Abstract
The activation function-2 (AF-2) domain of the thyroid hormone (TH) receptor (TR)-β is a TH-dependent binding site for nuclear coactivators (NCoA), which modulate TH-dependent gene transcription. In contrast, the putative AF-1 domain is a TH-independent region interacting with NCoA. We determined the specificity of the AF-2 domain and NCoA interaction by evaluating thyroid function in mice with combined disruption of the AF-2 domain in TRβ, due to a point mutation (E457A), and deletion of one of the NCoAs, steroid receptor coactivator (SRC)-1. The E457A mutation was chosen because it abolishes NCoA recruitment in vitro while preserving normal TH binding and corepressor interactions resulting in resistance to TH. At baseline, disruption of SRC-1 in the homozygous knock-in (TRβE457A/E457A) mice worsened the degree of resistance to TH, resulting in increased serum T4 and TSH. During TH deprivation, disruption of AF-2 and SRC-1 resulted in a TSH rise 50% of what was seen when AF-2 alone was removed, suggesting that SRC-1 was interacting outside of the AF-2 domain. Therefore, 1) during TH deprivation, SRC-1 is necessary for activating the hypothalamic-pituitary-thyroid axis; 2) ligand-dependent repression of TSH requires an intact AF-2; and 3) SRC-1 may interact with the another region of the TRβ or the TRα to regulate TH action in the pituitary. This report demonstrates the dual interaction of NCoA in vivo: the TH-independent up-regulation possibly through another domain and TH-dependent down-regulation through the AF-2 domain.
Differential regulation of TSH expression in the absence or presence of TH is due to SRC-1 interaction with different domains on the thyroid hormone receptor.
Thyroid hormone (TH) action is mediated by coupling of T3 with the ligand-binding domain (LBD) of the nuclear TH receptor (TR). Stabilization of function of the T3-TR complex is in conjunction with a variety of nuclear coactivators (NCoA) and a coregulator (retinoid X receptor) regulating expression of a TH-responsive gene (1,2,3). Genes that are up-regulated by TH have a positive TH response element that interacts with the T3-TR complex (4,5,6,7). In the absence of T3, the TR is associated with a nuclear corepressor (NCoR), which prevents gene transcription from occurring. In the presence of T3, the NCoR is released from the T3-TR complex and NCoA are recruited, which have histone acetyltransferase activity resulting in gene transcription. In negatively regulated genes, the model is less straightforward. The negatively regulated TRE interacts with the T3-TR complex, but the NCoA must function as a NCoR and vice versa in the absence of T3. Although much is known about TH-mediated gene transcription, no single model is able to explain how TH up-regulates some genes and, with the same TR and cofactors, down-regulates others. Furthermore, negatively regulated TRE have not been identified on the down-regulated TRH and TSH genes. Therefore, to understand ligand-dependent negative regulation, some authors have suggested a model of a DNA-independent mechanism for down-regulation (8,9,10). Experimental evidence, however, suggests that TR DNA binding is required both in vitro (11) and in vivo (12) for down-regulation. Therefore, the explanation of how T3 down-regulates TSH remains unexplained.
The site of interaction of NCoAs on the TR is at the highly conserved activation function-2 (AF-2) domain located in the carboxyl-terminal portion of the ligand-binding domain. This domain is important for protein-protein interaction and represents a ligand-dependent transactivation domain. In addition, in the N-terminal domain, there is the AF-1 domain, which allows for hormone-independent protein-protein interaction with the receptor (13).
It has been demonstrated that substitution of the normal glutamic acid 457 with an alanine (E457A) in the AF-2 domain of the TRβ completely abolishes NCoA recruitment in vitro while preserving normal T3 binding and NCoR interaction (14,15,16). Furthermore, mice homozygous for the knock-in mutation at E457, resulting in disruption of the AF-2, have high serum TSH and TH levels, consistent with resistance to TH (RTH) and demonstrating that the AF-2 domain is required for up-regulated and down-regulated TH action (17).
We investigated the specificity of the TRβ AF-2 domain for a particular NCoA, namely steroid receptor coactivator (SRC)-1. We studied mice with disruption of the AF-2 domain by TRβ E457A knock-in (TRβE457A/E457A) in combination with the mice completely deficient in SRC-1, or SRC-1 knockout (KO). The absence of the AF-2 domain has been previously shown to exhibit severe RTH (17). The importance of SRC-1 function was previously demonstrated by mild RTH seen in SRC-1 KO mice (18,19). The RTH phenotype was aggravated with combined deletion of TRβ (20). The pituitary-thyroid axis at baseline and in response to TH deprivation and treatment was assessed in mice with combined AF-2 and SRC-1 disruption and compared with either genotype alone. We demonstrate that interaction of TR with SRC-1 did not require an intact AF-2 domain for ligand-independent positive regulation but rather that the TR may interact with SRC-1 via another domain such as AF-1. However, TH-dependent repression of TSH requires an intact AF-2 and may or may not involve SRC-1.
Materials and Methods
Mice
The SRC-1 KO mouse was produced and genotyped as described previously (21).
The TRβE457A/E457A mouse was produced by introducing the E457A mutation into the Thrb locus via homologous recombination in embryonic stem cells (exon 7) (17). Genotyping of tail DNA from E457A animals was performed by PCR.
