Abstract
BACKGROUND
Prostate cancer progression is partly facilitated by tumor-stroma interactions. We recently reported that protease-activated receptors (PAR-1 and PAR-2) are overexpressed in prostate cancer, and PAR-1 expression in peritumoral stroma is associated with biochemical recurrence. However, the nature of PAR expression in prostate tumor microenvironment is not fully understood. We therefore evaluated PAR-1 and PAR-2 expression in primary prostate cancer and bone metastasis.
METHODS
PAR-1 and PAR-2 expression in normal, primary prostate cancer and the corresponding bone metastatic tissues were examined by immunohistochemistry, and double-label immunohistochemistry with the use of additional markers.
RESULTS
PAR-1 was expressed in peritumoral stroma in the majority of primary cancer tissues (83%). Serial sections and double-label immunohistochemistry determined that these PAR-1 expressing stromal cells were predominantly myofibroblasts, the primary cell type in reactive stroma. Analysis of cancer glands revealed that PAR-1 expression was significantly increased in the reactive stroma around higher Gleason grade cancers. PAR-2 was predominantly expressed in the primary cancer cells as well as smooth muscle cells but not in reactive stroma. In bone metastasis, PAR-1 expression in cancer cells was elevated compared to the primary site from the same patient. In the bone reactive stroma, PAR-1 was present in vascular endothelial cells and fibroblasts, while both PAR-1 and PAR-2 were expressed in osteoblasts and osteoclasts.
CONCLUSIONS
In primary prostate cancer and bone metastasis, PAR-1 is upregulated in reactive stroma and PAR-2 is uniformly overexpressed in carcinoma cells, suggesting these receptors may play potentially different roles in prostate cancer development and metastasis.
Keywords: PAR-1, PAR-2, Prostate Cancer, Bone Metastasis, Reactive Stroma
INTRODUCTION
Prostate cancer is one of the most common malignancies of men, with approximately 220,000 new cases and 29,000 deaths annually in the United States [1, 2]. Hormone therapy failure follows an androgen-independent state which leads to widespread metastasis [3]. Prostate cancer primarily metastasizes to bone, which can be a major cause of morbidity and mortality [4]. Therefore novel strategies for effective treatment are needed. An area of research with promise is the study of reciprocal interactions between cancer cells and the surrounding tumor microenvironment (TME) [5]. For example, cancer cell growth may depend on extracellular matrix (ECM), angiogenesis, and a variety of stromal cells.
In order to understand the stromal mechanisms in prostate cancer development, it may be helpful to characterize the specific cells adjacent to the cancer epithelial cells. Prostate stroma is mainly composed of fibroblasts and smooth muscle cells, and between which is an intermediate cell type described as myofibroblast [6]. During wound repair, fibroblasts switch their phenotype to myofibroblast-like, and remodel the ECM through angiogenesis and increased protease activity. Tumors have similarities with wounds that do not heal in that this stromal reaction also exists with an analogous pattern [7]. These highly proliferative stromal cells immediately surrounding malignant glands have been described as “reactive stroma” [6, 8] or “carcinoma-associated fibroblast” (CAF) [9]. While this may be a very important phenomenon, an effective marker of reactive stroma is not yet available. Because serine proteases can have significant roles in tissue remodeling, including cancer invasion [6, 10], it is possible that the receptors activated by serine proteases (protease-activated receptors, PARs) may be potential regulators of the reactive stroma.
PARs are G-protein-coupled receptors that are implicated in cancer progression by regulating multiple cellular processes. PARs are activated via cleavage of their extracellular amino terminus by serine proteases: PAR-1, -3 and -4 by thrombin and PAR-2 by trypsin [11, 12]. PARs, especially PAR-1 and PAR-2, are overexpressed in several malignancies, including breast cancer [13, 14], colon cancer [15, 16], gastric cancer [17, 18] and prostate cancer [19, 20]. Once activated, PARs stimulate cancer progression through a variety of mechanisms. For example, PAR-1 and PAR-2 promote colon cancer [15, 16] and gastric cancer [17] cell proliferation by transactivating epidermal growth factor receptor (EGFR). In prostate cancer, PAR-1 and PAR-2 stimulation results in the activation of RhoA and Rac1/Cdc42 signaling with resultant cytoskeletal changes and enhanced migration in the LNCaP cells [19, 21]. PAR-1 and PAR-2 also can upregulate oncogenic genes such as vascular endothelial growth factor (VEGF) [22-24]. Furthermore, increased PAR-1 and PAR-2 expression is observed in proliferating stromal fibroblasts surrounding the breast carcinoma cells but not in normal or benign tissues [14], indicating PARs in stromal fibroblasts may also contribute to carcinogenesis.
