 0630 to 1830h, and room temperature and humidity were maintained at approximately 23°C and 30−70%, respectively.
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Graduate School Animal Care and Use Committee of UW-Madison. WNPRC is accredited by AAALAC as part of the UW-Madison Graduate School.
2.2. Design
The experimental design is summarized in Table 1. We collected data on each female marmoset during a 10-day period, beginning when her youngest infants were 23−24 days old. Animals in the cortisol condition received an injection of hydrocortisone sodium succinate (Solu-Cortef, Pfizer, New York, NY; 40 mg/kg body weight in 0.8 ml/kg saline, SC) on day 1, followed by daily injections of hydrocortisone acetate (Sigma-Aldrich, St. Louis, MO; 90 mg, SC suspended in 0.45 ml sesame oil) on days 2−8. Initial doses were based on published studies on the squirrel monkey (Saimiri sciureus, another small-bodied New World monkey; Lyons et al., 2000, 2004), and modified based on pilot tests on female marmosets (unpublished data). We aimed to produce circulating cortisol concentrations in between peak levels occurring at the height of the circadian cycle (∼275−400 μg/dl; L.M. George and D.H. Abbott, unpublished data) and those occurring in response to stress (e.g., ∼550 μg/dl following social group formation and wounding, Saltzman et al., 1994; ∼830 μg/dl during periods of inter-female aggressive interactions, L.M. George and D.H. Abbott, unpublished data) in female common marmosets. Hydrocortisone sodium succinate produces a marked, although relatively transient, elevation in circulating cortisol concentrations within 1 hour, whereas hydrocortisone acetate produces delayed but more prolonged elevations in circulating cortisol levels (Lyons et al., 2000, 2004; unpublished data). Serial application of the two cortisol preparations allowed rapid induction of elevated circulating cortisol levels on the first day of treatment, permitting behavioral testing during acute cortisol excess, as well as appropriate daily maintenance of hypercortisolemia, permitting subsequent behavioral testing during chronic cortisol excess. Control animals received an injection of 0.9% saline (0.8 ml/kg, SC) on day 1 and daily injections of sesame oil (0.45 ml, SC) on days 2−8. All injections were given at 0800−0830h.
Table 1.
Schedule of experimental procedures.
| Day of Experiment | Injections (Cortisol Group / Control Group) | Observations | Blood Samples |
|---|---|---|---|
| Day 0 | --- | --- | 0900−0930ha,b,c 1515−1545ha |
| Day 1 | Hydrocortisone succinate/saline 0800−0830h |
Air-horn test 1100−1230h |
1115−1230ha,b |
| Day 2 | Hydrocortisone acetate/oil 0800−0830h |
--- | --- |
| Day 3 | Hydrocortisone acetate/oil 0800−0830h |
Home cage 1430−1530h |
0900−0930ha |
| Day 4 | Hydrocortisone acetate/oil 0800−0830h |
Home cage 0930−1030h |
1515−1545ha |
| Day 5 | Hydrocortisone acetate/oil 0800−0830h |
--- | --- |
| Day 6 | Hydrocortisone acetate/oil 0800−0830h |
Home cage 1430−1530h |
0900−0930ha |
| Day 7 | Hydrocortisone acetate/oil 0800−0830h |
Home cage 0930−1030h |
1515−1545ha |
| Day 8 | Hydrocortisone acetate/oil 0800−0830h |
Air-horn test 1100−1230h |
1115−1230ha,b |
| Day 9 | --- | --- | 0900−09300a,b,c |
Plasma was assayed for cortisol.
Plasma was assayed for ACTH.
Plasma was assayed for progesterone.
Each female marmoset underwent an air-horn test (see below) with one of her infants, followed immediately by blood sample collection, at 1100−1230h on day 1 of treatment, 3−4 h after the first injection (hydrocortisone sodium succinate or saline), to characterize the acute effects of elevated cortisol concentrations on maternal behavior and responsiveness to stress. Marmosets subsequently underwent a second, identical air-horn test on day 8 to characterize the effects of chronically elevated cortisol levels. To further characterize the effects of cortisol treatment on maternal behavior, we collected behavioral data on each female with one of her infants in their home cage (see below) at 1430−1530h on days 3 and 6 and at 0930−1030h on days 4 and 7. For females with two infants, we alternated which infant was used in stress tests and home-cage observations.
Basal blood samples were collected (see below) at 0900−0930h on days 0 (the day before the first injection), 3, 6, and 9 (approximately 25 h after the final injection), and at 1515−1545h on days 0, 4, and 7. All blood samples were assayed for plasma cortisol, and the morning samples collected on days 0 and 9 as well as the samples collected immediately after air-horn tests on days 1 and 8 were additionally assayed for plasma adrenocorticotropic hormone (ACTH). Because marmosets ovulate and conceive approximately 10−20 days postpartum (Saltzman et al., 1997a; Tardif et al., 2003), we assayed samples collected on days 0 and 9 for progesterone, to determine whether or not subjects were in either the luteal phase of the ovarian cycle or early pregnancy. Finally, we weighed each mother and infant at 0800−0930h on days 0, 5, and 9 to obtain a gross index of body condition.
2.3. Home-cage observations
Approximately 5 min prior to the beginning of observations, the subject's mate and offspring were captured in a nest box and released into a cage in another room; the female subject remained in her home cage. We then released one of her infants back into the home cage with its mother and immediately collected behavioral data on the mother and infant for 15 min.
Behavioral data were collected on a laptop computer using the JWatcher event-recorder program (Blumstein et al., 2007) by a trained observer sitting in full view of the animals, which had been habituated to the observer during the preceding two weeks. Table 2 describes the behaviors that were scored and analyzed. A variety of behaviors were recorded each time they were performed by the mother (solicit, inspect, lick, vocal threat, attack, bite, cuff, approach, scratch self, autogroom, long-call, chirp) or the infant (ngä). For several other behaviors we scored the duration of each bout performed by the mother (carry, reject, bristle strut). In addition, every minute, upon an audible signal from a timer, we scored whether the mother was locomoting, whether she was in proximity to the infant, and whether the infant was suckling. Several additional behaviors (approach by infant, ear-tufts flick, facial submit, genital present, groom infant, huddle, lipsmack, squawk, avoid, scent mark; see Saltzman et al., 1997b for definitions) were observed very rarely and were therefore omitted from analyses.
Table 2.
