Abstract
Hepatitis due to hepatitis B virus reactivation after cytotoxic or immunosuppressive therapy is a serious cause of liver-related morbidity and mortality. With the characterization of the underlying pathogenesis, much progress in the management of this important clinical problem has been made in the past 2 decades. By year 2008, it is mandatory to screen for hepatitis B surface antigen status before initiating intensive chemotherapy or immunosuppressive therapy. All those who are hepatitis B surface antigen positive should be started on preemptive nucleos(t)ide analogues. However, there remains important issues, such as the type and duration of nucleos(t)ide analogue therapy, which need to be understood. As not all hepatitis B surface antigen-positive patients will suffer from HBV reactivation, it is therefore useful to identify risk factors related to HBV reactivation so that patients will not be treated unnecessarily with nucleos(t)ide analogues. To date, a high baseline level of viral replication, as reflected by high serum HBV DNA level, positive serum hepatitis B e antigen, and a high intrahepatic covalently closed circular DNA level, is the most important predictor for HBV reactivation. Recently, there has been an increased awareness of reactivation of occult hepatitis B virus, especially in hepatitis B virus endemic area, such as the Asia-Pacific region. Careful epidemiological study will be needed to clarify the impact of occult hepatitis B infection in patients treated with cytotoxic or immunosuppressive therapy.
Keywords: HBV infection, Reactivation, Chemotherapy, Preemptive lamivudine
How important is the problem?
Over the past 20 years of clinical practice at Queen Mary Hospital, we have seen a lot of progress in the management of hepatitis due to HBV reactivation in hepatitis B surface antigen carriers [1–5]. In the past, we saw patients who died of fulminant hepatic failure after being administered a few courses of cytotoxic therapy to treat their life-threatening lymphoma [6–9] and breast cancer [10–12] if they were also hepatitis B surface antigen positive. At that time, the literature report of this important clinical issue was scanty. The first report in the field was published by Wand et al. [13] who studied the effects of antitumor chemotherapeutic agents on hepatitis antigen (HBAg) and antibody (HBAb) in 25 patients with myeloproliferative and in 60 patients with lymphoproliferative disorders. This elegant study was performed at the time when only antigen and antibody associated with viral hepatitis could be semiquantified [14]. In those patients who had HBAg at the time of initiation of chemotherapy, bone marrow suppression by chemotherapeutic agents was associated with a marked increase in HBAg titer, which was then followed by hepatocellular damage, as manifested by an elevation in serum transaminase enzymes [13]. Since then, this important observation was further confirmed in other studies [8, 15, 16], with the rate of HBV reactivation ranging from 19% to 48%. Among them, one-quarter to half would be complicated, with severe hepatitis, hepatic failure, and even death [1–6, 17–20].
With the numerous clinical research performed in the past 20 years, hepatitis due to reactivation of HBV is now a well-recognized complication in patients with chronic HBV infection receiving cytotoxic or immunosuppressive therapy. This is particularly a problem in Asia owing to the high hepatitis B surface antigen carrier rate, compounded by the recent increase in use of cytotoxic or immunosuppressive therapy for the treatment of a wide variety of clinical diseases [21]. With the increasing prevalence of HIV infection, HBV reactivation has also been observed in HBV-infected subjects with advanced immune deficiency due to HIV infection [22–27]. Hepatitis due to HBV reactivation has not only been reported in HBsAg-positive patients, HBeAg-positive [6, 28–30] or HBeAg-negative [31–41] subjects who were treated with chemotherapy and transplantation but also in HBsAg-negative patients who had past HBV infection (hepatitis B surface antibody; anti-HBs positive and hepatitis B core antibody; anti-HBc positive) [42–49], especially those treated with rituximab or alemtuzumab-containing chemotherapy [50–55].
Chemotherapy or immunosuppressive therapy related to HBV reactivation
The most commonly reported types of chemotherapy related to HBV reactivation are those used for the treatment of hematological malignancy, such as acute leukemia, myeloproliferative disorders, lymphoproliferative disorders, and plasma cell dyscrasias [5, 6, 8, 13]. Almost all these patients had intense marrow suppression with a drastic reduction of white cell count, and the rebound of the white cell with immune recovery correlates with the initiation of liver damages [56]. Severe hepatitis due to HBV reactivation has also been reported in HBV-infected patients treated with chemotherapy for other malignancies such as breast cancer [10–12], hepatocellular carcinoma [18, 57, 58], small-cell lung cancer [59], and nasopharyngeal cancer [60]. The relative lack of report in other type of malignancy is probably related to their lower incidence in HBV endemic area, such as the Asia-Pacific region. In the transplant setting, such as bone marrow transplant [1–3, 19, 20, 61], heart transplant [62], and kidney transplant [63, 64], the use of immunosuppressive therapy is mandatory to prevent graft rejection and HBV reactivation has been well characterized. Recently, the advance in therapies based on mechanisms that target critical molecular pathways of tumors has evoked considerable interest and among them, rituximab (anti-CD20) [50–54], alemtuzumab (anti-CD52) [55], infliximab (anti-TNF) [65], have been associated with HBV reactivation in HBsAg-positive as well as HBsAg-negative patients. These agents cause profound and long-lasting immunosuppression, which may account for the risk of HBV reactivation following treatment. Also, immunosuppressive agents increase HBV replication and antigen expression. As the host immune response to the virus plays a pivotal role in controlling HBV infection [66, 67], suppression of such immune responses would increase viral replication. On the other hand, immunosuppressive agents may have a more direct stimulatory effect on viral replication. Exceptionally, one immunosuppressive agent, mycophenolate mofetil (MMF), has been shown to suppress the expression of HBsAg and HBeAg as well as the replication of HBV DNA in the 2.2.15 cell in a dose-dependent manner [68, 69]. In the transplant setting, steroids, azathioprine, cyclosporine, and tacrolimus (FK 506) are commonly used to prevent graft rejection. In vitro, corticosteroid increases HBV DNA and RNA production by stimulating HBV transcription [70, 71], by binding to the glucocorticoid responsive element (GRE), and augmenting the HBV enhancer I. In addition, the use of rituximab can effectively remove or suppress anti-HBs producing B cells and cause reactivation of HBV even in HBV immune patients. This may account for the enhanced risk of rituximab-containing chemotherapy regimen in causing HBV reactivation [54].
Pathogenesis and diagnosis of HBV reactivation
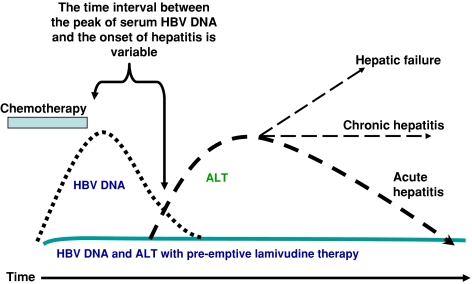
With careful prospective serial serological testing, it is now known that the liver damage due to HBV reactivation is a 2-stage process. Initially, during intense cytotoxic or immunosuppressive therapy, there is a markedly enhanced viral replication, as reflected by increases in serum levels of HBV DNA, hepatitis B e antigen (HBeAg), and HBV DNA polymerase, resulting in widespread infection of hepatocytes. With the subsequent restoration of immune function due to the withdrawal of cytotoxic or immunosuppressive therapy, there is a rapid immune-mediated destruction of HBV-infected hepatocytes, which is manifested clinically as hepatitis, hepatic failure, and even death (Fig. 1) [1–5].
