Abstract
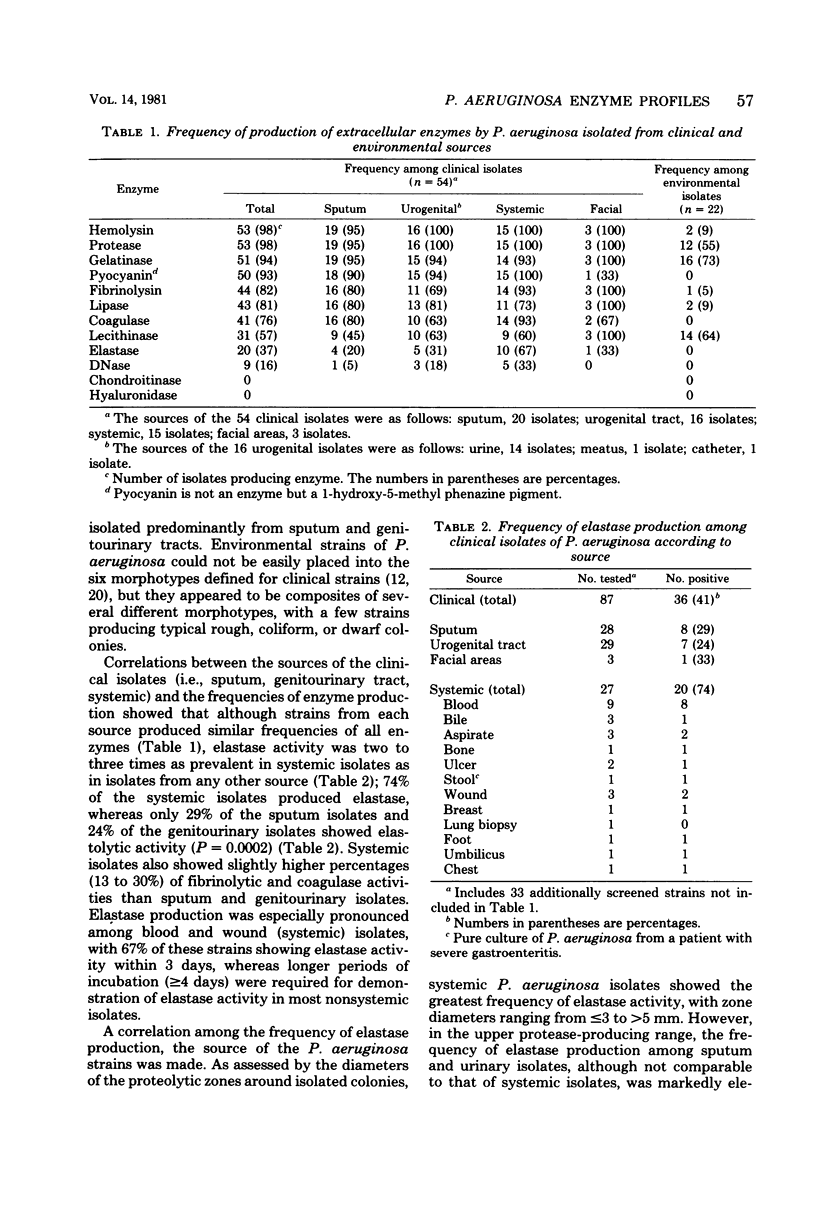
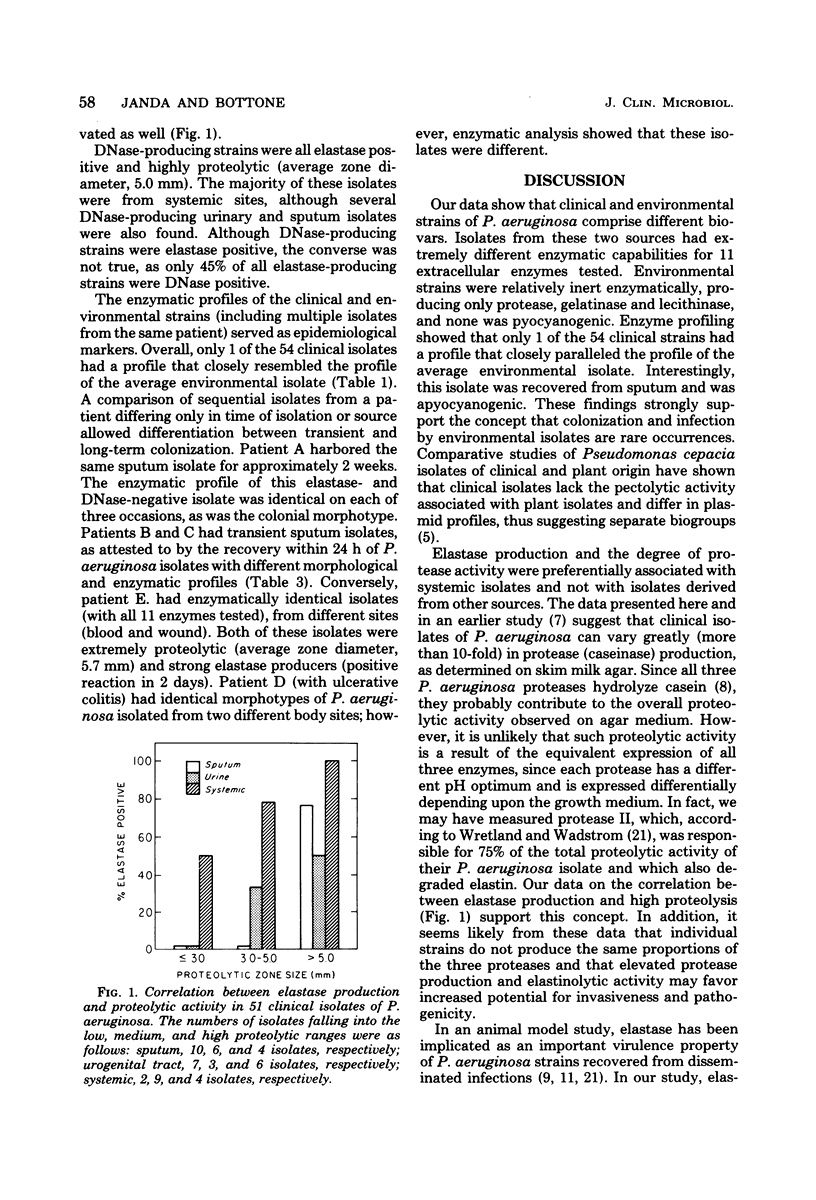
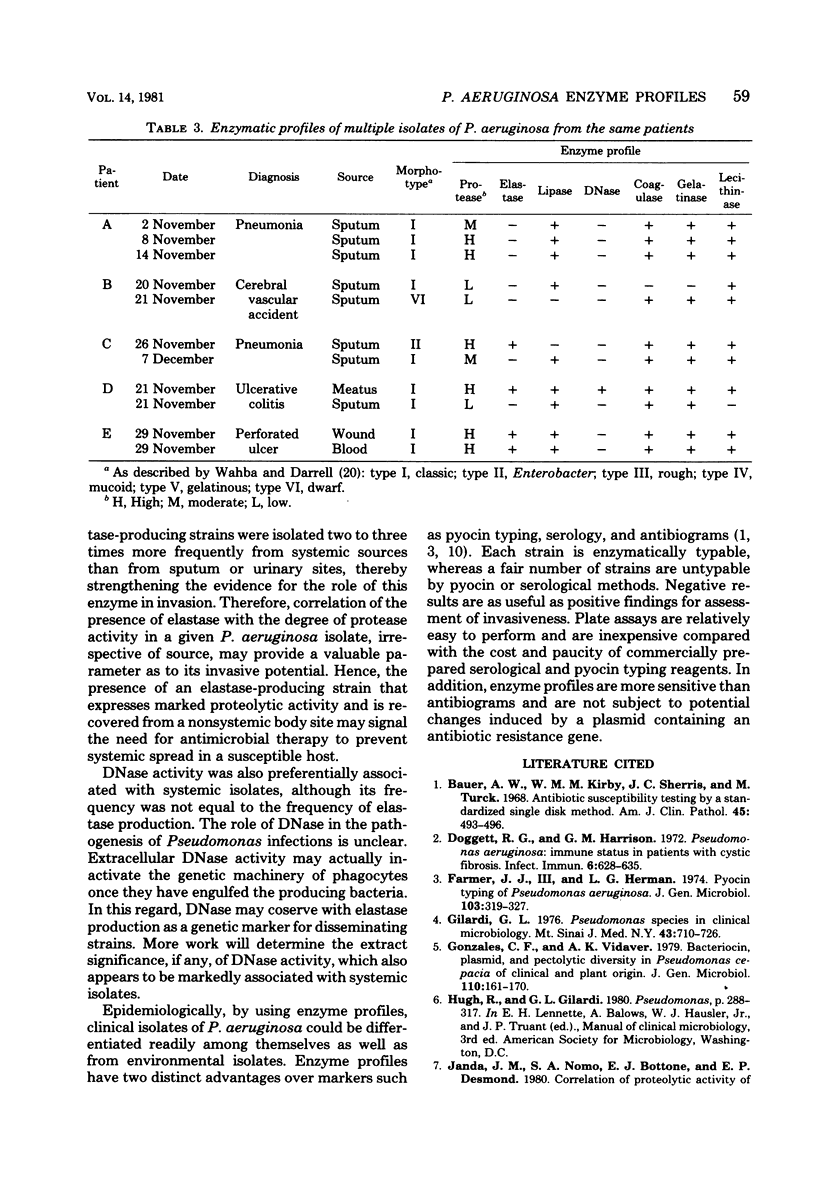
The enzymatic potential of 54 clinical and 22 environmental isolates of Pseudomonas aeruginosa from soil and water were evaluated by substrate plate assays. Clinical isolates produced substantial levels of 9 of the 11 enzymes assayed, whereas strains recovered from soil or water were relatively inert enzymatically. Elastase, deoxyribonuclease, and elevated protease activities were associated preferentially with clinical isolates of systemic origin; these activities were found twice as frequently in clinical isolates as in strains derived from sputum or the urogenital tract. Our data suggest that these factors may play an important role in the dissemination of P. aeruginosa from local or superficial sites. A comparison of the enzyme profiles of the environmental and clinical isolates indicated that colonization or infection by environmental strains of P. aeruginosa is a rare event and that environmental and clinical strains comprise separate biovars. Epidemiologically, enzyme profiles permitted the fingerprinting and differentiation of clinical strains from various sources.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M. Pseudomonas aeruginosa: immune status in patients with cystic fibrosis. Infect Immun. 1972 Oct;6(4):628–635. doi: 10.1128/iai.6.4.628-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Pseudomonas species in clinical microbiology. Mt Sinai J Med. 1976 Nov-Dec;43(6):710–726. [PubMed] [Google Scholar]
