Abstract
ADP-ribosylation of cell surface proteins in mammalian cells is a post-translational modification by which ecto-ADP-ribosyltransferases (ARTs) transfer ADP-ribose from extracellular NAD to protein targets. The ART2 locus at murine chromosome 7 encompasses the tandem Art2a and Art2b genes that encode the distinct ART2.1 and ART2.2 proteins. Although both ecto-enzymes share 80% sequence identity, ART2.1 activity is uniquely regulated by an allosteric disulfide bond that is reducible in the presence of extracellular thiols, such as cysteine and glutathione, that accumulate in hypoxic and ischemic tissues. Previous studies have characterized the expression of ART2.1 and ART2.2 in murine T lymphocytes but not in other major classes of lymphoid and myeloid leukocytes. Here, we describe the expression of ART2.1 activity in a wide range of freshly isolated or tissue-cultured murine myeloid and lymphoid leukocytes. Spleen-derived macrophages, dendritic cells (DC), and B cells constitutively express ART2.1 as their predominant ART while spleen T cells express both ART2.1 and the thiol-independent ART2.2 isoform. Although bone-marrow-derived macrophages (BMDM) and dendritic cells (BMDC) constitutively express ART2.1 at low levels, it is markedly up-regulated when these cells are stimulated in vitro with IFNβ or IFNγ. ART2.1 expression and activity in splenic B cells is modestly up-regulated during incubation in vitro for 24 h, a condition that promotes B cell apoptosis. This increase in ART2.1 is attenuated by IL-4 (a B cell survival factor), but is not affected by IFNβ/γ, suggesting a possible induction of ART2.1 as an ancillary response to B cell apoptosis. In contrast, ART2.1 and ART2.2, which are highly expressed in freshly isolated splenic T cells, are markedly down-regulated when purified T cells are incubated in vitro for 12–24 h. Studies with the BW5147 mouse thymocyte line verified basal expression of ART2.1 and ART2.2, as in primary spleen T cells, and demonstrated that both isoforms can be up-regulated when T cells are maintained in the presence of IFNs. Comparison of the surface proteins which are ADP-ribosylated by ART2.1 in the different leukocyte subtypes indicated both shared and cell-specific proteins as ART2.1 substrates. The LFA-1 integrin, a major target for ART2.2 in T cells, is also ADP-ribosylated by the ART2.1 expressed in macrophages. Thus, ART2.1, in contrast to ART2.2, is expressed in a broad range of myeloid and lymphoid leukocytes. The thiol redox-sensitive nature of this ecto-enzyme suggests an involvement in purinergic signaling that occurs in the combined context of inflammation and hypoxia/ischemia.
Keywords: NAD, ADP-ribosylation, Ecto-enzyme, Antigen presenting cell, Macrophage, Dendritic cell, B cell, T cell, Redox signaling
Introduction
Mono-ADP-ribosylation represents a post-translational protein modification catalyzed by toxin-related ADP-ribosyltransferases (ARTs) that are expressed as GPI-anchored or secreted ecto-enzymes in mammalian cells. These ecto-ARTs transfer the ADP-ribose moiety from extracellular NAD to arginine and cysteine residues on target protein substrates with resultant changes in activity [1–4]. The ability of extracellular NAD, acting through ecto-ARTs, to modulate the function of multiple cell surface proteins comprises an alternative route of purinergic signaling that operates in parallel with the well-characterized pathways targeted by P2 and P1 receptors. Based on sequence homology, the ecto-ART family in mammals includes four human subtypes (ART1, 3, 4, 5) and six murine subtypes (ART1, 2.1, 2.2, 3, 4, 5). Mammalian ART3 and ART4 lack measurable enzyme activity and have presumably acquired other functions as cell surface proteins. Although the other ARTs possess similar catalytic activities, they are characterized by distinct patterns of tissue-selective expression and utilization of different target proteins as predominant substrates [5].
The ART2 ecto-enzymes have been particularly well-characterized in rodent T lymphocytes. ART2 gene products are expressed as GPI-anchored proteins on the surfaces of murine T cells and catalyze ADP-ribosylation of CD8, CD43, CD44, CD45, and LFA-1 to regulate cell surface receptor clustering, cell trafficking, and signaling [6–8]. Additionally, ART2 mediates the NAD-induced transactivation of the P2X7 purinergic receptor (P2X7R) in murine T cells via ADP-ribosylation of Arg-125 on that receptor; this results in gating of Ca2+ influx, macropore formation, phosphatidylserine exposure, CD62L shedding and accelerated cell death [9, 10].
The ART2 locus at murine chromosome 7 encompasses the tandem Art2a and Art2b genes that encode the distinct ART2.1 and ART2.2 proteins; these share 80% identity in sequence and both function as ecto-ADP-ribosyltransferases or NAD glycohydrolases. However, an additional pair of unique cysteines (Cys-80 and Cys-201) in ART2.1 can result in disulfide bond formation that allosterically suppresses catalytic activity of this isoform. This inhibited state of ART2.1 is readily reversed in the presence of thiol reductants, such as exogenous dithiothreitol or endogenous cysteine or glutathione, that accumulate in the extracellular compartments of inflamed or hypoxically stressed tissues [11, 12]. The additional layer of allosteric regulation for ART2.1, but not ART2.2, indicates that these isoforms are not simply redundant gene products; this is also consistent with their differential expression in various inbred mouse strains [13–15]. These differences in allosteric regulation and effect of genetic background further suggest that ART2.1 versus ART2.2 may be selectively utilized for signaling by different subpopulations of leukocytes, or during particular inflammatory/immune responses that occur in the context of hypoxia and ischemia.
We have recently reported that bone-marrow-derived macrophages (BMDM) lack significant expression of any of the murine ecto-ART subtypes, but selectively up-regulate ART2.1 (but not ART2.2 or other subtypes) in response to multiple inflammatory mediators [16]. In contrast, freshly isolated T cells from the same mice basally express both ART2.1 and ART2.2 [17–23]. Given these striking differences in expression of ART2.1 and ART2.2 in different leukocyte subsets and under in vivo versus in vitro conditions, this study was designed to address two major questions: (1) Is ART2.1 is expressed in leukocyte types other than macrophages and T cells? (2) Is regulation of ART2.1 (or ART2.2) expression by cytokines and other local factors a general characteristic of ART2 biology in other leukocytic backgrounds, including B cells, dendritic cells, and T cells? Our experiments demonstrate that the thiol-sensitive ecto-ART2.1 is expressed as the functionally predominant ecto-ART subtype in myeloid and lymphoid cell types including B lymphocytes, dendritic cells, and tissue macrophages. The expression of ART2.1 was markedly higher in freshly isolated versus in vitro cultured populations of macrophages, dendritic cells, or T cells. This suggests that the robust expression of ART2.1 in vivo is maintained by local or humoral factors that are absent or diluted during in vitro cell culture. Additional studies indicate that in vitro ART2.1 expression is increased when macrophages, dendritic cells, and T cells, (but not B cells) are cultured in the presence of type 1 and type 2 interferons.
Materials and methods
Reagents LPS (E. coli serotype 01101:B4) was from List Biological Laboratories. Recombinant murine interferon-γ (IFN-γ) was from Boehringer Mannheim Biochemica, recombinant murine interferon-β (IFN-β) from US Biologicals, and recombinant murine IL-4 from R&D. ERK kinase inhibitor U0126 was from Calbiochem. NAD, etheno-NAD, ADP-ribose and TRIzol were from Sigma-Aldrich. Oligo dT primer was from Promega. AMV reverse transcriptase was from Roche. Taq DNA polymerase was from New England BioLabs. 1G4 mouse monoclonal antibody was prepared as previously described [16]. Goat polyclonal anti-actin (sc1615) antibody and all HRP-conjugated secondary antibodies were from Santa Cruz Biotechnology. APC-conjugated anti-F4/80 was from eBioscience and PE-conjugated anti-CD11c was from Pharmingen. PE- and FITC-conjugated Anti-CD3 and anti-CD19 were also from BD Pharmingen/BD Biosciences [23]. Mouse CD11c mAb was a generous gift from Dr. Clifford Harding (Case Western Reserve University).
