Abstract
Cervical cancer, a potentially preventable disease, remains the second most common malignancy in women worldwide. Human papillomavirus (HPV) is the single most important etiological agent in cervical cancer, contributing to neoplastic progression through the action of viral oncoproteins, mainly E6 and E7. Cervical screening programs using Pap smear testing have dramatically improved cervical cancer incidence and reduced deaths, but cervical cancer still remains a global health burden. The biomarker discovery for accurate detection and diagnosis of cervical carcinoma and its malignant precursors (collectively referred to as high-grade cervical disease) represents one of the current challenges in clinical medicine and cytopathology.
Keywords: Cervical cancer, Human papillomavirus (HPV), biomarker
Introduction
Worldwide, cervical cancer is the second most common cancer in women; and is estimated to cause over 470,000 new cases and 233,000 deaths each year. Based on strong epidemiological evidence, supported by basic experimental findings, there is no doubt that persistent infections with high-risk types of human papillomavirus (HPV) represent a necessary cause of cervical cancer (Walboomers et al. 1999). HPVs infect epithelial cells and cause a variety of lesions ranging from common warts to cervical neoplasia and cancer. Over 100 different HPV types have been identified so far, with a subset of these being classified as high risk. High-risk HPV DNA is found in almost all cervical cancers (>99.7%), with HPV16 being the most prevalent type in both low-grade disease and cervical neoplasia. Productive infection by high-risk HPV types is manifest as cervical flat warts or condyloma that shed infectious virions from their surface. Viral genomes are maintained as episomes in the basal layer, with viral gene expression being tightly controlled as the infected cells move towards the epithelial surface. The pattern of viral gene expression in low-grade cervical lesions resembles that seen in productive warts caused by other HPV types. High-grade neoplasia represents an abortive infection in which viral gene expression becomes deregulated, and the normal life cycle of the virus cannot be completed. Most cervical cancers arise within the cervical transformation zone at the squamous/columnar junction, and it has been suggested that this is a site where productive infection may be inefficiently supported (Doorbar, 2006).
Although HPV infection is widespread, few people even know they are infected as the symptoms are seldom noticeable. It is even less well known is that nearly all cervical cancers (99.7%) are directly linked to previous infection with one or more of the oncogenic types of HPV (Walboomers et al. 1999). It is estimated that for every 1 million women infected, a hundred thousand (about 10%) will develop precancerous changes in their cervical tissue. Of these, about 8% of them will develop early carcinoma limited to cervical epithelium (carcinoma in situ; CIS) and a few of them will develop invasive cancer unless the precancerous lesions are detected and treated with such cases having been found to carry the oncogenic HPVs (e.g. types 16 and 18) that cause cervical cancer.
The HPV genome consists of 8 kb, and is a double-stranded DNA molecule. The relative arrangement of the 8–10 open reading frames (ORFs) within the genome is the same in all papillomavirus types, and a particular characteristic of papilloma viruses is that the partly overlapping ORFs are arranged on only one DNA strand. The genome can be divided into three regions: the long control region (LCR) without coding potential; the region of early proteins (E1–E8); and the region of late proteins (L1 and L2) (Walter and Philip, 2004). E6 and E7 are the most important oncogenic proteins. These proteins have pleiotropic functions, such as transmembrane signaling, regulation of the cell cycle, transformation of established cell lines, immortalization of primary cell line and regulation of chromosomal stability. Both E6 and E7 proteins can bind to multiple cellular targets. The interactions that are thought to be most relevant for their transforming functions are E6 binding, via the cellular protein E6-AP, to the tumor suppressor gene product p53, and E7 binding to the retinoblastoma tumor suppressor gene product pRb and its related pocket proteins, p107 and p130 (Dyson et al. 1989; Davies, 1993). The first interaction results in rapid ubiquitin-dependent proteolytic degradation of p53, which prevents cells from undergoing p53-mediated apoptosis (Thomas, 1999). A consequence of E7-pRb interaction is interfering with cell cycle control. In combination, the E6-p53 and E7-pRb interactions seem to compromise the accuracy of mitosis. In addition, HPV E6 can activate the telomere lengthening enzyme telomerase independent of p53 binding, and E7 can induce abnormal centrosome duplication through a mechanism independent of inactivation of pRb and its family member. It is likely that these latter properties also contribute to the transforming characteristic of these viral oncoproteins.
HPV infection causes changes in expression of host cervical cell cycle regulatory proteins. Such differentially expressed host proteins and nucleic acids may have a role as ‘biomarker’ of dysplastic cells. Investigation of potential biomarkers may also help to unravel new pathways involved in the HPV-mediated pathogenesis of cervical dyskaryosis.
Cervical Cancer Screening
For more than 50 years the Pap smear has been the mainstay of cervical screening resulting in a dramatic decrease in death from cervical cancer. However, the Pap smear has certain disadvantages (Table 1). It has a low sensitivity and high false negative rate. The data reveals that some of the false negative Pap smears rarely contain any abnormal cells on the slide (DeMay, 1996; Spitzer, 2002). So far, an effort to seek an explanation for this matter has been focused on either the incomplete transfer of cells from collection devices to the slide or inadequate sampling. This results in the development of liquid-based cytology technique (DeMay, 1996; McGoogan et al. 1998). Additionally, one of the emerging explanations is the lack of exfoliation of dysplastic cell (shedder and nonshedder hypothesis) (Felix et al. 2002; Felix, 2003). Data from some studies have been shown to the effect that there is an abnormal expression of the adhesion molecules in a subset of dysplastic lesions of the cervix (et al. 2002; Felix, 2003). It can prevent detection by any test requiring exfoliated abnormal cell, including liquid-based technique. Despite the nonshedding behavior, those lesions can be identified by visual test (Felix, 2003). There have been a number of visual tests which investigated for primary screening or used as adjunctive test of cytology method. These tests include cervicography, visual inspection with acetic acid (VIA), speculoscopy. At the present time, cervicography has a limited role as a primary screening or an adjunct to Pap smear (Schneider et al. 1999; Autier et al. 1999; Costa et al. 2000). However, as a triaging strategy for patients with ASCUS Pap smear, it is still a promising technique (Ferris et al. 2001; Brotzman, 2002). Direct inspection is the other method based on applying acetic acid to the cervix and then visualizing it. It can be done under incandescent light with or without magnification or the chemiluminescent light (Wright et al. 2002; Parham, 2003). This chemiluminescent light is of low intensity. It is diffuse and produces minimal reflective glare from normal tissue. There are studies showing that the use of chemiluminescent light allows the examiner to identify acetowhitening better than the incandescent light does (Lonky and Edwards, 1992; Mann et al. 1993; Lonky, 2002; Parham, 2003). Speculoscopy is developed for cervical screening by using chemiluminescence and low-power magnification to examine the cervix after applying an acetic acid. It can detect acetowhite dysplastic lesions and has been reported to be effective in detecting cervical intraepithelial lesions when combined with the Pap smear (Lonky, 2002; Wright et al. 2002; Parham, 2003).
