Abstract
In this study the mRNA levels of five EGFR indirectly related genes, EGFR, HB-EGF, ADAM17, PTEN, and MMP9, have been assessed by Real-time PCR in a panel of 37 glioblastoma multiforme specimens and in 5 normal brain samples; as a result, in glioblastoma, ADAM17 and PTEN expression was significantly lower than in normal brain samples, and, in particular, a statistically significant inverse correlation was found between PTEN and MMP9 mRNA levels. To verify if this correlation was conserved in gliomas, PTEN and MMP9 expression was further investigated in an additional panel of 16 anaplastic astrocytoma specimens and, in parallel, in different human normal and astrocytic tumor cell lines. In anaplastic astrocytomas PTEN expression was significantly higher than in glioblastoma multiforme, but no significant correlation was found between PTEN and MMP9 expression. PTEN and MMP9 mRNA levels were also employed to identify subgroups of specimens within the different glioma malignancy grades and to define a gene expression-based diagnostic classification scheme. In conclusion, this gene expression survey highlighted that the combined measurement of PTEN and MMP9 transcripts might represent a novel reliable tool for the differential diagnosis of high-grade gliomas, and it also suggested a functional link involving these genes in glial tumors.
1. Introduction
Glioblastoma multiforme is the most malignant brain tumor among astrocytic gliomas with a typical prognosis of about 12 months in spite of current therapeutic approaches that include neurosurgery followed by combined chemotherapy and radiotherapy [1]. Recently, the development of massive screening genome technologies, such as gene expression profiling, has prompted new attempts to the classification of glioblastoma subgroups on molecular basis in order to identify new diagnostic or prognostic tools. At present the search for potential molecular markers among aberrant signal transduction pathways in glioblastoma is actively exploited for the optimization of existing therapies or the development of innovative drugs [2]. However, the accomplishment of this ambitious task is severely hindered by the extreme heterogeneity of glioblastoma tumor samples and by the subsequent variability of possibly identified molecular markers. One way to overcome this limit could be represented by the concomitant analysis of the mRNA expression of several selected genes, already known to be functionally involved in the cellular malignant transformation. This analysis could highlight differences in gene expression levels among high-grade gliomas, or at the same time it could reveal relationships within glioma subtypes between the genes analyzed in order to improve their reliability as prognostic or diagnostic markers.
The epidermal growth factor (EGF) receptor (EGFR or ErbB1) plays a pivotal role in cancer physiology because its activation, elicited by at least six different endogenous peptidergic EGF-like ligands, leads to the activation of intracellular signalling pathways that modulate cell proliferation, metastasis, and angiogenesis [3]. About 40%–50% of glioblastoma cases are characterized by EGFR gene amplification or overexpression, together with the expression of the mutated and constitutively active EGFR isoform EGFRvIII [3]. Upregulation of the EGFR pathway could also result from an increased availability of EGFR endogenous agonists belonging to the family of EGF-like growth factors.
Heparin-binding epidermal growth factor (HB-EGF) acts as a potent proliferative agent in many different cell types via the activation of EGFR or the other EGF-like receptor ErbB4 [4]. HB-EGF is initially synthesized as the membrane-spanning protein proHB-EGF and then is proteolytically cleaved by “A Disintegrin And Metalloproteinase” (ADAM) family members that release the soluble form (sHB-EGF) in the extracellular space. The ADAM isoform responsible for this process appears to be cell type dependent, since in different experimental models ADAM 10, 12, and 17 have been involved in proHB-EGF shedding [3]. The overexpression of ADAM17, also named “tumor necrosis factor-alpha-converting enzyme” (TACE), seems to be involved in the malignant potential of cancer cells [5], and, notably, this metalloprotease modulates HB-EGF shedding and cell proliferation in U373-MG glioblastoma cell line [5].
In the clinical practice only 10%–20% of glioblastoma patients respond to EGFR kinase inhibitors, and this poor response has been ascribed to a combination of EGFR overexpression and loss or mutation of the Phosphatase and TEN sin homolog deleted from chromosome 10 (PTEN) tumor suppressor protein [6]. The PTEN phosphatase reduces the levels of the second messenger phosphatidylinositol 3,4,5-triphosphate (PI3K) and regulates the activity of the downstream PI-3K/AKT- and mammalian target of rapamacyn- (mTOR-) dependent pathways [7]. Notably, PTEN functional loss or mutation is present in 60%–70% of high-grade gliomas and is associated with malignant phenotypic changes such as migration capability, probably by modulation of FAK activity [8]. Moreover, since PTEN loss appears to accelerate the formation of high-grade gliomas [6], it could potentially represent a valid candidate gene to discriminate between high- and low-grade gliomas.
One typical feature of glioblastoma is its high ability to disseminate and spread to distant brain areas. Proteases expressed by glioma cells appear to play a significant role in these processes because selective matrix metalloproteinases like MMP2 or MMP9 degrade the extracellular environment in order to facilitate tumor cell growth and migration. Expression of these proteases appears to increase with glioma grade and in vitro studies showed that modifications of their expression levels resulted in altered migratory properties [9]. Although some previous reports have examined the expression of EGFR [3], PTEN [10], HB-EGF [9], ADAM17 [11], and MMP9 [12] in astrocytoma samples, at present the transcriptional expression of these five EGFR pathway-related genes has not yet been simultaneously investigated in glioma.
Therefore, in this study we have evaluated by quantitative Real-time PCR the expression of ADAM17, EGFR, HB-EGF, PTEN, and MMP9 mRNAs in a panel of glioblastoma and anaplastic astrocytomas specimens and cell lines, and we have finally compared them to normal control samples to ascertain whether these expression profiles might provide additional tools in glioma diagnosis and in tumor subtypes identification.