Animals and cells BALB/c and C57BL/6 mice were purchased from Taconic, Inc. BALB/c mice with single knockout of ART2.1 (ART2.1KO), were generated and characterized as previously described [24]. All experiments and procedures using these various mouse strains were approved by the Institutional Animal Use and Care Committees of Case Western Reserve University, or Hamburg University Hospital. Bone-marrow-derived macrophages (BMDM) and bone-marrow-derived dendritic cells (BMDC) or splenocytes, including splenic T cells, B cells, DCs, and macrophages, were respectively prepared from the bone marrow or spleens of CO2-euthanized animals. For BMDM or BMDC, femurs and tibia were isolated from the euthanized mouse and the marrow cavity plugs were washed out by sterile PBS. BALB/c BMDM were expanded and grown in the presence of M-CSF as previously described [16]. For generation of BMDC, bone marrow cells were cultured in DMEM (Sigma-Aldrich) supplemented with 2 ng/ml GM-CSF (PeproTech), 10% calf serum (HyClone Laboratories), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies) in 150-mm dishes, in the presence of 10% CO2 at 37°C. The culture media were changed on the fourth and seventh day after the initial plating. The resulting BMDC were replated into six-well plates on the ninth day. Greater than 50% of these BMDC expressed high levels of CD11c as monitored by flow cytometry (data not shown) and the cells were used within 6 days. For isolation of B- and T lymphocytes, spleens from euthanized mice were cut into small pieces and passed through nylon mesh (100 μm) to generate single cell suspensions. The suspension was further processed by removal of erythrocytes, granulocytes, and debris using gradient centrifugation on Histopaque-1077 (Sigma-Aldrich). The mononuclear leukocyte layer at the gradient interface was used for purification of B- and T lymphocytes. B cells were isolated from the flow-through of Dynal mouse B cell negative selection magnetic beads (Invitrogen). T cells were isolated from the flow-through of Dynal mouse T cell negative selection magnetic beads (Invitrogen). Following isolation, B cells or T cells were either assayed immediately for ART expression or were placed in short-term (12–24 h) cell culture in RPMI-1640 medium supplemented with 10% calf serum, plus or minus other cytokines as indicated. BW5147 thymocytes were cultured as previously described [25].
RT-PCR analyses Total RNA was isolated using TRIzol reagent according to manufacturer’s instructions and quantified by optical density at 260 nm. Three micrograms of RNA and 1 µg of oligo dT primer were added into one RT reaction catalyzed by AMV reverse transcriptase (50 units). The RT reaction was incubated at 42°C for 60 min. The RT products were then used for PCR amplification in appropriate optimal cycling conditions for each primer set. The resulting PCR amplicons were electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining. The primers and PCR conditions for the six murine ecto-ARTs, iNOS, IRF-1, IFN-β and GAPDH were identical to those previously described [16]. Additionally, CD38 was used as a marker for the B cell population with the primer set: forward: 5′-GCAACATCACAAGAGAAGACTACGC-3′; reverse: 5′-ACACACTGAAGAAACCTGGCAGGCC-3′. The PCR with these CD38 primers was run at: 94°C, 45 s; 50°C, 30 s; 72°C, 60 s for 40 cycles.
1G4 mAb-based ART activity assay and Western blot protocols ART activity was assayed in BMDC, BMDM, freshly isolated tissue macrophages, splenic T lymphocytes, splenic B lymphocytes, or BW5147 thymoma cells using the 1G4 antibody [26] that detects etheno-ADP-ribosylated proteins as previously described [16]. Briefly, intact cells were transferred to basic salt solution (BSS) containing 130 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 25 mM HEPES (pH 7.5), 5 mM glucose and 1% BSA, supplemented with the indicated concentrations of ε-NAD substrate and 1 mM ADP-ribose (ADP-R) plus or minus 2 mM dithiothreitol (DTT). The ADP-ribosylation reaction time was 15 min at 37°C and the reactions were terminated by removal of incubation media. The cells were washed with PBS and lysed on ice for 20 min in PBS containing 1% TritonX-100, 1 mM DTT, 2 µg/ml leupeptin, 100 µg/ml PMSF, and 2.5 µg/ml aprotinin. The collected lysates were centrifuged at 15, 000×g for 10 min at 4°C and the supernatants were transferred to new tubes. Proteins were denatured by addition of 4× SDS gel loading buffer and boiling for 5 min. The samples were electrophoresed on 15% SDS-PAGE gels and transferred (24 V for 54 min) to PVDF membranes (Millipore) in Tris–Glycine buffer (9.09 g Tris, 43.2 g glycine, 600 mL methanol, 2.4 L H2O). The membranes were blocked in immunoblot (IB) buffer (10 mM Tris (pH 7.4), 0.9% NaCl, 0.05% Tween 20, and 1 mM EDTA) containing 4% nonfat milk for 1 h and incubated with primary antibodies (1G4 at 75 μg/ml; anti-actin at 0.4 μg/ml) at 4°C for 12–15 h. After five washes in IB buffer, the membranes were incubated with appropriate horseradish-peroxidase-conjugated secondary antibodies (anti-mouse at 80 ng/ml; anti-goat at 160 ng/ml) at room temperature for 1 h and washed five times in IB buffer. The membranes were developed by chemiluminescent reagents (SuperSignal, Pierce) and exposed to Eastman Kodak X-ray film.
Immunoprecipitation and analysis of ADP-ribosylated LFA-1 integrin Intact BALB/c BMDM were incubated in fresh DMEM containing 15% calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in the presence of 100 ng/ml LPS and 10 μg/ml U0126 for 24 h. The primed cells were transferred to basic salt solution (BSS) containing 130 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 25 mM HEPES (pH 7.5), 5 mM glucose and 0.1% BSA. The cells were then incubated at 37°C for 15 min with the 50 μM ε-NAD substrate and 1 mM ADP-ribose (ADP-R) plus 2 mM dithiothreitiol (DTT). Washed cells were lysed in 200 µl ice-cold PBS containing 1% TritonX-100 (Sigma-Aldrich), 2 mM DTT, 2 µg/ml leupeptin, 100 µg/ml PMSF, and 2.5 µg/ml aprotinin. Insoluble material was pelleted by high-speed centrifugation (15 min, 13,000×g). Two micrograms per milliliter anti-LFA-1 Ab (Santa Cruz) was added into the lysate and incubated 2 h at 4°C, and lysates were further incubated with protein G PLUS agarose beads (20 μl beads/lysates from 106 cells) for 60 min at 4°C. Immunoprecipitates were washed three times using TX-100 buffer. The final product was eluted into 30 μl 2× SDS binding buffer and boiled for 5 min. The samples were loaded on 15% SDS-PAGE gels and ADP-ribosylated proteins were detected by 1G4 Ab-based Western blot.
Fluorescence-activated cell-sorting analyses and 1G4 mAb-based FACS assays Leukocytes were isolated from the spleens of BALB/c mice. B cells and T cells were respectively stained with fluorochrome-conjugated antibodies against CD19 and CD3 for 30 min at 4°C. Splenocytes negative for CD3 and CD19 were gated and analyzed for expression of CD11c and F4/80 to define the dendritic cell (DC) and macrophage populations, respectively. The defined leukocyte subsets were then stained with antibodies specific for human ART4 (isotype control), ART2.1, or ART2.2. Staining for ART2.1 was performed with Alexa488-labeled mAb Gugu2-22 for 30 min at 4°C. Staining for ART2.2 was performed with Alexa488-labeled mAb Nika102 for 30 min at 4°C. Etheno-ADP-ribosylation of cell surface proteins was monitored using Alexa488- or Alexa467-conjugated, etheno-adenosine specific mAb 1G4 following incubation with 10 µM etheno-NAD as previously described [26]. Stained cells were washed and analyzed on a FACS-Canto II using Diva (Becton Dickinson) and FlowJo (Treestar) software. Gating was performed on living cells on the basis of propidium iodide exclusion. For FACS-based analyses, the cells were incubated with 10 µM ε-NAD and 1 mM ADPR in the presence or absence of DTT for 15 min.