Behaviors scored in home-cage observations and air-horn tests.
| Behavior | Measure | Definition |
|---|---|---|
| Approach | Frequency | Move to within 10 cm of infant |
| Solicit | Frequency | Position body directly above or against infant and/or attempt to pull infant onto body |
| Carry | Duration | Infant has all four limbs on any part of female's body |
| Reject | Duration | Rub, pull, or otherwise try to force juvenile off body (excludes biting) |
| Inspect | Frequency | Push face against or toward infant and/or use hands to investigate infant (excludes grooming) |
| Lick | Frequency | Common usage; lick any part of infant's body |
| Vocal threat (erh-erh) | Frequency | Low-pitched, staccato chattering; performed in the context of offensive or defensive aggression (Epple, 1968; Stevenson and Poole, 1976) |
| Attack | Frequency | Lunge at or pounce on infant aggressively |
| Bite | Frequency | Direct biting motions towards infant |
| Cuff | Frequency | Swift, superficial blow, scratch, or push performed aggressively |
| Bristle strut | Duration | Arching posture and/or strut locomotion and/or general piloerection |
| Scratch self | Frequency | Common usage; direct scratching toward any part of body |
| Autogroom | Frequency | Use hands and/or mouth to pick through own fur, mouth, or other body part |
| Long-call (phee) | Frequency | Long, high-pitched, whistle-like contact call; most commonly performed during separation from a familiar groupmate(s) (Epple, 1968) |
| Chirp | Frequency | Any tsee, tsik, twitter, or chirp vocalization; associated with high arousal; may be used as alarm/mobbing calls (Epple, 1968; Cross and Rogers, 2006) |
| Ngä (infant) | Frequency | Infant emits relatively low-pitched, atonal, infantile squeal; associated with distress or used as a contact call |
| Locomotion | 1-min scan | Engaged in locomotion or other whole-body movement |
| Proximity | 1-min scan | Any part of female's body is <10 cm from infant |
| Suckling | 1-min scan | Infant's mouth is on female's nipple and/or infant's face is in vicinity of female's nipple |
2.4. Air-horn tests
The female subject and one of her infants were captured from their home cage and released into a standard housing cage (61 × 91 × 183 cm) inside a small room containing no other animals, for 15 min. Immediately prior to testing, the room was sprayed with air freshener (Vanilla Indulgence, AirWick, Parsippany, NJ) to increase the novelty of the environment and to provide a comparable presentation of the testing environment across animals. Approximately 5.5, 7.5 and 12.5 min after initial release of the animals into the test cage, a celebration air horn (Unique Inc., Philadelphia, PA; ∼115 dB) was blasted once (duration ∼1 sec) by an observer sitting next to the cage, approximately 1 m from the test animals. A similar, unpredictable noise paradigm has been used previously to induce stress in pregnant rhesus monkeys, and was found to elevate plasma glucocorticoid levels significantly in the mothers as well as to induce persistent alterations in brain catecholamine activity, hypothalamic-pituitary-adrenal axis function, and behavior in their offspring (Clarke and Schneider, 1993; Clarke et al., 1994; Schneider et al., 1998). Behavioral data were collected throughout the 15-min test by a second, trained observer seated behind a one-way mirror, using the JWatcher program as described above. After 15 min had elapsed, the mother was manually captured from the test cage and a blood sample was collected (see below). The mother and infant were subsequently reunited with their family in their home cage.
2.5. Collection of blood samples
Marmosets were manually captured from their home cage (or, following air-horn tests, from the test cage) and restrained in a marmoset restraint device (Hearn, 1977) while 0.1−0.4 ml blood was collected from the femoral vein into a heparinized syringe and immediately placed on ice. Less than 3 min elapsed from initial disturbance to the animal (investigator's entry into the home cage or test room) until the completion of blood collection for 94% of the samples. The remaining eight samples were collected in 4.5 ± 0.7 min (mean ± SEM) of disturbance to the animal. These blood-sampling procedures do not elevate plasma cortisol concentrations in marmosets in our colony that have previously undergone frequent blood collection (Saltzman et al., 1994, 1998). Blood samples to be assayed for ACTH were processed as described by Orth (1979): samples were initially centrifuged at 4200 rpm for 15 min at 4°C, and the plasma fraction was removed and centrifuged again at 9000 rpm for 10 min at 4°C. The plasma fraction was again removed and stored at −80°C. Samples to be assayed only for cortisol (or for cortisol and progesterone) were centrifuged at 2000 r.p.m. for 10 min, and the plasma fraction was removed and stored at −20°C.
2.6. Hormone assays
All blood samples were assayed in duplicate for plasma cortisol using an antibody-coated-tube radioimmunoassay (RIA) kit (GammaCoat, DiaSorin Corp., Stillwater, MN) that had been fully validated for use with marmoset plasma, as previously described (Saltzman et al., 1994). Assay sensitivity at 90% binding was 0.1 ng/tube (1.0 μg/dl), and intra- and inter-assay coefficients of variation (CVs) of a plasma pool assayed in quadruplicate in each assay (36% binding) were 9.6% and 10.6%, respectively.
Plasma ACTH concentrations were measured by an RIA that had been fully validated for marmoset plasma (Saltzman et al., 2004). All samples were run in a single assay. Assay sensitivity at 90% binding was 0.5 pg/tube (6.7 pg/ml), and the intra-assay CV of a plasma pool assayed in triplicate (33% binding) was 4.6%.
Plasma progesterone concentrations were measured in duplicate using a heterologous enzymeimmunoassay that was fully validated for marmoset plasma (Saltzman et al., 1994). Assay sensitivity at 90% binding was 3.6 pg/tube (2.7 ng/ml), and intra- and inter-assay CVs of a plasma pool assayed in duplicate in each assay (40% binding) were 4.8% and 13.5%, respectively.
2.7. Analysis
Plasma cortisol, ACTH, and progesterone concentrations were analyzed by ANOVA, with day and time of day treated as repeated measures and treatment condition (cortisol, vehicle) as a between-groups variable. Marmosets were considered to be pregnant or in the luteal phase of the ovarian cycle if plasma progesterone concentrations exceeded 10 ng/ml (Harlow et al., 1983). Mothers’ and infants’ body weights were similarly analyzed by ANOVA. For mothers with two infants, we used the twins’ mean weight for each time point.
Most of the behavioral data were not normally distributed and contained many zero scores; therefore, behavior was analyzed nonparametrically. For behaviors scored in home-cage observations, we first performed a Friedman test on each treatment group separately to examine possible differences across the four observations (days 3, 4, 6, and 7). If behavior scores did not show a clear pattern of change across days for either group, we compared the two groups’ mean scores from all four observations using a Mann-Whitney U test. For behaviors scored in each air-horn test (days 1 and 8), we compared data from the cortisol and control groups using Mann-Whitney U tests, with separate analyses performed for the first 5.5 min (prior to the first noise exposure) and the subsequent 9.5 min (during and after the three noise exposures). We also compared behavior scores between the pre- and post-noise periods for each group separately using Wilcoxon tests.
Analyses were performed using Systat 5 for the Macintosh and Systat 12 for Windows (San Jose, CA). Results were evaluated at the 0.05 level (two-tailed).
3. Results
3.1. Cortisol
On the day before the first cortisol or vehicle injection, basal plasma cortisol concentrations were significantly higher in the morning than in the afternoon, as expected (F[1,12] = 40.858, p<0.001), but did not differ between female marmosets in the cortisol and control groups (main effect of group: F[1,12] = 0.726, p = 0.411; groups × time interaction: F[1,12] = 0.139, p = 0.716; Figs. 1A, 1B).
Fig. 1.