2. Materials and Methods
2.1. Human Biopsy Samples
Biopsy samples, obtained from Azienda Ospedaliera Universitaria di Parma (Parma, Italy) after informed consent of the patients, were placed in ice-cold Trizol reagent (Invitrogen, Paisley, UK) and immediately processed for RNA extraction. Sections of samples were independently histologically and morphologically evaluated by different neuropathologists and classified as grade IV (glioblastoma multiforme) or grade III (anaplastic astrocytoma), according to WHO guidelines [13]. Clinical data of glioblastoma patients are reported in Table 1, and they included 19 females and 18 males (age range 23–84 years, mean 57.8 ± 13.3). The anaplastic astrocytoma patients (Table 2) included 7 males and 9 females (age 28–68, mean 50.7 ± 13.9). Total RNA samples extracted from human postmortem normal brain (NB) cortical regions, as reported in Table 3, were purchased from Ambion (Foster City, Calif, USA): these included 2 females and 3 males (age range 50–71 years, mean 60.6 ± 9.3).
Table 1.
Age, gender, mRNA expression values (in femptograms), and anatomical location of glioblasytoma multiforme samples.
| Age | Sex | EGFR | ADAM17 | HB-EGF | PTEN | MMP9 | Location |
|---|---|---|---|---|---|---|---|
| 68 | M | 2.78 | 0.31 | 0.69 | 1.85 | 6.39 | Parietal |
| 84 | F | 155.97 | 0.31 | 4.43 | 6.81 | 31.13 | Parietal |
| 23 | M | 95.25 | 3.54 | 19.01 | 5.15 | 0.04 | Cerebellum |
| 50 | M | 579.69 | 0.87 | 9.28 | 8.97 | 43.32 | Frontal dx |
| 71 | F | 7.11 | 0.08 | 0.82 | 2.27 | 0.21 | Frontal dx |
| 58 | M | 236.18 | 0.91 | 8.98 | 15.05 | 183.29 | Parietal dx |
| 66 | M | 12.85 | 0.13 | 1.38 | 0.41 | 0.04 | Temporal sx |
| 50 | M | 150.97 | 3.08 | 4.87 | 1.65 | 3.09 | Temporal dx |
| 38 | F | 60.10 | 2.23 | 9.23 | 7.01 | 30.41 | Occipital dx |
| 61 | F | 2.88 | 0.41 | 1.75 | 1.96 | 0.72 | Frontal sx |
| 68 | F | 4.87 | 0.63 | 8.71 | 0.82 | 0.82 | Occipital dx |
| 70 | M | 23.38 | 2.13 | 2.82 | 3.92 | 3.51 | Frontal dx |
| 59 | M | 425.51 | 1.28 | 4.32 | 4.84 | 8.04 | Frontal sx |
| 67 | F | 1268.24 | 2.99 | 13.92 | 12.68 | 32.58 | Temporal dx |
| 31 | M | 13.33 | 1.87 | 52.08 | 6.66 | 13.33 | Frontal sx |
| 68 | F | 0.77 | 0.59 | 12.85 | 0.62 | 1.04 | Parietal dx |
| 39 | F | 6.87 | 13.33 | 0.21 | 3.14 | 0.16 | Frontoinsular |
| 39 | M | 2.37 | 0.42 | 3.96 | 4.79 | 0.21 | Parietal sx |
| 45 | F | 274.74 | 0.66 | 7.14 | 1.25 | 2.52 | Frontal dx |
| 63 | M | 1284.37 | 13.33 | 0.21 | 3.12 | 10.62 | Temporal dx |
| 71 | M | 30.11 | 5.42 | 10.62 | 2.92 | 2.08 | Frontal sx |
| 60 | M | 73.96 | 17.71 | 7.54 | 3.33 | 4.37 | Temporal sx |
| 44 | F | 2562.92 | 6.87 | 18.96 | 3.33 | 8.33 | Thalamus sx |
| 73 | F | 38.75 | 13.12 | 7.53 | 3.33 | 1.04 | Paratrigonal sx |
| 47 | F | 16.88 | 4.79 | 7.29 | 3.13 | 1.04 | Frontal sx |
| 63 | M | 12.01 | 12.31 | 28.22 | 3.85 | 3.28 | Occipital dx |
| 77 | F | 239.85 | 8.72 | 22.56 | 2.92 | 6.31 | Cortical anterior |
| 55 | F | 22.36 | 2.51 | 5.38 | 1.54 | 0.05 | Parietal dx |
| 70 | F | 13.18 | 9.18 | 12.31 | 3.08 | 0.16 | Parietal dx |
| 60 | F | 8.92 | 7.95 | 16.56 | 1.54 | 0.23 | Temporal sx |
| 54 | M | 1071.43 | 15.49 | 16.56 | 2.56 | 1.64 | Temporal sx |
| 55 | M | 371.49 | 9.54 | 19.49 | 1.54 | 1.85 | Temporal sx |
| 55 | F | 27.54 | 9.23 | 5.13 | 1.54 | 0.15 | Temporal sx |
| 58 | M | 21.38 | 20.87 | 17.38 | 3.08 | 1.23 | Temporal sx |
| 53 | F | 8.1 | 7.23 | 9.74 | 2.05 | 0.15 | Occipital sx |
| 69 | F | 4.66 | 12.56 | 26.69 | 3.08 | 1.69 | Frontal sx |
| 70 | F | 29.49 | 5.23 | 7.08 | 7.23 | 0.61 | Parietal dx |
Table 2.
Age, gender, PTEN, and MMP9 expression values (in femtograms), and anatomical location of anaplastic astrocytoma samples.
| Age | Sex | PTEN | MMP9 | Location |
|---|---|---|---|---|
| 44 | M | 1.72 | 1.25 | Temporal sx |
| 68 | F | 7.66 | 0.57 | Thalamus sx |
| 67 | F | 2.25 | 37.06 | Frontal dx |
| 60 | F | 2.44 | 1.62 | Frontal sx |
| 50 | M | 92.46 | 0.79 | Temporal dx |
| 39 | M | 79.25 | 32.92 | Parietal dx |
| 64 | F | 33.82 | 43.21 | Parietal dx |
| 28 | F | 25.64 | 26.24 | Temporal sx |
| 55 | F | 12.47 | 0.32 | Temporal sx |
| 31 | M | 19.81 | 1.95 | Temporal sx |
| 52 | F | 50.12 | 4.37 | Temporal sx |
| 71 | F | 17.98 | 0.28 | Frontal cortex |
| 62 | M | 20.41 | 0.03 | Total cortex |
| 50 | M | 60.72 | 0.76 | Occipital cortex |
| 52 | M | 45.11 | 0.45 | Total cortex |
| 68 | F | 25.30 | 0.23 | Frontal cortex |
Table 3.
