Abstract
Rationale: Increased oxidative stress and decreased superoxide dismutase (SOD) activity in the asthmatic airway are correlated to airflow limitation and hyperreactivity. We hypothesized that asthmatic individuals with higher levels of oxidative stress may have greater loss of SOD activity, which would be reflected systemically in loss of circulating SOD activity and clinically by development of severe asthma and/or worsening airflow limitation. Methods: To investigate this, serum SOD activity and proteins, the glutathione peroxidase/glutathione antioxidant system, and oxidatively modified amino acids were measured in subjects with asthma and healthy control subjects. Results: SOD activity, but not Mn-SOD or Cu,Zn-SOD protein, was lower in asthmatic serum as compared with control, and activity loss was significantly related to airflow limitation. Further, serum SOD activity demonstrated an inverse correlation with circulating levels of 3-bromotyrosine, a posttranslational modification of proteins produced by the eosinophil peroxidase system of eosinophils. Exposure of purified Cu,Zn-SOD to physiologically relevant levels of eosinophil peroxidase-generated reactive brominating species, reactive nitrogen species, or tyrosyl radicals in vitro confirmed that eosinophil-derived oxidative pathways promote enzyme inactivation. Conclusion: These findings are consistent with greater oxidant stress in asthma leading to greater inactivation of SOD, which likely amplifies inflammation and progressive airflow obstruction.
Keywords: asthma, superoxide dismutase, glutathione, pulmonary functions, peroxidase
Asthma is a chronic inflammatory disorder of the airways involving a complex interaction of cells and mediators, most of which result in increased reactive oxygen and nitrogen species (ROS and RNS, respectively) in the airways (1–5). Current regimens for asthma therapy usually maintain normal to near-normal pulmonary function and prevent chronic symptoms, but in rare cases asthma is severe or refractory to antiinflammatory therapies, including corticosteroids (6). The reasons for variable severity of asthma are unclear, but evidence suggests that ROS/RNS excess and antioxidant deficiency in the lung are related to severity of airflow limitation and hyperreactivity (3, 7–11). Although in some experimental systems of lung inflammation, antioxidants increase in response to oxidant stress and minimize oxidant-induced damage (10, 12, 13), antioxidant defense is impaired in the asthmatic airway (7–9).
Antioxidant enzymes crucial for protection of the airway against oxidant stress include superoxide dismutases (EC 1.15.1.1, copper-zinc superoxide dismutase [Cu,Zn-SOD] in the cytosol; manganese SOD [Mn-SOD] in the mitochondria; and extracellular SOD [EC-SOD]) and glutathione peroxidases (GPx; EC 1.11.1.9) (10, 12–14). SODs convert superoxide to hydrogen peroxide, whereas glutathione peroxidase removes hydrogen peroxide and organic hydroperoxides in a reaction that consumes the tripeptide glutathione. Exquisitely sensitive to inactivation by ROS and RNS (15–17), SOD activity is reduced in the oxidant-rich environment of the asthmatic airway (11) and during asthma exacerbation further loss of SOD activity occurs with enhanced production of oxygen radicals by inflammatory cells (7, 18, 19). Moreover, SOD activity in the lung is related to airway hyperreactivity and airflow limitation (9, 11). Despite evidence that localized inactivation of SOD activity occurs within the inflamed asthmatic airways, the relationship of systemic levels of SOD activity to quantitative measures of asthma severity is unknown. We hypothesized that asthmatic individuals with higher levels of oxidative stress may have greater loss of SOD activity, which would be reflected systemically in loss of circulating SOD activity and clinically by development of severe asthma and/or worsening airflow limitation. To investigate this hypothesis, we undertook a study with cross-sectional samples obtained throughout the United States and England to assess systemic antioxidant enzyme activities for SOD and the GPx–glutathione system, and the relationship between antioxidants and asthma severity. Finally, potential mechanisms of SOD inactivation were examined in model systems, while in parallel, serum enzyme activity levels in subjects were related to circulating levels of molecular markers of distinct oxidative pathways known to be increased in asthma, such as those produced by eosinophil peroxidase–generated reactive brominating species, nitric oxide–derived oxidants, and tyrosyl radical (3, 4, 20).
Some of the results of these studies have been previously reported in the form of an abstract (21).
METHODS
See the online supplement for further detail on methods.
Study Population
To evaluate SOD in serum, the study population included 134 individuals composed of 20 healthy nonsmoking individuals and 115 individuals with asthma (75 with nonsevere asthma and 40 with severe asthma) contacted through the National Heart, Lung, and Blood Institute Severe Asthma Research Program. In this study, severe asthma was defined by the proceedings of the American Thoracic Society Workshop on Refractory Asthma (6), with major and minor characteristics (see the online supplement for definitions of major and minor characteristics and detailed methods). Separate from the subclasses of nonsevere and severe asthma, we also evaluated all individuals with asthma on the basis of severity of airflow limitation, as classified by %FEV1 and FEV1/FVC ratio. Spirometry was performed with an automated spirometer, consistent with American Thoracic Society standards with National Health and Nutrition Examination Survey (NHANES III) reference. Allergy skin testing was performed with the following: cat allergen, dog hair, Dermatophagoides pteronyssinus, Dermatophagoides farinae, cockroach, tree mix, ragweed mix, common weed mix, molds (including Alternaria, Aspergillus, and Cladosporium), normal saline as negative control, and histamine as positive control. Allergy or atopy was defined as two or more positive skin tests in the presence of positive histamine reaction. Methacholine challenge testing was performed only on volunteers with a baseline %FEV1 greater than 55%.
