Abstract
Positive-strand RNA virus genomes are substrates for translation, RNA replication, and encapsidation. To identify host factors involved in these functions, we used the ability of brome mosaic virus (BMV) RNA to replicate in yeast. We report herein identification of a mutation in the essential yeast gene DED1 that inhibited BMV RNA replication but not yeast growth. DED1 encodes a DEAD (Asp-Glu-Ala-Asp)-box RNA helicase required for translation initiation of all yeast mRNAs. Inhibition of BMV RNA replication by the mutant DED1 allele (ded1–18) resulted from inhibited expression of viral polymerase-like protein 2a, encoded by BMV RNA2. Inhibition of RNA2 translation was selective, with no effect on general cellular translation or translation of BMV RNA1-encoded replication factor 1a, and was independent of p20, a cellular antagonist of DED1 function in translation. Inhibition of RNA2 translation in ded1–18 yeast required the RNA2 5′ noncoding region (NCR), which also conferred a ded1–18-specific reduction in expression on a reporter gene mRNA. Comparison of the similar RNA1 and RNA2 5′ NCRs identified a 31-nucleotide RNA2-specific region that was required for the ded1–18-specific RNA2 translation block and attenuated RNA2 translation in wild-type yeast. Further comparisons and RNA structure predictions suggest a modular arrangement of replication and translation signals in RNA1 and RNA2 5′ NCRs that appears conserved among bromoviruses. The 5′ attenuator and DED1 dependence of RNA2 suggest that, despite its divided genome, BMV regulates polymerase translation relative to other replication factors, just as many single-component RNA viruses use translational read-through and frameshift mechanisms to down-regulate polymerase. The results show that a DEAD-box helicase can selectively activate translation of a specific mRNA and may provide a paradigm for translational regulation by other members of the ubiquitous DEAD-box RNA helicase family.
On entering the cytoplasm, the messenger-sense genomic RNAs of all positive-strand RNA viruses first are translated to produce viral proteins including RNA replication factors. For eukaryotic translation initiation on most capped viral and cellular mRNAs, the cap-binding complex eIF4F and other factors recruit the 40S ribosome to scan from the mRNA 5′ end to the initiating AUG. This scanning requires canonical translation initiation factor eIF4A, a DEAD (Asp-Glu-Ala-Asp)-box RNA helicase, to unwind secondary structure in the 5′ noncoding region (NCR) (1).
DEAD-box proteins like eIF4A form a large family of established and putative RNA helicases sharing seven conserved amino acid motifs (2). They are found in bacteria, eukaryotes, and their viruses and are involved in many aspects of RNA metabolism including rRNA processing and mRNA splicing, export, translation, and decay. Some DEAD-box proteins show tissue-specific expression, suggesting possible regulatory functions. In addition to eIF4A, a second DEAD-box protein, Ded1p, encoded by the essential DED1 gene is required in yeast for translation initiation (3). Ded1p has RNA-dependent ATPase and ATP-dependent RNA helicase activities (4) and is antagonistic with p20, a general negative regulator of yeast translation (5). Higher eukaryotes including humans encode DEAD-box proteins that can replace DED1 in yeast translation initiation (3).
One model for viral gene expression, RNA replication, and virus–host interactions in positive-strand RNA viruses is brome mosaic virus (BMV). BMV is a member of the alphavirus-like superfamily of human, animal, and plant viruses. The BMV genome consists of three capped RNAs (Fig. 1A). RNA1 and RNA2 encode RNA replication factors 1a and 2a. Factor 1a protein contains a C-terminal DEAD-box helicase domain and an N-terminal domain with 7-methylguanosine (m7G) methyltransferase and covalent m7GMP binding activities (6, 7) required for viral RNA capping (7). Factor 2a protein contains an RNA-dependent RNA polymerase domain. These three domains are conserved in all alphavirus-like viruses. RNA3 encodes cell-to-cell movement and coat proteins that direct systemic infection in BMV's natural plant hosts. Coat protein is not translated from RNA3 but only from a subgenomic mRNA, RNA4, initiated internally on negative-strand RNA3.
Figure 1.
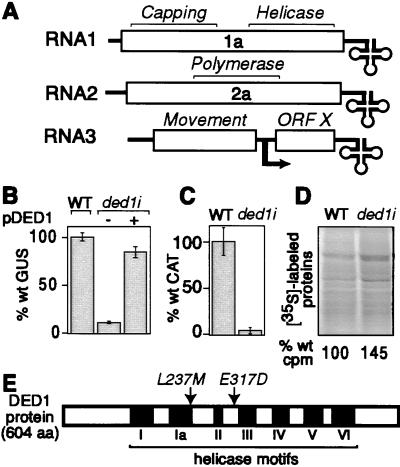
Identification of DED1. (A) Schematic diagram of the BMV genome shows ORFs (open boxes), noncoding regions (single lines), tRNA-like 3′ ends (cloverleaves), and the subgenomic mRNA start site (bent arrow). (B) BMV-directed GUS expression in 1a- and 2a-expressing wt yeast, ded1i yeast, and ded1i yeast complemented with wt DED1 expressed from pDED1 is shown. (C) BMV-directed CAT expression in 1a- and 2a-expressing wt and ded1i yeast transfected with B3CAT in vitro transcripts. (D) Autoradiographs and acid-precipitable counts (below lanes) from 35S-labeled protein extracts from wt and ded1i yeast are shown. (E) Schematic diagram of Ded1p showing conserved helicase motifs (roman numerals) and substitutions in the BMV-inhibiting ded1–18 allele.
