Abstract
Despite the fact that we possess highly effective tools for the primary and secondary prevention of myocardial infarction and other complications of atherosclerosis, coronary heart disease remains the most common cause of death in our society. Arterial inflammation and endothelial dysfunction play central roles in the pathogenesis of atherosclerosis and adverse cardiovascular (CV) events. Therapeutic lifestyle changes in conjunction with an aggressive multidrug regimen targeted toward the normalization of the major CV risk factors will neutralize the atherogenic milieu, reduce vascular inflammation, and markedly decrease the risk of adverse CV events and need for revascularization procedures. Specific CV risk factors and optimal therapies for primary and secondary prevention are discussed.
ACC = American College of Cardiology; AHA = American Heart Association; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; BP = blood pressure; CHD = coronary heart disease; CKD = chronic kidney disease; CV = cardiovascular; DHA = docosahexaenoic acid; DM = diabetes mellitus; EPA = eicosapentaenoic acid; FDA = Food and Drug Administration; HbA1C = glycated hemoglobin; HDL-C = high-density lipoprotein cholesterol; HF = heart failure; hsCRP = high-sensitivity C-reactive protein; HTN = hypertension; LDL-C = low-density lipoprotein cholesterol; LVH = left ventricular hypertrophy; MetS = metabolic syndrome; MI = myocardial infarction; PCI = percutaneous coronary intervention; PSS = psychosocial stress; RR = relative risk; TZD = thiazolidinedione
A paradigm shift, the seeds of which were planted decades ago, is now in full bloom and is drastically altering the way in which we diagnose and treat stable coronary heart disease (CHD). The traditional and intuitively logical plumbing paradigm hypothesized that the likelihood of major adverse cardiovascular (CV) events, such as myocardial infarction (MI) and CV death, were directly related to the angiographic severity of the atherosclerotic stenoses. Under this paradigm, the definitive means of favorably altering the prognosis of patients with CHD was to eliminate the angiographically significant stenoses with revascularization procedures, namely coronary artery bypass graft surgery and percutaneous coronary intervention (PCI). Despite the inherent commonsense plausibility of this theory, the prospective trials have repeatedly shown that revascularization procedures improve neither the short- nor long-term prognosis of patients with stable CHD, except for those with high-grade left main stenosis or severe proximal 3-vessel disease.1
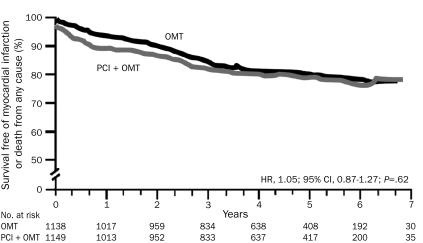
Among the culprit lesions that cause acute coronary syndromes, studies indicate that only about 15% have a luminal diameter stenosis greater than 70% immediately before the rupture and thrombosis that precipitate the acute arterial occlusion.1 When viewed in this light, it is not surprising that the revascularization of 1 or more high-grade lesions does not lower the risks of MI or death for a patient with stable CHD. A recent meta-analysis of all 17 randomized trials that compared PCI with medical therapy in patients with stable CHD showed that, although revascularization improves angina, it does not alter the incidence of MI or death compared with aggressive medical therapy alone1-3 (Figure 1). Impressively, in the COURAGE trial (for expansion of all trial names, see Glossary), optimal medical therapy improved anginal status during the course of the 5-year trial to such a degree that, by study end, 70% of patients were angina free, which was equivalent to the proportion free from angina in the PCI plus optimal medical therapy arm of the study.3 Similarly, the BARI 2D study found that aggressive medical therapy in patients with type 2 diabetes mellitus (DM) was as effective as elective coronary revascularization for reducing death and adverse CV events.4
FIGURE 1.
Patients randomly assigned to optimal medical therapy (OMT) did equally well during follow-up as those assigned to percutaneous coronary intervention (PCI) plus OMT with respect to the primary end point (nonfatal myocardial infarction or death from any cause). CI = confidence interval; HR = hazard ratio. From N Engl J Med,3 with permission. ©2007 Massachusetts Medical Society. All rights reserved.
In the new paradigm, arterial inflammation and endothelial dysfunction, which play central roles in determining the prognosis and angina status of patients with stable CHD, are best treated with an aggressive multidrug regimen coupled with therapeutic lifestyle changes in an effort to normalize the major CV risk factors (eg, dyslipidemia, hypertension [HTN], smoking, sedentary lifestyle, obesity, hyperglycemia). This strategy neutralizes the atherogenic milieu and reduces vascular inflammation, thereby markedly decreasing the risk of adverse CV events and the need for revascularization procedures.5
Atherosclerosis typically develops, progresses, and festers for decades in a clinically silent fashion; however, when it finally manifests itself, it often does so as a life-threatening catastrophe, such as sudden cardiac arrest, MI, or stroke, often in persons who previously would have been classified as at low or intermediate risk by the Framingham Risk score.6 Thus, the SHAPE national task force has endorsed the screening of asymptomatic middle-aged patients with either computed tomography for coronary artery calcification or ultrasonography for carotid artery plaque.7 Indeed, the coronary artery calcium score has proved to be superior to the Framingham Risk score for identifying persons at risk of CHD.8
Although CHD remains highly prevalent among westernized populations and continues to be the leading cause of death in the United States, the age-adjusted CV death rates have decreased almost 50% during the past 25 years.9 Recent studies indicate that improvements in CV risk factors, especially cholesterol, HTN, and cigarette smoking, have accounted for most of this dramatic decrease in risk of CV death.9 Although many of the major CV risk factors (eg, HTN, DM, dyslipidemia) are influenced by a person's genetic constitution, most studies have not found widespread genetic screening to be practical or clinically useful for identifying those at high risk of CHD.10 Indeed, the INTERHEART study suggests that the risk of MI is almost entirely attributable to modifiable CV risk factors.11 This large case-control study identified 9 modifiable risk factors, which in aggregate accounted for more than 90% of the variability of acute MI. These risk factors were dyslipidemia, smoking, HTN, psychosocial stress (PSS), DM, increased waist-hip ratio, physical inactivity, poor diet, and abstinence from alcohol. These CV risk factors often cluster together, creating a synergistic effect that multiplies risk of MI. Randomized trial data and clinical experience indicate that aggressive multimodal therapy targeting the modifiable CV risk factors in a reasonably adherent patient dramatically reduces and by some accounts almost eliminates the occurrence of adverse CV events.12 Nevertheless, recent statistics suggest that the United States, with its twin epidemics of obesity and DM (sometimes referred to as diabesity), may soon see a reversal in the dramatic progress made against CV disease in recent decades.13 Obesity also accounts for the increasing prevalence of younger patients presenting with acute MI.14
HYPERTENSION
The lifetime incidence of HTN has been increasing in recent decades and is now 90% in the United States.15 Recent reports indicate that 70% of patients with HTN are aware of their condition and 59% are receiving treatment; however, HTN is controlled in only 30% of patients. Most patients will require 2 or more HTN medications to achieve control,16,17 and systolic blood pressure (BP) is the most important parameter both for prognosis and as a target for treatment. Very large meta-analyses show a 50% increase in long-term CV mortality risk for every 20-mm Hg increase in systolic BP above 115 mm Hg.18 Lowering elevated BP will lower risk of major CV events, regardless of age, race, sex, or other factors.19,20 Aggressive HTN therapy targeted to achieve BP below 130/85 mm Hg is especially important in the presence of comorbid conditions, such as chronic kidney disease (CKD), heart failure (HF), and DM.20
Renin-Angiotensin-Aldosterone System
A large body of evidence supports the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) as first- or second-line agents in the treatment of HTN. These drugs have also been shown to reduce the risk of CV events. In the HOPE study,21 9297 patients with CV disease or DM were randomized to ramipril (10 mg/d) or placebo and followed up for 5 years. Use of ACEIs significantly reduced the rates of death from CV causes (relative risk [RR], 0.74; P<.001), MI (RR, 0.80; P<.001), stroke (RR, 0.68; P<.001), and all-cause mortality (RR, 0.84; P=.005); it also reduced the incidence of revascularization procedures, cardiac arrest, HF, and DM-related complications. Similarly, in the EUROPA study,22 which enrolled 12,000 patients with CHD but without HF, those randomized to perindopril (8 mg/d) experienced a 20% (P=.0003) RR reduction in CV death, MI, and cardiac arrest compared with those assigned to placebo.
ONTARGET23 showed that the ARB telmisartan (80 mg/d) was equivalent to ramipril (10 mg/d) for the reduction of CV events in patients with established CV disease or DM with target organ damage. In this very large randomized trial, the combination of an ACEI and an ARB did not further reduce CV events but increased adverse effects and serum creatinine levels compared with either agent alone. The LIFE study,24 which included 9193 patients with moderate-to-severe HTN and left ventricular hypertrophy (LVH), showed that 50 mg/d of losartan reduced the RR of death, MI, or stroke by 13% (P=.021) compared with 50 mg/d of atenolol, despite similar reductions in BP. In patients with HTN, ACEIs may be superior to diuretic agents for first-line therapy. The ANBP2 study,25 which randomized 6083 patients with HTN to therapy with ACEIs or thiazide diuretic agents, found that, over the course of the 4.1-year trial, ACEI therapy significantly reduced the RR of major CV events by 11% (P=.05) compared with thiazide diuretic therapy, despite similar reductions in BP; the specific drugs and doses for the ACEIs and thiazide diuretic agents were not specified in the protocol.