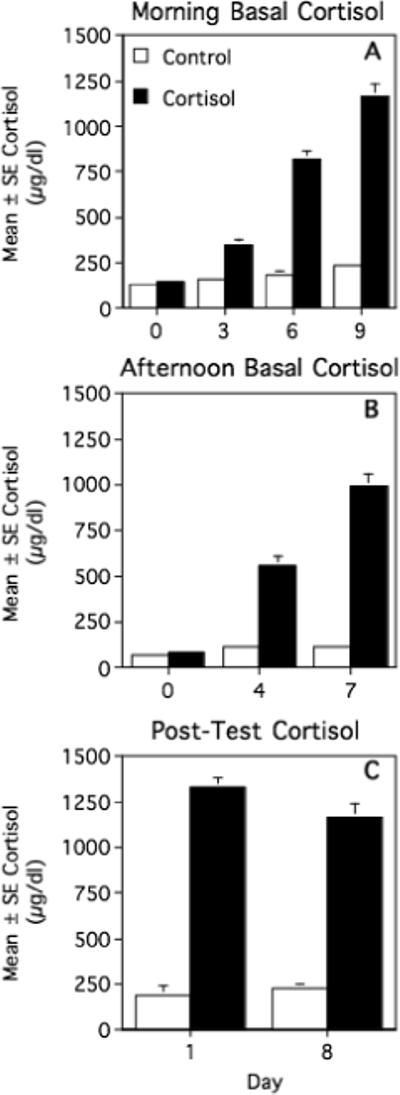
Mean ± SEM morning basal (A), afternoon basal (B), and post-air-horn test plasma cortisol concentrations (C) in cortisol-treated and vehicle-treated (control) female marmosets. See text for statistical results.
Cortisol treatment successfully elevated marmosets’ circulating cortisol levels. Morning (0900−0930h) plasma cortisol concentrations differed significantly between the two treatment groups (F[1,12] = 196.197, p<0.001) and across days (days 0, 3, 6, and 9; F[3,36] = 124.829, p<0.001), and showed a significant groups × days interaction (F[3,36] = 84.477, p<0.001; Fig. 1A). Morning cortisol levels increased progressively in both cortisol-treated (F[3,18] = 123.215, p<0.001) and control females (F[3,18] = 6.416, p = 0.004), but this pattern was much more pronounced in the cortisol-treated animals. Compared to their baseline levels on day 0, cortisol-treated marmosets exhibited a 7-fold increase in morning cortisol levels by day 9, whereas controls exhibited only a 0.8-fold increase.
Afternoon cortisol levels were similarly influenced by treatment condition (F[1,11] = 195.326, p<0.001), days (days 0, 4, and 7; F[2,22] = 80.374, p<0.001), and a groups × days interaction (F[2,22] = 69.525, p<0.001; Fig. 1B). When analyzed separately, cortisol-treated animals exhibited a progressive rise in afternoon cortisol concentrations across days (F[2,12] = 92.949, p<0.001) whereas control animals did not (F[2,10] = 2.486, p = 0.133). Compared to afternoon baseline levels on day 0, afternoon cortisol concentrations on day 7 increased by 11-fold in cortisol-treated marmosets but only by 0.6-fold in controls. Consequently, following the initiation of cortisol or vehicle treatment, mean morning plasma cortisol levels were significantly higher than mean afternoon levels in control animals (T = 8.271, df = 6, p<0.001) but not in cortisol-treated animals (T = 0.094, df = 6, p = 0.929).
Plasma cortisol concentrations immediately following air-horn tests did not differ consistently between days 1 and 8 of treatment (F[1,12] = 1.848, p = 0.199; Fig. 1C). Post-test cortisol levels were significantly higher in cortisol-treated animals than in controls (F[1,12] = 375.356, p<0.001), however, and the two groups tended to show different patterns of change from day 1 to day 8: cortisol-treated marmosets tended to have lower post-test cortisol levels on day 8 than day 1, whereas the control animals showed the opposite pattern. This difference was not quite significant, though (F[1,12] = 4.274, p = 0.061). We were not able to assess the magnitude of stress-induced changes in cortisol concentrations, as we did not determine cortisol levels at the same time of day under baseline conditions.
3.2. ACTH
Comparison of morning basal ACTH concentrations on day 0 (the day before the first hydrocortisone or vehicle injection) and day 9 (the day after the final injection) revealed a significant groups x days interaction (F[1,12] = 10.631, p = 0.007; Fig. 2A): ACTH levels decreased across days in cortisol-treated animals (T = 3.399, df = 6, p = 0.015) but did not change reliably in controls (T = −1.579, df = 6, p = 0.166). Nonetheless, basal ACTH concentrations did not differ significantly between the two treatment groups on either day 0 (T = −1.617, df = 12, p = 0.132) or day 9 (T = 1.751, df = 12, p = 0.105).
Fig. 2.
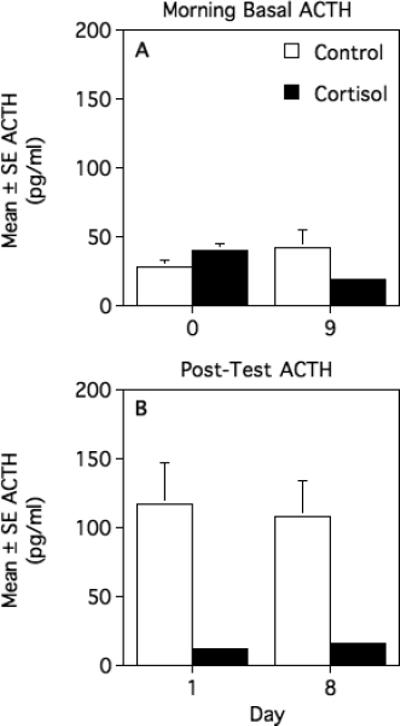
Mean ± SEM morning basal (A) and post-air-horn test plasma ACTH concentrations (B) in cortisol-treated and vehicle-treated (control) female marmosets. See text for statistical results.
Plasma ACTH concentrations immediately after air-horn tests did not differ between day 1 and day 8 (main effect of days: F[1,11] = 0.053, p = 0.822; groups × days interaction: F[1,11] = 0.269, p = 0.614) but, as expected, were significantly lower in cortisol-treated animals than in controls (F[1,11] = 16.806, p = 0.002; Fig. 2B). We were not able to characterize the magnitude of stress-induced changes in ACTH concentrations, as we did not determine ACTH levels at the same time of day under baseline conditions.
3.3. Progesterone
Each female's plasma progesterone concentrations on days 0 and 9 were well above 10 ng/ml (range: 44.7−165.3 ng/ml), indicating that all females had undergone a postpartum ovulation prior to the beginning of data collection and were still pregnant or in the luteal phase of the ovarian cycle at the end of data collection. Progesterone concentrations increased significantly from day 0 to day 9 (76.95 ± 7.68 vs. 107.09 ± 7.06 ng/ml, respectively; F[1,12] = 12.640, p = 0.004), typical of the early to mid-luteal phase in this species (Harlow et al., 1983). Progesterone levels did not, however, differ reliably between cortisol-treated and vehicle-treated marmosets (main effect of group: F[1,12] = 0.064, p = 0.804; groups × days interaction: F[1,12] = 0.257, p = 0.622).
3.4. Behavior in the home cage
When tested in their home cage with one of their own infants, cortisol-treated females did not show any significant behavioral changes across the four days of observations. Control females showed significant changes only in frequency of chirping (Friedman test; chi-square = 8.786, df = 3, p = 0.032) and time spent carrying their infant (Friedman test statistic; chi-square = 7.971, df = 3, p = 0.047); however, these behaviors did not show a clear pattern of change across time. Therefore, for each behavior we compared total scores from the four observation periods between the two groups. Results are summarized in Table 3.