In alphavirus-like viruses with a single genomic RNA, viral polymerase expression is inhibited relative to other RNA replication factors by translational read-through or frameshift (8, 9). Because BMV encodes 1a and 2a on separate genomic RNAs, it has been unclear whether RNA2-specific mechanisms exist to down-regulate 2a polymerase translation.
BMV also directs RNA replication, subgenomic mRNA synthesis, and encapsidation in the yeast Saccharomyces cerevisiae, reproducing all known features of BMV replication in plant cells (10). As in plant cells, BMV RNA replication in yeast localizes to the endoplasmic reticulum; depends on 1a, 2a, and specific cis-acting RNA signals; and generates excess positive- to negative-strand RNA (10–13). Because of the facility of yeast genetics, BMV replication in yeast can assist identification and study of viral and host functions in RNA replication and gene expression (refs. 13–15 and W. M. Lee and P.A., unpublished results).
Herein we identify a DED1 mutant allele that strongly suppresses BMV RNA replication in yeast by selectively inhibiting expression of the polymerase-like 2a protein. We also identify RNA2 5′ NCR subdomains that are required for this inhibition and attenuate 2a translation in wild-type (wt) cells. In addition to their implications for viral replication, these results show that a member of the ubiquitous DEAD-box RNA helicases can selectively activate translation of a specific mRNA.
Materials and Methods
Yeast and Plasmids.
Standard yeast genetic techniques and media were used (16, 17). YMI04 yeast (MATα ura3–52 lys2–801∷B3GUS can1∷B3URA3 ade2–101 trp1-Δ63 his3-Δ200 leu2-Δ1) was as described (14). BMV RNA3 and B3CPfs were expressed from pB3RQ39 (18) and pB3MS82 (19).
BMV 1a and 2a were expressed from ADH1 promoters using pB1CT19 and pB2CT15 (10) and from GAL1 promoters using pB1YT3 and pB2YT5 (19). wt BMV RNA2 was expressed from pB2NR3 (J.C., A.N., and P.A., unpublished results). pB1YT1 (provided by Y. Tomita, M. Ishikawa, and S. Naito, Hokkaido Univ.) is a CEN4 centromeric plasmid with the TRP1 marker, expressing 1a from the GAL1 promoter. To express wt RNA1, the wt RNA1 5′ NCR and 3′ NCR (followed by a hepatitis δ virus ribozyme) were generated by PCR from pB1TP3 (20) and used to replace the nonviral sequences flanking 1a in pB1YT1, creating pB1AON1.
pDED1/URA and pDED1/TRP were constructed by cloning a 2.5-kb XhoI–XmnI fragment containing the DED1 gene from a yeast genomic library clone (21) into the XhoI–SmaI sites of CEN4 plasmids pRS316 and pRS314 (22), which contain URA3 and TRP1, respectively. To construct pDED1, pDED1/TRP was cleaved with BsrBI, and the resulting 2.6-kb fragment containing DED1 was cloned into the unique SmaI site of a Ycplac22 (23) derivative with the TRP1 marker replaced by LYS2.
RNA and Protein Analysis.
Total yeast RNA isolation, Northern blot analysis, and total protein extractions and Western blot analysis were as described (10, 13, 14). Synthesis of capped in vitro transcripts, RNA transfections, and chloramphenicol acetyltransferase (CAT), β-glucuronidase (GUS), and luciferase assays were as described (10, 24) except that CAT and GUS activities were measured with FAST CAT (Molecular Probes) and GUS-Light (Tropix) kits. β-Galactosidase (lacZ) assays were performed with a Gal-Screen kit (Tropix, Bedford, MA). Chemiluminescence was measured with a PharMingen monolight model 3010 luminometer.
Secondary Structure Analysis.
Secondary structure analysis was performed with mfold version 2.3 (25) with the following parameters: 30°C folding temperature, 5% suboptimality, and no limits on distance between paired bases. The entire BMV RNA2 sequence (2,865 nt) and first 220 nt of the other RNAs discussed were analyzed.
Results
DED1 Mutation Inhibits BMV-Directed Gene Expression.
Yeast strain YMI04 contains chromosomally integrated, galactose-inducible expression cassettes of RNA3 derivatives B3URA3 and B3GUS, with the coat gene replaced by the URA3 and GUS genes, respectively (14). YMI04 yeast containing a plasmid expressing 1a and 2a was mutagenized with ethyl methanesulfonate, and yeast chromosomal mutants inhibiting BMV-directed URA3 and GUS expression were isolated as for UV mutagenesis (14). For one such yeast mutant, screening a wt yeast genomic library showed that BMV-directed GUS expression was partially complemented by plasmids bearing the wt DED1 gene. Partial complementation suggested that this strain contained one or more additional BMV-inhibiting mutations or that DED1 suppressed a mutation at a different locus.
To determine whether DED1 contributed to BMV replication and gene expression, a PCR mutagenesis approach was used to target mutations to DED1 (26). Yeast strain YAON01 was constructed from YMI04 by deleting the chromosomal DED1 gene and providing the essential DED1 gene on pDED1/URA3, a plasmid also containing URA3. Next, mutagenized copies of the DED1 ORF were synthesized by error-prone PCR, introduced into pDED1/TRP containing the TRP1-selectable marker, and transformed into YAON01 also containing a plasmid expressing 1a and 2a. The resulting Trp+ colonies were replica plated onto medium with 5-fluoroorotic acid, which selects against URA3. These steps replaced the wt pDED1/URA3 plasmid with the mutagenized pDED1/TRP plasmid, which still must provide DED1 functions essential for yeast growth.
