Abstract
Although functional MRI (fMRI) based on blood oxygenation-level dependent (BOLD) signal changes is a sensitive tool for mapping brain activation, quantitative studies of the physiological effects of pharmacological agents using fMRI alone are difficult to interpret due to the complexities inherent in the BOLD response. Hypercapnia calibrated-BOLD methodology is potentially a more powerful physiological probe of brain function, providing measures of the changes in cerebral blood flow (CBF) and the cerebral metabolic rate of oxygen (CMRO2). In this study, we implemented a quantitative R2* approach for assessing the BOLD response to improve the stability of repeated measurements, in combination with the calibrated-BOLD method, to examine the CBF and CMRO2 responses to caffeine ingestion. Ten regular caffeine consumers were imaged before and after a 200mg caffeine dose. A dual echo arterial spin labeling technique was used to measure CBF and BOLD responses to visual stimulation, caffeine consumption and mild hypercapnia. For a region of interest defined by CBF activation to the visual stimulus, the results were: hypercapnia increased CBF (+46.6%, ±11.3, mean and standard error), visual stimulation increased both CBF (+47.9%, ±2.9) and CMRO2 (+20.7%, ±1.4), and caffeine decreased CBF (-34.5%, ±2.6) with a non-significant change in CMRO2 (+5.2%, ±6.4). The coupling between CBF and CMRO2 was significantly different in response to visual stimulation compared to caffeine consumption. A calibrated-BOLD methodology using R2* is a promising approach for evaluating CBF and CMRO2 changes in response to pharmacological interventions.
Keywords: functional magnetic resonance imaging (fMRI), blood oxygenation level dependent (BOLD), cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), calibrated-BOLD, caffeine
Introduction
The blood oxygen level-dependent (BOLD) signal is typically used in functional MRI (fMRI) experiments to detect areas of brain activity. However, the BOLD signal is a complex function of changes in cerebral blood flow (CBF), cerebral blood volume (CBV) and cerebral metabolic rate of oxygen consumption (CMRO2). The calibrated-BOLD technique (Davis et al., 1998) aims to resolve some of the ambiguities of the BOLD signal. In this approach, the CBF and BOLD responses to mild hypercapnia as well as task-induced activation are measured. The hypercapnic period is assumed to elicit no changes in CMRO2, and is used to calibrate the BOLD signal by quantifying the contribution of CBF changes to the BOLD response in the absence of CMRO2 changes. A mathematical model is then used to combine the CBF and BOLD activation responses with the hypercapnia-calibrated relationship between BOLD and CBF to estimate the CMRO2 change due to activation (Davis et al., 1998). The coupling between changes in blood flow and oxygen metabolism can be described by the ratio, n, of the fractional CBF change to the fractional CMRO2 change. Previous calibrated-BOLD studies have typically reported a strong coupling between CBF and CMRO2 changes during brain activation, with n values in the range 2-3 observed in the visual cortex in response to visual stimulation (Chiarelli et al., 2007a; Davis et al., 1998; Hoge et al., 1999; Kim et al., 1999; Leontiev and Buxton, 2007a). Regional differences in n across the brain have also been reported (Chiarelli et al., 2007a).
Calibrated-BOLD fMRI can provide quantitative, physiologically meaningful measures of brain function, and can be used to study the effects of drug and behavioral interventions on the brain (St Lawrence et al., 2003). One substance that has been the subject of previous fMRI research is caffeine, which is one of the most widely consumed neural stimulants in the world. Caffeine is lipid-soluble and freely crosses the blood-brain barrier. Its main action is to bind to adenosine receptors and block the actions of agonists at these receptors (Fredholm et al., 1999), causing vasoconstriction. The resulting decrease in baseline CBF has been demonstrated using various measurement techniques in humans (Behzadi and Liu, 2006; Bendlin et al., 2006; Cameron et al., 1990; Field et al., 2003; Liu et al., 2004; Mathew and Wilson, 1985). In addition, fMRI has previously been used to study the effects of caffeine on the amplitude (Bendlin et al., 2006; Laurienti et al., 2002, 2003; Mulderink et al., 2002) and temporal dynamics (Behzadi and Liu, 2006; Liu et al., 2004) of the BOLD response, typically detecting a speeding up of the BOLD response that is compatible with a model for the vasculature in which vasoconstrictive agents cause the arterioles to become more responsive to stimulation (Behzadi and Liu, 2005).
Although caffeine is generally believed to enhance mental alertness and energy, its effect on baseline cerebral oxygen and glucose metabolism is not clear. Animal studies are inconclusive, reporting both regional increases (Nehlig and Boyet, 2000) and no changes (Gotoh et al., 2001) in cerebral glucose metabolism (CMRGlc) in response to caffeine. Several studies in humans have also focused on the metabolic response to caffeine, again with mixed findings. Dager and colleagues (Dager et al., 1999) measured brain lactate changes as a measure of the metabolic response to caffeine using spectroscopic imaging and found no significant difference between the brain lactate/NAA ratios among regular caffeine users, suggesting that CMRGlc and CMRO2 remained matched in these subjects. However, lactate/NAA ratio did increase within a caffeine intolerant group and within a group of regular users who had abstained for 1-2 months, suggesting a larger increase in CMRGlc than CMRO2 in these subjects. The animal and human studies are not conclusive, though an uncoupling of CBF and energy metabolism in the brain’s response to caffeine is suggested.
The prevalence of caffeine consumption makes it important to characterize its physiological effects for future neuroimaging studies, as well as a basic understanding of the effects of caffeine on energy metabolism. Investigation of the impact of caffeine on CBF, the BOLD signal and CMRO2 is particularly important for the correct interpretation of fMRI and calibrated-BOLD studies, and will enable appropriate control for caffeine effects in future studies. In addition, caffeine presents a useful model system for further development of the calibrated-BOLD methodology as a tool for evaluating the effects of pharmacological agents.