All mice studied were in C57BL/6 background (more than 10 backcrosses). Heterozygous, TRβE457A/WT SRC-1+/− mice were interbred to generate litters containing TRβE457A/WT, TRβE457A/E457A, TRβE457A/WT SRC-1 KO, TRβE457A/E457A SRC-1 KO, TRβ wild-type (WT) SRC-1 KO, and TRβ WT SRC-1 WT. Eight to 15 male mice, aged 60–80 d, were evaluated. Experiments were terminated by exsanguination through eye vein puncture under anesthesia. Serum was separated by centrifugation and stored at −20 C until analyzed in the same assay for each experiment. Mice were housed in a controlled environment at 19 C and under 12 h alternating darkness and artificial light cycles. All animal experiments were performed according to protocols approved by The University of Chicago Animal Care and Use Committee.
Measurements of TH and TSH concentrations in serum
Serum TSH was measured in 50 μl serum using a sensitive, heterologous, disequilibrium double-antibody precipitation RIA (22), and results are expressed in bioassayable TSH units. Serum total T4 and total T3 concentrations were measured by a double-antibody precipitation RIA (Diagnostic Products Corp., Los Angeles, CA) using 25 and 50 ml serum, respectively. All samples were individually analyzed for each mouse.
TH withdrawal and treatment
TH deficiency was induced by feeding with a low-iodine (LoI) diet supplemented with 0.15% propylthiouracil (PTU) (Harlan Teklad, Madison, WI). On the 11th day, groups of seven to 11 mice from each genotype were injected once daily for 4 d with the vehicle only (1× PBS), and others received 0.8 μg l-T3/100 g body weight · d while maintained on the LoI/PTU diet. Fourteen to 16 h after the final injection, experiments were terminated by exsanguination. l-T3, dissolved in PBS and 0.002% human serum albumin as a vehicle was given by ip injection in a total volume of 0.1–0.3 ml. A stock of l-T3 (Sigma Chemical Co., St. Louis, MO) at a concentration of 1 mg/ml was prepared in a solution of 50% ethanol/50% 1× PBS containing 5 mm NaOH and kept at −20 C, protected from light. The concentration of l-T3 was confirmed by RIA (Diagnostic Products). Blood samples were obtained at baseline, on the 10th day after the initiation of the LoI/PTU diet, and at the termination of the experiment on d 14.
The dose of l-T3 given to intact and TH-deficient animals were derived from previous experiments (20). It was optimized to achieve a replacement and normalization of serum TSH in WT mice as well as to make evident the differences between the latter and transgenic mice (19,23).
Isolation of tissue mRNA
Pituitary glands and liver from mice were immediately frozen on dry ice and stored at −80 C. For RNA extraction, approximately 80 mg tissue from individual mice were homogenized in 1 ml TRIzol (Life Technologies, Rockville, MD) with a Polytron tissue homogenizer. Total RNA was extracted according to the protocol provided with the TRIzol reagent. Concentration (A260) of the total RNA was determined, and RNA was stored in 1/10 vol 3 m NaOAc and 3 vol 100% ethanol at −80 C.
Quantitative RT-PCR of TRβ1, TRβ2, TRα1, TRα2, SRC-1, -2, and -3, and TSHβ in pituitary
To quantitate mRNAs in pituitary of different genotypes, 2 μg total mRNA were reverse transcribed with the First-Strand Synthesis Superscript Kit (Life Technologies, Rockville, MD) using random hexamer, according to the provided protocol. cDNAs thus obtained were diluted with ribonuclease-free water to a concentration of 1 ng/μl. TaqMan fluorescent probe/primer sets were designed using Primer Express 1.5 (Applied Biosystems, Foster City, CA), and mRNA sequences were obtained from GenBank. Specificity was confirmed by BLAST search. Primer/probe sets were then obtained for mTRα1, mTRα2, mTRβ1, mTRβ2, and mSRC-1, -2, and -3 (MegaBases, Evanston, IL) (24). Equal loading of wells was controlled using a commercially available probe/primer set for 18S rRNA or RPII (Applied Biosystems). Detection of mRNA was performed with Sequence Detector Software and ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA), capable of reading two fluorophores simultaneously (FAM-specific probe and VIC-ribosomal control). Ten nanograms of reverse-transcribed cDNA sample were run in duplicate, and the reaction was performed using TaqMan Universal Mix and 96-well optical plates (Applied Biosystems). Each duplicate sample represents reverse-transcribed total RNA from an individual mouse pituitary. Assays were repeated at least three times, and the data were normalized and merged.
Quantitative RT-PCR of Dio1 and GST in liver
mRNAs in liver of TH-responsive genes Dio1 and GST were quantified as previously described (25).
Statistical analysis
Values are reported as mean ± se. The number of mice is indicated. ANOVA was used to test interaction, and P values were calculated by Student’s t test. The value of the limit of assay sensitivity was assigned to samples with undetectable TSH and T4 concentration.
