There is a significant difference in the cardiovascular disease (CVD) risk between men and women (1). Epidemiological studies have shown that the risk of atherosclerotic disease is low in premenopausal women and increases dramatically after menopause (1). The basis of differences in CVD risk has been attributed to declining levels of estrogens in women (2). In this issue, Schreckenberg et al. (3) provide new insights into the mechanism of action of estrogens on heart tissue, showing that PTHrP is a crucial downstream mediator. The significance of their findings in the context of hormone replacement therapy (HRT) use is discussed below.
After the onset of menopause, levels of estrogens slowly ebb to equilibrate with those of age-matched men. Because of the disparity in CVD rates before and after menopause, estrogens in general were long touted as agents capable of preventing CVD (4). This claim was substantiated by numerous case-controlled and laboratory studies in the 1980s and 1990s (5); these studies demonstrated that estrogens could reduce CVD risk via a variety of mechanisms, including producing more favorable lipid profiles, causing vascular dilatation, and augmenting endothelial repair after damage (4). The composition of HRT, with conjugated estrogens, combined with progestin for those women with a uterus, is eloquently reviewed by Blaustein (2); because of its beneficial effects on the heart, the use of HRT moved from being a symptomatic treatment for peri- and postmenopausal vasomotor symptoms to a general therapy for the prevention of all menopausal-related pathology (6). The logic for using HRT as a preventative treatment was based on the aforementioned evidence for its protective effect on the cardiovascular system as well as copious studies dating back to the 1960s showing that estrogen deficiency has a causal role in postmenopausal osteoporosis (7). The mechanism whereby estradiol-17β prevented osteoporosis was shown to involve augmenting osteoblast survival and decreasing pro-osteoclastic signals (8).
The beneficial effects of HRT were challenged in 2002 with the publication of two studies not only showing a lack of benefit for HRT on CVD but also suggesting the HRT could worsen CVD and lead to strokes and deep venous thrombi (7). One study in particular, the Women’s Health Initiative (WHI) (7), was widely publicized, with the New York Times commenting on the “Women’s Health Initiative findings of increased risks of heart disease, breast cancer, strokes and gallbladder disease” (9).
These publications had a substantial negative impact on HRT use and left clinicians and patients confused as to the role of HRT (9). Several hypotheses were rapidly put forward to explicate the negative results of HRT on CVD risk and in turn justify its use in certain patient segments. One was the timing hypothesis, which asserted that the crucial timing of action for estrogens needed to happen before the deficiency-induced changes in the vasculature have irreversibly manifested themselves (10,11). Secondary analyses of major studies have shown that there is indeed some truth to this hypothesis and that starting HRT during the late perimenopausal period or early postmenopausal period leads to mild decreases in CVD risk (12). However, the effect is not generalizable to the whole cardiovascular system, because there is still a significantly increased risk of stroke regardless of the timing of HRT initiation (12).
Another hypothesis was that the action of estrogens alone is cardioprotective and that when combined with progestin, which is known to have detrimental effects on the vasculature, the progestin blocked estrogens’ protective effects (10). This hypothesis, however, has not held, because there is no protection against CVD with prolonged therapy consisting only of conjugated estrogens, albeit the dramatically increased CVD risk observed for combination therapy is not seen for those on estrogens alone (10).
Because the issue of CVD risk and estrogens is not entirely clear, the use of HRT as a preventative measure is no longer clinically recommended for CVD prevention, although confusingly, it remains so for osteoporosis (13). Surprisingly, the WHI demonstrated only a modest prevention of osteoporotic fractures in those women who initiate HRT after the menopause (7). In 2006, new research re-highlighted the fact that the perimenopausal period is the crucial time period for menopausal bone loss, with approximately half of a women’s total bone loss occurring during the perimenopausal period alone. Studies using genetically modified mice have suggested that the observed rapid perimenopausal bone loss may be, in part, caused by dramatic increases in FSH levels and not solely the continual decline in levels of estrogens (14,15). FSH is now known to directly augment the bone-resorbing activity of human osteoclasts (14,15). Thus, the fact that the WHI study showed only modest declines in the fracture rates for those women initiating HRT long after the onset f menopause is not surprising; ongoing randomized trials with HRT initiation during the perimenopausal period should shed some light onto the full potential of HRT in bone loss and fracture prevention.