- Gonzalez C. F., Vidaver A. K. Bacteriocin, plasmid and pectolytic diversity in Pseudomonas cepacia of clinical and plant origin. J Gen Microbiol. 1979 Jan;110(1):161–170. doi: 10.1099/00221287-110-1-161. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Atang-Nomo S., Bottone E. J., Desmond E. P. Correlation of proteolytic activity of Pseudomonas aeruginosa with site of isolation. J Clin Microbiol. 1980 Oct;12(4):626–628. doi: 10.1128/jcm.12.4.626-628.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Meinke G., Barum J., Rosenberg B., Berk R. In Vivo Studies with the Partially Purified Protease (Elastase) from Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):583–589. doi: 10.1128/iai.2.5.583-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. R., Young V. M., Kenton D. M., Vermeulen G. D. Pseudomonas aeruginosa in a center for cancer research. I. Distribution of intraspecies types from human and environmental sources. J Infect Dis. 1972 Feb;125(2):95–101. doi: 10.1093/infdis/125.2.95. [DOI] [PubMed] [Google Scholar]
- Mull J. D., Callahan W. S. The role of the elastase of Pseudomonas aeruginosa in experimental infection. Exp Mol Pathol. 1965 Dec;4(6):567–575. doi: 10.1016/0014-4800(65)90037-7. [DOI] [PubMed] [Google Scholar]