In this study we investigated the effect of caffeine on baseline CMRO2 using calibrated-BOLD fMRI. The CBF and BOLD responses from a defined baseline state to three conditions were measured: mild hypercapnia, visual stimulation and post-caffeine consumption. The BOLD signal changes were derived from measured changes in the apparent transverse relaxation rate, R2*, which allowed estimation of the effect of caffeine on baseline CMRO2. The coupling of blood flow and oxygen metabolism in response to visual stimulation and caffeine ingestion were compared. This approach is an extension to the methods used in a previous calibrated-BOLD drug study, where the CMRO2 response to activation was compared before and after indomethacin administration (St Lawrence et al., 2003). In a subsequent comment on that study, Uludag and Buxton (Uludag and Buxton, 2004) pointed out that the measured data also could be used to estimate the change in CMRO2 due to the drug itself. In the present study we further develop this approach using R2* measurements, rather than raw BOLD signal measurements, to help control for signal drifts. The stability of the baseline CBF and R2* measurements between two scan sessions was investigated in a separate set of reliability experiments, in which caffeine was not administered.
Methods
Participants
Data were acquired on 10 healthy adults (5 males; mean age 33 years, standard deviation 7 years; mean weight 154 pounds, standard deviation 32 pounds). The study was approved by the institutional review board at the University of California San Diego, and written informed consent was obtained from all participants. All subjects reported a moderate daily caffeine intake of between 100 and 250 mg, and abstained from caffeine consumption for at least 12 hours prior to participating in the study. Eight of the 10 subjects (3 male; mean age 35 years, standard deviation 6 years) also took part in an additional study investigating R2* and CBF baseline stability. All studies were performed at approximately the same time of day (between 8am and noon).
Imaging Protocol
A schematic diagram of the full experimental design is shown in Figure 1. All data were acquired on a GE Signa Excite 3T whole body system with a body transmit coil and an 8-channel receive-only head coil. Each imaging protocol consisted of two sessions, between which the subject ingested an over-the-counter tablet containing 200 mg caffeine and remained outside the scanner for approximately 30 mins to allow the caffeine to take effect before returning to the scanner for the post-dose session (Fredholm et al., 1999). Care was taken when positioning each subject: the laser landmark and stationary landmarks on the head coil were used to ensure that the positioning of the subject for the post-dose scan was as closely matched as possible to their pre-dose scan. The impact of errors in this alignment is discussed in later sections and further addressed in the stability study.
Figure 1.
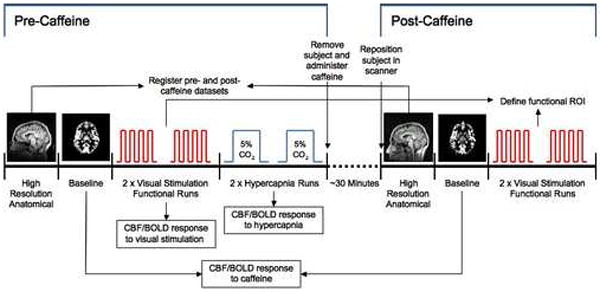
Schematic of the experimental design for the caffeine study, showing the order of data acquisition. The baseline measurement stability protocol was identical, except that no caffeine was administered during the break between the two scan sessions, and no hypercapnia data were acquired.
During both the pre and post-dose sessions, a dual echo arterial spin labeling (ASL) PICORE QUIPSS II sequence (Wong et al., 1998) with spiral readout was used to acquire simultaneous BOLD and CBF data during one baseline, two functional and two hypercapnia (pre-dose only) scans. Full ASL sequence parameters were: six 5mm slices aligned with the calcarine sulcus, TR 2.5 s, TI1/TI2 600/1500 ms, TE1 2.9 ms, TE2 24 ms, 90 degree flip angle, FOV 240 mm, matrix 64×64. During the baseline scan, 100 tag/control image pairs were acquired (total scan time 8min 20s) while the subject was instructed to fixate on a small stationary white square projected onto a gray screen which could be comfortably viewed through a mirror. Each functional run was 6 min 50s, starting with a 60s rest period followed by 4 cycles of 20s task/60s rest, and a final 30s rest period. The task consisted of a black and white checkerboard flickering at 8Hz while numbers appeared in the center of the checkerboard. The numbers changed at a rate of 2Hz, and subjects were instructed to fixate on the center of the checkerboard and tap their fingers in the order cued by the numbers. The rest condition consisted of a small stationary white square on a gray background. The checkerboard was intended to activate the visual cortex, and the finger tapping was included to ensure that the subject remained alert and attentive to the stimulus throughout.
At the end of the pre-dose session only, two 7 minute hypercapnia scans were acquired; the subjects wore a non-rebreathing mask that could be connected to a gas mixture enriched with 5% CO2. Subjects breathed room air for 2 min, followed by 5% CO2 for 3 min, followed by room air for 2 min. In addition, a cerebral spinal fluid (CSF) reference scan and a minimum contrast scan were acquired for use in quantifying CBF. The CSF scan consisted of a single-echo, single repetition scan acquired at full relaxation and TE 2.9 ms. The same in-plane parameters as the ASL scan were used, but the number of slices was increased to cover the lateral ventricles. The minimum contrast scan was acquired with TR=2 s, TE=11 ms to ensure little contrast between gray matter, white matter and CSF. Two 8-interleave repetitions were acquired using the same slice prescription as the CSF scan.
A high resolution structural image was also acquired at the start of each session, using a magnetization prepared 3D fast spoiled gradient acquisition in the steady state (FSPGR) sequence (172 sagittal slices, 1 mm slice thickness, TI 450 ms, TR 7.9 ms, TE 3.1 ms, 12 degree flip angle, FOV 25 cm, matrix 256×256).
Throughout scanning, cardiac pulse and respiratory effort data were monitored using a pulse oximeter (InVivo) and a respiratory effort transducer (BIOPAC), respectively. Scanner TTL pulse data were also recorded to synchronize the physiological data to the acquired images.
Baseline Measurement Stability Protocol
The stability experiments were designed to assess the stability of the baseline R2* and CBF measurements between the pre and post-dose sessions, i.e. to quantify the variability in R2* and CBF caused by removing the subject from the scanner between sessions. The protocol was identical to that described above, but no caffeine was administered in the 30 minute break between the two imaging sessions. In addition, no hypercapnia scans were acquired.
Preprocessing and General Linear Model Analysis for ROI selection
The first four images of each ASL scan were excluded from data analysis to allow the MRI signal to reach steady state. All functional runs were motion corrected and registered to the first functional run using AFNI software (Cox, 1996). For each dataset, the pre and post-dose high-resolution structural data were used to determine the rotation matrices required to align the post-dose ASL data to the pre-dose ASL data.