Table 1.
The single Pap smear test has limited sensitivity and specificity.
| Limitations of Pap smear screening |
|---|
|
Presently, new technologies such as liquid-based cytology, HPV DNA test have been introduced. This test is used to detect the HPVs, which is considered the primary cause of virtually all cervical cancers. There are at least 30 different types of HPV strains that target the genital area, and are transmitted through sexual, skin-to-skin contact. Of these, approximately 13 are considered to be ‘high risk’ because they can trigger the development of abnormal cells associated with cervical cancer. The remaining ‘low-risk’ types can cause genital warts. Although the Pap smear can pick up the cellular changes caused by high-risk types of HPV, it’s not as sensitive as the HPV test, which specifically detects the viral DNA. The HPV test is not yet routinely used by the majority of doctors, in part because it is more expensive than a regular Pap test. Therefore, it would be important to improve the cost-effectiveness of screening and reduce the psychologic burden of benign positive test results.
Molecular Biomarkers in Cervical Cancer
HPV E6
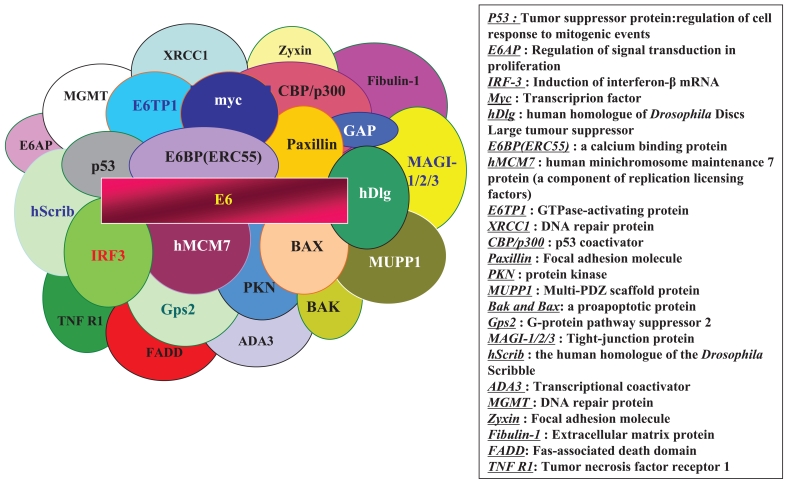
The E6 oncoproteins of high risk HPV interfere with the function of the cellular tumor suppressor protein p53 through the induction of increased proteasome-dependent p53 degradation. High risk HPV E6 proteins target the cellular E3 ubiquitin ligase E6-AP to p53, resulting in the transfer of ubiquitin peptides from E6-AP to p53, which marks p53 for degradation by the 26S proteasome. Low risk and cutaneous epithelia-infecting HPV E6 proteins are unable to target the cellular p53 protein for degradation through the proteasome pathway. Although E6-induced loss of p53 is an important element of E6-induced cellular transformation, recent studies have identified a number of additional cellular targets of E6 that may also play an important role. These included the following (Filippova et al. 2004; Yim et al. 2004): proteins involved in the regulation of transcription and DNA replication, such as p300/CBP (Huang and McCance, 2002), Gps2 (Degenhardt and Silverstein, 2001), IRF-3 (Ronco et al. 1998), hMcm7 (Kukimoto et al. 1998), E6TP1 (Gao et al. 1999) and ADA3 (Kumar et al. 1999); proteins, involved in apoptosis and immune evasion, such as Bak (Thomas and Banks, 1998), Bax (Bemard et al. 2003), TNF receptor 1 (TNF R1), FADD (Filippova et al. 2002) and c-Myc (Chen and Defendi, 1992); proteins involved with epithelial organization and differentiation, such as paxillin (Tong and Howley, 1997), E6BP/ERC-55 (Chen et al. 1995), zyxin (Degenhardt and Silverstein, 2001) and fibulin-1 (Du et al. 2002); proteins involved in cell-cell adhesion, polarity and proliferation control, which contain a PDZ-binding motif, such as hDLG (Kiyono et al. 1997), hScrib (Nakagawa and Huibregtse, 2000), PKN (Gao et al. 2000), MAGI-1 (Glaunsinger et al. 2000), MAGI-2, MAGI-3 (Thomas et al. 2002) or MUPP1 (Lee et al. 2000); and proteins involved in DNA repair, such as XRCC1 (Iftner et al. 2002) and 6-O-methylguanine-DNA methyltransferase (MGMT) (Strivenugopal and Ali-Osman, 2002) (Figure 1).
Figure 1.
Cellular binding partners for HPV E6.