Antioxidants and Oxidative Modifications
SOD and GPx, total glutathione (reduced plus oxidized glutathione), and extracellular glutathione peroxidase protein (eGPx) were determined as previously described (22, 23). Cu,Zn-SOD protein and Mn-SOD protein were measured with ELISAs (Cu,Zn-SOD ELISA from Calbiochem/EMD Biosciences, San Diego, CA; Mn-SOD ELISA from SCETI, Tokyo, Japan). Protein-bound 3-nitrotyrosine, 3-bromotyrosine, and o,o′-dityrosine were determined by stable isotope dilution liquid chromatography–tandem mass spectrometry on a triple quadrupole mass spectrometer (Quattro Ultima; Micromass U.K. Limited, Manchester, UK) interfaced to an Aria LX Series HPLC multiplexing system (Cohesive Technologies, Franklin, MA) as previously described (24, 25). Cu,Zn-SOD (Calbiochem/EMD Biosciences) with specific activity of 3.78 U/μg protein was exposed to the eosinophil peroxidase (120 nM)–H2O2 (100 μM) system in the presence of either sodium bromide, nitrite, or tyrosine (100 μM) for 30 minutes at 37°C in potassium phosphate buffer (15 mM, pH 7.0) supplemented with 200 μM diethylenetriaminepentaacetic acid. Reactions were quenched by addition of methionine (100 μM) and snap freezing in liquid nitrogen.
Statistical Analysis
Quantitative data are summarized as means ± SE unless otherwise noted; categorical data are summarized by frequencies. Two-tailed t test statistics, and analysis of variance (ANOVA) were used when appropriate, with the Bonferroni correction being applied to the significance criterion once pairwise comparisons were made among the study groups. Associations between SOD activity and age, sex, and medication were assessed by linear models and ANOVA, and these factors were included as covariates in linear models for the group comparisons. All tests and model fitting were performed with the open source R statistical language, version 1.9.0 (http://www.r-project.org/).
RESULTS
Subject Characteristics
A total of 134 patients (Wake Forest University, 47; Cleveland Clinic Foundation, 31; University of Wisconsin, 16; Imperial College, London [UK], 12; University of Pittsburgh, 12; National Jewish Medical and Research Center, 8; and Emory University, 8) were enrolled in the study. Baseline characteristics are shown in Table 1. On average, healthy control subjects and subjects with nonsevere asthma were younger than subjects with severe asthma (p < 0.05; Table 1). Duration of asthma in patients with severe asthma was 23 ± 2 years, and in patients with nonsevere asthma it was 17 ± 1 years (p = 0.024). As expected, airflow measurements were lower in subjects with severe asthma than in nonsevere or healthy control subjects.
TABLE 1.
Demographics, pulmonary function, and corticosteroid use for all subjects
| Control Subjects | Subjects with Nonsevere Asthma |
Subjects with Severe Asthma |
|
|---|---|---|---|
| n | 20 | 74 | 40 |
| Mean age, yr | 34.1 (12.3) | 33.3 (11.2) | 40.2 (13.8)* |
| Absolute FEV1 | 3.8 (0.9) | 2.9 (0.9) | 2.0 (0.6)* |
| FEV1, % | 100.4 (13.3) | 92.4 (18.5) | 65.4 (21.9)* |
| Absolute FVC | 4.7 (1.1) | 3.9 (1.1) | 3.1 (0.9)* |
| FVC, % | 101.5 (10.8) | 84.7 (18.8) | 80.1 (21.3)* |
| FEV1/FVC | 0.82 (0.1) | 0.77 (0.4) | 0.67 (0.1)* |
| Sex, F/M | 10/10 | 47/27 | 25/15 |
| Race, A/AA/AB/C/H/MR | 2/1/0/16/0/0/1 | 3/20/0/47/2/2 | 3/6/2/29/0/0 |
| Sinusitis, % | 0/20 (0%) | 26/74 (35.1%) | 25/40 (62.5%)* |
| Long-acting β2-agonist | 0/20 (0) | 7/74 (9.5%) | 33/40 (82.5%) |
| Corticosteroids | |||
| Inhaled | 0/20 (0%) | 18/74 (24.3%) | 39/40 (97.5%)* |
| High-dose inhalation | 0/20 (0%) | 7/74 (9.5%) | 36/40 (90%)* |
| Oral | 0/20 (2.7%) | 3/74 (4.1%) | 20/40 (50%)* |
| Injected | 0/20 (0%) | 2/74 (2.7%) | 4/40 (10%)* |
| Atopy | 6/17 (46%) | 56/74 (75.6%) | 30/40 (75%)* |
| Smoking | 0/20 | 0/74 | 0/40 |
| Total cells, × 106 | 6.3 (1.9) | 6.6 (2.1) | 7.6 (3.3)* |
| Neutrophils, % | 55.7 (10.4) | 56.4 (11.2) | 58.8 (16.3) |
| Lymphocytes, % | 34.5 (8.2) | 31.9 (9.5) | 29.4 (11.5) |
| Eosinophils, % | 2.5 (1.3) | 3.9 (2.9) | 3.7 (3.0) |
| Basophils, % | 0.47 (0.39) | 0.5 (0.6) | 0.5 (0.5) |
| Monocytes, % | 7.5 (2.6) | 7.4 (2.4) | 6.1 (2.4)* |
| IgE level, IU/ml | 58 (24) | 198 (30) | 463 (258) |
Definition of abbreviations: A = Asian; AA = African American; AB = African British; C = white (Caucasian); F = female; H = Hispanic; M = male; MR = multiple race.
High-dose corticosteroids = fluticasone propionate exceeding 800 μg/day or equivalent. Data are presented as means (SD). Total cells and differentials are from whole blood.
p < 0.05.
Evaluation of Antioxidants
Analysis of antioxidants.