Table 3.
Behavior scores (median, range) of cortisol-treated and vehicle-treated (control) female marmosets when tested in their home cage with one of their own infants on days 3, 4, 6, and 7 of treatment.
| Behavior | Cortisol Group | Control Group | P (Mann-Whitney U test) |
|---|---|---|---|
| Approach infanta | 0.033 (0.000−0.767) |
0.000 (0.000−0.000) |
0.025 |
| Carry infantb | 0.992 (0.242−0.997) |
0.996 (0.994−0.998) |
0.025 |
| Reject infantb | 0.002 (0.000−0.234) |
0.000 (0.000−0.019) |
0.056 |
| Inspect infanta | 0.633 (0.183−3.267) |
0.267 (0.033−1.333) |
0.064 |
| Proximity to infantc | 1.000 (0.364−1.000) |
1.000 (1.000−1.000) |
0.062 |
| Lick infanta | 0.167 (0.000−0.367) |
0.017 (0.000−0.267) |
0.137 |
| Solicit infanta | 0.067 (0.017−0.800) |
0.067 (0.033−0.067) |
0.256 |
| Contact aggression (bite + cuff + attack)a | 0.000 (0.00−0.333) |
0.000 (0.000−0.050) |
0.230 |
| Vocal threata | 0.300 (0.000−0.517) |
0.100 (0.000−1.783) |
0.797 |
| Chirpa | 1.767 (0.117−32.617) |
0.700 (0.100−5.200) |
0.277 |
| Long-calla | 0.917 (0.000−3.083) |
0.567 (0.000−3.083) |
0.898 |
| Autogrooma | 0.017 (0.000−0.083) |
0.017 (0.000−0.617) |
0.547 |
| Scratcha | 0.133 (0.000−0.500) |
0.133 (0.000−0.833) |
0.846 |
| Bristle strutb | 0.341 (0.034−0.711) |
0.141 (0.014−0.965) |
0.749 |
| Locomotionc | 0.153 (0.033−0.309) |
0.073 (0.000−0.172) |
0.085 |
| Suckling by infantc | 0.034 (0.000−0.333) |
0.241 (0.000−0.527) |
0.396 |
| Ngä by infanta | 0.000 (0.000−1.333) |
0.000 (0.000−0.000) |
0.062 |
Number of occurrences per minute across all four observations
Proportion of time across all four observations
Proportion of instantaneous scans across all four observations
Cortisol-treated mothers spent less time carrying their infants (Mann-Whitney U = 42.000, N = 7,7, p = 0.025) but approached their infants more often than control mothers (Mann-Whitney U = 10.500, N = 7,7, p = 0.025). Mothers in both treatment groups generally retrieved their infants within several seconds of the infants’ reintroduction into the home cage; however, control mothers consistently carried their infants throughout the remainder of each observation period, whereas cortisol-treated mothers were more likely to reject and retrieve their infants repeatedly, sometimes up to 20 times in a single 15-min observation period. Cortisol-treated females tended to spend less time in proximity to their infants, to spend more time rejecting their infants, to inspect their infants more frequently, and to engage in locomotion more frequently than control females; however, these differences did not quite reach statistical significance (see Table 3). Moreover, infants of cortisol-treated mothers showed a non-significant trend toward emitting ngä vocalizations more often than infants of control mothers.
All forms of contact aggression toward the infant (attack, bite, cuff) occurred very infrequently and were therefore summed for analysis (“contact aggression”). Frequencies of contact aggression, as well as frequencies of licking the infant, soliciting the infant, vocal threats, chirping long-calling, autogrooming, scratching, and locomotion, by the mother, frequency of suckling by the infant, and amount of time spent bristle strutting by the mother, did not differ between the two groups (Table 3). Three of the four mothers that performed contact aggression, however, were cortisol-treated, comprising 43% of cortisol-treated mothers versus 14% of controls.
3.5. Behavior during air-horn tests
When tested in a novel cage on day 1, several hours after the first cortisol or vehicle injection, most mothers in both treatment groups exhibited behavioral inhibition both before and after exposure to the noise stressor, engaging in few overt behaviors except vocalizations (Table 4). All mothers carried their infants throughout the entire test, but most of them engaged in few other behavioral interactions with their infants. Cortisol-treated females emitted more chirp vocalizations per min during the 5.5 min before the first noise exposure than during the 9.5 min after (Wilcoxon test, Z = −2.023, N = 6, p = 0.043), whereas control females long-called more frequently after than before the first noise exposure (Wilcoxon test, Z = 2.023, N = 6, p = 0.043). No other behavior differed significantly between the pre- and post-test periods for either group. The only significant behavioral difference between groups was that after (but not before) the first noise exposure, cortisol-treated mothers inspected their infants more frequently than controls (Mann-Whitney U = 7.000, N = 6,6, p = 0.050). Mothers did not orient in any particular direction on hearing the air horn.
Table 4.
Behavior scores (median, range) of cortisol-treated and vehicle-treated (control) female marmosets in a test cage with one of their own infants before (pre) and after (post) the first of three exposures to a noise stressor on days 1 and 8 of treatment.
| Behavior | Test Day | Cortisol Gp Pre | Control Gp Prea | P-Value Prea | Cortisol Gp Post | Control Gp Post | P-Value Posta |
|---|---|---|---|---|---|---|---|
| Inspect infantb | Day 1 | 0.472 (0.000−3.220) |
0.000 (0.000−1.278) |
0.475 | 0.675 (0.000−5.864) |
0.000 (0.000−0.315) |
0.050 |
| Day 8 | 0.195 (0.000−4.918) |
0.364 (0.000−1.441) |
0.690 | 0.104 (0.000−3.294) |
0.000 (0.000−1.138) |
0.549 | |
| Chirpb | Day 1 | 4.074e (0.000−14.428) |
1.700 (0.000−12.061) |
0.370 | 1.084 (0.000−9.255) |
2.063 0.000−5.523) |
0.872 |
| Day 8 | 0.000 (0.000−17.827) |
0.000 (0.00−12.741) |
0.724 | 0.104 (0.000−8.511) |
0.104 (0.000−3.575) |
0.641 | |
| Long-callb | Day 1 | 1.887 (0.000−6.612) |
2.364e (0.000−6.856) |
0.872 | 1.499 0.623−3.188) |
4.951 0.000−7.497) |
0.337 |
| Day 8 | 0.000 (0.000−4.494) |
0.360 (0.000−3.399) |
0.946 | 0.206 (0.000−3.387) |
0.000 (0.000−3.092) |
0.322 | |
| Carry infantc | Day 1 | 0.969 (0.929−0.996) |
0.992 (0.958−0.997) |
0.173 | 1.000 (1.000−1.000) |
1.000 (1.000−1.000) |
1.000 |
| Day 8 | 0.993 (0.979−0.997) |
0.992 (0.963−0.995) |
0.700 | 1.000 (1.000−1.000) |
1.000 (1.000−1.000) |
1.000 | |
| Bristle strutc | Day 1 | 0.941 (0.764−0.988) |
0.965 (0.907−0.986) |
0.522 | 1.000 (0.684−1.000) |
1.000 (0.000−1.000) |
0.902 |
| Day 8 | 0.963 (0.000−0.987) |
0.934 (0.000−0.985) |
0.480 | 1.000 (0.809−1.000) |
0.664 (0.000−1.000) |
0.119 | |
| Locomotiond | Day 1 | 0.100 (0.000−0.600) |
0.200 (0.000−0.600) |
0.613 | 0.111 (0.100−0.222) |
0.211 (0.000−0.333) |
0.255 |
| Day 8 | 0.000 (0.00−0.400) |
0.000 (0.00−0.400) |
1.000 | 0.111 (0.00−0.333) |
0.111 (0.00−0.222) |
0.843 | |
| Suckling by infantd | Day 1 | 0.000 (0.000−1.000) |
0.000 (0.000−0.800) |
0.528 | 0.000 (0.000−0.259) |
0.000 (0.000−0.333) |
0.674 |
| Day 8 | 0.000 (0.000−0.200) |
0.000 (0.000−1.000) |
0.424 | 0.000 (0.000−0.000) |
0.000 (0.000−0.900) |
0.142 |
Results of Mann-Whitney U test comparing cortisol and control groups pre or post
Number of occurrences per minute
Proportion of time
Proportion of instantaneous scans
Significantly different from corresponding "post" value (p<0.05, Wilcoxon test)
When re-tested with the noise stressor on day 8 of treatment, all mothers again carried their infants throughout the entire test but performed few other overt behaviors except vocalizations (Table 4). No behaviors differed significantly between the pre- and post-stressor periods for either treatment group or between cortisol-treated and control animals (Table 4).
