Abstract
The renin-angiotensin system (RAS) plays a critical role in kidney development. Mutations in the genes encoding components of the RAS cause a spectrum of congenital abnormalities of the kidney and renal collecting system ranging from hypoplasia of the renal medulla and hydronephrosis in mice to renal tubular dysgenesis in humans. However, the mechanisms by which an intact RAS controls proper renal system development and how aberrations in the RAS result in abnormal kidney and renal collecting system development are poorly understood. The renal collecting system originates from the ureteric bud (UB). A number of transcription and growth factors regulate UB branching morphogenesis to ultimately form the ureter, pelvis, calyces, medullary and cortical collecting ducts. Importantly, UB morphogenesis is a key developmental process that controls organogenesis of the entire metanephros. This review emphasizes emerging insights into the role for the RAS in UB morphogenesis and explores the mechanisms whereby RAS regulates this important process. A conceptual framework derived from recent work indicates that cooperation of angiotensin II AT1 receptor and receptor tyrosine kinase signaling performs essential functions during renal collecting system development via control of UB branching morphogenesis.
Keywords: kidney development, ureteric bud, renin-angiotensin, GDNF, c-Ret, EGF receptor
INTRODUCTION
Branching morphogenesis of the ureteric bud (UB) is a key developmental process that controls not only formation of the renal collecting system, but organogenesis of the entire metanephros. Derangements in UB morphogenesis cause a spectrum of congenital abnormalities of the kidney and urinary tract (CAKUT). Many instances of CAKUT have a genetic basis and are associated with hereditary human syndromes (1). The inheritance pattern of some nonsyndromic cases of CAKUT (eg., autosomal-dominant or recessive polycystic kidney disease) is well known (2). In other nonsyndromic forms of CAKUT (eg., obstructive uropathy, vesico-ureteral reflux, kidney hypodysplasia, duplex kidneys), the hereditary and molecular mechanisms remain to be determined.
Mutations in the genes encoding components of the renin-angiotensin system (RAS) or pharmacological inhibition of RAS in animals or humans cause diverse malformations of the renal excretory system that include papillary and medullary hypodysplasia, hydronephrosis, collapsed collecting ducts, aberrant UB budding, duplicated collecting system, and urinary concentrating defect (3–10). Since CAKUT are the major cause of renal failure in childhood (11), identification of the molecular mechanisms that lead to diverse forms of CAKUT under conditions of disrupted RAS has important clinical implications.
OVERVIEW OF METANEPHRIC KIDNEY DEVELOPMENT
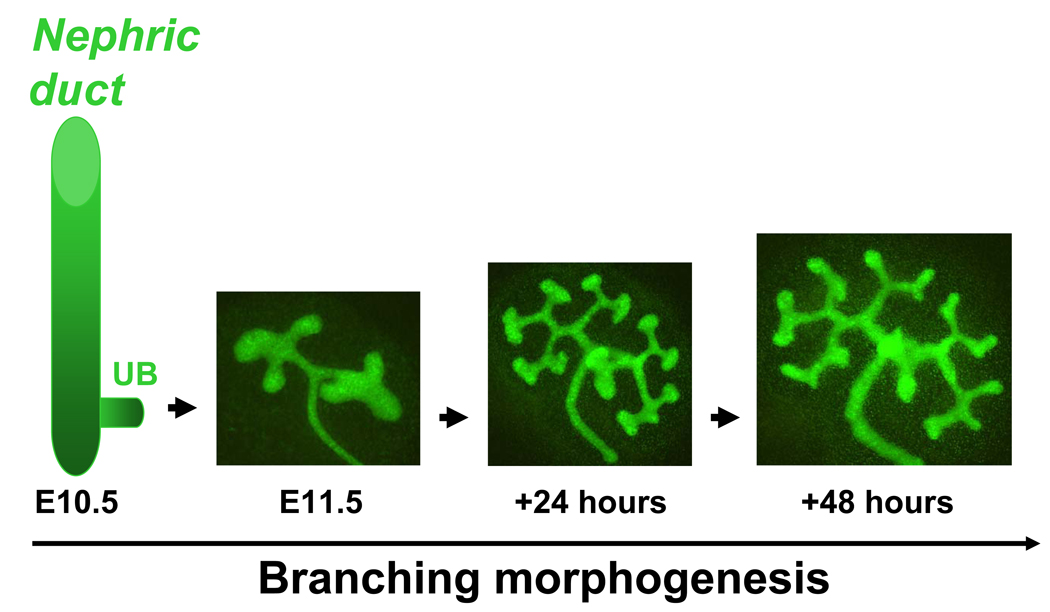
The development of the metanephric kidney begins when the nephric duct (ND) gives rise to ureteric bud (UB) on embryonic (E) day E10.5 in mice and E28 in humans (12). UB outgrowth from the ND is followed by its repetitive branching, growth and remodeling, a process called branching morphogenesis (Fig. 1), to eventually form the renal collecting system (13, 14). Initial generations of UB branches will be remodeled into the ureter, renal pelvis and calyces, whereas subsequent generations of UB branches will give rise to collecting ducts. The ureter will translocate from the ND to fuse with the bladder (15, 16). Collecting ducts will subsequently undergo patterning to contribute importantly to the renal papilla and medulla. Each UB tip is capable of inducing the adjacent metanephric mesenchyme to undergo mesenchymal-to-epithelial transition (MET) and form nephrons (from the glomerulus through the distal tubule) (13). Even subtle defects in the efficiency of UB branching result in a significant decrease in nephron endowment (17). In turn, decreased nephron endowment is linked to renal hypodysplasia, CAKUT, hypertension and eventual progression to chronic renal failure (11, 18, 19).