Age, gender, mRNA expression values (in femtograms), and anatomical location of normal brain specimens.
| Age | Sex | EGFR | ADAM17 | HB-EGF | PTEN | MMP9 | Location |
|---|---|---|---|---|---|---|---|
| 71 | F | 32.58 | 10.74 | 14.84 | 17.98 | 0.28 | Frontal cortex |
| 62 | M | 16.70 | 12.76 | 10.41 | 20.41 | 0.03 | Total cortex |
| 50 | M | 57.94 | 18.35 | 30.41 | 60.72 | 0.76 | Occipital cortex |
| 52 | M | 25.74 | 13.87 | 28.55 | 45.11 | 0.45 | Total cortex |
| 68 | F | 46.98 | 17.20 | 7.60 | 25.30 | 0.23 | Frontal cortex |
2.2. Cell Lines
Normal human astrocytes (NHAs) were purchased from Cambrex (East Rutherford, NJ, USA) and cultivated in the specific astrocyte AGM medium (Cambrex) according to the manufacturer's specifications. Human glioma cell line U138-MG, derived from a glioblastoma multiforme patient and widely employed, was purchased from ATCC (Rockville, Md, USA); PRT-HU2 cells, previously described [14], were analogously derived from a glioblastoma multiforme patient. U138-MG and PRT-HU2 cells were cultivated in D-MEM medium supplemented with 10% FBS, 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 1% L-glutamine (Invitrogen, Paisley, UK).
2.3. Real Time Quantitative PCR
Total RNA was extracted as previously described [14] and accurately quantified using spectrophotometric and fluorimetric (Quant-it RNA Assay, Invitrogen) approaches. The gene-specific primers were designed using the “Primer3 input” software (http://frodo.wi.mit.edu/primer3/), and their specificity was verified using the Primer-BLAST software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome/). GeneBank accession numbers of the five genes examined, their respective primer pairs sequences, and PCR products lengths were reported in Table 5. Quantitative Real-time PCR analysis was performed as previously described [14]. For the absolute quantification of specific cDNA, standard curves were derived using different concentrations of EGFR, ADAM17, MMP9, HB-EGF, and PTEN DNA-sequenced templates, prepared by reamplifying the PCR purified products obtained from Real-time PCR. The second derivative maximum method in the Light Cycler software was used to calculate the crossing point (Cp) value, and the concentrations of each specific cDNA were determined. All results were quantitative expressed in femtograms (fg) of cDNA, normalized to a total RNA input of 1 microgram.
Table 5.
GeneBank accession numbers, PCR primer sequences, and products.
| Gene | Accession number | PCR primer sequences (5′-3′) | PCR product (bp) |
|---|---|---|---|
| EGFR | NM_005228 | AGGAAGAAGCTTGCTGGTAGC | 88 |
| CTCTGGAAGACTTGTGGCTTG | |||
| ADAM17 | NM_003183 | CAAGTCATTTGAGGATCTCACG | 96 |
| TCTTTGCTGTCAACACGATTCT | |||
| HB-EGF | NM_001945 | GCCTAGGCGATTTTGTCTACC | 119 |
| GCCCAACCTCTTCTGAGACTT | |||
| PTEN | NM_000314 | CAGCAGTGGCTCTGTGTGTAA | 98 |
| ATGGACATCTGATTGGGATGA | |||
| MMP9 | NM_004994 | AAAGCCTATTTCTGCCAGGAC | 105 |
| GCACTGCAGGATGTCATAGGT |
2.4. Statistical and Bioinformatics Analysis
All results were expressed as mean ± standard deviation of each biopsy specimens, assayed in duplicate. The Instat v.3 software (GraphPad Software Inc., Mass, USA) was used for the statistical analysis of differences in gene expression between groups by one-way ANOVA and for the analysis of correlations among gene expression profiles, using the Pearson coefficient r. A P-value less than .05 was considered statistically significant. The dendrogram and the classification tree analysis were performed using the Orange data mining software (http://www.ailab.si/orange/). In particular, for the hierarchical clustering analysis, an Euclidean distance matrix was adopted. Statistical trends were obtained from the PTEN and MMP9 average quantitative expression within normal control, anaplastic astrocytoma, and glioblastoma multiforme diagnostic classes, using the Excel software with an exponential setting (Microsoft Word Package 2003, Redmond, Wash, USA).
3. Results
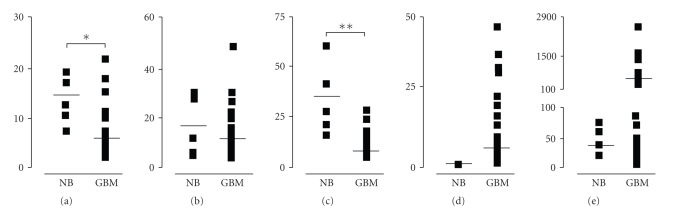
The mRNA expression of the investigated genes of glioblastoma multiforme and normal brain specimens were analytically reported in Tables 1 and 2 and depicted in Figures 1 and 2. On the basis of their expression patterns in glioblastoma and using the mean values of normal samples as cut-off, the investigated genes were roughly classified in three different subgroups, the first including ADAM17, HB-EGF, and PTEN, whose average levels in glioblastoma were below the controls, the second comprising MMP9, whose majority of values in glioblastoma specimens were higher than in controls and, finally, the last one constituted by the EGFR gene, displaying the widest variation and data dispersion (Figure 1).
Figure 1.
The mRNA expression levels of (a) ADAM17, (b) HB-EGF, (c) PTEN, (d) MMP9, and (e) EGFR in 37 human glioblastoma samples (GBM) and in five normal brain cortex samples (NB). Horizontal lines represent the mean values. *P < .002, **P < .0001.