Fig. 1.
Hepatitis due to HBV reactivation is a 2-phase process. The initial phase is related to intense immunosuppression caused by the cytotoxic therapy and is characterized by an enhanced HBV viral replication. There is a marked increase in serum HBV DNA level and viral protein expression in the hepatocytes. The second phase occurs during immune restoration on withdrawal of the chemotherapy and is marked by a much enhanced host immune response against HBV-laden hepatocytes, resulting in liver damages of varying severity, from hepatitis to hepatic failure and even death (solid line). The use of nucleoside analogues with anti-HBV activity in the initial phase could effectively prevent viral replication enhancement and therefore reduce the incidence of HBV-related hepatitis (dotted line)
Based on the understanding of the pathogenesis, HBV reactivation is best defined as an increase of HBV viral replication from a low to high replicative level in patients with chronic or past HBV infection. The key issue is the demonstration of increased HBV replication in patients with serological evidence of chronic or past HBV infection. First, serological evidence of HBV reactivation, such as anti-HBe positive or HBeAg negative to HBeAg positive, and anti-HBs positive to HBsAg positive, should be obtained. However, HBsAg-positive patients could suffer from reactivation even if they remain HBeAg negative. Sequence analysis of the HBV isolated from these patients had demonstrated the presence of point mutation in the precore region that inhibited the synthesis of HBeAg [59, 72–74]. Therefore, it would be more reliable to demonstrate the presence of HBV reactivation by showing an increase of serum HBV DNA by quantitation. At the moment, the most reliable and easily performed test is the quantitation of serum HBV DNA and to track the temporal relationship of the rise in HBV DNA titers with hepatitis and chemotherapy administration [1–5, 54]. However, owing to the wide variation of the sensitivity and linearity of the different assay, the reported incidence varies. For example, the earlier branched DNA hybridization assay (Quantiplex HBV DNA assay; Chiron, Berkeley, CA) has a detection limit of 0.7 × 106 copies/ml [20], whereas recent real-time polymerase chain reaction assays have a lower detection limit down to 11 copies/ml [75]. Second, the patient should have serological evidence of chronic HBV infection. In other words, they should be HBsAg positive, preferably for at least 6 months, before HBV reactivation. In practice, this might be difficult if testing of HBV markers is not a routine before the institution of cytotoxic or immunosuppressive therapy. This is particularly the case if the patient is taking herbs, which might contain immunosuppressive elements, such as steroids, and is seen by alternate medical professionals. HBV reactivation had also been reported in HBsAg-negative patients but had serological evidence of past infection (anti-HBc positive) after immunosuppressive therapy. In HBsAg-negative patients strongly suspected to have HBV reactivation, testing of HBsAg just by monoclonal antibody-based ELISA might not be adequate as mutation in the major neutralizing epitope cluster might render a false-negative result with these assays [76]. In these instances, polyclonal assay and testing of HBV DNA should be performed [77]. Other serological tests, such as serum IgM antibody to hepatitis B core antigen (anti-HBc), are not specific enough to differentiate between acute HBV infection and HBV reactivation in patients with chronic HBV infection [78–80].
Hepatitis should be defined to be due to HBV reactivation if it was preceded or accompanied by enhanced HBV viral replication. The time interval between the peak of HBV DNA viral load and the onset of hepatitis is variable (Fig. 1) [1–5]. In the setting of allogeneic bone marrow transplantation, other causes of liver derangement, such as venoocclusive disease (VOD), GVHD, organ rejection, or superinfection with cytomegalovirus or herpes simplex virus, should be excluded [81, 82]. Because the hepatitis is preceded or accompanied by HBV virological reactivation, its diagnosis relied heavily on the serial quantification of serum HBV viral load. In our center, hepatitis was defined as a more than 3-fold increase of serum ALT on 2 consecutive determinations at least 5 days apart. Icteric hepatitis was defined as hepatitis associated with clinical jaundice and a serum bilirubin level that exceeded 30 μmol/l. HBV reactivation is defined as an increase of serum HBV DNA to more than 1 log higher than that of the preexacerbation baseline or the serum HBV DNA turned from negative to positive [1–3, 19, 20, 54, 56, 83–88]. Histological evidence of the reappearance or enhancement of active necroinflammation was not obtained in our patients because most of them had thrombocytopenia, for which percutaneous liver biopsy carried an increased risk. Moreover, from our previous experience, hepatic histological proof of reappearance or enhancement of active necroinflammation (obtained by transjugular liver biopsy) is not necessary if there is a more than 3-fold increase of serum ALT on 2 consecutive determinations at least 5 days apart, in the absence of clinical features suggestive of infection by cytomegalovirus or herpes simplex virus. In addition, hepatic failure was defined as the presence of hepatic encephalopathy and deranged blood coagulation (prothrombin time prolonged for ≥10 s).
Risk factors involved in HBV reactivation
Not all HBsAg-positive patients treated with cytotoxic chemotherapy or immunosuppressive therapy will suffer from HBV reactivation. Even in the setting of bone marrow transplantation (BMT), only half of the patients will suffer from hepatitis due to HBV reactivation. Moreover, for those who were treated with cyclic cytotoxic chemotherapy, HBV reactivation usually will not occur until the second or third course of therapy [6, 56]. At present, it is not at all clear what is the viral and host determinant factors to explain who will suffer from HBV reactivation. Clinically, a good understanding of the risk factors associated with HBV reactivation in HBsAg-positive patients treated with intense cytotoxic or immunosuppressive therapy, is of paramount importance. This would give us guidance as when to use preemptive nucleos(t)ide analogue therapy and also help to elucidate the complex virus–host balance in causing HBV-related hepatic necroinflammation. Despite its clinical importance, data on the risk factors for HBV reactivation after chemotherapy are limited. As hepatitis due to HBV reactivation is preceded by enhanced HBV viral replication [1–5], a high prechemotherapy HBV viral load has been consistently found to be the most important risk factor for postchemotherapy HBV reactivation, by multivariate analysis (Table 1). In our center, by studying 137 consecutive autologous BMT patients, we first described that pre-BMT HBV DNA level >105 copies/ml (by Digene Hybrid Capture II assay) was the only significant risk factor [20] associated with post-BMT HBV reactivation. Subsequently, in breast cancer patients receiving standard cytotoxic chemotherapy, a high HBV viral load prior to the administration of cytotoxic chemotherapy was also identified as the most significant predictive factor for the development of HBV reactivation. The optimal cut-off was found to be at serum HBV DNA level of 3 × 105 copies/ml (by real-time PCR assay), which gave a sensitivity of 81% and a specificity of 85% [90]. In addition, it was recently shown that in Asian patients with hepatocellular carcinoma, a high HBV viral load prior to systemic cytotoxic chemotherapy was an adverse factor, not only with severe hepatitis but with also survival [91].
Table 1.