Figure 1.
Ureteric bud branching morphogenesis in the mouse. Ureteric bud (UB) emerges from the nephric duct on embryonic (E) day E10.5. Metanephros isolated on E11.5 from Hoxb7-GFP transgenic mouse embryo and grown ex vivo for 48 hours. Hoxb7 promoter directs expression of green fluorescent protein (GFP) to the UB. The number of branching structures increases progressively with time. Adapted from (62) (with permission from the American Society of Nephrology).
GROWTH FACTORS IN UB MORPHOGENESIS
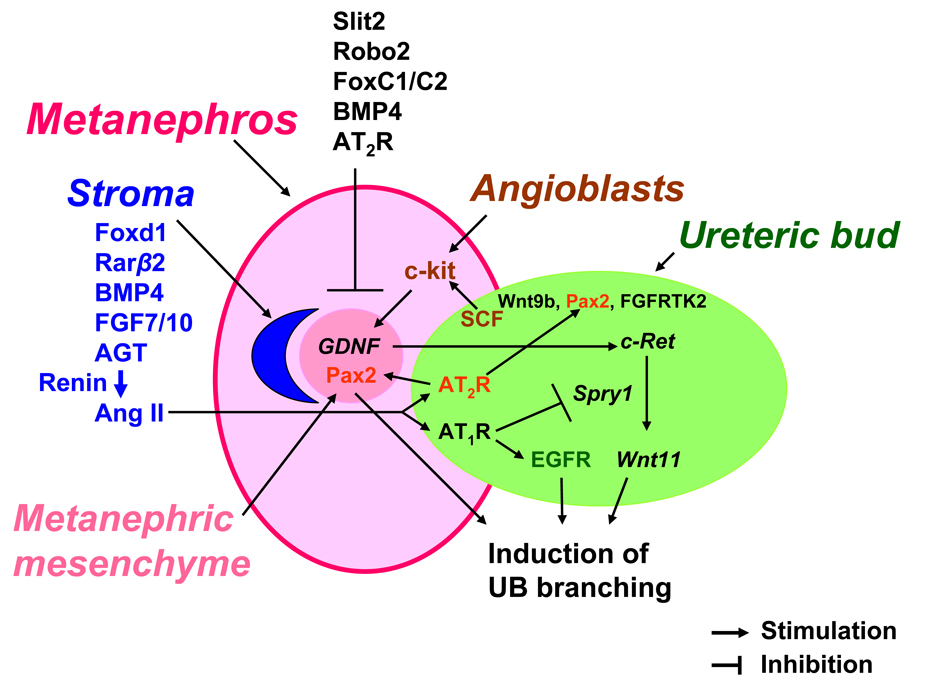
UB initiation, branching and patterning is regulated by reciprocal interactions of transcription and growth factors expressed in metanephric mesenchyme, stroma, angioblasts and UB itself (20) (Fig. 2). Signals from the metanephric mesenchyme, stroma and angioblasts induce the UB to originate from the ND, invade the mesenchyme and undergo branching morphogenesis. Metanephric mesenchymal cells secrete glial-derived neurotrophic factor (GDNF) which interacts with the c-Ret tyrosine kinase receptor expressed in the UB tip cells to induce branching (21). GDNF or c-Ret expression is activated by paired box 2 (Pax-2), a transcription factor present in the metanephric mesenchyme (22, 23). Stromal cells provide critical signals to regulate UB branching via forkhead/winged helix transcription factor d1 (Foxd1) or retinoic acid. Foxd1 or combined retinoic acid receptor (RAR) α/β2 deficiency in mice compromises UB branching (24, 25). Interestingly, UB tip cells in RARα/β2-deficient mice express less c-Ret and genetic overexpression of c-Ret in these mice rescues UB branching and stromal patterning. Thus, not only stroma-derived signals regulate UB morphogenesis, but c-Ret-dependent UB output is important in stromal development. In addition, UB signaling is critical for the induction and maintenance of nephrogenesis. For example, conditional inactivation of Wnt9b, normally expressed in the UB epithelium, leads to defects in formation of renal vesicles (26). This is accompanied by the absence of the early nephron markers such as Wnt4, Fgf8 and Pax8. UB branching in Wnt9b-deficient mice is also disrupted possibly due to decreased levels of GDNF in the mesenchyme and of Wnt11- in the UB tip cell domain (26).
Figure 2.