HPV E7
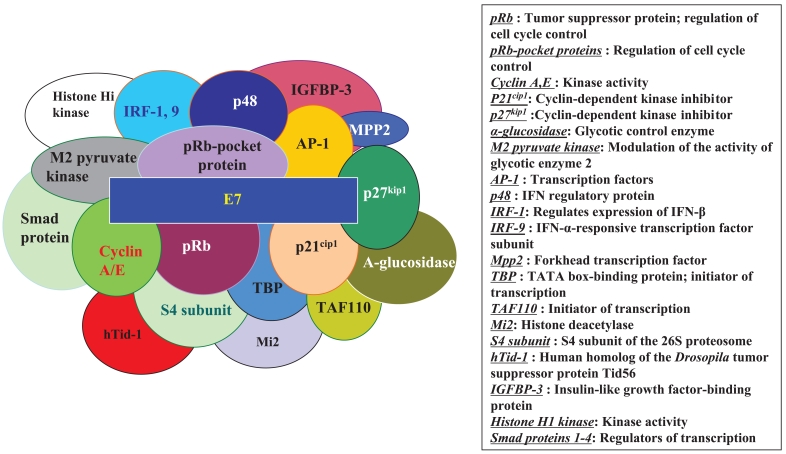
HPV E7 proteins interact with the so-called ‘pRb-associated pocket proteins,’ including the retinoblastoma protein pRb, which are negative cell cycle regulators involved in the G1/S and G2/M transitions. The interaction between high-risk E7 and pRb results in enhanced phosphorylation and degradation. pRb destruction leads to the release of E2F family of transcription factors and the subsequent activation of genes promoting cell proliferation. However, the stimulatory effect of E7 upon cell proliferation not only depends on its association with pRb, since E7 targets the function of a plethora of cell cycle regulators, including cyclin A (Dyson et al. 1992), E (McIntyre et al. 1996) and cyclin-dependent kinase inhibitor p21Cip1 (Jones et al. 1997) and p27kip1 (Zerfass-Thome et al. 1996) together with the metabolic regulators, acid-α-glucosidase (Zwerschke et al. 2000) and M2 pyruvate kinase (Zwerschke et al. 1999). HPV E7 also interferes with the activity of a variety of cellular transcription factors, such as AP-1 (Antinore et al. 1996), p48 (Bamard and McMillan, 1999), interferon regulatory factor-1 (IRF-1) (Part et al. 2000), forkhead transcription factor MPP2 (Luscher-Firzlaff et al. 1999), TATA- box binding protein (TBP) and TATA-box binding protein- associated factor (TAF110) (Mazzarelli et al. 1995), as well as with the Mi2 histone deacetylase (Brehm et al. 1999). Also, E7 interacts with the S4 subunit of the 26S proteasome (Duensing and Munger, 2003), a human homolog of the Drosophila tumor suppressor protein Tid56 (hTid-1) (Schilling et al. 1998), interferon regulatory factor-9 (IRF-9) (Antonsson et al. 2006), Smad protein (Habig et al. 2006), insulin-like growth factor binding protein (IGFBP-3) (Mannhardt et al. 2000) and histone H1 kinase (Davies et al. 1993) (Figure 2).
Figure 2.
Cellular binding partners for HPV E7.
Mini chromosome maintenance (MCM)
DNA replication occurs only once in a single normal cell cycle, due to a mechanism known as ‘licensing’ of DNA replication. This process requires the assembly of a protein complex which includes the mini chromosome maintenance (MCM) proteins and the cell division cycle protein 6 (CDC6) (Cook et al. 2002; Shin et al. 2003). Disassembly of this complex prevents repetitive replication during the same cell cycle (Lei and Tye, 2001). Changes in the expression pattern of DNA ‘licensing’ proteins are frequently observed in dysplastic cells. In comparison with be present only during the cell cycle in normal cells, MCM proteins and CDC6 have been demonstrated to be overexpressed in dysplastic cells.
In normal cervical epithelium, MCM protein staining is limited to the basal proliferating layer and is absent in differentiated and quiescent cells. In cervical glandular and squamous dysplasia, however, MCM expression is dramatically increased, suggesting its potential as a biomarker of cervical dysplasia (Ohta et al. 2001; Stoeber et al. 2002; Going et al. 2002; Alison et al. 2002; Davies et al. 2002; Davidson et al. 2003). MCM5 has been the focus of much of this research, but MCM7 is also a highly informative marker of cervical cancer. The number of nuclei positive for MCM5 at the surface of dysplastic epithelium correlates with the severity of dysplasia (Williams et al. 1998; Freeman et al. 1999) (Table 2).
Table 2.
MCM5 and HPV oncoprotein expression.
| MCM5 |
|---|
|
Cell division cycle protein 6 (CDC6)
Both MCM5 and CDC6 play essential roles in the regulation of eukaryotic DNA replication. CDC6 was first identified in 1998 as a marker of cervical dysplastic cells in cervical biopsies and in smears using polyclonal antibodies. Not only MCM5 but also CDC6 protein expression are present in proliferating cells and absent in differentiated or quiescent cells. In normal cervical epithelium, CDC6 staining is absent or limited to the basal proliferative layer. However, CDC6 protein expression is dramatically up-regulated in squamous and glandular cervical carcinomas. Several studies have illustrated a linear increase in CDC6 expression observed in normal cervix, preinvasive neoplasia and invasive cervical carcinoma. CDC6 was preferentially expressed in areas exhibiting histological HPV changes. Interestingly, the expression pattern of CDC6 closely mirrors that of the high-risk HPV E6 oncoprotein, which is mainly expressed in higher grade lesions and invasive carcinomas (Table 3).
Table 3.
CDC6 and HPV oncoprotein expression.
| CDC6 |
|---|
|
p16INK4A
p16INK4A is a tumor supressor gene and a key regulator of the cell cycle. The expression pattern of p16INK4A in dysplastic squamous and glandular cervical cells in tissue sections and in cervical smears has been extensively investigated (Sano et al. 1998; Klaes et al. 2001; Bibbo et al. 2002). In all normal cervical tissues examined, no p16INK4A staining is evident. Additionally, all normal regions adjacent to cervical intraepithelial neoplasia (CIN) lesions do not show any detectable expression of p16INK4A. While p16INK4A identified dysplastic squamous and glandular lesions with a sensitivity rate of 99.9% and a specificity rate of 100% in cervical biopsy sections, only a few studies have examined the possible prognostic value of p16INK4A in cervical lesions (Murphy et al. 2003). It is now widely accepted that p16INK4A is a sensitive and specific marker of squamous and glandular dysplastic cells of the cervix and also a surrogate marker of high risk human papillomavirus, suggesting a valuable adjunctive test in cervical cancer screening (Table 4).
