Abstract
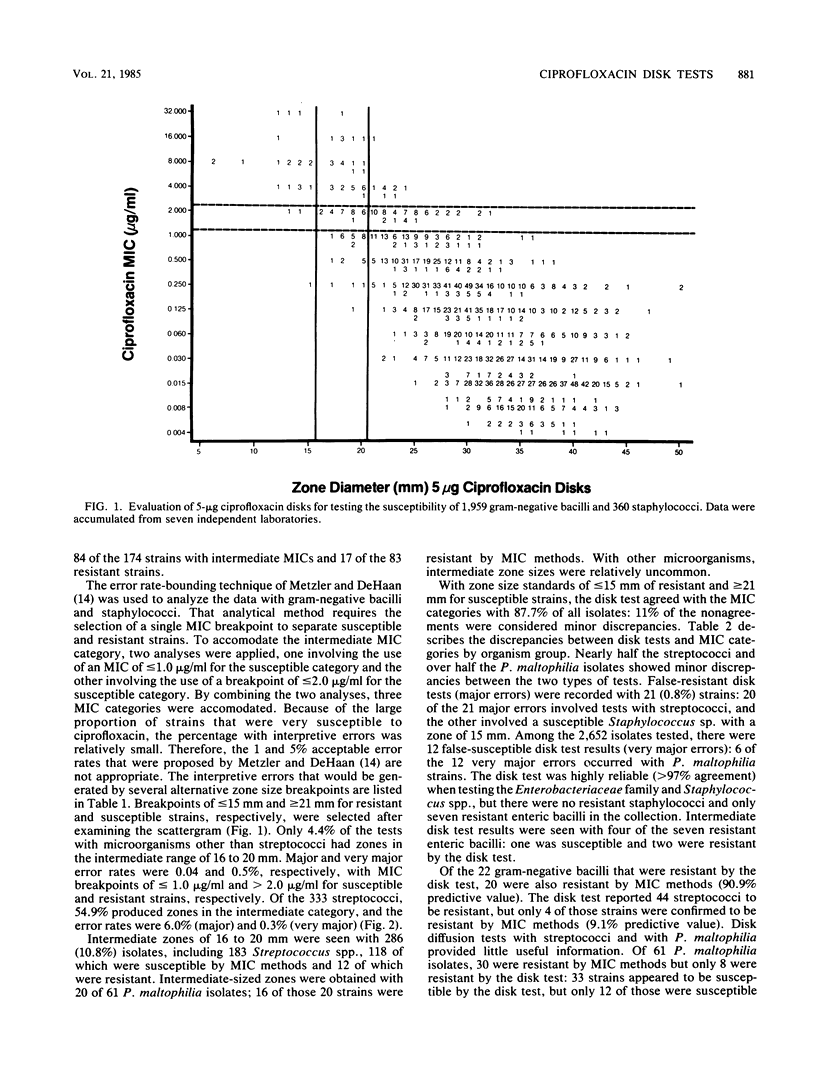
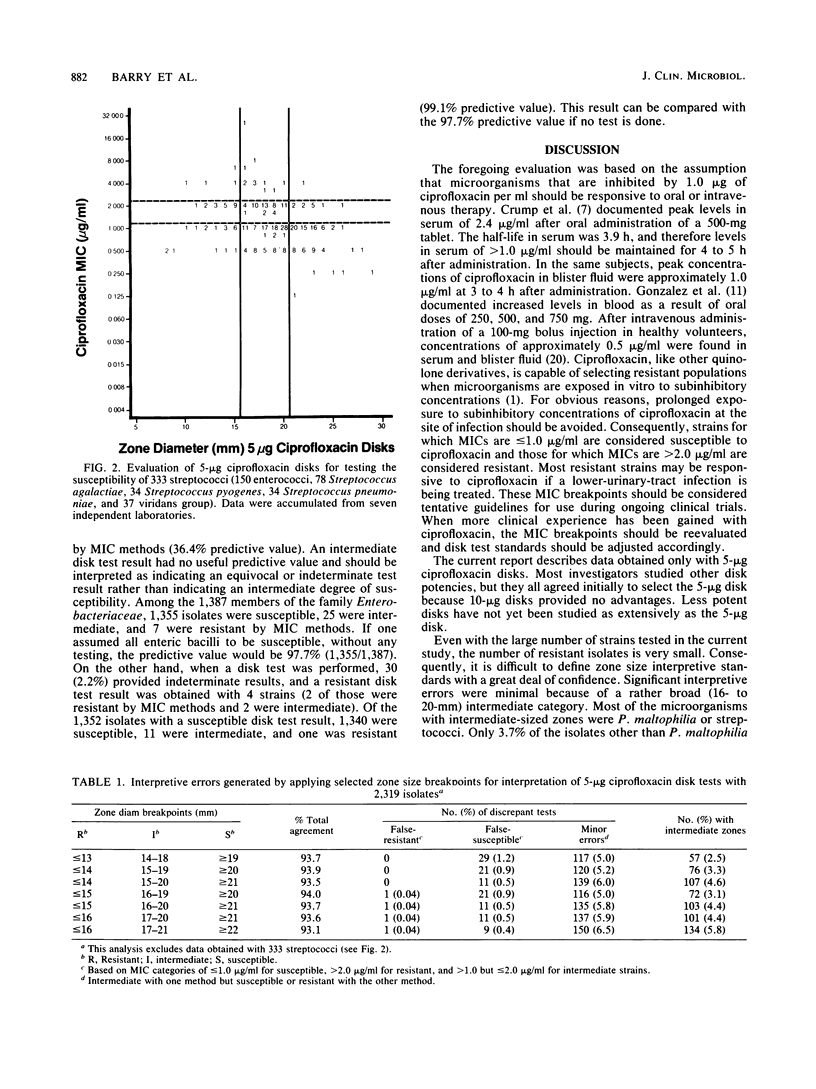
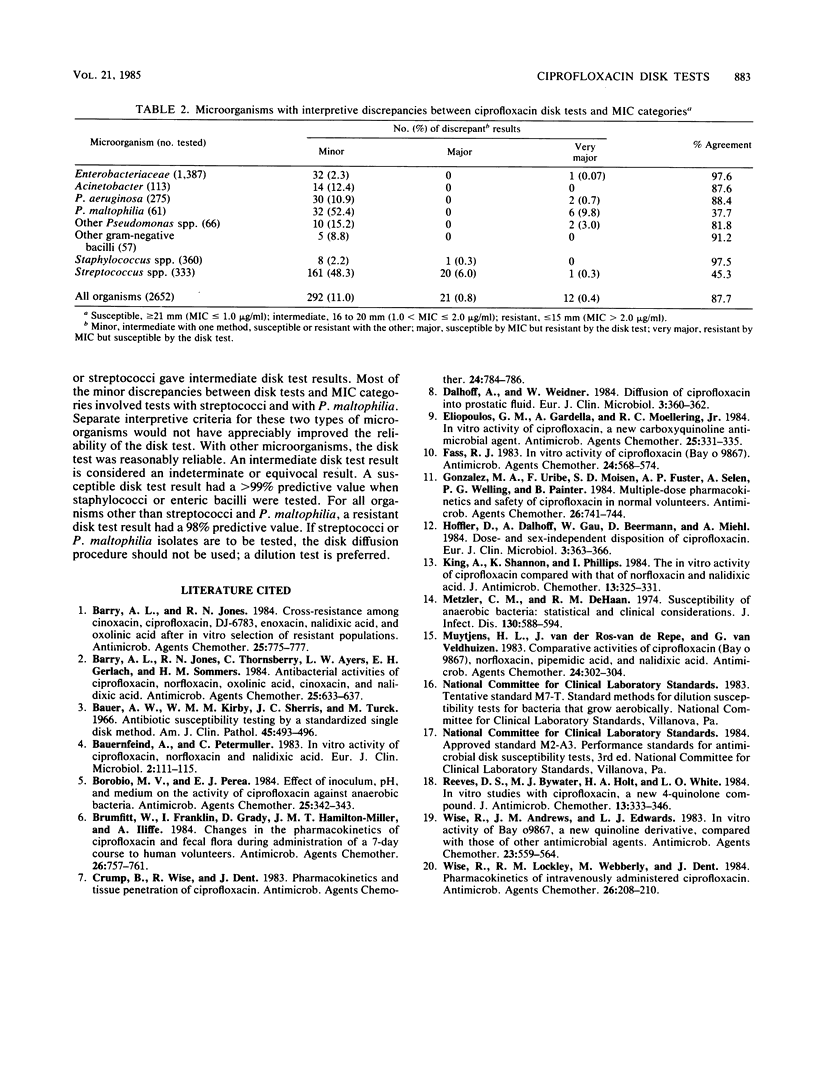
Evaluations of 5-microgram ciprofloxacin disk diffusion susceptibility tests were performed independently by seven different investigators. The results of the separate tests were combined to increase the number of resistant strains in the challenge set of microorganisms. Based on data with 2,652 isolates, the following interpretive breakpoints are tentatively proposed for use in ongoing clinical trials of ciprofloxacin: less than or equal to 15 mm, resistant (MIC greater than 2.0 micrograms/ml); 16 to 20 mm, intermediate (1.0 less than MIC less than or equal to 2.0 micrograms/ml); and greater than or equal to 21 mm, susceptible (MIC less than or equal to 1.0 micrograms/ml). Disk tests with Streptococcus spp. and with Pseudomonas maltophilia were not reliable; other microorganisms were accurately categorized by the disk diffusion test.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N. Cross-resistance among cinoxacin, ciprofloxacin, DJ-6783, enoxacin, nalidixic acid, norfloxacin, and oxolinic acid after in vitro selection of resistant populations. Antimicrob Agents Chemother. 1984 Jun;25(6):775–777. doi: 10.1128/aac.25.