Statistical analysis of the functional data was performed using a general linear model (GLM) approach for the analysis of ASL data (Mumford et al., 2006; Restom et al., 2006). The first echo data were used for the analysis of CBF activity, and the second echo data for the analysis of BOLD activity. The stimulus-related regressor in the GLM was obtained by convolving the block design stimulus pattern with a gamma density function (Boynton et al., 1996). The measured cardiac and respiratory data were included in the GLM as regressors to account for the modulation of the ASL signal caused by physiological fluctuations (Glover et al., 2000; Restom et al., 2006). A constant and a linear term were also included as nuisance regressors. Pre-whitening was performed using an autoregressive AR(1) model (Burock and Dale, 2000; Woolrich et al., 2001). The data from the two functional runs were concatenated for the GLM analysis as described in (Restom et al., 2006).
An anatomical mask that included the visual cortex was drawn for each subject, and further analysis was restricted to this area to avoid the possible inclusion of motor areas activated by the finger-tapping task. Voxels exhibiting CBF or BOLD activation were detected after correcting for multiple comparisons using the AFNI AlphaSim program (Cox, 1996; Forman et al., 1995), using an overall significance threshold of p=0.05. For each subject, an active visual cortex region of interest (ROI) was defined as those voxels exhibiting CBF activation on both the pre and post-dose functional runs. To test for potential bias due to ROI selection, a second ROI was defined as those voxels active on both CBF and BOLD time courses, again both pre and post caffeine. The two ROIs are subsequently referred to as the CBF and CBF/BOLD ROIs.
CBF and BOLD Responses to Caffeine and Visual Stimulation
The CBF and BOLD responses to caffeine ingestion were calculated using the pre and post-dose baseline scans. CBF time series were computed by taking the surround subtraction of the tag/control image series from the first echo data (Liu and Wong, 2005). These time series were then corrected for inhomogeneities in the coil sensitivity profiles using the smoothed minimum contrast images (Wang et al., 2005), and converted to physiological units (ml/100ml/min) using the CSF image as a reference signal (Chalela et al., 2000). Mean baseline pre and post-dose CBF values were calculated by averaging over all data points and voxels within each of the two ROIs.
The BOLD response to caffeine was estimated by using the dual-echo baseline scans to calculate the change in the apparent transverse relaxation rate, R2*, in each of the two ROIs. The voxelwise surround average of both the first and second echo data (Liu and Wong, 2005) was calculated and averaged over each of the ROIs. These mean signals (Secho1(t) and Secho2(t)) were then combined to give a mean R2* time course using the relation R2*(t) = ln(Secho1(t)/Secho2(t))/ΔTE, where ΔTE is the difference between the first and second echo times. This was then converted to a BOLD signal using the relation:
| [1] |
where ΔR2*(t) is the change in R2* between the pre and post-dose baseline scans.
The CBF and BOLD responses to visual stimulation were calculated using the pre-dose functional scans only, since responses to all stimuli (caffeine, visual stimulation and hypercapnia) were measured relative to the pre-caffeine baseline state. For each voxel, the physiological noise components estimated with the GLM were first removed from the data. The average CBF response was calculated by averaging the first echo data (after performing a surround subtraction) over each ROI, and normalizing to the baseline value (determined by averaging over the initial 1 minute rest period of the functional scans). The BOLD response to visual stimulation was calculated in the same manner as the BOLD response to caffeine, i.e. by measuring the change in R2* The mean R2* time course over each ROI was normalized to its baseline value and converted to a BOLD time course using Eq [1].
We defined the mean amplitude of each functional response as the mean response over the second 10 seconds of the task period and the subsequent 5 seconds (7 time points in total).
CMRO2 Responses to Caffeine and Visual Stimulation
The effects of caffeine and visual stimulation on CMRO2 were investigated using a mathematical model that relates the BOLD signal to changes in CBF and CMRO2 (Davis et al., 1998):
| [2] |
Where M is a proportionality constant that reflects baseline deoxy-hemoglobin content and is proportional to CBV, the baseline oxygen extraction fraction, the magnetic field strength and TE. The parameters α and β are empirically determined and are assumed to be global properties; α is the exponent in an assumed power law relationship between CBF and CBV, and a value of 0.38 is typically used (Grubb et al., 1974; Mandeville et al., 1998). β is typically taken to be 1.5 (Davis et al., 1998). The prime superscript represents the activated state.
First the BOLD scaling factor, M, was calculated using the hypercapnia data. For each subject, the two hypercapnia runs were averaged after removing the physiological noise components from the data. The mean CBF and BOLD time series (again using R2* to calculate the BOLD signal) were calculated for each ROI. The percent CBF and BOLD responses were estimated by normalization to the initial 2 minute rest period, and the response amplitudes were calculated as the mean response over the last 2 minutes of CO2 inhalation. M was then calculated by applying Eq [2] under the assumption that hypercapnia induces no CMRO2 change.
Equation [2] was then used to estimate the CMRO2 changes associated with caffeine consumption and visual stimulation, using the CBF and BOLD responses determined from the baseline and functional runs, respectively. The calculations were performed for both the CBF and CBF/BOLD ROIs, using the individually determined M values for each subject. Since it is not known whether M is the same over subjects, with differences between measurements being due to measurement error, or whether M values can vary between individuals, the subject-wise CMRO2 calculations were repeated using a group mean M value.
All measured CBF, BOLD and CMRO2 changes were tested for significance as well as compared between the two ROIs using two-tailed paired t-tests. Significance was accepted at the p<0.05 level.
Baseline Measurement Stability Analysis
Analysis of the stability data was identical to that described above. However, no hypercapnia data were collected and so no calculations of M or CMRO2 changes were performed.
Results
Caffeine
Tables 1 and 2 report the results of the hypercapnia and visual stimulation experiments, respectively, performed within the pre-caffeine scan session. In the hypercapnia experiment, end-tidal pCO2 increased by 8.4 ± 1.8 Torr. Hypercapnia led to robust CBF and BOLD increases in all subjects, and the calculated M values showed no significant differences between the two ROIs. These M values (either applied individually, or using a group mean M for each subject) were used to calculate the CMRO2 changes in response to visual stimulation given in Table 2. The CMRO2 increases were very consistent over subjects, with a mean increase of approximately 20%. Using the mean M rather than individually determined M values did not significantly affect the results.