Despite a better understanding of how changes in perimenopausal hormones impacts bone loss, most clinicians, on deciding whether to start HRT or not, will lean toward the latter if there are no vasomotor symptoms (e.g. hot flashes) (9). Because CVD remains the number one cause of death for women, acceptance of HRT as a therapy hinges crucially on a better understanding of the effects of estrogens on the cardiovascular system. To date, there remains a vast discrepancy between the potentially negative CVD effects of HRT observed in clinical trials and experimental human and animal studies showing a clear benefit of estrogens in the prevention of CVD (1,10,16).
Studies suggest that estrogens are cardioprotective, at least in part, by decreasing serum cholesterol levels (1). Interestingly, although the results from the WHI trial confirm that there are significant reductions in low-density lipoprotein (LDL) levels with HRT, it paradoxically showed that these changes failed to bring about any cardioprotective effects (7). Another explanation for estrogens’ cardioprotective effects relates to its ability to induce coronary vascular dilatation (1). The vasodilatation occurs 5–20 min after administration of estradiol-17β and is not dependent upon gene transcription (1). Estradiol-17β’s mechanism of action for vasodilatation is likely through augmented nitric oxide (NO) production, and specifically through phosphatidylinositol 3-kinase (PI3K) and Akt signaling to activate endothelial NO synthase (eNOS) (16). Despite a clear theoretical benefit from expanding lumen diameter, it should be noted that acute coronary vasodilatation with NO analogs, used in acute coronary syndromes for vasodilatation, has never produced a demonstrated mortality benefit and may actually be harmful (17,18); this suggests that acute administration of nitric oxide contributes little to the overall pathophysiology and provides only symptomatic relief.
Although the effects of estrogens on vessel dilatation are theoretically appealing, an often underappreciated effect of estrogens is their action on cardiac musculature. Long-term administration of estradiol-17β increases the tolerance of cardiac tissue to vasculature injury (19); thus, they help preserve the functional capacity of ischemic cardiac tissue. ER-β expression in the myocardium is induced by vascular injury (10). Through the traditional ER, estrogens act to protect the myocardium partly through chronic NO production. This effect occurs over a period of hours to days (i.e. is long term) and is dependent upon changes in gene expression that ironically may involve NO-induced changes (10). Most evidence now points to long-term genomic changes induced by estrogens as the critical mediator of estrogens’ protective action on the cardiovascular system (10,16).
How does chronic NO production protect the heart, or conversely, how does the loss of chronic NO production predispose to CVD? One factor released from the endothelium during ischemia is PTHrP, which both vasodilates the coronary arteries and improves the contractility of the postischemic heart (20). In noncardiac tissue, it is known that a deficiency of estradiol-17β decreases PTHrP expression (21). New research by Schreckenberg et al. (3) in this issue of the journal has now demonstrated that this deficiency, manifested by a chronic lack of NO production, alters the response of the myocardium to PTHrP via TGFβ-correlated reduction in PTH receptor expression (3). Interestingly, in these studies, PTHrP was still able to induce vasodilatation in NO-deficient animals, suggesting that its mechanism of vasodilatation is independent of NO (3). When comparing the results of chronic NO inhibition to those induced by ovariectomy, Schreckenberg et al. (3) showed similar findings, thus providing a mechanism to explain how loss of estrogens manifest as differences in cardiac function.
Could PTHrP be the crucial mediator of estradiol-17β action in the myocardium of premenopausal women and conversely negatively impact cardiac function when HRT is initiated long after menopause? Studies in rodents demonstrate that female hearts have a markedly increased basal expression of PTHrP compared with males (22). Inhibition of endogenously released PTHrP, as would occur with a deficiency of estrogens, impairs the postischemic recovery of the myocardium (23). Moreover, in comparing older animals to young adults, PTHrP’s action on the myocardium switches from one that is protective to one that is detrimental in the myocardium’s ability to tolerate ischemic insults (20).
The findings on the role of PTHrP in postmenopausal changes in cardiac function open up novel mechanisms of therapy and help refocus the timing of HRT. It could be envisioned that PTHrP infusion can serve as a protective agent against ischemic heart damage, especially given that the only common complication is transient hypercalcemia (24). Most of the studies looking at PTHrP and myocardial ischemia have highlighted the role that PTH and PTHrP play in inducing release of cardiac-repairing stem cells from the bone marrow (24). Further studies are needed to evaluate how the effects of PTHrP on cardiac tissue switch from increasing to decreasing ischemic tolerance as individuals age. From an osteoporosis perspective, studies have demonstrated that PTHrP can be used in postmenopausal women as a cyclically administered bone-anabolic agent, similar in action to PTH1-34 (25,26). Although PTH1-34 has not been used widely for treatment of osteoporosis because of the fear of osteosarcoma (27), this adverse effect, observed only in rats, has never been documented in humans. Thus, it is likely that future studies will address the possibility of using PTH or PTHrP as a potential menopausal treatment.