Schematic representation of the concept of angiotensin II-growth factor cross-talk in ureteric bud morphogenesis. Metanephric mesenchyme and stroma interact reciprocally with the ureteric bud (UB) and angioblasts to form the metanephros. Multiple gene regulatory networks have been shown to regulate UB elongation, branching and nephron formation. Signaling molecule Slit2 and its receptor Robo2, forkhead/winged helix transcription factors C1 and C2 (FoxC1/C2), bone morphogenetic protein (BMP) 4 and angiotensin (Ang) II AT2R act to restrict glial derived neurotrophic factor (GDNF) expression anteriorly and specify correct site of UB outgrowth from the nephric duct (46, 57). GDNF/c-Ret/Wnt11 pathway is the major positive regulator of UB development (22). Stromal factors Foxd1, retinoic acid receptor (Rar) β2, BMP4, fibroblast growth factors (FGF) 7 and 10 play an essential role in UB morphogenesis (77). FGF7/10 act via FGF receptor tyrosine kinase 2 (RTK2). Angiotensinogen (AGT) and Ang II are emerging stromal factors that regulate UB morphogenesis (60). Since renin-expressing cells are present in the stroma on E12 (61), co-localization of AGT (60) and renin in the stroma suggests that Ang II is generated around the UB and acts in a paracrine manner on the AT1R and AT2R expressed in the UB to regulate branching. Ang II AT1R cross-talks with GDNF/c-Ret/Wnt11 pathway and EGFR. Stimulation of the AT1R by Ang II inhibits Spry1 gene expression and thereby releaves inhibition of signaling via the GDNF/c-Ret/Wnt11 pathway (66). In addition, Ang II enhances tyrosine phosphorylation of EGFR in UB cells (62). Inhibition of EGFR tyrosine kinase activity abrogates Ang II-induced UB morphogenesis (62). Coupling of Ang II with the AT2R upregulates paired box 2 (Pax2) and stimulates UB branching (63). Stem cell factor (SCF) is expressed in the UB and acts via its receptor c-kit to induce GDNF expression in the metanephric mesenchyme and thereby modulate UB branching (31).
Wnt11 cooperates with GDNF/c-Ret pathway to regulate UB morphogenesis. Metanephroi of Wnt11-null mice express less GDNF and have fewer nephrons and UB branches compared to wild-type littermates (27). Moreover, c-Ret and Wnt11 interact genetically to induce UB branching. Thus, Wnt11 acts as a critical component of GDNF/c-Ret/Wnt11 signaling loop to stimulate UB branching (28). Augmenting effects of GDNF/c-Ret/Wnt11 pathway on UB development are antagonized by Spry1. Spry1 acts to inhibit c-Ret receptor tyrosine kinase (RTK) activity, prevent expansion of GDNF and Wnt11 domains thus decreasing UB branching (28). Recent studies demonstrated that Wnt-dependent UB branching is mediated via the canonical β-catenin pathway (29). Targeted inactivation of β-catenin in UB cell lineage causes decreased expression of transcription factors Emx2, Pax2, Lim1 followed by downregulation of GDNF/c-Ret/Wnt11 and results in disrupted branching morphogenesis (29). Stem cell factor (SCF) represents yet another signaling molecule released by the UB. SCF interacts with RTK c-kit located in Foxd1-negative interstitial cells to induce UB branching (30). Interestingly, a subset of c-kit-positive cells express angioblast markers Flk-1 and Podx1. Antagonism of c-kit RTK activity inhibits UB branching, reduces the number of angioblasts and downregulates Flk-1 gene expression. Angioblasts can also modulate UB branching by regulating GDNF expression in the metanephric mesenchyme (31).
In addition to GDNF, a number of other RTK-stimulating growth factors have been shown to regulate UB morphogenesis. While fibroblast growth factors (FGF) 7 and 10 or insulin-like growth factor (IGF-1) promote, TGF-β and activin A inhibit UB budding (32, 33, 34, 35). Genetic inactivation of FGF7 or FGF10 in mice results in a reduced number of UB branches, medullary collecting ducts and leads to papillary hypodysplasia (32, 34). Both FGF7 and FGF10 are present in the stroma and bind FGF RTK2 which is expressed exclusively in the UB (36, 37, 38). UB-specific inactivation of the FGF RTK2, but not FGF RTK1, in mice reduces UB branching and nephron number (37). Epidermal growth factor receptor (EGFR) is expressed in renal collecting ducts (39). Stimulation of the EGFR by its ligand enhances branching tubule formation in mouse inner medullary collecting duct (IMCD-3) cells grown in three-dimensional collagen matrix gels in vitro (40). In vivo studies demonstrate that EGFR-mutant mice have abnormal collecting ducts (41). As RTK-stimulating growth factors, GDNF, FGF7/FGF10 or EGF may signal via shared downstream pathways to stimulate UB branching. Moreover, GDNF/c-Ret-dependent pathway can be bypassed by other morphogenetic growth factors. In this regard, addition of FGF7 along with activin A, an inhibitor of TGF-β superfamily, induces branching in isolated nephric ducts in vitro (42). Therefore, there is a functional redundancy between growth factor signaling during UB morphogenesis.