Results
Expression of thiol-sensitive ecto-ART2.1 in freshly isolated spleen macrophages, dendritic cells, and B cells
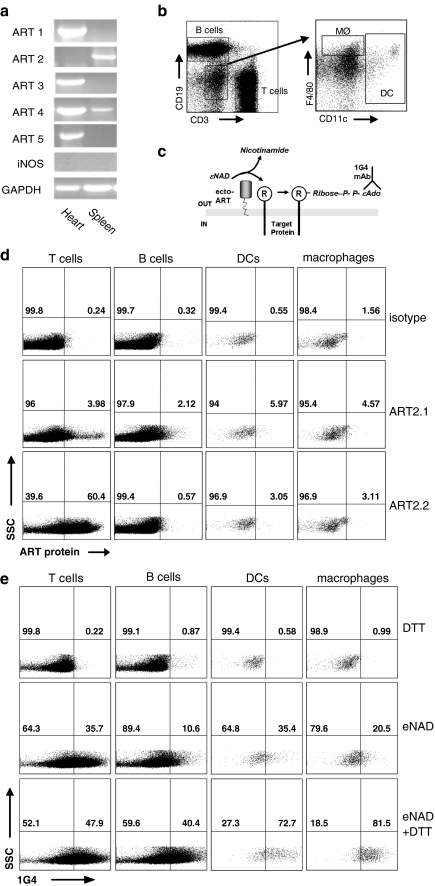
A previous study reported that ecto-ART functional activity was present in splenic T cells but not in splenic B cells or peritoneal macrophages from C57BL/6 mice [8]. This is consistent with the well-characterized expression of ART2.2 in most T cell-lineage subsets from this and other murine strains. However, because C57BL/6 cells cannot express functional ART2.1 protein [27], these earlier results implied that ART2.2 is likely absent in B cells and macrophages, but left open the possibility that ART2.1 might be expressed by these latter leukocyte subsets in mouse strains other than C57BL/6. In this regard, we recently reported that ART2.1 is selectively expressed as a cytokine-inducible ecto-ART in tissue-cultured bone-marrow-derived macrophages from BALB/c mice [16]. Given that splenic T lymphocytes from BALB/c mice constitutively express both ART2.1 and ART2.2, we tested whether the resident macrophages and dendritic cells (DC), as well as the B cell population, in the spleens of this murine strain also express ART2.1 or other ecto-ARTs. RT-PCR analysis indicated ART2, and ART4 mRNA, but not ART1, ART3, or ART5, was present in whole spleen extracts (Fig. 1a) and we have previously reported that BALB/c splenocytes express both ART2.1 and ART2.2 mRNA [16]. Because murine ART4 lacks enzymatic activity, these data indicated that only ART2.1 or ART2.2 can catalyze the ecto-ART reactions observed in any of the leukocyte subpopulations within the spleen. We used mAbs selective for ART2.1 or ART2.2 to detect expression of the corresponding enzymes on the surface of various leukocyte subpopulations within the spleen as reported in previous studies with T cells [16]. The gating strategy used to define T cell, B cell, macrophage, and splenic DC populations of splenocytes is illustrated in Fig. 1b. As expected from previous studies, significant levels of ART2.2 were only detected on the surface of T cells, although a low level of staining slightly above the background defined by an isotype control was also observed on the other cell populations studied (Fig. 1d lower panels). ART2.1 protein was detected on all cell populations, albeit at low levels (middle panels). Importantly, while ART2.2 was expressed to much higher levels than ART2.1 on T cells, ART2.1 expression was higher than that of ART2.2 on B cells, dendritic cells and macrophages. We next assayed the ecto-ART activity in the different spleen leukocyte subtypes by FACS analyses with the 1G4 mAb. As schematically indicated in Fig. 1c, this mAb selectively recognizes the ε-ADP-ribose moieties covalently attached to target proteins by ART-dependent ADP-ribosylation in the presence of εNAD, an NAD analog [26]. We used a FACS-based protocol to distinguish 1G4-positive cells within the CD3high T cell, CD19high B cell, F4/80high macrophage, and CD11chigh DC subpopulations of BALB/c splenocytes incubated with εNAD in the absence or presence of DTT (Fig. 1e). As expected, T cells showed substantial ART activity, as measured by 1G4 fluorescence intensity upon incubation with εNAD, both in the presence and absence of DTT, consistent with expression of thiol-independent ART2.2 by this cell population. Cell surface ART activity was also detected on B cells, macrophages, and DC. Notably, and in contrast to the situation observed for T cells, cell surface ART activity in these cell populations was much more robust (by two- to fourfold) in the presence of DTT. DTT-dependent ART activity was highest on macrophages, followed by CD11c+ splenic DCs, and B cells. Furthermore, it is of note that DTT-dependent ART activity was observed on the above-mentioned cell populations regardless of whether the experiments were performed in the presence or absence of ADP-ribose, a known inhibitor of the CD38 NADase (data not shown). This indicates that competition for the NAD substrate by CD38 may modulate, but does not completely block ART activity on these cells (also see Fig. 6b). These data indicate that functional ecto-ART activity is not limited to T cells but is also basally expressed in tissue resident DCs, macrophages, and B cells. However, in contrast to T cells, ecto-ART activity in these latter cell populations is largely dependent on the presence of a reducing agent suggesting that it is mediated by the thiol-dependent ART2.1.
Fig. 1.
FACS analysis reveals basal expression of thiol-sensitive ecto-ART2.1 in the B lymphocyte, macrophage, and DC populations from mouse spleen. a cDNAs from spleen and heart of BALB/c mice were analyzed by RT-PCR for ART1, ART2, ART3, ART4, and ART5 mRNA content. b Fluorescence-activated cell sorting (FACS) gating strategy used to identify T and B lymphocytes, DCs and macrophages. Splenocytes negative for CD3 and CD19 (left panel) were analyzed for expression of CD11c and F4/80 to define dendritic cells and macrophages, respectively (right panel). c Schematic illustrating the 1G4-based assay for detection of ADP-ribosylated proteins. In the presence of etheno-NAD and active ART, etheno-ADP-ribose is incorporated into target proteins. This moiety is detected by the monoclonal antibody 1G4, specific for etheno-adenosine. d FACS detection of ART2 proteins on the surface of spleen cell populations. The cell populations defined in b were stained with antibodies specific for human ART4 (isotype control), ART2.1, or ART2.2. e Cell surface ART activity detected on different spleen cell populations by the 1G4-based FACS assay. Freshly isolated spleen cells were incubated for 10 min with 10 µM ε-NAD in the absence or presence of 2 mM DTT. Relevant cell populations were identified by gating as shown in b, and ART activity was determined by staining with Alexa488-conjugated 1G4. The results are representative of observations from three independent experiments
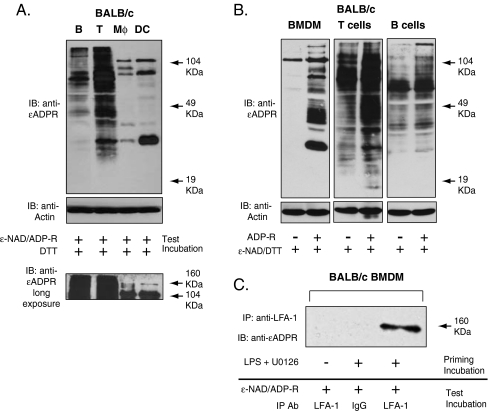
Fig. 6.
Integrins and other cell surface proteins as substrates for the ART2.1 expressed in lymphoid and myeloid leukocytes. a Major ADP-ribosylation targets for ART2.1 in freshly isolated spleen B and T cells versus IFN-γ (100 u/ml; 24 h) primed cultures of BMDM and BMDC (normalized for identical protein content (40 μg) per lane). Thiol-dependent ecto-ART activity was assayed as described in Fig. 4c. Results are representative of three independent experiments. b Effect of exogenous ADP-ribose on the relative accumulation of ADP-ribosylated proteins in freshly isolated spleen B and T cells versus IFN-γ (100 u/ml; 24 h) primed cultures of BMDM. c BALB/c BMDM were primed without or with 100 ng/ml LPS and 10 μg/ml U0126 for 24 h and then incubated for 15 min with 50 μM ε-NAD, 1 mM ADP-ribose (ADP-R), and 2 mM DTT. The cells were then lysed and processed for immunoprecipitation with anti-LFA or irrelevant IgG as described in the “Materials and methods” section. ADP-ribosylated proteins in the IPs were detected by probing the Western blot with 1G4 mAb. Results are representative of two experiments
ART2.1 is the predominant ecto-ART subtype in bone-marrow-derived macrophages
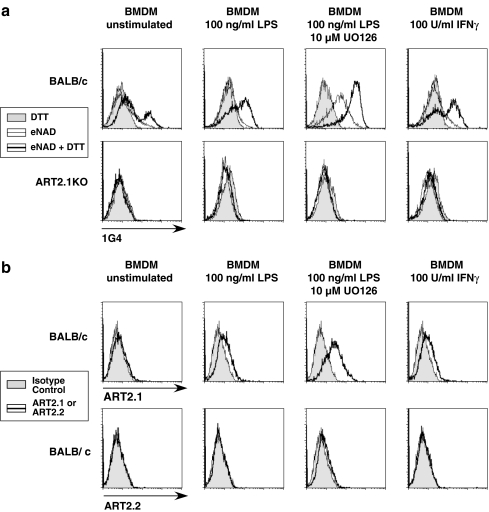
The significant basal expression of ecto-ART activity in resident tissue macrophages (Fig. 1) contrasts with the very low basal ecto-ART expression in cultured bone-marrow-derived macrophages (BMDM) that we recently reported [16]. That study indicated BALB/c BMDM selectively express ART2.1 as a predominant ecto-ART, but only after activation by IFN or LPS. To further characterize the selective expression of ART2.1 in macrophage-lineage cells, we measured the basal and cytokine/LPS-inducible ecto-ART activities in bone-marrow-derived macrophages (BMDM) from control BALB/c mice versus BMDM from an ART2.1-knockout BALB/c strain. DTT-dependent ART activity was increased when control BMDM were stimulated with either LPS or IFN-γ (Fig. 2a); the effect of LPS was further potentiated by simultaneous inhibition (with U0126) of the MEK/ERK signaling pathway as previously reported [16]. In contrast, the ART2.1-knockout BMDM expressed little ecto-ART activity, in the absence or presence of DTT, either before or after induction with LPS/IFN-γ. Consistent with these ecto-ART activity profiles, no anti-ART2.2 staining was observed in the control BALB/c BMDM either before or after pro-inflammatory stimulation (Fig. 2b). As with the freshly isolated spleen macrophages, ART2.1 staining was detected in the BMDM but only after induction with LPS or IFN-γ.
