Abstract
The mRen2.Lewis congenic strain is an estrogen-sensitive model of hypertension whereby estrogen depletion produces a significant and sustained increase in blood pressure. The recent identification of G protein-coupled receptor 30 (GPR30) as a third estrogen receptor isotype prompted us to test the hypothesis that this novel receptor exhibits beneficial cardiovascular actions in the hypertensive female mRen2.Lewis rat. Intact female, ovariectomized female (OVX), and male mRen2.Lewis rats were treated with the selective GPR30 agonist G-1 or vehicle via osmotic minipump for 2 wk. G-1 significantly reduced systolic blood pressure in OVX (178 ± 7 to 142 ± 10 mm Hg, P < 0.001, n = 8) but not intact female (144 ± 3 to 143 ± 5 mm Hg, P > 0.05, n = 5) or male mRen2.Lewis rats (207 ± 7 to 192 ± 5 mm Hg, P > 0.05, n = 7). G-1 did not alter uterine or body weight in OVX, suggesting activation of a receptor distinct from estrogen receptor-α and -β. In isolated aortic rings from OVX, G-1 reduced constriction in response to angiotensin II. Vascular angiotensin-converting enzyme and angiotensin type 1 receptor mRNA were also lower, whereas angiotensin-converting enzyme-2 mRNA was increased. G-1 treatment in OVX was not associated with alterations in either endothelial nitric oxide synthase expression or acetylcholine-induced relaxation. Immunohistochemical staining for GPR30 was evident in both the intima and media of the aorta. We conclude that the novel estrogen receptor GPR30 may contribute to the beneficial cardiovascular actions of estrogen in female mRen2.Lewis rats through regulation of vascular components of the renin-angiotensin system.
The estrogen receptor GPR30 contributes to the estrogen-dependent regulation of blood pressure in mRen2.Lewis female rats, perhaps by differential regulation of vascular components of the renin-angiotensin system.
Female mRen2.Lewis rats, a congenic model that expresses the mouse renin 2 gene, exhibit higher blood pressures than Lewis controls but display only mild hypertension compared to male mRen2.Lewis (1). We recently showed that male mRen2.Lewis rats exhibit significantly higher levels of the vasopressor hormone angiotensin (Ang) II in the circulation and in both cortical and medullary regions of the kidney (1). The blood pressure of mRen2.Lewis females, however, is markedly increased after removal of circulating estrogen via ovariectomy (OVX), whereas blood pressure is not altered by OVX in the background Lewis strain (2). Moreover, chronic administration of 17β-estradiol or the Ang II type 1 (AT1) receptor antagonist olmesartan reduces blood pressure in OVX mRen2.Lewis (2). These studies in the hypertensive mRen2.Lewis strain suggest that estrogen may provide an inhibitory influence on the Ang II-angiotensin-converting enzyme (ACE)-AT1 receptor axis. Indeed, we and others have shown that estrogen depletion is associated with increased expression of ACE and the AT1 receptor (2,3,4,5). Estradiol replacement also blunts the development of hypertension in male and female stroke-prone spontaneously hypertensive rats as well as the female Dahl salt-sensitive strain (6,7).
Estrogen receptor (ER) subtypes ERα and ERβ are classical nuclear hormone receptors, which bind estrogen and translocate to the nucleus to produce genomic actions. However, studies using arterial rings from ERα and ERβ knockout mice show that neither blockade nor removal of these receptors completely inhibits estradiol-induced vasorelaxation, suggesting that estrogen’s acute actions occur via a different pathway (8,9,10). Recently an estradiol-binding membrane receptor distinct from the classical estrogen receptors was identified as G protein-coupled receptor 30 (GPR30) (11). In contrast to the long-term genomic effects of nuclear ERα and ERβ, GPR30 is localized to the plasma membrane and/or endoplasmic reticulum and may mediate estrogen’s direct actions on the vasculature (12). This receptor binds estradiol at a similar affinity as ERα and ERβ and exerts comparable actions on calcium mobilization and phosphoinositide 3-kinase activation (13).