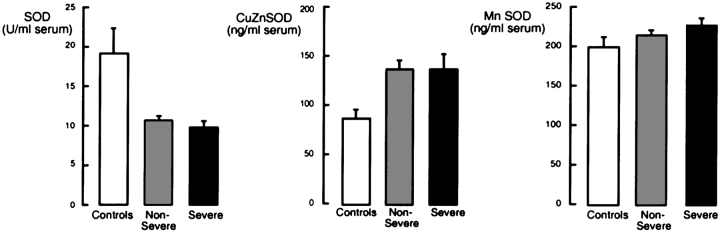
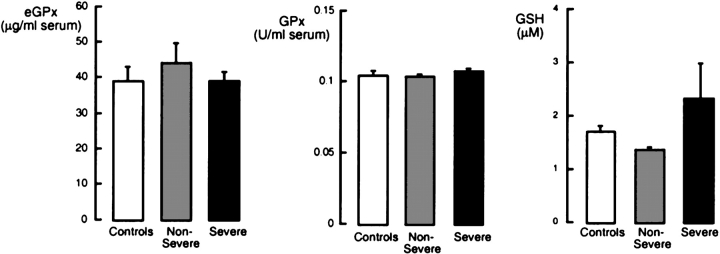
To investigate whether oxidative stress in asthmatic airways influences systemic antioxidants, GPx activity, eGPx, total glutathione, and total SOD activity were measured in serum. Total SOD activity was significantly different between the asthma and control groups (p = 0.001), whereas there was no significant difference in Mn-SOD protein levels among subjects with asthma and control subjects (Mn-SOD, ng/ml serum [mean ± SE]: control subjects, 198 ± 16; subjects with nonsevere asthma, 214 ± 8; subjects with severe asthma, 225 ± 12; ANOVA, p = 0.416; Figure 1). Cu,Zn-SOD protein levels tended to be higher when comparing all subjects with asthma with control subjects (Cu,Zn-SOD, ng/ml serum [mean ± SE]: all subjects with asthma, 136 ± 10; control subjects, 86 ± 10; p = 0.057) but were not significantly different among severe asthma, nonsevere asthma, and control groups (Cu,Zn-SOD, ng/ml serum [mean ± SE]: control subjects, 86 ± 10; subjects with nonsevere asthma, 135 ± 13; subjects with severe asthma, 137 ± 18; ANOVA, p = 0.165; Figure 1). Serum glutathione levels were similar among groups (glutathione [μM]: control, 1.69 ± 0.19; nonsevere asthma, 1.35 ± 0.09; severe asthma, 2.30 ± 0.73; p = 0.193; Figure 2). GPx activity and eGPx protein were also not significantly different among the three groups (p > 0.05; Figure 2). There was no relationship between loss of SOD activity or glutathione levels among former smokers and nonsmokers, and no effect of second-hand smoke was found.
Figure 1.
Cu,Zn-SOD (superoxide dismutase) protein, Mn-SOD protein, and total SOD activity in serum of control subjects (n = 20), subjects with nonsevere asthma (n = 74), and subjects with severe asthma (n = 40). Subjects with asthma have decreased SOD activity compared with control subjects (analysis of variance [ANOVA], p = 0.001), and SOD activity levels of subjects with severe and nonsevere asthma are similarly reduced. There is no significant difference in Mn-SOD (ANOVA, p = 0.416) or Cu,Zn-SOD levels among nonsevere, severe asthma, or control groups (ANOVA, p = 0.165), although subjects with asthma as a group tend to have more Cu,Zn-SOD than do control subjects (t test, p = 0.057).
Figure 2.
Evaluation of eGPx protein, GPx activity, and total glutathione (reduced glutathione [GSH] plus oxidized glutathione [GSSG]) in serum of control subjects (n = 20), individuals with nonsevere asthma (n = 74), and individuals with severe asthma (n = 40). eGPx protein, GPx activity, and total serum GSH are not significantly different among the groups (all p > 0.05).
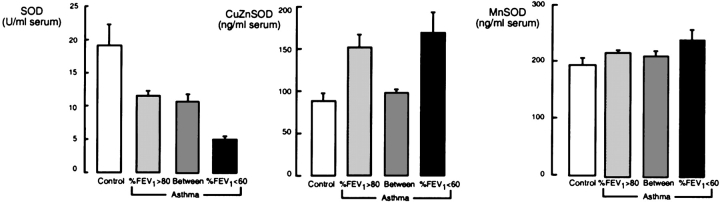
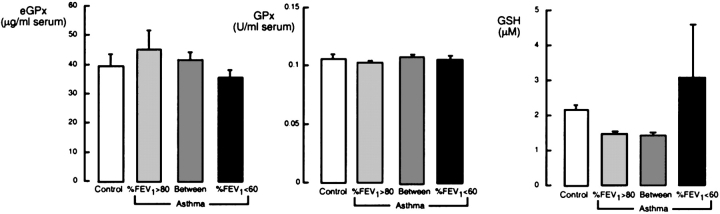
Analysis of antioxidants by severity of airflow limitation in asthma.
Antioxidants were also evaluated on the basis of %FEV1 measurements (%FEV1 > 80, %FEV1 between 60 and 80, and %FEV1 < 60; Figures 3 and 4). SOD activity was significantly related to airflow limitation (ANOVA, p = 0.005) but Mn-SOD protein was not (ANOVA, p = 0.453; Figure 3). Interestingly, airflow limitation was related to Cu,Zn-SOD protein at higher levels in subjects with asthma with %FEV1 > 80 or %FEV1 < 60 (ANOVA, p = 0.03; Figure 3). GPx activity and eGPx protein were similar among the groups (Figure 4). Glutathione tended to be higher in subjects with asthma with severe airflow limitation (%FEV1 < 60) than in control subjects or other subjects with asthma (ANOVA, p = 0.08; Figure 4).
Figure 3.
Analysis of SOD activity and proteins in individuals with asthma, based on airflow limitation. SOD activity is significantly lower in individuals with asthma with FEV1 lower than 60% of predicted (ANOVA, p = 0.005; %FEV1 < 60, n = 19; %FEV1 between 60 and 80, n = 36; %FEV1 > 80, n = 59). Mn-SOD protein is not different among the groups (ANOVA, p > 0.05), whereas Cu,Zn-SOD protein is significantly different among the groups, with higher levels in individuals with asthma with %FEV1 < 60 or > 80% (ANOVA, p = 0.03).
Figure 4.