Results
Parameters of thyroid function at baseline in adult TRβE457A/E457A mice with and without SRC-1
Serum TH concentrations
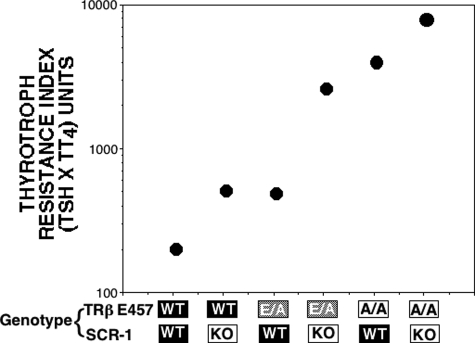
Results of thyroid function tests in untreated mice heterozygous or homozygous for the TRβ AF-2 disruption with and without SRC-1 are shown in Table 1. In general, significant increases in the three iodothyronines (T4, T3, and rT3) were observed in all groups of transgenic mice compared with the WT animals. However, significant increases in TSH were observed only in homozygous TRβE457A/E457A mice and in heterozygous TRβE457A/WT in combination with SRC-1 KO. More specifically, and as previously reported (19), mild RTH was observed in the SRC-1 KO, characterized by slight elevations of the TSH (not significant in this group of animals) and total T4 (TT4), TT3, and TrT3 compared with WT mice (56, 62, and 40%, respectively) (19). Also, as previously reported (17), there was severe resistance noted in homozygous TRβE457A/E457A mice (TSH, TT4,and TT3 were 418, 407, and 287% of WT). Although mice heterozygous for the TRβE457A/WT had intermediate resistance, removal of SRC-1 worsened the resistance in both the heterozygous and homozygous TRβE457 mice. The influence of the number of mutant TRβE457A and deleted SRC-1 alleles is demonstrated by the thyrotroph T4 resistance index (TT4RI) (Fig. 1). TT4RI, a measure of relative central resistance to TH, is the product of the serum TSH and T4 (26). The fewer WT SRC-1 alleles and the more TRβE457A mutant alleles resulted in a greater TT4RI value. TSH measured by RIA was in agreement with the bioactivity for all genotypes studied based on thyroid weights (data not shown).
Table 1.
Baseline thyroid function tests in transgenic mice
| Genotype
|
Baseline
|
||||
|---|---|---|---|---|---|
| TRβ | SRC-1 | TSH (mU/liter) | TT4 (μg/dl) | TT3 (ng/dl) | TrT3 (ng/dl) |
| WT | WT | 62 ± 14 (8) | 3.4 ± 0.3 (8) | 100 ± 3.7 (8) | 17 ± 1.6 (8) |
| WT | KO | 97 ± 15 (10) | 5.5 ± 0.4a (12) | 139 ± 7.3a (12) | 29 ± 2.6a (10) |
| E457A/WT | WT | 106 ± 15 (11) | 4.8 ± 0.2a (11) | 109 ± 5.2 (11) | 35 ± 2.5a (11) |
| E457A/WT | KO | 280 ± 74a,b,c (14) | 9.8 ± 0.6a,b,c (15) | 191 ± 13a,b,c (15) | 68 ± 3.9a,b,c (10) |
| E457A/E457A | WT | 259 ± 58a (9) | 16.0 ± 0.5a (10) | 281 ± 19a (10) | 162 ± 9.8a (9) |
| E457A/E457A | KO | 410 ± 44a,d (10) | 20.0 ± 0.9a,d (10) | 417 ± 22a,d (10) | 177 ± 10a (8) |
Data are expressed as mean ± se. Number of mice is shown in parentheses.
a–d P < 0.05:
vs. WT;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO;
SRC-1 KO vs. TRβE457A/WT-SRC-1 KO;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO.
Figure 1.
TT4RI. The product of serum TSH and TT4 is plotted against each genotype in the order of increasing number of deficient/mutant alleles (SRC-1 KO and TRβ E457A/E457A). The higher the TT4RI, the more severe is the resistance. Homozygotes TRβ E457A/E457A are shown as A/A and heterozygotes TRβE457A/WT as E/A. n = 8–15 for each group.
Pituitary expression of mRNA for TRs, SRCs, and TSHβ
The abundance of TR isoform mRNA levels in the pituitary is shown in Table 2 relative to that of 18S mRNA levels. It should be noted the TRβ mRNA sequence that specifically amplifies from WT and mutant TRβ samples are not distinguished by this method of measurement. TRα2 mRNA was relatively reduced in all groups of TRβE457A mice (46–70%), and no changes in TRα1 were seen with any of the genotypes.
Table 2.
Pituitary mRNA expression at baseline in transgenic mice: TR
| Genotype
|
mRNA
|
||||
|---|---|---|---|---|---|
| TRβ | SRC-1 | TRα1/18s | TRα2/18s | TRβ1/18s | TRβ2/18s |
| WT | WT | 100 ± 9.9 (6) | 100 ± 7.8 (6) | 100 ± 6 (5) | 100 ± 9.5 (5) |
| WT | KO | 93 ± 9 (6) | 93 ± 14 (6) | 136 ± 14a (5) | 99 ± 15 (6) |
| E457A/WT | WT | 105 ± 8.7 (6) | 70 ± 7.7a (6) | 115 ± 12 (6) | 122 ± 16 (6) |
| E457A/WT | KO | 78 ± 3.8 (6) | 52 ± 4.8a,b,c (5) | 105 ± 13 (6) | 101 ± 13 (6) |
| E457A/E457A | WT | 73 ± 9.5 (6) | 46 ± 3.7a,b,c (5) | 101 ± 19 (6) | 142 ± 15a (5) |
| E457A/E457A | KO | 107 ± 12 (6) | 64 ± 10a (6) | 175 ± 20a,b (5) | 185 ± 14a,b,c (5) |
Data are expressed as mean ± se. Number of mice is shown in parentheses.
a–d P < 0.05:
vs. WT;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO;
SRC-1 KO vs. TRβE457A/WT-SRC-1 KO;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO.