Fig. 2.
FACS analysis reveals that cytokine-inducible ART2.1 is the predominant ecto-ART in murine bone-marrow-derived macrophages from BALB/c mice. a BMDM from wildtype BALB/c mice or ART2.1 knockout mice (BALB/c background) were transferred to M-CSF-free medium and then stimulated with or without 100 ng/ml LPS in the absence or presence of 10 µM U0126, or with 100 U/ml IFN-γ for 20 h. Aliquots of the control and primed cells were incubated for 10 min with 50 µM εNAD in the absence or presence of 2 mM DTT and then stained with Alexa488-conjugated, ε-adenosine specific mAb 1G4 followed by FACS analysis. B) Parallel aliquots of control and LPS- or IFN-γ-primed wildtype BALB/c BMDM were stained with anti-ART2.1 or anti-ART2.2 mAbs and assayed by FACS analysis as described in the “Materials and methods” section and Fig. 1 legend. The gray histograms show staining with an isotype control antibody (anti-human ART4)
Interestingly, bone-marrow-derived macrophages stimulated with LPS/U0126 express measurable ecto-ART activity even in the absence of exogenously added reducing agents (Fig. 2a). This activity must be due to ART2.1, as it is absent on ART2.1 single-knockout cells. This suggests that these cells secrete endogenous thiols, such as glutathione or cysteine that mimic the ability of exogenously added DTT to support ART2.1 activity.
ART2.1 is the predominant ecto-ART in murine dendritic cells
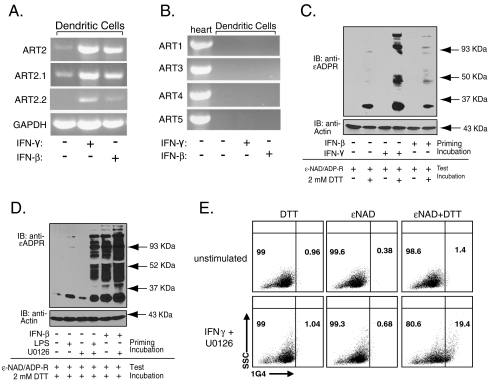
Macrophages and dendritic cells are derived from a common set of myeloid progenitor cells in bone marrow; these progenitors differentiate into BMDM in the presence of M-CSF but become bone-marrow-derived dendritic cells (BMDC) in the presence of GM-CSF. Given this shared lineage from common marrow progenitors, we tested whether BMDM and BMDC exhibit a similar pattern of ecto-ART expression, particularly with regard to ART2.1. GM-CSF-expanded BMDC basally expressed ART2.1 mRNA (Fig. 3a), but not any of the other ecto-ART subtypes (Fig. 3b). RT-PCR analyses further indicated that the genes for ART2 isoforms (Fig. 3a), but not other ART subtypes (Fig. 3b), were selectively induced when BMDC were stimulated with IFNs. IFN-γ increased both ART2.1 and ART2.2 mRNA in BMDC. In contrast, IFN-β stimulation augmented the amount of ART2.1 mRNA, but induced only minor increases in ART2.2 expression. In both control and IFN-stimulated BMDC, functional ART activity was observed as indicated by the presence of ε-ADP-ribosylated proteins in cell lysates following incubation of intact cells with εNAD (Fig. 3c). The accumulation of these ADP-ribosylated protein bands during incubation of intact BMDC with ε-NAD substrate was dependent on the presence of extracellular DTT. This indicated that the thiol-dependent ART2.1, rather than the thiol-independent ART2.2, catalyzed ADP-ribosylation. In the absence of IFN stimulation, the basal ART activity was predominantly limited to ADP-ribosylation of a single 34-KDa substrate, which may indicate auto-ADP-ribosylation of ART2.1 itself [28]. In BMDC stimulated with IFNs for 12 or 24 h, additional higher molecular weight proteins were ADP-ribosylated in a time-dependent manner (Fig. 3c and d). This increase in ADP-ribosylation of multiple surface proteins in IFN-primed cells correlated with the increased expression of ART2.1 mRNA. Given the critical role of GM-CSF in DC differentiation [29], we tested whether the presence or absence of GM-CSF per se modulates ART2.1 expression or activity in BMDC. Removal of GM-CSF from the culture medium for 24 h modestly increased basal ART2.1 activity in BMDC (data not shown). We also tested the effect of LPS on the induction of ART2.1 activity in BMDC. By itself, a 24-h exposure to LPS induced only a minor increase in ART activity as indicated by the intensity of the 34-KDa ADP-ribosylated protein band. Similar to our previous finding that the LPS-induced ART2.1 expression in BMDM is potentiated by the suppression of the ERK1/2 pathway (Fig. 2 and [16]), blockade of this pathway by the MEK1 inhibitor U0126 also markedly increased induction of ART2.1 activity by LPS and IFN-β in BMDC (Fig. 3d).
Fig. 3.
Cytokine-inducible ART2.1 is the predominant ecto-ART in murine bone-marrow-derived dendritic cells. a BMDC (from BALB/c mice) were stimulated with IFN-β (100 U/ml) or IFN-γ (100 U/ml) for 24 h before extraction and RT-PCR analysis for ART2 (total), ART2.1, ART2.2, and GAPDH mRNA content. b Aliquots of cDNAs from the experiments in panel a were analyzed by RT-PCR for ART1, ART3, ART4, and ART5 mRNA content. As controls, expression of ART1, 3, 4, or 5 mRNA were assayed by RT-PCR in freshly isolated heart from BALB/c mice. c BMDC were stimulated with IFN-β (100 U/ml) or IFN-γ (100 U/ml) for 12 h (priming incubation). Cells were incubated with εNAD before Western blot analysis for εADP-ribosylated proteins. d BMDC were stimulated with LPS (100 ng/ml) or IFN-β (100 U/ml) for 24 h in the presence or absence of 10 μM U0126 (priming incubation). Cells were incubated with εNAD before Western blot analysis for εADP-ribosylated proteins as in c. e BMDC incubated for 24 h in the presence or absence of IFN-γ (100 U/ml) and 10 µM U0126 were analyzed by FACS for ecto-ART activity by 1G4 staining as in Fig. 1
We used the previously described FACS-based analyses (Fig. 1) to characterize 1G4 staining (Fig. 3e) in individual cells from control BMDC cultures versus BMDC stimulated with IFN-γ plus U0126. These measurements verified the expression of a thiol-dependent ART reactivity in individual BMDC but only after priming with an inflammatory cytokine. Staining with antibodies specific for ART2.1 or ART2.2 failed to reveal detectable levels of either isoform on these cells (data not shown). Nonetheless, the clear dependence of the observed ART activity on the presence of a reducing agent strongly suggests that ART2.1 is the responsible enzyme.