We previously found that overexpressed PAR-1 in the prostate cancer peritumoral stroma is associated with higher rates of biochemical recurrence [19]. However, another recent study reported that PAR-1 was expressed predominantly on endothelial cells but not in tumor stroma in prostate cancer [20]. Therefore, comprehensive characterization of PAR-1 localization in prostate cancer would be beneficial in clarifying the stromal expression of PAR-1. With regards to metastatic prostate cancer, Chay et al. [25] reported the increased PAR-1 expression in the cell lines derived from bone metastasis, but the in situ expression of PARs in bone metastasis was not studied. Since PAR-1 and PAR-2 may facilitate cancer dissemination, we applied additional immunohistochemical markers in this study to further characterize PAR-1 and PAR-2 localization in normal, primary prostate cancer and the corresponding bone metastatic tissues.
MATERIALS AND METHODS
Patients
Tissue samples used in this study were ten histologically normal prostates obtained at autopsy with no history of reproductive or endocrine-related diseases, and twenty four patients who died from advanced prostate cancer with bone metastasis between 1998 and 2004. Rapid autopsies were performed under the aegis of the Prostate Cancer Donor Program at the University of Washington Medical Center (UWMC) as previously described [3]. Primary prostate cancer tissue and bone metastatic tissue from the same patient were analyzed. The clinical information obtained for each patient including age at diagnosis, Gleason score, final serum PSA level, androgen independence years, and intervals of time after diagnosis to first bone metastasis is summarized in table I.
Table I.
Patients Clinical Data
| Clinical Data | No. of cases (%) | mean |
|---|---|---|
|
| ||
| Age at Diagnosis (years) | 63.5 | |
| <65 | 14 (58.3%) | |
| ≥65 | 10 (41.7%) | |
| Final Serum PSA (ng/mL) | 597.95 | |
| <300 | 9 (37.5%) | |
| ≥300 | 15 (62.5%) | |
| Gleason Score | 7.9 | |
| 4-6 | 5 (20.8%) | |
| 7-10 | 19 (79.2%) | |
| Androgen Independence (years) | 1.94 | |
| <1.5 | 13 (54.2%) | |
| ≥1.5 | 11 (45.8%) | |
| Androgen Ablation Duration (years) | 3.75 | |
| <4 | 13 (54.2%) | |
| ≥4 | 11 (45.8%) | |
| Survival (years) | 5.4 | |
| <5 | 14 (58.3%) | |
| ≥5 | 10 (41.7%) | |
| Bone metastasis delay from diagnosis (years) | 3.33 | |
| <3 | 14 (58.3%) | |
| ≥3 | 10 (41.7%) | |
Tissues
Tissue samples were routinely fixed in 10% buffered formalin for 2 days and embedded in paraffin. Following fixation, bone samples were decalcified in a 10% formic acid solution until an assay for free calcium in solution was negative, and then processed for paraffin embedding. Three micrometer (primary tissue) or five micrometer (bone tissue) serial sections from each block were cut on a microtome (Reichert-Jung/Leica, Wetzlar, Germany) and mounted on pre-charged slides (VWR Scientific, West Chester, PA). After baking for 2 hours in a 58°C incubator, sections were deparaffinized and rehydrated in xylene and a series of graded alcohols.
Antibodies
Mouse anti-human PAR-1 monoclonal (clone number ATAP2, dilution 1:50), mouse anti-human PAR-2 monoclonal (SAM11, 1:50) and normal mouse IgG antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-human α-smooth muscle actin (α-SMA) monoclonal (1A4, 1:200) and mouse anti-human desmin monoclonal (DE-U-10, 1:150) antibodies were from Sigma (St. Louis, MO). Mouse anti-human vimentin monoclonal (VIM 3B4, 1:200), mouse anti-human CD34 monoclonal (QBEnd-10, 1:200), and mouse anti-human proliferating cell nuclear antigen (PCNA) monoclonal (PC 10, 1:400) antibodies were from Dako (Glostrup, Denmark).
Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously described in detail [26]. To accurately characterize PAR-1 and PAR-2 positive cells, double-label IHC was performed to simultaneously detect PAR-1 or PAR-2 expression with combination of the proliferation marker (PCNA), α-smooth muscle actin (SMA) and desmin. Briefly, after antigen retrieval, sections were incubated with avidin/biotin blocking solution (Vector Laboratories, Burlingame, CA) to block endogenous biotin activity. After incubation with 5% normal horse-chicken-goat serum (Vector Laboratories) for 1 hour, the sections were incubated with appropriately diluted primary antibodies at 4°C overnight and then incubated with biotinylated secondary antibodies at room temperature for 30 minutes. For visualization, avidin-biotin complex (Vectastain Elite ABC kit, Vector Laboratories) was applied at room temperature for 30 minutes followed by using either SG or DAB (Vector Laboratories) as the chromogen. Before the second stain, 3% hydrogen peroxide was used to quench the remaining horseradish peroxidase, and avidin/biotin solution was used to block the remaining biotin activity. Sections were incubated with 5% normal serum for 1 hour, and then with the second primary antibody for 1 hour at room temperature, followed by incubation with biotinylated secondary antibodies and amplification with avidin-biotin complex. Sections was developed using DAB or SG as the chromogen. Positive stained cells exhibited the deposition of gray precipitate (SG) and/or brown precipitate (DAB). To better differentiate the two reaction products, no counterstaining was performed in double-label IHC.