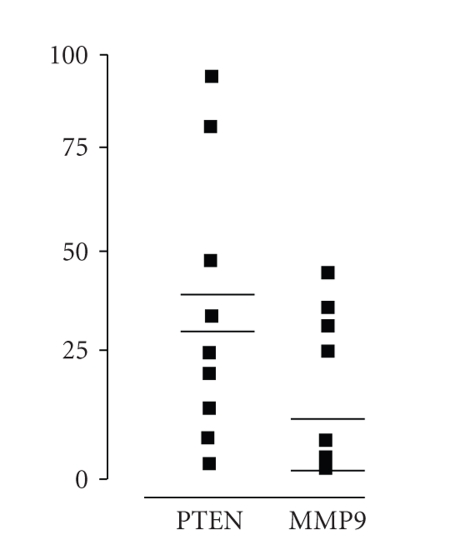
Figure 2.
The mRNA expression levels of PTEN and MMP9 in 16 human grade III astrocytoma samples (AA). Bold horizontal lines represent the mean values, and thin lines represent the mean normal brain cortex samples (NB) values.
The mRNA levels of ADAM17 metallo-protease in glioblastoma (mean 5.88 fg ± 5.71) were significantly lower (P < .002) than in controls (14.51 fg ± 3.15). In particular, in 33 out of 37 cases (89.19%), ADAM17 expression was lower than in control samples. For HB-EGF gene, although 31 out of 37 (83.78%) glioblastoma samples displayed mRNA levels lower than controls, statistical analysis revealed that the difference between controls (18.36 fg ± 10.52) and glioblastoma specimens (10.96 fg ± 10.13) was not statistically significant (P < .434).
PTEN expression pattern showed statistically lower mRNA levels in all glioblastoma samples compared to controls. Notably, the highest PTEN expression level among all glioblastoma had a quantitative expression (15.05 fg) that was less than 50% of the mean controls values (33.91 fg), with a control normalized ratio ranging from 2.25 to 82.72 fg. As expected, there was a very high statistically significant difference (P < .0001) of PTEN mRNA expression between glioblastoma (3.86 fg ± 3.15) and controls (33.91 fg ± 18.44).
MMP9 was overexpressed in the majority of glioblastoma specimens (72.22%) and, furthermore, in a subgroup of 24 cases (64.86%) the expression level was at least twofold higher than in controls, with a control normalized ratio within this subgroup ranging from 1.74 to 123.71 fg. However, the mean expression level of MMP9 mRNA in glioblastoma (5.11 fg ± 8.78) was not significantly different (P = .238) from control samples (0.35 fg ± 0.27).
EGFR mRNA transcript levels, due to their amplitude in expression, were arbitrarily divided into two glioblastoma subgroups. The former, ranging from 0.77 to 95.25 fg, included 25 samples (67.63%); the latter, had EGRF absolute quantitative values ranging from 150.97 to 2562.92 fg. All control samples showed EGFR expression values below 100 fg, with a mean value of 35.98 fg ± 16.51. It was evident that for EGFR there was no statistically significant difference (P < .371) between glioblastoma (247.60 fg ± 517.45) and control samples (35.98 fg ± 16.52).
Within glioblastoma samples, a highly statistically significant negative correlation (P < .0001; Person coefficient r = −0.776) was related to the expression of PTEN and MMP9; in a different manner, a statistically significant positive correlation (P < .05; Pearson coefficient, r = 0.9221) was scored for the same genes within the control samples. The inverse correlation found between PTEN and MMP9 mRNA expression in glioblastoma compared to control samples, prompted us to investigate whether this correlation was also detectable in other glioma grades of malignancy. Therefore, PTEN and MMP9 expression, reported in Table 2 and illustrated in Figure 2, was investigated in 16 histological confirmed anaplastic astrocytoma specimens, previously classified as WHO grade III. In these samples, PTEN mRNA normalized quantitation (29.72 fg ± 31.62) was not significantly different from control (P = .792), but this value was significantly higher compared to glioblastoma (P < .0001). MMP9 expression (13.60 fg ± 17.28) was neither significantly different from the control (0.35 fg ± 0.27, P = .113) nor from glioblastoma specimens (P = .103). Notably, no statistically significant correlation (P = .709) was found between the mRNA levels of these two genes in anaplastic astrocytoma samples. Differently from glioblastoma, no inverse correlation in PTEN and MMP9 expression was found comparing anaplastic astrocytoma and control samples (Pearson coefficient, r = 0.127).
Then, PTEN and MMP9 mRNA levels were comparatively examined in two different human established glioblastoma cell lines (PRT-HU2 and U138-MG) and in a primary culture of embryonic normal human astrocytes (NHA), as shown in Table 4. In these cells, PTEN mRNA levels were much variable comparing to MMP9 expression; similarly to the above examined control samples, normal astrocytes exhibited the highest PTEN expression.
Table 4.
MMP9 and PTEN absolute quantitative expression in different human normal (NHA) and glioma (PRT-HU2 and U138-MG) cell lines. Expression is indicated in femptograms.
| Cell line | MMP9 | PTEN |
|---|---|---|
| NHA | 0.59 | 117.97 |
| PRT-HU2 | 0.56 | 45.62 |
| U138-MG | 3.09 | 4.08 |
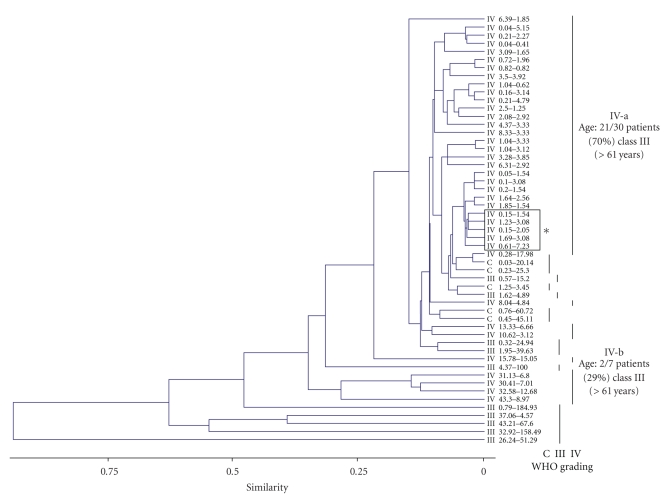
We next exploited if PTEN and MMP9 expression might be more tightly related to the glioma tumor progression. To this purpose, different bioinformatics analyses were performed using the experimental data set reported in Tables 1, 2, 3, and 4 and considering the WHO grading of the specimens. We therefore performed hierarchical clustering, a standard unsupervised learning method [10] of the tumor specimens. The WHO grading was simultaneously compared to PTEN and MMP9 expression values, to identify homogeneous clusters. As reported in the dendrogram of Figure 3, different groups of samples were created, according to the above mentioned criteria. Using Euclidean distances, WHO grades IV and III and normal control samples were roughly classified into different clusters. Of note, a major subset of the glioblastoma specimens was identified (Figure 3, subgroup IV-a), showing the lowest and almost similar levels of both PTEN and MMP9 transcripts; the remaining glioblastoma samples clustered within subgroup IV-b. The association of tumor anatomical localization and age of the patients with the levels of PTEN and MMP9 expression was also investigated. According to the clinical data (Tables 1–3), ages of the patients were divided into three classes (i.e., I, 20–40; II, 41–60; III, >61 years): as reported in the dendrogram, the age-class III patients exhibited a statistically significant difference in distribution between subgroups IV-a and IV-b (P < .01, Anova one-way). Differently, no clear associations between levels of PTEN and MMP9 expression and tumor localization were highlighted, with the exception of a single cluster of four class-III patients with a temporal tumor localization within subgroup IV-a.