Calcium Channel Blockers
Randomized trial data consistently show that calcium channel blockers significantly improve CV prognosis in the setting of HTN; among the various agents in this class, amlodipine has by far the most randomized trial data supporting its effectiveness. The ASCOT-BPLA trial26 showed that 5 to 10 mg/d of amlodipine plus an ACEI (perindopril, 4-8 mg/d) was superior to 50 to 100 mg/d of atenolol plus 1.25 to 2.50 mg/d of bendroflumethiazide in reducing major adverse CV events, all-cause mortality, and new-onset DM. ALLHAT27 was a large HTN trial that compared amlodipine (2.5-10 mg/d), lisinopril (10-40 mg/d), and chlorthalidone (12.5-25 mg/d), finding them to be equivalent for reducing CV events. In the VALUE trial,28 there was no difference in fatal and nonfatal cardiac events at 4.2 years among patients randomized to 5 mg/d of amlodipine or to 80 mg/d of the ARB valsartan. However, the amlodipine-based regimen was associated with more rapid control of HTN and fewer CV events during the first year of the trial.
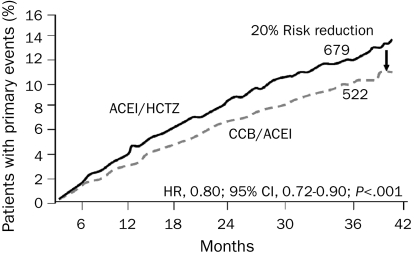
The cumulative randomized trial data indicate that an ideal strategy for improving prognosis in patients with HTN may be the use of an ACEI or an ARB in combination with a calcium channel blocker. In the ACCOMPLISH trial,29 11,506 patients with HTN who had CV disease, CKD, or other target organ damage were randomized to the ACEI benazepril (20-40 mg/d) plus hydrochlorothiazide (12.5-25.0 mg/d) or to benazepril (20-40 mg/d) plus amlodipine (5-10 mg/d). After 36 months, there was a 20% RR reduction in primary end point (death from CV causes, nonfatal MI, nonfatal stroke, hospitalization for angina, resuscitation after sudden cardiac arrest, and coronary revascularization) with benazepril plus amlodipine compared with benazepril plus hydrochlorothiazide (hazard ratio, 0.80; P<.001)30 (Figure 2). Calcium channel blockers, ACEIs, and ARBs are neutral to beneficial for glucose metabolism and tend to have fewer adverse effects compared with diuretic agents and traditional vasoconstricting β-adrenergic blockers, which are more frequently discontinued because of adverse effects and which worsen insulin resistance, thereby increasing the risk of type 2 DM.31
FIGURE 2.
Kaplan-Meier curves for time to first adverse cardiovascular event for patients treated with benazepril plus amlodipine (calcium channel blocker [CCB]/angiotensin-converting enzyme inhibitor [ACEI]) compared with benazepril plus hydrochlorothiazide (HCTZ). CI = confidence interval; HR = hazard ratio. From N Engl J Med,30 with permission. ©2008 Massachusetts Medical Society. All rights reserved.
Thiazide Diuretic Agents
Most major HTN clinical trials testing diuretic agents have used either hydrochlorothiazide or chlorthalidone.32 Although chlorthalidone was as effective as either amlodipine or lisinopril at reducing nonfatal CHD and MI in the ALLHAT study, it increased the risk of developing new-onset DM.16 Chlorthalidone has some advantages over hydrochlorothiazide, including a longer half-life (45-60 hours vs 8-15 hours) and more potent BP reductions. A small randomized head-to-head trial comparing these 2 agents showed chlorthalidone to be more effective in lowering BP.33 Additionally, the largest HTN trials have primarily used chlorthalidone27,34,35 as the diuretic agent, and these have generally reported significant CV event reduction.
Most patients without CKD respond to lower doses of thiazide diuretic agents, and the higher doses can worsen insulin resistance, lower levels of high-density lipoprotein cholesterol (HDL-C) and potassium, and increase triglyceride and uric acid levels without much additional HTN control.36 Indapamide has been shown to improve the prognosis of patients with HTN in multiple large randomized trials,29 and it is less likely than thiazide diuretic agents to increase glucose levels or lower potassium levels. Additionally, spirolactone and eplerenone are aldosterone receptor blockers that lower BP and improve prognosis for patients with CV disease without causing the adverse metabolic consequences noted with thiazides.37,38 These 2 agents are particularly helpful in the setting of refractory HTN and/or for regressing LVH.
β-Adrenergic Blockers
Recent studies have indicated that traditional β-adrenergic blockers may have substantial shortcomings when used for the treatment of HTN.39 Older β-adrenergic blockers such as atenolol and metoprolol are generally associated with peripheral vasoconstriction, reduced cardiac output, and worsening insulin resistance. Atenolol is still the most commonly prescribed β-adrenergic blocker for HTN and was the fourth most common prescription written in the United States in 2008. However, when used for treating HTN, atenolol is no better than placebo for improving prognosis in patients with CV disease, possibly because it increases risk of type 2 DM by 25% and does not lower central aortic pressure, which appears to be a better predictor of adverse CV outcomes than brachial BP.40 A systematic review of 9 randomized trials showed that, for patients with primary HTN, atenolol was associated with increased rates of stroke and both CV and all-cause mortality compared with other HTN drugs such as ACEIs, ARBs, and diuretic agents.41
Carvedilol, a potent nonselective β-adrenergic blocker, also induces α-1 blockade, stimulating arteriolar vasodilatation. Thus, unlike older β-adrenergic blockers, carvedilol does not reduce cardiac output. It has also been shown to be superior to the vasoconstricting β-adrenergic blockers with respect to its effects on cardiac output, proteinuria, insulin resistance, oxidative stress, glucose metabolism, and lipid levels.39 In addition, carvedilol (target dose, 25 mg twice daily), when compared with metoprolol (target dose, 50 mg twice daily), has been shown to reduce all-cause mortality by 17%, new-onset DM by 22%, and stroke death by 67% in COMET,42 a 5-year randomized trial involving more than 3000 patients with HF. Because African Americans and older patients respond poorly to vasoconstricting β-adrenergic blockers, vasodilating β-adrenergic blockers such as carvedilol or nebivolol are generally a better choice for these populations.43 Nebivolol is the most β-1-selective agent in this class and has vasodilating effects, potentially making it better tolerated than other β-adrenergic blockers.
A β-adrenergic blocker is a logical agent to use for many patients with HTN who also have CHD or HF and/or have findings to suggest excess sympathetic tone, such as those with a rapid resting heart rate or atrial or ventricular ectopy. Indeed, β-adrenergic blockers have been shown to favorably alter prognosis in patients with CHD, reduce risk of sudden death, improve left ventricular function, and slow atherosclerotic progression.44 However, when using β-blocker therapy for HTN, a vasodilating drug such as carvedilol or nebivolol is probably superior to a vasoconstricting agent such as metoprolol or atenolol.
For most patients with HTN, ACEIs or ARBs are logical choices as first-line antihypertensive therapy. A calcium channel blocker, particularly amlodipine, is also an excellent first- or second-line therapy. A diuretic agent or a vasodilating β-blocker such as carvedilol can be added if further BP lowering is required. However, the treatment of HTN must be customized to each patient on the basis of compelling indications, comorbid conditions, and interpatient variability in response to therapy and tolerability.
DYSLIPIDEMIA
In the INTERHEART study, the single most powerful CV risk factor was dyslipidemia; the ratio of apolipoprotein B to apolipoprotein A-1 (a more precise measure of the total cholesterol/HDL-C ratio) accounted for greater than 50% of the attributable risk of MI.11 The effectiveness of statin therapy for improving the prognosis of patients with CHD is supported by more long-term, high-quality, randomized controlled trial data than is the effectiveness of virtually any other CV treatment. The lipid hypothesis has been repeatedly confirmed, demonstrating conclusively that lowering low-density lipoprotein cholesterol (LDL-C) improves the prognosis of patients with CHD.45
Lowering LDL-C Levels Reduces CV Risk
A meta-analysis of more than 30 trials involving diet, drugs, or surgery to lower cholesterol levels has shown that for every 1 mg/dL reduction in LDL-C (to convert to mmol/L, multiply by 0.0259), total mortality is reduced by 1%.46,47 Statins are the drug class of choice for treating dyslipidemia and improving the prognosis of patients with CV disease. Statins lower LDL-C levels by 18% to 55%, reduce the number of small dense LDL-C particles, raise HDL-C levels by 4% to 9%, and lower triglyceride levels by 7% to 30%. In the HPS study, 20,536 patients with CHD or DM were randomized to 40 mg/d of simvastatin or placebo. After 5 years, simvastatin reduced the risk of CHD death by 18%, and the RR reduction was the same in patients with baseline LDL-C levels less than 100 mg/dL and in those with baseline LDL-C levels greater than 100 mg/dL.47
The normal LDL-C range is 40 to 70 mg/dL for native hunter-gatherers, healthy human neonates, free-living primates, and wild animals.48 Furthermore, large randomized trials have shown that intensive lowering of LDL-C levels to a range of 40 to 70 mg/dL improves the prognosis of patients with CV disease with minimal toxicity and no major safety concerns.49 The TNT trial50 randomly assigned 10,000 patients with clinically evident CHD and LDL-C levels of less than 130 mg/dL to 80 mg of atorvastatin or 10 mg of atorvastatin and followed them up for 4.9 years. The high-dose atorvastatin produced a 22% RR reduction (P<.001) in the primary end point of first major CV event. Furthermore, the risk of CV events and all-cause mortality decreased in direct proportion to the decrease in LDL-C levels achieved during treatment.51 The “lower is better” LDL-C strategy for improving the prognosis of patients with CHD was bolstered by several other randomized trials.47,48,52,53 To date, no lower threshold has been reached at which point further significant reductions in LDL-C levels have not produced lower rates of adverse CV events.