TRβ2 mRNA was increased in the homozygous TRβE457A/E457A mutant mice in the presence or absence of SRC-1. TRβ1 mRNA was increased in the SRC-1 KO mice with or without the mutant TRβ allele. There was an apparent compensatory increase in SRC-3 mRNA in the pituitary of two of three genotypes (SRC-1 KO and TRβE457A/E457A SRC-1 KO) lacking SRC-1 (137–148%, Table 3). As expected, SRC-1 was undetectable in the SRC-1 KO mice but was also significantly lower in the TRβE457A/E457A mice (48–72%, Table 3). SRC-2 could not be detected in any pituitary samples.
Table 3.
Pituitary mRNA expression at baseline in transgenic mice: coactivators
| Genotype
|
mRNA
|
||
|---|---|---|---|
| TRβ | SRC-1 | SRC-1/18S | SRC3/18S |
| WT | WT | 100 ± 4 (5) | 100 ± 4 (5) |
| WT | KO | ND | 137 ± 16a (5) |
| E457A/WT | WT | 72 ± 9a (6) | 94 ± 12 (6) |
| E457A/WT | KO | ND | 96 ± 8 (6) |
| E457A/E457A | WT | 48 ± 9a,b,c (6) | 74 ± 10a (6) |
| E457A/E457A | KO | ND | 148 ± 13a,b (5) |
Data are expressed as mean ± se. Number of mice is shown in parentheses. ND, Not detectable.
a–d P < 0.05:
vs. WT;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO;
SRC-1 KO vs. TRβE457A/WT-SRC-1 KO;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO.
Measurement of TSHβ mRNA demonstrated the same trends as seen in the serum TSH measurements among the genotypes, with the most profoundly affected being the homozygous TRβE457A/E457A mutant mice (Table 4).
Table 4.
Pituitary mRNA expression at baseline in transgenic mice: TSHβ
| Genotype
|
mRNA, TSHβ/RPII | |
|---|---|---|
| TRβ | SRC-1 | |
| WT/WT | WT | 100 ± 12.3 (6) |
| WT/WT | KO | 94 ± 6.8 (6) |
| E457A/WT | WT | 101 ± 85 (6) |
| E457A/WT | KO | 124 ± 6.8 (5) |
| E457A/E457A | WT | 199 ± 24a,b (5) |
| E457A/E457A | KO | 188 ± 12a (5) |
Data are expressed as mean ± se. Number of mice is shown in parentheses.
a–d P < 0.05:
vs. WT;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO;
SRC-1 KO vs. TRβE457A/WT-SRC-1 KO;
TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO.
The role of the AF-2 domain and SRC-1 on TSH regulation in the absence of TH
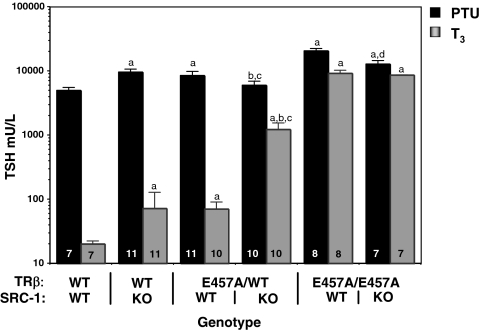
To determine the function of SRC-1 in the presence and absence of the AF-2 domain, we measured the up-regulation of TSH during TH deprivation and its supplementation (Fig. 2). In the presence of a normal AF-2 binding site, absence of SRC-1 increased the serum TSH level 192% compared with WT animals (4972 ± 514 compared with 9530 ± 1066 mU/liter, P < 0.005). This may be due to the small residual amount of TH in the PTU-treated animal, which in the presence of SRC-1 has more potent suppressive effect. On the other hand, the complete absence of an AF-2 binding domain in the homozygous E457A mutant mice (with or without SRC-1) results in greater ligand-independent activation of TSH secretion compared with mice with an intact AF-2 domain.
Figure 2.
Serum TSH after TH withdrawal and l-T3 treatment. Serum TSH concentrations are reported for each genotype after 14 d PTU/LoI diet (black bars) and then after 4 d l-T3 (0.8 μg/100 g) treatment (gray bars). The data are expressed as mean ± se. Number of mice per group is indicated inside the bar. Statistical significance at P ≤ 0.05: a, vs. WT; b, TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO; c, SRC-1 KO vs. TRβE457A/WT-SRC-1 KO; d, TRβE457A/E457A vs. TRβE457A/WT-SRC-1 KO.