Table 4.
p16INK4A and HPV oncoprotein expression.
| p16INK4A |
|---|
|
Squamous cell carcinoma antigen (SCC)
SCC belongs to the family of serine and cysteine protease inhibitors (Suminami et al. 1991). This antigen is present in normal cervix epithelium with an increased expression in proportion to dyspalstic lesion and cervical squamous cell carcinoma. Though SCC is not sufficient for use in screening, pretreatment serum SCC values works as an independent prognostic factor. Approximately 60% of patients with cervical cancer are detected with elevated levels of serum SCC at initial diagnosis, when all stages are included (Farghaly, 1992). Besides, serum SSC -> SCC levels correlate significantly with tumor stage (Crombach et al. 1989; Duk et al. 1990). More specifically -> If split with stage, serum SCC is elevated in 24–53% of patients with Stage IB or IIA squamous cell cervical cancer, and in 75–90% of patients with advanced stage (FIGO IIB and higher) disease (Gaarenstroom et al. 1995; Duk et al. 1996). Several studies have concluded that serum SCC is useful in monitoring the course of squamous cell cervical cancer following primary therapy (Bolli et al. 1994; Bonfrer et al. 1997). Persistently elevated and/or increasing serum SCC levels after and/or during treatment suggest tumor persistence or progressive disease (Brioschi et al. 1991). Patients with plateau SCC level revealed higher incidence of treatment failure after radiotherapy, indicating SCC levels provide useful information for the need of further work-up and management (Hong et al. 1998). In view of a strong correlation with the clinical course, SCC is suitable for monitoring the early detection of recurrent or progressive disease after primary treatment, and may therefore be useful in the management of patients. However, there is as yet no evidence that earlier detection of recurrent disease influences treatment outcome (Table 5).
Table 5.
Currently available and potentially useful serum marker squamous cell carcinoma (SCC).
| Squamous cell carcinoma (SCC) |
|---|
|
Cell proliferation markers
The rate of cell proliferation in a tumor is generally thought to be of prognostic importance, and until recently the only means available to the pathologist to assess this was to count the number of mitotic figures, a technique fraught with difficulties and pitfalls. A number of antigens have now been described, which are expressed specifically by proliferating cells and which, with the use of monoclonal antibodies, can be demonstrated immunocytochemically: demonstration of these antigens affords, in theory at least, a much more accurate estimate of the number of proliferating cells than does a mitotic count. The two proliferation antigens which have been most widely studied are proliferating cell nuclear antigen (PCNA), which is expressed during the G1 and early S phases of the proliferative cycle, and Ki-67, which is expressed during the G2 and mitotic phases of the cycle. Ki-67 is the more reliable indicator of the growth fraction of a tumor, largely because PCNA has a long half-life and may still be demonstrable in post-mitotic cells (Scott et al. 1991). The study of Ki-67 was originally, however, limited by the necessity to use fresh or snap frozen tissue (Hall and Levison, 1990), but the recently introduced antibody MIB-1 can be used to detect the antigen in fixed paraffin-embedded tissue (McCormick et al. 1993). The number of cell nuclei staining positively for these markers of proliferation can be estimated by simple counting or can be measured in an image analysis system. In cervical intraepithelial neoplasia both PCNA and Ki-67 expressions are, as compared to normal cervices, increased in the upper levels of the cervical epithelium (Konishi et al. 1991; Shurbaji et al. 1993; Mittal et al. 1993; Raju, 1994; McLuggage et al. 1996), and it is thought that this staining pattern, particularly that for Ki-67, may be of considerable value in distinguishing CIN from non-neoplastic lesions that may mimic CIN. Two studies of PCNA expression in cervical carcinoma have yielded conflicting results, one finding the PCNA index to be of considerable import (Oka et al. 1992) and another being unable to show that this index is of any prognostic value (Al-Nafussi et al. 1993). Investigations of Ki-67 expression in cervical carcinoma have generally failed to show any relationship between the number of positively stained cells and prognosis (Cole et al. 1992; Levine et al. 1995; Oka and Arai, 1996), though in one study the Ki-67 index was significantly related to tumor size, lymphatic spread, and disease-free interval in patients with stage I disease (Garzetti et al. 1995). In endometrial adenocarcinomas the PCNA index has been found to correlate with tumor grade, depth of myometrial invasion, and recurrence risk (Garzetti et al. 1996a), and it has been suggested that PCNA staining can be used as a method of pre-operative identification of high risk patients (Garzetti et al. 1996b). Ki-67expression in endometrial carcinomas was found to be correlated with grade but not with stage or depth of myometrial invasion in one study (Nielsen et al. 1994), but it emerged as a highly significant indicator of tumor recurrence in another (Geislet et al. 1996) (Table 6). By contrast, others have found neither staining for PCNA nor Ki-67 to be of any prognostic value in endometrial neoplasm (Hamel et al. 1996; Nordstrom et al. 1996).
Table 6.
Cell proliferation markers PCNA and Ki-67.
| Type | Limitation | |
|---|---|---|
| PCNA | Proliferation marker | Multiple factors affect staining intensity |
| Ki-67 | Proliferation marker | Multiple factors affect expression levels |
Although few new markers have reached the clinic in recent years, several reported cancer biomarkers have been found to have low sensitivity in that they are found only in a small subset of patients with a particular type of cancer.
Needs of Biomarker Discovery
The future of clinical cancer management belongs to the prognostic and predictive biomarkers of cancer. These markers are of utmost importance as they will be the used to make clinical decisions that will eventually save lives. In the future, biomarkers will guide decision making during cancer management. Biomarkers that correctly predict outcome in a specific disease and allow physicians and patients to make informed treatment decisions need to be developed. Biomarkers will not only help screen, detect, diagnose, help in prognostic evaluation, monitor treatment and predict recurrence, but also play a major role in clinical decision making.