Evaluation of serum eGPx protein, GPx activity, and total glutathione in individuals with asthma, based on airflow limitation. Levels of eGPx protein and GPx are similar among the groups (all p > 0.1), whereas GSH tends to be higher in subjects with asthma with severe airflow limitation (p = 0.08).
Age- and Sex-adjusted Group Effect within the Asthma Group
The mean age in the severe asthma group was higher than that of healthy control subjects. Therefore, difference among groups was tested with an ANOVA model that adjusted for age. SOD activity (p = 0.003) remained significantly different among the asthmatic and control groups when adjusted for age. Similarly, when using an ANOVA model that adjusted for sex, SOD activity remained significantly different (p = 0.004).
Multiple Linear Regression Analysis
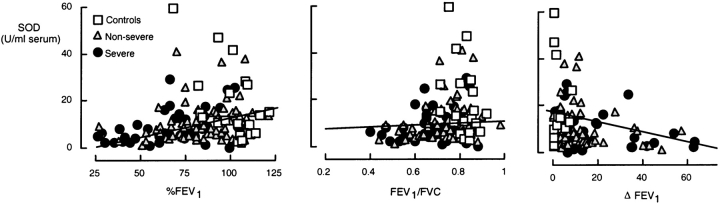
To investigate the quality of the relationship of antioxidants to airflow and hyperreactivity, regression analyses were performed, with %FEV1, FEV1/FVC, and change in FEV1 after bronchodilator administration (ΔFEV1; Figure 5). %FEV1 and ΔFEV1 were most strongly correlated to SOD activity (Table 2), whereas no correlations were found with other antioxidants (Table 3). These results suggest that serum SOD activity may serve as a global index of severity of asthma. As half of the subjects with severe asthma (19 of 40) could not undergo methacholine challenge testing because of an initial low %FEV1, SOD relation to the provocative concentration of bronchoconstrictor causing a 20% fall in FEV1 could not be evaluated.
Figure 5.
Correlations of serum SOD activity with airflow (%FEV1, FEV1/FVC, and ΔFEV1). SOD activity is directly correlated with %FEV1 (R = 0.312, p < 0.001) and FEV1/FVC (R = 0.296, p < 0.001), whereas SOD activity is inversely correlated with hyperresponsiveness, as determined by change in FEV1 after β-agonist administration (180 μg of albuterol; R = –0.334, p = 0.001).
TABLE 2.
Correlations of total superoxide dismutase activity with lung function
| All Groups | Control Subjects |
Subjects with Nonsevere Asthma |
Subjects with Severe Asthma |
|
|---|---|---|---|---|
| %FEV1* | R = 0.312 | R = −0.371 | R = 0.240 | R = 0.447 |
| p < 0.001 | p = 0.105 | p = 0.043 | p = 0.004 | |
| FEV1/FVC | R = 0.296 | R = 0.236 | R = 0.338 | R = 0.211 |
| p < 0.0001 | p = 0.407 | p = 0.004 | p = 0.191 | |
| ΔFEV1† | R = −0.334 | R = −0.245 | R = −0.243 | R = −0.449 |
| p < 0.001 | p = 0.292 | p = 0.040 | p = 0.004 |
%FEV1, prebronchodilator value, except five subjects with nonsevere asthma and two with severe asthma used β-agonist within 12 h of testing for clinical need.
ΔFEV1, percent change in FEV1 after two puffs of β-agonist inhaler (180 μg of albuterol).
TABLE 3.
Correlations of serum antioxidants with lung function in the three groups studied
| SOD | GSH + GSSG | GPx | eGPx | |
|---|---|---|---|---|
| Lung Function | (U/ml) | (μM) | (U/ml) | (ng/ml) |
| FEV1* | R = 0.295 | R = 0.07 | R = −0.01 | R = 0.157 |
| p < 0.001 | p = 0.94 | p = 0.94 | p = 0.07 | |
| %FEV1 | R = 0.312 | R = 0.113 | R = −0.057 | R = 0.124 |
| p = 0.001 | p = 0.19 | p = 0.51 | p = 0.15 | |
| %FVC | R = 0.113 | R = 0.146 | R = 0.19 | R = 0.122 |
| p = 0.2 | p = 0.09 | p = 0.022 | p = 0.16 | |
| FEV1/FVC | R = 0.296 | R = 0.163 | R = −0.048 | R = 0.01 |
| p < 0.001 | p = 0.06 | p = 0.58 | p = 0.9 | |
| ΔFEV1† | R = −0.334 | R = −0.05 | R = −0.04 | R = 0.01 |
| p < 0.001 | p = 0.58 | p = 0.63 | p = 0.94 |
Definition of abbreviations: eGPx = extracellular glutathione peroxidase; GPx = glutathione peroxidase; GSH = glutathione; GSH + GSSG = total glutathione; GSSG = oxidized glutathione; SOD = superoxide dismutase.
FEV1, prebronchodilator value, except five subjects with nonsevere asthma and two with severe asthma used β-agonist within 12 h of testing for clinical need.
ΔFEV1, percent change in FEV1 after two puffs of β-agonist inhaler (180 μg of albuterol).
Effect of Corticosteroids on SOD Response Adjusted for Age and Sex
Previously, corticosteroids were related to improvement of airflow and restoration of airway SOD activity in subjects with asthma (8). Overall, corticosteroids taken orally, inhaled, or injected did not have a clear influence on SOD activity in this study (p = 0.506). Difference among airflow limitation groups (%FEV1 < 60, %FEV1 between 60 and 80, and %FEV1 > 80) was also tested with an ANOVA model that adjusted for corticosteroid use. Despite the obvious relationship of corticosteroid use to the groups, SOD activity (p < 0.001) was still significantly different among the groups when adjusted for corticosteroid use. When evaluating corticosteroid use on the basis of method of administration (oral, inhaled, or injected), only the use of injected corticosteroids may influence systemic measures of SOD activity in subjects with asthma (Table 4).
TABLE 4.