- Phillips I. Identification of Pseudomonas aeruginosa in the clinical laboratory. J Med Microbiol. 1969 Feb;2(1):9–16. doi: 10.1099/00222615-2-1-9. [DOI] [PubMed] [Google Scholar]
- Pollack M. Pseudomonas aeruginosa exotoxin A. N Engl J Med. 1980 Jun 12;302(24):1360–1362. doi: 10.1056/NEJM198006123022410. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Small P. M., Shands J. W., Jr, Fischlschweiger W., Small P. A., Jr Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect Immun. 1980 Feb;27(2):614–619. doi: 10.1128/iai.27.2.614-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., GILFILLAN R. F., BARDAWIL W. A. A plate assay for elastase. Nature. 1960 Oct 22;188:322–323. doi: 10.1038/188322b0. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L. Identification of Pseudomonas species isolated from hospital environment and human sources. Appl Microbiol. 1968 Oct;16(10):1532–1538. doi: 10.1128/am.16.10.1532-1538.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHBA A. H., DARRELL J. H. THE IDENTIFICATION OF ATYPICAL STRAINS OF PSEUDOMONAS AERUGINOSA. J Gen Microbiol. 1965 Mar;38:329–342. doi: 10.1099/00221287-38-3-329. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Wadström T. Purification and properties of a protease with elastase activity from Pseudomonas aeruginosa. J Gen Microbiol. 1977 Dec;103(2):319–327. doi: 10.1099/00221287-103-2-319. [DOI] [PubMed] [Google Scholar]