Figure 3.
Dendrogram comparing PTEN and MMP9 absolute quantitative expression. Absolute quantifications are expressed in femtograms, as reported in Materials and Methods. WHO grades of malignancy (III, IV) and healthy brain control (C) specimens are indicated. Glioblastoma multiforme subgroups IV-a, including the majority of glioblastoma specimens, and IV-b, described in Results, are highlighted. The box (*) indicates subgrouping of patients sharing similar tumor anatomical localization (i.e., temporal). Subgroups IV-a and IV-b have a different and significant distribution of age-class III patients (>61 years, P < .01 Anova-one way).
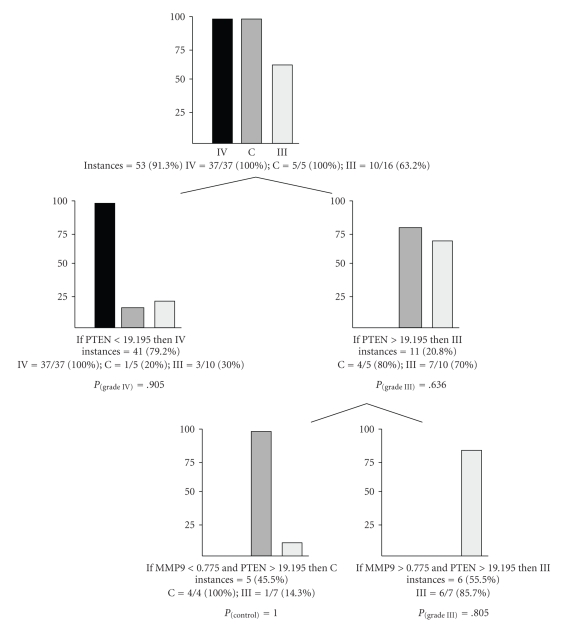
Then, a classification tree was produced, evaluating the performance of PTEN and MMP9 expression in predicting roots characterized by different WHO malignant grades; as reported in Figure 4, 53 out of 58 total samples, corresponding to 53 instances (i.e., 91.3%) fitted with the classification tree; in detail, all grade IV samples had PTEN normalized expression values below a quantity of 19.195 fg (37/37 instances, P = .905), while the large majority of control (80% of the total C instances) and anaplastic astrocytomas samples (70%, P = .636) were grouped with PTEN > 19.195 fg; in this subgroup, to further differentiate control and anaplastic astrocytomas, MMP9 < 0.775 fg clustered all control instances (P = 1.000).
Figure 4.
Classification tree derived from the combined measurement of PTEN and MMP9 quantitative expression. At each root, diagnostic classes are divided following the absolute quantification and expressed in femtograms. Probabilities values (P) for the different classes are reported.
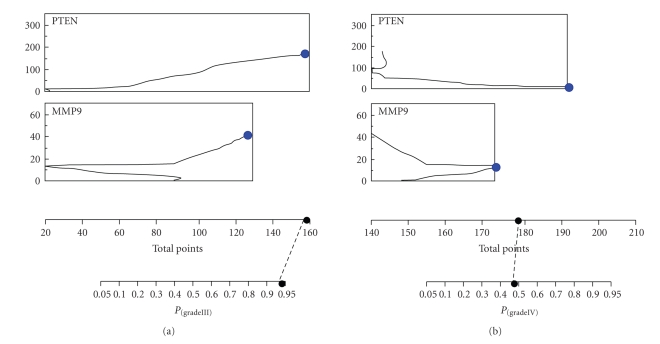
Nomogram analysis, performed within the same data set and reported in Figure 5 confirmed that PTEN and MMP9 expression analysis might be particularly helpful in the identification of anaplastic astrocytoma specimens having a 95% of probability (P(grade III) = .95) to correctly classify these samples, in correspondence of MMP9-normalized expression value of 19.195 fg, independently of PTEN expression level. Nomograms analysis with control and glioblastoma as target diagnostic classes showed less statistical significant probabilities (P(control) = .60 and P(grade IV) = .55).
Figure 5.
Nomogram analysis of PTEN and MMP9 expression within the different WHO grades of malignancy (III, IV) of the astrocytomas specimens. Points and total points axes indicate the points attributed to each variable value and the sum of the points for each variable, respectively. P axes indicate the predicted probability that relates PTEN or MMP9 quantitative expression to each WHO tumor malignancy grade.