Result evaluation and Statistical analysis
For immunohistochemical assessment, immunoreactions were evaluated manually based on the extent and intensity of staining. The proportion score was assigned to represent the extent of positive stained cells (0, none; 1, <10%; 2, 10%-50%; and 3, ≥50%). The intensity score was then assigned according to the average staining intensity of positive cells (0, none; 1, weak; 2, moderate; and 3, strong) [26, 27]. A total immunoreactivity score was calculated by addition of proportion score and intensity score [28]. Statistical analysis was performed using the chi-square test to assess the correlation between PAR-1 expression and Gleason pattern, and using Wilcoxon non-parametric test to evaluate the difference of PAR-1 or PAR-2 expression in normal, primary prostate cancer and bone metastasis. p<0.05 or p<0.01 was considered statistically significant.
RESULTS
PAR-1 expression in primary prostate cancer and bone metastasis
Localization of PAR-1 in primary prostate cancer
We performed IHC in tissues from 10 cases of normal prostate and 24 cases of prostate cancer. In normal prostate, PAR-1 was not or very weakly expressed in epithelial and stromal cells (Fig. 1A). PAR-1 immunolabeling was observed in most vascular endothelial cells (Fig. 1B). In primary cancer, PAR-1 expression was increased both in the epithelial and stromal compartments. In 20/24 (83.3%) of the primary cancer tissues, PAR-1 was highly expressed in stromal cells surrounding the malignant glands, especially the high Gleason grade glands (Fig. 1C, D). As a control, stromal cells immediately surrounding the benign glands did not overexpress PAR-1 (Fig. 1E, F). Besides these peritumoral stromal cells, PAR-1 was also expressed in some stromal cells in interstitial area (Fig. 1G). Consistent with our previous report [19], PAR-1 expression was also observed in some high Gleason grade cancers (Fig. 1H), and most of the low grade cancers (Fig. 1I). The specificity of these findings was confirmed using mouse IgG as a negative control (Fig. 1J).
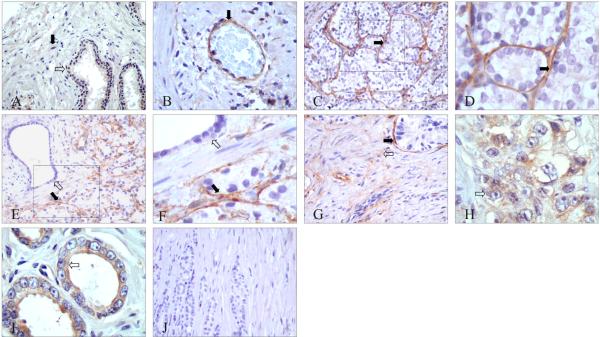
Figure 1. Representative immunohistochemical localization of PAR-1 in normal and primary prostate tumor tissues.
A: No or very weak PAR-1 expression in epithelial cells (indicated by white arrow) and stromal cells (black arrow) of normal prostate tissues. B: PAR-1 expression in endothelial cells (black arrow). C and D: In prostate cancer, peritumoral stromal cells surrounding high Gleason pattern glands showed predominant PAR-1 immunostaining (black arrow). E and F: Compared with benign glands nearby (white arrow), malignant glands had specific PAR-1 positive stromal cells around (black arrow). G: Besides peritumoral stromal cells (black arrow), PAR-1 was also expressed in some stromal cells in interstitial area (white arrow). H: PAR-1 immunostaining in high Gleason pattern glands (white arrow). I: PAR-1 immunostaining in low Gleason pattern glands (white arrow). J: Normal mouse IgG used as a negative control.
Original magnification: A, C, E, G and J ×200; B ×400; D, F, H, and I ×600.
PAR-1 is upregulated in reactive stroma of primary prostate cancer
To better define these PAR-1 positive peritumoral stromal cells, we further characterized these cells using immunohistochemical markers: vimentin, α-SMA and desmin. Expression of vimentin (a marker for cells of mesenchymal origin) [29] without α-SMA indicates a fibroblast phenotype [30]. Co-expression of α-SMA and desmin (a late stage smooth muscle marker) indicates smooth muscle cells. Co-expression of vimentin and α-SMA without expression of desmin is indicative of a myofibroblast phenotype, an intermediate between fibroblasts and smooth muscle cells [30]. IHC on serial sections showed that most of these peritumoral stromal cells were positive for PAR-1 (Fig. 2A), vimentin (Fig. 2B), α-SMA (Fig. 2C), but not desmin (Fig. 2D). This indicates that these PAR-1 expressing peritumoral stromal cells are mostly myofibroblasts, which have been defined as the main cell type in “reactive stroma” [30, 31]. To further confirm this finding, we performed double-label IHC on serial sections. Consistently, double IHC showed most of these peritumoral stromal cells were PAR-1 (+)/desmin (-) (Fig. 2E), but PAR-1 (+)/α-SMA (+) (Fig. 2F). As endothelial cells also express PAR-1, we then compared the expression of PAR-1 with CD34, an endothelial antigen [32]. As shown in figure 2G and 2H, CD34 labeled the endothelial cells in isolated capillaries around the malignant glands, but these endothelial cells are only a small portion of the PAR-1 positive stromal cells. We also performed double IHC using antibodies to PAR-1 and PCNA, which labels the nuclei of proliferating cells [33]. Co-localization of PAR-1 and PCNA was observed in reactive stromal cells (Fig. 2I). Taken together, these data demonstrated that PAR-1 is highly expressed in reactive stroma of prostate cancer.