3.6. Body weight
Mothers’ body weights did not differ reliably across days (days 0, 5, and 9; F[2,18] = 0.721, p = 0.500) or between cortisol-treated and control animals (main effect of group: F[2,9] = 0.719, p = 0.513; groups x days interaction: F[4,18] = 0.554, p = 0.699). Infant weights increased progressively from day 0 (51 ± 2 g) to day 5 (57 ± 3 g) to day 9 (62 ± 3 g; F[2,20] = 135.544, p<0.001); however, neither absolute weights (F[1,10] = 0.269, p = 0.616) nor the pattern of change across time (F[2,20] = 0.615, p = 0.551) differed between infants of cortisol-treated and control mothers.
4. Discussion
In this study we administered exogenous cortisol to multiparous female marmosets during the mid-lactational period and assessed the effects on maternal behavior under both baseline and test conditions. The results provide, to our knowledge, the first experimental evidence supporting the hypothesis that elevated cortisol concentrations alter – or specifically, disrupt – maternal behavior in a primate species, but indicate that these effects do not appear to be traumatic. Elevated cortisol induced an aspect of maternal neglect (spending less time carrying infants), but no significant infant abuse.
Effects of exogenous cortisol on maternal behavior were more pronounced under baseline conditions than during exposure to a noise stressor. When tested in their home cage with one of their own infants, cortisol-treated females spent significantly less time carrying the infant than did controls. At the same time, they approached the infant significantly more often and showed clear, although non-significant, tendencies to engage in more visual/olfactory inspection of the infant and to spend more time rejecting the infant. When marmosets were tested during exposure to a noise stressor in an unfamiliar environment, the only difference between groups was that cortisol-treated females inspected their infants more frequently than control females in the first of two stress tests. These latter findings should, however, be interpreted with caution. We were unable to characterize the incremental effects of the air-horn test on cortisol and ACTH concentrations, because we did not obtain baseline values at the same time of day, and therefore cannot definitively conclude that the test constituted an effective stressor.
Our results are consistent with correlational findings in several other primate species. In captive Western lowland gorillas, mothers’ postpartum urinary cortisol concentrations, divided by prepartum urinary cortisol levels (“postpartum stress index”), correlated negatively with the proportion of time that mothers spent in ventro-ventral supported contact with their infants, especially during locomotion (Bahr et al., 1998). Similarly, among captive savannah baboons, mothers with higher urinary cortisol concentrations postpartum spent less time maintaining contact with their infants than those with lower urinary cortisol levels (Bardi et al., 2004). Among captive Japanese macaques, in contrast, mothers’ fecal cortisol levels postpartum were not significantly associated with time spent in contact with the infant, but were positively correlated with the frequency of maternal rejection of infants (Bardi et al., 2003). Importantly, in all of these studies on nonhuman primates, as in the present study, all or virtually all of the animals studied were experienced (multiparous) mothers.
Correlational findings in women have been more variable. In a comprehensive series of studies, Fleming and her colleagues found that circulating or salivary cortisol concentrations correlated with aspects of maternal behavior, maternal attitudes, or maternal mood, but that the specific correlations differed with women's age and parity. During the first few days postpartum, for example, salivary or circulating cortisol levels of first-time mothers were positively associated with the mothers’ frequency of affectionate contact with their infants and attraction to infant odors, whereas cortisol concentrations of experienced mothers were associated with increased caretaking activities and an enhanced ability to recognize their own infants’ odors, but also with increased anxiety (Fleming et al., 1987, 1997). Moreover, among primiparous mothers, salivary cortisol concentrations approximately six weeks postpartum correlated negatively with instrumental care-taking activities and fatigue in teenaged mothers, correlated positively with affectionate behavior towards infants in “young mothers” (19−25 years old) and did not correlate with maternal behavior, but were positively associated with fatigue and negative moods in “mature mothers” (26−40 years old; Krpan et al., 2005).
In sum, these findings from humans and nonhuman primates suggest that elevated cortisol concentrations may have generally stimulatory effects on maternal behavior in younger, less experienced mothers, especially in terms of increasing mothers’ attraction to and affectionate behavior towards infants. Among older, more experienced mothers, in contrast, elevated cortisol levels may have fewer and qualitatively different effects on maternal behavior and may be more closely associated with negative affect. In non-human primates, including the marmosets in our study, high cortisol levels specifically appear to inhibit experienced mothers’ contact with and carrying of infants.
Although the present study focused primarily on effects of chronic cortisol elevations, the design additionally permitted us to examine the effects of acute cortisol treatment, as we first observed each mother and infant in an air-horn test 3−4 h after the initial cortisol or vehicle injection. At this time point, cortisol-treated mothers already had significantly higher plasma cortisol levels and significantly lower ACTH levels than controls. Nonetheless, cortisol-treated females showed no behavioral differences from controls during the first 5 min in the test cage, prior to their first exposure to the noise stressor, and only one significant difference (increased rates of investigating their infant) during/after noise exposure. These results suggest that acute, unlike chronic, cortisol elevations may have limited effects on maternal behavior in this primate. It is important to note, however, that we did not evaluate maternal behavior under undisturbed conditions in the home cage shortly after the first cortisol or vehicle treatment.