Contrary to the effect of SRC-1 in the presence of an intact AF-2 domain, SRC-1, in the partial or complete absence of a functional AF-2 binding domain, augments the increase in TSH in LoI/PTU-treated mice. In fact, the presence of SRC-1 enhances the TSH increase in homozygous TRβE457A/E457A mice (20,676 ± 1,737 vs. 12,706 ± 1,908 mU/liter, P < 0.05) as well as in heterozygote TRβE457A/WT (9420 ± 804 vs. 5891 ± 941 mU/liter, P < 0.05). In other words when AF-2 is available for interaction with SRC-1, the latter functions as a corepressor, but when AF-2 is completely or partially absent, there is interaction of SRC-1 with another binding site on the TRβ or with a site on the TRα or another cofactor that allows it to function as a ligand-independent coactivator.
Sensitivity to TH as determined by the administration of l-T3
The role of SRC-1 and its interaction with the AF-2 domain in mediating pituitary thyrotroph sensitivity to TH is suggested by the higher baseline concentration of serum TSH in TRβE457A/E457A mice and SRC-1 KO mice, despite concomitant elevation of serum T4 and T3 levels. Because the concentration of TH is different among genotypes, the degree of resistance to TH cannot be assessed based on TSH concentrations. Therefore, equal amounts of TH were given to all groups after they were deprived for sufficient time from their endogenous TH. Treatment with 0.2 μg T3/mouse · d for 4 d resulted in clearly more profound suppression of TSH in WT mice (20 ± 2.3 mU/liter, 0.4% of the level before TH treatment). In the absence of SRC-1, there was still suppression of TSH to 1.8% of the level before TH treatment, but it was 8.6-fold higher than in the presence of SRC-1. The severe resistance to TH seen in the TRβE457A/E457A mice with only minimal suppression of the TSH was not significantly different with or without SRC-1, indicating that for ligand-dependent suppression, SRC-1 function requires the AF-2 domain of TRβ. This is further suggested by the marked resistance observed in the heterozygous TRβE457A/WT mice when SRC-1 is absent (TSH after T3 suppression 70 ± 20 compared with 1218 ± 324, P < 0.005, with and without SRC-1, respectively).
Absence of SRC-1 does not affect TH action in the liver
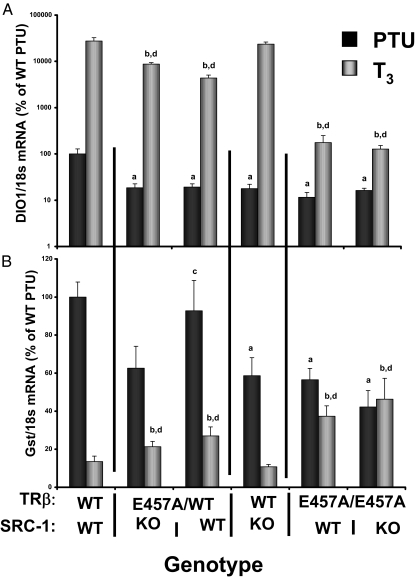
Dio1 and GSTα mRNA, which are TH up-regulated and down-regulated, respectively, were measured in the transgenic mice (Fig. 3, A and B). Dio1 mRNA increased 351-fold in response to TH treatment in WT mice and was considerably less (18-fold) in TRβE457A/E457A mice. This was not affected by the absence of SRC-1. Expression of GSTα was down-regulated to 13.5% of the value with PTU treatment in response to TH in the WT mice. TH treatment of TH-deprived mice did not change significantly in the TRβ E457A/E457A mice. This was also not affected by the absence of SRC-1. These data are consistent with the minor effect of SRC-1 on TH action in peripheral tissues (27), where other NCoA may be responsible for mediating TH action, suggesting that the mechanism of ligand-independent gene up-regulation is different in the pituitary and liver. In other words, the AF-1 effect may not be as strong in the peripheral tissues as in the pituitary action of TRβ-mediated TH action.
Figure 3.
Expression of Dio1 (A) and Gst (B) in liver of transgenic mice. Real-time PCR was used to measure mRNA in the liver of various groups of transgenic mice. Livers were extracted from mice after 14 d PTU/LoI diet and then after 4 d l-T3 (0.8 μg/100 g) treatment. Values are reported relative to the mean value ± se of WT mice after PTU treatment expressed as 100%. n = 5–6 mice per group. Statistical significance at P ≤ 0.05: a, vs. WT PTU treated; b, vs. WT T3 treated; c, vs. SRC-1 KO PTU treated; d, vs. SRC-1 KO T3 treated.
Discussion
TRβ AF-2, AF-1, and SRC-1
SRC-1 is a member of the class of the P160 family of NCoA. These proteins function as remodelers of chromatin by virtue of their ability to allow histone acetylation and thereby interaction of the RNA polymerase with other transcription factors (28). The NCoA directly interacts with the TR at the distal ligand-binding region (29). When T3 binds to the TR, there is a change in the position of helix 12 (where the AF-2 domain is located), and this results in displacement of the NCoR and recruitment of the NCoA (30,31). That SRC-1 plays a role in positive and negative regulation of TSH by T3 in vivo was previously demonstrated (18,19). That the AF-2 region is important in the T3-mediated positive and negative regulation of TSH in vivo has also been demonstrated (17). The purpose of this study was to determine whether 1) SRC-1 acts solely via the AF-2 region and 2) whether other NCoA may interact with the AF-2 region responsible for TH action.
