Abstract
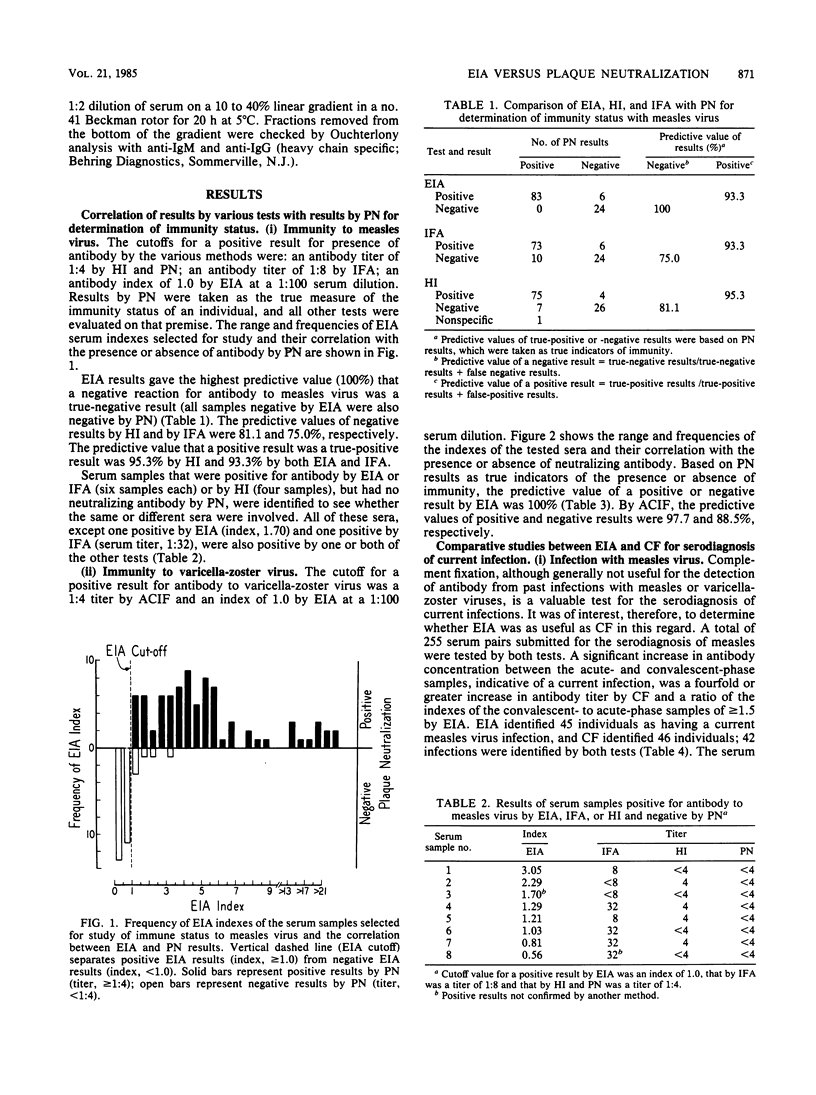
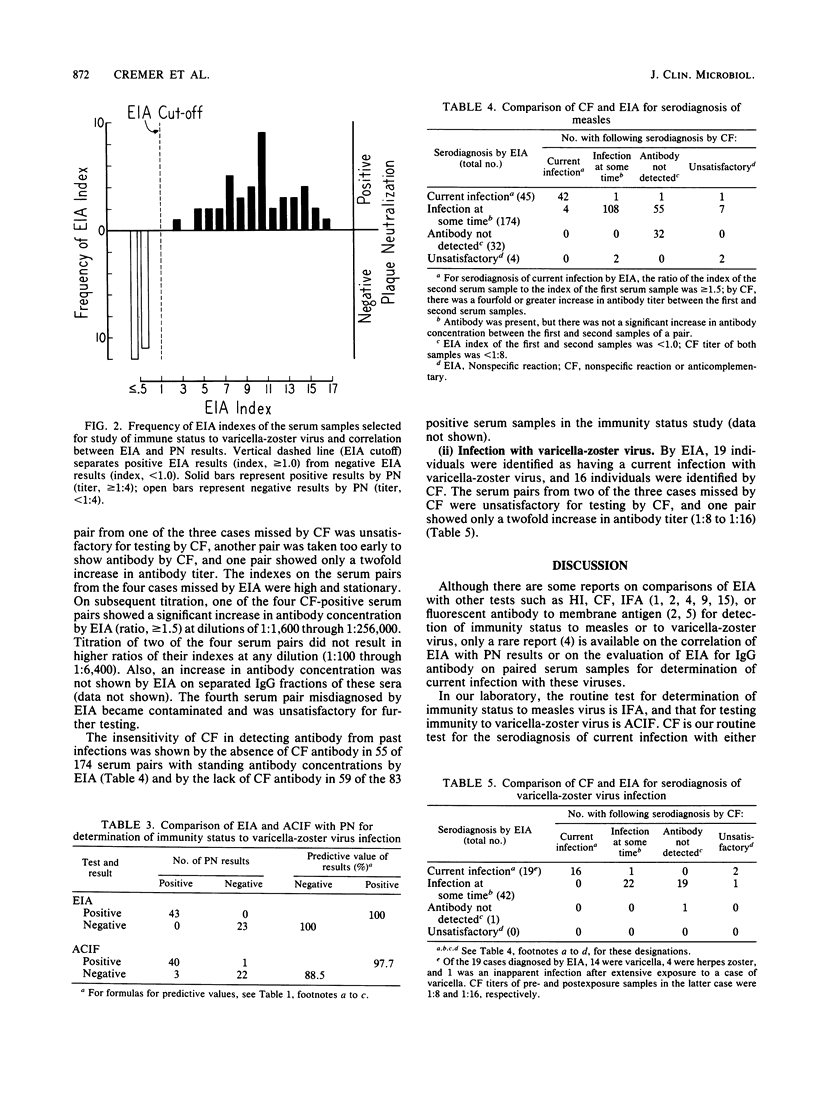
Results by an enzyme immunoassay method (EIA) performed at one serum dilution and results by indirect immunofluorescence (IFA) and hemagglutination inhibition (HI) tests performed at step dilutions were correlated with results by a neutralization test (50% plaque neutralization [PN]) performed at step dilutions on single serum samples for serologic evaluation of immunity status to measles virus. PN results were taken as true indicators of immunity, and the other tests were evaluated on that basis. The predictive value of a positive result being positive also by PN was 95.3% for HI and 93.3% for EIA and IFA. The predictive value of a negative result being negative also by PN was 81.1% for HI, 100% for EIA, and 75.0% for IFA. A similar study on immunity status to varicella-zoster virus by EIA and by an anticomplement immunofluorescence test versus PN showed a 100% predictive value of a positive or negative result by EIA. By the anticomplement immunofluorescence test, the predictive value of a positive result was 97.7%, and that of a negative result was 88.5%. Studies on the comparative ability of EIA versus complement fixation (CF) to detect significant changes in antibody concentration between acute-phase and convalescent-phase serum samples indicative of a current infection were also done. Both tests were satisfactory for the serodiagnosis of measles or varicella-zoster virus infections. However, EIA was preferable to CF because it was less technically difficult, less labor intensive, and could be performed on sera that were anticomplementary in CF reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwell D. E., Bartlett A., Voller A. Enzyme immunoassays for viral diseases. J Infect Dis. 1977 Oct;136 (Suppl):S274–S278. doi: 10.1093/infdis/136.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Moloney M. B., Herrington R. W., Hampson A. W., Hurrell J. G. Enzyme immunoassay for antibodies to membrane associated antigen of varicella zoster virus. J Virol Methods. 