Risk factors associated with HBV reactivation in HBsAg-positive patients treated with cytotoxic or immunosuppressive therapy, identified by multivariate analysis
| Author | Underlying diseases | Rate of HBV reactivation (%) | Risk factors |
|---|---|---|---|
| Yeo et al. [18] | HCC | 36 | Elevated serum ALT level |
| Lau et al. [20] | Lymphoma | 45 | Serum HBV DNA>105 copies/ml |
| Jang et al. [89] | HCC | 22 | Positive serum HBeAg |
| Nagamatsu et al. [58] | HCC | 24 | Positive serum HBeAg |
| Zhong et al. [90] | Various malignancies | 26 | Lymphoma/breast Ca, Anthracycline/steroid Serum HBV DNA>103 copies/ml |
| Hui et al. [83] | Lymphoma | 41 | High intrahepatic cccDNA |
To obtain a better predictor for postchemotherapy HBV reactivation, we recently investigated the effect of prechemotherapy intrahepatic covalently closed circular DNA (cccDNA) on HBV reactivation. This is because HBV cccDNA serves as the template for the production of HBV pregenomic RNA (pgRNA) [92] and a higher number of replication templates should increase the rate of HBV reactivation under immunosuppression. Using receiver-operating characteristics, the overall accuracy of using intrahepatic cccDNA to predict HBV reactivation was 88.9% (95% confidence interval [CI]: 73.2–100.0%); the optimal cut-off value being 2.1 copies per cell with the sensitivity, specificity, positive predictive value, and negative predictive value of 77.8% (95% CI: 40.0–97.2%), 100% (95% CI: 75.3–100%), 100% (95% CI: 59.0–100%), and 86.7% (95% CI: 59.5–98.3%), respectively. Therefore, quantifying intrahepatic cccDNA will help identify patients at a high risk for HBV reactivation after chemotherapy so that they could be treated with preemptive anti-HBV therapy. However, the facilities and technique required for quantifying intrahepatic cccDNA are not widely available and could only be performed in a few research centers. Also, not all patients could withstand percutaneous liver biopsy as they might have clotting derangement, especially those with hematological malignancy [83]. Another viral factor that has been investigated is hepatitis B e antigen (HBeAg) but the results are conflicting [6, 10, 58, 89, 93–95]. This is probably related to the presence of the precore/core promoter HBV mutants (i.e., HBeAg-negative/hepatitis B e antigen-positive chronic hepatitis B infection) [96]. Studies on the clinical significance of HBV genotypes indicate that genotypes may correlate with key clinical events [97, 98]. However, data are scarce on the incidence of HBV reactivation after chemotherapy, in relationship to HBV genotypes.
Apart from viral factors, the rate of HBV reactivation varies with the nature, duration, and degree of immunosuppression related to the cytotoxic or immunosuppressive agents used. Among the chemotherapeutic agents used, corticosteroids and anthracyclines [1–7] are most frequently associated with HBV reactivation. The HBV DNA contains a glucocorticoid-responsive element that facilitates replication [70, 71], while anthracyclines have been shown in vitro to stimulate HBV DNA secretion [99]. Hence, “steroid free” chemotherapy has been proposed to minimize the risk of HBV reactivation. In a prospective study of 50 patients with aggressive non-Hodgkin lymphoma (NHL) (75% diffuse large B-cell NHL in both the steroid-free and the steroid-inclusive arms, the use of steroid-free chemotherapy resulted in a significant decrease in the rate of HBV reactivation (73% vs. 38%, P = 0.03). However, patients in the steroid-free arm had a significantly lower rate of complete remission and shorter overall survival, presumably due to suboptimal therapy [100]. Although individual agents may be associated with HBV reactivation through specific mechanisms, the degree of immunosuppression as a consequence of combining these agents with others could also contribute to the development of the condition. This is exemplified in patients treated with BMT, where there is a higher incidence of HBV reactivation when compared with the more commonly used, standard dose of chemotherapy [1–5]. Also, patients with gastrointestinal malignancies who undergo cytotoxic chemotherapy, mainly consisting of less-immunosuppressive agents (fluorouracil and folinic acid), have a lower risk of developing viral reactivation [71], and in HBV-infected patients with hepatocellular carcinoma, the incidence of HBV reactivation appears to correlate with the level of immunosuppression of the anticancer therapy administered; viral reactivation was reported in 40, 25, and 2% of patients who underwent systemic chemotherapy, transatrial chemotherapy, and percutaneous ethanol injection or surgical resection, respectively, in descending order of immunosuppressive effects [58, 89, 101].
Preemptive use of nucleoside analogues
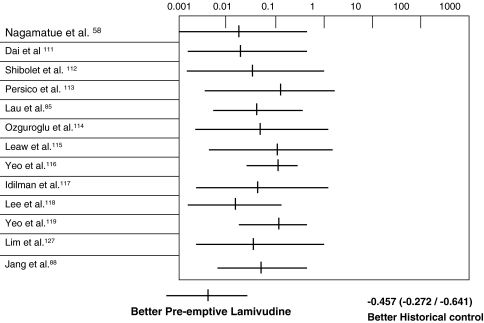
As hepatitis due to HBV virological reactivation is associated or preceded by enhanced HBV viral replication during the immunosuppressive phase, the initiation of antiviral treatment such as lamivudine [102–109] and famciclovir [84, 110] only after there are major biochemical abnormalities is not entirely satisfactory. This may not be effective in reducing liver injury by this time, because the immunologic events causing the flare have already been activated and viral elimination is ongoing [1–6]. Thus, a more proactive approach may be necessary. Recently, many clinical researchers have reported the effectiveness of preemptive lamivudine in reducing the incidence of hepatitis due to HBV reactivation in HBsAg-positive patients treated with immunosuppressive or cytotoxic therapy. From 2002 to 2006, in the literature, there were 13 reports on the use of preemptive lamivudine with untreated controls (Table 2). A meta-analysis of these 13 studies with 702 HBsAg-positive patients (237 treated with preemptive lamivudine and 465 untreated controls), showed that the incidence of hepatitis due to HBV reactivation was 3.3% in the treated group and 35.0% in the untreated control group (odds ratio [OR]: 0.083; 95% CI: 0.045–0.155, P < 0.0001). Hence, there is a strong suggestion of a beneficial effect on the administration of preemptive lamivudine in reducing the hepatitis due to HBV reactivation in HBsAg-positive patients treated with cytotoxic or immunosuppressive therapy (Fig. 2). However, the design of these studies was mostly retrospective using untreated controls for comparison. It remains unclear on the timing of when to start preemptive lamivudine therapy. One can administer lamivudine before or at the initiation of cytotoxic therapy and cover the entire period of immunosuppression with anti-HBV nucleoside analogues (early preemptive therapy). Alternatively, one can monitor closely for serological evidence of HBV reactivation and use anti-HBV nucleoside analogues only when there is evidence of HBV virological reactivation (deferred preemptive therapy) [56, 86]. Although early preemptive therapy reduces the need to closely monitor the serum HBV DNA level, it runs the risk of overtreating at least half of the patients with nucleoside analogues who would not develop HBV reactivation. In addition, the duration of therapy with nucleoside analogues, such as lamivudine, would be longer with this approach and this could increase the risk of developing HBV viral resistance, as the incidence of viral resistance increases with prolonged antiviral therapy [67]. On the other hand, although the latter approach is more scientific, there are a few shortcomings. First, patients could still have hepatitis due to HBV reactivation with this approach. Second, the need for very close monitoring of serum HBV DNA by expensive or labor-intensive quantitative assay could be a problem when there is a lack of such laboratory support. Third, with the poor understanding of viral and T-cell kinetics in hepatitis due to HBV reactivation, the frequency of monitoring has been poorly defined. To explore the optimal time to initiate lamivudine in these settings, we studied 30 consecutive HBsAg-positive lymphoma patients treated with intensive cytotoxic therapy and randomized (1:1) them to receive either lamivudine 100 mg daily 1 week before chemotherapy or to have this treatment deferred until there was serological evidence of HBV reactivation on the basis of serial 2-week-interval serum hepatitis B virus DNA monitoring by a Digene Hybrid Capture II assay. It was found that early is preferable to deferred preemptive lamivudine therapy for HBsAg-positive patients undergoing chemotherapy, with a significantly lower incidence of HBV virological reactivation after chemotherapy. Almost all patients (87.5%) in the “deferred” treatment group suffered from hepatitis (5 anicteric hepatitis, 1 icteric hepatitis, and 1 hepatic failure), despite being treated with lamivudine at the time of detection of HBV virological reactivation. Currently, this early preemptive approach with the use of lamivudine has been adopted by the major HBV treatment guidelines [120–122].