Table 1. Response to hypercapnia.
Mean responses (one standard error), measured in the pre-dose caffeine hypercapnia experiment (10 subjects), and calculated M values.
| CBF ROI | CBF/BOLD ROI | |
|---|---|---|
| ΔCBF (%) | +46.6 (11.3) | +46.6 (12.2) |
| ΔR2* (s-1) | -1.1 (0.2) | -1.2 (0.2) |
| ΔBOLD (%) | +2.6 (0.4) | +2.9 (0.5) |
| M (%) | 9.3 (1.2) | 9.7 (0.9) |
Table 2. Response to visual stimulation.
Mean responses (one standard error), measured in the pre-dose caffeine visual stimulation experiment (10 subjects). CMRO2 values are calculated using individual or mean M values from the hypercapnia experiment, and used to calculate 1/n.
| CBF ROI | CBF/BOLD ROI | |
|---|---|---|
| ΔCBF (%) | +47.9 (2.9) | +50.3 (3.4) |
| ΔR2* (s-1) | -0.49 (0.05) | -0.68 (0.06) |
| ΔBOLD (%) | +1.2 (0.1) | +1.6 (0.1) |
| ΔCMRO2 (%) | +20.7 (1.4) | +18.5 (1.7) |
| ΔCMRO2, mean M (%) | +22.2 (1.0) | +18.8 (1.4) |
| 1/n | 0.44 (0.03) | 0.37 (0.03) |
| 1/n, mean M | 0.47 (0.02) | 0.39 (0.02) |
Figure 2 compares the subject-wise CBF and R2* values measured in the pre- and post-caffeine baseline scans, and Table 3 gives the mean responses to caffeine over subjects. As predicted, all subjects showed a decrease in baseline CBF after caffeine administration, with an average decrease of 35%. However, this CBF decrease was accompanied by a substantial decrease in the BOLD signal (by 6.5% in the CBF ROI and 5.5% in the CBF/BOLD ROI), due to a significant increase in R2*. The decrease in both the CBF and BOLD signals post-caffeine resulted in no significant caffeine-induced CMRO2 change for either ROI.
Figure 2.
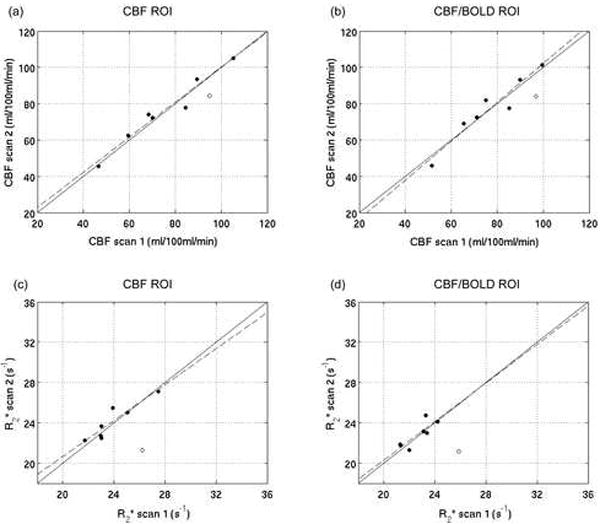
Comparison of the subject-wise baseline CBF and R2* values measured pre and post-caffeine for the CBF ROI (a, c) and the CBF/BOLD ROI (b, d). The identity lines are shown in solid black.
Table 3. Response to caffeine.
Mean values (one standard error), measured in the pre- and post-dose caffeine experiments (10 subjects). The four ΔCMRO2 and four 1/n estimates are not significantly different from zero (p>0.4 in all cases, denoted * in table).
| CBF ROI | CBF/BOLD ROI | |
|---|---|---|
| Pre-caffeine | ||
| CBF (ml/100ml/min) | 79.9 (5.0) | 81.4 (4.6) |
| R2* (s-1) | 25.1 (1.1) | 24.2 (0.9) |
| Post-caffeine | ||
| CBF (ml/100ml/min) | 52.0 (3.3) | 52.8 (3.2) |
| R2* (s-1) | 27.9 (1.1) | 26.6 (0.8) |
| Post-caffeine – Pre-caffeine | ||
| ΔCBF (%) | -34.5 (2.6) | -34.9 (2.6) |
| ΔR2* (s-1) | +2.8 (0.6) | +2.4 (0.5) |
| ΔBOLD (%) | -6.5 (1.2) | -5.5 (1.1) |
| ΔCMRO2 (%) | +5.2 (6.4)* | -2.7 (4.1)* |
| ΔCMRO2, mean M (%) | +2.7 (5.7)* | -3.1 (4.3)* |
| 1/n | -0.20 (0.21)* | 0.08 (0.13)* |
| 1/n, mean M | -0.10 (0.19)* | 0.09 (0.14)* |
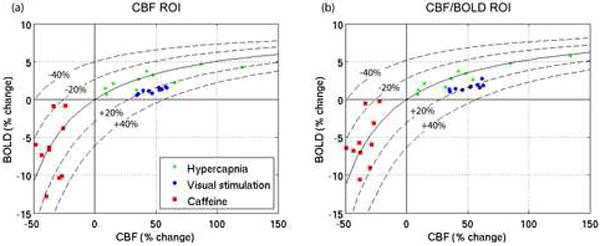
Figure 3 shows the measured CBF/BOLD data for all 10 subjects in response to hypercapnia, visual stimulation and caffeine consumption, for each of the two ROIs. Iso-CMRO2 contours, calculated using the mean M values are shown. The visual stimulation data are clustered tightly around the +20% CMRO2 contour, whereas the caffeine data are considerably more variable. Although the data are centered around the zero CMRO2 change contour, the change in CMRO2 for individual subjects was as large as ±20% in some cases. No correlations were found between individual subject’s CMRO2 responses to caffeine and subject gender, weight, baseline CBF or mean daily caffeine intake. In addition, no correlation was found between subject weight and measured CBF change due to caffeine (as a percentage or in absolute units).
Figure 3.