Importantly, we found no clear evidence that exogenous cortisol increased mothers’ aggression toward their infants. Common marmosets have been reported to show elevated rates of killing or abusing their own offspring under stressful conditions and to have markedly decreased infant survival rates during chronic stress (Johnson et al., 1991). In addition, female marmosets exhibit low attraction to and low tolerance of other females’ infants, and may commonly commit infanticide, during late pregnancy, when circulating cortisol concentrations are elevated (Saltzman, 2003; Saltzman and Abbott, 2005). In the present study, however, frequencies of most aggressive behaviors, including attack, bite, cuff, genital present, and ear-tufts flick, were extremely low. Most females were never observed performing any of these behaviors during either home-cage observations or air-horn tests, but three of the four females that performed contact aggression toward their infants were cortisol-treated. Mothers did occasionally perform vocal threats to their infants, but frequencies of this behavior did not differ between cortisol-treated and control females. Thus, our findings do not provide strong support for the hypothesis that elevated cortisol levels increase aggression towards infants by marmoset mothers, and suggest that stress-related increases in child- or infant abuse in this and other species (e.g., humans: Brockington, 1996; Tolan et al., 2005; macaques: Reite and Caine, 1983; Troisi and D’Amato, 1994; Maestripieri and Carroll, 1998a,b) may not be mediated by glucocorticiods alone.
The mechanisms by which cortisol may alter maternal behavior are not known. One possibility is that cortisol acts directly on neural regions involved in the control of maternal behavior. The neuroanatomical basis of maternal behavior has not been characterized in primates. In rodents, however, several key brain regions involved in the control of maternal behavior, such as the medial preoptic area, bed nucleus of the stria terminalis, and medial amygdala, contain type-II glucocorticoid receptors, which are activated when glucocorticoid levels are elevated (Ahima and Harlan, 1990; Numan, 2007). Additionally or alternatively, cortisol might act indirectly through actions on other hormone, neurotransmitter, or neuropeptide systems. In rats, for example, glucocorticoids increase the expression of corticotropin-releasing hormone (CRH) in the central nucleus of the amygdala (Schulkin et al., 2005). The amygdala plays a crucial role in the onset of maternal behavior in rats (Numan, 2007), and intracerebroventricular administration of CRH may inhibit aspects of maternal behavior in rodents (Pedersen et al., 1991; Gammie et al., 2004) as well as in common marmosets (W. Saltzman, C.A. Boettcher, J.L. Post, D.H. Abbott, unpublished data). Cortisol might also influence maternal behavior indirectly through broader effects on cognition, perception, emotionality, or arousal (Fleming et al., 1987, 1997). Finally, effects of exogenous cortisol on mothers’ behavior might be mediated via effects on their infants. We were unable to systematically examine effects of maternal cortisol treatment on infant behavior in this study, for logistical reasons; however, exposure to elevated glucocorticoid levels in breast milk has persistent effects on behavior, cognition, and neural development in rats (e.g., Catalani et al., 2000, 2002) and is thought to influence infant temperament in humans (Glynn et al., 2007).
Several aspects of our experimental design should be kept in mind when interpreting our results. First, we used experienced breeding females, each of which had successfully reared at least one infant prior to the litter used in this experiment. Experienced mothers in several species exhibit lower rates of infant abuse or neglect than first-time mothers and may therefore be less vulnerable to stress-induced disruptions of maternal behavior; however, no such differences have been found in common marmosets (e.g., Tardif et al., 1984; Bardi and Petto, 2002). Second, we collected data roughly halfway through the lactational period rather than during the early postpartum period. In rats, hormonal influences are more apparent in the initial onset of maternal behavior (i.e., during the early postpartum period) than in the subsequent maintenance of maternal behavior (Numan and Insel, 2003). In human mothers, however, cortisol levels have been found to correlate with aspects of maternal behavior as late as six weeks postpartum (Krpan et al., 2005), and in macaques and baboons, cortisol levels may correlate with maternal behavior over the first 2−3 months postpartum (Bardi et al., 2003; Ramirez et al., 2004). Thus, effects of cortisol on maternal behavior may not be restricted to the early postpartum period in primates.
A third caveat is that plasma cortisol concentrations in our cortisol-treated animals increased progressively across successive days of treatment and, by the end of the 8-day treatment period, were substantially higher than levels typically found at the peak of the circadian cycle or in response to stress in female common marmosets (see Methods). We have, however, documented endogenous plasma cortisol levels similar to those of our cortisol-treated animals, in female marmosets treated with a high dose of exogenous ACTH (Pattison et al., 2007). Moreover, plasma cortisol levels across most days of cortisol treatment in this study were within the physiological range (basal to stressed states), and we found very few behavioral changes across the four days of home-cage observations, suggesting that the exceptionally high cortisol levels at the end of the study did not affect behavior differently than the more physiological levels generated earlier.
It should also be noted that marmosets and other small-bodied New World monkeys exhibit several unusual features of the hypothalamic-pituitary-adrenal axis. Circulating cortisol concentrations are an order of magnitude higher than those in Old World primates, and virtually all of this cortisol circulates unbound or loosely bound to albumin, as marmosets, like other New World primates, have extremely low circulating levels of corticosteroid-binding globulin (Pugeat et al., 1984; Robinson et al., 1985; Klosterman et al., 1986). The high circulating cortisol levels appear to be compensated for by partial glucocorticoid resistance (Chrousos et al., 1986), associated with overexpression of the intracellular FK506-binding protein (FKBP51), which inhibits translocation to the nucleus of glucocorticoid bound to either the mineralocorticoid or glucocorticoid receptor (Denny et al., 2000; Scammell et al., 2001) and hence impairs functionality of the glucocorticoid receptor (Her et al., 2005).
In several species, treatment with exogenous glucocorticoids either raises or lowers concentrations of corticosteroid-binding globulin (CBG), presumably altering the bioavailability of glucocorticoids (e.g., Feldman et al., 1979; Schlechte and Hamilton, 1987; Berdusco et al, 1993). Marmosets, in contrast, do not have appreciable amounts of CBG, and cortisol circulates unbound or loosely bound to albumin (Pugeat et al., 1984; Robinson et al., 1985; Klosterman et al., 1986). Therefore, all of the exogenous (and endogenous) cortisol measured in our animals is likely to be biologically active, and the cortisol concentrations measured in our assays should be highly correlated with levels of cortisol available to the tissues.
In conclusion, our results provide the first experimental support for the hypothesis that cortisol can disrupt maternal behavior in primates, especially in terms of reducing mothers’ time in contact with or carrying their infants. This effect was apparent only under basal, rather than test, conditions, however, and we found no strong evidence that elevated cortisol levels increased mothers’ aggression towards infants. Thus, disruption of maternal behavior and escalation of infant neglect, but not necessarily infant abuse, by stress or stress-related psychiatric disorders, as reported in several primate species, might be mediated at least to some extent by elevated cortisol levels. Additional studies are needed to characterize the roles of other stress-responsive hormones, neurotransmitters, and neuropeptides, such as epinephrine, norepinephrine, CRH, and the endogenous opioids, in modulating primate maternal behavior under stressful conditions.
Acknowledgments
We thank C. Fitz , C. Boettcher, and K. Crosno for assistance with data collection; F.H. Wegner, D.J. Wittwer, and D.E. Green in the WNPRC Assay Services laboratories for assistance with hormone assays; and the WNRPC Veterinary Services and Animal Care staff for assistance with animals. We are grateful to two anonymous reviewers for constructive comments on earlier drafts of the manuscript.