Table 2.
Reports of preemptive lamivudine in HbsAg-positive patients to reduce HBV reactivation after cytotoxic chemotherapy, with historical controls
| Author | Underlying disease | Type of chemotherapy | Incidence of HBV reactivation |
|---|---|---|---|
| Treated* versus untreated controls | |||
| Nagamateu et al. [58] | Hepatocellular carcinoma | Transhepatic intra-arterial chemotherapy | 0/8 vs. 6/9 |
| Dai et al. [111] | Breast cancer | Systemic | 0/11 vs. 5/9 |
| Shibolet et al. [112] | Various malignancies | Systemic | 0/13 vs. 2/5 |
| Persico et al. [113] | Lymphoma | Systemic | 0/3 vs. 12/21 |
| Lau et al. [85] | Lymphoma | Systemic | 1/20 vs. 9/20 |
| Ozguroglu et al. [114] | Lymphoma | Systemic | 0/4 vs. 5/8 |
| Leaw et al. [115] | Lymphoma | Systemic | 0/11 vs. 17/61 |
| Yeo et al. [116] | Various malignancies | Systemic | 3/65 vs. 48/193 |
| Idilman et al. [117] | Hematological malignancies | Systemic | 0/8 vs. 5/10 |
| Lee et al. [118] | Lymphoma | Systemic | 1/11 vs. 17/20 |
| Yeo et al. [119] | Breast cancer | Systemic | 2/31 vs. 19/61 |
| Lim et al. [127] | Various malignancies | Systemic | 0/16 vs. 7/19 |
| Jang et al. [88] | Hepatocellular carcinoma | Transhepatic intra-arterial chemotherapy | 1/36 vs. 11/37 |
* Pre-emptive lamivudine therapy
Fig. 2.
A meta-analysis of 13 studies reported during 2002–2006 that compare preemptive use of lamivudine versus historical controls in hepatitis B surface antigen-positive patients treated with systemic chemotherapy or transhepatic intraarterial chemotherapy. Altogether, 702 hepatitis B surface antigen-positive patients (237 treated with preemptive lamivudine and 465 untreated controls) were recruited. Those patients treated with preemptive lamivudine had a significantly lower incidence of hepatitis due to HBV reactivation (3.3%) than the untreated controls (35.0%; OR 0.083; 95% CI: 0.045–0.155; p < 0.0001). Hence, there is a strong suggestion of a beneficial effect on the administration of preemptive lamivudine in reducing the hepatitis due to HBV reactivation in HBsAg-positive patients treated with cytotoxic or immunosuppressive therapy
It remains speculative whether monitoring the serum HBV DNA at a closer interval, with a more sensitive HBV DNA assay, would improve the situation [123]. The major concerns related to the use of this early preemptive approach have been the prolonged use of lamivudine and the unnecessary treatment for some patients who may not develop HBV reactivation. This will lead to the development of lamivudine resistance caused by point mutations, with substitution of either valine or isoleucine for the amino acid position 204 methionine (rtM204V or rtM204I, respectively) in the HBV DNA polymerase gene (tyrosine–methionine–aspartate–aspartate [YMDD] motif) [67]. Its risk increases as the therapy is prolonged, reaching a level of 67% after 4 years in nonimmunocompromised patients [67]. In particular, the fact that YMDD mutant infection is occasionally associated with rapid clinical deterioration after transplantation raised additional concern [124, 125]. To avoid this problem, one may use alternative nucleos(t)ides analogues that are associated with less viral resistance, such as adefovir, entecavir, and telbuvidine [67].
How long should we keep the patients on lamivudine?
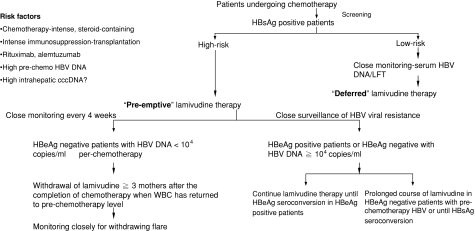
As the mechanism of nucleos(t)ide analogues in inhibiting viral replication is direct suppression of HBV polymerase activity and has little effect on the restoration on host immune control [67], its premature withdrawal could lead to rapid rebound of viral replication, resulting in liver-related morbidity and mortality [126]. On the other hand, prolonged therapy with nucleos(t)ide analogues is associated with an increased likelihood of developing lamivudine-resistant mutants. Hence, most cancer centers would aim at discontinuing or withdrawing preemptive lamivudine as soon as possible to limit the duration of antiviral therapy [57, 85, 86, 111–119, 127]. However, at the moment there is no available consensus on the optimal duration of lamivudine therapy. This is mostly due to the lack of data on occurrence of hepatic flares after the withdrawal of preemptive antiviral therapy in these patients. As HBV reactivation after cytotoxic or immunosuppressive therapy is usually accompanied by an upsurge of white cell counts from the nadir, our center has adopted a protocol of only withdrawing lamivudine once the total white cell count has normalized and at least 3 months after completion of chemotherapy [56]. The concept of this protocol or approach is to cover the entire period when the host interaction with HBV has been disturbed as a result of the cytotoxic or immunosuppressive therapy. This way, we would only be withdrawing lamivudine after the host’s immune system has recovered sufficiently to the prechemotherapy state. Recently, we examined the occurrence of hepatic flares after withdrawal of preemptive lamivudine and determined what factors are associated with hepatic flares after withdrawal of preemptive lamivudine. We studied forty-six consecutive HBsAg-positive patients treated with preemptive lamivudine, started one week before initiation of chemotherapy and continued for the entire duration of chemotherapy. Preemptive lamivudine was stopped at a median 3.1 (range 3.0–3.4) months after completion of chemotherapy. Patients were longitudinally followed up after withdrawal of preemptive lamivudine. Median time of follow-up after withdrawal of lamivudine was 25.7 (range 5.7–75.7) months. Eleven of the 46 patients (23.9%) developed HBV reactivation after withdrawal of preemptive lamivudine. Eight of 16 patients with high prechemotherapy HBV DNA (>104 copies/ml) compared with 3 of the 30 patients with low prechemotherapy HBV DNA (≤104 copies/ml) developed HBV reactivation (50.0% vs. 10.0%, respectively; P = 0.001). Hepatitis B e antigen-positive patients were also more likely to develop HBV reactivation (5/11 [45.5%] vs. 6/35 [17.1%], respectively; P = 0.041). A high prechemotherapy HBV DNA (>104 copies/ml) was the most important risk factor for HBV reactivation after withdrawal of preemptive lamivudine on Cox proportional hazards analysis (relative risk 16.13; 95% CI 2.99–87.01; P = 0.001). Hence, HBV reactivation is more likely to occur in patients with high prechemotherapy HBV DNA after withdrawal of preemptive lamivudine [87]. On the basis of these results, in our center we will keep those patients with high pretreatment HBV DNA level (>104 copies/ml) on nucleos(t)ide analogues for a longer period of time until there is evidence of restoration of host immune control on the virus, such as e-seroconversion or even s-seroconversion (Fig. 3).