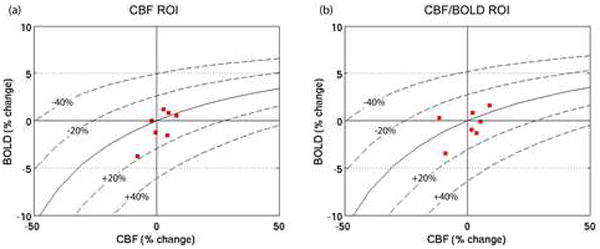
Subject-wise BOLD vs. CBF responses to hypercapnia (green), visual stimulation (blue) and caffeine (red) within (a) the CBF ROI, and (b) the CBF/BOLD ROI. The solid black line shows the contour for zero CMRO2 change, and the dashed lines show the contours for ±20%, ±40% CMRO2 changes. The contours are calculated using the mean M value for the group (see Table 1; corresponding CMRO2 changes with mean M are shown in Figures 4(c) and 4(d)).
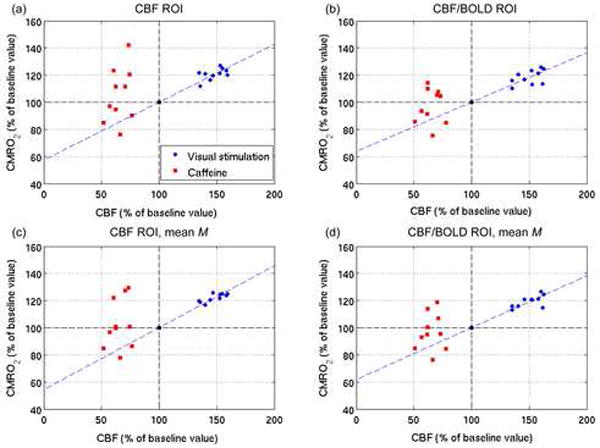
Figure 4 shows the visual stimulation and caffeine data in the CBF/CMRO2 plane. Also shown is the line of best-fit through the visual stimulation data, which is constrained to pass through the baseline state (both CBF and CMRO2 at 100% of their baseline values). The coupling, n, of CBF to CMRO2 changes in response to visual stimulation is given by the slope of this line; in the CBF ROI, n=2.3 using individual M values, and n=2.2 using a group mean M. In the CBF/BOLD ROI, n=2.7 using individual M values, and n=2.6 using a group mean M. In order to compare the coupling of blood flow and metabolism in response to visual stimulation with that in response to caffeine consumption, a measure of the coupling, 1/n, in response to each stimulus was calculated for each subject and compared using paired t-tests (see Tables 2 and 3). The use of 1/n avoided n values approaching infinity for cases in which ΔCMRO2~0. There was a significant difference (p<0.05) between the two sets of 1/n values for the CBF ROI (when using either individual or mean M values), as well as for the CBF/BOLD ROI when using individually determined M values. For the CBF/BOLD ROI with mean M values, the difference was approaching significance (p=0.052).
Figure 4.
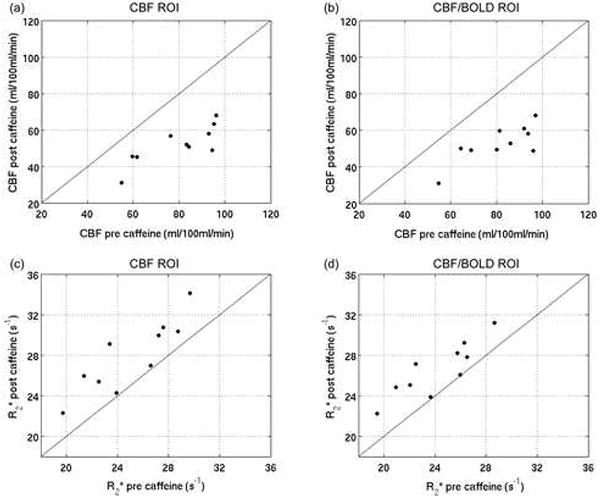
CBF vs CMRO2 responses to visual stimulation and caffeine within CBF (a, c) and CBF/BOLD (b, d) ROIs. The CMRO2 values are calculated using both individual (a, b) and mean (c, d) M values The dashed green lines show the best fit of the visual stimulation data; the slope gives the CBF/CMRO2 coupling under visual stimulation.
Baseline Measurement Stability
Figure 5 compares the subject-wise CBF and R2* values between the baseline runs in the two scan sessions (similar to Fig 2, but without caffeine administration). There is a good correspondence between sessions, indicating that absolute CBF and R2* measurements are robust even when a subject is removed from and then repositioned in the scanner between subsequent measurements. Table 4 reports the mean baseline CBF and R2* values over subjects, as well as the percentage difference between sessions, for both ROIs. One of the eight subjects (shown as an open circle in Fig 5) is excluded from the mean data presented in the table because a large change in R2*, and therefore the BOLD signal, was measured between the two sessions (BOLD changes of +12.0% in the CBF ROI and +12.6% in the CBF/BOLD ROI). This BOLD change is more than 7 standard deviations away from the mean over the remaining seven subjects. This outlying subject was excluded from all further analysis but will be discussed later. For the remaining seven subjects, there was no significant difference in the R2* values between the two sessions, which led to no significant change in the BOLD signal between sessions, and no significant change in baseline CBF. If the outlying subject is included in the analysis, the mean ΔBOLD for both the CBF ROI (+1.1%, ±1.7, mean and standard error) and the CBF/BOLD ROI (+1.1%, ±1.6) remain not significantly different from zero (p>0.5). Likewise, the mean ΔCBF for the CBF ROI (-0.03%, ±2.3) and the CBF/BOLD ROI (-1.4%, ±3.0) remain non-significant (p>0.5). The main difference between the two ROIs studied was the magnitude of the BOLD response to visual stimulation, which was expected to be larger in the CBF/BOLD ROI. Otherwise the results for the two ROIs were similar (see Table 4).
Figure 5.
Baseline measurement stability data. Comparison of the subject-wise baseline CBF and R2* measurements in the two scan sessions (‘scan 1’ and ‘scan 2’) for the CBF ROI (a, c) and the CBF/BOLD ROI (b, d). The identity lines (solid) and the best fit lines to the data (dashed) are shown. The subject defined as an outlier due to large R2* differences between the two scans is shown as an open circle and is not included in the CBF or R2* best-fit analyses. In all cases the y-intercepts for the best-fit lines are not significantly different from zero, and the slopes are not significantly different from one. The R2 values are (a) 0.96, (b) 0.92, (c) 0.85, (d) 0.70.