Role of the Funding Source
This research was supported by NIH grant MH075973 and was conducted at a facility (the National Primate Research Center at the University of Wisconsin - Madison) constructed with support from NIH Research Facilities Improvement Program grants RR15459−01 and RR020141−01. NIH had no further role in design of the experiment; in collection, analysis, and interpretation of the study; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Both authors declare that they have no conflict of interest regarding this research.
References
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Bahr NI, Pryce CR, Döbeli M, Martin RD. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol. Behav. 1998;64:429–437. doi: 10.1016/s0031-9384(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Bailham D, Joseph S. Post-traumatic stress following childbirth: a review of the emerging literature and directions for research and practice. Psychol. Health Med. 2003;8:159–168. [Google Scholar]
- Banyard VL, Williams LM, Siegel JA. The impact of complex trauma and depression on parenting: an exploration of mediating risk and protective factors. Child Maltreat. 2003;8:334–349. doi: 10.1177/1077559503257106. [DOI] [PubMed] [Google Scholar]
- Bardi M, French JA, Ramirez SM, Brent L. The role of the endocrine system in baboon maternal behavior. Biol. Psychiatry. 2004;55:724–732. doi: 10.1016/j.biopsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Bardi M, Petto AJ. Parental failure in captive common marmosets (Callithrix jacchus): a comparison with tamarins. Folia Primatol. 2002;73:46–48. doi: 10.1159/000060418. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum cortisol levels and mother-infant interactions in Japanese macaques. Am. J. Phys. Anthropol. 2003;120:298–304. doi: 10.1002/ajpa.10150. [DOI] [PubMed] [Google Scholar]
- Berdusco ET, Hammond GL, Jacobs RA, Grolla A, Akagi K, Langlois D, Challis JR. Glucocorticoid-induced increase in plasma corticosteroid-binding globulin levels in fetal sheep is associated with increased biosynthesis and alterations in glycosylation. Endocrinology. 1993;132:2001–2008. doi: 10.1210/endo.132.5.8477651. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Quanitifying Behavior the JWatcher Way. Sinauer; Sunderland, MA: 2007. [Google Scholar]
- Brockington I. Motherhood and Mental Health. Oxford University Press; Oxford: 1996. [Google Scholar]
- Brockington I. Postpartum psychiatric disorders. Lancet. 2004;363:303–310. doi: 10.1016/S0140-6736(03)15390-1. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Cigliana G, Scaccianoce S, Consoli C, Cinque C, Zuena AR, Angelucci L. Maternal corticosterone influences behavior, stress response and corticosteroid receptors in the female rat. Phamacol. Biochem. Behav. 2002;73:105–114. doi: 10.1016/s0091-3057(02)00755-4. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Loriaux DL, Tomita M, Brandon DD, Renquist D, Albertson B, Lipsett MB. The new world primates as animal models of glucocorticoid resistance. Adv. Exp. Med. Biol. 1986;196:129–144. doi: 10.1007/978-1-4684-5101-6_9. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev. Psychobiol. 2003;26:293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev. Psychobiol. 1994;27:257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- Cohen LR, Hien DA, Batchelder S. The impact of cumulative maternal trauma and diagnosis on parenting behavior. Child Maltreat. 2008;13:27–38. doi: 10.1177/1077559507310045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R, Rogers LJ. Mobbing vocalizations as a coping response in the common marmoset. Horm. Behav. 2006;49:237–245. doi: 10.1016/j.yhbeh.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman SB, Panagiotides H, Hessl D, Self J, Yamada E, Embry L. Preschool outcomes of children of depressed mothers: role of maternal behavior, contextual risk, and children's brain activity. Child Dev. 2003;74:1158–1175. doi: 10.1111/1467-8624.00599. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Digby LJ, Ferrari SF, Saltzman W. Callitrichines: the role of competition in cooperatively breeding species. In: Campbell CJ, Fuentes AF, Mackinnon KC, Panger M, Bearder S, editors. Primates in Perspective. Oxford University Press; Oxford: 2007. pp. 85–106. [Google Scholar]
- Epple G. Comparative studies on vocalization in marmoset monkeys (Hapalidae). Folia Primatol. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Feldman D, Mondon CE, Horner JA, Weiser JN. Glucocorticoid and estrogen regulation of corticosteroid-binding globulin production by rat liver. Am. J. Physiol. 1979;237:E493–E499. doi: 10.1152/ajpendo.1979.237.6.E493. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Anderson V. Hormonal and attitudinal correlates ofmaternal behaviour during the early postpartum period in first-time mothers. J. Reprod. Inf. Psychol. 1987;5:193–205. [Google Scholar]
- Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm. Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav. Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Hum. Dev. 2007;83:675–681. doi: 10.1016/j.earlhumdev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Graham MD, Rees SL, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on maternal memory in postpartum rats. Horm. Behav. 2006;49:353–361. doi: 10.1016/j.yhbeh.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Gems S, Hodges JK, Hearn JP. The relationship between plasma progesterone and the timing of ovulation and early embryonic development in the marmoset monkey (Callithrix jacchus). J. Zool. 1983;201:273–282. [Google Scholar]
- Hearn JP. Restraining device for small monkeys. Lab. Anim. 1977;11:261–262. doi: 10.1258/002367777780936459. [DOI] [PubMed] [Google Scholar]
- Her S, Patel PD, Schatzberg AF, Lyons DM. Mutations in squirrel monkey glucocorticoid receptor impair nuclear translocation. J. Steroid Biochem. Mol. Biol. 2005;94:319–326. doi: 10.1016/j.jsbmb.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter S, Gold PW, Chrousos GP. “Environmental stress” and reproductive success in the common marmoset (Callithrix jacchus jacchus). Am. J. Primatol. 1991;25:191–201. doi: 10.1002/ajp.1350250306. [DOI] [PubMed] [Google Scholar]
- Klosterman LL, Murai JT, Siiteri PK. Cortisol levels, binding, and properties of corticosteroid-binding globuulin in the serum of primates. Endocrinology. 1986;118:424–434. doi: 10.1210/endo-118-1-424. [DOI] [PubMed] [Google Scholar]
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kotch JB, Browne DC, Ringwalt CL, Stewart PW, Ruina E, Holt K, Lowman B, Jung J-W. Risk of childabuse or neglect in a cohort of low-income children. Child Abuse Negl. 1995;19:1115–1130. doi: 10.1016/0145-2134(95)00072-g. [DOI] [PubMed] [Google Scholar]
- Krpan KM, Coombs R, Zinga D, Steiner M, Fleming AS. Experiential and hormonal correlates of maternal behavior in teen and adult mothers. Horm. Behav. 2005;47:112–122. doi: 10.1016/j.yhbeh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Léonhardt M, Matthews SG, Meaney MJ, Walker C-D. Psychological stressors as a model of maternal adversity: diurnal modulation of corticosterone responses and changes in maternal behavior. Horm. Behav. 2007;51:77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin. Psych. Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Schatzberg AF. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J. Neuroscience. 2000;20:7816–7821. doi: 10.1523/JNEUROSCI.20-20-07816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Eliez S, Reiss AL, Schatzberg AF. Cognitive correlates of white matter growth and stress hormones in female squirrel monkey adults. J. Neurosci. 2004;24:3655–3662. doi: 10.1523/JNEUROSCI.0324-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Behavioral and environmental correlates of infant abuse in group-living pigtail macaques. Inf. Behav. Dev. 1998a;21:603–612. [Google Scholar]