New Biomarker Development
Concern remains as to whether the tools available are well suited to provide the technological support to meet the demands of new biomarker development. Until recently, the discovery of cancer biomarkers has been a slow approach to identify proteins that are dysregulated as a consequence of the disease and shed into the body fluids such as serum, urine or saliva. Unfortunately, this approach is arduous and prolonged as each candidate markers must be identified among thousands of proteins. The recent advancements in genomic and proteomic technologies including gene array technology, serial analysis of gene expression (SAGE) improved 2-DE and new mass spectrometric techniques coupled with advancements in bioinformatic tools, shows great promise of meeting the demand for the discovery of a variety of new biomarkers that are both sensitive and specific (Chatterjee and Zetter, 2005). Like these, high-throughput approaches are useful in cancer biomarker discovery and clinical diagnostics. The combined use of proteomics, genomics and bioinformatics tools may hold promise for early detection of disease by proteomic patterns, diagnosis based on proteomic signatures as a complement to histopathology, individualized selection of therapeutic combinations that best target the entire disease-specific protein network, rational modulation of therapy based on changes in the diseased protein network associated with drug resistance and understanding of carcinogenesis.
Acknowlegement
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A06-0079-AA1018-06N1-00010A).
References
- Alison MR, Hunt T, Forbes SJ. Minichromosome maintenance (MCM) proteins may be pre-cancer markers. Gut. 2002;50:290–1. doi: 10.1136/gut.50.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nafussi AI, Klys HS, Rebello G, et al. The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in the uterine cervix and cervical squamous neoplasia. Int J Gynecol Cancer. 1993;3:154–8. doi: 10.1046/j.1525-1438.1993.03030154.x. [DOI] [PubMed] [Google Scholar]
- Antinore MJ, Birrer MJ, Patel D, et al. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 1996;15:1950–60. [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Payne E, Hengst K, et al. The Human Papillomavirus Type 16 E7 Protein Binds Human Interferon Regulatory Factor-9 via a Novel PEST Domain Required for Transformation. J Interferon Cytokine Res. 2006;26:455–61. doi: 10.1089/jir.2006.26.455. [DOI] [PubMed] [Google Scholar]
- Autier P, Coibion M, De Sutter P, et al. Cytology alone versus cytology and cervicography for cervical cancer screening: a randomized study. Obstet Gynecol. 1999;93:353–8. doi: 10.1016/s0029-7844(98)00472-4. [DOI] [PubMed] [Google Scholar]
- Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999;259:305–13. doi: 10.1006/viro.1999.9771. [DOI] [PubMed] [Google Scholar]
- Bemard B, Pretet JL, Charlot JF, et al. Human papillomaviruses type 16+ and 18+ cervical carcinoma cells are sensitive to staurosporine-mediated apoptosis. Biol Cell. 2003;95:17–26. doi: 10.1016/s0248-4900(02)01220-0. [DOI] [PubMed] [Google Scholar]
- Bibbo M, Klump WJ, DeCecco J, et al. Procedure for immunocytochemical detection of P16INK4 antigen in thin-layer, liquid-based specimens. Acta Cytologica. 2002;46:25–9. doi: 10.1159/000326711. [DOI] [PubMed] [Google Scholar]
- Bolli JN, Doering DL, Bosscher JR, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–73. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- Brehm A, Nielsen SJ, Miska EA, et al. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 1999;18:2449–58. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioschi PA, Bischof P, Delafosse C, et al. Squamous cell carcinoma antigen (SCC-A) values related to clinical outcome of pre-invasive and invasive cervical carcinoma. Int J Cancer. 1991;47:376–9. doi: 10.1002/ijc.2910470311. [DOI] [PubMed] [Google Scholar]
- Brotzman GL, Spitzer M. Adjunctive testing: cervicography. In: Apgar BS, Brotzman GL, Spitzer M, editors. Colposcopy: Principles and practice. Philadelphia: Saunders; 2002. pp. 73–84. [Google Scholar]
- Chatterjee SK, Zetter BR. Cancer biomarkers: knowing the present and predicting the future. Future Oncol. 2005;1:37–50. doi: 10.1517/14796694.1.1.37. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Reid CE, Band V, et al. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–31. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- Chen TM, Defendi V. Functional interaction of p53 with HPV18 E6, c-myc and H-ras in 3T3 cells. Oncogene. 1992;7:1541–7. [PubMed] [Google Scholar]
- Cole DJ, Brown DC, Crossley F, et al. Carcinoma of the cervix uteri: An assessment of the relationship of tumor proliferation to prognosis. Br J Cancer. 1992;65:783–5. doi: 10.1038/bjc.1992.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JG, Park CH, Burke TW, et al. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc Natl Acad Sci USA. 2002;99:1347–52. doi: 10.1073/pnas.032677499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S, Sideri M, Syrjanen K, et al. Combined Pap smear, cervicography and HPV DNA testing in the detection of cervical intraepithelial neoplasia and cancer. Acta Cytol. 2000;44:310–8. doi: 10.1159/000328471. [DOI] [PubMed] [Google Scholar]
- Crombach G, Würz H, Herrmann F, et al. Bedeutung des SCC-antigens in der diagnostik und verlaufskontrolle des zervixkarzinoms. Dtsch Med Wochenschr. 1989;114:700–5. doi: 10.1055/s-2008-1066658. [DOI] [PubMed] [Google Scholar]
- Davidson EJ, Morris LS, Scott IS, et al. Minichromosome maintenance (Mcm) proteins, cyclin B1 and D1, phosphohistone H3 and in situ DNA replication for functional analysis of vulval intraepithelial neoplasia. Br J Cancer. 2003;88:257–62. doi: 10.1038/sj.bjc.6600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RJ, Freeman A, Morris LS, et al. Analysis of minichromosome maintenance proteins as a novel method for detection of colorectal cancer in stool. Lancet. 2002;369:1917–9. doi: 10.1016/S0140-6736(02)08739-1. [DOI] [PubMed] [Google Scholar]