The localization of GPR30 mRNA to human blood vessels and the increased blood pressure displayed in GPR30 knockout mice implies a role for this receptor in the cardiovascular system (14,15). Therefore, we hypothesized that the novel estrogen receptor GPR30 plays a role in the estrogen sensitivity of blood pressure in female mRen2.Lewis congenic rats. We used the specific GPR30 agonist G-1, which has an affinity for GPR30 (11 nm) that is similar to that of estradiol (5.7 nm) but does not compete for ERα or ERβ at doses up to 1 μm (16). Specifically, we determined whether GPR30 activation in vivo decreases blood pressure and influences expression of the vascular renin-angiotensin system (RAS) or nitric oxide (NO) components.
Materials and Methods
Animals
Animals were derived from the Wake Forest University School of Medicine Hypertension Center transgenic breeding colony, and all protocols were approved by the university Animal Care and Use Committee. The congenic mRen2.Lewis strain was obtained by a backcross of the (mRen2)27 transgenic Sprague Dawley rat, which expresses the mouse renin 2 gene, with the Lewis strain for nine generations to obtain an inbred population (2). Insertion of the mouse renin 2 gene results in extrarenal expression of renin and is confirmed by measurement of mouse plasma renin concentration after addition of exogenous angiotensinogen at pH 8.5 (17). Rats were housed in a temperature-controlled room (22 ± 2 C) with a 12 h light, 12-h dark cycle and free access to food and water. OVX was performed in females at 4 wk of age under 4% isoflurane via nosecone as described previously (2). The GPR30 agonist G-1 (EMD Chemicals, Gibbstown, NJ) was dissolved in 50% dimethylsulfoxide and delivered via osmotic minipump implanted sc at the dorsum of the neck at a dose of 400 μg/kg · d for 2 wk (model 2ML2; Alza Corp., Palo Alto, CA). This dose of G-1 was based on the concentration used for estradiol replacement in OVX mRen2.Lewis females (2). G-1 treatment was administered in males and females from 13 to 15 wk of age. In some animals, after 2 wk of G-1 treatment, losartan was administered for 1 additional week in the drinking water at a dose of 10 mg/kg · d (18). Animals were randomly assigned to experimental groups: intact males+vehicle (n = 4), intact males+G-1 (n = 8), intact females (n = 7), intact female+G-1 (n = 5), OVX+vehicle (n = 8), and OVX+G-1 (n = 8). Two days before the animals were killed, urine was collected over a 24-h period in metabolic cages.
Blood pressure
Systolic blood pressure was monitored biweekly from 9 to 15 wk of age using an automated tail-cuff system (Narco Bio-Systems, Houston, TX) while warming at 35 C under slight restraint (19). Multiple measurements were taken for each animal, and the last four readings were recorded as mean systolic blood pressure. In our hands, the use of tail cuff has produced consistent and reproducible results.
Measurement of hormones and glucose
Plasma concentrations of Ang II and Ang-(1–7) were measured by RIA as previously described (20). Briefly, plasma was obtained from trunk blood collected after decapitation into chilled Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) containing a mixture of peptidase inhibitors, as follows (in millimoles): 25 EDTA, 0.44 o-phenanthroline, 1 4-chloromercuribenzoic acid, 0.12 pepstatin A, and 0.003 acetyl-His-Pro-Phe-Val-statine-Leu-Phe, a specific rat renin inhibitor. After 20 min, samples were centrifuged at 3000 rpm for 10 min, and plasma was removed without disturbing the cell pellet. Aliquots of plasma were stored at −80 C until RIA of angiotensin peptides by the departmental core laboratory (21). Serum glucose was measured using the Freestyle blood glucose monitoring system (Abbott, Alameda, CA).
Vascular reactivity
A portion of the thoracic aorta was carefully dissected from surrounding fat and cut into 2- to 3-mm segments. Endothelium-intact or denuded aortic rings were suspended from isometric force transducers (Grass Technologies, West Warwick, RI) in organ baths filled with Krebs solution containing (in millimoles): 118 NaCl, 25 NaHCO3, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 11 glucose (pH 7.4) and bubbled continuously with 95% O2-5% CO2 at 37 C. An optimal passive tension of 2 g was determined by contraction to 60 mm KCl at successively greater lengths until the change in active tension was less than 10%. The responses to 1 μm Ang II (Bachem Americas, Torrance, CA), 100 nm phenylephrine, and 1 μm acetylcholine (both from Sigma-Aldrich, St. Louis, MO) were assessed. Rings with greater than 50% relaxation were considered endothelium-intact. Rings were washed and preconstricted with 100 nm phenylephrine before completing a dose-response curve to G-1 (10 nm to 3 μm).