Inflammation in CHD
Inflammation is a key element in the pathogenesis of CHD. A plaque with extensive inflammation and a thin fibrous cap that renders it susceptible to ulceration, rupture, and thrombosis is typically the underlying cause of an acute coronary syndrome. Although aggressive long-term reduction of LDL-C levels can slow or halt progression, or even cause regression of atherosclerosis, this is a slow process that occurs over years. In contrast, the CV benefits of statins appear quickly, within days to a few weeks after initiation of therapy, possibly owing to their anti-inflammatory properties and ability to stabilize plaque and improve endothelial function. In this capacity, statins appear to work by resorbing oxidized lipid deposits in macrophages and the extracellular matrix, decreasing neointimal inflammation and enhancing fibrous cap integrity.36 In the MIRACL trial,54 80 mg of atorvastatin initiated 24 to 96 hours after an acute coronary syndrome reduced the primary end point (a composite of adverse CV events) by 16% during the 4-month placebo-controlled trial.
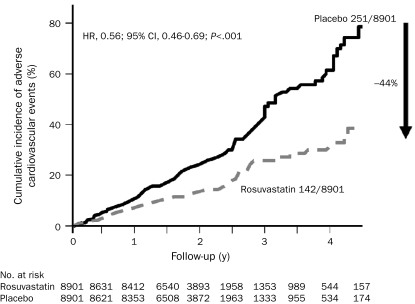
The level of high-sensitivity C-reactive protein (hsCRP), a nonspecific marker for systemic inflammation, is an important independent predictor of adverse CV events and progression of coronary atherosclerosis.55 The JUPITER trial56 was designed to test whether 20 mg/d of rosuvastatin could reduce the rate of the first major CV event in patients without atherosclerosis, high cholesterol, or DM, but who were at higher risk of CHD because of an elevated hsCRP level. Rosuvastatin significantly lowered LDL-C levels by 50% (from a baseline level of only 109 mg/dL) and hsCRP levels by 37%, thereby conferring substantial benefits, including a 44% reduction in the primary end point of major adverse CV events56 (Figure 3), a 47% reduction in hard CV events (MI, stroke, and CV death), a 43% reduction in venous thromboembolism,57 and a 20% decrease in all-cause mortality. The mean LDL-C level during treatment was 55 mg/dL in the JUPITER trial, the lowest achieved yet in a large outcome study. Other studies support the use of statins for high-risk primary prevention.56,58,59 This study also suggests that hsCRP levels should be monitored in patients similar to those of the JUPITER trial, such as those at intermediate or high risk of CHD without a preexisting indication for statin therapy.60 A recent article suggested that an additional 6.5 million US adults would qualify for statin therapy using the JUPITER eligibility criteria.61 Importantly, therapeutic lifestyle changes, particularly exercise training and weight loss, can also significantly lower levels of hsCRP.62-64
FIGURE 3.
Cumulative incidence of adverse cardiovascular events of rosuvastatin compared with placebo. CI = confidence interval; HR = hazard ratio. From N Engl J Med,56 with permission. ©2008 Massachusetts Medical Society. All rights reserved.
Atherosclerotic Progression
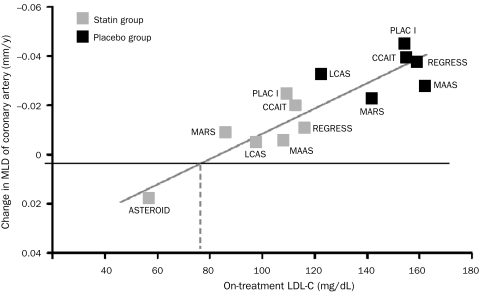
Treating dyslipidemia has also been shown to favorably affect the development and progression of atherosclerosis. Meta-regression analyses have shown that atherosclerotic progression slows in proportion to LDL-C reduction; at approximately 70 to 75 mg/dL, atherosclerotic growth is halted. Regression of coronary atherosclerosis is possible when the LDL-C level decreases to less than 70 mg/dL (Figure 4).65 In the ASTEROID study, 40 mg/d of rosuvastatin lowered LDL-C levels by more than 50% to 64 mg/dL, resulting in mean regression of coronary atherosclerotic stenoses as assessed by intravascular ultrasonography.66 In the METEOR67 investigation, 40 mg/d of rosuvastatin lowered LDL-C levels to 78 mg/dL and halted the growth of maximum carotid intima-medial thickness for 2 years, whereas that growth continued in patients receiving placebo. Similarly, the REVERSAL trial55 showed that intensive treatment with atorvastatin at 80 mg (to a goal LDL-C level during treatment of 79 mg/dL) halted the progression of coronary atherosclerosis, whereas 40 mg of pravastatin (to a goal LDL-C level during treatment of 110 mg/dL) did not.
FIGURE 4.
Highly significant association between atherosclerotic progression and on-treatment low-density lipoprotein cholesterol (LDL-C). ASTEROID = A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden; CCAIT = Canadian Coronary Atherosclerosis Intervention Study; LCAS = Lipoprotein and Coronary Atherosclerosis Study; MAAS = Multicentre Anti Atheroma Study; MARS = Monitored Atherosclerosis Regression Study; MLD = minimum lumen diameter; PLAC I = Pravastatin Limitation of Atherosclerosis in the Coronary Arteries; REGRESS = Regression Growth Evaluation Statin Study. SI conversion factor: To convert LDL-C values to mmol/L, multiply by 0.0259. From Circulation,65 with permission from Wolters Kluwer Health.
Ezetimibe is an agent that reduces the absorption of dietary cholesterol by acting on a receptor in the brush border of the small intestine. One small trial, the ENHANCE study,68 did not support the “lower is better” theory of LDL-C levels and caused a great deal of confusion among the lay and medical communities.69,70 Although the ENHANCE trial had limitations, it served as a reminder that no definitive outcome data are yet available to suggest that 10 mg/d of ezetimibe, either alone or when added to a statin, will reduce adverse CV events or atherosclerosis. Until confirmatory randomized trial data are available, ezetimibe should be added only after up-titration of statins has failed to achieve LDL-C goals.
The SEARCH71 study randomized 12,064 MI survivors to intensive high-dose (80 mg/d) or low-dose (20 mg/d) simvastatin. The high-dose simvastatin produced only a 14 mg/dL LDL-C reduction, which was not sufficient to decrease major adverse CV events but resulted in an increased risk of serious myopathy (53 cases vs 3 cases, respectively). These and other data indicate that the 80 mg dose of simvastatin is at the threshold of a toxic level and thus should be used with caution, especially in patients with multiple comorbid conditions who have complex medication regimens.72
Lipid Guidelines
In patients with established CHD or a CHD risk equivalent, the American College of Cardiology (ACC) and the American Heart Association (AHA) have promoted a target LDL-C level below 100 mg/dL with an optional goal of 70 mg/dL for high-risk patients as a class 1A indication.73 Most experts think that the LDL-C goal of less than 70 mg/dL is appropriate for patients at high risk of CV events, including those with evidence of atherosclerosis of the coronary arteries or other arterial beds, DM, or hsCRP levels greater than 2.0 mg/L (to convert to nmol/L, multiply by 9.524). Titration to maximal tolerable dose of a potent statin in an attempt to achieve the LDL-C goal should be the initial strategy. When and if this fails, a second agent can be added (eg, ezetimibe, niacin, bile acid sequestrant, fibrate). The bile acid sequestrant colesevelam, when added to a statin, will further lower LDL-C levels by 16% and hsCRP levels by 23%.74 This drug has also been approved by the Food and Drug Administration (FDA) for reducing hyperglycemia and is thus particularly useful for patients with CHD and/or dyslipidemia who also have metabolic syndrome (MetS) or type 2 DM.75
Triglyceride and HDL-C Levels
The Adult Treatment Panel III defines a fasting triglyceride goal of less than 150 mg/dL (to convert to mmol/L, multiply by 0.0113), but a level of less than 100 mg/dL is probably ideal.73 Triglyceride values of 150 to 199 mg/dL can be treated with lifestyle modifications, glycemic control, omega-3 fatty acids, and reduction in LDL-C levels. In patients with triglyceride values of 200 to 499 mg/dL, primary therapy is directed at decreasing LDL-C levels, with a secondary goal of normalizing triglyceride levels. Drug therapy with omega-3 fatty acids, niacin, fibrates, and statins can be used either alone or in combination to achieve triglyceride goals. When triglyceride values are greater than 400 to 500 mg/dL, the primary goal of lipid therapy is lowering of triglyceride levels to prevent acute pancreatitis.
Atherogenic dyslipidemia is characterized by elevated triglyceride levels (≥150 mg/dL), depressed HDL-C levels (<40 mg/dL; to convert to mmol/L, multiply by 0.0259), and elevated numbers of small dense LDL-C particles. These LDL-C particles are highly atherogenic and lead to rapid progression of CHD. Hypertriglyceridemia, especially after a meal, is an independent risk factor for CHD because of associated elevations in atherogenic particles, such as very-low-density lipoprotein, remnants, and small dense LDL-C particles. Effective nonpharmacologic strategies for increasing HDL-C levels include smoking cessation, weight loss, exercise, and diets high in monounsaturated fats, omega-3 fatty acids, and lean protein and with restricted intakes of high glycemic index carbohydrates.62,76
Agents such as omega-3 fatty acids, fibrates, niacin, or combination therapy (eg, a statin with an omega-3 fatty acid or niacin) are pharmacologic ways to improve HDL-C values. Niacin can shift small dense LDL-C particles toward larger, less atherogenic buoyant particles. Additionally, niacin has been noted to reduce major CV events in patients with DM.77 Niacin can worsen both insulin resistance and hyperglycemia in some patients with DM78; however, most large studies have not indicated that niacin significantly increases glycated hemoglobin (HbA1C) concentrations.78-80 Caution must be taken when combining a statin plus a fibrate (especially gemfibrozil) because of the potential for increased risk of muscle pain, myopathy, and rhabdomyolysis. However, a recently reformulated fenofibric acid has been approved by the FDA for use in combination with a statin.