Bone morphogenetic proteins (BMP), members of TGF-β superfamily, play an important role in UB morphogenesis. BMP growth factors signal via activin-like kinase (ALK) type I serine/threonine kinase receptors coupled to downstream cytoplasmic signal-transducing Smad proteins (43). BMP2, BMP4 and BMP7 are most clearly involved in regulating UB branching. Treatment of embryonic kidney explants or isolated UBs with exogenous BMP2, BMP4 or high doses of BMP7 inhibits UB branching in vitro (44, 45, 46, 47). BMP4 is expressed exclusively in the stroma. BMP4+/− embryos exhibit duplicated UBs suggesting that BMP4 acts to suppress ectopic UB budding (46). UB-specific inactivation of ALK3 receptor subtype in mice causes aberrant UB and collecting duct morphogenesis resulting in hypoplasia of the renal medulla (48). Since these mice exhibit a decrease in the expression of Pax2 in the UB and metanephric mesenchyme, initial decrease in UB branching may be due, in part, to a reduced Pax2-dependent signaling.
RENIN-ANGIOTENSIN SYSTEM-GROWTH FACTOR CROSS-TALK IN UB MORPHOGENESIS
Angiotensin (Ang) II [Ang-(1–8)] is the principal effector peptide growth factor of the renin-angiotensin system (RAS) which acts via two major types of G protein-coupled receptors (GPCR): AT1R and AT2R. AT1R is a mitogen for renal vascular, mesangial and tubular cells (49). In contrast, AT2R inhibits growth and proliferation in mesangial cells (50). Ang-(1–7) is a newly discovered member of the RAS. Ang-(1–7) is formed from Ang II by angiotensin-converting enzyme 2 (ACE2) and acts via GPCR Mas to oppose Ang II-AT1R–mediated effects (51, 52). Genetic inactivation of the AT1R, AT2R and other RAS components in mice or pharmacologic antagonism of the RAS cause a spectrum of abnormalities in the development of the renal collecting system (3–10). Angiotensinogen, angiotensin-converting enzyme (ACE) or AT1R-deficient mice manifest hydronephrosis, hypoplastic medulla and papilla. Studies from Dr. Ichikawa’s laboratory have suggested that medullary hypoplasia and hydronephrosis in AT1R-deficient mice may be due to a hypoplastic ureteral smooth muscle layer which exhibits impaired peristalsis (53). Functionally, ACE- and AT1R-null animals have a reduced ability to concentrate urine (6, 7). Use of ACE inhibitors and AT1R blockers in humans cause oligohydramnios and anuria (54, 55). Therefore, these drugs should not be used by pregnant women. AT2R-mutant mice demonstrate a decrease in the rate of apoptosis of mesenchymal cells around the nascent UB (56) and increased incidence of duplex ureters and vesicoureteral reflux (57). Thus, it is conceivable that absence of timely apoptosis of mesenchymal cells may cause an aberrant UB budding and lead to duplicated ureters. In addition, AT2R may act as restricting signal to limit anterior expansion of the GDNF domain (Fig. 2). The role of ACE2-Ang-(1–7)-Mas axis in kidney development and UB morphogenesis remains to be determined. However, based on the findings that ACE2 homolog collectrin is expressed in the UB branches as early as on E13 in the mouse (58) and that Ang-(1–7), acting via Mas, inhibits Ang II-induced phosphorylation of MAP kinase in kidney proximal tubular cells (52), such role is likely.
Mutations in the genes encoding for angiotensinogen, renin, ACE or AT1R in humans lead to renal tubular dysgenesis (RTD) (10, 59). In RTD, renal cortex exhibits a paucity of proximal tubules. In the medulla, collapsed collecting ducts and abundant interstitial fibrosis are observed (59). Importantly, RTD is characterized by perinatal death due to anuria combined with pulmonary hypoplasia. The more severe outcome in humans than in mice without the functional RAS may be due to the temporal difference in completion of nephrogenesis. In mice, nephrogenesis is completed 2 weeks after birth, whereas in humans- at 38 weeks of gestation. Collectively, the observed abnormalities in UB/medullary development imply that UB-derived structures are targets for Ang II actions during metanephric development in both mice and humans.
We recently demonstrated that angiotensinogen and Ang II AT1R are present in both UBs and stroma on E12 in the mouse and that their expression increases progressively from E12 to E16 (60). Importantly, AT1R immunoreactivity is present on both luminal and basolateral aspects of UB branches. Since renin-producing cells originate from the mesenchyme on E11-E12, at a time when UB branching is just beginning (61), Ang II may be generated locally in the mesenchyme to act in a paracrine fashion on the adjacent AT1R-expressing UBs to regulate branching (Fig. 2). In accord with this hypothesis, we further demonstrated that Ang II, acting via the AT1R, stimulates UB growth and branching in the intact whole embryonic (E) day E11.5 metanephroi grown ex vivo and studied at 24 and 48 hours after Ang II treatment (62). These findings indicate that Ang II promotes morphogenesis of the renal collecting system. Ang II can stimulate UB branching directly or through interaction with other transcription and growth factors present in the metanephric mesenchyme, stroma, angioblasts or the UB that are known to regulate UB morphogenesis (Fig. 2). For example, Ang II may stimulate Pax2 present in the UB or mesenchyme and thereby enhance UB branching. This possibility is supported by the ability of Ang II to increase Pax2 gene expression in metanephric kidney (63). Since such RTK-stimulating growth factors as FGF7 or EGF enhance the ability of GDNF to elicit ectopic UB budding (42), Ang II may induce UB branching by enhancing sensitivity of the UB to GDNF, EGF, or FGF7. In addition, Ang II may alter the expression of competence growth factors in favor of UB branching. In keeping with this hypothesis, renal papilla of angiotensinogen- and AT1R-deficient mice exhibits reduced EGF mRNA levels (4). Moreover, ACE inhibition during neonatal nephrogenesis in the rat decreases IGF-1 mRNA and protein expression in the renal medulla (64). Both AT1R and FGF7 are expressed in the stromal mesenchyme, and Ang II increases FGF7 mRNA levels in luteal cells (65). These observations indicate that RAS interacts with EGF and IGF-1 and may induce FGF7 during medullary development.