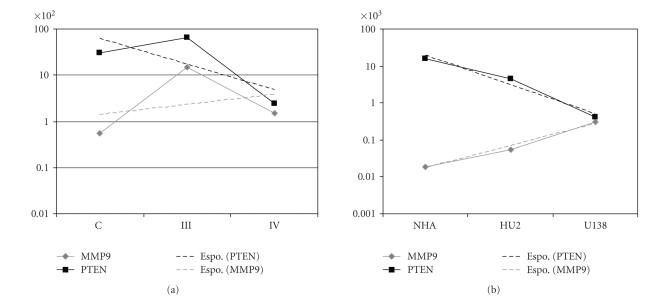
To further elucidated if PTEN and MMP9 expression had an expression trend that reflected the malignant grade of the specimens (i.e., normal, anaplastic astrocytomas, and glioblastoma multiforme), a statistical trend analysis was performed (Figure 6); the analysis reported in Panel A showed that PTEN and MMP9 differences in their normalized expression clearly decreased following the malignant glioma grading, with the lowest values corresponding to glioblastoma multiforme specimens; similarly, the trend analysis, reported in Figure 6 Panel B, highlighted that, within the investigated cell lines, normal human astrocytes exhibited the largest differences in PTEN and MMP9 expression levels, while, on the contrary, U138-MG cells, isolated from a glioblastoma multiforme patient, showed nearly coincident PTEN and MMP9 expression values; the glioblastoma derived PRT-HU2 cell line showed values of PTEN and MMP9 expression trends, intermediate between NHA and U138-MG.
Figure 6.
Statistical trends of PTEN (black dotted line) and MMP9 (grey dotted line) expression in control (C), anaplastic astrocytomas (III) and glioblastoma multiforme (IV) specimens (Panel A), and in normal human astrocytes (NHA), glioma PRT-HU2 (HU2), and U138-MG (U138) cells (Panel B). Trends are calculated using average quantitative expression of PTEN and MMP9, and adopting exponential (Espo) models of prediction as follows: PTEN (C, III, IV average expression samples), y = 2148e−1.29x; MMP9 (C, III, IV), y = 83716e0.50x; PTEN (NHA, HU2, U138), y = 118596e−1.82x; MMP9 (NHA, HU2, U138), y = 42439e1.40x.
4. Discussion
The new challenge in cancer biology is to move from one purely morphological classification of cancer to one that is based on the integration of histological and molecular criteria [15]. Among several cancer specific investigated genes, the epidermal growth factor receptor (EGFR) plays a pivotal pathological role through the activation of downstream intracellular signalling pathways that can directly modulate cell proliferation, metastasis, and angiogenesis [8]. Glial tumors, in particular, due to processes of gene-amplification or mutation, showed altered EGFR-related functional pathways [3]. Within this context, in order to find clinically relevant correlations between gene expression and tumor malignant progression, a cohort of glioma specimens was analyzed for the expression of several genes, that is, ADAM-17, PTEN, MMP9, EGFR, and HB-EGF, indirectly involved in the EGFR-dependent signaling pathway, In fact, the concurrent measurement of the transcript levels of the different genes could potentially represent a useful tool to identify a dysregulation of receptor activation or of downstream signaling pathways or might also suggest functional links between these genes in pathological conditions [16].
Comparing a cohort of glioblastoma specimens and controls, only two genes, ADAM-17 and PTEN, had expression levels that significantly changed between the two WHO classes; on the contrary, considering the expression of each transcript separately, we did not elucidate any association between clinical status and EGFR, HB-EGF, and MMP9 expression profiles. In particular, the wide range of variation for the EGFR gene, found in glioblastoma specimens, was in partial agreement with previous studies carried out in glial tumor samples [8]. In general, nearly 50% of glioblastoma multiforme cases express amplified EGFR, and about 40% of them also express the constitutively activated mutated EGFRvIII isoform [11]. Since the primers we used in our experiments did not discriminate between EGFR and EGFRvIII, it is very likely that the EGFR mRNA levels found in our samples reflected the combined contribution of both the transcripts.
Since chemotherapic treatments enhance the EGFR-mediated proliferative responses via an increased HB-EGF expression and shedding [17], previous studies have suggested a prominent role for HB-EGF in tumorigenic processes. In fact, in glioma cell lines, the inducible expression of EGFRvIII can enhance HB-EGF expression and can activate EGFR-dependent pathways via a positive feedback autocrine loop [18]. The only previous published study, based on a semiquantitative assessment by Northern blot analysis, found an increased expression of HB-EGF in glioblastoma compared to control samples [9]. On the opposite, our data seemed to suggest that HB-EGF is not upregulated in glioblastoma; however, our results agreed with microarray gene expression profiles studies that showed no significant differences in HB-EGF expression between glioblastoma and control samples [19].
Recent mounting evidence showed that the expression of MMP9 might play a critical role in brain neoplastic tissue invasion, metastasis, and angiogenesis [12]. Even if not completely statistically supported, our findings were in good agreement with a previous work showing an upregulation of MMP9 mRNA levels in glioblastoma compared to controls and suggesting a close relationship between MMP9 expression and tumor malignant progression [12].
Although ADAM17 expression has been reported in normal human brain tissue and in cell lines [5], its expression at mRNA level has been poorly investigated in brain tumors. Functional studies in U373-MG glioma cells have demonstrated that cannabinoids induced cell proliferation through a two-step mechanism involving ADAM17-mediated shedding of proHB-EGF and subsequent EGFR stimulation [5]. Our finding that ADAM17 mRNA levels in glioblastoma are statistically lower compared to controls is in contrast with a previous work that reported an increased expression of ADAM17 in glioblastoma specimens [20]. This might reflect differences in tumor sampling or a consequence of glioblastoma multiforme cellular and molecular heterogeneity.
Our finding showing that glioblastoma expressed statistically significant lower PTEN mRNA levels compared to control samples confirmed previous reports [21], showing that PTEN expression variations were detectable only in a low fraction of anaplastic astrocytoma and were almost absent in low-grade brain tumors and controls. Taken together, these observations strengthen the hypothesis that an impairment of PTEN expression, together with a consequent aberrant activity of the PI3K-dependent pathway, might represent a typical hallmark of glioblastoma multiforme. A functional confirmation of this hypothesis was that, in a mouse astrocytoma model with genetic inactivation of the Nf1 and p53 tumor suppressor genes, the loss of PTEN heterozygosity and the Akt activation contributed to the brain tumor malignant progression [6].