1984 Feb;8(1-2):137–145. doi: 10.1016/0166-0934(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Dennis J. Antibody assays for varicella-zoster virus: comparison of enzyme immunoassay with neutralization, immune adherence hemagglutination, and complement fixation. J Clin Microbiol. 1978 Nov;8(5):545–552. doi: 10.1128/jcm.8.5.545-552.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A. A., Frey H. M., Steinberg S. P., Seeman M. D., Bidwell D., Voller A. Determination of immunity to varicella using an enzyme-linked-immunosorbent-assay. Arch Virol. 1981;70(2):169–172. doi: 10.1007/BF01315011. [DOI] [PubMed] [Google Scholar]
- Kramer S. M., Cremer N. E. Detection of IgG antibodies to measles virus using monoclonal antibodies for antigen-capture in enzyme immunoassay. J Virol Methods. 1984 May;8(3):255–263. doi: 10.1016/0166-0934(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Norrby E., Enders-Ruckle G., Meulen V. Differences in the appearance of antibodies to structural components of measles virus after immunization with inactivated and live virus. J Infect Dis. 1975 Sep;132(3):262–269. doi: 10.1093/infdis/132.3.262. [DOI] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Identification of measles virus-specific hemolysis-inihibiting antibodies separate from hemagglutination-inhibiting antibodies. Infect Immun. 1975 Feb;11(2):231–239. doi: 10.1128/iai.11.2.231-239.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Hammarskjöld B. Structural components of measles virus. Microbios. 1972 Jan;5(17):17–29. [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Neutralizing antibody responses to varicella-zoster virus. Infect Immun. 1975 Sep;12(3):606–613. doi: 10.1128/iai.12.3.606-613.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani L., Haikin H., Sarov I. A rapid immunoperoxidase assay for determination of IgG antibodies to measles virus. J Immunol Methods. 1981;40(3):359–365. doi: 10.1016/0022-1759(81)90367-7. [DOI] [PubMed] [Google Scholar]
- Weller T. H. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983 Dec 1;309(22):1362–1368. doi: 10.1056/NEJM198312013092205. [DOI] [PubMed] [Google Scholar]
- de Savigny D., Voller A. The communication of ELISA data from laboratory to clinician. J Immunoassay. 1980;1(1):105–128. doi: 10.1080/01971528008055779. [DOI] [PubMed] [Google Scholar]