ART2.1 is the predominant ecto-ART in murine B cells
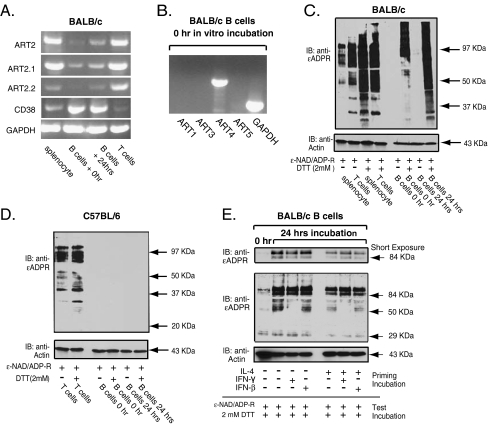
Immunomagnetic beads were used to separate the B cell and T cell populations from BALB/c splenocytes for in vitro characterization of ART subtype expression. RT-PCR analyses indicated that the B cells basally express mRNA transcripts for ART2.1 and ART4 but not ARTs 1, 3, or 5 (Fig. 4a and b). Consistent with previous studies [23], the B cells expressed abundant transcripts for CD38. In contrast, the T cell fraction contained high levels of mRNA for both ART2.1 and ART2.2 but little CD38 (Fig. 4a). Consistent with the basal expression of ART2.1 mRNA, the freshly isolated intact B cells also showed robust ART activity but only in the presence of DTT (Fig. 4c). In contrast, unfractionated splenocytes or purified T cells exhibited similarly strong ART activities when assayed in the absence or presence of DTT Fig. 4c). The absence of ADP-ribosylation activity in splenic B cells isolated from C57BL/6 mice, strongly suggested that the thiol-dependent ART activity observed in the purified BALB/c splenic B cells was due to the ART2.1 enzyme and was not due to contamination by residual T cells (Fig. 4d). Thiol-independent ART activity was observed in C57BL/6 T cells, consistent with their normal expression of ART2.2 (Fig. 4d). In vitro incubation of the BALB/c B cells for 24 h resulted in significant increases in both ART2.1 mRNA and thiol-dependent ART activity (Fig. 4a, c, and e). Note that the Western blot in Fig. 4e was developed for a shorter time than that in Fig. 4c to facilitate comparison of relative signal strengths in the major ART substrate proteins at ∼55 kDa, 85 KDa, and 105 KDa. Naïve splenic B cells incubated in vitro in the absence of survival factors undergo rapid apoptosis with ∼50% of the population becoming TUNEL-positive within 24 h [30]. Inclusion of IL-4, which suppresses B cell apoptosis [30], in the culture medium modestly attenuated the observed increase in ART2.1 activity (Fig. 4e). Thus, the increased ART2.1 expression in short-term cultures of B cells may be a secondary consequence of apoptotic induction. Previous studies have reported that naive B cells cultured in the presence of IFN-α or IFN-β, but not IFN-γ, exhibit markedly increased expression of CD69, CD86, and other activation markers [31]. However, no obvious decrease or increase in ADP-ribosylation of the major ART substrate proteins was observed when B cells were incubated in vitro in the presence of either IFN-β or IFN-γ (Fig. 4e).
Fig. 4.
ART2.1 is the predominant ecto-ART in splenic B lymphocytes. a BALB/c splenocytes were used for isolation of B cells and T cells as described in the “Materials and methods” section. B cells were processed for RNA isolation immediately after isolation or after incubation in vitro for 24 h, while splenocyte and purified T cells were processed immediately after isolation. RNA was converted to cDNA and used for RT-PCR analysis for ART2 (total), ART2.1, ART2.2, CD38, and GAPDH mRNA content. b The cDNA from freshly isolated B cells was assayed by RT-PCR for ART1, 3, 4, or 5. c Splenocytes, purified B cells, or purified T cells were used immediately for ecto-ART assays or, for B cells only, were also incubated in vitro for 24 h prior to ecto-ART analysis. The various lymphocyte subsets were incubated with 50 µM ε-NAD and 1 mM ADP-ribose in the presence or absence of 2 mM DTT at 37°C for 15 min (test incubation) before extraction for SDS-PAGE and Western blot analysis for 1G4 reactive εADP-ribosylated proteins. d B cells and T cells isolated from C57BL/6 mice were assayed for ecto-ART activity as described in panel C. e Isolated BALB/c B lymphocytes were incubated without or with the indicated mixtures of IL-4 (4 ng/ml), IFN-β (100 U/ml), and IFN-γ (100 U/ml) for 0 or 24 h (priming incubation) and then assayed for ecto-ART activity as in c. The top panel indicates a shorter film exposure of the 80–100-KDa region of the blotted membrane. Results in all panels are representative of observations from two to three independent experiments
Regulated expression of ART2.1 and ART2.2 in murine T cells
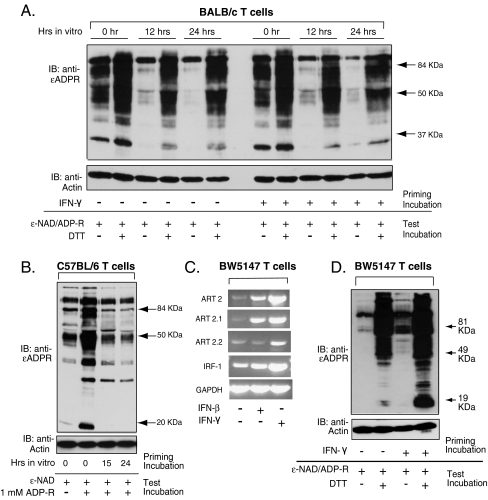
ART2.1 is significantly up-regulated by IFNs in macrophages (Fig. 2 and [16]) and dendritic cells (Fig. 3) but not in B cells (Fig. 4). This suggested that the IFN induction of ART2.1 expression might be limited to myeloid leukocytes and absent in the lymphoid leukocytes. However, ART2.1 was induced in purified B cells as a likely response to the absence of extrinsic survival factors when cultured in vitro (Fig. 4). Thus, we tested whether the robust ART2.1 and ART2.2 expression observed in freshly isolated spleen T cells is modulated either upon removal of in vivo factors or when supplemented with exogenous IFN-γ, a known modulator of T cell proliferation and survival [32]. T cells from the spleens of BALB/c mice were purified by negative selection on immunomagnetic beads and then incubated for 12–24 h in the absence or presence of IFN-γ. DTT-independent ecto-ART activity was assayed as an index of ART2.2 expression while activity in the presence of added DTT provided a measure of combined ART2.1 and ART2.2 expression. Compared to freshly isolated cells, T cells incubated in standard serum-supplemented culture medium for 12–24 h were characterized by markedly decreased ART2.2 activity, but only a modest decrease in the combined ART2.1/ART2.2 activity supported by DTT (Fig. 5a). A time-dependent decrease in DTT-independent ART activity was also observed when freshly purified splenic T cells from C57BL/6 mice were cultured in vitro (Fig. 5b). This further suggests that ART2.2, rather than ART2.1, is the predominant isoform that is down-regulated during culture of BALB/c T cells.
Fig. 5.
Basal and cytokine-modulated expression of ART2.1 and ART2.2 in splenic T cells and BW5147 thymocytes. a T cells were isolated from BALB/c spleens by negative selection (immuno-depletion of B cells and myeloid cells) and were either assayed for ecto-ART activity immediately after isolation or after in vitro incubation for 12 or 24 h in the absence or presence of IFN-γ (100 U/ml). T cells were assayed for ecto-ART activity as described in Fig. 4c. b T cells were isolated from C57BL/6 spleens by negative selection and were assayed for ecto-ART activity immediately after isolation or after in vitro incubation for 15 or 24 h. T cells were assayed for ecto-ART activity as described in Fig. 4c. c BW5147 T cells were stimulated without or with IFN-β (100 U/ml) or IFN-γ (100 U/ml) for 24 h before extraction and RT-PCR analysis for ART2 (total), ART2.1, ART2.2, IRF-1, and GAPDH mRNA content. d BW5147 T cells were incubated without or with IFN-γ (100 U/ml) for 24 h (priming incubation) prior assay of ecto-ART activity as described in Fig. 4c. All results are representative of observations from two to three independent experiments
Neither DTT-independent nor DTT-dependent ADP-ribosylation of surface proteins was significantly modulated by inclusion of IFN-γ in the culture medium (Fig. 5a). However, the rapid down-regulation of ART2.1 and ART2.2 during short-term incubation of splenic T cells made it difficult to gauge the contribution of any possible stimulatory effect of IFN-γ on ART2.1/ART2.2 expression. To further test whether IFNs can enhance ART2.1 and/or ART2.2 expression in T cell-lineage leukocytes, we utilized the BW5147 murine T cell line. RT-PCR analyses confirmed that BW5147 cells, like primary BALB/c spleen T cells, constitutively express both ART2.1 and ART2.2 mRNA (Fig. 5c). The ART2.1 mRNA level in these cells was markedly up-regulated by both IFN-β and IFN-γ while ART2.2 expression was enhanced by IFN-γ, but not IFN-β. IRF-1 up-regulation by IFN-β/γ was correlated with the increased ART2 expression. As in primary T cells, BW5147 cells also express DTT-dependent and DTT-independent ART activities on their extracellular surface (Fig. 5d). IFN-γ stimulation caused marked increases in the DTT-sensitive and DTT-independent ART activities (Fig. 5d). IFN-β priming (data not shown) resulted in a marked up-regulation of the DTT-sensitive ART2.1 function but only a modest increase in DTT-independent ART activity; these relative changes in ART activities were consistent with the observed changes in ART2.1 and ART2.2 mRNA levels (Fig. 5c).