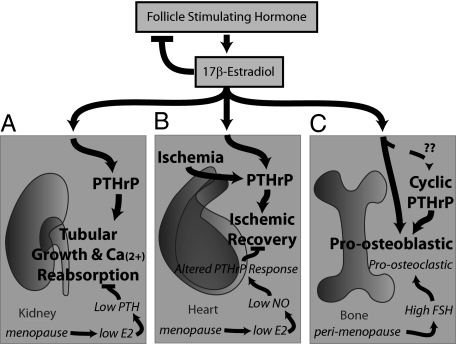
Figure 1 demonstrates the role of estrogens, PTHrP, and FSH in the pathological features of menopause. Because one of the remaining primary reasons for HRT use is osteoporosis prevention, and because greater than half of total bone loss happens rapidly in the perimenopausal period, refocusing HRT to the early perimenopausal period instead of waiting for menopause to fully manifest might not only finally demonstrate a cardioprotective effect for estrogens on CVD but, importantly, also help prevent the rapid declines in bone mineral density. The evidence for this assertion relating to osteoporosis comes from studies demonstrating that FSH inhibition can blunt hypogonadal bone loss (8). It is becoming increasingly clear that many of the pathological features of menopause are attributed to a variety of different hormones and mechanisms, not just a deficiency of estrogens. Future research on the role that PTHrP and FSH play in the many tissues affected by menopause should bring about exciting discoveries.
Figure 1.
Estrogen action on the kidneys, heart, and bone. The anterior pituitary hormone FSH acts on the ovaries to induce production of estrogens in premenopausal women; estrogens serve to inhibit FSH production through feedback mechanisms that include inhibins (28). During the perimenopausal period, ovarian failure is sensed by the pituitary, which dramatically increases its production of FSH in an attempt to keep levels of estrogens within normal limits. Menopause is heralded by the failure of estrogenic output from the ovaries. A, Estrogens act in the kidney to augment activation of vitamin D and induce PTHrP production; PTHrP, in turn, augments the growth of and calcium reabsorption by the renal tubular cells. During menopause, the lack of estrogens causes decreases in circulating PTH and may affect renal PTHrP production (21); these effects may ultimately lead to calcium wasting. B, Estrogens act on the heart to chronically stimulate NO production, which in turn stimulates PTHrP production; chronically produced PTHrP, as well as ischemia-triggered PTHrP, serve to augment recovery after an ischemic insult. During menopause, a decrease in chronic NO production may cause a dramatic decrease in basal PTHrP production. For reasons that remain unclear, ischemically triggered PTHrP no longer serves to augment recovery from an insult but rather is detrimental. C, Estrogens act on osteoblasts to directly augment their formation and matrix-producing capacity. It remains unclear whether there is a connection between the action of estrogens on osteoblasts/osteocytes and PTHrP. Cyclically injected PTH1-34 or PTHrP augment osteoblastic bone formation through yet unclear mechanisms. During the perimenopausal period, high circulating FSH induces increases in osteoclastic bone resorption (14,15). During menopause, decreases in estrogens negatively impact osteoblast function.
Acknowledgments
We are grateful to Ms. Mary Jo Sweeney for expert editorial assistance and thank Jeffrey Blaustein for his helpful comments.
Footnotes
J.I. acknowledges the American Federation for Aging Research (AFAR). M.Z. acknowledges the National Institutes of Health (AG14197, AG23176, and DK70526).
Disclosure Summary: J.S. has nothing to declare. M.Z. is on speaker panels/advisory boards for Proctor and Gamble, Novartis, Roche, GlaxoSmithKline, and Sanofiaventis.
For article see page 3735
Abbreviations: CVD, Cardiovascular disease; HRT, hormone replacement therapy; WHI, Women’s Health Initiative.