Differently from the above mentioned investigated genes, the analysis of PTEN and MMP9 expression, using a combination of unsupervised and supervised algorithms, provided interesting results: firstly, as reported in the dendrogram analysis, the expression profiling derived novel subsets of astrocytomas. This hierarchically clustering analysis clarified that tumor classification based even on a quantization of two genes could generate a patient stratification, clinically relevant and more informative than a single conventional histological classification. The PTEN and MMP9 expression-generated subgroups produced also a different distribution of the patients according to their age: in particular subgroup IV-a, differently from IV-b, was enriched in age-class III patients (i.e., >61 years); this result might suggest that in glioblastoma multiforme tumor specimens PTEN and MMP9 expression levels might be partly related with the elderly of the patients. On the contrary, in anaplastic astrocytoma and in control patients no association between age-related classes and PTEN/MMP9 expression levels was evidenced. Furthermore, the originated dendrogram does not reflect a classification of samples according to their anatomical tumor localization. However, in the light of the development of new pharmacological treatments, the identification of patient subsets with specific molecular signatures within tumor malignancy grades is becoming more and more relevant [22]. A finer analysis of PTEN and MMP9 expression was also employed to derive a parsimonious classification tree of the investigated samples into their tumor malignancy grades. Specific PTEN and MMP9 expression values significantly addressed the specimens into a specific diagnostic class, that is, glioblastoma or anaplastic astrocytomas. However, the significance and the sensitivity of this classification might be further refined with the increasing of specimens and through the identification of novel tumor-diagnostic markers. Basing on the average expression of PTEN and MMP9, the observed statistical trends clearly differentiate the control, anaplastic astrocytomas, and glioblastoma multiforme diagnostic classes; a similar expression trend for PTEN and MMP9 genes was documented comparing normal versus astrocytic tumor cell lines. These results, in particular, reinforced the concept that anaplastic astrocytomas were intermediate-grade tumors, showing detectable mitotic activity, absent in low-grade astrocytomas, but not necrosis and prominent vascular proliferation, characteristic of glioblastoma multiforme [23, 24].
An additional interesting finding emerging from our study was the significant negative correlation between PTEN and MMP9 mRNA expression in glioblastoma multiforme. Notably, not only was this negative correlation absent in anaplastic astrocytoma samples, but it was reversed in control samples. It was evident that differences in PTEN gene expression mainly account for these correlations because its levels in glioblastoma were significant higher compared to anaplastic astrocytoma samples, whilst no statistical difference was found for MMP9 mRNA levels. The positive correlation between PTEN and MMP9 in controls is derived essentially from an overexpression of PTEN rather than a low expression of MMP9 compared to glioblastoma. The functional significance of these correlations is currently unknown, and future functional studies aimed at elucidating possible interplays of these genes in glioblastoma are clearly warranted. The negative correlation in glioblastoma between MMP9 and PTEN could imply a functional interplays between these two genes, as already documented. It has been reported that PTEN modulates the expression and secretion of MMP2 and MMP9, thereby modifying tumor cell invasiveness [9, 25]. Notably, recent reports have clearly demonstrated that, in glioblastoma, PTEN may regulate migration via a PI3K-independent pathway [26]. In this model, the lack or functional loss of PTEN not only potentiates the migration induced by EGFR- and beta-integrin-dependent pathways but also enhances cell migration via a still largely unclear mechanism. On this regard, a recent report suggested that integrins could be a converging point in the mechanism supporting tumor invasion and migration of cancer cells with PTEN loss and MMP9 overexpression.
The intrinsic genetic heterogeneity and redundant overlapping aberrant signalling transduction pathways underlie the failure of monotherapies in glioblastoma [8]. Therefore a sensitive and reliable method to measure gene expression, such as Real-time PCR, may greatly ameliorate diagnostic tools and eventually address the pharmacological approach using multitarget kinase inhibitors or combination of therapies based on multiple single-targeted receptor or intracellular kinase inhibitors. Some researchers have proposed that the combination of PTEN loss and EGFR hyperfunctionality could be predictive of the ineffectiveness of therapies with EGFR inhibitors [4] because these two pathways might synergize to enhance glioblastoma malignancy. This hypothesis has been elegantly supported by the recent observation that the pharmacological inhibition of PI3K-alpha and mTOR augments the antiproliferative activity of the EGFR inhibitor erlotinib in glioblastoma cell lines [7]. The inverse correlation between PTEN and MMP9 expression reported here raised the issue whether the concomitant hyperactivation of the PI3K-alpha and MMP9-dependent pathways might be instrumental in devising or refining combined pharmacological therapies in glioblastoma. The modest efficacy of mTOR inhibitors alone in clinical trials was greatly enhanced when these compounds were administered in combination with the EGFR inhibitor Gefitinib [27]. Although monotherapy regimens with MMPs inhibitors in clinical trials have been quite disappointing, the relevance of MMPs as valid target has been reevaluated by the recent finding that the combined use of MMPs, COX2, and EGFR inhibitors reduced human breast cancer tumor growth [11]. We therefore speculate that the pivotal role of MMPs in glioma invasion and angiogenesis deserves future in vitro and in vivo experiments using MMP inhibitors in combination with PI3K inhibitors alone or with these latter compounds plus EGFR inhibitors.
The analyses of gene expression at transcriptional level in biopsy tissue samples are instrumental in delineating abnormal gene expression signature of brain tumors, but it should be mentioned that these studies suffer some pitfalls and limitations: in particular, the use of supervised approaches, based on the assumption that the grouping (i.e., the histological tumor diagnoses) is correct, may not be a valid assumption for all the clinical cases examined; additionally, the intrinsic heterogeneity of glioblastoma, together with the presence of nontumor cells in the samples, probably accounts for the variability found in transcripts levels and may represent a critical factor and a limitation in the interpretation of our results. From a technical point of view, a main difference of our contribution, compared to other reports, deals with the criteria adopted to express transcript levels in the investigated specimens. The majority of clinical gene expression profile studies performed by Real-time PCR normalized data using an internal housekeeping gene as a reference, but great caution in choosing this normalization method is necessary especially when analyzing tumor biopsy samples [28]. The tumorigenic process itself, via genomic mutations or amplifications, could induce modifications of housekeeping genes levels [29], and hence the choice of unreliable housekeeping genes may lead to interpretation errors and bias in experimental results [30]. Our attempts to use GAPDH, ACTB, and HPRT as reference genes were unsuccessful due to the great variations among all the samples (data not shown); therefore, we decided to express gene expression as absolute amount of femtograms (fg) of transcripts, normalized to the total amount of RNA employed, through accurate quantification using the combination of spectrofotometric and fluorimetric approaches.