Table 4. Baseline measurement stability results.
Mean values (one standard error), measured in the stability experiments (7 subjects). The baseline signal changes between the pre and post scans are not significantly different from zero (p>0.4; denoted * in table).
| CBF ROI | CBF/BOLD ROI | |
|---|---|---|
| Scan 1 | ||
| CBF (ml/100ml/min) | 74.9 (7.5) | 76.9 (6.1) |
| R2* (s-1) | 23.9 (0.7) | 22.7 (0.4) |
| Scan 2 | ||
| CBF (ml/100ml/min) | 75.8 (7.4) | 77.4 (6.8) |
| R2* (s-1) | 24.1 (0.7) | 22.8 (0.5) |
| Scan 2 – Scan 1 | ||
| ΔCBF (%) | +1.5 (2.0)* | +0.3 (2.8)* |
| ΔR2* (%) | +1.1 (1.2)* | +0.9 (1.2)* |
| ΔBOLD (%) | -0.6 (0.7)* | -0.4 (0.6)* |
Figure 6 shows the measured change in the baseline CBF and BOLD signals between scan sessions for all subjects, excluding the outlying subject for display purposes. Included in this plot are approximations of the 0, ±20% and ±40% ΔCMRO2 contours, created using Eq [2] and the mean M values determined from the caffeine experiments. These contours are included to give a sense of how instability in the CBF and BOLD measurements propagates to errors in the estimates of CMRO2 changes. The variability in the BOLD signal most likely reflects the instability in the R2* measurement caused by small differences in subject position between scan sessions. This is discussed in detail later.
Figure 6.
Baseline measurement stability data. Subject-wise BOLD vs. CBF changes between the baseline scans in the two scan sessions within (a) the CBF ROI, and (b) the CBF/BOLD ROI. The solid black line shows the approximate contour for zero CMRO2 change, estimated using the mean M values for the group from the caffeine study (from Table 1). The dashed lines show the approximate contours for ±20%, ±40% CMRO2 changes. The outlying subject is not displayed (see text for further details).
Discussion
Functional MRI based on the BOLD response provides a sensitive tool for mapping brain activation by detecting small changes in the MR signal in response to a neural stimulus. Because the method is noninvasive and can easily be applied in patient populations, it has a potentially important role in the assessment of the effects of pharmacological agents, both for drug evaluation and for monitoring the response to therapy. However, the BOLD response is a measure of the signal change between two states, and for a slow-acting agent this is difficult to measure because the signal changes due to the agent are easily overwhelmed by scanner or physiology related drift of the MR signal. Conventional fMRI overcomes this problem with multiple stimulus presentations to measure repeated responses, but this is not possible when the goal is to assess the effects of a single administration of the agent. For this reason, most applications of fMRI to the evaluation of pharmacological agents have focused on how the presence of the agent alters the BOLD response to a standard stimulus compared to the response prior to administration of the agent. Our goal in this report was to test the feasibility of instead measuring the effect of the agent itself on blood flow and oxygen metabolism, rather than how the agent affects neural responses, using a calibrated-BOLD technique.
The calibrated-BOLD method offers a potentially powerful paradigm for fMRI by providing a quantitative probe of brain physiology (discussed further in (Brown et al., 2007)). Previously, St Lawrence and colleagues (St Lawrence et al., 2003) used the calibrated-BOLD approach to investigate the effects of a drug, indomethacin, on CBF and CMRO2 responses to motor activation. In a subsequent letter, Uludag and Buxton (Uludag and Buxton, 2004) argued that the data collected in that study could also be used to estimate the change in CMRO2 between the baseline states (pre-and post-indomethacin). They concluded that the baseline changes were consistent with a coupled reduction of CBF and CMRO2, in response to indomethacin, with about the same ratio as was observed in response to neural activation. In the current study, we implemented a quantitative R2* approach to make possible BOLD response measurements between sessions, and combined this with a calibrated-BOLD approach to investigate the effect of caffeine on baseline CBF and CMRO2. Previous studies have shown significant reductions in CBF with caffeine, but it is unknown whether this is an uncoupling of CBF and CMRO2, with only CBF decreasing, or a coupled change in both CBF and CMRO2.
Hypercapnia data were acquired for determination of the BOLD scaling factor, M, with results similar to previously reported values (Chiarelli et al., 2007a; Davis et al., 1998; Leontiev and Buxton, 2007a). Functional runs were acquired to enable the determination of an ROI within the visual cortex and to allow comparison of the coupling of CBF and CMRO2 under the different conditions of visual stimulation and caffeine consumption. As has previously been reported, we measured a tight coupling between CBF and CMRO2 in response to visual stimulation, with CMRO2 increases of ~20% that were consistent between subjects. The coupling values (n in the range 2.2-2.7) are in good agreement with previous work investigating CBF and CMRO2 responses in the human visual cortex (Chiarelli et al., 2007a; Davis et al., 1998; Hoge et al., 1999; Kim et al., 1999; Leontiev and Buxton, 2007a), and the higher n in the CBF/BOLD ROI reflects the bias introduced by requiring that each voxel in the ROI has a significant positive BOLD response (Brown et al., 2007; Chiarelli et al., 2007b; Leontiev et al., 2007b).
A robust CBF decrease of 35% in response to caffeine consumption was observed, in good agreement with previous work (Behzadi and Liu, 2006; Cameron et al., 1990; Field et al., 2003; Liu et al., 2004). However, we measured an accompanying decrease in the BOLD signal which, when analyzed in the context of a mathematical model of the BOLD effect (Davis et al., 1998), suggests that the CBF decrease is not accompanied by a change in CMRO2. This led to the observation of a significant difference between the coupling of flow and metabolism changes in response to visual stimulation compared with the coupling in response to caffeine consumption. This uncoupling of CBF and CMRO2 changes is consistent with caffeine acting primarily on adenosine receptors to constrict blood vessels and lower CBF, with a considerably weaker effect on energy metabolism.