After transfer to galactose medium to induce B3URA3 and B3GUS transcription, four yeast mutants were isolated with inhibited BMV-directed URA3 and GUS expression. The DED1-containing plasmids from these mutant yeast were recovered, reintroduced into YAON01, and shown to reproduce the BMV-inhibiting phenotype. Mutant allele ded1–18, exhibiting the most severe BMV inhibition, was selected for further study. To eliminate variations in expression associated with varying plasmid copy numbers, we replaced the DED1 chromosomal locus of wt strain YMI04 with the ded1–18 allele, creating isogenic strain ded1i. This strain supported BMV-directed GUS expression to only 12% of wt (Fig. 1B) but was complemented to near wt levels (85%) by pDED1 containing the LYS2 selectable marker. Different complementation levels were obtained by different DED1-containing plasmids, possibly because of differences in plasmid copy number. pDED1/TRP, e.g., complemented BMV-dependent GUS expression in ded1i yeast to higher levels than wt yeast.
To determine whether DED1 inhibition of BMV was independent of in vivo DNA-directed transcription or nucleocytoplasmic transport of BMV RNA3 transcripts, wt and ded1i yeast expressing 1a and 2a were transfected with in vitro-transcribed B3CAT, an RNA3 derivative with the coat gene replaced by the CAT gene. BMV-directed CAT expression in ded1i yeast was inhibited to 5% of that in wt yeast (Fig. 1C), a result similar to results with B3GUS RNA expressed from the nucleus (Fig. 1B).
Normal Growth and Translation in ded1i Yeast.
Sequencing ded1–18 revealed two nucleotide changes causing conservative amino acid substitutions L237 M (TTG → ATG) and E317D (GAA → GAT) (Fig. 1E). Although these changes are in the helicase domain required for DED1's essential translation function, the mutant allele retained the function(s) critical for cell growth. ded1i yeast grew robustly, with a cell doubling rate indistinguishable from that of isogenic wt yeast. Overall cellular translation was wt or even enhanced in the mutant. Incorporation of [35S]methionine/[35S]cysteine into acid-precipitable peptides in ded1i yeast averaged 50% higher than in wt cells (Fig. 1D). Similarly, levels of 35S-labeled proteins observed on an SDS/polyacrylamide gel were wt or slightly higher in ded1i yeast (Fig. 1D).
ded1i Inhibits BMV RNA Synthesis.
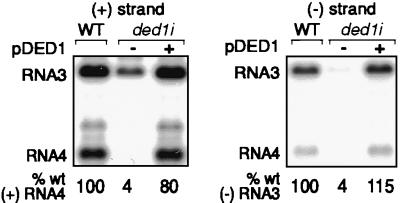
To determine whether inhibition of BMV-directed gene expression in ded1i yeast was caused by inhibition of subgenomic mRNA translation or synthesis, we tested the accumulation of BMV RNA replication products. Wt and ded1i yeast expressing 1a and 2a were transformed with a plasmid expressing RNA3 derivative B3CPfs, which contains a frame-shifted coat protein gene (19). This derivative was used to separate ded1i effects on RNA replication from any effects on coat protein production, which encapsidates and stabilizes BMV RNAs in yeast (27). However, similar results were obtained when WT RNA3 was used (data not shown). Fig. 2 shows that positive-strand RNA4 and negative-strand RNA3 levels were reduced to 4% of those in wt yeast. The mutant phenotype was complemented to near-wt levels (80–115% of wt) by pDED1.
Figure 2.
Inhibited BMV RNA synthesis in ded1i yeast shown by Northern blot analysis of RNA3 and RNA4 replication products in 1a- and 2a-containing yeast. Blots were hybridized with positive-strand-specific (Left) and negative-strand-specific (Right) RNA probes from the coat protein gene. Average accumulation of positive-strand RNA4 and negative-strand RNA3 from three or more experiments is shown at the bottom.
Selective Inhibition of 2a Protein Accumulation in ded1i Yeast.
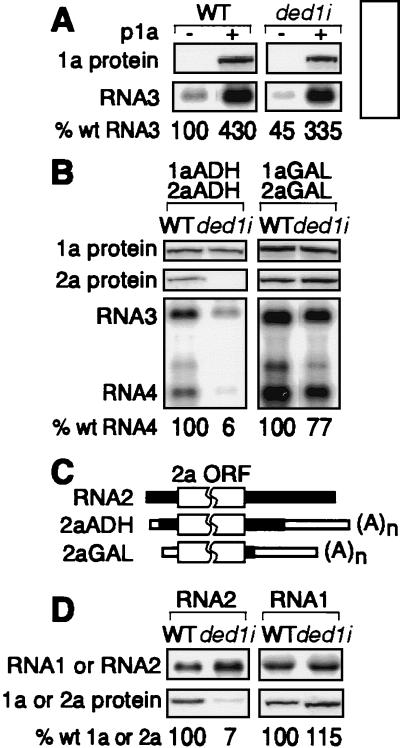
Next we compared the accumulation and function of replication factors 1a and 2a in wt and ded1i yeast. As a prerequisite for negative-strand RNA3 synthesis, 1a inhibits RNA3 translation, dramatically increasing RNA3 stability (13, 28). Factor 1a protein-induced stabilization of RNA3 transcripts in wt and ded1i yeast was measured by comparing steady-state levels of RNA3 with or without 1a. As shown in Fig. 3A, 1a accumulated in ded1i yeast to nearly normal levels (85% of wt) and was similarly functional in stimulating RNA3 accumulation.