- Davies R, Hicks R, Crook T, et al. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J Virol. 1993;67:2521–8. doi: 10.1128/jvi.67.5.2521-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt YY, Silverstein SJ. Gps2, a protein partner for human papillomavirus E6 proteins. J Virol. 2001;75:151–60. doi: 10.1128/JVI.75.1.151-160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt YY, Silverstein SJ. Interaction of zyxin, a focal adhesion protein, with the e6 protein from human papillomavirus type 6 results in its nuclear translocation. J Virol. 2001;75:11791–802. doi: 10.1128/JVI.75.23.11791-11802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMay RM. Cytopathology of false negativespreceding cervical carcinoma. Am J Obstet Gynecol. 1996;175:1110–3. doi: 10.1016/s0002-9378(96)70013-3. [DOI] [PubMed] [Google Scholar]
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–41. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- Du M, Fan X, Hong E, et al. Interaction of oncogenic papillomavirus E6 proteins with fibulin-1. Biochem Biophys Res Commun. 2002;296:962–9. doi: 10.1016/s0006-291x(02)02041-7. [DOI] [PubMed] [Google Scholar]
- Duensing S, Munger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J Virol. 2003;77:12331–5. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duk JM, De Bruijn HWA, Groenier KH, et al. Cancer of the uterine cervix: sensitivity and specificity of serum squamous cell carcinoma antigen determinations. Gynecol Oncol. 1990;39:186–94. doi: 10.1016/0090-8258(90)90430-s. [DOI] [PubMed] [Google Scholar]
- Duk JM, Groenier KH, De Bruijn HWA, et al. Pretreatment serum squamous cell carcinoma antigen: a newly identified prognostic factor in early-stage cervical carcinoma. J Clin Oncol. 1996;14:111–8. doi: 10.1200/JCO.1996.14.1.111. [DOI] [PubMed] [Google Scholar]
- Dyson N, Guida P, Münger K, et al. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N, Howley PM, Münger K, et al. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–40. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Farghaly SA. Tumor markers in gynecologic cancer. Gynecol Obstet Invest. 1992;34:65–72. doi: 10.1159/000292728. [DOI] [PubMed] [Google Scholar]
- Felix JC. The science behind the effectiveness of in vivo screening. Am J Obstet Gynecol. 2003;188:8–12. doi: 10.1067/mob.2003.235. [DOI] [PubMed] [Google Scholar]
- Felix JC, Lonky NM, Tamura K, et al. Aberrant expression of E-cadherin in cervical intraepithelial neoplasia correlates with a falsenegative Papanicolaou smear. Am J Obstet Gynecol. 2002;186:1308–14. doi: 10.1067/mob.2002.123732. [DOI] [PubMed] [Google Scholar]
- Ferris DG, Schiffman M, Litaker MS. Cervicography for triage of women with mildly abnormal cervical cytology results. Am J Obstet Gynecol. 2001;185:939–43. doi: 10.1067/mob.2001.117485. [DOI] [PubMed] [Google Scholar]
- Filippova M, Parkhurst L, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. J Biol Chem. 2004;279:25729–44. doi: 10.1074/jbc.M401172200. [DOI] [PubMed] [Google Scholar]
- Filippova M, Song H, Connolly JL, et al. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J Biol Chem. 2002;277:21730–9. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- Freeman A, Morris LS, Mills AD, et al. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res. 1999;5:121–32. [PubMed] [Google Scholar]
- Gaarenstroom KN, Bonfrer JMG, Kenter GG, et al. Clinical value of pre-treatment serum Cyfra 21–1, tissue polypeptide antigen, and squamous cell carcinoma antigen levels in patients with cervical cancer. Cancer. 1995;76:807–13. doi: 10.1002/1097-0142(19950901)76:5<807::aid-cncr2820760515>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gao Q, Kumar A, Srinivasan S, et al. PKN binds and phosphorylates human papillomavirus E6 oncoprotein. J Biol Chem. 2000;275:14824–30. doi: 10.1074/jbc.275.20.14824. [DOI] [PubMed] [Google Scholar]
- Gao Q, Srinivasan S, Boyer SN, et al. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–44. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzetti GG, Ciavittini A, Goteri G, et al. Proliferating cell nuclear antigen (PCNA) immunoreactivity in stage I endometrial cancer: A new prognostic factor. Int J Gynecol Cancer. 1996a;6:186–92. [Google Scholar]
- Garzetti GG, Ciavittini A, Goteri G, et al. Proliferating cell nuclear antigen in endometrial adenocarcinoma: Pre-treatment identification of high risk patients. Gynecol Oncol. 1996b;61:16–21. doi: 10.1006/gyno.1996.0089. [DOI] [PubMed] [Google Scholar]
- Garzetti GG, Ciavittini A, Lucarini G, et al. MIB1 immunostaining in stage 1 squamous cervical carcinoma: relationship with natural killer cell activity. Gynecol Oncol. 1995;58:23–33. doi: 10.1006/gyno.1995.1179. [DOI] [PubMed] [Google Scholar]
- Geisler JP, Wiemann MC, Zhou Z, et al. Proliferation index determined by MIB-1 and recurrence of endometrial carcinoma. Gynecol Oncol. 1996;61:373–7. doi: 10.1006/gyno.1996.0159. [DOI] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, et al. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19:5270–80. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Going JJ, Keith WN, Neilson L, et al. Aberrant expression of minichromosome maintenance proteins 2 and 5, and Ki-67 in dysplastic squamous oesophageal epithelium and Barrett’s mucosa. Gut. 2002;50:373–7. doi: 10.1136/gut.50.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Aebersold R. Mass spectrometry and proteomics. Curr Opin Chem Biol. 2000;4:489–94. doi: 10.1016/s1367-5931(00)00121-6. [DOI] [PubMed] [Google Scholar]