Immunohistochemistry
Aortic segments were paraffin embedded and stained using the avidin-biotin method. Tissue sections were blocked with 0.1% Tween 20, 1% BSA, and 5% normal donkey serum. Anti-GPR30 (1:300; MBL, Woburn, MA) and biotinylated goat antirabbit (1:400) were diluted in the blocking buffer. Antibody binding was detected using Vectastain Elite avidin-biotin complex kit (Vector Laboratories, Burlingame, CA) and 0.1% diaminobenzene (Sigma-Aldrich).
Protein isolation and Western blot hybridization
Aortic segments were flash frozen in liquid nitrogen and lysed in radioimmunoprecipitation assay buffer (Pierce, Rockford, IL). Protein concentration was determined by the bicinchoninic acid method using BSA as the standard. Precast 10% sodium dodecyl sulfate gels (Bio-Rad, Hercules, CA) were loaded with 50 μg protein in Laemmli buffer, separated by electrophoresis, and transferred to polyvinyl difluoride. Anti-GPR30 (1:500; MBL) and secondary antirabbit (1:5000) were applied, and chemiluminescence was visualized using SuperSignal (Pierce).
Nitric oxide measurements
Nitric oxide metabolites were measured quantitatively in both serum and urine using the total nitric oxide assay kit (Pierce). Both nitrite and nitrate were measured, using nitrate reductase to convert nitrate to nitrite for the latter assay. Nitrite was detected by colorimetric assay at 540 nm with the Benchmark Plus microplate spectrophotometer and Microplate Manager software version 5.2.1 (Bio-Rad). Urine measurements were converted to daily excretion rate.
RNA isolation and reverse transcriptase/real-time PCR
RNA was isolated from tissue with TRIZOL reagent (Invitrogen, Carlsbad, CA) as directed by the manufacturer. The quantity and quality of RNA was measured using an Agilent 2100 bioanalyzer with RNA 6000 Nano LabChip (Agilent Technologies, Palo Alto, CA). First-strand cDNA was synthesized from approximately 1 μg of RNA using the reverse transcriptase method in a 20-μl reaction mixture containing deoxyribonucleotides, random hexamers, and ribonuclease inhibitor and halted by heating to 95 C. Two microliters of the resultant cDNA were added to TaqMan universal PCR master mix with commercially available primer/probe sets (Applied Biosystems, Foster City, CA) with the exception of ACE2 (forward primer 5′-CCCAG AGAACAGTGGACCAAAA-3′; reverse primer 5′-GCTCCACCACACCAACG AT-3′; and probe 5′-FAM-CTCCCGCTTCAT CTCC-NFQ-3′), as previously described (22). Real-time PCRs were analyzed with ABI Prism 7000 (Applied Biosystems). All reactions were performed in triplicate and 18S ribosomal RNA, amplified using the TaqMan ribosomal RNA control kit (Applied Biosystems), served as an internal control. Relative gene expression was expressed as the ratio of target to18S rRNA threshold cycle values.
Statistics
All measurements were expressed as the mean ± sem. Data were analyzed using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA). Paired t test was used to analyze pre- and posttreatment blood pressures within groups. One-way ANOVA with Newman-Keuls multiple comparison test was used to compare posttreatment blood pressures and physiological parameters between groups. Two-way ANOVA was used to analyze the dose response data. One-way ANOVA and Tukey’s or Dunnett’s posttest was used for all other comparisons with a confidence limit of 95% considered significant.