Integrins and other cell surface proteins as substrates for the ART2.1 expressed in lymphoid and myeloid leukocytes
The studies described above indicate that the thiol-sensitive ART2.1, in contrast to ART2.2, is expressed in a broad range of myeloid and lymphoid leukocytes. This raises questions as to whether common and/or cell-specific surface proteins are preferentially targeted by ART2.1 in these various leukocyte subsets. Figure 6a compares the major ADP-ribosylation targets for ART2.1 in freshly isolated spleen B and T cells versus IFN-γ primed cultures of BMDM and BMDC (normalized for identical protein content per lane); the T cell lane necessarily includes proteins targeted by ART2.1 and/or ART2.2. Several common protein substrates at ∼105, 80, and 35 kDa were apparent in all four leukocyte extracts, albeit at different intensities which likely reflect cell-specific differences in the copy number of ART2.1 and/or the copy number of the protein substrate. However, other ribosylated bands, e.g., in the 19–35-kDa region, were unique to lymphoid-lineage leukocytes.
Other ecto-enzymes that modulate the efficiency of NAD-dependent ADP-ribosylation of cell surface proteins can also be differentially expressed in leukocyte subsets. These include: (1) the CD38 NAD glycohydrolases and CD203 nucleotide pyrophosphatases that metabolize extracellular NAD and thereby reduce substrate drive to the ARTs; and (2) the ADP-ribose hydrolases (ARHs) that de-ADP-ribosylate proteins and thereby reverse the effects of ARTs (2). As indicated in previous figures, extracellular ADP-ribose (1 mM) was routinely added with NAD to minimize the actions of these other ecto-enzymes. We also assessed the relative contributions of these latter reactions in macrophages, T cells, and B cells by comparing the accumulation of ADP-ribosylated proteins in the absence or presence of ADP-ribose (Fig. 6b). Inclusion of ADP-ribose strongly facilitated accumulation of modified proteins by macrophages while only modestly increasing the labeling of T cell proteins and exerting an intermediate effect in B cells. Interestingly, the ART2.2-selective modification of surface proteins in C57BL/6 T cells showed a greater sensitivity to exogenous ADP-ribose (Fig. 5b) than the combined ART2.1/ART2.2-catalyzed ADP-ribosylation patterns observed in BALB/c T cells (Fig. 6b).
Notably, high-molecular-mass substrates in the 160–180-kDa region were very prominent in both B and T cells and were also evident in the macrophage and DC extracts when the immunoblot peroxidase reactions were over-developed (Fig. 6a, lower panel). The 100 and 160–180-kDa ADP-ribosylated bands suggest that β and α subunits of certain integrins may be common targets for the ART2.1 expressed in all leukocyte subsets. Previous studies have identified LFA-1 (CD11a/CD18) as a major ART2.2 substrate in T cells that results in ADP-ribosylation of both the CD11a α subunit and the CD18 β subunit [8, 33]. We used anti-LFA-1 mAb to immunoprecipitate protein in a lysate prepared from LPS/U0126-primed BMDM that were acutely incubated with ε-NAD for 15 min prior to extraction and immunoprecipitation (Fig. 6c). These data indicate that the 180-kDa α subunit of LFA-1 is also a substrate for the ART2.1 expressed in inflammatory macrophages.
Discussion
These studies demonstrate that the ART2.1 ecto-enzyme is basally expressed at functionally significant levels in murine macrophages, dendritic cells, and B lymphocytes. Moreover, both myeloid leukocytes (BMDC and BMDM) and lymphoid T cells, but not B cells, respond to type I and type II IFNs with marked increases in expression of ART2.1 mRNA and activity. These findings add to the existing body of data regarding expression of other ecto-ART subtypes in different leukocyte populations. Human neutrophils constitutively express ART1 stored in an intracellular pool which can be rapidly mobilized to the cell surface in response to activation by formyl-Met-Leu-Phe [34]. Human monocytes basally express ART3 mRNA and inducibly express ART4 mRNA in response to LPS priming [35, 36]; however, neither ART3 nor ART4 appear to act as functionally active ADP-ribosyltransferases. As noted previously, the expression and function of the ART2 gene products have been extensively characterized in murine T cells [9, 17, 33, 37] and we recently reported that ART2.1 is selectively expressed in murine BMDM stimulated with IFNs or LPS [16]. These new data conclusively demonstrate that ART2.1 is expressed as a functional ecto-enzyme in a much broader range of leukocyte subtypes (dendritic cells, macrophages, B cells, and T cells) than is ART2.2 which appears restricted to T cell subsets.
The observed differences in extent and inducibility of ART2.1 expression in tissue myeloid cells, bone-marrow-derived myeloid cells, and lymphoid-lineage cells suggest that the basal expression of this gene product may vary with proliferation or differentiation state as well as lineage. Freshly isolated splenic macrophages and splenic dendritic cells exhibited robust DTT-dependent ART activity (Fig. 1e) despite the very modest expression of immunoreactive ART2.1 protein that could be detected by FACS (Fig. 1d). However, tissue-cultured BMDC (Fig. 3) and BMDM (Fig. 2 and [16]) were characterized by modest basal ART2.1 expression that was markedly up-regulated in response to inflammatory activation.
The strong basal expression of ART2.1 and ART2.2 in freshly isolated T cells (Figs. 1 and 5) and an established T cell line (Fig. 5) indicates that these genes are constitutively active in the T cell background. This also suggests that basal ART2 expression may involve regulation by T-cell-specific transcription factors. The robust in vivo expression of ART2.1 and ART2.2 in freshly isolated spleen T cells suggests that extrinsic in vivo factors may amplify or sustain the activity of such transcription factors. This possibility is supported by our finding that the activities of ART2.2 and ART2.1 (to a lesser extent) markedly decreased when purified spleen T cells were placed in short-term cell culture (Fig. 5a). Although expression of ART2.1 and 2.2 was not obviously modulated during short-term (24 h) in vitro culture of primary T cells, both ecto-enzymes were up-regulated in BW5147 thymocytes cultured in the presence of IFNs. With the caveat that BW5147 are a transformed T cell line, these studies suggest that the expression of both ART2.1 and ART2.2 can be modulated by cytokines and possibly other extrinsic factors in T lymphocytes at different developmental stages.
In contrast to T cells, expression of only ART2.1, but not ART2.2, is constitutive in B cells (Fig. 4) and neither gene appeared to be inducible by interferons. However, modest increases in ART2.1 activity during short-term culture of isolated B cells were reversed by IL-4 suggesting a possible induction of ART2.1 as an ancillary response to B cell apoptosis. Finally, in myeloid leukocytes, the ART2.1 (and perhaps ART2.2) gene product is constitutively expressed at a low level and is induced to varying degrees in response to tissue-specific environmental factors that may include extracellular matrix, chemokines, cytokines, and direct contact with other non-myeloid cell types.
In some experiments with macrophages or DCs, we observed some ecto-ART activity even in the absence of added DTT. This nominally DTT-insensitive activity was most likely due to ART2.1 and not ART2.2 since it was not found in leukocytes from BALB/c knockout mice which lack the thiol-sensitive ART2.1 but express the thiol-insensitive ART2.2. Additionally, ART activity in the absence of exogenous DTT was also observed in leukocytes from NZW mice which are genetically defective in ART2.2 expression (data not shown). We tentatively conclude that ART2.1 activity can be supported by endogenous thiols, such as cysteine and glutathione, released from leukocyte suspensions, particularly when incubated with NAD at high cell concentrations. Extracellular thiols in circulating plasma include 8–10 μM cysteine, ∼40 μM cystine, and 2–4 μM glutathione (GSH) [38]. In the fasting state, GSH is released from the liver and skeletal muscle into the circulation; extracellular GSH is steadily metabolized by cell surface γ-glutamyl transferases to yield free cysteine. Significantly, the concentration of extracellular cysteine within inflammatory loci can rise to submillimolar levels due to increased release and degradation of GSH by activated macrophages [12, 39]. Depending on local oxygenation, the accumulated cysteine will be variably oxidized to cystine to set the local redox ratio of Cys/CysSS, and this ratio will be increased in interstitial compartments with low oxygen tension as occurs in ischemic or hypoxic tissue. Thus, ART2.1 activity will be facilitated in leukocytes by the hypoxic conditions that often characterize inflamed or damaged tissues.
It should be noted that some ART2.2 mRNA, but no measurable thiol-independent ART activity, can be measured in tissue-cultured B cells (Fig. 4), BMDC (Fig. 3), and BMDM from BALB/c mice, as well as BMDM from C57BL/6 animals [16]. However, we were unable to measure any significant DTT-independent ART activity in BMDM isolated BALB/c mice with a selective knockout of ART2.1 expression (Fig. 2). Additional experiments are required to ascertain whether ART2.2 significantly contributes to overall ART2 activity in myeloid leukocytes and B cells under in vivo conditions.