DIABETES
Patients with DM are at markedly increased risk of CHD, cerebrovascular disease, and peripheral arterial disease and thus are considered to have a CHD risk equivalent. Cardiovascular disease continues to be the leading cause of mortality and morbidity among the DM population, accounting for 70% of all deaths.81 Persons with DM are at increased risk of silent myocardial ischemia and MI: up to one-third of patients with DM who experience an acute MI do not manifest chest pain. Compared with non-DM patients, 1-month mortality rates after acute MI are 50% higher, and by 5 years the cumulative mortality among patients with DM is twice the rate of that observed in non-DM post-MI patients.36 Optimization of the dismal CV prognosis associated with DM requires aggressive, longitudinal, multimodal CV risk factor treatment.
Glycemic Control
The UKPDS study82 has shown that regimens reducing average HbA1C levels to less than 7% resulted in sustained reductions in retinopathy, nephropathy, and neuropathy. More recent studies indicate that very aggressive regimens targeting HbA1C levels to less than 6.5% might increase risk of CV events, especially in persons with CHD who are experiencing episodes of severe hypoglycemia.83 Thus, oral therapies that do not cause hypoglycemia (eg, metformin, dipeptidyl peptidase-4 [DPP-4] inhibitors, glucosidase inhibitors, thiazolidinediones [TZDs]) are logical agents to use, particularly in patients with recently diagnosed DM. When concurrent use of 3 or 4 oral agents does not achieve adequate glycemic control, insulin therapy is usually required.36
Metformin is generally considered the first-line therapy for newly diagnosed type 2 DM and is also an excellent choice for use in combination therapy for DM.84 It is an inexpensive generic drug that is reasonably well tolerated and very safe. Metformin tends to produce a small decrease in weight because it has a mild anorexic effect. In the UKPDS study,82 753 obese patients with diabetes were randomized to metformin (2 g/d) or usual care (diet and exercise). Metformin was associated with RR reductions for DM-related complications of 32% (P=.002) and DM-related deaths of 42% (P=.017) at 10.7 years despite only a 0.6% mean reduction in HbA1C levels. Metformin also reduced the progression from impaired glucose tolerance to DM by 31% at 2.8 years in the Diabetes Prevention Program, with an even more significant benefit in younger patients.85
In the ADOPT trial, 4360 treatment-naive patients with type 2 DM were randomized to 4 years of treatment with 8 mg/d of rosiglitazone, 2 g/d of metformin, or 15 mg/d of glyburide. The cumulative incidence of monotherapy failure (fasting plasma glucose level >180 mg/dL [to convert to mmol/L, multiply by 0.055]) at 5 years was significantly less for rosiglitazone (15%) compared with either metformin (21%) or glyburide (34%), representing risk reductions of 32% and 63%, respectively (P<.001 for both comparisons).86 The TZDs (rosiglitazone and pioglitazone) have been shown to be useful in improving insulin resistance and preserving β-cell insulin secretory function. However, in predisposed persons TZDs can cause fluid retention and worsen HF.87
Postprandial Dysmetabolism
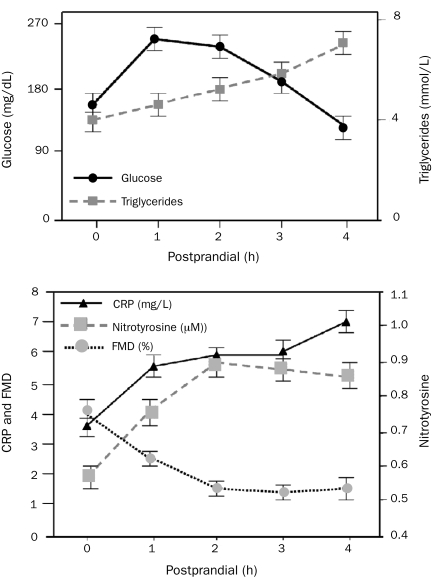
Traditional risk factors only account for a portion of the occurrence of CV events in persons with type 2 DM, MetS, or glucose intolerance. Postprandial levels of both glucose and triglycerides correlate better with future CV risk than do fasting levels, even in persons without DM.88 Exaggerated supraphysiological postprandial spikes in plasma levels of glucose and lipids stimulate immediate and proportional increases in reactive oxygen species (free radicals), which in turn induce oxidative stress, endothelial dysfunction, vasoconstriction, atherosclerosis, hypercoagulation, and sympathetic hyperactivity76,89,90 (Figure 5).
FIGURE 5.
The immediate deleterious effects of 700 kcal/m2 consumed as a beverage containing 75 g of glucose mixed with whipping cream on glucose, triglycerides, oxidant stress (nitrotyrosine), inflammation (C-reactive protein [CRP]), and arterial flow-mediated dilation (FMD). From J Am Coll Cardiol,76 with permission from Elsevier.
Glucosidase inhibitors have been shown to improve postprandial dysmetabolism and subsequently to reduce atherosclerosis and CV events.89 The STOP-NIDDM study randomly assigned 1429 participants to receive 100 mg of acarbose twice daily or placebo and followed them up for 3.3 years. Significant reductions were noted in both the development of DM (25%) and in CV events (49%).91 Other therapies that are effective in blunting postprandial glucose spikes include exercise, diet, DPP-4 inhibitors, incretin analogs, nateglinide, repaglinide, TZDs, and ultrafast-acting insulins. Such agents are beyond the scope of this review and have been discussed in detail elsewhere.76
Lipid Treatment in Patients With Type 2 DM
For patients with type 2 DM, optimizing the lipid profile is the single most important intervention for reducing the risk of CHD death, MI, stroke, and revascularization procedures. The Adult Treatment Panel III update recommends treatment to reduce LDL-C levels by at least 30% to 40% for patients with type 2 DM, regardless of the baseline LDL-C level.36 The ACC/AHA guidelines indicate that an LDL-C goal of less than 70 mg/dL is reasonable for patients with DM. Several studies indicate strong support for intensive LDL-C lowering in patients with type 2 DM.59,92,93 In CARDS,59 2838 patients were randomized to 10 mg/d of atorvastatin or placebo (LDL-C level was 119 mg/dL at baseline and 73 mg/dL during atorvastatin treatment). At 4 years, atorvastatin reduced the RR of a major CV event by 37% (5.8% vs 9.0%; P<.001).
Diabetes and Hypertension
Persons with DM are prone to HTN because of excess activity in the renin-angiotensin-aldosterone system, salt retention, and increased sympathetic tone. In the UKPDS study, each 10-mm Hg reduction in mean systolic BP decreased the RR of DM-related death by 15%, MI by 11%, and microvascular complications by 13%.94 In the setting of type 2 DM, the goal of therapy is to reduce BP to less than 130/80 mm Hg, with an optimal BP of less than 120/75 mm Hg. On average, 3.2 medications are required to achieve target BP in the setting of diabetic HTN.
In the HOPE trial, 10 mg/d of ramipril conferred RR reductions of 37% for CV-related deaths and 24% for the development of nephropathy in the cohort of 3577 patients with DM.95 Because of their proven CV-protective and renoprotective properties, an ACEI or an ARB drug should be the first-line BP therapy for most patients with DM.21,96 The ACCOMPLISH trial,29 60% of whose study patients had DM, demonstrated that combination of benazepril plus a calcium channel blocker was superior to benazepril plus hydrochlorothiazide. Nonetheless, the addition of a thiazide diuretic agent will potentiate ARBs and ACEIs and offset their tendency to induce hyperkalemia. Thus, diuretic agents continue to have a role in the treatment of HTN in patients with DM; however, they should probably be relegated to third- or fourth-line agents.
Excess sympathetic tone is frequently associated with diabetes, predisposing those with diabetes to increased risk of CV events, including sudden cardiac arrest. The GEMINI trial31 evaluated carvedilol (6.25 mg twice daily) vs metoprolol (50 mg titrated to maximum 25 or 200 mg twice daily) in patients with both HTN and DM. Mean HbA1C levels (the primary end point of the trial) significantly worsened with metoprolol but were unchanged with carvedilol. In addition, progression of microalbuminuria was less frequent with carvedilol than with metoprolol. Carvedilol and nebivolol are the 2 β-adrenergic blockers that do not cause vasoconstriction, reduce cardiac output, or worsen glycemic control and thus are the preferred agents in this class for treating patients with diabetes who have HTN.97
Antiplatelet Therapy
The US Preventive Services Task Force, an independent panel convened by the US Department of Health and Human Services, recently published consensus guidelines for aspirin use in primary prevention.98 They recommended that low-dose daily aspirin be considered for men beginning at age 45 years (primarily to protect against MI) and for women beginning at age 55 years (primarily to protect against stroke). For both sexes, aspirin, when indicated, should be used at a dose of 81 mg/d because larger doses increase risks of bleeding and other complications without adding further benefit. However, daily aspirin therapy in primary prevention does not appear to affect either CV-related or all-cause mortality.99 Furthermore, a recent large meta-analysis found that the risks of aspirin therapy often outweigh the benefits in the setting of primary prevention.100 Thus, the use of aspirin in primary prevention should be considered on a case-by-case basis after discussion with the patient.