Given that GDNF/c-Ret signaling pathway is a major positive regulator of UB branching morphogenesis program (22), we recently tested whether Ang II enhances GDNF and c-Ret expression during active UB branching. Treatment with Ang II induced expression of GDNF mRNA in the metanephric mesenchyme (66). This was accompanied by increased expression of c-Ret, GDNF RTK, and of Wnt11, a downstream target of c-Ret signaling, in the UB tip cells. Moreover, Ang II caused preferential proliferation of UB tip cells, whereas inhibition of endogenous AT1R signaling inhibited UB tip cell apoptosis (66). Therefore, Ang II not only induced GDNF/c-Ret expression during critical period of UB morphogenesis, but induced mitogenic and prosurvival effects in the UB tip cells leading to UB growth and branching. To determine the mechanism of Ang II-induced upregulation of GDNF/c-Ret signaling, we next examined the effect of Ang II and its AT1R on the expression of Spry1, an endogenous inhibitor of c-Ret RTK signaling (28). Ang II decreased Spry1 in an AT1R-dependent manner (66). These findings indicate that AT1R-GDNF/c-Ret cross-talk involves downregulation of Spry1. This in turn facilitates c-Ret RTK signaling leading to activation of the GDNF/Ret/Wnt11 positive feedback loop. It is conceivable that Ang II induces focal bursts of proliferation of UB tip cells, and together with decreased apoptosis, plays an important role in the expansion of the ampulla, subsequent branching and directional bud elongation.
Alternatively or additionally, stimulation of the AT1R by its ligand can transactivate RTK to enhance morphogenetic signaling without altering the expression levels of a given positive growth factor or its cognate RTK. To test this hypothesis, we recently investigated the ability of Ang II to transactivate EGFR in UB cells and examined the contribution of EGFR activation to Ang II-induced UB branching. Ang II increased tyrosine phosphorylation of EGFR in UB cells (56). Moreover, inhibition of EGFR tyrosine kinase activity abrogated Ang II-induced UB branching in the intact metanephros (62). These findings indicate that cross-talk between the AT1R and EGFR is crucial in UB morphogenesis and that cooperation of AT1R and EGFR signaling promotes the development of the renal collecting system.
MECHANISMS OF ANGIOTENSIN II-GROWTH FACTOR INTERACTIONS DURING UB MORPHOGENESIS
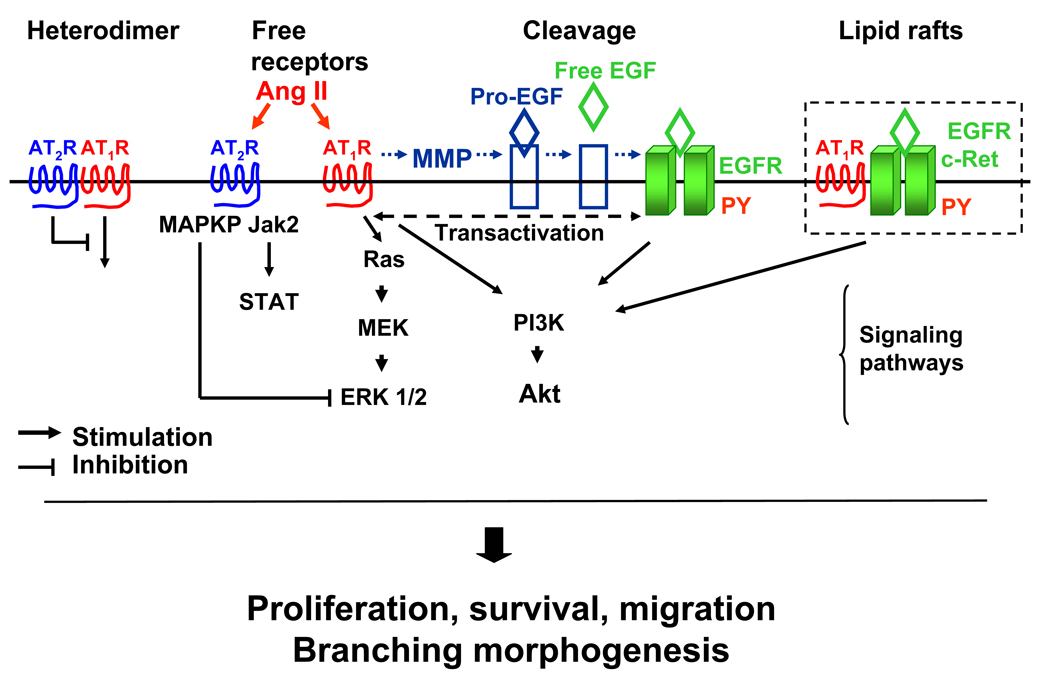
Cross-talk may be defined as a functional interaction between different signaling pathways. These interactions enhance the ability of the cell to integrate, process and respond to the information received from its microenvironment. The consequences of cross-talks are that the expression of a given set of target genes is regulated by multiple signaling pathways. Thus, the expression of the UB morphogenetic program genes and UB growth depend on complex network of regulatory pathways and signaling effectors. Ang II may cross-talk with RTK and their cognate ligands via intracellular signaling pathways, induction of cleavage of membrane-bound RTK proligand, facilitation of physical interaction between AT1R/AT2R and RTK or other mechanisms. For example, inhibition of PI3K blocks GDNF-dependent UB branching and migration of c-Ret-transfected MDCK cells (67, 68). Stimulation of Ang II AT1R activates PKC (69) leading to MAP kinase (MAPK)-dependent upregulation of transcription of cell