Results
Baseline systolic blood pressures in OVX rats (183 ± 7 mm Hg, n = 16) were markedly higher than in intact animals (148 ± 5 mm Hg, P < 0.005, n = 7), consistent with previous studies in the female mRen2.Lewis (2). Two-week treatment with G-1 significantly reduced blood pressure by 36 mm Hg (178 ± 7 to 142 ± 10 mm Hg, P < 0.001, n = 8). As shown in Table 1, the blood pressure after G-1 treatment was not different from age-matched intact females (151 ± 3 mm Hg, P > 0.05). Vehicle treatment in OVX females did not alter blood pressure (189 ± 11 to 176 ± 9 mm Hg, P > 0.05, n = 8). In addition, G-1 did not reduce blood pressure in either intact female (144 ± 3 to 143 ± 5 mm Hg, P > 0.05, n = 5) or intact male mRen2.Lewis rats (207 ± 7 to 192 ± 5 mm Hg, P > 0.05, n = 7). Vehicle treatment also did not alter blood pressure in intact males (208 ± 6 to 195 ± 9, P > 0.05, n = 4). A subset of male mRen2.Lewis were subsequently treated with the AT1 receptor antagonist losartan for 1 wk, and the resultant reduction in blood pressure confirmed that these animals were responsive to AT1 receptor inhibition (194 ± 4 to 142 ± 5 mm Hg, P < 0.001, n = 4).
Table 1.
Posttreatment blood pressures, organ weights, and plasma and urinary indexes in female mRen2.Lewis rats at 15 wk of age
| Intact | OVX+veh | OVX+G-1 | |
|---|---|---|---|
| n | 7 | 8 | 8 |
| Systolic blood pressure (mm Hg) | 151 ± 3 | 176 ± 9a | 142 ± 10b |
| Body weight (mg) | 216 ± 4 | 272 ± 5a | 265 ± 8a |
| Uterine weight (mg) | 657 ± 48 | 69 ± 18a | 39 ± 7a |
| Heart rate (beats/min) | 233 ± 8 | 227 ± 9 | 227 ± 10 |
| Heart weight to BW (mg/g) | 3.3 ± 0.07 | 3.3 ± 0.18 | 3.1 ± 0.15 |
| Left ventricular weight/BW(mg/g) | 2.8 ± 0.07 | 2.8 ± 0.21 | 2.5 ± 0.15 |
| Kidney weight to BW (mg/g) | 3.8 ± 0.06 | 3.0 ± 0.07a | 3.1 ± 0.04a |
| Urine volume (ml/d) | 8.4 ± 0.6 | 7.7 ± 0.9 | 10.0 ± 2.4 |
| Protein excretion (mg/d) | 1.6 ± 0.1 | 2.0 ± 0.6 | 1.5 ± 0.2 |
| Ang II (pmol/liter) | 13 ± 3 | 30 ± 19 | 31 ± 11 |
| Ang-(1–7) (pmol/liter) | 24 ± 6 | 39 ± 9 | 25 ± 4 |
| Glucose (mmol/liter) | 9.0 ± 0.2 | 8.7 ± 0.2 | 8.9 ± 0.3 |
Values are mean ± sem. n, Number of animals per group; veh, vehicle; BW, body weight.
P < 0.05 vs. intact;
P < 0.05 vs. OVX+veh.
Body weight was significantly increased and uterine weight reduced in OVX vs. intact female mRen2.Lewis (Table 1). Neither of these physiological parameters was altered by G-1 treatment. No differences in heart rate, heart weight, or left ventricular weight were evident between groups. Kidney weight was significantly reduced in the OVX+vehicle and OVX+G-1 groups, although urine volume and proteinuria were not changed. Neither circulating Ang II and Ang-(1–7) nor fasting glucose levels were altered by OVX or G-1 (Table 1).
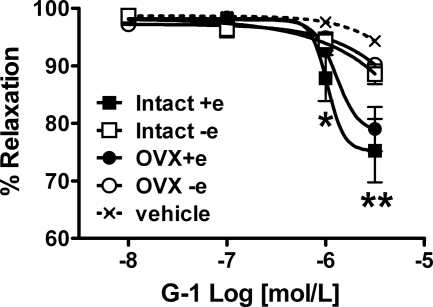
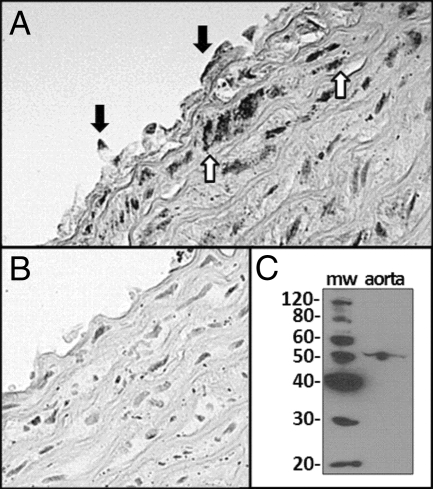
In aortic rings isolated from intact and OVX females, exogenous G-1 administration induced vasorelaxation that was abolished by removal of the endothelium (Fig. 1). Positive immunostaining for GPR30 in aortic segments was localized to both endothelial and smooth muscle cells (Fig. 2A), whereas staining was absent in tissue sections that lacked the primary antibody (Fig. 2B). The immunoblot of a full-length gel revealed a single band corresponding to the appropriate molecular weight of GPR30 in aortic homogenates (Fig. 2C).