Figure 3.
(A) RNA3 stabilization by factor 1a protein in wt and ded1i yeast in the absence of factor 2a protein. Western and Northern blot analyses of wt and ded1i yeast expressing wt RNA3 with or without 1a. Northern blots were hybridized with a positive-strand-specific RNA probe from the coat protein gene. Averages from three or more experiments are shown. (B) Accumulation of 1a and 2a proteins expressed from ADH1 (Left)- or GAL1 (Right)-driven mRNAs and wt RNA3 replication in wt and ded1i yeast. Northern blots were hybridized with a positive-strand-specific RNA probe from the coat protein gene. Percent wt RNA4 values are averages of three or more experiments. (C) Schematic diagram of BMV RNA2, 2aADH mRNA, and 2aGAL mRNA shows BMV RNA2-derived (solid bars) and non-BMV (open bars) 5′ and 3′ NCR sequences. (D) Defective 2a but not 1a expression from wt RNA2 and RNA1, respectively. (Left) 2a protein expression from RNA2 in wt and ded1i yeast. A Northern blot was hybridized with a positive-strand-specific RNA probe from the 2a ORF. (Right) Data are similar to Left, except that wt and ded1i yeast expressed 1a from RNA1. Percent wt 1a or 2a values are averages of three or more experiments.
In contrast, 2a was undetectable in ded1i yeast (Fig. 3B Left). Because 2a expression can be substantially reduced without inhibiting BMV RNA replication (29), it was unclear whether low 2a expression was the sole or primary cause of ded1i defects in RNA replication. In the above experiments, 2a was expressed from an ADH1 promoter-driven mRNA, designated 2aADH mRNA. Expression from an alternate GAL1 promoter-driven mRNA, designated 2aGAL mRNA (15), restored 2a accumulation and BMV RNA replication in ded1i yeast in parallel (Fig. 3C). Thus, ded1i inhibited BMV RNA replication by inhibiting 2a accumulation. The reasons for defective 2a accumulation in ded1i yeast from 2aADH mRNA but not 2aGAL mRNA and the relevance of this defect to wt BMV RNA2 translation are addressed below.
Defective 2a Accumulation from wt RNA2 in ded1i Yeast.
In 2aADH mRNA, ADH1 promoter- and terminator-derived sequences replaced the first 46 nt of the 103-nt 5′ NCR and last 194 nt of the 293-nt 3′ NCR, creating chimeric BMV/ADH1 NCRs (Fig. 3C). In 2aGAL mRNA, the entire 5′ NCR and all but 22 nt of the 3′ NCR of RNA2 were replaced by GAL1 promoter and ADH1 terminator-derived sequences, respectively. Because defective 2a expression from 2aADH mRNA in ded1i could result from the chimeric nature of the 2aADH NCRs or from BMV-sequence-specific effects, we tested 2a accumulation when expressed from wt RNA2 (Fig. 3D). wt RNA2 transcript levels in ded1i yeast were as high or higher than in those in wt yeast. However, 2a accumulation in ded1i yeast was inhibited 14-fold.
As discussed below, BMV RNA1 and RNA2 have similar 5′ and 3′ NCRs, a feature conserved across other bromoviridae. For comparison, we tested 1a expression from wt RNA1. Similar to RNA2, RNA1 transcript levels were equal in wt and ded1i yeast. However, unlike 2a, 1a levels in ded1i yeast averaged 115% of those in wt yeast (Fig. 3D).
Defective 2a Accumulation Requires RNA2 5′ NCR.
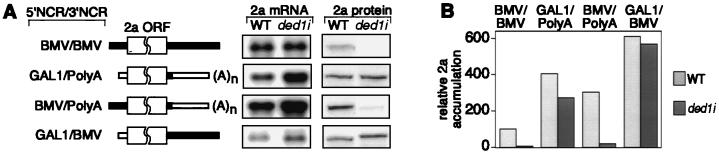
To identify sequences responsible for defective translation of wt RNA2 in ded1i yeast, the 5′ and 3′ NCRs of 2aGAL mRNA and RNA2 were interchanged to produce RNAs containing a wt RNA2 5′ and polyadenylated 3′ NCR [BMV/poly(A)] and a GAL1 5′ and wt RNA2 3′ NCR (GAL1/BMV). To measure the translational activity of each mRNA in wt and ded1i yeast, relative 2a accumulation was calculated as the ratio between 2a protein and 2a mRNA levels (Fig. 4).
Figure 4.
Factor 2a protein accumulation defect requires RNA2 5′ NCR. (A) Schematic diagram of 2a mRNAs shows 2a ORF, wt RNA2 NCR sequences (solid bars), and GAL1 message NCRs (open bars). Northern (Left) and Western (Right) blot analyses of wt and ded1i yeast containing these mRNAs are shown to the right. Northern blots were hybridized with a positive-strand-specific RNA probe from the 2a ORF. Western blots were incubated with anti-2a antibody (11). (B) Relative accumulation of 2a protein expression from mRNAs in A. Relative 2a accumulation was defined as the ratio between 2a protein and mRNA levels and was normalized to relative 2a accumulation for wt RNA2 (BMV/BMV) in wt yeast (100%). Data are averages of three or more experiments.