Influence of corticosteroid use on superoxide dismutase activity
| Oral
|
Inhaled
|
Injected
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Never | Some/Daily | p Value | Never | Some/Daily | p Value | Never | Some/Daily | p Value | |
| SOD, U/ml | 10.2 ± 0.8 | 9 ± 2 | 0.259 | 10 ± 1 | 10.0 ± 0.9 | 0.734 | 9.7 ± 0.7 | 16 ± 5 | 0.10 |
Definition of abbreviation: SOD = superoxide dismutase.
Some/daily: use of corticosteroid at least once per week to twice per day; p values are Wald p values (two-tailed comparison).
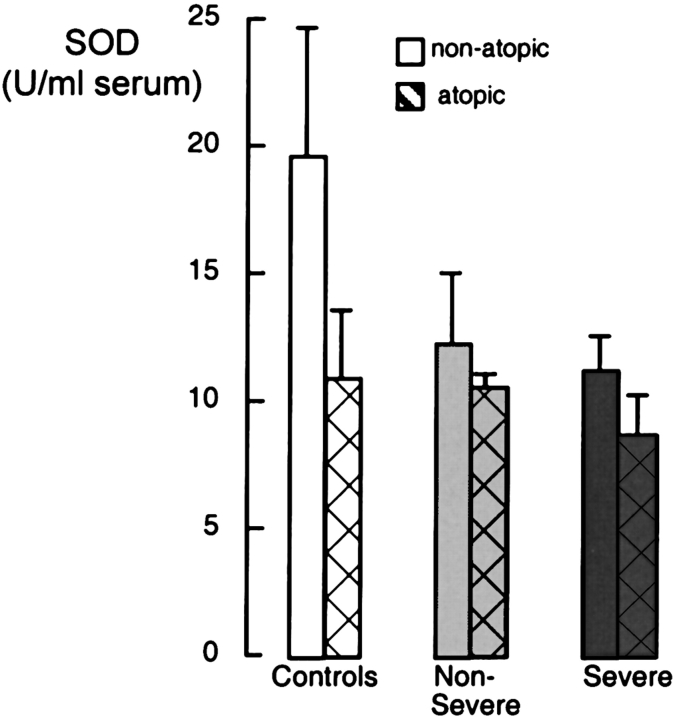
Loss of SOD Related to Atopy
Although atopy is implicated in the cause of asthma, the relationship between antioxidant status and atopy has not been investigated. When comparing all nonatopic versus all atopic subjects, atopic individuals (subjects with asthma and control subjects together) have lower SOD levels than do nonatopic subjects (p = 0.002). Although SOD in nonatopic subjects is noticeably higher than in atopic subjects in each group (Figure 6), the individual t tests do not reach significance for any group (all p > 0.1). Furthermore, adjustment for atopy does not alter the statistically significant difference in SOD between control subjects and subjects with asthma (p = 0.02). Thus, the differences between SOD activities in control subjects and subjects with asthma are not explained simply by a different balance of atopic and nonatopic individuals within the groups.
Figure 6.
Analysis of SOD activity corrected for atopy. Individuals with asthma and control individuals with allergies have lower levels of serum SOD activity, but no significant difference was found among nonatopic and atopic individuals within the same group (control subjects: nonatopic, n = 11; atopic, n = 6; subjects with nonsevere asthma: nonatopic, n = 18; atopic, n = 56; subjects with severe asthma: nonatopic, n = 10; atopic, n = 30; all p > 0.1).
Mechanism of SOD Inactivation
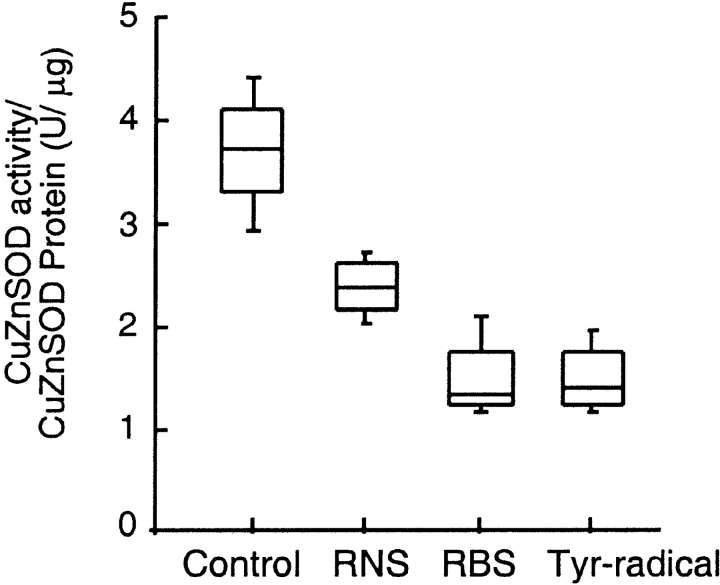
In the context of significant correlation of SOD activity to physiologic parameters of asthma and atopy, we investigated potential mechanisms of SOD inactivation in asthma. Previous in vitro studies indicate that SOD is exquisitely susceptible to oxidative modification and inactivation (15, 17, 26–28). Notably, eosinophil peroxidase-generated oxidants have been identified as specific participants in oxidative injury in both allergic and severe asthma, with 3-bromotyrosine, a specific protein modification generated by eosinophil peroxidase–catalyzed oxidation (3, 4, 29, 30). The Cu,Zn-SOD structure does not contain tyrosine residues, but oxidative modification of the enzyme may occur through effects on alternative susceptible target amino acids such as methionine, cysteine, histidine, tryptophan, arginine, and lysine. Cu,Zn-SOD was therefore exposed to physiologically relevant levels of eosinophil peroxidase–generated reactive brominating species, RNS, or tyrosyl radicals to assess the potential role of oxidative pathways that might contribute to enzyme inactivation. All reactive species lead to loss of specific SOD activity (Figure 7). The magnitude of effect by reactive brominating species supports a potential role for eosinophil peroxidase-catalyzed inactivation in vivo. To test this hypothesis, plasma samples from asthmatic patients were analyzed by mass spectrometry to quantify levels of protein-bound bromotyrosine, as a marker of eosinophil-derived reactive brominating species; dityrosine, an oxidative cross-link generated via a tyrosyl radical intermediate (24); and 3-nitrotyrosine, a stable protein modification generated by nitric oxide–derived oxidants (3). Systemic levels of bromotyrosine demonstrated statistically significant inverse correlations with serum SOD activity (R = –0.404; p = 0.049), consistent with loss of SOD activity as a consequence of reactive brominating species. Interestingly, no such relationship was observed with either nitrotyrosine or dityrosine, suggesting that neither nitric oxide–derived oxidants nor tyrosyl radical-mediated oxidative cross-links participate in SOD inactivation in vivo.