References
- Mendelsohn ME, Karas RH 1999 The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- Blaustein JD 2008 An estrogen by any other name. Endocrinology 149:2697–2698 [DOI] [PubMed] [Google Scholar]
- Schreckenberg R, Wenzel S, da Costa Rebelo RM, Rothig A, Meyer R, Schluter K 2009 Cell-specific effects of nitric oxide deficiency on parathyroid hormone-related peptide (PTHrP) responsiveness and PTH1 receptor expression in cardiovascular cells. Endocrinology 150:3735–3741 [DOI] [PubMed] [Google Scholar]
- Ouyang P, Michos ED, Karas RH 2006 Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol 47:1741–1753 [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH 1991 Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med 325:756–762 [DOI] [PubMed] [Google Scholar]
- Foster RH, Balfour JA 1997 Estradiol and dydrogesterone. A review of their combined use as hormone replacement therapy in postmenopausal women. Drugs Aging 11:309–332 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Zaidi M, Blair HC, Iqbal J, Zhu LL, Kumar TR, Zallone A, Sun L 2007 Proresorptive actions of FSH and bone loss. Ann NY Acad Sci 1116:376–382 [DOI] [PubMed] [Google Scholar]
- Speroff L 2004 A clinician’s review of the WHI-related literature. Int J Fertil Womens Med 49:252–267 [PubMed] [Google Scholar]
- Xing D, Nozell S, Chen YF, Hage F, Oparil S 2009 Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol 29:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Clarkson T, Heward CB, Hecht HS, Karas RH, Judelson DR, Naftolin F 2004 Is the WHI relevant to HRT started in the perimenopause? Endocrine 24:195–202 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML 2007 Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477 [DOI] [PubMed] [Google Scholar]
- Aubuchon M, Santoro N 2004 Lessons learned from the WHI: HRT requires a cautious and individualized approach. Geriatrics 59:22–26 [PubMed] [Google Scholar]
- Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M 2006 Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci USA 103:14925–14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M 2006 FSH directly regulates bone mass. Cell 125:247–260 [DOI] [PubMed] [Google Scholar]
- Kim JK, Levin ER 2006 Estrogen signaling in the cardiovascular system. Nucl Recept Signal 4:e013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Pope TW, Moore SA, Campbell CL 2007 The tragedy of TRIUMPH for nitric oxide synthesis inhibition in cardiogenic shock: where do we go from here? Am J Cardiovasc Drugs 7:337–345 [DOI] [PubMed] [Google Scholar]
- Kantha SS 1997 Could nitroglycerine poisoning be the cause of Alfred Nobel’s anginal pains and premature death? Med Hypotheses 49:303–306 [DOI] [PubMed] [Google Scholar]
- Tastan I, Schreckenberg R, Mufti S, Abdallah Y, Piper HM, Schlüter KD 2009 Parathyroid hormone improves contractile performance of adult rat ventricular cardiomyocytes at low concentrations in a non-acute way. Cardiovasc Res 82:77–83 [DOI] [PubMed] [Google Scholar]
- Ross G, Schlüter KD 2005 Cardiac-specific effects of parathyroid hormone-related peptide: modification by aging and hypertension. Cardiovasc Res 66:334–344 [DOI] [PubMed] [Google Scholar]
- Cros M, Silve C, Graulet AM, Morieux C, Ureña P, de Vernejoul MC, Bouizar Z 1998 Estrogen stimulates PTHrP but not PTH/PTHrP receptor gene expression in the kidney of ovariectomized rat. J Cell Biochem 70:84–93 [PubMed] [Google Scholar]
- Grohé C, van Eickels M, Wenzel S, Meyer R, Degenhardt H, Doevendans PA, Heinemann MP, Ross G, Schlüter KD 2004 Sex-specific differences in ventricular expression and function of parathyroid hormone-related peptide. Cardiovasc Res 61:307–316 [DOI] [PubMed] [Google Scholar]
- Jansen J, Gres P, Umschlag C, Heinzel FR, Degenhardt H, Schlüter KD, Heusch G, Schulz R 2003 Parathyroid hormone-related peptide improves contractile function of stunned myocardium in rats and pigs. Am J Physiol Heart Circ Physiol 284:H49–H55 [DOI] [PubMed] [Google Scholar]
- Zaruba MM, Huber BC, Brunner S, Deindl E, David R, Fischer R, Assmann G, Herbach N, Grundmann S, Wanke R, Mueller-Hoecker J, Franz WM 2008 Parathyroid hormone treatment after myocardial infarction promotes cardiac repair by enhanced neovascularization and cell survival. Cardiovasc Res 77:722–731 [DOI] [PubMed] [Google Scholar]
- Horwitz MJ, Tedesco MB, Gundberg C, Garcia-Ocana A, Stewart AF 2003 Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 88:569–575 [DOI] [PubMed] [Google Scholar]
- Yang D, Singh R, Divieti P, Guo J, Bouxsein ML, Bringhurst FR 2007 Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone 40:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JF 2001 The bone growth-stimulating PTH and osteosarcoma. Medscape Womens Health 6:7 [PubMed] [Google Scholar]
- Gaddy D 2008 Inhibin and the regulation of bone mass. Curr Osteoporos Rep 6:51–56 [DOI] [PubMed] [Google Scholar]