Figure 1.
Response to in vitro G-1 or vehicle in endothelium-intact (+e) and denuded (−e) aortic rings preconstricted with phenylephrine. Data shown represent the mean ± sem from intact or OVX mRen2.Lewis females (n = 7–8 per group). *, P < 0.01, intact+e vs. vehicle; **, P < 0.001 intact+e and OVX+e vs. vehicle.
Figure 2.
GPR30 immunostaining in a cross section of the thoracic aorta. A, Representative slide from an OVX mRen2.Lewis female using a primary antibody against GPR30 shows staining in endothelial cells (closed arrows) and smooth muscle cells (open arrows). B, Representative control slide stained in the absence of the primary antibody. C, Full-length immunoblot of aortic tissue showing the specificity of the primary antibody as evidenced by a single band corresponding to the appropriate molecular weight (mw) of GPR30 (∼50).
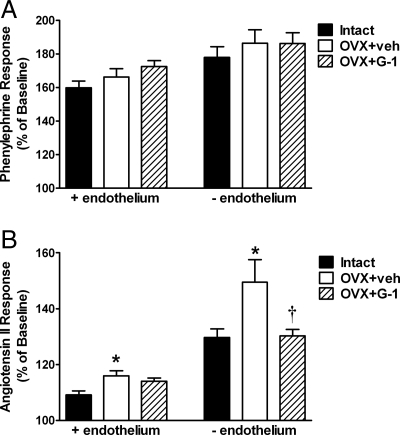
Although the response to phenylephrine in both intact and denuded aortic rings was not different between groups (Fig. 3A), Ang II-induced vasoconstriction was significantly increased by OVX in both endothelium-intact (109 ± 1 vs. 116 ± 2%, P < 0.01; Fig. 3B) and denuded rings (130 ± 3 vs. 149 ± 8%, P < 0.05). In endothelium-intact rings, the OVX+G-1 group was not significantly different from intact controls (114 ± 1%, P > 0.05). In endothelium-denuded rings, G-1 treatment significantly reduced the Ang II response to a similar level as intact controls (130 ± 2%, P < 0.05).
Figure 3.
Vascular reactivity in aortic rings from intact, OVX+vehicle (veh), and OVX+G-1 rats. Bars represent the mean ± sem (n = 7–8 per group). A, Response to 100 nm phenylephrine in endothelium-intact (+endothelium) and denuded (−endothelium) rings. B, Vasoconstriction in response to 1 μm Ang II. *, P < 0.05 vs. Intact; †, P < 0.05 vs. OVX+veh.
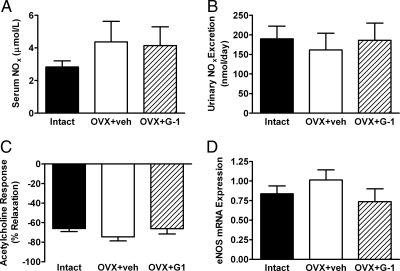
Neither OVX nor G-1 significantly altered the serum concentration (Fig. 4A) or daily urinary excretion rate (Fig. 4B) of NO metabolites. Receptor-mediated NO release, as measured by relaxation of aortic rings in response to 1 μm acetylcholine, was also similar between groups (Fig. 4C). In addition, neither OVX nor G-1 altered endothelial NO synthase (eNOS) gene expression in aortic tissue (Fig. 4D).
Figure 4.
Effects of OVX and G-1 on components of the nitric oxide pathway. Bars represent the mean ± sem (n = 7–8 per group). A, Concentration of nitric oxide metabolites (NOx) in the serum. B, Daily urinary excretion of NOx. C, Response to 1 μm acetylcholine in phenylephrine-constricted aortic rings. D, Endothelial nitric oxide synthase (eNOS) gene expression in aortic tissue, expressed as the ratio of eNOS mRNA to 18S ribosomal RNA. veh, Vehicle.