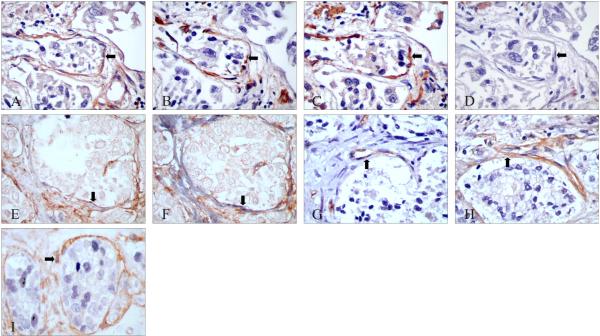
Figure 2. Immunohistochemical characterization of PAR-1 localization in reactive stroma.
A-D: Serial sections stained with PAR-1, vimentin, α-SMA and desmin antibodies, respectively. The peritumoral stromal cells were PAR-1 positive (A, black arrow), vimentin positive (B), α-SMA positive (C) but desmin negative (D). E*, F*: Serial sections with double staining of PAR-1 (brown) & desmin (gray) (E), and PAR-1 (brown) & α-SMA (gray) (F). The peritumoral stromal cells were PAR-1 and α-SMA positive, but desmin negative (black arrows). G, H: Serial sections stained with CD34 (G, black arrow) or PAR-1 (H, black arrow). I*: Co-expression of PAR-1 (in brown) and PCNA (in gray) in reactive stromal cells (black arrow).
Original magnification: A to I ×400
*No counterstaining was performed in double-label IHC.
As we observed that PAR-1 tends to be expressed in reactive stromal cells surrounding higher Gleason pattern glands than lower ones, we randomly chose 523 individual cancer glands to investigate the correlation between PAR-1 expression and Gleason patterns. As summarized in table II, PAR-1 expression was significantly increased in reactive stromal cells around higher Gleason grade glands (patterns 4-5) than lower ones (patterns 1-3) (81.7% vs. 18.3%). In cancer cells, PAR-1 expression was significantly increased in lower Gleason grade glands than higher ones (79.7% vs. 20.3%).
Table II.
Correlation of Gleason patterns with PAR-1 Expression in Malignant Glands and the Surrounding Reactive Stroma
| PAR-1 Expression |
|||
|---|---|---|---|
| Carcinoma Cells | Surrounding Reactive Stroma | Total | |
| Lower Grade Glands* | 212 | 47 | 259 |
|
| |||
| Higher Grade Glands** | 54 | 210 | 264 |
|
| |||
| Total | 266 | 257 | 523 |
Glands with Gleason patterns 1~3
Glands with Gleason patterns 4~5
X2= 197.2012, p<0.01
PAR-1 expression is elevated in bone metastasis
We further studied PAR-1 expression in bone metastasis. PAR-1 was expressed both in metastatic cancer cells and bone stromal cells (Fig. 3A, B). In 14/24 (58.3%) of bone metastasis, PAR-1 immunoreactivity in cancer cells (Fig. 3B) was increased compared to the primary cancer tissue from the same patient (Fig. 3C). We further analyzed this elevation by comparing the PAR-1 staining intensity scores. The result showed PAR-1 expression in cancer cells was increased in bone metastasis than the primary cancer tissues (p<0.01) (Fig. 3D), suggesting PAR-1 may play a role in prostate cancer bone metastasis. As vascular endothelial cells contribute to the formation of a favorable cancer cell bone microenvironment, we compared PAR-1 with CD34 by serial section staining. PAR-1 was expressed in both cancer cells and bone stromal cells (Fig. 3E), while CD34 labeled some of the PAR-1 positive stromal cells but not cancer cells (Fig. 3F). These data suggest that PAR-1 expression in reactive stroma in the bone is predominantly composed of endothelial cells and fibroblasts. We also observed that PAR-1 was apparently expressed in osteoclasts (Fig. 3A) and osteoblasts (Fig. 3G), which are the essential cells to the bone reaction in metastasis [34].
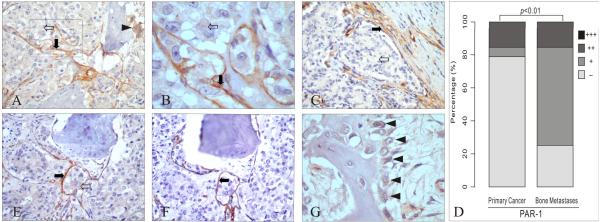
Figure 3. PAR-1 expression in prostate cancer bone metastasis.