cycle progression genes, such as cyclin D1, through activation of the transcription factor AP-1 (70). Thus, one of the possible mechanisms facilitating Ang II-EGFR/c-Ret interactions and UB morphogenesis may involve EGFR- or c-Ret-mediated stimulation of MAPK and PI3K/Akt pathways. Whether effects of AT1R and EGFR/c-Ret result in synergistic stimulation of MAPK/PI3K and UB branching remains to be determined. AT2R-dependent mesenchymal apoptosis (35) may result from activation of MAP kinase phosphatase 1 leading to inactivation of ERK1/2 (50). AT2R-dependent activation of Pax2 in whole metanephroi and mesenchymal cells is mediated by JAK2/STAT pathway (63).
Another mechanism by which Ang II may activate EGFR is by stimulating the proteolytic cleavage of pro-heparin-binding EGF at the cell membrane (71). Coupling of Ang II with AT1R activates extracellular metalloproteinase which cleaves transmembrane EGF precursor and releases mature EGF (71). Mature EGF interacts with the ectodomain of the EGFR and activates intracellular signal. Facilitation of direct physical interaction between Ang II receptors and RTK may involve clustering in lipid rafts. For example, caveolae/lipid rafts are essential for Ang II–induced transactivation of EGFR (72). Interestingly, EGF induces phosphorylation of AT1R and leads to formation of a multireceptor complex containing AT1R and activated EGFR (73). These findings suggest that cross-talk between the RAS and RTK can be bi-directional. One mechanism whereby Ang II may transactivate RTK during UB branching is the balance between AT1R- and AT2R-mediated actions. In this regard, recent reports suggest that AT2R offsets the growth-promoting effects of the AT1R (74) and that part of the antagonism between the two receptor types might be due to the formation of AT1R/AT2R heterodimers whereby the AT2R directly inhibits AT1R (75). Since AT2R expression in the metanephros progressively decreases and of AT1R increases with gestation (76), it is conceivable that increased levels of AT1R not complexed with AT2R may facilitate AT1R-growth factor/RTK cross-talk to stimulate UB morphogenesis.
Future studies investigating the effect of AT1R and AT2R null mutations on the expression of the critical growth factors and signaling networks known to regulate UB branching will provide essential information regarding the nature of events culminating in the formation of the mature metanephros.
Figure 3.
Proposed mechanisms of angiotensin II-growth factor cross-talk in ureteric bud branching morphogenesis. Heterodimerization of AT1R and AT2R leads to inhibition of AT1R signaling by AT2R (75). AT1R- and AT2R-mediated activation of multiple signaling pathways involving Jak2/STAT (63), Ras/ERK1/2 (69, 70), PI3K/Akt (67, 68), epidermal growth factor receptor (EGFR) transactivation (62) may stimulate UB cells proliferation, survival, migration, and promote UB morphogenesis. Ang II may stimulate cleavage of EGFR proligand (71). Facilitation of a direct physical interaction between Ang II receptors and RTK (EGFR, c-Ret) may involve clustering in lipid rafts (72).
ACKNOWLEDGMENTS
The author would like to thank colleagues who have contributed in various ways to our studies in this area, particularly Mercedes Schroeder, Mary Kate Boh, Melissa Spera, Renfang Song and Samir El-Dahr. The original work was supported by NIH Grants P20 RR17659 and DK071699-01 (Ihor Yosypiv).