It is important to note that in comparison to the CMRO2 response to visual stimulation, substantially greater variability was seen in the CMRO2 response to caffeine across subjects, which was not explained by subject weight, gender, caffeine usage or baseline CBF. This issue was investigated using stability experiments performed in a subset of the caffeine study participants (discussed later). Previous metabolic studies of caffeine effects have focused on CMRGlc rather than CMRO2 changes, making a direct comparison with our results difficult. However, our findings could be consistent with no change in CMRGlc, in line with the observation of no lactate changes in regular caffeine consumers (Dager et al., 1999).
Our results suggest that with caffeine consumption, the metabolic demands of the brain in terms of oxygen consumption remain stable, and the CBF decrease must therefore be compensated by increased oxygen extraction. By mass balance, these physiological quantities are related by:
| [3] |
where CA denotes the arterial concentration of oxygen, and E is the oxygen extraction fraction (Buxton et al., 2004). It is this increase in E that leads to an increase in deoxyhemoglobin concentration, and the observed reduction in the BOLD signal. The increased extraction fraction is also expected to reduce the average partial pressure of oxygen (pO2) within the capillary bed, and the pO2 difference between the capillary bed and the tissue determines the driving gradient for O2 flux from blood to tissue. An increase in E with caffeine implies a reduction in the capillary pO2, and therefore a reduction in the tissue pO2 given a constant CMRO2. In short, our findings could imply that caffeine pushes the tissue toward hypoxia (lower tissue pO2). However, it has been suggested that with regular caffeine use, the body adjusts its baseline CBF, such that the CBF of regular caffeine users after caffeine consumption is similar to non-caffeine users without caffeine (Field et al., 2003). In that case, regular caffeine users who have abstained from caffeine (as in the present study) would have a higher than normal CBF, resulting in a reduced E and an elevated tissue pO2. Indeed, in the present study the measured CBF values are towards the high end of the typically reported range for gray matter (Calamante et al., 1999), despite the fact that our reported values most likely underestimate the true gray matter CBF due to partial voluming with white matter in the large voxels used. It is therefore possible that caffeine reduces both CBF and pO2 to normal levels in these regular users.
The stability study was carried out to investigate the variability in the baseline R2* and CBF measurements caused by the repositioning of the subject between the two scan sessions. The results typically showed good consistency between the two scan sessions (see Fig 5), with no significant change in the mean CBF or BOLD signals (see Table 4). However, the measured differences in R2* translate to BOLD signal changes of up to several percent, which can lead to calculated CMRO2 changes of up to 20% (see Fig 6). The measured changes in the BOLD signal are most likely due to small differences in the subject position in the scanner between the pre and post-dose sessions; small changes in the angle and/or positioning of the head can lead to subtle changes in R2* due to relative positioning of other brain structures and differences in scanner hardware characteristics, e.g. shimming, between the pre and post dose session. The sensitivity of the CMRO2 calculation to small changes in the BOLD signal makes accurate repositioning of the subject between the pre and post-dose scans crucial.
Intra-venous drug administration is an alternative methodological approach that avoids these issues by not requiring the removal of the subject from the scanner between sessions, and was employed in the study of indomethacin using calibrated-BOLD fMRI (St Lawrence et al., 2003), and more recently in an fMRI study of caffeine effects (Chen and Parrish, 2007). Caffeine has also previously been administered orally in liquid form (Bendlin et al., 2006; Dager et al., 1999) whilst the subject remained in position in the scanner. Oral caffeine administration was not possible in the present study since the participants were wearing a mask for CO2 administration. Further, liquid administration presents a potentially hazardous situation in which the subject is somewhat confined in a supine position by a head coil while swallowing. Movement artifacts are also likely due to swallowing. Intravenous or liquid administration whilst the subject remains in situ is only appropriate for drugs that work on short time scales, and even then, the subject is likely to be required to withstand an extended period in the scanner to allow time for all pre and post-dose imaging as well as waiting for the drug to take effect.
In this study we chose to remove the subject from the scanner for caffeine administration and subsequently reposition them for post-dose imaging, in order to minimize subject discomfort. This approach has previously been employed in several caffeine fMRI studies (Behzadi and Liu, 2006; Laurienti et al., 2002, 2003; Liu et al., 2004; Mulderink et al., 2002) and is a more widely applicable approach that can be extended to testing pharmacologic agents that work over longer timescales, as well as to pre and post interventions such as surgery. Stability experiments were performed to investigate the impact of subject repositioning on the baseline CBF and R2* measurements. Data from one of the subjects in this stability study were discarded since BOLD changes of >12% between the pre and post sessions were measured. Visual comparison of the pre and post localizer scans for each subject revealed that this subject had the largest discrepancy in head position between the two sessions. This may have led to changes in the shimming characteristics and therefore to the large change in R2* observed, and further highlights the importance of consistent subject positioning. An alternative to R2* measurement is the use of a spin-echo BOLD sequence to measure R2, which may be less sensitive to subject head position due to its reduced sensitivity to magnetic field perturbations due to susceptibility effects. Aside from this one subject, the stability experiments showed good consistency between the baseline CBF and R2* measurements in the two scan sessions. However, small errors can lead to the measurement of significant CMRO2 changes when the Davis model is applied (see Fig 6), such that measurement error could explain much of the between subject variability in the CMRO2 responses to caffeine (Fig 3).
Previous fMRI studies have typically measured and compared pre and post-caffeine task-related BOLD responses. However, measuring changes in baseline signals between two scan sessions requires the measurement of absolute tissue or physiological properties, e.g. CBF, rather than relative signals that can be affected by variation in the exact acquisition parameters between scans, e.g. transmit and receiver gains and shimming parameters. For this reason, measurement of the BOLD time series as the magnitude of a T2* weighted image series is not appropriate if the BOLD signal is to be compared between scans. In this study we therefore calculated the BOLD signal using R2* measurements obtained from dual echo data. R2* is a direct measurement that should, in theory, be independent of scanner settings and the exact value of the measured signals. In addition, the temporal drift often observed in the BOLD signal is reduced when using R2*-based measurements, since they are based on the ratio of the dual echo data. It should be remembered, however, that subject positioning and shim values will impact the R2* measured, as discussed above. In line with previous studies, we also converted our perfusion time series to absolute CBF values using CSF signal as an intensity reference to enable comparison between scan sessions.