Fig. 3.
Algorithm for management of hepatitis B surface antigen-positive patients treated with a cytotoxic or immunosuppressive agent. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen
Future direction
In the future, education of the medical profession to screen for HBsAg-positive status before initiation of cytotoxic chemotherapy or immunosuppressive therapy should be enhanced. On the research side, identification of viral or host determinants of HBV reactivation would further facilitate and improve the management of this important clinical problem, especially in the Asia-Pacific region. In addition, the application of other nucleos(t)ide analogues with more potent antiviral effect and less resistance will need to be explored.
References
- 1.Liang R, Lau GKK, Kwong YL. Chemotherapy and bone marrow transplantation for cancer patient who are also chronic hepatitis B carriers: a review of the problem. J Clin Oncol. 1999;17:394–98. [DOI] [PubMed]
- 2.Luo XR, Yan AW, Liang R, Lau GKK. Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy-pathogenesis and management. Rev Med Virol. 2001;11(5):287–9. [DOI] [PubMed]
- 3.Lau GKK, Lee CK, Liang R. Hepatitis B and bone marrow transplantation. Crit Rev Oncol Hematol. 1999;31:71–6. [DOI] [PubMed]
- 4.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43(2):209–20. [DOI] [PubMed]
- 5.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136(5):699–712. [DOI] [PubMed]
- 6.Lok ASF, Liang RHS, Chiu EKW, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–8. [DOI] [PubMed]
- 7.Liang RHS, Lok ASF, Lai CL, Chan TK, Todd O, Chiu EKW. Hepatitis B infection in patients with lymphomas. Hematol Oncol. 1990;8:261–70. [DOI] [PubMed]
- 8.Lau JY, Lai CL, Lin HJ, Lok ASF, Liang RHS, Wu PC, et al. Fatal reactivation of chronic hepatitis B virus infection following withdrawal of chemotherapy in lymphoma patients. Q J Med. 1989;73:911–7. [PubMed]
- 9.Soh LT, Ang PT, Sng I, Chua EJ, Ong YW. Fulminant hepatic failure in non- Hodgkin’s lymphoma patients treated with chemotherapy. Eur J Cancer. 1992;28A:1338–9. [DOI] [PubMed]
- 10.Yeo W, Chan PKS, Hui P, HoWM, Lam KC, Kwan WH, et al. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553–61. [DOI] [PubMed]
- 11.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, and fluorouracil in node-positive breast cancer. The results of 20 years follow-up. N Engl J Med. 1995;332:901–6. [DOI] [PubMed]
- 12.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9. [DOI] [PubMed]
- 13.Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68:105–12. [PubMed]
- 14.GN Vyas, Rao KR, Ibrahim AB. Australia antigen (hepatitis B antigen): a conformational antigen dependent on disulfide bonds. Science. 1972;178:1300–1. [DOI] [PubMed]
- 15.Galbraith RM, Eddleston ALWF, Wiiliams R, Zuckerman AJ, Bagshawe KD. Fulminant hepatic failure in leukemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet. 1975;2:528–30. [DOI] [PubMed]
- 16.Hoofnagle JH, Dusheiko GM, Schafer DF, Jones EA, Micetich KC, Young RC, et al. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Int Med. 1982;96:447–9. [DOI] [PubMed]
- 17.Kumagai K, Takagi T, Nakamura S, Sawada U, Kura U, Kodama F, et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol. 1997;8(Suppl 1):107–9. [PubMed]
- 18.Yeo W, Lam KC, Zee B, Chan PSK, Mo FKF, Ho WM, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–6. [DOI] [PubMed]
- 19.Lau GKK, Liang RHS, Chiu EKW, Lee CK, Lam SK. Hepatic events after bone marrow transplantation in patients with hepatitis B infection: a case-controlled study. Bone Marrow Transplant. 1997;19:795–9. [DOI] [PubMed]
- 20.Lau GKK, Leung YH, Fong DYT, Au WY, Kwong YL, Lie A, et al. High HBV DNA viral load is the most significant risk factor for hepatitis B reactivation in hepatitis B surface antigen positive patients treated with autologous hematopoietic cell transplantation. Blood. 2002;99:2324–30. [DOI] [PubMed]
- 21.Cheng VCC, Lo CM, Lau GKK. Current issues and treatment of fulminant hepatic failure including transplantation in Hong Kong and Far East. Sem Liver Dis. 2003;23(3):239–50. [DOI] [PubMed]
- 22.Waite J, Gilson RJ, Weller IV, Lacey CJ, Hambling MH, Hawkins A, et al. Hepatitis B virus reactivation or reinfection associated with HIV-1 infection. AIDS. 1988;2(6):443–8. [DOI] [PubMed]
- 23.Manegold C, Hannoun C, Wywiol A, Dietrich M, Polywka S, Chiwakata CB, et al. Reactivation of hepatitis B virus replication accompanied by acute hepatitis in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2001;32(1):144–8. [DOI] [PubMed]
- 24.Bani-Sadr F, Maillard A, Ponscarme D, Scieux C, Molina JM. Reactivation of HBV replication in HIV-HBV infected patients. Am J Med. 2003;114(9):768–9. [DOI] [PubMed]
- 25.Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS. 2006;20(12):817–22. [DOI] [PubMed]
- 26.Barros MF, Piedade J, Nunes G, Canas-Ferreira W, Silva AP, Champalimaud JL, et al. Active replication of hepatitis B virus (HBV) in HIV type 1 and in HIV type 2 infected patients. Rev Inst Med Trop Sao Paulo. 1996;38(4):253–8. [DOI] [PubMed]
- 27.Clark SJ, Creighton S, Horner M, Smith HM, Portmann B, Taylor C, et al. Reactivation of latent hepatitis B virus infection with HIV-related immunosuppression. Int J STD AIDS. 2006;17(1):67–9. [DOI] [PubMed]
- 28.Ustun C, Koc H, Karayalcin S, Akyol G, Gurman G, Ilhan O, et al. Hepatitis B virus infection in allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;20:289–96. [DOI] [PubMed]
- 29.Yeo W, Steinberg JL, Tam JS, Chan PK, Leung NW, Lam KC, et al. Lamivudine in the treatment of hepatitis B virus reactivation during cytotoxic chemotherapy. J Med Virol. 1999;59:263–9. [DOI] [PubMed]
- 30.Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology. 1999;46:2925–30. [PubMed]
- 31.Yagci M, Sucak GT, Haznedar R. Fludarabine and risk of hepatitis B virus reactivation in chronic lymphocytic leukemia. Am J Hematol. 2000;64:233–4. [DOI] [PubMed]
- 32.Silvestri F, Sperotto A, Ermacora A, Fanin R, Damiani D, Baccarani M. Lamivudine for the prevention of hepatitis B virus reactivation during autologous stem cell transplantation. A case report. Haematologica. 2000;85:327–9. [PubMed]
- 33.Maguire CM, Crawford DH, Hourigan LF, Clouston AD, Walpole ET, Powell EE. Case report: lamivudine therapy for submassive hepatic necrosis due to reactivation of hepatitis B following chemotherapy. J Gastroenterol Hepatol. 1999;14:801–3. [DOI] [PubMed]