Deletion of the LBD of other steroid hormone receptors results in constitutively active expression in reporter genes. This led to the identification of a hormone-independent AF-1 (activation function 1) in the N-terminal domain (13). The AF-1 domain contrasts with the AF-2 activity in the LBD, which is ligand dependent. The AF-1 domain has been demonstrated in vitro for TRβ2 (32) as well as other members of the steroid hormone receptor class: androgen receptor (33,34), glucocorticoid receptor (35), mineralocorticoid receptor (36,37), estrogen receptor-α and -β (38,39,40), and the progesterone receptor (41,42). It remained unclear, however, what transcription factor or factors were responsible for mediating the AF-1 function in vivo. Our results indicate that the AF-1 domain may be responsible for SRC-1-mediated up-regulation of TSH subunit gene expression (Fig. 4).
Figure 4.
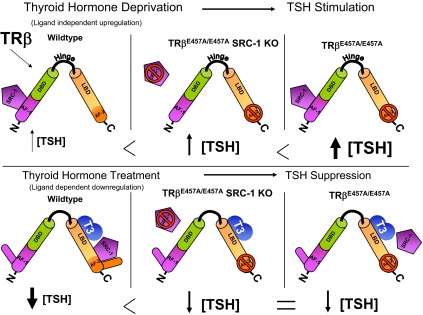
Proposed mechanism of SRC-1 and AF-2 interaction in ligand-independent TRβ-mediated up-regulation of TSH (upper) and ligand-dependent down-regulation of TSH (lower). The model shown in this diagram illustrates that in mice with disruption of the AF-2 domain and an intact SRC-1, the amount of TSH up-regulation during TH withdrawal (upper) is greater than when SRC-1 is absent. During TH treatment (lower), the disruption of the AF-2 domain with or without the presence of SRC-1 results in a similar resistance to TSH suppression. See Discussion for more details. DBD, DNA-binding domain.
The hypothalamus plays an important role along with the pituitary in regulation of TH levels. In mouse models of RTH and in humans, an intact pituitary and hypothalamus are required to elevate TH levels (43). In addition, SRC-1 has been demonstrated in the paraventricular nucleus, and this factor also likely plays a role in the hypothalamic regulation of TH (44).
TH deprivation (ligand-independent positive regulation of TSH): possible role of AF-1 domain
At baseline, the gradations of resistance caused by deletion of the cofactors and AF-2 sites are demonstrated in Fig. 1 and Table 1. During TH deprivation in the E457A mutant mice, the increase in serum TSH was more robust when SRC-1 was present. This suggested that SRC-1 may interact with the TRβ outside of the AF-2 domain to increase the TSH transcription (ligand-independent positive regulation). One possible site of SRC-1 interaction with TRβ is in the AF-1 domain, and it was previously reported that the TRβ2 AF-1 domain binds SRC-1 in vitro (45). The interaction at this site is known to be ligand independent in other systems and supports the role of SRC-1/AF-1 interaction in up-regulation of serum TSH in the absence of TH.
The role of both AF-2 and AF-1 domains can be appreciated by comparing the present experiments with data obtained in mice absent of both AF-2 and AF-1 domains, namely the mouse TRβ KO model. When mice with double KO of SRC-1 and TRβ were subject to TH withdrawal, the increase in serum TSH was 1.6-fold higher compared with TRβ KO with SRC-1 present (20). In the current study, when SRC-1 was absent with the AF-1 domain intact, the increase in TSH was 40% lower than when SRC-1 was present (see Fig. 2 E457A/E457A with and without SRC-1). In both the KO and knock-in models, the TRα and other cofactors are intact. Therefore, we hypothesize that the ligand-independent up-regulation of TSH is TRβ dependent, most likely via the AF-1 domain, although direct evidence is lacking.
TH treatment: SRC-1 and AF-2 are both involved in TH-dependent negative regulation
T3 treatment after TH deprivation showed that the absence of SRC-1 impaired the ligand-dependent negative T3 regulation of serum TSH levels, although this impairment was mild compared with the absence of AF-2 alone. Moreover, combined disruption of both SRC-1 and AF-2 demonstrated no greater resistance to T3 suppression than absence of AF-2 function alone. This suggests that the AF-2 domain is the dominant protein-protein interacting domain of the TRβ in the presence of ligand. Although one cannot definitively conclude from these studies that the SRC-1/AF-2 interaction is absolutely necessary for the negative T3 regulation, results in heterozygous animals certainly support this hypothesis. For example, heterozygous knock-in mice, TRβE457A/WT, demonstrated significantly lesser suppression with T3 in the absence of SRC-1 than in the presence of SRC-1 (Fig. 2). However, other cofactors must also be involved in negative T3 regulation because the SRC-1 KO alone did not reproduce the phenotype of animals lacking only the AF-2 function.
Conclusion
This report demonstrates the dual interaction of NCoA in vivo, the TH-independent up-regulation through one as yet unknown domain, and TH-dependent down-regulation through the AF-2 domain. In summary, 1) during TH deprivation, SRC-1 is necessary for fully activating the hypothalamic-pituitary-thyroid axis, and the activation may be mediated by another domain when the AF-2 domain is absent; 2) ligand-dependent repression of TSH requires an intact AF-2 and likely involves SRC-1; 3) SRC-1 may interact with another region of the TRβ or the TRα to regulate TH action in the pituitary; and 4) These effects and interactions do not apply to all genes negatively regulated by TH.