REFERENCES
- 1.Yosypiv IV. Hypothesis: a novel role for the renin-angiotensin system in ureteric bud branching. Organogenesis. 2004;1:26–32. doi: 10.4161/org.1.1.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374–1380. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 3.Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75:745–753. [PubMed] [Google Scholar]
- 4.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 6.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;7:953–965. [PubMed] [Google Scholar]
- 7.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazaki Y, Tsuchida S, Fogo A, Ichikawa I. The renal lesions that develop in neonatal mice during angiotensin inhibition mimic obstructive nephropathy. Kidney Int. 1999;55:1683–1695. doi: 10.1046/j.1523-1755.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Gribouval O, Gonzales M, Neuhaus T. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 11.North American Pediatric Renal Trials and Collaborative Studies. NAPRTCS Annual report. 2006 [Google Scholar]
- 12.Saxen L. Organogenesis of the kidney. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 13.Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989;3:2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- 14.Grobstein C. Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse metanephros. Science. 1953;118:52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- 15.Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet. 2002;32:109–115. doi: 10.1038/ng952. [DOI] [PubMed] [Google Scholar]
- 16.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–1089. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai H, Nigam S. In vitro branching tubulogenesis: implications for developmental and cystic disorders, nephron number, renal repair, and nephron engineering. Kidney Int. 1998;54:14–26. doi: 10.1046/j.1523-1755.1998.00969.x. [DOI] [PubMed] [Google Scholar]
- 18.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 19.Lisle SJ, Lewis RM, Petry CJ, Ozanne SE, Hales CN, Forhead AJ. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr. 2003;90:33–39. doi: 10.1079/bjn2003881. [DOI] [PubMed] [Google Scholar]
- 20.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 21.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 22.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- 23.Clarke JC, Patel SR, Raymond RM, Jr, Andrew S, Robinson BG, Dressler GR, Brophy PD. Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet. 2006;15:3420–3428. doi: 10.1093/hmg/ddl418. [DOI] [PubMed] [Google Scholar]
- 24.Hatini A, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes and Development. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 25.Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126:1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- 26.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- 28.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 29.Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317:83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development. 2005;132:5437–5449. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- 32.Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development. 1999;126:547–554. doi: 10.1242/dev.126.3.547. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai H, Barros EJ, Tsukamoto T, Barasch J, Nigam SK. An in vitro tubulogenesis system using cell lines derived from the embryonic kidney shows dependence on multiple soluble growth factors. Proc Natl Acad Sci U S A. 1997;94:6279–6284. doi: 10.1073/pnas.94.12.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]; 34 Ritvos O, Tuuri T, Erämaa M, Sainio K, Hildén K, Saxén L, Gilbert SF. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mech Dev. 1997;50:229–245. doi: 10.1016/0925-4773(94)00342-k. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai H, Tsukamoto T, Kjelsberg CA, Cantley LG, Nigam SK. Department EGF receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol. 1997;273:F463–F472. doi: 10.1152/ajprenal.1997.273.3.F463. [DOI] [PubMed] [Google Scholar]
- 36.Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123–135. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Center Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Department Receptor specificity of the fibroblast growth factor family The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernardini N, Bianchi F, Lupetti M, Dolfi A. Immunohistochemical localization of the epidermal growth factor, transforming growth factor alpha, and their receptor in the human mesonephros and metanephros. Dev Dyn. 1996;206:231–238. doi: 10.1002/(SICI)1097-0177(199607)206:3<231::AID-AJA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai H, Tsukamoto T, Kjelsberg CA, Cantley LG, Nigam SK. EGF receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol. 1997;273:F463–F472. doi: 10.1152/ajprenal.1997.273.3.F463. [DOI] [PubMed] [Google Scholar]
- 41.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 42.Maeshima A, Vaughn DA, Choi Y, Nigam SK. Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol. 2006;295:473–485. doi: 10.1016/j.ydbio.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Cain JE, Hartwig S, Bertram JF, Rosenblum ND. Bone morphogenetic protein signaling in the developing kidney: present and future. Differentiation. 2008 doi: 10.1111/j.1432-0436.2008.00265.x. (in press) [DOI] [PubMed] [Google Scholar]
- 44.Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Piscione TD, Yager TD, Gupta IR, Grinfeld B, Pei Y, Attisano L, Wrana JL, Rosenblum ND. BMP-2 and OP-1 exert direct and opposite effects on renal branching morphogenesis. Am J Physiol. 1997;273:F961–F975. doi: 10.1152/ajprenal.1997.273.6.F961. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki Y, Oshima K, Fogo A, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cain JE, Nion T, Jeulin D, Bertram JF. BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro. Kidney Int. 2005;67:420–431. doi: 10.1111/j.1523-1755.2005.67098.x. [DOI] [PubMed] [Google Scholar]