In secondary prevention, antiplatelet therapy has been shown to reduce ischemic nonfatal MI, stroke, and vascular death by 25% in high-risk patients. The American Diabetes Association recommends low-dose aspirin (75-162 mg/d) as a secondary prevention strategy in men and women with DM who have evidence of atherosclerotic vascular disease or who are at increased CV risk. However, in a recently published randomized trial of 2500 patients with diabetes who did not have CV disease, aspirin therapy (80-100 mg/d) showed only an insignificant trend toward benefit.101 Recent data suggest that when used in combination with clopidogrel, the aspirin dose should not exceed 81 mg/d because of increased bleeding risks with no clear incremental benefit at higher doses.102
Smoking
The toxic effects of long-term tobacco smoking on CV and general health are profound.103 A graded relationship exists between the number of cigarettes smoked and the risk of MI.11 Persons who smoke 1 pack of cigarettes per day are twice as likely to experience an MI or stroke compared with aged-matched nonsmokers. Within 12 to18 months after successful smoking cessation, most of the increased CV risk disappears; by 3 to 5 years, the risk of CV events is no different than that of a nonsmoker.36 Among all the modifiable CV risk factors evaluated in the INTERHEART study,104 current smoking was second only to dyslipidemia in importance for determining attributable risk of MI.
A systematic review showed a 36% RR reduction in mortality in patients with CHD who quit compared with those who continued smoking.103 Smoking cessation has proven to be a very cost-effective intervention. The US Public Health Service recommends that clinicians counsel all patients who use tobacco to permanently quit. The AHA/ACC secondary prevention guidelines also recommend complete avoidance of tobacco smoke at work and home.105 Repeated interventions are often needed to help patients maintain abstinence. Many effective medications are available, and patients should be encouraged to use them unless contraindicated.106 Seven first-line (FDA-approved) medications (bupropion sustained-release, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, nicotine patch, and varenicline) and 2 second-line (non—FDA-approved for tobacco use treatment) medications (clonidine and nortriptyline) are recommended as being effective for long-term abstinence from smoking.106 Despite the risks associated with potentially worsening depression (and theoretically, the risk of suicides), varenicline has the best randomized trial data regarding long-term smoking cessation.51,107
Obesity
An estimated 1.1 billion adults worldwide are overweight or obese,108 including 70% of US adults (130 million).109 Abdominal obesity has been linked with serious metabolic abnormalities, such as insulin resistance, hyperinsulinemia, hypertriglyceridemia, HTN, and DM, likely as a result of disrupting normal hormonal balance and increasing systemic inflammation. Waist circumference has a continuous, graded, and highly significant direct correlation with CV risk.110 Among 360,000 European adults, waist circumference was strongly associated with the risk of death.111 Body mass index has shown a modest graded association with MI; however, waist circumference is more strongly associated with metabolic risk factors, incident CV events, and death.110,112 Waist size has also been shown to independently predict the risk of type 2 DM.113 Some data suggest that waist-to-height ratio may be a better marker of CV risk than either body mass index or waist circumference.114 An ideal waist circumference is less than half of the height, which is a goal that is easily communicated to and understood by patients and the general public. The current status of obesity as a CV risk factor and the safety and efficacy of weight reduction for reducing risk have been reviewed recently.13
Physical Activity
The role of physical activity and exercise training has been discussed in detail in this symposium, including the important role of cardiac rehabilitation in secondary prevention.62 Clearly, in addition to improving most of the established CV risk factors, regular exercise likely also independently improves CV risk.
Diet
The calorie-dense, nutrient-depleted, highly processed diet currently favored in the United States is at the root of the parallel epidemics of obesity and type 2 DM. This eating pattern often leads to exaggerated postprandial spikes in glucose and lipid levels that can lead to an excess of free radicals and can trigger a cascade of endothelial dysfunction and sympathetic hyperactivity.115,116 Even a single meal high in easily digestible carbohydrates and saturated fat will cause immediate increases in glucose, triglycerides, chylomicrons, and remnant lipoproteins with proportional increases in oxidative stress and inflammation. This state, known as postprandial dysmetabolism, is an independent predictor of future CV events76 and is commonly associated with insulin resistance and MetS.76 Nutrition plans that include a vast array of fresh unprocessed plants, lean protein, and beneficial fats (omega-3 and monounsaturated fats), are rich in antioxidants, and allow for only small amounts of processed carbohydrates, saturated fats, and transfats will improve postprandial glucose and lipid levels.117 The traditional Mediterranean and Okinawan diets, which are low in processed and calorie-dense foods and high in nutrient-rich fresh plant foods, have been associated with improved CV health.117,118 Nuts, such as almonds, walnuts, and pecans, are often included in the Mediterranean diet and have been shown to lower risk of death from CHD. The DASH eating plan has been helpful in controlling HTN, with its emphasis on consumption of fruits, vegetables, and nonfat or low-fat dairy products and intake of only limited amounts of saturated and total fat. Dietary sodium should be reduced to no more than 2.4 g/d.119
Omega-3 Fatty Acids
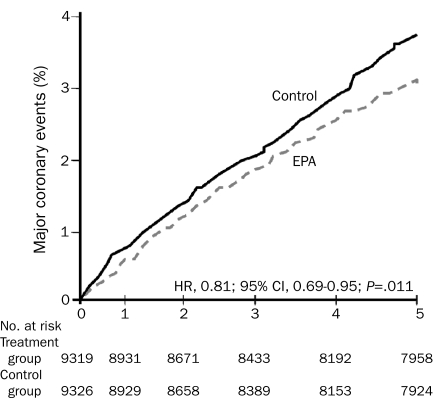
Many studies have documented the CV protective benefit of omega-3 fatty acids, specifically docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA).120,121 Dietary supplementation with omega-3 fatty acids121 has been shown to reduce arrhythmias, lower BP, improve arterial and endothelial function, reduce platelet aggregation, and improve autonomic tone, in addition to other effects. Major, large-scale, randomized controlled trials have shown benefits for primary and secondary prevention of CHD and, most recently, for patients with advanced HF122 (Figure 6). The AHA currently recommends consumption of 1 g/d of a DHA and EPA combination in patients with established CHD. In patients without CHD, at least 500 mg/d of EPA plus DHA is recommended; this goal can be met by eating 2 fish meals per week, with an emphasis on fatty fish, such as salmon, herring, and sardines. Triglyceride reductions of 30% to 50% require 3 to 4 g/d of EPA plus DHA, which can be achieved by consuming a highly purified and concentrated fish oil such as prescription omega-3.
FIGURE 6.
Cumulative incidence of major coronary events in patients treated with eicosapentaenoic acid (EPA) plus statin compared with statin alone. CI = confidence interval; HR = hazard ratio. From Lancet,122 with permission from Elsevier.
Alcohol
An impressive body of literature, largely observational in nature, suggests that light-to-moderate alcohol consumption may confer CV protection.123 A large meta-analysis involving more than 1 million persons showed that consumption of one-half to 1 drink daily by women and 1 or 2 drinks daily by men reduced total mortality by 18%.124 One drink per day is consistently associated with an approximately 30% reduction in the risk of acute MI.125 Daily low-dose alcohol has been shown to improve HDL-C levels, insulin sensitivity, postprandial glucose levels, and excess sympathetic tone, while also decreasing inflammation as measured by levels of hsCRP, tumor necrosis factor α, and interleukin 6.123,126 However, alcohol has also been shown to increase risk of HTN, cancer, stroke, HF, dementia, and DM in a dose-dependent fashion when more than 2 drinks are consumed daily.127 Alcohol intake should be limited to not more than 1 drink in women and 2 drinks in men, with 1 drink being defined as 12 oz of beer, 5 oz of wine, or 1.5 oz of 80-proof liquor. A recent meta-analysis of 1.3 million women showed a dose-dependent carcinogenic effect of ethanol for women that became significant even at 1 drink per day.128
Vitamin D Deficiency
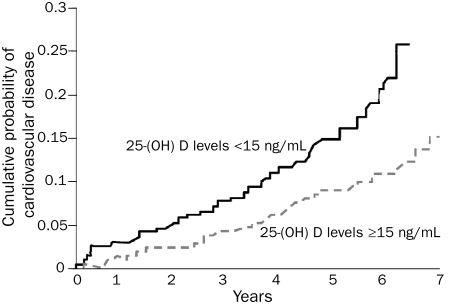
A growing body of scientific data suggests that vitamin D deficiency is associated with increased CV risk.129 Persons with vitamin D deficiency have been shown to be predisposed to HTN, DM (both types 1 and 2), MetS, LVH, HF, and chronic vascular inflammation.130 Long-term prospective observational studies, including the US Physicians Health Study and the Framingham Offspring Study, have linked vitamin D deficiency with a 2-fold increased risk of MI131,132 (Figure 7). Similarly, in a cohort of 3258 German adults who were followed up for 7.7 years after an elective cardiac catheterization, those in the lowest quartile for baseline serum 25-hydroxyvitamin D had a 2-fold increased risk of CV death compared with those in the highest quartile.133 A meta-analysis including 18 randomized controlled trials comprising 57,000 participants showed that an intake of vitamin D greater than 500 IU/d was associated with a reduction in all-cause mortality, in part because of a lower incidence of CV deaths.134
FIGURE 7.
Kaplan-Meier curve showing the probability of major adverse cardiovascular events in participants with 25 hydroxyvitamin D (25-[OH] D) levels ≥15 ng/mL vs 25-(OH) D levels <15 ng/mL. SI conversion factor: To convert 25-(OH) D values to nmol/L, multiply by 2.496. From J Am Coll Cardiol,132 with permission from Elsevier.
Although a general consensus regarding the optimal levels of serum 25-hydroxyvitamin D does not yet exist, most experts consider a level of less than 20 ng/mL (to convert to nmol/L, multiply by 2.496) deficient and a level of 21 to 29 ng/mL insufficient.132 Large randomized controlled trials are needed to clarify the relevance of vitamin D status to CV health. In the interim, monitoring of vitamin D levels in patients with CV disease or CV risk factors (eg, HTN, DM, elevated hsCRP levels) is suggested, and repletion of suboptimal levels is clearly indicated for optimizing musculoskeletal health. Vitamin D deficiency is present in approximately 90% of persons who report symptoms of myalgias while receiving statin therapy.135 In a nonrandomized series from our Preventive Cardiology Clinic at the Mid America Heart Institute, about 80% of such patients can be successfully maintained on a statin when their vitamin D level is normalized via a vitamin D supplement.