Figure 7.
Loss of specific Cu, Zn-SOD activity occurs after protein is exposed to eosinophil peroxidase–generated reactive nitrogen species (RNS), reactive brominating species (RBS), or tyrosyl radicals (·Tyr) in vitro (p = 0.001).
DISCUSSION
Previous reports show that localized decreases in SOD activity occur within asthmatic airway epithelial cells, bronchoalveolar lavage fluid, and bronchoalveolar lavage cells in proportion to airflow limitation and asthmatic exacerbations (7, 9, 11). Whether systemic reductions in activity of the antioxidant protective enzyme occur during asthma has not yet been examined. The present study provides evidence to support global inhibition of systemic SOD catalytic activity in asthma that is related to airflow limitation. The relationship of circulating SOD activity measures to plasma bromotyrosine levels, an oxidative modification characteristic of eosinophil peroxidase–generated brominating oxidants, is consistent with an oxidant mechanism of inactivation. The present study suggests that systemic measures of SOD inactivation may serve as a sensitive and quantitative functional measure of global oxidative stress in asthma.
In contrast to loss of SOD activity, Cu,Zn-SOD and Mn-SOD protein levels were not reduced in asthmatic serum. In fact, there may be an increase in Cu,Zn-SOD protein in subgroups of patients with asthma with mild to no airflow limitation, and/or in those with severe airflow obstruction. The intracellular enzymes Cu,Zn-SOD and Mn-SOD are released to the circulation during normal turnover of cells and account for serum SOD activity. Although EC-SOD is found predominantly in the extracellular matrix space, it is bound to heparan sulfate proteoglycans of endothelial cell surfaces and so less than 1% of EC-SOD is found in the serum (31, 32). Taken together, this supports the notion that the loss of serum SOD activity is not related to decrease in SOD proteins. We speculate that the lower systemic SOD activity in asthma may derive from airway inflammation and injury, cell turnover, and release of inactive SOD. However, earlier studies in adults have indicated that increased oxidative stress also occurs in the circulation of asthmatic subjects (33–36). For example, blood eosinophils and monocytes produce more ROS in patients with asthma as compared with control subjects (33, 34), and asthmatic leukocytes have increased levels of eosinophil peroxidase in peripheral blood (37). Loss of serum SOD activity in asthma may thus reflect a greater magnitude and/or ongoing systemic oxidative stress in subjects with asthma with severe airflow limitation, with a consequent greater oxidative modification of SOD systemically. Allergen-triggered inflammatory pathways may participate in loss of systemic SOD activity, perhaps through increasing oxidative stress (3, 4, 38), and subsequent inactivation of SOD. Activated peripheral blood monocytes of atopic individuals produce superoxide when IgE binds to membrane receptors (39) and serum eosinophil cationic protein, a biomarker of eosinophil activation, is increased with atopy and asthma severity indices (40). Whether or not SOD inactivation occurs locally in the lung or in the systemic circulation, the association of SOD activity with asthmatic obstructive lung disease is clearly present and may be unique, given that systemic antioxidant capacity in chronic obstructive pulmonary diseases is unrelated to airflow limitation (41–43).
ROS and RNS can react with many amino acid targets including methionine, tyrosine, histidine, tryptophan, lysine, and cysteine, profoundly altering the function of proteins by posttranslational oxidative modification. All SOD enzymes are sensitive to oxidative modification and inactivation (15–17, 44). In vitro studies have shown that ROS/RNS lead to oxidative and nitrative modification of tyrosine and inactivation of Mn-SOD and EC-SOD, whereas Cu,Zn-SOD can be inactivated by RNS through targeting of susceptible histidine residues (17, 27, 45). We have shown that oxidative modification/inactivation of Mn-SOD is present in asthmatic airway epithelial cells (11). Quantitative data on Mn-SOD oxidation/nitration in lungs of patients with mild asthma with near-normal lung function show that Mn-SOD tetramers possess at least one oxidative modification, which would lead to as much as 7% inactivation of Mn-SOD (11). Here, evidence consistent with SOD inactivation in the circulation due to oxidative/nitrative modifications is presented by the correlation of SOD activity with the levels of plasma bromotyrosine, an eosinophil-generated oxidative marker. Evidence consistent with a causative relationship between increased oxidants and SOD inactivation is supported by quantitative assay of Cu,Zn-SOD activity after exposures to eosinophil peroxidase–generated reactive species in vitro.
The glutathione system is a central mechanism for reducing organic and inorganic hydroperoxides. The key enzyme in the redox cycle responsible for the reduction of peroxides is GPx, which requires reduced glutathione to serve as the electron donor (46). The glutathione system is altered in lung inflammatory conditions, such as asthma. Many reports have shown alterations of glutathione in asthmatic airways (7–9, 47, 48). Furthermore, the total glutathione pool is increased in erythrocytes of individuals with asthma (49, 50). In the present study, individuals with asthma with severe airflow limitation have a tendency to increased serum glutathione. In vitro studies have shown that exposure to oxidative stress, such as superoxide or H2O2, causes a transient depletion of glutathione, followed later by prolonged elevation of intracellular glutathione levels (22, 51). Further studies are needed to evaluate the relationship of serum glutathione to airway inflammation and oxidative stress. The present data show that serum eGPx protein and GPx activity are not upregulated in individuals with asthma when compared with control subjects, although prior studies demonstrate that eGPx is upregulated in asthmatic airway epithelial cells and in epithelial lining fluid (22). The localized increase in lung eGPx, but absence of increase in serum levels, supports the concept that alterations in serum SOD levels may reflect systemic effects of oxidants.