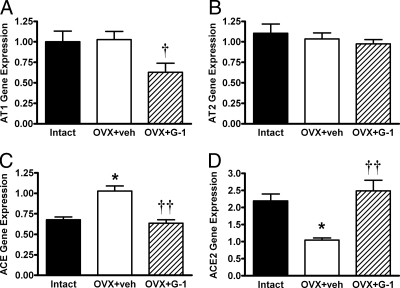
Finally, we assessed whether differences in the vascular response to Ang II were due to alterations in the local expression of RAS components. G-1 significantly reduced aortic AT1 receptor gene expression by 38% (Fig. 5A), whereas expression of the Ang II type 2 receptor was not altered (Fig. 5B). Aortic ACE mRNA was 51% greater in OVX animals, and G-1 treatment significantly reduced ACE mRNA to the level of intact controls (Fig. 5C). Conversely, OVX decreased aortic ACE2 mRNA by 52%, and G-1 reversed this effect (Fig. 5D).
Figure 5.
Effects of OVX and G-1 on relative gene expression in aortic tissue. All data are expressed as the ratio of target mRNA to 18S ribosomal RNA, and bars represent the mean ± sem (n = 7–8 per group). A, Angiotensin II type 1 receptor (AT1) mRNA. B, Angiotensin II type 2 receptor (AT2) mRNA. C, Angiotensin-converting enzyme (ACE) mRNA. D, Angiotensin-converting enzyme 2 (ACE2) mRNA. *, P < 0.005 vs. Intact, †, P < 0.05 vs. OVX+veh, ††, P < 0.001 vs. OVX+veh. veh, Vehicle.
Discussion
In the present study, we show that in vivo activation of the novel ER GPR30 significantly decreased blood pressure in OVX mRen2.Lewis rats. The decrease in blood pressure was associated with a reduction in Ang II-induced vasoconstriction and alterations in expression of local RAS components, suggesting that G-1 reduced blood pressure by attenuating vascular Ang II signaling. Our results are consistent with earlier findings from this laboratory showing that either estradiol or the AT1 receptor antagonist olmesartan significantly reduces blood pressure in OVX mRen2.Lewis females (2).
The blood pressure effects of GPR30 activation were present only in the estrogen-depleted female mRen2.Lewis. We attribute the lack of an effect in intact females to the presence of the endogenous ligand for GPR30. In contrast to our results in OVX females, G-1 did not decrease blood pressure in male mRen2.Lewis rats. Estrogen-sensitivity has not been established in these congenic males or their transgenic predecessors but has been demonstrated in other animal models of hypertension, such as the deoxycorticosterone acetate plus salt model (23). The lack of a G-1 effect may be due to decreased GPR30 expression in males, although a recent study detected GPR30 mRNA in human blood vessels from both males and females (14). Alternatively, the presence of circulating androgens may mask the effects of GPR30 activation, as evidenced by the blood pressure-lowering actions of estradiol in castrated but not intact normotensive male rats (24). Additional studies are required to address the extent of sex differences in GPR30 expression and the effect of G-1 on blood pressure in gonadectomized male mRen2.Lewis rats.
Whereas GPR30 activation mimicked the effects of estradiol in terms of blood pressure (2), G-1 did not induce estrogenic effects on body and uterine weight, corroborating findings in GPR30 knockout mice (15,25). OVX mRen2.Lewis females exhibited an increase in body weight, an effect observed in other models of hypertension (26). Estrogen counteracts weight gain by inducing changes in feeding, adiposity, and physical activity, which is mediated by both ERα and ERβ as evidenced by higher body weights in receptor knockout mice (27,28). The higher body weights in OVX animals offsets an increase in cardiac hypertrophy induced by high blood pressure, resulting in no difference in the tissue to body weight ratio. In addition, the decreased kidney to body weight ratios in OVX animals may be attributed to the lack of estrogenic inhibition on weight gain. In the female mRen2.Lewis, OVX significantly decreased uterine weight, an effect primarily attributed to ERα because estradiol does not stimulate uterotrophic effects in mice deficient in this receptor (29). Chronic G-1 treatment did not influence total body weight or uterine weight, suggesting that the blood pressure-lowering actions of the agonist were ERα and ERβ independent.