- Habig M, Smola H, Dole VS, et al. E7 proteins from high- and low-risk human papillomaviruses bind to TGF-beta-regulated Smad proteins and inhibit their transcriptional activity. Arch Virol. 2006;19 doi: 10.1007/s00705-006-0768-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hall PA, LEvison DA. Assessment of cellular proliferation in histological material. J Clin Pathol. 1990;43:184–92. doi: 10.1136/jcp.43.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel NW, Sebo TJ, Wilson TO, et al. Prognostic value of p53 and proliferating cell nuclear antigen expression in endometrial carcinoma. Gynecol Oncol. 1996;62:192–8. doi: 10.1006/gyno.1996.0214. [DOI] [PubMed] [Google Scholar]
- Hong JH, Tsai CS, Chang JT, et al. The prognostic significance of pre-and post-treatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:823–30. doi: 10.1016/s0360-3016(98)00147-3. [DOI] [PubMed] [Google Scholar]
- Huang SM, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J Virol. 2002;76:8710–21. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftner T, Elbel M, Schopp B, et al. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. EMBO J. 2002;21:4741–8. doi: 10.1093/emboj/cdf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Alani RM, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11:2101–11. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Hiraiwa A, Fujita M, et al. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–6. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- Konishi I, Jujii S, Nonogaki H, et al. Immunohistochemical analysis of estrogen receptors, progesterone receptors, Ki-67 antigen, and human papillomavirus DNA in normal and neoplastic epithelium of the uterine cervix. Cancer. 1991;68:1340–50. doi: 10.1002/1097-0142(19910915)68:6<1340::aid-cncr2820680626>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kukimoto I, Aihara S, Yoshiike K, et al. Human papillomavirus oncoprotein E6 binds to the C-terminal region of human minichromosome maintenance 7 protein. Biochem Biophys Res Commun. 1998;249:258–62. doi: 10.1006/bbrc.1998.9066. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhao Y, Meng G, et al. Human papillomavirus oncoprotein E6 inactivates the transcriptional coactivator human ADA3. Mol Cell Biol. 2002;22:5801–12. doi: 10.1128/MCB.22.16.5801-5812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Tye BK. Initiating DNA synthesis: from recruiting to activating the MCM complex. J Cell Sci. 2001;114:1447–54. doi: 10.1242/jcs.114.8.1447. [DOI] [PubMed] [Google Scholar]
- Lee SS, Glaunsinger B, Mantovani F, et al. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74:9680–93. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine EL, Renehan A, Gossiel R, et al. Apoptosis, intrinsic radiosensitivity and prediction of radiotherapy response in cervical carcinoma. Radiother Oncol. 1995;37:1–9. doi: 10.1016/0167-8140(95)01622-n. [DOI] [PubMed] [Google Scholar]
- Lonky NM, Edwards G. Chemiluminescent light versus incandescent light in the visualization of acetowhite epithelium. Am J Gynecol Health. 1992;6:11–5. [Google Scholar]
- Lonky NM. Adjunctive testing: cervical screening with in vivo and in vitro modalities: speculoscopy combined with cytology. In: Apgar BS, Brotzman GL, Spitzer M, editors. Colposcopy: Principles and practice. Philadelphia: Saunders; 2002. pp. 98–106. [Google Scholar]
- Luscher-Firzlaff JM, Westendorf JM, Zwicker J, et al. Interaction of the fork head domain transcription factor MPP2 with the human papilloma virus 16 E7 protein: enhancement of transformation and transactivation. Oncogene. 1999;18:5620–30. doi: 10.1038/sj.onc.1202967. [DOI] [PubMed] [Google Scholar]
- Mann W, Lonky N, Massad S, et al. Papanicolaou smear screening augmented by a magnified chemiluminescent exam. Int J Gynecol Obstet. 1993;43:289–96. doi: 10.1016/0020-7292(93)90518-2. [DOI] [PubMed] [Google Scholar]
- Mannhardt B, Weinzimer SA, Wagner M, et al. Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol. 2000;20:6483–95. doi: 10.1128/mcb.20.17.6483-6495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarelli JM, Atkins GB, Geisberg JV, et al. The viral oncoproteins Ad5 E1A, HPV16 E7 and SV40 TAg bind a common region of the TBP-associated factor-110. Oncogene. 1995;11:1859–64. [PubMed] [Google Scholar]
- McCormick D, Chong H, Hobbs C, et al. Detection of the Ki67 antigen in fized and wax embedded sections with the monoclonal antibody MIB1. Histopathology. 1993;22:355–60. doi: 10.1111/j.1365-2559.1993.tb00135.x. [DOI] [PubMed] [Google Scholar]
- McGoogan E, Colgan TJ, Ramzy I, et al. Cell preparation methods and criteria for sample adequacy. IAC Task Force summary. Diagnostic cytology towards the 21st century: An international expert conference and tutorial. Acta Cytol. 1998;42:25–32. doi: 10.1159/000331532. [DOI] [PubMed] [Google Scholar]
- McIntyre MC, Ruesch MN, Laimins LA. Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology. 1996;215:73–82. doi: 10.1006/viro.1996.0008. [DOI] [PubMed] [Google Scholar]
- McLuggage WG, Buhidma M, Tang L, et al. Monoclonal antibody MIB1 in the assessment of cervical squamous intraepithelial lesions. Int J Gynecol Pathol. 1996;15:131–6. doi: 10.1097/00004347-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Mittal KR, Demopoulos RJ, Goswami S. Proliferating cell nuclear antigen (cyclin) expression in normal and abnormal squamous epithelia. Am J surg Pathol. 1993;17:117–22. doi: 10.1097/00000478-199302000-00003. [DOI] [PubMed] [Google Scholar]
- Murphy N, Ring M, Killalea AG, et al. p16INK4 as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56:56–63. doi: 10.1136/jcp.56.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–53. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm RS, Verma M, Srivastava S. The promise of biomarkers in cancer screening and detection. Trends Mol Med. 2002;8:288–93. doi: 10.1016/s1471-4914(02)02353-5. [DOI] [PubMed] [Google Scholar]
- Nielsen AL, Nyholm HCJ, Engel P. Expressionof MIB-1 (paraffin ki-67) and AgNOR morphology in endometrial adenocarcinomas of endometrioid type. Int J Gynecol Pathol. 1994;13:37–44. doi: 10.1097/00004347-199401000-00005. [DOI] [PubMed] [Google Scholar]