- 34.Alexopoulos CG, Vaslamatzis M, Hatzidimitriou G. Prevalence of hepatitis B virus marker positivity and evolution of hepatitis B virus profile, during chemotherapy, in patients with solid tumours. Br J Cancer. 1999;81:69–74. [DOI] [PMC free article] [PubMed]
- 35.Clark FL, Drummond MW, Chambers S, Chapman BA, Patton WN. Successful treatment with lamivudine for fulminant reactivated hepatitis B infection following intensive therapy for high-grade non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:385–7. [DOI] [PubMed]
- 36.Mertens T, Kock J, Hampl W, Schlicht HJ, Tillmann HL, Oldhafer KJ, et al. Reactivated fulminant hepatitis B virus replication after bone marrow transplantation: clinical course and possible treatment with ganciclovir. J Hepatol. 1996;25:968–71. [DOI] [PubMed]
- 37.Lau JY, Bird GL, Gimson AE, Alexander GJ, Williams R. Treatment of HBV reactivation after withdrawal of immunosuppression. Lancet. 1991;337:80249. [DOI] [PubMed]
- 38.Reed EC, Myerson D, Corey HT, Meyers JD. Allogeneic marrow transplantation in patients positive for hepatitis B surface antigen. Blood. 1991;77:195–200. [PubMed]
- 39.Flowers MA, Heathcote J, Wanless IR, Sherman M, Reynolds WJ, Cameron RG, et al. Fulminant hepatitis as a consequence of reactivation of hepatitis B virus infection after discontinuation of low-dose methotrexate therapy. Ann Intern Med. 1990;12:381–2. [DOI] [PubMed]
- 40.Rostoker G, Rosenbaum J, Ben Maadi A, Nedelec G, Deforge L, Vidaud M, et al. Reactivation of hepatitis B virus by corticosteroids in a case of idiopathic nephrotic syndrome. Nephron. 1990;16:224. [DOI] [PubMed]
- 41.Pariente EA, Goudeau A, Dubois F, Degott C, Gluckman E, Devergie A, et al. Fulminant hepatitis due to reactivation of chronic hepatitis B virus infection after allogeneic bone marrow transplantation. Dig Dis Sci. 1988;33:1185–91. [DOI] [PubMed]
- 42.Nagington J, Cossart YE, Cohen BJ. Reactivation of hepatitis B after transplantation operations. Lancet. 1977;68:105–12. [DOI] [PubMed]
- 43.Grotz W, Rasenack J, Benzing T, Berthold H, Peters T, Walter E, et al. Occurrence and management of hepatitis B virus reactivation following kidney transplantation. Clin Nephrol. 1998;49:385–8. [PubMed]
- 44.Blanpain C, Knoop C, Delforge ML, Antoine M, Peny MO, Liesnard C, et al. Reactivation of hepatitis B after transplantation in patients with pre-existing anti-hepatitis B surface antigen antibodies: report on three cases and review of the literature. Transplantation. 1998;66:883. [DOI] [PubMed]
- 45.Ahmed A, Keeffe EB. Lamivudine therapy for chemotherapy-induced reactivation of hepatitis B virus infection. Am J Gastroenterol. 1999;94:249–51. [DOI] [PubMed]
- 46.Romand F, Michallet M, Pichoud C, Trepo C, Zoulim F. Hepatitis B virus reactivation after allogeneic bone marrow transplantation in a patient previously cured of hepatitis B. Gastroenterol Clin Biol. 1999;23:770–4. [PubMed]
- 47.Dhedin N, Douvin C, Kuentz M, Saint Marc MF, Reman O, Rieux C, et al. Reverse seroconversion of hepatitis B after allogeneic bone marrow transplantation: a retrospective study of 37 patients with pretransplant anti-HBs and anti-HBc. Transplantation. 1998;66:616. [DOI] [PubMed]
- 48.Schnepf N, Sellier P, Bendenoun M, Zini JM, Sanson-le Pors MJ, Mazeron MC. Reactivation of lamivudine-resistant occult hepatitis B in an HIV-infected patient undergoing cytotoxic chemotherapy. J Clin Virol. 2007;39(1):48–50. [DOI] [PubMed]
- 49.Chamorro AJ, Casado JL, Bellido D, Moreno S. Reactivation of hepatitis B in an HIV-infected patient with antibodies against hepatitis B core antigen as the only serological marker. Eur J Clin Microbiol Infect Dis. 2005;24(7):492–4. [DOI] [PubMed]
- 50.Tsutsumi Y, Tanaka J, Kawamura T, Miura T, Kanamori H, Asaka M, et al. Possible efficacy of lamivudine treatment to prevent hepatitis B virus reactivation due to rituximab therapy in a patient with non-Hodgkin’s lymphoma. Ann Hematol. 2003;83:58–60. [DOI] [PubMed]
- 51.Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001;344:68–9. [DOI] [PubMed]
- 52.Westhoff TH, Jochimsen F, Schmittel A, Stoffler-Meilicke M, Schafer JH, Zidek W, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood. 2003;102:1930. [DOI] [PubMed]
- 53.Perceau G, Diris N, Estines O, Derancourt C, Lévy S, Bernard P. Late lethal hepatitis B virus reactivation after rituximab treatment of low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2006;155(5):1053–6. [DOI] [PubMed]
- 54.Hui CK, Cheung WWW, Au WY, Zhang HY, Yueng YH, Leung N, et al. Rituximab increases the risk of de novo hepatitis B infection in hepatitis B surface antigen negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131(1):59–68. [DOI] [PubMed]
- 55.Iannitto E, Minardi V, Calvaruso G, Mule A, Ammatuna E, Trapani RD, et al. Hepatitis B virus reactivation and alemtuzumab therapy. Eur J Haematol. 2005;74:254–8. [DOI] [PubMed]
- 56.Lau GK, Yiu HHY, Fong DYT, Cheng HC, Au WY, Lai LSY, et al. “Early” is superior to “deferred” pre-emptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–9. [DOI] [PubMed]
- 57.Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol. 2004;99:2369–75. [DOI] [PubMed]
- 58.Nagamatsu H, Kumashiro R, Itano S, Matsugaki S, Sata M. Investigation of associating factors in exacerbation of liver damage after chemotherapy in patients with HBV-related HCC. Hepatol Res. 2003;26:293–301. [DOI] [PubMed]
- 59.Steinberg JL, Yeo W, Zhong S, Chan JY, Tam JS, Chan PK, et al. Hepatitis B virus reactivation in patients undergoing cytotoxic chemotherapy for solid tumours: precore/core mutations may play an important role. J Med Virol. 2000;60:249–55. [PubMed]
- 60.Yeo W, Hui EP, Chan AT, Ho WM, Lam KC, Chan PK, et al. Prevention of hepatitis B virus reactivation in patients with nasopharyngeal carcinoma with lamivudine. Am J Clin Oncol. 2005;28(4):379–84. [DOI] [PubMed]
- 61.Locasciulli A, Bacigalupo A, Van Lint MT, Chemello L, Pontisso P, Occhini D, et al. Hepatitis B virus (HBV) infection and liver disease after allogeneic bone marrow transplantation: a report of 30 cases. Bone Marrow Transplant. 1990;6(1):25–9. [PubMed]
- 62.Zampino R, Marrone A, Ragone E, Costagliola L, Cirillo G, Karayiannis P, et al. Heart transplantation in patients with chronic hepatitis B: clinical evolution, molecular analysis, and effect of treatment. Transplantation. 2005;80(9):1340–3. [DOI] [PubMed]