A, B: PAR-1 expression in cancer cells (white arrow), bone stromal cells (black arrow) and osteoclasts (arrowhead). C: PAR-1 expression in primary prostate cancer tissue from the same patient of figure 3A and 3B: epithelial cells were PAR-1 negative (white arrow) while stromal cells were positive (black arrow). D: The intensity of PAR-1 staining in cancer cells was increased in bone metastasis compared to the primary cancer in the same patient (p < 0.01). E, F: Serial sections of bone metastasis stained with PAR-1 and CD34: PAR-1 (E) was expressed in cancer cells (white arrow) and bone stromal cells (black arrow), while CD34 (F) was expressed only in endothelial cells (black arrow). G: PAR-1 expression in osteoblasts (black arrowheads).
Original magnification: A, C, E and F ×200; B ×600; G: ×400.
PAR-2 expression in primary prostate cancer and bone metastasis
Similar to PAR-1, very weak PAR-2 immunolabeling was observed in normal prostate tissues (Fig. 4A). In primary cancer, PAR-2 was uniformly and strongly expressed in cancer cells in both low and high Gleason grade glands (Fig. 4B, C). PAR-2 was distinctly increased in malignant glands compared to benign glands (Fig. 4D-F). PAR-2 expression in 24 primary prostate cancer specimens was significantly elevated compared to 10 normal prostate specimens (p<0.01). PAR-2 was also present in some stromal cells, but without the peritumoral characteristics as observed for PAR-1 (Fig. 4G). Double IHC showed that these PAR-2 positive stromal cells are mostly smooth muscle cells by expressing α-SMA (Fig. 4H). Unlike PAR-1, PAR-2 was not expressed in the endothelial cells (Fig. 4I).
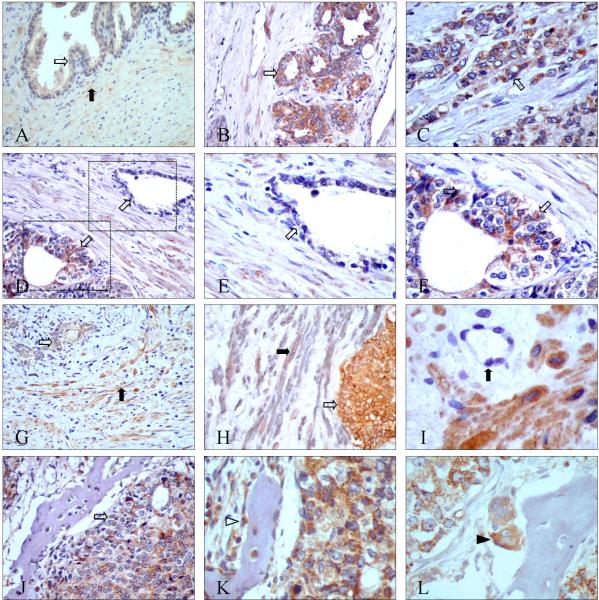
Figure 4. Representative immunohistochemical localization of PAR-2 in normal, primary prostate cancer, and bone metastasis.
A: Normal prostate tissues showed weak PAR-2 expression in epithelial cells (white arrow) and stromal cells (black arrow); B, C: PAR-2 expression in epithelial cancer cells (white arrows) in low Gleason pattern glands (B) and high Gleason pattern glands (C); D-F: PAR-2 was highly expressed in malignant glands (bottom-left corner of D, magnified in figure F) but not in benign glands (top-right corner of D, magnified in figure E); G: Besides cancer cells (white arrow), PAR-2 expression was also observed in some stromal cells (black arrow); H*: Double IHC of PAR-2 (in brown) and α-SMA(in gray): cancer cells were PAR-2 positive (white arrow), and some of the stromal cells co-expressed PAR-2 and α-SMA(black arrow); I: PAR-2 was not present in endothelial cells (black arrow); J: PAR-2 was highly expressed in cancer cells in bone metastasis (white arrow); K: PAR-2 was present in osteoblasts (white arrowhead); L: PAR-2 was present in osteoclasts (black arrowhead).
Original magnification: A, B, D, G, and J ×200; C, E, F, H, K, and L×400; I×600
*No counterstaining was performed in double-label IHC.
In bone metastasis, PAR-2 was consistently and highly expressed in cancer cells (Fig. 4J). Compared to normal prostate tissues, PAR-2 expression in bone metastasis was dramatically increased (p<0.01), while no significant difference was found when compared to the primary cancers (p>0.05). Similar to PAR-1, PAR-2 immunolabeling was also observed in osteoblasts (Fig. 4K) and osteoclasts (Fig. 4L).