In wt yeast, 2a protein accumulation did not correlate with message abundance. Wt RNA2 accumulated to intermediate levels but expressed 2a least efficiently. Replacing the 5′ or 3′ NCR of RNA2 with non-BMV sequences increased 2a accumulation in wt yeast, but these effects were not additive.
In ded1i yeast, the levels of all 2a transcripts except wt RNA2 increased over those in wt yeast, with the greatest increase seen for the GAL1/poly(A) message. In transcripts with the GAL1 5′ NCR [GAL1/poly(A) and GAL1/BMV], the increased transcript level was accompanied by an equivalent increase in 2a protein, yielding similar relative 2a accumulation in wt and ded1i yeast. In contrast, for transcripts containing the RNA2 5′ NCR [RNA2 = BMV/BMV and BMV/poly(A)], 2a accumulation was inhibited in ded1i yeast to 7% of that in wt yeast. Thus, the wt RNA2 5′ NCR inhibited 2a accumulation in ded1i yeast irrespective of the 3′ NCR.
RNA2 5′ NCR Subdomains Inhibit 2a Accumulation.
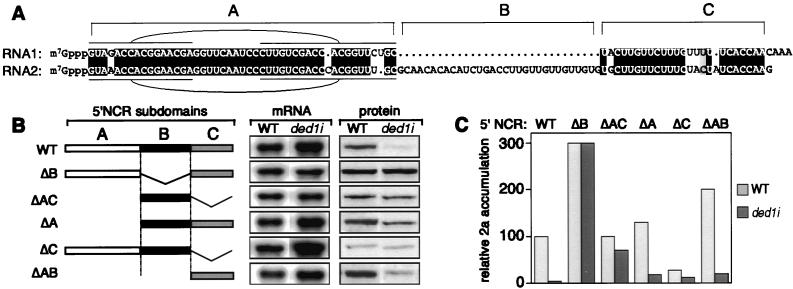
Comparison of RNA1 and RNA2 5′ NCRs defined three subdomains (Fig. 5A): subdomain A, a region implicated in RNA replication (30, 31) that is 92% identical between RNAs 1 and 2; subdomain B, a 31-nt region specific to RNA2; and subdomain C, a pyrimidine-rich sequence 80% identical between RNAs 1 and 2. Secondary structure analysis revealed that only subdomain A folded into a stable stem–loop structure that was described (31) and has a moderate free energy of −13.6 kcal/mol (Fig. 5A). Similar 5′-terminal hairpins with conserved apical loops are found in RNAs 1 and 2 from the related cowpea chlorotic mottle and cucumber mosaic viruses (31) and broad bean mottle virus (unpublished results).
Figure 5.
Contribution of RNA2 subdomains to 2a accumulation defect. (A) Alignment of RNA1 and RNA2 5′ NCR sequences shows subdomains A, B, and C. Identical residues are highlighted in black and similar residues (purines or pyrimidines) are on a shaded background. Sequences predicted to be paired in subdomain A are overlined or underlined. (B) Schematic diagram of deletions in 2a mRNA 5′ NCRs and Northern and Western blot analyses are shown as in Fig. 4A of wt and ded1i yeast containing these mRNAs. (C) Relative accumulation of 2a protein expression from mRNAs diagramed in B is shown. Data are averages of three or more experiments.
Deletion mapping of the RNA2 5′ NCR was performed in tandem with wt RNA2 and the BMV/poly(A) derivative of Fig. 4, with equivalent results. Fig. 5 presents data from 5′ NCR deletions in BMV/poly(A), because the 3′ poly(A) improved translation efficiency and thus 2a quantitation for all derivatives, including those with low 2a expression levels. Use of BMV/poly(A) also allowed separating 5′ NCR contributions from possible effects of the wt RNA2 3′ NCR.
Because wt RNA1 strongly expressed 1a in ded1i yeast (Fig. 3D) and the major difference between RNAs 1 and 2 5′ NCRs was subdomain B, an exact deletion of subdomain B was constructed in RNA2. This ΔB deletion had two striking effects (Fig. 5 B and C): (i) It stimulated 2a expression levels in wt yeast 3-fold. (ii) It suppressed all ded1i-specific inhibition of 2a expression, so that 2a levels in ded1i yeast matched the enhanced level in wt yeast. Interestingly, a 5′ NCR containing subdomain B alone (ΔAC) only mildly inhibited 2a accumulation in ded1i yeast. Thus, subdomain B attenuated 2a expression in wt yeast and was required but not sufficient for DED1-dependent 2a expression.
Subdomains B and C retained most of the DED1 dependence in 2a expression, because after deleting subdomain A (ΔA), 2a accumulation in ded1i yeast remained at only 14% of wt levels. Deleting subdomain C alone reduced relative 2a accumulation in wt yeast by 4-fold, complicating interpretation of its behavior in ded1i yeast. Nevertheless, in ded1i yeast this ΔC derivative retained 50% or more of its wt 2a accumulation, further suggesting a possible role for subdomain C in DED1 dependence. Consistent with this observation, a derivative retaining only subdomain C in the 5′ NCR (ΔAB) showed clear DED1 dependence, expressing 2a in ded1i yeast to only 10% the level in wt yeast. Thus, both 5′ NCR subdomains B and C contribute to the inhibition of 2a expression in ded1i yeast. Further interactions between 5′ NCR subdomains are considered in the Discussion.
RNA2 5′ NCR Directs ded1i-Specific Inhibition of lacZ Expression.