It has been suggested that corticosteroids have a beneficial effect on antioxidants (8). Previous reports have shown that treatment with corticosteroid reduces oxidative stress and restores intracellular SOD activity levels in mild asthma (8, 52). In this study, inhaled or oral corticosteroids were not correlated with serum SOD activity measures in subjects with asthma, but parenteral corticosteroid revealed a tendency for improved SOD activity measures in patients with asthma. Lack of improvement with corticosteroids may suggest either relative corticosteroid resistance or perhaps noncompliance with therapy. Further studies are necessary to evaluate whether high-dose systemic corticosteroids improve antioxidant capacity in asthma.
There has also been considerable interest in the association between dietary intake of antioxidants, systemic oxidative stress, and lung function in individuals with asthma. It has been speculated that a diet rich in antioxidants may help to prevent asthma in individuals who have a susceptibility to asthma (53). Several studies have shown alterations in systemic nonenzymatic antioxidants in asthma as compared with healthy control subjects (50, 53, 54). Rubin and coworkers (53) showed that selenium and both vitamin C and β-carotene are inversely associated with asthma prevalence, suggesting a potential role of serum antioxidants in either the prevention of asthma onset and/or the progression of asthma. Vitamin E levels also show a strong correlation with pulmonary functions (54). Furthermore, selenium and protein containing selenocysteines, such as GPx, are profoundly influenced by dietary supply, which differs considerably between various regions of the world. For example, the population of New Zealand has a low intake of dietary selenium related to soil content, and a marked increase in prevalence and mortality due to asthma, with a sixfold increased risk of asthma for individuals with low GPx blood activity (55). Furthermore, animal models of asthma provide evidence of a link between antioxidants and airway hyperresponsiveness. For example, transgenic mice that overexpress SOD have decreased allergen-induced physiologic changes in the airway in comparison with wild-type control animals (56). In other studies, treatment with SOD mimics reduces the magnitude of allergen-induced airway hyperresponsiveness in sensitized guinea pig or mouse models of asthma (57, 58). Further studies are needed to determine the relationship of systemic SOD activity, oxidative modification of proteins, and/or glutathione levels to lower airway inflammation. However, systemic measures of antioxidants and oxidatively modified proteins may serve as easily quantifiable circulating biomarkers to assess overall magnitude of oxidative stress, which the present studies reveal are related to severity of airflow limitation in asthma.
Supplementary Material
Acknowledgments
The authors thank A. Janocha with assistance with SOD EIA, D. Schmitt for help with mass spectrometry studies, and J. Hammel for assistance with statistical analyses.
Supported by HL69170, AI70649, HL04265, HL61878, HL 07649, HL69130, HL69116, HL69174, HL69155, HL69167, P01/U01HL67663, U10HL74225, HL69349, and M01 RR018390 from the National Center for Research Resources.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. [DOI] [PubMed] [Google Scholar]
- 2.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 2001;98:2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol 2001;166:5763–5772. [DOI] [PubMed] [Google Scholar]
- 4.Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest 2000;105:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haahtela T. Airway remodelling takes place in asthma: what are the clinical implications? Clin Exp Allergy 1997;27:351–353. [PubMed] [Google Scholar]
- 6.American Thoracic Society. Proceedings of the ATS Workshop on Refractory Asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351. [DOI] [PubMed] [Google Scholar]
- 7.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 2000;355:624. [DOI] [PubMed] [Google Scholar]
- 8.De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, Secic M, Thomassen MJ, Erzurum SC. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol 1997;272:L148–L154. [DOI] [PubMed] [Google Scholar]
- 9.Smith LJ, Shamsuddin M, Sporn PH, Denenberg M, Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med 1997;22:1301–1307. [DOI] [PubMed] [Google Scholar]
- 10.Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, Mossman BT. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem 1991;266:24398–24403. [PubMed] [Google Scholar]
- 11.Comhair SA, Xu W, Ghosh S, Thunnissen FB, Almasan A, Calhoun WJ, Janocha AJ, Zheng L, Hazen SL, Erzurum SC. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol 2005;166:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erzurum SC, Danel C, Gillissen A, Chu CS, Trapnell BC, Crystal RG. In vivo antioxidant gene expression in human airway epithelium of normal individuals exposed to 100% O2. J Appl Physiol 1993;75:1256–1262. [DOI] [PubMed] [Google Scholar]
- 13.Hass MA, Iqbal J, Clerch LB, Frank L, Massaro D. Rat lung Cu,Zn superoxide dismutase: isolation and sequence of a full-length cDNA and studies of enzyme induction. J Clin Invest 1989;83:1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 2003;167:1600–1619. [DOI] [PubMed] [Google Scholar]
- 15.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem 1990;265:11919–11927. [PubMed] [Google Scholar]
- 16.Sharonov BP, Churilova IV. Inactivation and oxidative modification of Cu,Zn superoxide dismutase by stimulated neutrophils: the appearance of new catalytically active structures. Biochem Biophys Res Commun 1992;189:1129–1135. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med 2004;37:813–822. [DOI] [PubMed] [Google Scholar]
- 18.Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med 1994;123:131–136. [PubMed] [Google Scholar]
- 19.Jarjour NN, Busse WW, Calhoun WJ. Enhanced production of oxygen radicals in nocturnal asthma. Am Rev Respir Dis 1992;146:905–911. [DOI] [PubMed] [Google Scholar]