Estradiol-induced vasorelaxation is maintained in arterial rings from ERα and ERβ knockout mice, suggesting the presence of a third estrogen receptor (8,9). Whereas the novel receptor GPR30 was postulated to be a mediator of estrogen’s direct effects on the vasculature, our results in aortic rings show nominal vasodilation in response to G-1, even though GPR30 staining was evident in both endothelial and smooth muscle cells. The lack of a potent effect in our studies may reflect the use of a conduit vessel, and future experiments are required to address the issue of G-1 sensitivity in resistance vessels.
The ability of in vivo estradiol administration to down-regulate vascular AT1 receptors was recently shown in OVX transgenic (mRen2)27 rats and was associated with increased eNOS expression (30). In vascular smooth muscle cells, estradiol down-regulates AT1 receptors via an NO-dependent pathway (31). Our study revealed that G-1 decreased aortic AT1 mRNA but had no effect on NO metabolites, eNOS expression, or receptor-mediated NO release. These results are in agreement with findings by Martensson et al. (15) showing no alterations in the responses to NO synthase inhibition or acetylcholine in GPR30 knockout mice.
Previous studies show that aortic ACE mRNA and activity is decreased by estradiol, whereas renal and uterine ACE2 expression is elevated during pregnancy (4,32,33,34). Although there were no changes in circulating Ang II and Ang-(1–7), OVX and G-1 treatment influenced the local expression of ACE and ACE2 in the mRen2.Lewis. OVX increased ACE mRNA and decreased ACE2 mRNA in the aorta, which may lead to elevated local concentrations of Ang II. In contrast, G-1 decreased ACE and increased ACE2, which may shift the balance from Ang II to Ang-(1–7) within the vasculature, favoring a reduction in vascular tone (35). Future studies will measure protein expression of these RAS components in the vasculature because changes in mRNA may not always correlate with alterations in protein.
Premenopausal females have a lower incidence of cardiovascular disease, but it is unknown which estrogen receptor mediates these beneficial effects. The present studies are the first to demonstrate that the novel receptor GPR30 contributes to the estrogen-dependent regulation of blood pressure in mRen2.Lewis females, perhaps by differential regulation of vascular RAS components. Although the translation of these experimental findings to the complex and, at times, contrasting actions of estrogen in peri- and postmenopausal women awaits further study, the contribution of GPR30 to the physiological spectrum of estrogenic influences on the cardiovascular system should be considered.
Acknowledgments
We thank Nancy Pirro and Brian Westwood for their technical expertise.
Footnotes
This work was supported by the National Heart Lung and Blood Institute, National Institutes of Health Grants HL-56973 and HL-51952, American Heart Association Grants 0825515E, and unrestricted grants from the Unifi Corp. (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 16, 2009
Abbreviations: ACE, Angiotensin-converting enzyme; Ang, angiotensin; AT1, Ang II type 1 receptor; eNOS, endothelial NO synthase; ER, estrogen receptor; GPR30, G protein-coupled receptor 30; NO, nitric oxide; OVX, ovariectomy; RAS, renin-angiotensin system.