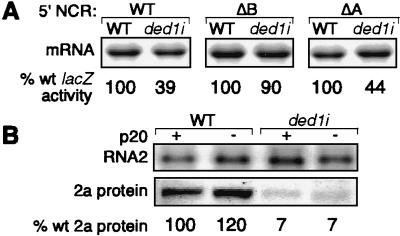
To see whether the wt RNA2 5′ NCR could inhibit expression of other genes in ded1I-yeast, this NCR was placed in front of the lacZ ORF. The RNA2 5′ NCR-lacZ fusion was expressed from the yeast GAL1 promoter and followed by a polyadenylation signal to produce wt-lacZ RNA. This plasmid was transformed into wt and ded1i yeast, and lacZ activity was assayed. As seen in Fig. 6A, lacZ expression in ded1i yeast was inhibited to 39% of that in wt yeast. Because lacZ expression in ded1i yeast was not inhibited as efficiently as 2a accumulation, we tested whether defective lacZ expression also depended on subdomain B. As observed for the wt RNA2 derivatives expressing 2a, deleting subdomain B but not A suppressed the mutant phenotype.
Figure 6.
(A) RNA2 5′ NCR-directed lacZ expression is shown by Northern blot analysis and relative lacZ activity in wt and ded1i yeast containing wt-lacZ, ΔB-lacZ or ΔA-lacZ mRNA. Northern blots were hybridized with an RNA probe from the lacZ ORF, and relative lacZ activity is expressed as the ratio of lacZ activity and lacZ mRNA levels. Values given are averages of three or more experiments. (B) Relative accumulation of 2a expressed from RNA2 in wt or ded1i yeast with or without p20. Northern blots were hybridized with a positive-strand-specific RNA probe from the 2a ORF. Values given are averages of three or more experiments.
ded1i-Specific Inhibition Is Independent of p20.
The yeast p20 protein encoded by CAF20 inhibits cap-dependent translation (5, 32). p20 acts antagonistically with Ded1p: deleting CAF20 partially suppresses the growth defect caused by the ded1/spp81–3 mutant, whereas overexpressing p20 decreases growth rate in that mutant (5). To see whether the 2a accumulation defect in ded1i yeast depended on p20, CAF20 was disrupted by URA3 insertion in wt and ded1i yeast. In wt yeast, 2a expression from RNA2 was wt or slightly higher when CAF20 was disrupted (Fig. 6B). However, in ded1i yeast, disrupting CAF20 had no effect on 2a accumulation, which remained at 7% of that in wt yeast.
Discussion
The results presented herein show that mutation of the essential yeast gene DED1 can inhibit BMV replication by selectively inhibiting translation of the viral polymerase gene. The unusual, specific dependence of RNA2 translation on DED1 required an RNA2-specific region in the 5′ NCR. These results suggest that BMV polymerase expression may be regulated at the level of translation initiation and that general initiation factor DED1 and perhaps other RNA helicases may be involved in regulating the translation of particular mRNAs.
Translation Regulation and Helicase Function.
Like eIF4A, Ded1p is an essential, general translation initiation factor. It has RNA-dependent ATPase and RNA helicase activities required for its translational function in vivo (4). To the limits of present detection, loss of DED1 function inhibits translation initiation of all yeast mRNAs (3). Genetic data suggest that DED1 and eIF4A play independent roles in translation initiation (5). Thus, as in splicing, RNA helicases might play multiple roles in translation initiation, such as unwinding or rearranging RNA structures and disrupting RNA–protein or protein–protein interactions.
The ability of the ded1–18 mutation to selectively inhibit BMV RNA2 translation while supporting general cell translation and growth may reflect at least two possibilities. (i) DED1 might have regulatory functions distinct from its role in general translation initiation. (ii) The mutant ded1–18 allele might have reduced helicase activity that is above the threshold for cell growth but not for translating RNA2 (see below). The possibility of reduced helicase activity is consistent with the location of the ded1–18 mutations in the conserved helicase region of Ded1p.
Our results with DED1 and BMV RNA2 show that a DEAD-box RNA helicase can selectively activate translation of a specific mRNA. This may be a paradigm for selective activation of other mRNAs by DEAD-box RNA helicases, which include a number of developmentally regulated and tissue-specific proteins. One example is the mammalian DEAD-box protein gene PL10. PL10 can substitute for DED1 in yeast (3) but is expressed only in the male germ line during certain stages of spermatogenesis in mice (33).
Role of 5′ NCR Subdomains in Regulating RNA2 Translation.
Exchanges between wt RNA2 and a GAL1-promoted 2a mRNA showed that inhibition of RNA2 translation in ded1i yeast depended on the wt RNA2 5′ NCR. Sequence comparisons and secondary structure analysis of the RNA2 and RNA1 5′ NCRs revealed a modular arrangement of three subdomains; A and C are common to RNAs 1 and 2, and B is unique to RNA2 (Fig. 5A). Subdomain A consists of a 5′ terminal stem–loop structure conserved in RNAs 1 and 2 of all sequenced bromoviruses (ref. 31 and unpublished results) and implicated in RNA replication (ref. 30 and J.C., A.N., and P.A., unpublished results). Subdomains B and C have fewer recognizable structural characteristics but possess pyrimidine-rich segments.
In wt yeast, all three 5′ NCR subdomains affected RNA2 translation (Fig. 5 B and C). Region B attenuates RNA2 translation, because its deletion increased RNA2 translation in wt yeast 3-fold. Region C may help overcome translation-inhibiting effects of the region A stem–loop structure, because deleting C alone inhibited RNA2 translation in wt yeast but this inhibition was suppressed if A was deleted with C. In keeping with this observation, deleting region A alone modestly increased RNA2 translation in wt yeast, even in the presence of region C.