- 48.Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND. BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol. 2008;19:117–124. doi: 10.1681/ASN.2007010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf G, Thaiss F, Schoeppe W, Stahl RA. Angiotensin II-induced proliferation of cultured murine mesangial cells: inhibitory role of atrial natriuretic peptide. J Am Soc Nephrol. 1992;3:1270–1278. doi: 10.1681/ASN.V361270. [DOI] [PubMed] [Google Scholar]
- 50.Goto M, Mukoyama M, Suga S, Matsumoto T, Nakagawa M, Ishibashi R, Kasahara M, Sugawara A, Tanaka I, Nakao K. Growth-dependent induction of angiotensin II type 2 receptor in rat mesangial cells. Hypertension. 1997;30:358–362. doi: 10.1161/01.hyp.30.3.358. [DOI] [PubMed] [Google Scholar]
- 51.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos RA, Ferreira AJ. Angiotensin-(1–7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 53.Miyazaki Y, Tsuchida S, Nishimura H, Pope JC, 4th, Harris RC, McKanna JM, Inagami T, Hogan BL, Fogo A, Ichikawa I. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J Clin Invest. 1998;102:1489–1497. doi: 10.1172/JCI4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaefer C. Angiotensin II-receptor-antagonists: further evidence of fetotoxicity but not teratogenicity. Part A Clin Mol Teratol. Birth Defects Res. 2003;67:591–594. doi: 10.1002/bdra.10081. [DOI] [PubMed] [Google Scholar]
- 55.Tabacova S, Little R, Tsong Y, Vega A, Kimmel CA. Adverse pregnancy outcomes associated with maternal enalapril antihypertensive treatment. Pharmacoepidemiol Drug Saf. 2003;12:633–646. doi: 10.1002/pds.796. [DOI] [PubMed] [Google Scholar]
- 56.Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, Hogan BM, Fogo A, Brock JW, 3rd, Inagami T, Ichikawa I. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- 57.Oshima K, Miyazaki Y, Brock JW, Adams MC, Ichikawa I, Pope JC. Angiotensin type II receptor expression and ureteral budding. J Urol. 2001;166:1848–1852. [PubMed] [Google Scholar]
- 58.Zhang H, Wada J, Hida K, Tsuchiyama Y, Hiragushi K, Shikata K, Wang H, Lin S, Kanwar YS, Makino H. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem. 2001;276:17132–17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- 59.Lacoste M, Yi Cai Y, Liliane Guicharnaud, et al. Renal Tubular Dysgenesis, a Not Uncommon Autosomal Recessive Disorder Leading to Oligohydramnios: Role of the Renin-Angiotensin System. J Am Soc Nephrol. 2006;17:2253–2263. doi: 10.1681/ASN.2005121303. [DOI] [PubMed] [Google Scholar]
- 60.Iosipiv, Schroeder M. A role for angiotensin II AT1 receptors in ureteric bud cell branching. Am J Physiol. 2003;285:F199–F207. doi: 10.1152/ajprenal.00401.2002. [DOI] [PubMed] [Google Scholar]
- 61.Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol. 2001;281:F345–F356. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- 62.Yosypiv IV, Schroeder M, El-Dahr SS. AT1R–EGFR crosstalk regulates ureteric bud branching morphogenesis. J Am Soc of Nephrol. 2006;17:1005–1014. doi: 10.1681/ASN.2005080803. [DOI] [PubMed] [Google Scholar]
- 63.Zhang SL, Moini B, Ingelfinger JR. Angiotensin II increases Pax-2 expression in fetal kidney cells via the AT2 receptor. J Am Soc Nephrol. 2004;15:1452–1465. doi: 10.1097/01.asn.0000130567.76794.58. [DOI] [PubMed] [Google Scholar]
- 64.Nilsson AB, Nitescu N, Chen Y, Guron GS, Marcussen N, Matejka GL, Friberg P. IGF-I treatment attenuates renal abnormalities induced by neonatal ACE inhibition. Am J Physiol. 2000;279:R1050–R1060. doi: 10.1152/ajpregu.2000.279.3.R1050. [DOI] [PubMed] [Google Scholar]
- 65.Stirling D, Magness RR, Stone R, Waterman MR, Simpson ER. Angiotensin II inhibits luteinizing hormone-stimulated cholesterol side chain cleavage expression and stimulates basic fibroblast growth factor expression in bovine luteal cells in primary culture. J Biol Chem. 1990;265:5–8. [PubMed] [Google Scholar]
- 66.Yosypiv IV, Boh MK, Spera M, El-Dahr SS. Downregulation of Spry-1, an inhibitor of GDNF/Ret, as a mechanism for angiotensin II-induced ureteric bud branching. Kidney International. 2008 doi: 10.1038/ki.2008.378. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- 68.Kim D, Dressler GR. PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol. 2007;307:290–299. doi: 10.1016/j.ydbio.2007.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol. 2001;281:H2337–H2365. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe G, Lee RJ, Albanese C, Rainey WE, Batle D, Pestell RG. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- 71.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 72.Ushio-Fukai M, Hilenski L, Santanam N, et al. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- 73.Olivares-Reyes JA, Shah BH, Hernandez-Aranda J, Garcia-Gaballero A, Farshori MP, Gatcia-Sainz JA, Catt KJ. Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors. Mol Pharmacol. 2005;68:356–364. doi: 10.1124/mol.104.010637. [DOI] [PubMed] [Google Scholar]
- 74.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Villalba P, Denkers ND, Wittwer CT, Wittwer CT, Hoff C, Nelson RD, Mauch TJ. Real-time PCR quantification of AT1 and AT2 angiotensin receptor mRNA expression in the developing rat kidney. Nephron. Exp Nephrol. 2003;94:e154–e159. doi: 10.1159/000072499. [DOI] [PubMed] [Google Scholar]
- 77.Yosypiv IV. A new role for the renin-angiotensin system in ureteric bud branching. Organogenesis. 2004;1:26–32. doi: 10.4161/org.1.1.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]