Abstract
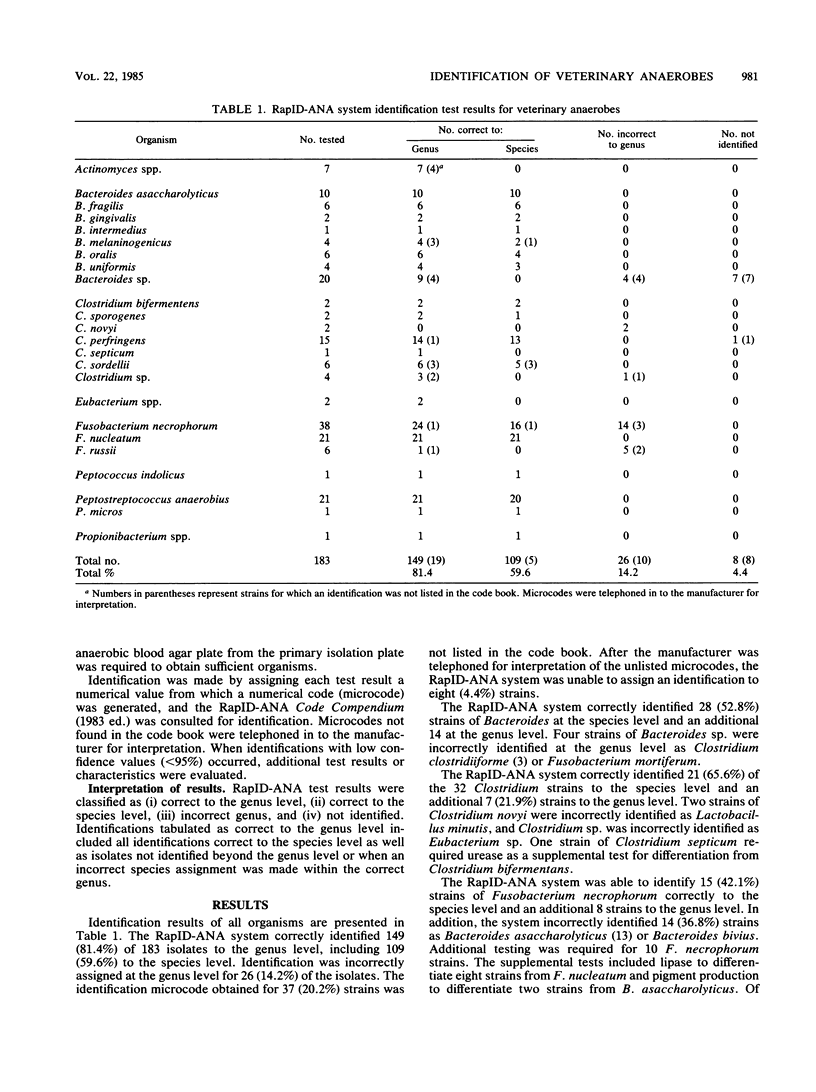
This study evaluated the ability of the RapID-ANA system (Innovative Diagnostic Systems, Inc., Atlanta, Ga.) to accurately identify a spectrum of freshly isolated veterinary anaerobes. A total of 183 isolates were tested and included 7 Actinomyces spp., 53 Bacteroides spp., 32 Clostridium spp., 2 Eubacterium spp., 65 Fusobacterium spp., 1 Peptococcus spp., 22 Peptostreptococcus spp., and 1 Propionibacterium spp. All isolates were initially identified by conventional biochemical testing and gas-liquid chromatography of short-chain fatty acid metabolites. Additional tests were performed as required by the RapID-ANA system. Of these isolates, 81.4% were correctly identified to the genus level, including 59.6% to the species level, 14.2% were incorrectly identified at the genus level, and 4.4% were not identified. Initially, 20.2% of the strains were not identified because the microcodes were not in the code book. The majority of the incorrect identifications were caused by the misidentification of Fusobacterium spp. as Bacteroides spp. Errors also occurred when veterinary anaerobes not included in the data base were assigned an identification from the existing data base. The RapID-ANA system appears to be a promising new method for rapid identification of veterinary anaerobes; however, further evaluation with an extended data base is needed before the system can accurately identify all clinically significant anaerobes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C., Kaufmann C. S., Depenbusch J. W. Accuracy and reproducibility of a four-hour method for anaerobe identification. J Clin Microbiol. 1985 Jun;21(6):894–898. doi: 10.1128/jcm.21.6.894-898.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. N., Fales W. H., Scanlan C. M. Occurrence of anaerobic bacteria in diseases of the dog and cat. Am J Vet Res. 1979 Jun;40(6):876–881. [PubMed] [Google Scholar]
- Berkhoff G. A., Redenbarger J. L. Isolation and identification of anaerobes in the veterinary diagnostic laboratory. Am J Vet Res. 1977 Jul;38(7):1069–1073. [PubMed] [Google Scholar]
- Hirsh D. C., Biberstein E. L., Jang S. S. Obligate anaerobes in clinical veterinary practice. J Clin Microbiol. 1979 Aug;10(2):188–191. doi: 10.1128/jcm.10.2.188-191.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D. C., Indiveri M. C., Jang S. S., Biberstein E. L. Changes in prevalence and susceptibility of obligate anaerobes in clinical veterinary practice. J Am Vet Med Assoc. 1985 May 15;186(10):1086–1089. [PubMed] [Google Scholar]
- Karachewski N. O., Busch E. L., Wells C. L. Comparison of PRAS II, RapID ANA, and API 20A systems for identification of anaerobic bacteria. J Clin Microbiol. 1985 Jan;21(1):122–126. doi: 10.1128/jcm.21.1.122-126.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F. Identification of some anaerobic bacteria in nonspecific anaerobic infections in animals. Can J Comp Med. 1979 Apr;43(2):194–199. [PMC free article] [PubMed] [Google Scholar]