Notably, in addition to ART2.1 mRNA and functional activity, we also observed ART4 mRNA transcripts in both total spleen extracts (Fig. 1) and purified B cells (Fig. 4). In contrast to the inducible expression of ART4 mRNA reported in human alveolar epithelial cells [40] and human monocytes [35, 36], murine B cells express ART4 mRNA in their basal state (Fig. 4b). Despite the expression of ART4 mRNA in B cells, monocytes, and epithelial cells, the activity of this protein as an ADP-ribosyltransferase in mammalian leukocytes has not been established [35, 36]. Indeed, purified recombinant mammalian ART4 expressed in transfected insect cells shows no ADP-ribosyltransferase activity even when tested with the arginine-analog model substrate agmatine [5]; this contrasts with the readily measured activities of recombinant ART1, ART2, or ART5 in identical assays. Interestingly, the recently cloned avian orthologue of ART4 is an arginine-specific ART that is highly expressed in hematopoietic cells [41]. This suggests that mammalian ART4 has accumulated mutations which suppress its function as an ADP-ribosyltransferase while not affecting its utilization in other biological roles. For example, ART4 is the basis for the Dombrock blood group antigens in humans [42, 43]. That we observed no DTT-independent ART activity in BALB/c B cells minimally indicates that ART4 cannot function as a thiol-independent ecto-ART in these lymphocytes (Fig. 4c).
When normalized for cell number, fresh T cells clearly express much higher total ecto-ART activity than fresh B cells, primed BMDM, or primed BMDC (Fig. 6a). This reflects in part the very high expression of ART2.2, in addition to ART2.1, in T cells. Moreover, comparative FACS analyses with anti-ART2.1 mAbs have indicated that the copy number of ART2.1 is greater in T cells (see Fig. 7c in Ref. [16]) than in B cells (Fig. 1) or the myeloid leukocytes even after inflammatory activation (Figs. 2 and 3). A major limitation of our studies is the relatively low avidity of the anti-ART2.1 mAbs used for FACS analysis as well as the absence of anti-ART2.1 antibodies suitable for Western blot analysis of ART2.1 protein expression. While FACS analysis indicated that only 2–6% of rigorously gated spleen B cells, DCs, or macrophages expressed significant levels of immunoreactive ART2.1, the 1G4 mAb-based activity analyses demonstrated that 40–80% of the identically gated splenocyte subsets were positive for ADP-ribosylated cell surface protein. This suggests that high reaction rates in a small pool of ecto-ART2.1 enzymes on a single leukocyte are sufficient to drive significant accumulation of covalently modified cell surface proteins. The efficacy of this limited pool of ART2.1 to support accumulation of ADP-ribosylated proteins in individual leukocytes will be further modulated by differential expression of the CD38 and CD203 ecto-enzymes that compete with ARTs for local pools of extracellular NAD.
Several cell surface proteins in the different leukocyte subsets appeared to be common substrates for ART2 ecto-enzymes (Fig. 6a and b). We specifically identified the LFA-1 integrin (Fig. 6c) as a common substrate for the ART2.1 in myeloid leukocytes and the ART2.2 in T cells. In other preliminary studies we have observed that the P2X7 receptor is ADP-ribosylated by ART2.1 on the same arginine residues that are modified by ART2.2. Identification of other target proteins modified by ART2.1 is an important goal for future experiments.
In conclusion, these studies suggest that the thiol-dependent ART2.1 is widely expressed as a basal or cytokine-inducible ecto-enzyme in all murine leukocyte types tested. This general expression may underlie a potential role for ART2.1 in innate and adaptive immune responses occurring under conditions (hypoxia, tissue damage, microbial invasion) that will favor both NAD release to extracellular compartments and reduction of the allosteric disulfide bond on ART2.1. The consequent activation of ART2.1 catalytic function under such conditions may target other cell surface proteins of the host leukocytes as well as arginine-rich, secreted anti-microbial proteins such as the α-defensins [44–46].
Acknowledgments
This work was supported by NIH grants GM36387 (to G.R.D.), DFG grant No310/6 (to F.H. and F. K-N.), and American Heart Association Fellowship 0715129B (to S.H.).
Disclosures The authors have no financial conflicts of interest.
References
- 1.Corda D, Di Girolamo M (2003) Functional aspects of protein mono-ADP-ribosylation. EMBO J 22:1953–1958. doi:10.1093/emboj/cdg209 [DOI] [PMC free article] [PubMed]
- 2.Koch-Nolte F, Adriouch S, Bannas P, Krebs C, Scheuplein F, Seman M, Haag F (2006) ADP-ribosylation of membrane proteins: unveiling the secrets of a crucial regulatory mechanism in mammalian cells. Ann Med 38:188–199. doi:10.1080/07853890600655499 [DOI] [PubMed]
- 3.Seman M, Adriouch S, Haag F, Koch-Nolte F (2004) Ecto-ADP-ribosyltransferases (ARTs): emerging actors in cell communication and signaling. Curr Med Chem 11:857–872. doi:10.2174/0929867043455611 [DOI] [PubMed]
- 4.Zhao Z, Gruszczynska-Biegala J, Zolkiewska A (2005) ADP-ribosylation of integrin α7 modulates the binding of integrin α7β1 to laminin. Biochem J 385:309–317. doi:10.1042/BJ20040590 [DOI] [PMC free article] [PubMed]
- 5.Glowacki G, Braren R, Firner K, Nissen M, Kuhl M, Reche P, Bazan F, Cetkovic-Cvrlje M, Leiter E, Haag F, Koch-Nolte F (2002) The family of toxin-related ecto-ADP-ribosyltransferases in humans and the mouse. Protein Sci 11:1657–1670. doi:10.1110/ps.0200602 [DOI] [PMC free article] [PubMed]
- 6.Liu ZX, Yu Y, Dennert G (1999) A cell surface ADP-ribosyltransferase modulates T cell receptor association and signaling. J Biol Chem 274:17399–17401. doi:10.1074/jbc.274.25.17399 [DOI] [PubMed]
- 7.Nemoto E, Stohlman S, Dennert G (1996) Release of a glycosylphosphatidylinositol-anchored ADP-ribosyltransferase from cytotoxic T cells upon activation. J Immunol 156:85–92 [PubMed]
- 8.Okamoto S, Azhipa O, Yu Y, Russo E, Dennert G (1998) Expression of ADP-ribosyltransferase on normal T lymphocytes and effects of nicotinamide adenine dinucleotide on their function. J Immunol 160:4190–4198 [PubMed]
- 9.Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F (2003) NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 19:571–582. doi:10.1016/S1074-7613(03)00266-8 [DOI] [PubMed]
- 10.Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F (2008) ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J 22:861–869. doi:10.1096/fj.07-9294com [DOI] [PubMed]
- 11.Hara N, Badruzzaman M, Sugae T, Shimoyama M, Tsuchiya M (1999) Mouse Rt6.1 is a thiol-dependent arginine-specific ADP-ribosyltransferase. Eur J Biochem 259:289–294. doi:10.1046/j.1432-1327.1999.00039 [DOI] [PubMed]
- 12.Hara N, Terashima M, Shimoyama M, Tsuchiya M (2000) Mouse T-cell antigen Rt6.1 has thiol-dependent NAD glycohydrolase activity. J Biochem 128:601–607 [DOI] [PubMed]
- 13.Adriouch S, Ohlrogge W, Haag F, Koch-Nolte F, Seman M (2001) Rapid induction of naive T cell apoptosis by ecto-nicotinamide adenine dinucleotide: requirement for mono(ADP-ribosyl) transferase 2 and a downstream effector. J Immunol 167:196–203 [DOI] [PubMed]
- 14.Koch-Nolte F, Klein J, Hollmann C, Kuhl M, Haag F, Gaskins HR, Leiter E, Thiele HG (1995) Defects in the structure and expression of the genes for the T cell marker Rt6 in NZW and (NZB x NZW) F1 mice. Int Immunol 7:883–890. doi:10.1093/intimm/7.5.883 [DOI] [PubMed]