References
- Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC 2008 Sex differences in circulating and renal angiotensins of hypertensive mRen(2).Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol 295:H10–H20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB 2003 Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen(2).Lewis rat. Hypertension 42:781–786 [DOI] [PubMed] [Google Scholar]
- Krishnamurthi K, Verbalis JG, Zheng W, Wu Z, Clerch LB, Sandberg K 1999 Estrogen regulates angiotensin AT1 receptor expression via cytosolic proteins that bind to the 5′ leader sequence of the receptor mRNA. Endocrinology 140:5435–5438 [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB 1999 Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 33:323–328 [DOI] [PubMed] [Google Scholar]
- Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M 1998 Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 97:2197–2201 [DOI] [PubMed] [Google Scholar]
- von Eiff AW, Lutz HM, Gries J, Kretzschmar R 1985 The protective mechanism of estrogen on high blood pressure. Basic Res Cardiol 80:191–201 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ohno Y, Otsuka K, Suzawa T, Suzuki H, Saruta T 2000 Oestrogen attenuates the increases in blood pressure and platelet aggregation in ovariectomized and salt-loaded Dahl salt-sensitive rats. J Hypertens 18:911–917 [DOI] [PubMed] [Google Scholar]
- Cruz MN, Douglas G, Gustafsson JA, Poston L, Kublickiene K 2006 Dilatory responses to estrogenic compounds in small femoral arteries of male and female estrogen receptor-β knockout mice. Am J Physiol Heart Circ Physiol 290:H823–H829 [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS 1997 Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest 99:2429–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freay AD, Curtis SW, Korach KS, Rubanyi GM 1997 Mechanism of vascular smooth muscle relaxation by estrogen in depolarized rat and mouse aorta. Role of nuclear estrogen receptor and Ca2+ uptake. Circ Res 81:242–248 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB 2008 The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol 109:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M 2007 Differential effects of 17β-estradiol on function and expression of estrogen receptor α, estrogen receptor β, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 49:1358–1363 [DOI] [PubMed] [Google Scholar]
- Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM 2009 Deletion of the G protein-coupled receptor GPR30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–698 [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Bohlender J, Ménard J, Edling O, Ganten D, Luft FC 1998 Mouse and rat plasma renin concentration and gene expression in (mRen2)27 transgenic rats. Am J Physiol 274:H1450–H1456 [DOI] [PubMed] [Google Scholar]
- Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM 2006 Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol 291:H2166–H2172 [DOI] [PubMed] [Google Scholar]
- Chappell MC, Yamaleyeva LM, Westwood BM 2006 Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol 291:R1557–R1563 [DOI] [PubMed] [Google Scholar]
- Allred AJ, Chappell MC, Ferrario CM, Diz DI 2000 Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 279:F636–F645 [DOI] [PubMed] [Google Scholar]
- Chappell MC, Brosnihan KB, Diz DI, Ferrario CM 1989 Identification of angiotensin-(1–7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem 264:16518–16523 [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann Tallant E, Smith RD, Chappell MC 2005 Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int 68:2189–2196 [DOI] [PubMed] [Google Scholar]
- Crofton JT, Share L 1997 Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension 29:494–499 [DOI] [PubMed] [Google Scholar]
- Fischer GM, Swain ML 1977 Effect of sex hormones on blood pressure and vascular connective tissue in castrated and noncastrated male rats. Am J Physiol 232:H617–H621 [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH 2009 GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41 [DOI] [PubMed] [Google Scholar]
- Zheng W, Ji H, Maric C, Wu X, Sandberg K 2008 Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 294:H1508–H1513 [DOI] [PubMed] [Google Scholar]
- Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C 1999 Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERβ(−/−) mice. J Clin Invest 104:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, Karl M 2007 Gender-specific effects of endogenous testosterone: female α-estrogen receptor-deficient C57BL/6J mice develop glomerulosclerosis. Kidney Int 72:464–472 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnihan KB, Li P, Figueroa JP, Ganten D, Ferrario CM 2008 Estrogen, nitric oxide, and hypertension differentially modulate agonist-induced contractile responses in female transgenic (mRen2)27 hypertensive rats. Am J Physiol Heart Circ Physiol 294:H1995–H2001 [DOI] [PubMed] [Google Scholar]
- Nickenig G, Strehlow K, Wassmann S, Bäumer AT, Albory K, Sauer H, Böhm M 2000 Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation 102:1828–1833 [DOI] [PubMed] [Google Scholar]
- Brosnihan KB, Li P, Ganten D, Ferrario CM 1997 Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol 273:R1908–R1915 [DOI] [PubMed] [Google Scholar]
- Neves LA, Stovall K, Joyner J, Valdés G, Gallagher PE, Ferrario CM, Merrill DC, Brosnihan KB 2008 ACE2 and ANG-(1–7) in the rat uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol 294:R151–R161 [DOI] [PubMed] [Google Scholar]
- Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM 2003 Enhanced renal immunocytochemical expression of ANG-(1–7) and ACE2 during pregnancy. Hypertension 42:749–753 [DOI] [PubMed] [Google Scholar]
- Chappell MC 2007 Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS receptor axis: more than regulation of blood pressure? Hypertension 50:596–599 [DOI] [PubMed] [Google Scholar]