Footnotes
This work was supported by National Institutes of Health Grants DK17050 and DK20595 to S.R. and R01 DK 42916, R01 DK R0153036, and P60 DK79637 to F.E.W.). Support is acknowledged from Esformes Family and Abroms Family Foundation.
Disclosure Summary: The authors of this manuscript have nothing to declare.
First Published Online April 30, 2009
Abbreviations: AF-2, Activation function-2; KO, knockout; LoI, low iodine; LBD, ligand-binding domain; NCoA, nuclear coactivator; NCoR, nuclear repressor; PTU, propylthiouracil; RTH, resistance to TH; SRC, steroid receptor coactivator; TH, thyroid hormone; TR, TH receptor; TT4, total T4; TT4RI, thyrotroph T4 resistance index; WT, wild type.
References
- Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- Lazar MA 2003 Thyroid hormone action: a binding contract. J Clin Invest 112:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Bodenner DL, Mroczynski MA, Weintraub BD, Radovick S, Wondisford FE 1991 A detailed functional and structural analysis of a major thyroid hormone inhibitory element in the human thyrotropin β-subunit gene. J Biol Chem 266:21666–21673 [PubMed] [Google Scholar]
- Hollenberg AN, Monden T, Wondisford FE 1995 Ligand-independent and -dependent functions of thyroid hormone receptor isoforms depend upon their distinct amino termini. J Biol Chem 270:14274–14280 [DOI] [PubMed] [Google Scholar]
- Näär AM, Boutin JM, Lipkin SM, Yu VC, Holloway JM, Glass CK, Rosenfeld MG 1991 The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell 65:1267–1279 [DOI] [PubMed] [Google Scholar]
- Sasaki S, Lesoon-Wood LA, Dey A, Kuwata T, Weintraub BD, Humphrey G, Yang WM, Seto E, Yen PM, Howard BH, Ozato K 1999 Ligand-induced recruitment of a histone deacetylase in the negative-feedback regulation of the thyrotropin β gene. EMBO J 18:5389–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison LD, Ahlquist JA, Rogers SD, Jameson JL 1993 Negative regulation of the glycoprotein hormone α gene promoter by thyroid hormone: mutagenesis of a proximal receptor binding site preserves transcriptional repression. Mol Cell Endocrinol 94:129–136 [DOI] [PubMed] [Google Scholar]
- Tagami T, Madison LD, Nagaya T, Jameson JL 1997 Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol 17:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Park Y, Jameson JL 1999 Mechanisms that mediate negative regulation of the thyroid-stimulating hormone α gene by the thyroid hormone receptor. J Biol Chem 274:22345–22353 [DOI] [PubMed] [Google Scholar]
- Shibusawa N, Hashimoto K, Nikrodhanond AA, Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen RN, Wondisford FE 2003 Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J Clin Invest 112:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibusawa N, Hollenberg AN, Wondisford FE 2003 Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. J Biol Chem 278:732–738 [DOI] [PubMed] [Google Scholar]
- Lavery DN, McEwan IJ 2005 Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J 391:449–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingwood TN, Rajanayagam O, Adams M, Wagner R, Cavaillès V, Kalkhoven E, Matthews C, Nystrom E, Stenlof K, Lindstedt G, Tisell L, Fletterick RJ, Parker MG, Chatterjee VK 1997 A natural transactivation mutation in the thyroid hormone β receptor: impaired interaction with putative transcriptional mediators. Proc Natl Acad Sci USA 94:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Gu WX, Peairs PT, West BL, Jameson JL 1998 A novel natural mutation in the thyroid hormone receptor defines a dual functional domain that exchanges nuclear receptor corepressors and coactivators. Mol Endocrinol 12:1888–1902 [DOI] [PubMed] [Google Scholar]
- Tone Y, Collingwood TN, Adams M, Chatterjee VK 1994 Functional analysis of a transactivation domain in the thyroid hormone β receptor. J Biol Chem 269:31157–31161 [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Shibusawa N, Nikrodhanond A, Oliveira KJ, Machado DS, Liao XH, Cohen RN, Refetoff S, Wondisford FE 2005 Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface. J Clin Invest 115:2517–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Zhang XY, Ying H, Kato Y, Willingham MC, Xu J, O'Malley BW, Cheng SY 2003 Modulation by steroid receptor coactivator-1 of target-tissue responsiveness in resistance to thyroid hormone. Endocrinology 144:4144–4153 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S 1999 Mice deficient in the steroid receptor coactivator-1 (SRC-1) are resistant to thyroid hormone. EMBO J 18:1900–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadow PM, Koo E, Chassande O, Gauthier K, Samarut J, Xu J, O'Malley BW, Seo H, Murata Y, Weiss RE 2003 Thyroid hormone receptor-specific interactions with steroid receptor coactivator-1 in the pituitary. Mol Endocrinol 17:882–894 [DOI] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW 1998 Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S 1999 Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- Macchia PE, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, Weiss RE, Refetoff S 2001 Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor α. Proc Natl Acad Sci USA 98:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadow PM, Chassande O, Koo EK, Gauthier K, Samarut J, Xu J, O'Malley BW, Weiss RE 2003 Regulation of expression of thyroid hormone receptor isoforms and coactivators in liver and heart by thyroid hormone. Mol Cell Endocrinol 203:65–75 [DOI] [PubMed] [Google Scholar]