Repletion of vitamin D should be initiated with 50,000 IU of vitamin D2 or D3 once or twice weekly for a period of 8 to 12 weeks. Vitamin D can be supplemented by sunlight (about 3000 IU of vitamin D3 per 5-10 minutes of midday sun) or 1000 to 5000 IU/d of vitamin D. Among foods, oily fish such as wild salmon have the highest content of vitamin D, ranging from 100 to 1000 IU per 3.5 oz.132
Psychosocial Stress
Although emotions emanate from the brain, they resonate strongly and immediately with the heart and CV system. A growing body of data indicates that a happy and socially connected heart is generally healthier than a heart burdened by depression, loneliness, anger, or anxiety. The INTERHEART study reported that PSS accounted for approximately 30% of the population's attributable risk of acute MI, placing it behind only lipids and smoking in importance among the 9 major modifiable CHD risk factors.11 Pathologic depression, social isolation, cynical distrust, hostility, pessimism, and a sense of hopelessness have each been linked to adverse CV events.136-139 Spikes in the incidence of CV deaths, lethal arrhythmias, and MIs have been documented in the affected populations immediately after anxiety-provoking disasters such as earthquakes and terrorist attacks.136 In contrast, factors that have been associated with both lowered stress levels and reduced rates of CV mortality and morbidity include social connection, exercise, optimism, humor, altruism, animal companionship, and involvement in organized religion.62,136
The toxicity of PSS is markedly exacerbated when a person has insufficient control over the source of the stress and limited emotional support and resources.136,138 Psychosocial stressors are thought to increase CV risk by activating the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis, resulting in sympathetic predominance and increased cortisol levels, respectively.137,138 Excess stress can adversely affect BP as well as blood glucose and lipid levels; it can also increase atherosclerotic progression and inflammation140 and predispose emotionally stressed persons to endothelial dysfunction and central obesity.8,141
Because PSS is common both as a cause and a complication of symptomatic CHD, screening tools are important for identifying patients at risk. A scientific consensus does not exist regarding the optimal tool for quantifying PSS. Open-ended questions as outlined in the Table can be helpful in screening for PSS. The AHA has recently recommended that patients with CHD be screened for depression and treated if it is present62,138,142-144 (Table).
TABLE.
Suggested Questions for Assessing Psychosocial Stress
The ideal approach to lowering PSS and improving stress-related adverse prognoses is unclear. Pharmacologic therapy is the standard approach to stress reduction; although antidepressants improve symptoms of depression in patients with CHD, they have not been shown to favorably alter the prognosis of patients with CV disease. In contrast, exercise training has been associated with reductions in PSS and its related mortality.62,145,146 A prescription for a structured exercise program, including formal cardiac rehabilitation, is a logical and practical therapy for emotionally distressed persons with or at risk of CHD and has been discussed in detail in this symposium.
Stress reduction training using meditation, relaxation breathing, yoga, and other techniques can improve subjective and objective measures of PSS. Additionally, altruism, pets (animal companionship), and organized religion or spirituality can counteract PSS and might contribute to CV health; however, such a contribution currently remains speculative.
CONCLUSION
Coronary atherosclerosis and its complications are eminently preventable. The assessment of CHD risk factors and screening for asymptomatic coronary or carotid atherosclerosis are helpful in identifying individuals at risk of adverse CV events. Aggressive normalization of CV risk factors such as dyslipidemia, hypertension, sedentary lifestyle, and tobacco smoking is the most effective means of improving prognosis in the setting of chronic coronary artery disease.
Supplementary Material
Acknowledgments
The authors wish to thank Lori J. Wilson for her help in preparation of the submitted manuscript.
Glossary of Clinical Trials
- ACCOMPLISH
Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension
- ADOPT
A Diabetes Outcome Progression Trial
- ALLHAT
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
- ANBP2
Second Australian National Blood Pressure Study
- ASCOT-BPLA
Blood Pressure Lowering Arm of the Anglo-Scandinavian Cardiac Outcomes Trial
- ASTEROID
A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden
- BARI 2D
Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes
- CARDS
Collaborative Atorvastatin Diabetes Study
- COMET
Carvedilol or Metoprolol European Trial
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation
- DASH
Dietary Approaches to Stop Hypertension
- ENHANCE
Effect of Ezetimibe Plus Simvastatin Versus Simvastatin Alone on Atherosclerosis in the Carotid Artery
- EUROPA
European Trial on Reduction of Cardiac Events With Perindopril in Stable Coronary Artery Disease
- GEMINI
Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives
- HOPE
Heart Outcomes Prevention Evaluation
- HPS
Heart Protection Study
- INTERHEART
A Global Case-Control Study of Risk Factors for Acute Myocardial Infarction
- JUPITER
Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin
- LIFE
Losartan Intervention for Endpoint Reduction
- METEOR
Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin 40 mg
- MIRACL
Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering
- ONTARGET
Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial
- REVERSAL
Reversal of Atherosclerosis With Aggressive Lipid Lowering
- SEARCH
Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine
- SHAPE
Screened Health Assessment and Pacer Evaluation
- STOP-NIDDM
Study to Prevent Non-Insulin-Dependent Diabetes Mellitus
- TNT
Treating to New Targets
- UKPDS
UK Prospective Diabetes Study
- VALUE
Valsartan Antihypertensive Long-term Use Evaluation
On completion of this article, you should be able to (1) understand the importance of aggressive multimodal risk factor modification for improving the prognosis of patients with coronary heart disease, (2) recognize that aggressive low-density lipoprotein cholesterol reduction is required to optimize cardiovascular prognosis for patients with coronary heart disease, and (3) recognize that postprandial triglyceride and glucose levels are important cardiovascular risk factors.
Footnotes
A glossary of clinical trials appears at the end of this article.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Schömig A, Mehilli J, de Waha A, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52(11):894-904 [DOI] [PubMed] [Google Scholar]
- 2.Katritsis DG, Ioannidis JP. PCI for stable coronary disease [letter]. N Engl J Med. 2007;357(4):414-415 [DOI] [PubMed] [Google Scholar]
- 3.Boden WE, O'Rourke RA, Teo KK, et al. COURAGE Trial Research Group Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007April12;356(15):1503-1516 Epub 2007 Mar 26 [DOI] [PubMed] [Google Scholar]
- 4.Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Study Group A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009June11;360(24):2503-2515 Epub 2009 Jun 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. J Nucl Med. 2007November;48(11):1800-1815 Epub 2007 Oct 17 [DOI] [PubMed] [Google Scholar]
- 6.Lauer MS. Primary prevention of atherosclerotic cardiovascular disease: the high public burden of low individual risk [editorial]. JAMA 2007March28;297(12):1376-1378 Epub 2007 Mar 25 [DOI] [PubMed] [Google Scholar]
- 7.Naghavi M, Falk E, Hecht HS, et al. SHAPE Task Force From vulnerable plaque to vulnerable patient, Part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006July17;98(2A):2H-15H Epub 2006 Jun 12 [DOI] [PubMed] [Google Scholar]
- 8.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345 [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356(23):2388-2398 [DOI] [PubMed] [Google Scholar]
- 10.Morgan TM, Krumholz HM, Lifton RP, Spertus JA. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study [published correction appears in JAMA. 2007;298(9):973] JAMA 2007;297(14):1551-1561 [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937-952 [DOI] [PubMed] [Google Scholar]
- 12.Clendenning R. The optimal low-density lipoprotein is 50 to 70 mg/dl [letter]. J Am Coll Cardiol. 2005;45(10):1732 [DOI] [PubMed] [Google Scholar]
- 13.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925-1932 [DOI] [PubMed] [Google Scholar]
- 14.Madala MC, Franklin BA, Chen AY, et al. CRUSADE Investigators Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52(12):979-985 [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [published correction appears in JAMA. 2003;290(2):197] JAMA 2003May21;289(19):2560-2572 Epub 2003 May 14 [DOI] [PubMed] [Google Scholar]
- 16.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) [published correction appears in JAMA. 2002;288(23):2976] JAMA 2000;283(15):1967-1975 [PubMed] [Google Scholar]
- 17.Cushman WC, Ford CE, Cutler JA, et al. ALLHAT Collaborative Research Group Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich) 2002;4(6):393-404 [DOI] [PubMed] [Google Scholar]
- 18.Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies [published correction appears in Lancet. 2003;361(9362):1060] Lancet 2002;360(9349):1903-1913 [DOI] [PubMed] [Google Scholar]
- 19.Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008May17;336(7653):1121-1123 Epub 2008 May 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Treatment of hypertension in adults with diabetes. Diabetes Care 2003;26(suppl 1):S80-S82 [DOI] [PubMed] [Google Scholar]
- 21.Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [published corrections appear in N Engl J Med. 2000;342(18):1376 and 2000;342(10):748] N Engl J Med. 2000;342(3):145-153 [DOI] [PubMed] [Google Scholar]
- 22.EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362(9386):782-788 [DOI] [PubMed] [Google Scholar]