- 63.Berger A, Preiser W, Kachel HG, Stürmer M, Doerr HW. HBV reactivation after kidney transplantation. J Clin Virol. 2005;32(2):162–5. [DOI] [PubMed]
- 64.Degos F, Lugassy C, Degott C, Debure A, Carnot F, Theirs V, et al. Hepatitis B virus and hepatitis B-related viral infection in renal transplant recipients. A prospective study of 90 patients. Gastroenterology. 1988;94(1):151–6. [DOI] [PubMed]
- 65.Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53(9):1363–5. [DOI] [PMC free article] [PubMed]
- 66.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. [DOI] [PubMed]
- 67.Hui CK, Lau GK. The present and future therapy for chronic hepatitis B infection. Semin Liver Dis. 2006;26(2):192–7. [DOI] [PubMed]
- 68.Wu J, Xie HY, Jiang GP, Xu X, Zheng SS. The effect of mycophenolate acid on hepatitis B virus replication in vitro. Hepatobiliary Pancreat Dis Int. 2003;2(3):410–3. [PubMed]
- 69.Gong ZJ, De Meyer S, Clarysse C, Verslype C, Neyts J, De Clercq E, et al. Mycophenolic acid, an immunosuppressive agent, inhibits HBV replication in vitro. J Viral Hepat. 1999;6(3):229–36. [DOI] [PubMed]
- 70.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986;83:1627–31. [DOI] [PMC free article] [PubMed]
- 71.Liaw YF. Hepatitis viruses under immunosuppressive agents. J Gastroenterol Hepatol. 1998;13:14–20. [DOI] [PubMed]
- 72.McIvor C, Morton J, Bryant A, Cooksley WG, Durrant S, Walker N. Fatal reactivation of precore mutant hepatitis B virus associated with fibrosing cholestatic hepatitis after bone marrow transplantation. Ann Intern Med. 1994;121:274–5. [DOI] [PubMed]
- 73.Yoshiba M, Sekiyama K, Sugata F, Okamoto H, Yamamoto K, Yotsumoto S. Reactivation of precore mutant hepatitis B virus leading to fulminant hepatic failure following cytotoxic treatment. Dig Dis Sci. 1992;37:1253–9. [DOI] [PubMed]
- 74.Talbodec N, Loriot MA, Gigou M, Guigonis V, Boyer N, Bezeaud A, et al. Hepatitis B virus precore mutations and HBeAg negative reactivation of chronic hepatitis B after interferon therapy. Liver. 1995;15:93–8. [DOI] [PubMed]
- 75.Allice T, Cerutti F, Pittaluga F, Varetto S, Gabella S, Marzano A, et al. COBAS AmpliPrep-COBAS TaqMan hepatitis B virus (HBV) test: a novel automated real-time PCR assay for quantification of HBV DNA in plasma. J Clin Microbiol. 2007;45(3):828–34. [DOI] [PMC free article] [PubMed]
- 76.Carman WF, Korula J, Wallace L, MacPhee R, Mimms L, Decker R. Fulminant reactivation of hepatitis B due to envelope protein mutant that escaped detection by monoclonal HBsAg ELISA. Lancet. 1995;345:1406–7. [DOI] [PubMed]
- 77.Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212–5. [DOI] [PubMed]
- 78.Kryger P. Significance of anti-HBc IgM in the differential diagnosis of viral hepatitis. J Virol Methods. 1985;10:283–9. [DOI] [PubMed]
- 79.Chau KH, Hargie MP, Decker RH, Mushahwar IK, Overby LR. Serodiagnosis of recent hepatitis B infection by IgM class anti-HBc. Hepatology. 1983;3:142–9. [DOI] [PubMed]
- 80.Colloredo Mels G, Bellati G, Leandro G, Brunetto MR, Vicari O, Piantino P, et al. Role of IgM antibody to hepatitis B core antigen in the diagnosis of hepatitis B exacerbations. Arch Virol Suppl. 1993;8:203–11. [DOI] [PubMed]
- 81.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. [DOI] [PubMed]
- 82.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. [DOI] [PubMed]
- 83.Hui CK, Bowden S, Jackson K, Au WY, Fong DY, Lie AK, et al. Clinical significance of intrahepatic hepatitis B virus covalently closed circular DNA in chronic hepatitis B patients who received cytotoxic chemotherapy. Blood. 2005;105:2616–7. [DOI] [PubMed]
- 84.Lau GKK, Liang RHS, Wu PC, Lee CK, Lim WL, Au WY. Use of famciclovir to prevent HBV reactivation in HBsAg-positive recipients after allogeneic bone marrow transplantation. J Hepatol. 1998;28:359–68. [DOI] [PubMed]
- 85.Lau GK, He ML, Fong DY, Bartholomeusz A, Au WY, Lie AK, et al. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology. 2002;36:702–9. [DOI] [PubMed]
- 86.Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis. 2007;11(4):965–91. [DOI] [PubMed]
- 87.Hui CK, Cheung WWW, Au WY, Lie AWK, Zhang HY, Yueng YH, et al. Hepatitis B reactivation after withdrawal of preemptive lamivudine in patients with hematological malignancy upon completion of cytotoxic chemotherapy. Gut. 2005;54(11):1597–603. [DOI] [PMC free article] [PubMed]
- 88.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43(2):233–40. [DOI] [PubMed]
- 89.Jang JW, Choi JY, Bae SH, Kim CW, Yoon SK, Cho SH, et al. Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma. J Hepatol. 2004;41:427–35. [DOI] [PubMed]
- 90.Zhong S, Yeo W, Schroder C, Chan P, Wong W, Ho WM, et al. High hepatitis B virus (HBV) DNA viral load is an important risk factor for HBV reactivation in breast cancer patients undergoing cytotoxic chemotherapy. J Viral Hepat. 2004;11:55–9. [DOI] [PubMed]
- 91.Yeo W, Mo FK, Chan SL, Leung NW, Hui P, Lam WY, et al. Hepatitis B viral load predicts survival of HCC patients undergoing systemic chemotherapy. Hepatology. 2007;45(6):1382–9. [DOI] [PubMed]
- 92.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–8. [DOI] [PubMed]
- 93.Yeo W, Zhong S, Chan PKS, Ho WM, Wong HTM, Chan ASK, et al. Sequence variations of precore/core and precore promoter regions of hepatitis B virus in patients with or without viral reactivation during cytotoxic chemotherapy. J Viral Hepat. 2000;7:448–58. [DOI] [PubMed]
- 94.Picardi M, Pane F, Quintarelli C, De Renzo A, Del Giudice A, De Divitiis B, et al. Hepatitis B virus reactivation after fludarabine-based regimens for indolent non-Hodgkin’s lymphomas: high prevalence of acquired viral genomic mutations. Haematologica. 2003;88:1296–303. [PubMed]
- 95.Liao CA, Lee CM, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin’s lymphoma. Br J Haematol. 2002;116:166–9. [DOI] [PubMed]
- 96.Carman WF, Fagan EA, Hadziyannis S, Karayiannis P, Tassopoulos NC, Williams R, et al. Association of a precore genomic variant of HBV with fulminant hepatitis. Hepatology. 1991;14:219–22. [PubMed]
- 97.Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97(4):265–72. [DOI] [PubMed]
- 98.Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B, progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43(3):411–7. [DOI] [PubMed]