There are several drawbacks to the present study: only moderate, regular caffeine consumers were included; a uniform 200mg of caffeine was administered; and only the visual cortex was studied. Our study was confined to moderate caffeine users in an attempt to make our subject pool homogeneous; caffeine tolerance has been shown to have an effect on the physiological response of the brain (Dager et al., 1999), and whether similar findings would result from studying caffeine abstainers or heavy caffeine users is not known. The use of a uniform 200mg caffeine dose is in line with many other studies (Behzadi and Liu, 2006; Bendlin et al., 2006; Laurienti et al., 2002; Liu et al., 2004; Mulderink et al., 2002). Alternatively, a per kg dose can be administered. In the current study, no correlation between the change in CBF (percent or absolute change) with subject weight was observed. The visual cortex was studied since it has been the target of several previous calibrated-BOLD studies (Chiarelli et al., 2007a; Davis et al., 1998; Hoge et al., 1999; Kim et al., 1999; Leontiev and Buxton, 2007a) and our CBF/CMRO2 coupling results could therefore be validated by comparison with the literature. However, previous studies have shown regional effects, e.g. Nehlig and colleagues (Nehlig and Boyet, 2000) found a more widespread glucose metabolic response in rats for high doses of caffeine compared to low doses. Therefore other brain regions as well as higher caffeine doses may be expected to provide different results.
In conclusion, this study found that the coupling between CBF and CMRO2 in response to visual stimulation is significantly different to that in response to caffeine consumption. Our data showed a significant reduction in CBF in human visual cortex due to caffeine consumption, but no accompanying change in CMRO2. In addition, we demonstrated the feasibility of using an R2*-based calibrated-BOLD approach for comparing longitudinal data that may prove useful in other studies of medication effects.
Acknowledgments
This work was supported by NIH grants NS-36722, NS-42069, and NS-51661. The authors thank Beau Ances, Christine Liang and Susan Hopkins for their help with this study, and Khaled Restom and Yashar Behzadi for help with data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behzadi Y, Liu TT. An arteriolar compliance model of the cerebral blood flow response to neural stimulus. Neuroimage. 2005;25:1100–1111. doi: 10.1016/j.neuroimage.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Liu TT. Caffeine reduces the initial dip in the visual BOLD response at 3 T. Neuroimage. 2006;32:9–15. doi: 10.1016/j.neuroimage.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Trouard TP, Ryan L. Caffeine attenuates practice effects in word stem completion as measured by fMRI BOLD signal. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17:107–125. doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23(Suppl 1):S220–233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19:701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Modell JG, Hariharan M. Caffeine and human cerebral blood flow: a positron emission tomography study. Life Sci. 1990;47:1141–1146. doi: 10.1016/0024-3205(90)90174-p. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Chen Y, Parrish TB. Activation induced BOLD and CBF responses vary with caffeine dose. Proc ISMRM. 2007:112. doi: 10.1016/j.neuroimage.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Gallichan D, Piechnik SK, Wise R, Jezzard P. Flow-metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med. 2007a;57:538–547. doi: 10.1002/mrm.21171. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Piechnik S, Jezzard P. Sources of systematic bias in hypercapnia-calibrated functional MRI estimation of oxygen metabolism. Neuroimage. 2007b;34:35–43. doi: 10.1016/j.neuroimage.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dager SR, Layton ME, Strauss W, Richards TL, Heide A, Friedman SD, Artru AA, Hayes CE, Posse S. Human brain metabolic response to caffeine and the effects of tolerance. Am J Psychiatry. 1999;156:229–237. doi: 10.1176/ajp.156.2.229. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AS, Laurienti PJ, Yen YF, Burdette JH, Moody DM. Dietary caffeine consumption and withdrawal: confounding variables in quantitative cerebral perfusion studies? Radiology. 2003;227:129–135. doi: 10.1148/radiol.2271012173. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WP, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster–size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to it widespread use. Pharmcological reviews. 1999;51:83–133. [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gotoh J, Kuang TY, Nakao Y, Cohen DM, Melzer P, Itoh Y, Pak H, Pettigrew K, Sokoloff L. Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am J Physiol Heart Circ Physiol. 2001;280:H821–829. doi: 10.1152/ajpheart.2001.280.2.H821. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HBW, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Dietary caffeine consumption modulates fMRI measures. Neuroimage. 2002;17:751–757. [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Relationship between caffeine-induced changes in resting cerebral perfusion and blood oxygenation level-dependent signal. AJNR Am J Neuroradiol. 2003;24:1607–1611. [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated-BOLD fMRI. Neuroimage. 2007a;35:175–184. doi: 10.1016/j.neuroimage.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO(2) coupling measured with calibrated BOLD fMRI: Sources of bias. Neuroimage. 2007b doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Behzadi Y, Restom K, Uludag K, Lu K, Buracas GT, Dubowitz DJ, Buxton RB. Caffeine alters the temporal dynamics of the visual BOLD response. Neuroimage. 2004;23:1402–1413. doi: 10.1016/j.neuroimage.2004.07.061. [DOI] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Caffeine induced changes in cerebral circulation. Stroke. 1985;16:814–817. doi: 10.1161/01.str.16.5.814. [DOI] [PubMed] [Google Scholar]
- Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 2002;15:37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Hernandez-Garcia L, Lee GR, Nichols TE. Estimation efficiency and statistical power in arterial spin labeling fMRI. Neuroimage. 2006;33:103–114. doi: 10.1016/j.neuroimage.2006.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Boyet S. Dose-response study of caffeine effects on cerebral functional activity with a specific focus on dependence. Brain Res. 2000;858:71–77. doi: 10.1016/s0006-8993(99)02480-4. [DOI] [PubMed] [Google Scholar]
- Restom K, Behzadi Y, Liu TT. Physiological noise reduction for arterial spin labeling functional MRI. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.01.026. [DOI] [PubMed] [Google Scholar]
- St Lawrence KS, Ye FQ, Lewis BK, Frank JA, McLaughlin AC. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med. 2003;50:99–106. doi: 10.1002/mrm.10502. [DOI] [PubMed] [Google Scholar]
- Uludag K, Buxton RB. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med. 2004;51:1088–1089. doi: 10.1002/mrm.20067. author reply 1090. [DOI] [PubMed] [Google Scholar]
- Wang J, Qiu M, Constable RT. In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med. 2005;53:666–674. doi: 10.1002/mrm.20377. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]