In conclusion, the combined analysis of the transcripts of PTEN and MMP9 genes in biopsy specimens could represent a reliable diagnostic and prognostic marker of human glial tumor. Further epidemiological and functional in vitro studies are required to establish the reliability of PTEN and MMP9 genes as possible valid molecular targets in the pharmacological strategies aimed at controlling human glioma malignant progression.
Acknowledgments
This study was supported by grants from the Italian MIUR, “Progetti di Ricerca di Rilevante Interesse Nazionale (2005).” A. A. is granted by Fondo Sociale Europeo (F.S.E.). The authors are particularly grateful to Dr. Eugenio Benericetti (Azienda Ospedaliera di Parma, Italy) for providing WHO graded glioma specimens. Orange Software is released under General Programming License (GPL). The authors are therefore particularly grateful to Orange Program's authors, Demsar J, Zupan B, Leban G: (2004) Orange: From Experimental Machine Learning to Interactive Data Mining, White Paper (http://www.ailab.si/orange/), Faculty of Computer and Information Science, University of Ljubljana (Slovenia).
References
- 1.Louis DN. Molecular pathology of malignant gliomas. Annual Review of Pathology. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. American Journal of Pathology. 2007;170(5):1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashiyama S, Nanba D. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochimica et Biophysica Acta. 2005;1751(1):110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto S, Yagi H, Yotsumoto F, Kawarabayashi T, Mekada E. Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Science. 2006;97(5):341–347. doi: 10.1111/j.1349-7006.2006.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor α-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Research. 2004;64(6):1943–1950. doi: 10.1158/0008-5472.can-03-3720. [DOI] [PubMed] [Google Scholar]
- 6.Kwon C-H, Zhao D, Chen J, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Research. 2008;68(9):3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Q-W, Cheng CK, Nicolaides TP, et al. A dual phosphoinositide-3-kinase α/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Research. 2007;67(17):7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston JB, Navaratnam S, Pitz MW, et al. Targeting the EGFR pathway for cancer therapy. Current Medicinal Chemistry. 2006;13(29):3483–3492. doi: 10.2174/092986706779026174. [DOI] [PubMed] [Google Scholar]
- 9.Kim M-S, Park M-J, Moon E-J, Kim S-J, Lee C-H, Yoo H. Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to enhance the motility of human glioma cells. Cancer Research. 2005;65(3):686–691. [PubMed] [Google Scholar]
- 10.Hastie T, Tibshirani R, Botstein D, Brown P. Supervised harvesting of expression trees. Genome Biology. 2001;2(1, article research0003.1-0003.12) doi: 10.1186/gb-2001-2-1-research0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. The New England Journal of Medicine. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu K, Nakanishi Y, Nemoto N, Hori T, Sawada T, Kobayashi M. Expression and quantitative analysis of matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor Pathology. 2004;21(3):105–112. doi: 10.1007/BF02482184. [DOI] [PubMed] [Google Scholar]
- 13.Kleihues P, Cavenee WK. World Health Organization Classification of Tumors of the Nervous System. Lyon, France: 2000. [Google Scholar]
- 14.Paolillo M, Barbieri A, Zanassi P, Schinelli S. Expression of endothelins and their receptors in glioblastoma cell lines. Journal of Neuro-Oncology. 2006;79(1):1–7. doi: 10.1007/s11060-005-9111-z. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Holland EC, Cairncross JG. Glioma classification: a molecular reappraisal. American Journal of Pathology. 2001;159(3):779–786. doi: 10.1016/S0002-9440(10)61750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Y, Diehn M, Watson N, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(16):5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Liu R, Lee SW, Sloss CM, Couget J, Cusack JC. Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene. 2007;26(14):2006–2016. doi: 10.1038/sj.onc.1209999. [DOI] [PubMed] [Google Scholar]
- 18.Ramnarain DB, Park S, Lee DY, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Research. 2006;66(2):867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 19.Rickman DS, Bobek MP, Misek DE, et al. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Research. 2001;61(18):6885–6891. [PubMed] [Google Scholar]
- 20.Wildeboer D, Naus S, Sang Q-XA, Bartsch JW, Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. Journal of Neuropathology and Experimental Neurology. 2006;65(5):516–527. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- 21.Knobbe CB, Merlo A, Reifenberger G. Pten signalling in gliomas. Neuro-Oncology. 2002;4(3):196–211. [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller GN, Hess KR, Rhee CH, et al. Molecular classification of human diffuse gliomas by multidimensional scaling analysis of gene expression profiles parallels morphology-based classification, correlates with survival, and reveals clinically-relevant novel glioma subsets. Brain Pathology. 2002;12(1):108–116. doi: 10.1111/j.1750-3639.2002.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichimura K, Ohgaki H, Kleihues P, Collins VP. Molecular pathogenesis of astrocytic tumours. Journal of Neuro-Oncology. 2004;70(2):137–160. doi: 10.1007/s11060-004-2747-2. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed Rasheed BK, Wiltshire RN, Bigner SH, Bigner DD. Molecular pathogenesis of malignant gliomas. Current Opinion in Oncology. 1999;11(3):162–167. doi: 10.1097/00001622-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa K, Kumon Y, Harada H, et al. PTEN gene transfer suppresses the invasive potential of human malignant gliomas by regulating cell invasion-related molecules. International Journal of Oncology. 2006;29(1):73–81. [PubMed] [Google Scholar]
- 26.Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303(5661):1179–1181. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- 27.Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110(1):12–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 28.Tricarico C, Pinzani P, Bianchi S, et al. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Analytical Biochemistry. 2002;309(2):293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 29.Waxman S, Wurmbach E. De-regulation of common housekeeping genes in hepatocellular carcinoma. BMC Genomics. 2007;8:243–250. doi: 10.1186/1471-2164-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aerts JL, Gonzales MI, Topalian SL. Selection of appropriate control genes to assess expression of tumor antigens using real-time RT-PCR. BioTechniques. 2004;36(1):84–91. doi: 10.2144/04361ST04. [DOI] [PubMed] [Google Scholar]