- 23.Mann JF, Schmieder RE, McQueen M, et al. ONTARGET investigators Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372(9638):547-553 [DOI] [PubMed] [Google Scholar]
- 24.Dahlöf B, Devereux RB, Kjeldsen SE, et al. LIFE Study Group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359(9311):995-1003 [DOI] [PubMed] [Google Scholar]
- 25.Wing LM, Reid CM, Ryan P, et al. Second Australian National Blood Pressure Study Group A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348(7):583-592 [DOI] [PubMed] [Google Scholar]
- 26.Dahlöf B, Sever PS, Poulter NR, et al. ASCOT Investigators Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366(9489):895-906 [DOI] [PubMed] [Google Scholar]
- 27.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published corrections appear in JAMA. 2003;289(2):178 and 2004;291(18):2196] JAMA 2002;288(23):2981-2997 [DOI] [PubMed] [Google Scholar]
- 28.Julius S, Kjeldsen SE, Weber M, et al. VALUE trial group Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363(9426):2022-2031 [DOI] [PubMed] [Google Scholar]
- 29.Cleland JG, Coletta AP, Yassin A, et al. Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta-analysis of Cox-2 studies, HAT, ON-TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON-HF and REVERSE. Eur J Heart Fail 2008June;10(6):614-620 Epub 2008 May 27 [DOI] [PubMed] [Google Scholar]
- 30.Jamerson K, Weber MA, Bakris GL, et al. ACCOMPLISH Trial Investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417-2428 [DOI] [PubMed] [Google Scholar]
- 31.Bakris GL, Fonseca V, Katholi RE, et al. GEMINI Investigators Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004;292(18):2227-2236 [DOI] [PubMed] [Google Scholar]
- 32.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004January;43(1):4-9 Epub 2003 Nov 24 [DOI] [PubMed] [Google Scholar]
- 33.Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006March;47(3):352-358 Epub 2006 Jan 23 [DOI] [PubMed] [Google Scholar]
- 34.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265(24):3255-3264 [PubMed] [Google Scholar]
- 35.Multiple Risk Factor Intervention Trial Research Group Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention Trial. Circulation 1990;82(5):1616-1628 [DOI] [PubMed] [Google Scholar]
- 36.O'Keefe JH, Bell DS, Wyne KL. Diabetes Essentials 4th ed.Sudbury, MA: Jones and Bartlett Publishers; 2009:191 [Google Scholar]
- 37.Pitt B, Remme W, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med. 2003;348(22):2271] N Engl J Med. 2003April3;348(14):1309-1321 Epub 2003 Mar 31 [DOI] [PubMed] [Google Scholar]
- 38.Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709-717 [DOI] [PubMed] [Google Scholar]
- 39.Klapholz M. β-Blocker use for the stages of heart failure. Mayo Clin Proc. 2009;84(8):718-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan NM. Beta-blockers in hypertension: adding insult to injury [editorial]. J Am Coll Cardiol. 2008;52(18):1490-1491 [DOI] [PubMed] [Google Scholar]
- 41.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice [published correction appears in Lancet. 2005;365(9460):656]?Lancet 2004;364(9446):1684-1689 [DOI] [PubMed] [Google Scholar]
- 42.Poole-Wilson PA, Swedberg K, Cleland JG, et al. COMET Investigators Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362(9377):7-13 [DOI] [PubMed] [Google Scholar]
- 43.Saunders E, Smith WB, DeSalvo KB, Sullivan WA. The efficacy and tolerability of nebivolol in hypertensive African American patients. J Clin Hypertens (Greenwich) 2007;9(11):866-875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sipahi I, Tuzcu EM, Wolski KE, et al. β-Blockers and progression of coronary atherosclerosis: pooled analysis of 4 intravascular ultrasonography trials. Ann Intern Med. 2007;147(1):10-18 [DOI] [PubMed] [Google Scholar]
- 45.Lavie CJ, Milani RV. Shedding light on high-density lipoprotein cholesterol: the post-ILLUMINATE era [editorial]. J Am Coll Cardiol. 2008;51(1):56-58 [DOI] [PubMed] [Google Scholar]
- 46.Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371(9607):117-125 [DOI] [PubMed] [Google Scholar]
- 47.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7-22 [DOI] [PubMed] [Google Scholar]
- 48.O'Keefe JH, Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43(11):2142-2146 [DOI] [PubMed] [Google Scholar]
- 49.Wiviott SD, Cannon CP, Morrow DA, Ray KK, Pfeffer MA, Braunwald E, PROVE IT-TIMI 22 Investigators Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy [published correction appears in J Am Coll Cardiol. 2006;47(2):472] J Am Coll Cardiol. 2005;46(8):1411-1416 [DOI] [PubMed] [Google Scholar]
- 50.LaRosa JC, Grundy SM, Waters DD, et al. Treating to New Targets (TNT) Investigators Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005April7;352(14):1425-1435 Epub 2005 Mar 8 [DOI] [PubMed] [Google Scholar]
- 51.Bybee KA, Lee JH, O'Keefe JH. Cumulative clinical trial data on atorvastatin for reducing cardiovascular events: the clinical impact of atorvastatin. Curr Med Res Opin. 2008April;24(4):1217-1229 Epub 2008 Mar 20 [DOI] [PubMed] [Google Scholar]
- 52.Pedersen TR, Faergeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial [published correction appears in JAMA. 2005;294(24):3092] JAMA 2005;294(19):2437-2445 [DOI] [PubMed] [Google Scholar]
- 53.de Lemos JA, Blazing MA, Wiviott SD, et al. A to Z Investigators Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004September15;292(11):1307-1316 Epub 2004 Aug 30 [DOI] [PubMed] [Google Scholar]
- 54.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285(13):1711-1718 [DOI] [PubMed] [Google Scholar]
- 55.Nissen SE, Tuzcu EM, Schoenhagen P, et al. REVERSAL Investigators Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291(9):1071-1080 [DOI] [PubMed] [Google Scholar]
- 56.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008November20;359(21):2195-2207 Epub 2008 Nov 9 [DOI] [PubMed] [Google Scholar]
- 57.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009April30;360(18):1851-1861 Epub 2009 Mar 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sever PS, Dahlöf B, Poulter NR, et al. ASCOT investigators Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149-1158 [DOI] [PubMed] [Google Scholar]
- 59.Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS investigators Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364(9435):685-696 [DOI] [PubMed] [Google Scholar]
- 60.Musunuru K, Kral BG, Blumenthal RS, et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008October;5(10):621-635 Epub 2008 Aug 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michos ED, Blumenthal RS. Prevalence of low low-density lipoprotein cholesterol with elevated high sensitivity C-reactive protein in the U.S. J Am Coll Cardiol. 2009;53(11):931-935 [DOI] [PubMed] [Google Scholar]
- 62.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84(4):373-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milani RV, Lavie CJ. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92(1):50-54 [DOI] [PubMed] [Google Scholar]
- 64.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43(6):1056-1061 [DOI] [PubMed] [Google Scholar]
- 65.Ballantyne CM, Raichlen JS, Nicholls SJ, et al. ASTEROID Investigators Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation 2008May;117(19):2458-2466 Epub 2008 Mar 31 [DOI] [PubMed] [Google Scholar]
- 66.Nissen SE, Nicholls SJ, Sipahi I, et al. ASTEROID Investigators Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006April5;295(13):1556-1565 Epub 2006 Mar 13 [DOI] [PubMed] [Google Scholar]
- 67.Crouse JR, III, Raichlen JS, Riley WA, et al. METEOR Study Group Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 2007March28;297(12):1344-1353 Epub 2007 Mar 25 [DOI] [PubMed] [Google Scholar]
- 68.O'Keefe JH, Bybee KA, Lavie CJ. Intensive lipid intervention in the post-ENHANCE era [editorial]. Mayo Clin Proc. 2008;83(8):867-869 [DOI] [PubMed] [Google Scholar]
- 69.Gerber TC, Taylor AJ. Carotid intima-media thickness: can it close the “detection gap” for cardiovascular risk [editorial]? Mayo Clin Proc. 2009;84(3):218-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lester SJ, Eleid MF, Khandheria BK, Hurst RT. Carotid intima-media thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proc. 2009;84(3):229-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.SEARCH Study Collaborative Group Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am Heart J. 2007November;154(5):815-823, 823.e1-6. Epub 2007 Sep 6 [DOI] [PubMed] [Google Scholar]
- 72.O'Keefe JH, Carter MD, Lavie CJ, Bell DS. The gravity of JUPITER (Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin). Postgrad Med. 2009;121(3):113-118 [DOI] [PubMed] [Google Scholar]
- 73.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update [published correction appears in Circulation. 2006;113(22):e847] Circulation 2006;113(19):2363-2372 [DOI] [PubMed] [Google Scholar]
- 74.Bays HE, Davidson M, Jones MR, Abby SL. Effects of colesevelam hydrochloride on low-density lipoprotein cholesterol and high-sensitivity C-reactive protein when added to statins in patients with hypercholesterolemia [published correction appears in Am J Cardiol. 2006;98(3):428] Am J Cardiol. 2006April15;97(8):1198-1205 Epub 2006 Mar 3 [DOI] [PubMed] [Google Scholar]
- 75.Bell DS, O'Keefe JH. Rediscovering bile acid sequestrants. Diabetes Obes Metab In press [DOI] [PubMed]
- 76.O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249-255 [DOI] [PubMed] [Google Scholar]
- 77.Canner PL, Furberg CD, McGovern ME. Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol. 2006February15;97(4):477-479 Epub 2005 Dec 21 [DOI] [PubMed] [Google Scholar]
- 78.Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc. 2008;83(4):470-478 [DOI] [PubMed] [Google Scholar]
- 79.Vittone F, Chait A, Morse JS, Fish B, Brown BG, Zhao XQ. Niacin plus simvastatin reduces coronary stenosis progression among patients with metabolic syndrome despite a modest increase in insulin resistance: a subgroup analysis of the HDL-Atherosclerosis Treatment Study (HATS). J Clin Lipidol. 2007;1(3):203-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardenas GA, Lavie CJ, Cardenas V, Milani RV, McCullough PA. The importance of recognizing and treating low levels of high-density lipoprotein cholesterol: a new era in atherosclerosis management. Rev Cardiovasc Med. 2008;9(4):239-258 [PubMed] [Google Scholar]