In ded1i yeast, selective inhibition of RNA2 translation was strongest with the wt 5′ NCR, for which 2a expression was only 7% of that in wt yeast. The most striking effect on RNA2 translation in ded1i yeast resulted from deleting subdomain B (Fig. 5 B and C). This deletion not only produced the highest 2a levels in wt yeast but also gave equal translation in ded1i yeast, corresponding to a greater than 40-fold stimulation of RNA2 translation in the mutant strain. By comparison, deleting subdomain A or C only increased RNA2 expression 2- to 2.5-fold in ded1i yeast. Nevertheless, the contribution of region B to the DED1 dependence of RNA2 translation depended on A and C, because ΔAC showed that subdomain B alone did not confer significant DED1 dependence on RNA2. Moreover, ΔAB, leaving region C as the sole 5′ NCR, resulted in a 10-fold inhibition of RNA2 translation in ded1i yeast relative to that of wt yeast. Thus, both domains B and C contribute to the DED1 dependence of RNA2 translation, possibly through their shared pyrimidine-rich domains (see below).
These RNA2 5′ NCR deletion results correspond well with results from hybrid 2a mRNAs (Figs. 3 and 4). The ADH1-promoted 2a mRNA, in which the RNA2 5′ NCR subdomain A (nucleotides 1–46) was replaced with ADH1 5′ NCR sequences but subdomains B and C were retained, showed severe translation inhibition in ded1i yeast (Fig. 3B). The GAL1-promoted 2a mRNA, in which the entire RNA2 5′ NCR was replaced with that of GAL1, expressed 2a efficiently in ded1i yeast (Figs. 3B and 4). Similarly, wt RNA1, whose 5′ NCR shares substantial identity with subdomains A and C but lacks subdomain B (Fig. 5A), was expressed at wt efficiency in ded1i yeast (Fig. 3D).
Secondary structure in 5′ NCRs can inhibit translation, presumably by blocking scanning by the 43S preinitiation complex (34, 35). Although the RNA2 5′ NCR was required for selective translation dependence on the DED1 RNA helicase, it is unclear to what extent RNA secondary structure contributes to these effects. Computer analysis of RNA2 secondary structure predicts that the 5′ NCR does not base pair with interior portions of RNA2. The most stable predicted structure within the 5′ NCR is the conserved stem–loop structure of subdomain A with a free energy of −13.6 kcal/mol, which was dispensable for inhibition of RNA2 or lacZ translation in ded1i yeast (Figs. 5 and 6A). Subdomain B, whose deletion eliminated ded1-dependent inhibition, has the potential to form some alternate, mutually exclusive, stem–loop structures, but the strongest has a free energy of only −7.6 kcal/mol.
As an alternative to secondary structure, the RNA2 5′ NCR may cause high-level DED1 dependence by binding one or more proteins. This possibility is consistent with the pyrimidine-rich sequences in subdomains B and C and the role of polypyrimidine binding proteins in viral and cellular translation (36–38). Such a mechanism might also contribute to subdomain B-dependent translation attenuation in wt yeast.
The RNA2 5′ NCR inhibited lacZ translation in ded1i yeast and, as with RNA2, this inhibition was independent of subdomain A but dependent on subdomain B (Fig. 6A). Because lacZ inhibition in ded1i yeast was not as pronounced as inhibition of 2a expression, the 5′ NCR might not be the only RNA2 determinant of DED1-dependent translation. However, further work will be needed to determine whether the 2a ORF negatively affects its own translation, perhaps in conjunction with the 5′ NCR, or whether the lacZ ORF positively affects translation. Differences in 2a and lacZ protein stability might also contribute to these effects (39).
Regulated Viral Polymerase Expression.
BMV is a member of the alphavirus-like superfamily, which shares a conserved core of RNA replication genes. Many viruses in this superfamily with single-component genomes use translational read-through or frameshift mechanisms to attenuate polymerase production with respect to other replication proteins. Sindbis virus and tobacco mosaic virus, e.g., regulate the ratio of 2a- to 1a-like proteins by read-through of leaky termination codons. In both cases, engineered mutations that equalize polymerase expression to that of the other replication proteins quickly revert to regain polymerase attenuation (40, 41). Our results showing that RNA2 translation has specialized dependence on DED1 and is attenuated by the RNA2-specific 5′ NCR subdomain B even in wt yeast suggest that BMV polymerase may also be subject to translational down-regulation. Such mechanisms may be conserved in other tripartite RNA viruses because sequenced bromoviruses and cucumoviruses conserve all recognizable features of RNA1 and RNA2 5′ NCRs, including the subdomain A 5′ stem–loop structure and an RNA2-specific, pyrimidine-rich sequence of approximately 30 nt after this stem–loop (ref. 42 and unpublished results).