- 20.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med 2003;35:213–225. [DOI] [PubMed] [Google Scholar]
- 21.Comhair SAA, Ricci KS, Hazen SL, Erzurum SC. Systemic loss of superoxide dismutase activity in asthma is related to asthma severity [abstract]. Proc Am Thorac Soc 2005;2:A240. [Google Scholar]
- 22.Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J 2001;15:70–78. [DOI] [PubMed] [Google Scholar]
- 23.Nebot C, Moutet M, Huet P, Xu JZ, Yadan JC, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem 1993;214:442–451. [DOI] [PubMed] [Google Scholar]
- 24.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 2002;277:17415–17427. [DOI] [PubMed] [Google Scholar]
- 25.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002;296:2391–2394. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol 2003;285:H1396–H1403. [DOI] [PubMed] [Google Scholar]
- 27.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys 1999;366:82–88. [DOI] [PubMed] [Google Scholar]
- 28.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med 2001;31:1603–1608. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Chen Y, Hazen SL. Eosinophil peroxidase nitrates protein tyrosyl residues: implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem 1999;274:25933–25944. [DOI] [PubMed] [Google Scholar]
- 30.Wu W, Chen Y, d'Avignon A, Hazen SL. 3-Bromotyrosine and 3,5-dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: potential markers for eosinophil-dependent tissue injury in vivo. Biochemistry 1999;38:3538–3548. [DOI] [PubMed] [Google Scholar]
- 31.Sandstrom J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem 1994;269:19163–19166. [PubMed] [Google Scholar]
- 32.Karlsson K, Marklund SL. Heparin-, dextran sulfate- and protamine-induced release of extracellular-superoxide dismutase to plasma in pigs. Biochim Biophys Acta 1988;967:110–114. [DOI] [PubMed] [Google Scholar]
- 33.Vachier I, Damon M, Le Doucen C, de Paulet AC, Chanez P, Michel FB, Godard P. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis 1992;146:1161–1166. [DOI] [PubMed] [Google Scholar]
- 34.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis 1992;145:317–325. [DOI] [PubMed] [Google Scholar]
- 35.Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Garg ML. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids 2000;35:967–974. [DOI] [PubMed] [Google Scholar]
- 36.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med 1999;160:216–220. [DOI] [PubMed] [Google Scholar]
- 37.Sanz ML, Parra A, Prieto I, Dieguez I, Oehling AK. Serum eosinophil peroxidase (EPO) levels in asthmatic patients. Allergy 1997;52:417–422. [DOI] [PubMed] [Google Scholar]
- 38.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol 2002;110:349–356. [DOI] [PubMed] [Google Scholar]
- 39.Demoly P, Vachier I, Pene J, Michel FB, Godard P, Damon M. IgE produces monocyte superoxide anion release: correlation with CD23 expression. Comparison of patients with asthma, patients with rhinitis, and normal subjects. J Allergy Clin Immunol 1994;93:108–116. [DOI] [PubMed] [Google Scholar]
- 40.Joseph-Bowen J, de Klerk N, Holt PG, Sly PD. Relationship of asthma, atopy, and bronchial responsiveness to serum eosinophil cationic proteins in early childhood. J Allergy Clin Immunol 2004;114:1040–1045. [DOI] [PubMed] [Google Scholar]
- 41.Rahman I, Swarska E, Henry M, Stolk J, MacNee W. Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease? Thorax 2000;55:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrin-Nadif R, Auburtin G, Dusch M, Porcher JM, Mur JM. Blood antioxidant enzymes as markers of exposure or effect in coal miners. Occup Environ Med 1996;53:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comhair SA, Lewis MJ, Bhathena PR, Hammel JP, Erzurum SC. Increased glutathione and glutathione peroxidase in lungs of individuals with chronic beryllium disease. Am J Respir Crit Care Med 1999;159:1824–1829. [DOI] [PubMed] [Google Scholar]
- 44.Mamo LB, Suliman HB, Giles BL, Auten RL, Piantadosi CA, Nozik-Grayck E. Discordant extracellular superoxide dismutase expression and activity in neonatal hyperoxic lung. Am J Respir Crit Care Med 2004;170:313–318. [DOI] [PubMed] [Google Scholar]
- 45.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA 1996;93:11853–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol 2002;283:L246–L255. [DOI] [PubMed] [Google Scholar]
- 47.Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis 1993;147:1461–1464. [DOI] [PubMed] [Google Scholar]
- 48.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999;354:482–483. [DOI] [PubMed] [Google Scholar]
- 49.Mak JC, Leung HC, Ho SP, Law BK, Lam WK, Tsang KW, Ip MS, Chan-Yeung M. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol 2004;114:260–264. [DOI] [PubMed] [Google Scholar]
- 50.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 2003;111:72–78. [DOI] [PubMed] [Google Scholar]
- 51.Bracci R, Benedetti PA, Ciambellotti V. Hydrogen peroxide generation in the erythrocytes of newborn infants. Biol Neonate 1970;15:135–141. [DOI] [PubMed] [Google Scholar]
- 52.Majori M, Vachier I, Godard P, Farce M, Bousquet J, Chanez P. Superoxide anion production by monocytes of corticosteroid-treated asthmatic patients. Eur Respir J 1998;11:133–138. [DOI] [PubMed] [Google Scholar]
- 53.Rubin RN, Navon L, Cassano PA. Relationship of serum antioxidants to asthma prevalence in youth. Am J Respir Crit Care Med 2004;169:393–398. [DOI] [PubMed] [Google Scholar]
- 54.Schunemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med 2001;163:1246–1255. [DOI] [PubMed] [Google Scholar]
- 55.Flatt A, Pearce N, Thomson CD, Sears MR, Robinson MF, Beasley R. Reduced selenium in asthmatic subjects in New Zealand. Thorax 1990;45:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol 2000;279:L350–L359. [DOI] [PubMed] [Google Scholar]
- 57.Chang LY, Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med 2002;33:379–386. [DOI] [PubMed] [Google Scholar]
- 58.Masini EVA, Bani D, Mannaioni PF, Salvemini D. Prevention of antigen-induced early obstructive reaction by inhaled M40419 in actively sensitized guinea-pigs [abstract]. Am J Respir Crit Care Med 2001;163:A813. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.