Differential Expression of PAR-1 and PAR-2
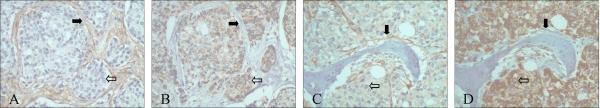
As described above, the expression pattern of PAR-1 and PAR-2 appears to be different, especially in high Gleason grade cancers. Therefore, we compared PAR-1 and PAR-2 localization in advanced prostate cancer and bone metastasis by serial sections. In primary cancer with high Gleason grade, PAR-1 was localized predominantly in reactive stroma (Fig. 5A), while PAR-2 was in cancer cells (Fig. 5B). Similarly, in bone metastasis, PAR-1 was highly expressed in reactive stroma and to a lesser extent in cancer cells (Fig. 5C), while PAR-2 was strongly expressed in cancer cells (Fig. 5D). This differential expression pattern of PAR-1 and PAR-2 suggests they may play different roles in prostate cancer progression.
Figure 5. Differential Expression of PAR-1 and PAR-2.
A, B: Serial sections of primary prostate cancer with high Gleason pattern stained with PAR-1 and PAR-2: PAR-1 (A) was localized in reactive stroma (black arrow) but not in epithelial cancer cells (white arrow); while PAR-2 (B) was present in cancer cells (white arrow) but not in reactive stroma (black). C, D: Serial sections of bone metastasis stained with PAR-1 and PAR-2: PAR-1 (C) was highly expressed in reactive stroma (black arrow) and lightly expressed in epithelial cancer cells (white arrow); while PAR-2 (D) was highly present in cancer cells (white arrow) but not in reactive stroma (black).
Original magnification: A-D ×200
DISUCSSION
One of the possible mechanisms of prostate cancer progression may be due to the complex interplay between the cancer epithelium and the surrounding stromal cells. Instead of having contact inhibition to the cancer cells, the adjacent stromal cells may actually be promoting cancer growth through the release of growth factors. But how these stromal cells acquire mitogenic properties is mostly unknown. It is possible that cancer cells may be stimulating stromal cells via expression of soluble proteases [10, 35, 36]. Recently, there has been increased attention on PAR-1, which is over-expressed in prostate cancer cells and peritumoral stromal cells. Therefore, the analysis of PAR-1 expression in stromal cells associated with prostate cancer may add unique information regarding their roles in prostate cancer progression. Here we show that PAR-1 is upregulated in reactive stroma in primary prostate cancer and also in bone metastatic tissues.
The exact nature of PAR-1 expression in prostate cancer is not fully understood. We previously observed that PAR-1 is expressed predominantly in the stromal cells surrounding malignant glands [19], whereas Kaushal and colleagues reported that PAR-1 expression is localized to endothelial cells but not tumor stroma [20]. However, their conclusion was not based on any characterization of the PAR-1 expressing cells with immunohistochemical markers for stromal cells. In their samples (their Fig. 4), the positive stromal expression may have been misinterpreted as background staining because of weaker staining [20]. Our staining technique may have provided a more clear stromal PAR-1 expression. In this study, we further confirmed our previous observation that PAR-1 expression in prostate cancer is predominantly in tumor stroma. Moreover, the positive expression of α-SMA, vimetin and PCNA but not desmin by these PAR-1 positive stromal cells shows a myofibroblast phenotype, the primary cell type in reactive tumor stroma [6]. Reactive stroma has been observed in various types of malignancies, including breast, lung, colon, gastric and prostate [7]. These cells are associated with all stages of cancer progression by increasing the expression of some extracellular matrix glycoproteins and growth factors. For example, myofibroblasts derived from invasive breast carcinomas enhance the growth of tumor cells by secreting stromal cell-derived factor-1 (SDF-1) [37]. In prostate cancer, the cancer associated fibroblasts have been shown to promote tumor growth in a mice tissue recombination study by Olumi et al. [9]. There are several potential markers of the activated fibroblast phenotype, including tenascin-C in breast cancer [38] and fibroblast-activation protein in colon cancer [39, 40]. In the current study, the PAR-1 expression by these cells suggests that PAR-1 could also be a potential new marker for the reactive stroma in prostate cancer.
The expression of PAR-1 and PAR-2 in epithelial cancers (including breast, gastric, and lung) as well as the surrounding stromal fibroblasts has been previously reported by D'Andrea et al, but prostate cancer was not evaluated [14]. Here, it was determined that PAR-1, but not PAR-2, is expressed in the reactive stroma of primary prostate cancer as well as bone metastasis. Moreover, PAR-1 expression is significantly increased in the reactive stroma around higher Gleason grade glands than lower ones. This association of reactive stroma and high grade disease is consistent with a previous study, in which Tuxhorn et al. reported the proportion of myofibroblasts in reactive stroma increases from moderately differentiated to poorly differentiated prostate carcinoma [31]. This could be a unique finding for prostate cancer, in part due to the fact that prostate epithelium produces significant amount of serine proteases. D'Andrea et al. theorized that in many epithelial cancers, the TME produces tissue factor, which in combination with coagulation factor VIIa may activate the serine protease, thrombin, the main physiologic activator of PAR-1 [14]. Our recent data on prostate cancer epithelial cells demonstrates a different mechanism whereby prostate-associated serine proteases (such as kallikreins-2, -4) can directly activate PAR-1 and/or PAR-2 on prostate cancer cells [41]. The consequence of this stimulation includes activation of RhoA signaling which leads to cytoskeletal changes and increase migration [19, 42]. Therefore the presence of PAR-1 may be important for both the cancer epithelium and the surrounding stromal cells.