- Nordstrom B, Strang P, Bergstrom R, et al. A comparison of proliferation markers and their prognostic value for woman with endometrial carcinoma: Ki-67, proliferating cell nuclear antigen and flow cytometric S-phase factor. Cancer. 1996;78:1942–51. [PubMed] [Google Scholar]
- Ohta S, Koide M, Tokuyama T, et al. Cdc6 expression as a marker of proliferative activity in brain tumors. Oncol Rep. 2001;8:1063–6. doi: 10.3892/or.8.5.1063. [DOI] [PubMed] [Google Scholar]
- Oka K, Arai T. MIB1 growth fraction is not related to prognosis in cervical squamous cell carcinoma treated with radiotherapy. Int J Gynecol Pathol. 1996;15:23–7. doi: 10.1097/00004347-199601000-00004. [DOI] [PubMed] [Google Scholar]
- Oka K, Hoshi T, Arai T. Prognostic significance of the PC10 index as a prospective assay for cervical cancer treated with radiation therapy alone. Cancer. 1992;70:1545–50. doi: 10.1002/1097-0142(19920915)70:6<1545::aid-cncr2820700617>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Parham GP. Comparison of cell collection and direct visualization cervical cancer screening adjuncts. Am J Obstet Gynecol. 2003;188:S13–20. doi: 10.1067/mob.2003.234. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim EJ, Kwon HJ, et al. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275:6764–9. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- Raju GC. Expression of the proliferating cell nuclear antigen in cervical neoplasia. Int J Gynecol Pathol. 1994;13:337–41. doi: 10.1097/00004347-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Ronco LV, Karpova AY, Vidal M, et al. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–72. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Oyama T, Kashiwabara K, et al. Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. Pathol Int. 1998;48:580–5. doi: 10.1111/j.1440-1827.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Schilling B, De-Medina T, Syken J, et al. A novel human DnaJ protein, hTid-1, a homolog of the Drosophila tumor suppressor protein Tid56, can interact with the human papillomavirus type 16 E7 oncoprotein. Virology. 1998;247:74–85. doi: 10.1006/viro.1998.9220. [DOI] [PubMed] [Google Scholar]
- Schneider DL, Herrero R, Bratti C, et al. Cervicography screening for cervical cancer among 8,460 women in a high-risk population. Am J Obstet Gynecol. 1999;180:290–8. doi: 10.1016/s0002-9378(99)70202-4. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Hall PA, Haldane JS, et al. A comparison of immunohistochemical markers of cell proliferation with experimentally determined growth fraction. J Pathol. 1991;165:173–8. doi: 10.1002/path.1711650213. [DOI] [PubMed] [Google Scholar]
- Shin JH, Grabowski B, Kasiviswanathan R, et al. Regulation of minichromosome maintenance helicase activity by Cdc6. J Biol Chem. 2003;278:38059–67. doi: 10.1074/jbc.M305477200. [DOI] [PubMed] [Google Scholar]
- Shurbaji MS, Brooks SK, Thurmond TS. Proliferating cell nuclear antigen: Immunoreactivity in cervical intraepithelial neoplasia and benign cervical epithelium. Am J Clin Pathol. 1993;100:22–6. doi: 10.1093/ajcp/100.1.22. [DOI] [PubMed] [Google Scholar]
- Spitzer M. In vitro conventional cytology: Historical strengths and current limitations. Obstet Gynecol Clin North Am. 2002;29:673–83. doi: 10.1016/s0889-8545(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Srivenugopal KS, Ali-Osman F. The DNA repair protein, O(6)-methylguanine-DNA methyltransferase is a proteolytic target for the E6 human papillomavirus oncoprotein. Oncogene. 2002;21:5940–5. doi: 10.1038/sj.onc.1205762. [DOI] [PubMed] [Google Scholar]
- Stoeber K, Swinn R, Prevost AT, et al. Diagnosis of genitor-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst. 2002;94:1071–9. doi: 10.1093/jnci/94.14.1071. [DOI] [PubMed] [Google Scholar]
- Suminami Y, Kishi F, Sekiguchi K, et al. Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun. 1991;181:51–8. doi: 10.1016/s0006-291x(05)81380-4. [DOI] [PubMed] [Google Scholar]
- Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999;80:1513–7. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- Thomas M, Laura R, Hepner K, et al. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–96. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Thomas M, Pim D, Banks L. The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- Tong X, Howley PM. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc Natl Acad Sci USA. 1997;94:4412–7. doi: 10.1073/pnas.94.9.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wilkins MR, Sanchez JC, Gooley JC, et al. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Gene Eng Rev. 1995;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- Williams GH, Romanowski P, Morris L, et al. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci USA. 1998;95:14932–7. doi: 10.1073/pnas.95.25.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TC, Jr, Denny L, Kuhn L, et al. Use of visual screening methods for cervical cancer screening. Obstet Gynecol Clin N Am. 2002;29:701–34. doi: 10.1016/s0889-8545(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Yim EK, Meoyng J, Namakoong SE, et al. Genomic and proteomic expression patterns in HPV-16 E6 gene transfected stable human carcinoma cell lines. DNA Cell Biol. 2004;23:826–35. doi: 10.1089/dna.2004.23.826. [DOI] [PubMed] [Google Scholar]
- Zerfass-Thome K, Zwerschke W, Mannhardt B, et al. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13:2323–30. [PubMed] [Google Scholar]
- Zwerschke W, Mannhardt B, Massimi P, et al. Allosteric activation of acid alpha-glucosidase by the human papillomavirus E7 protein. J Biol Chem. 2000;275:9534–41. doi: 10.1074/jbc.275.13.9534. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Massimi P, et al. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1999;96:1291–6. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]