- 81.Conaway DG, O'Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363-365 [DOI] [PubMed] [Google Scholar]
- 82.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [published correction appears in Lancet. 1998;352(9139):1558] Lancet 1998;352(9131):854-865 [PubMed] [Google Scholar]
- 83.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008June12;358(24):2545-2559 Epub 2008 Jun 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rendell M. Advances in diabetes for the millennium: drug therapy of type 2 diabetes. MedGenMed. 2004;6(3 suppl):9 [PMC free article] [PubMed] [Google Scholar]
- 85.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kahn SE, Haffner SM, Heise MA, et al. ADOPT study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy [published correction appears in N Engl J Med. 2007;356(13):1387-1388] N Engl J Med. 2006December7;355(23):2427-2443 Epub 2006 Dec 4 [DOI] [PubMed] [Google Scholar]
- 87.Modi P. Diabetes beyond insulin: review of new drugs for treatment of diabetes mellitus. Curr Drug Discov Technol. 2007;4(1):39-47 [DOI] [PubMed] [Google Scholar]
- 88.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26(3):881-885 [DOI] [PubMed] [Google Scholar]
- 89.Bell DS, O'Keefe JH, Jellinger P. Postprandial dysmetabolism: the missing link between diabetes and cardiovascular events? Endocr Pract. 2008;14(1):112-124 [DOI] [PubMed] [Google Scholar]
- 90.O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007September1;100(5):899-904 Epub 2007 Jun 26 [DOI] [PubMed] [Google Scholar]
- 91.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359(9323):2072-2077 [DOI] [PubMed] [Google Scholar]
- 92.Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361(9374):2005-2016 [DOI] [PubMed] [Google Scholar]
- 93.Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005;28(5):1151-1157 [DOI] [PubMed] [Google Scholar]
- 94.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 [published correction appears in BMJ. 1999;318(7175):29] BMJ 1998;317(7160):703-713 [PMC free article] [PubMed] [Google Scholar]
- 95.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy [published correction appears in Lancet. 2000;356(9232):860] Lancet 2000;355(9200):253-259 [PubMed] [Google Scholar]
- 96.ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008April10;358(15):1547-1559 Epub 2008 Mar 31 [DOI] [PubMed] [Google Scholar]
- 97.Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1-68 [DOI] [PubMed] [Google Scholar]
- 98.US Preventive Services Task Force Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(6):396-404 [DOI] [PubMed] [Google Scholar]
- 99.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150(6):405-410 [DOI] [PubMed] [Google Scholar]
- 100.Antithrombotic Trialists' (ATT) Collaboration Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373(9678):1849-1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ogawa H, Nakayama M, Morimoto T, et al. Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial [published correction appears in JAMA. 2009;301(18):1882] JAMA 2008November12;300(18):2134-2141 Epub 2008 Nov 9 [DOI] [PubMed] [Google Scholar]
- 102.Steinhubl SR, Bhatt DL, Brennan DM, et al. CHARISMA Investigators Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150(6):379-386 [DOI] [PubMed] [Google Scholar]
- 103.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290(1):86-97 [DOI] [PubMed] [Google Scholar]
- 104.Teo KK, Ounpuu S, Hawken S, et al. INTERHEART Study Investigators Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet 2006;36(9536):647-658 [DOI] [PubMed] [Google Scholar]
- 105.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47(10):2130-2139 [DOI] [PubMed] [Google Scholar]
- 106.The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee JH, Jones PG, Bybee KA, O'Keefe JH. A longer course of varenicline therapy improves smoking cessation rates. Prev Cardiol. 2008;11(4):210-214 [DOI] [PubMed] [Google Scholar]
- 108.Haslam DW, James WP. Obesity. Lancet 2005;366(9492):1197-1209 [DOI] [PubMed] [Google Scholar]
- 109.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288(14):1723-1727 [DOI] [PubMed] [Google Scholar]
- 110.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366(9497):1640-1649 [DOI] [PubMed] [Google Scholar]
- 111.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105-2120 [DOI] [PubMed] [Google Scholar]
- 112.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007April;28(7):850-856 Epub 2007 Apr 2 [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555-563 [DOI] [PubMed] [Google Scholar]
- 114.Schneider HJ, Glaesmer H, Klotsche J, et al. DETECT Study Group Accuracy of anthropometric indicators of obesity to predict cardiovascular risk. J Clin Endocrinol Metab. 2007February;92(2):589-594 Epub 2006 Nov 14 [DOI] [PubMed] [Google Scholar]
- 115.Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 2006May;49(5):846-854 Epub 2006 Mar 11 [DOI] [PubMed] [Google Scholar]
- 116.Weissman A, Lowenstein L, Peleg A, Thaler I, Zimmer EZ. Power spectral analysis of heart rate variability during the 100-g oral glucose tolerance test in pregnant women. Diabetes Care 2006;29(3):571-574 [DOI] [PubMed] [Google Scholar]
- 117.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006July4;114(1):82-96 Epub 2006 Jun 19 [DOI] [PubMed] [Google Scholar]
- 118.Fitó M, Guxens M, Corella D, et al. PREDIMED Study Investigators Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167(11):1195-1203 [DOI] [PubMed] [Google Scholar]
- 119.Parikh A, Lipsitz SR, Natarajan S. Association between a DASH-like diet and mortality in adults with hypertension: findings from a population-based follow-up study. Am J Hypertens. 2009April;22(4):409-416 Epub 2009 Feb 5 [DOI] [PubMed] [Google Scholar]
- 120.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases—a fish tale with growing credibility. J Am Coll Cardiol. In press [DOI] [PubMed]
- 121.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection [published correction appears in Mayo Clin Proc. 2008;83(6):730] Mayo Clin Proc. 2008;83(3):324-332 [DOI] [PubMed] [Google Scholar]
- 122.Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA Lipid Intervention Study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis [published correction appears in Lancet. 2007;370(9583):220] Lancet 2007;369(9567):1090-1098 [DOI] [PubMed] [Google Scholar]
- 123.O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50(11):1009-1014 [DOI] [PubMed] [Google Scholar]
- 124.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437-2445 [DOI] [PubMed] [Google Scholar]
- 125.Beulens JW, Rimm EB, Ascherio A, Spiegelman D, Hendriks HF, Mukamal KJ. Alcohol consumption and risk for coronary heart disease among men with hypertension. Ann Intern Med. 2007;146(1):10-19 [DOI] [PubMed] [Google Scholar]
- 126.Zairis MN, Ambrose JA, Lyras AG, et al. GENERATION Study Group C Reactive protein, moderate alcohol consumption, and long term prognosis after successful coronary stenting: four year results from the GENERATION study. Heart 2004;90(4):419-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beilin LJ, Puddey IB. Alcohol and hypertension: an update. Hypertension 2006June;47(6):1035-1038 Epub 2006 Apr 3 [DOI] [PubMed] [Google Scholar]
- 128.Allen NE, Beral V, Casabonne D, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009March4;101(5):296-305 Epub 2009 Feb 24 [DOI] [PubMed] [Google Scholar]
- 129.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373 [DOI] [PubMed] [Google Scholar]
- 130.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281 [DOI] [PubMed] [Google Scholar]
- 131.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949-1956 [DOI] [PubMed] [Google Scholar]
- 133.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340-1349 [DOI] [PubMed] [Google Scholar]
- 134.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730-1737 [DOI] [PubMed] [Google Scholar]
- 135.Barton Duell P, Connor WE. Vitamin D deficiency is associated with myalgias in hyperlipidemic subjects taking statins [abstract 3701]. Circulation 2008;118:S470 [Google Scholar]
- 136.O'Keefe JH, Jr, Poston WS, Haddock CK, Moe RM, Harris W. Psychosocial stress and cardiovascular disease: how to heal a broken heart. Compr Ther. 2004;30(1):37-43 [DOI] [PubMed] [Google Scholar]
- 137.Shen BJ, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol. 2008;51(2):113-119 [DOI] [PubMed] [Google Scholar]
- 138.Das S, O'Keefe JH. Behavioral cardiology: recognizing and addressing the profound impact of psychosocial stress on cardiovascular health. Curr Atheroscler Rep. 2006;8(2):111-118 [DOI] [PubMed] [Google Scholar]
- 139.Brummett BH, Helms MJ, Dahlstrom WG, Siegler IC. Prediction of all-cause mortality by the Minnesota Multiphasic Personality Inventory Optimism-Pessimism Scale scores: study of a college sample during a 40-year follow-up period. Mayo Clin Proc. 2006;81(12):1541-1544 [DOI] [PubMed] [Google Scholar]
- 140.Ranjit N, Diez-Roux AV, Shea S, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167(2):174-181 [DOI] [PubMed] [Google Scholar]
- 141.Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute working group report. Ann Behav Med. 2006;32(2):121-126 [DOI] [PubMed] [Google Scholar]
- 142.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation 2008October21;118(17):1768-1775 Epub 2008 Sep 29 [DOI] [PubMed] [Google Scholar]
- 143.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637-651 [DOI] [PubMed] [Google Scholar]
- 144.Narayan SM, Stein MB. Do depression or antidepressants increase cardiovascular mortality? The absence of proof might be more important than the proof of absence [editorial]. J Am Coll Cardiol. 2009;53(11):959-961 [DOI] [PubMed] [Google Scholar]
- 145.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007;120(9):799-806 [DOI] [PubMed] [Google Scholar]
- 146.Milani RV, Lavie CJ. Reducing psychosocial stress: a novel mechanism of improving survival from exercise training. Am J Med. In press [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.