In contrast to PAR-1, PAR-2 may have a separate biological role in prostate cancer. First, the expression pattern is different. We found that PAR-2 is expressed in cancer cells and some smooth muscle cells, but not present in the reactive stroma. Secondly, the specific agonist-activator(s) may be different. Although PAR-2 is typically activated by trypsin and mast cell tryptase [12], the widespread tissue distribution of PAR-2 indicates that more activating proteases for PAR-2 could be found in the TME. Recent studies suggest some trypsin-like serine proteases, such as kallikreins and TMPRSS2, could be endogenous activators of PAR-2 [36, 43, 44]. We recently reported that both hK2 and hK4 can activate PAR-2 and increase DU-145 prostate cancer cells proliferation [41], suggesting an important role of PAR-2 in prostate cancer growth. Therefore, PAR-1 may be a stromal activator while PAR-2 may be an activator for prostate cancer epithelium.
Bone metastasis is a critical problem because it is the second most frequent site of prostate cancer metastasis and effective treatment is not yet available [45, 46]. One potential strategy may be to utilize the finding that PAR-1 is over-expressed by bone metastatic tissue. Functional PAR-1 has been shown to be overexpressed by a cell line derived from bone metastasis [25]. But the expression of PAR-1 in human prostate metastatic tissue had not been previously reported, to our knowledge. We found that PAR-1 is upregulated in bone metastatic tissue compared to the primary sites on the same patient in the majority of cases (14/24). The increased PAR-1 may be an important mediator of bone metastasis but the mechanism is complex. In particular, we found that both osteoblasts (bone forming cells) and osteoclasts (bone lysis cells) express PAR-1. On one hand, osteoblasts may be stimulated through PAR-1 while osteoclasts may paradoxically be inhibited. Song et al. showed that osteoblasts will proliferate when stimulated with thrombin [47]. In contrast, PAR-1 knock out mice exhibit increased osteoclasts suggesting that PAR-1 is inhibitory to osteoclast differentiation [48]. Clearly, further research is required to determine the mechanism of PAR-1 mediated bone metastasis.
In summary, this study showed that PAR-1 and PAR-2 are upregulated in primary prostate cancer and bone metastasis. Our finding of PAR-1 overexpression in reactive stroma supports the hypothesis that the interactions between cancer cells and the surrounding TME play important roles during tumor progression. In contrast to PAR-1, PAR-2 has less demonstrable change at the metastatic sites compared to the primary sites. The differential expression of PAR-1 and PAR-2 in prostate cancer may also suggest their different roles in prostate cancer development.
ACKNOWLEDGMENTS
We thank Dr. Paul H. Lange (Department of Urology, University of Washington) for his support of our prostate cancer research. We also thank Dr. Colm Morrissey and Greg Mize (Department of Urology, University of Washington) for critical reading of this manuscript, and Jennifer Noteboom and Adam Van Mason (Department of Urology, University of Washington) with the protocols for tissue preparation.
This work was supported in part by grants from the SMT foundation, Department of Defense (DAMD17-03-01-0039), the National Institutes of Health -Prostate Cancer Program Project (5P01CA085859-05) and Pacific Northwest Prostate Cancer SPORE CA97186.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri MB, Tu SM, Yu-Lee LY, Lin SH. The central role of osteoblasts in the metastasis of prostate cancer. Cancer Metastasis Rev. 2006;25:601–609. doi: 10.1007/s10555-006-9034-y. [DOI] [PubMed] [Google Scholar]
- 5.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 6.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 7.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 8.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 9.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70:506–521. doi: 10.1046/j.1432-0436.2002.700905.x. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 12.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 13.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 14.D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001;158:2031–2041. doi: 10.1016/S0002-9440(10)64675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol. 2003;162:1503–1513. doi: 10.1016/S0002-9440(10)64283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: trypsin- induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- 17.Caruso R, Pallone F, Fina D, Gioia V, Peluso I, Caprioli F, Stolfi C, Perfetti A, Spagnoli LG, Palmieri G, Macdonald TT, Monteleone G. Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation. Am J Pathol. 2006;169:268–278. doi: 10.2353/ajpath.2006.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto D, Hirono Y, Goi T, Katayama K, Hirose K, Yamaguchi A. Expression of protease activated receptor-2 (PAR-2) in gastric cancer. J Surg Oncol. 2006;93:139–144. doi: 10.1002/jso.20420. [DOI] [PubMed] [Google Scholar]