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C., Ayers L. W., Gerlach E. H., Sommers H. M. Antibacterial activities of ciprofloxacin, norfloxacin, oxolinic acid, cinoxacin, and nalidixic acid. Antimicrob Agents Chemother. 1984 May;25(5):633–637. doi: 10.1128/aac.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bauernfeind A., Petermüller C. In vitro activity of ciprofloxacin, norfloxacin and nalidixic acid. Eur J Clin Microbiol. 1983 Apr;2(2):111–115. doi: 10.1007/BF02001575. [DOI] [PubMed] [Google Scholar]
- Borobio M. V., Perea E. J. Effect of inoculum, pH, and medium on the activity of ciprofloxacin against anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):342–343. doi: 10.1128/aac.25.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Franklin I., Grady D., Hamilton-Miller J. M., Iliffe A. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):757–761. doi: 10.1128/aac.26.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff A., Weidner W. Diffusion of ciprofloxacin into prostatic fluid. Eur J Clin Microbiol. 1984 Aug;3(4):360–362. doi: 10.1007/BF01977495. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Gardella A., Moellering R. C., Jr In vitro activity of ciprofloxacin, a new carboxyquinoline antimicrobial agent. Antimicrob Agents Chemother. 1984 Mar;25(3):331–335. doi: 10.1128/aac.25.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. In vitro activity of ciprofloxacin (Bay o 9867). Antimicrob Agents Chemother. 1983 Oct;24(4):568–574. doi: 10.1128/aac.24.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Uribe F., Moisen S. D., Fuster A. P., Selen A., Welling P. G., Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffler D., Dalhoff A., Gau W., Beermann D., Michl A. Dose- and sex-independent disposition of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):363–366. doi: 10.1007/BF01977496. [DOI] [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 Apr;13(4):325–331. doi: 10.1093/jac/13.4.325. [DOI] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J., van Veldhuizen G. Comparative activities of ciprofloxacin (Bay o 9867), norfloxacin, pipemidic acid, and nalidixic acid. Antimicrob Agents Chemother. 1983 Aug;24(2):302–304. doi: 10.1128/aac.24.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A., White L. O. In-vitro studies with ciprofloxacin, a new 4-quinolone compound. J Antimicrob Chemother. 1984 Apr;13(4):333–346. doi: 10.1093/jac/13.4.333. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]