- Maestripieri D, Carroll KA. Child abuse and neglect: usefulness of the animal data. Psychol. Bull. 1998b;123:211–223. doi: 10.1037/0033-2909.123.3.211. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress. Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- Nonacs R. Postpartum mood disorders: diagnosis and treatment considerations. In: Pearson KH, Sonawalla SB, Rosenbaum JF, editors. Women's Health and Psychiatry. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 127–136. [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Orth DN. Adrenocorticotropic hormone (ACTH). In: Jaffe BM, Behrman HR, editors. Methods of Hormone Radioimmunoassay. 2nd ed. Academic Press; New York: 1979. pp. 245–278. [Google Scholar]
- Oyserman D, Mowbray CT, Meares PA, Firminger KB. Parenting among mothers with a serious mental illness. Am. J. Orthopsychiatry. 2000;70:296–315. doi: 10.1037/h0087733. [DOI] [PubMed] [Google Scholar]
- Pattison JC, Saltzman W, Abbott DH, Hogan BK, Nguyen AD, Husen B, Einspanier A, Conley AJ, Bird IM. Gender and gonadal status differences in zona reticularis expression in marmoset monkey adrenals: cytochrome b5 localization with respect to cytochrome P450 17,20-lyase activity. Mol. Cell. Endocinol. 2007;265−266:93–101. doi: 10.1016/j.mce.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, McGuire M, Evans DL. Corticotropin-releasing hormone inhibits maternal behavior and induces pup-killing. Life Sci. 1991;48:1537–1546. doi: 10.1016/0024-3205(91)90278-j. [DOI] [PubMed] [Google Scholar]
- Pugeat MM, Chrousos GP, Nisula BC, Loriaux DL, Brandon D, Lipsett MB. Plasma cortisol transport and primate evolution. Endocrinology. 1984;115:357–361. doi: 10.1210/endo-115-1-357. [DOI] [PubMed] [Google Scholar]
- Ramirez SM, Bardi M, French JA, Brent L. Hormonal correlates of changes in interest in unrelated infants across the peripartum period in female baboons (Papio hamadryas anubis sp.). Horm. Behav. 2004;46:520–528. doi: 10.1016/j.yhbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on maternal behavior in the postpartum rat. Horm. Behav. 2004;46:411–419. doi: 10.1016/j.yhbeh.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on induction of maternal behavior in the virgin female rat. Horm. Behav. 2006;49:337–345. doi: 10.1016/j.yhbeh.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Reite M, Caine NG. Child Abuse: The Nonhuman Primate Data. Alan R. Liss; New York: 1983. [Google Scholar]
- Robinson PA, Hawkey C, Hammond GL. A phylogenetic study of the structural and functional characteristics of corticosteroid binding globulin in primates. J. Endocrinol. 1985;104:251–257. doi: 10.1677/joe.0.1040251. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Saltzman W. Reproductive competition among female common marmosets (Callithrix jacchus): proximate and ultimate causes. In: Jones CB, editor. Sexual Selection and Reproductive Competition in Primates: New Perspectives and Directions. American Society of Primatologists; Norman, OK: 2003. pp. 197–229. [Google Scholar]
- Saltzman W, Abbott DH. Diminished maternal responsiveness during pregnancy in multiparous female common marmosets. Horm. Behav. 2005;47:151–163. doi: 10.1016/j.yhbeh.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Prudom SL, Schultz-Darken NJ, Wittwer DJ, Abbott DH. Social suppression of cortisol in female marmoset monkeys: role of circulating ACTH levels and glucocorticoid negative feedback. Psychoneuroendocrinology. 2004;29:141–161. doi: 10.1016/s0306-4530(02)00159-2. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Abbott DH. Familial influences on ovulatory function in common marmosets (Callithrix jacchus). Am. J. Primatol. 1997a;41:159–177. doi: 10.1002/(SICI)1098-2345(1997)41:3<159::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol. Behav. 1994;56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abott DH. Suppression of cortisol levels in subordinate female marmosets: reproductive and social contributions. Horm. Behav. 1998;33:58–74. doi: 10.1006/hbeh.1998.1436. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Severin JM, Schultz-Darken NJ, Abbott DH. Behavioral and social correlates of escape from suppression of ovulation in female common marmosets housed with the natal family. Am. J. Primatol. 1997b;41:1–21. doi: 10.1002/(SICI)1098-2345(1997)41:1<1::AID-AJP1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Schlechte JA, Hamilton D. The effect of glucocorticoids on corticosteroid binding globulin. Clin. Endocrinol. 1987;27:197–203. doi: 10.1111/j.1365-2265.1987.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Clarke AS, Kraemer GW, Roughton EC, Lubach GR, Rimm-Kaufman S, Schmidt D, Ebert M. Prenatal stress alters brain biogenic amine levels in primates. Dev. Psychopathol. 1998;10:427–440. doi: 10.1017/s0954579498001679. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Stevenson MF, Poole TB. An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim. Behav. 1976;24:428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Richter CB, Carson RL. Effects of sibling-rearing experience on future reproductive success in two species of Callitrichidae. Am. J. Primatol. 1984;6:377–380. doi: 10.1002/ajp.1350060408. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Comparison of infant care in family groups of the common marmoset (Callithrix jacchus) and the cotton-top tamarin (Saguinus oedipus). Am. J. Primatol. 1986;11:103–110. doi: 10.1002/ajp.1350110202. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus). Comp. Med. 2003;53:364–368. [PubMed] [Google Scholar]
- Tolan P, Gorman-Smith D, Henry D. Family violence. Annu. Rev. Psychol. 2006;547:557–583. doi: 10.1146/annurev.psych.57.102904.190110. [DOI] [PubMed] [Google Scholar]
- Troisi A, D'Amato FR. Mechanisms of primate infant abuse: the maternal anxiety hypothesis. In: Parmigiana S, vom Saal F, editors. Infanticide and Parental Care. Harwood, Chur; Switzerland: 1994. pp. 199–210. [Google Scholar]
- Tu MT, Lupien SJ, Walker C-D. Measuring stress responses in postpartum mothers: perspectives from studies in human and animal populations. Stress. 2005;8:19–34. doi: 10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- Windham AM, Rosenberg L, Fuddy L, McFarlane E, Sia C, Duggan AK. Risk of mother-reported child abuse in the first 3 years of life. Child Abuse Negl. 2004;28:645–667. doi: 10.1016/j.chiabu.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Ximenes MFFM, Sousa MBC. Family composition and the characteristics of parental care during the nursing phase of captive common marmosets (Callithrix jacchus). Primates. 1996;37:175–183. [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Restraint stress impaired maternal behavior in female mice lacking the neuromedin B receptor (NMB-R) gene. Neurosci. Lett. 2002;330:163–166. doi: 10.1016/s0304-3940(02)00771-1. [DOI] [PubMed] [Google Scholar]