- 19.Black PC, Mize GJ, Karlin P, Greenberg DL, Hawley SJ, True LD, Vessella RL, Takayama TK. Overexpression of protease-activated receptors-1,-2, and-4 (PAR-1, -2, and -4) in prostate cancer. Prostate. 2007;67:743–756. doi: 10.1002/pros.20503. [DOI] [PubMed] [Google Scholar]
- 20.Kaushal V, Kohli M, Dennis RA, Siegel ER, Chiles WW, Mukunyadzi P. Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2006;66:273–282. doi: 10.1002/pros.20326. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg DL, Mize GJ, Takayama TK. Protease-activated receptor mediated RhoA signaling and cytoskeletal reorganization in LNCaP cells. Biochemistry. 2003;42:702–709. doi: 10.1021/bi027100x. [DOI] [PubMed] [Google Scholar]
- 22.Yin YJ, Salah Z, Maoz M, Ram SC, Ochayon S, Neufeld G, Katzav S, Bar-Shavit R. Oncogenic transformation induces tumor angiogenesis: a role for PAR1 activation. FASEB J. 2003;17:163–174. doi: 10.1096/fj.02-0316com. [DOI] [PubMed] [Google Scholar]
- 23.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Mueller BM. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun. 2006;344:1263–1270. doi: 10.1016/j.bbrc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Chay CH, Cooper CR, Gendernalik JD, Dhanasekaran SM, Chinnaiyan AM, Rubin MA, Schmaier AH, Pienta KJ. A functional thrombin receptor (PAR1) is expressed on bone-derived prostate cancer cell lines. Urology. 2002;60:760–765. doi: 10.1016/s0090-4295(02)01969-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Zhang X, Mize GJ, Takayama TK. Protease-activated receptor-1 upregulates fibroblast growth factor 7 in stroma of benign prostatic hyperplasia. Prostate. 2008;68:1064–75. doi: 10.1002/pros.20767. [DOI] [PubMed] [Google Scholar]
- 27.Zeng G, Hu Z, Kinch MS, Pan CX, Flockhart DA, Kao C, Gardner TA, Zhang S, Li L, Baldridge LA, Koch MO, Ulbright TM, Eble JN, Cheng L. High-level expression of EphA2 receptor tyrosine kinase in prostatic intraepithelial neoplasia. Am J Pathol. 2003;163:2271–2276. doi: 10.1016/S0002-9440(10)63584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 29.Azumi N, Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987;88:286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- 30.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–4780. [PubMed] [Google Scholar]
- 31.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 32.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 33.Garcia RL, Coltrera MD, Gown AM. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989;134:733–739. [PMC free article] [PubMed] [Google Scholar]
- 34.Morrissey C, Vessella RL. The role of tumor microenvironment in prostate cancer bone metastasis. J Cell Biochem. 2007;101:873–886. doi: 10.1002/jcb.21214. [DOI] [PubMed] [Google Scholar]
- 35.Loberg RD, Gayed BA, Olson KB, Pienta KJ. A paradigm for the treatment of prostate cancer bone metastasis based on an understanding of tumor cell-microenvironment interactions. J Cell Biochem. 2005;96:439–446. doi: 10.1002/jcb.20522. [DOI] [PubMed] [Google Scholar]
- 36.Borgoño CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 37.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 38.Mackie EJ, Chiquet-Ehrismann R, Pearson CA, Inaguma Y, Taya K, Kawarada Y, Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A. 1987;84:4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, Chen WT, Cheng JD. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:173617–41. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 41.Mize GJ, Wang W, Takayama TK. Prostate-specific kallikreins-2,-4 enhance proliferation of DU145 prostate cancer cells through PAR-1 and PAR-2. Mol Cancer Res. 2008;6:1043–1051. doi: 10.1158/1541-7786.MCR-08-0096. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg DL, Mize GJ, Takayama TK. Protease-activated receptor mediated RhoA signaling and cytoskeletal reorganization in LNCaP cells. Biochemistry. 2003;42:702–709. doi: 10.1021/bi027100x. [DOI] [PubMed] [Google Scholar]
- 43.Wilson S, Greer B, Hooper J, Zijlstra A, Walker B, Quigley J, Hawthorne S. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem J. 2005;388:967–972. doi: 10.1042/BJ20041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsay AJ, Dong Y, Hunt ML, Linn M, Samaratunga H, Clements JA, Hooper JD. Kallikrein-related peptidase (KLK) 4 initiates intracellular signalling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J Biol Chem. 2008;283:12293–12304. doi: 10.1074/jbc.M709493200. [DOI] [PubMed] [Google Scholar]
- 45.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 46.Roudier MP, Vesselle H, True LD, Higano CS, Ott SM, King SH, Vessella RL. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin Exp Metastasis. 2003;20:171–180. doi: 10.1023/a:1022627421000. [DOI] [PubMed] [Google Scholar]
- 47.Song SJ, Pagel CN, Pike RN, Mackie EJ. Studies on the receptors mediating responses of osteoblasts to thrombin. Int J Biochem Cell Biol. 2005;37:206–213. doi: 10.1016/j.biocel.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Song SJ, Pagel CN, Campbell TM, Pike RN, Mackie EJ. The role of protease-activated receptor-1 in bone healing. Am J Pathol. 2005;166:857–868. doi: 10.1016/S0002-9440(10)62306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]