- 99.Hsu CH, Hsu HC, Chen HL, Gao M, Yeh PY, Chen PJ, et al. Doxorubicin activates hepatitis B virus (HBV) replication in HBV-harboring hepatoblastoma cells. A possible novel mechanism of HBV reactivation in HBV carriers receiving systemic chemotherapy. Anticancer Res. 2004;24(5A):3035–40. [PubMed]
- 100.Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, et al. Steroid- free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320–8. [DOI] [PubMed]
- 101.Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15(11):1661–6. [DOI] [PubMed]
- 102.Clark FL, Drummond MW, Chambers S, et al. Successful treatment with lamivudine for fulminant reactivated hepatitis B infection following intensive therapy for high-grade non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:385–87. [DOI] [PubMed]
- 103.Maguire CM, Crawford DH, Hourigan LF, Clouston AD, Walpole ET, Powell EE. Case report: lamivudine therapy for submassive hepatic necrosis due to reactivation of hepatitis B following chemotherapy. J Gastroenterol Hepatol. 1999;14:801–3. [DOI] [PubMed]
- 104.Ahmed A, Keeffe EB. Lamivudine therapy for chemotherapy-induced reactivation of hepatitis B virus infection. Am J Gastroenterol. 1999;94:249–51. [DOI] [PubMed]
- 105.Liao CA, Lee CM, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin’s lymphoma. Br J Haematol. 2002;116:166–9. [DOI] [PubMed]
- 106.Matsuo K, Takenaka K, Shimomura H, Fujii N, Shinagawa K, Kiura K, et al. Lamivudine and glycyrrhizin for treatment of chemotherapy-induced hepatitis B virus (HBV) hepatitis in a chronic HBV carrier with non-Hodgkin lymphoma. Leuk Lymphoma. 2001;41:191–5. [DOI] [PubMed]
- 107.Jung YO, Lee YS, Yang WS, Han DJ, Park JS, Park SK. Treatment of chronic hepatitis B with lamivudine in renal transplant recipients. Transplantation. 1998;66:733–7. [DOI] [PubMed]
- 108.Henkes M, Martin S, Einsele H, Aulitzky WE. Successful antiviral treatment for fulminant reactivated hepatitis B after autologous stem cell transplantation and prophylaxis during subsequent allogeneic stem cell transplantation. Ann Hematol. 2002;81:343–6. [DOI] [PubMed]
- 109.Saif MW, Little RF, Hamilton JM, Allegra CJ, Wilson WH. Reactivation of chronic hepatitis B infection following intensive chemotherapy and successful treatment with lamivudine: a case report and review of the literature. Ann Oncol. 2001;12:123–9. [DOI] [PubMed]
- 110.Tang S, Ho SK, Moniri K, Lai KN, Chan TM. Efficacy of famciclovir in the treatment of lamivudine resistance related to an atypical hepatitis B virus mutant. Transplantation. 2002;73:148–51. [DOI] [PubMed]
- 111.Dai MS, Wu PF, Lu JJ, Shyu RY, Chao TY. Preemptive use of lamivudine in breast cancer patients carrying hepatitis B virus undergoing cytotoxic chemotherapy: a longitudinal study. Support Care Cancer. 2004;12:191–6. [DOI] [PubMed]
- 112.Shibolet O, Ilan Y, Gillis S, Hubert A, Shouval D, Safadi R. Lamivudine therapy for prevention of immunosuppressive-induced hepatitis B virus reactivation in hepatitis B surface antigen carriers. Blood. 2002;100:391–6. [DOI] [PubMed]
- 113.Persico M, De Marino F, Russo GD, Morante A, Rotoli B, Torella R, et al. Efficacy of lamivudine to prevent hepatitis reactivation in hepatitis B virus-infected patients treated for non-Hodgkin lymphoma. Blood. 2002;99:724–5. [DOI] [PubMed]
- 114.Ozguroglu M, Bilici A, Turna H, Serdengecti S. Reactivation of hepatitis B infection with cytotoxic therapy in non-Hodgkin’s lymphoma. Med Oncol. 2004;21:67–72. [DOI] [PubMed]
- 115.Leaw SJ, Yen CJ, Huang WT, Chen TY, Su WC, Tsao CJ. Preemptive use of interferon or lamivudine for hepatitis B reactivation in patients with aggressive lymphoma receiving chemotherapy. Ann Hematol. 2004;83:270–5. [DOI] [PubMed]
- 116.Yeo W, Chan PKS, Ho WM, Zee B, Lam KC, Lei KIK, et al. Lamivudine for the prevention of hepatitis B virus reactivation in HBsAg seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;15:1661–6.
- 117.Idilman R, Arat M, Soydan E, Toruner M, Soykan I, Akbulut H, et al. Lamivudine prophylaxis for prevention of chemotherapy-induced hepatitis B virus reactivation in hepatitis B virus carriers with malignancies. J Viral Hepat. 2004;11:141–7. [DOI] [PubMed]
- 118.Lee GW, Ryu MH, Lee JL, Oh S, Kim E, Lee JH, et al. The prophylactic use of lamivudine can maintain dose-intensity of adriamycin in hepatitis-B surface antigen (HBsAg)-positive patients with non-Hodgkin’s lymphoma who receive cytotoxic chemotherapy. J Korean Med Sci. 2003;18:849–54. [DOI] [PMC free article] [PubMed]
- 119.Yeo W, Ho WM, Hui P, Chan PK, Lam KC, Lee JJ, et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2004;88(3):209–15. [DOI] [PubMed]
- 120.Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, et al. For the Asian Pacific Consensus update working party on chronic hepatitis B. Asian-Pacific Consensus statement on the management of chronic hepatitis B: A 2005 update. Liver Int. 2005;25(3):472–89. [DOI] [PubMed]
- 121.de Franchis R, Hadengue A, Lau GK, Lavanchy D, Lok A, McIntyre N, et al. EASL JuryEASL International Consensus Conference on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol. 2003;39(Suppl 1):S3–25. [PubMed]
- 122.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507–39. [DOI] [PubMed]
- 123.Hui CK, Bowden S, Lewin S, Locarnini S, Naoumov NV, Lau GK. The use of real-time PCR assays for monitoring hepatitis B virus DNA response to antiviral therapy. J Clin Microbiol. 2006;44(8):2983–7. [DOI] [PMC free article] [PubMed]
- 124.Lee YC, Young KC, Su WC, Tsao CJ, Chen TY. Emergence of YMDD mutant hepatitis B virus after allogeneic stem cell transplantation from a HBsAg-positive donor during lamivudine prophylaxis. Haematologica. 2004;89(4):ECR09. [PubMed]
- 125.Chan HL, Chui AK, Lau WY, Chan FK, Hui AY, Rao AR, et al. Outcome of lamivudine resistant hepatitis B virus mutant post-liver transplantation on lamivudine monoprophylaxis. Clin Transplant. 2004;18(3):295–300. [DOI] [PubMed]
- 126.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongasawat S, Cooksley G, et al. Peginterferon alfa-2a (40KD) (PEGASYS®) monotherapy and in combination with lamivudine is more effective than lamivudine monotherapy in HBeAg-positive chronic hepatitis B: results from a large, multinational study. N Engl J Med. 2005;352(26):2682–95. [DOI] [PubMed]
- 127.Lim LL, Wai CT, Lee YM, Kong HL, Lim R, Koay E, et al. Prophylactic lamivudine prevents hepatitis B reactivation in chemotherapy patients. Aliment Pharmacol Ther. 2002;16:1939–44. [DOI] [PubMed]