Acknowledgments
We thank Daniel Gehling for excellent technical assistance and J. Y. Sgro for assistance in secondary structure analysis. This work was supported by the National Institutes of Health through Grant GM35072. P.A. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- BMV
brome mosaic virus
- CAT
chloramphenicol acetyltransferase
- GUS
β-glucuronidase
- NCR
noncoding region
- wt
wild type
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240460897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240460897
References
- 1.McCarthy J. Microbiol Mol Biol Rev. 1998;62:1492–1553. doi: 10.1128/mmbr.62.4.1492-1553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A E, Koonin E V. Curr Opin Cell Biol. 1993;3:419–429. [Google Scholar]
- 3.Chuang R-Y, Weaver P L, Liu Z, Chang T-H. Science. 1997;275:1468–1471. doi: 10.1126/science.275.5305.1468. [DOI] [PubMed] [Google Scholar]
- 4.Iost I, Dreyfus M, Linder P. J Biol Chem. 1999;274:17677–17683. doi: 10.1074/jbc.274.25.17677. [DOI] [PubMed] [Google Scholar]
- 5.de la Cruz J, Iost I, Kressler D, Linder P. Proc Natl Acad Sci USA. 1997;94:5201–5206. doi: 10.1073/pnas.94.10.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong F, Sivakumaran K, Kao C. Virology. 1999;259:200–210. doi: 10.1006/viro.1999.9763. [DOI] [PubMed] [Google Scholar]
- 7.Ahola T, Ahlquist P. J Virol. 1999;73:10061–10069. doi: 10.1128/jvi.73.12.10061-10069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss E G, Rice C M, Strauss J H. Proc Natl Acad Sci USA. 1983;80:5271–5275. doi: 10.1073/pnas.80.17.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson W O, Lehto K M. Adv Virus Res. 1990;38:307–342. doi: 10.1016/S0065-3527(08)60865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janda M, Ahlquist P. Cell. 1993;72:961–970. doi: 10.1016/0092-8674(93)90584-d. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo-Hartwig M, Ahlquist P. J Virol. 1999;73:10303–10309. doi: 10.1128/jvi.73.12.10303-10309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Ahlquist P. J Virol. 2000;74:4310–4318. doi: 10.1128/jvi.74.9.4310-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan M, Ahlquist P. J Virol. 1999;73:2622–2632. doi: 10.1128/jvi.73.4.2622-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa M, Diez J, Restrepo-Hartwig M, Ahlquist P. Proc Natl Acad Sci USA. 1997;94:13810–13815. doi: 10.1073/pnas.94.25.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez J, Ishikawa M, Kaido M, Ahlquist P. Proc Natl Acad Sci USA. 2000;97:3913–3918. doi: 10.1073/pnas.080072997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthrie C, Fink G R. Methods in Enzymology. Vol. 194. San Diego, CA: Academic; 1991. [Google Scholar]
- 17.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1987. [Google Scholar]
- 18.Ishikawa M, Janda M, Krol M A, Ahlquist P. J Virol. 1997;71:7781–7790. doi: 10.1128/jvi.71.10.7781-7790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahola T, den Boon J, Ahlquist P. J Virol. 2000;74:8803–8811. doi: 10.1128/jvi.74.19.8803-8811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janda M, French R, Ahlquist P. Virology. 1987;158:259–262. doi: 10.1016/0042-6822(87)90265-0. [DOI] [PubMed] [Google Scholar]
- 21.Halbrook J, Hoekstra M F. Mol Cell Biol. 1994;14:8037–8050. doi: 10.1128/mcb.14.12.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gietz R D, Sugino A. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 24.Russell P J, Hambidge S J, Kirkegaard K. Nucleic Acids Res. 1991;19:4949–4953. doi: 10.1093/nar/19.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuker M. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- 26.Umen J G, Guthrie C. Genetics. 1996;143:723–739. doi: 10.1093/genetics/143.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krol M A, Olson N H, Tate J, Johnson J E, Baker T S, Ahlquist P. Proc Natl Acad Sci USA. 1999;96:13650–13655. doi: 10.1073/pnas.96.24.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janda M, Ahlquist P. Proc Natl Acad Sci USA. 1998;95:2227–2232. doi: 10.1073/pnas.95.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinant S, Janda M, Kroner P A, Ahlquist P. J Virol. 1993;67:7181–7189. doi: 10.1128/jvi.67.12.7181-7189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pogue G P, Marsh L E, Connell J P, Hall T C. Virology. 1992;188:742–753. doi: 10.1016/0042-6822(92)90529-x. [DOI] [PubMed] [Google Scholar]
- 31.Pogue G P, Hall T C. J Virol. 1992;66:674–684. doi: 10.1128/jvi.66.2.674-684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altmann M, Schmitz N, Berset C, Trachsel H. EMBO J. 1997;16:1114–1121. doi: 10.1093/emboj/16.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy P, Alzari P, Sassoon D, Wolgemuth D, Fellous M. Cell. 1989;57:549–559. doi: 10.1016/0092-8674(89)90125-6. [DOI] [PubMed] [Google Scholar]
- 34.Kozak M. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozak M. Proc Natl Acad Sci USA. 1986;83:2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Echevarria M J, Peltz S W. Cell. 2000;101:741–751. doi: 10.1016/s0092-8674(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 37.Gosert R, Chang K H, Rijnbrand R, Yi M, Sangar D V, Lemon S M. Mol Cell Biol. 2000;20:1583–1595. doi: 10.1128/mcb.20.5.1583-1595.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sickinger S, Schweizer M. Biol Chem. 1999;380:1217–1223. doi: 10.1515/BC.1999.154. [DOI] [PubMed] [Google Scholar]
- 39.Bachmair A, Finley D, Varshavsky A. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Rice C M. J Virol. 1989;63:1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa M, Meshi T, Motoyoshi F, Takamatsu N, Okada Y. Nucleic Acids Res. 1986;14:8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan M, Ahlquist P. Semin Virol. 1997;8:221–230. [Google Scholar]