- Sadow PM, Chassande O, Gauthier K, Samarut J, Xu J, O'Malley BW, Weiss RE 2003 Specificity of thyroid hormone receptor subtype and steroid receptor coactivator-1 on thyroid hormone action. Am J Physiol Endocrinol Metab 284:E36–E46 [DOI] [PubMed] [Google Scholar]
- Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S 1997 Resistance to thyroid hormone caused by two mutant thyroid hormone receptors β, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3′-triiodothyroinine binding affinity. J Clin Endocrinol Metab 82:1608–1614 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu J, O'Malley BW, Weiss RE, Refetoff S 2002 Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T3-regulated transcription of genes in vivo. Endocrinology 143:1346–1352 [DOI] [PubMed] [Google Scholar]
- Fondell JD, Guermah M, Malik S, Roeder RG 1999 Thyroid hormone receptor-associated proteins and general positive cofactors mediate thyroid hormone receptor function in the absence of the TATA box-binding protein-associated factors of TFIID. Proc Natl Acad Sci USA 96:1959–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Spencer TE, OñateSA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW 1997 Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res 52:141–164; discussion 164–165 [PubMed] [Google Scholar]
- Feng W, Ribeiro RC, Wagner RL, Nguyen H, Apriletti JW, Fletterick RJ, Baxter JD, Kushner PJ, West BL 1998 Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280:1747–1749 [DOI] [PubMed] [Google Scholar]
- Marimuthu A, Feng W, Tagami T, Nguyen H, Jameson JL, Fletterick RJ, Baxter JD, West BL 2002 TR surfaces and conformations required to bind nuclear receptor corepressor. Mol Endocrinol 16:271–286 [DOI] [PubMed] [Google Scholar]
- Langlois MF, Zanger K, Monden T, Safer JD, Hollenberg AN, Wondisford FE 1997 A unique role of the β-2 thyroid hormone receptor isoform in negative regulation by thyroid hormone. Mapping of a novel amino-terminal domain important for ligand-independent activation. J Biol Chem 272:24927–24933 [DOI] [PubMed] [Google Scholar]
- Jenster G, van der Korput HA, Trapman J, Brinkmann AO 1995 Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem 270:7341–7346 [DOI] [PubMed] [Google Scholar]
- Simental JA, Sar M, Lane MV, French FS, Wilson EM 1991 Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem 266:510–518 [PubMed] [Google Scholar]
- Hollenberg SM, Evans RM 1988 Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell 55:899–906 [DOI] [PubMed] [Google Scholar]
- Fuse H, Kitagawa H, Kato S 2000 Characterization of transactivational property and coactivator mediation of rat mineralocorticoid receptor activation function-1 (AF-1). Mol Endocrinol 14:889–899 [DOI] [PubMed] [Google Scholar]
- Govindan MV, Warriar N 1998 Reconstitution of the N-terminal transcription activation function of human mineralocorticoid receptor in a defective human glucocorticoid receptor. J Biol Chem 273:24439–24447 [DOI] [PubMed] [Google Scholar]
- Delaunay F, Pettersson K, Tujague M, Gustafsson JA 2000 Functional differences between the amino-terminal domains of estrogen receptors α and β. Mol Pharmacol 58:584–590 [DOI] [PubMed] [Google Scholar]
- McInerney EM, Katzenellenbogen BS 1996 Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem 271:24172-24178 [DOI] [PubMed] [Google Scholar]
- Metzger D, Ali S, Bornert JM, Chambon P 1995 Characterization of the amino-terminal transcriptional activation function of the human estrogen receptor in animal and yeast cells. J Biol Chem 270:9535–9542 [DOI] [PubMed] [Google Scholar]
- Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H 1992 A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem 267:10882–10887 [PubMed] [Google Scholar]
- Tung L, Shen T, Abel MG, Powell RL, Takimoto GS, Sartorius CA, Horwitz KB 2001 Mapping the unique activation function 3 in the progesterone B-receptor upstream segment. Two LXXLL motifs and a tryptophan residue are required for activity. J Biol Chem 276:39843–39851 [DOI] [PubMed] [Google Scholar]
- Abel ED, Kaulbach HC, Campos-Barros A, Ahima RS, Boers ME, Hashimoto K, Forrest D, Wondisford FE 1999 Novel insight from transgenic mice into thyroid hormone resistance and the regulation of thyrotropin. J Clin Invest 103:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, Steenbergen PJ, De Kloet ER 2000 Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology 141:2192–2199 [DOI] [PubMed] [Google Scholar]
- Oberste-Berghaus C, Zanger K, Hashimoto K, Cohen RN, Hollenberg AN, Wondisford FE 2000 Thyroid hormone-independent interaction between the thyroid hormone receptor β2 amino terminus and coactivators. J Biol Chem 275:1787–1792 [DOI] [PubMed] [Google Scholar]