- 15.Sardinha DF, Rajan TV (1999) Cis-acting regulation of splenic Art2 gene expression in inbred mouse strains. Immunogenetics 49:700–703. doi:10.1007/s002510050668 [DOI] [PubMed]
- 16.Hong S, Brass A, Seman M, Haag F, Koch-Nolte F, Dubyak GR (2007) Lipopolysaccharide, IFN-γ, and IFN-β induce expression of the thiol-sensitive ART2.1 Ecto-ADP-ribosyltransferase in murine macrophages. J Immunol 179:6215–6227 [DOI] [PubMed]
- 17.Liu ZX, Azhipa O, Okamoto S, Govindarajan S, Dennert G (2001) Extracellular nicotinamide adenine dinucleotide induces T cell apoptosis in vivo and in vitro. J Immunol 167:4942–4947 [DOI] [PubMed]
- 18.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G (2006) P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol 176:2152–2160 [DOI] [PubMed]
- 19.Kawamura H, Aswad F, Minagawa M, Malone K, Kaslow H, Koch-Nolte F, Schott WH, Leiter EH, Dennert G (2005) P2X7 receptor-dependent and -independent T cell death is induced by nicotinamide adenine dinucleotide. J Immunol 174:1971–1979 [DOI] [PubMed]
- 20.Aswad F, Kawamura H, Dennert G (2005) High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol 175:3075–3083 [DOI] [PubMed]
- 21.Koch-Nolte F, Duffy T, Nissen M, Kahl S, Killeen N, Ablamunits V, Haag F, Leiter EH (1999) A new monoclonal antibody detects a developmentally regulated mouse ecto-ADP-ribosyltransferase on T cells: subset distribution, inbred strain variation, and modulation upon T cell activation. J Immunol 163:6014–6022 [PubMed]
- 22.Kanaitsuka T, Bortell R, Stevens LA, Moss J, Sardinha D, Rajan TV, Zipris D, Mordes JP, Greiner DL, Rossini AA (1997) Expression in BALB/c and C57BL/6 mice of Rt6-1 and Rt6-2 ADP-ribosyltransferases that differ in enzymatic activity: C57BL/6 Rt6-1 is a natural transferase knockout. J Immunol 159:2741–2749 [PubMed]
- 23.Krebs C, Adriouch S, Braasch F, Koestner W, Leiter EH, Seman M, Lund FE, Oppenheimer N, Haag F, Koch-Nolte F (2005) CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J Immunol 174:3298–3305 [DOI] [PubMed]
- 24.Ohlrogge W, Haag F, Lohler J, Seman M, Littman DR, Killeen N, Koch-Nolte F (2002) Generation and characterization of ecto-ADP-ribosyltransferase ART2.1/ART2.2-deficient mice. Mol Cell Biol 22:7535–7542. doi:10.1128/MCB.22.21.7535-7542.2002 [DOI] [PMC free article] [PubMed]
- 25.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR (1998) Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol 54:22–32 [DOI] [PubMed]
- 26.Krebs C, Koestner W, Nissen M, Welge V, Parusel I, Malavasi F, Leiter EH, Santella RM, Haag F, Koch-Nolte F (2003) Flow cytometric and immunoblot assays for cell surface ADP-ribosylation using a monoclonal antibody specific for ethenoadenosine. Anal Biochem 314:108–115. doi:10.1016/S0003-2697(02)00640-1 [DOI] [PubMed]
- 27.Matthes M, Hollmann C, Bertuleit H, Kuhl M, Thiele HG, Haag F, Koch-Nolte F (1997) “Natural” RT6–1 and RT6–2 “knock-out” mice. Adv Exp Med Biol 419:271–274 [PubMed]
- 28.Bortell R, Rigby M, Stevens L, Moss J, Kanaitsuka T, Mordes J, Greiner D, Rossini A (1997) Mouse RT6 locus 1 and rat RT6.2 are NAD+. Arginine ADP-ribosyltransferases with auto-ADP-ribosylation activity. Adv Exp Med Biol 419:169–173 [PubMed]
- 29.Lutz MB (2004) IL-3 in dendritic cell development and function: a comparison with GM-CSF and IL-4. Immunobiology 209:79–87. doi:10.1016/j.imbio.2004.03.001 [DOI] [PubMed]
- 30.Dufort FJ, Bleiman BF, Gumina MR, Blair D, Wagner DJ, Roberts MF, Abu-Amer Y, Chiles TC (2007) Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol 179:4953–4957 [DOI] [PubMed]
- 31.Coro ES, Chang WL, Baumgarth N (2006) Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol 176:4343–4351 [DOI] [PubMed]
- 32.Feng CG, Zheng L, Jankovic D, Bafica A, Cannons JL, Watford WT, Chaussabel D, Hieny S, Caspar P, Schwartzberg PL, Lenardo MJ, Sher A (2008) The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-γ-induced cell death. Nat Immunol 9:1279–1287. doi:10.1038/ni.1653 [DOI] [PMC free article] [PubMed]
- 33.Nemoto E, Yu Y, Dennert G (1996) Cell surface ADP-ribosyltransferase regulates lymphocyte function-associated molecule-1 (LFA-1) function in T cells. J Immunol 157:3341–3349 [PubMed]
- 34.Kefalas P, Saxty B, Yadollahi-Farsani M, MacDermot J (1999) Chemotaxin-dependent translocation of immunoreactive ADP-ribosyltransferase-1 to the surface of human neutrophil polymorphs. Eur J Biochem 259:866–871. doi:10.1046/j.1432-1327.1999.00114.x [DOI] [PubMed]
- 35.Grahnert A, Friedrich M, Pfister M, Haag F, Koch-Nolte F, Hauschildt S (2002) Mono-ADP-ribosyltransferases in human monocytes: regulation by lipopolysaccharide. Biochem J 362:717–723. doi:10.1042/0264-6021:3620717 [DOI] [PMC free article] [PubMed]
- 36.Grahnert A, Friedrich M, Engeland K, Hauschildt S (2005) Analysis of mono-ADP-ribosyltransferase 4 gene expression in human monocytes: splicing pattern and potential regulatory elements. Biochim Biophys Acta 1730:173–186 [DOI] [PubMed]
- 37.Ablamunits V, Bridgett M, Duffy T, Haag F, Nissen M, Koch-Nolte F, Leiter H (2001) Changing patterns of cell surface mono (ADP-ribosyl) transferase antigen ART2.2 on resting versus cytopathically-activated T cells in NOD/Lt mice. Diabetologia 44:848–858. doi:10.1007/s001250100559 [DOI] [PubMed]
- 38.Moriarty-Craige SE, Jones DP (2004) Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 24:481–509. doi:10.1146/annurev.nutr.24.012003.132208 [DOI] [PubMed]
- 39.Yeh MW, Kaul M, Zheng J, Nottet HS, Thylin M, Gendelman HE, Lipton SA (2000) Cytokine-stimulated, but not HIV-infected, human monocyte-derived macrophages produce neurotoxic levels of L-cysteine. J Immunol 164:4265–4270 [DOI] [PubMed]
- 40.Balducci E, Micossi LG, Soldaini E, Rappuoli R (2007) Expression and selective up-regulation of toxin-related mono ADP-ribosyltransferases by pathogen-associated molecular patterns in alveolar epithelial cells. FEBS Lett 581:4199–4204. doi:10.1016/j.febslet.2007.07.061 [DOI] [PubMed]
- 41.Grahnert A, Richter S, Siegert F, Berndt A, Hauschildt S (2008) The orthologue of the “acatalytic” mammalian ART4 in chicken is an arginine-specific mono-ADP-ribosyltransferase. BMC Mol Biol 9:86. doi:10.1186/1471-2199-9-86 [DOI] [PMC free article] [PubMed]
- 42.Parusel I, Kahl S, Braasch F, Glowacki G, Halverson GR, Reid ME, Schawalder A, Ortolan E, Funaro A, Malavasi F, Hardie D, Halder S, Buckley CD, Haag F, Koch-Nolte F (2005) A panel of monoclonal antibodies recognizing GPI-anchored ADP-ribosyltransferase ART4, the carrier of the Dombrock blood group antigens. Cell Immunol 236:59–65. doi:10.1016/j.cellimm.2005.08.008 [DOI] [PubMed]
- 43.Gubin AN, Njoroge JM, Wojda U, Pack SD, Rios M, Reid ME, Miller JL (2000) Identification of the Dombrock blood group glycoprotein as a polymorphic member of the ADP-ribosyltransferase gene family. Blood 96:2621–2627 [PubMed]
- 44.Paone G, Stevens LA, Levine RL, Bourgeois C, Steagall WK, Gochuico BR, Moss J (2006) ADP-ribosyltransferase-specific modification of human neutrophil peptide-1. J Biol Chem 281:17054–17060. doi:10.1074/jbc.M603042200 [DOI] [PubMed]
- 45.Paone G, Wada A, Stevens LA, Matin A, Hirayama T, Levine RL, Moss J (2002) ADP ribosylation of human neutrophil peptide-1 regulates its biological properties. Proc Natl Acad Sci USA 99:8231–8235. doi:10.1073/pnas.122238899 [DOI] [PMC free article] [PubMed]
- 46.Rodriguez-Garcia M, Oliva H, Climent N, Garcia F, Gatell JM, Gallart T (2007) Human immature monocyte-derived dendritic cells produce and secrete α-defensins 1–3. J Leukoc Biol 82:1143–1146. doi:10.1189/jlb.0507295 [DOI] [PubMed]