Abstract
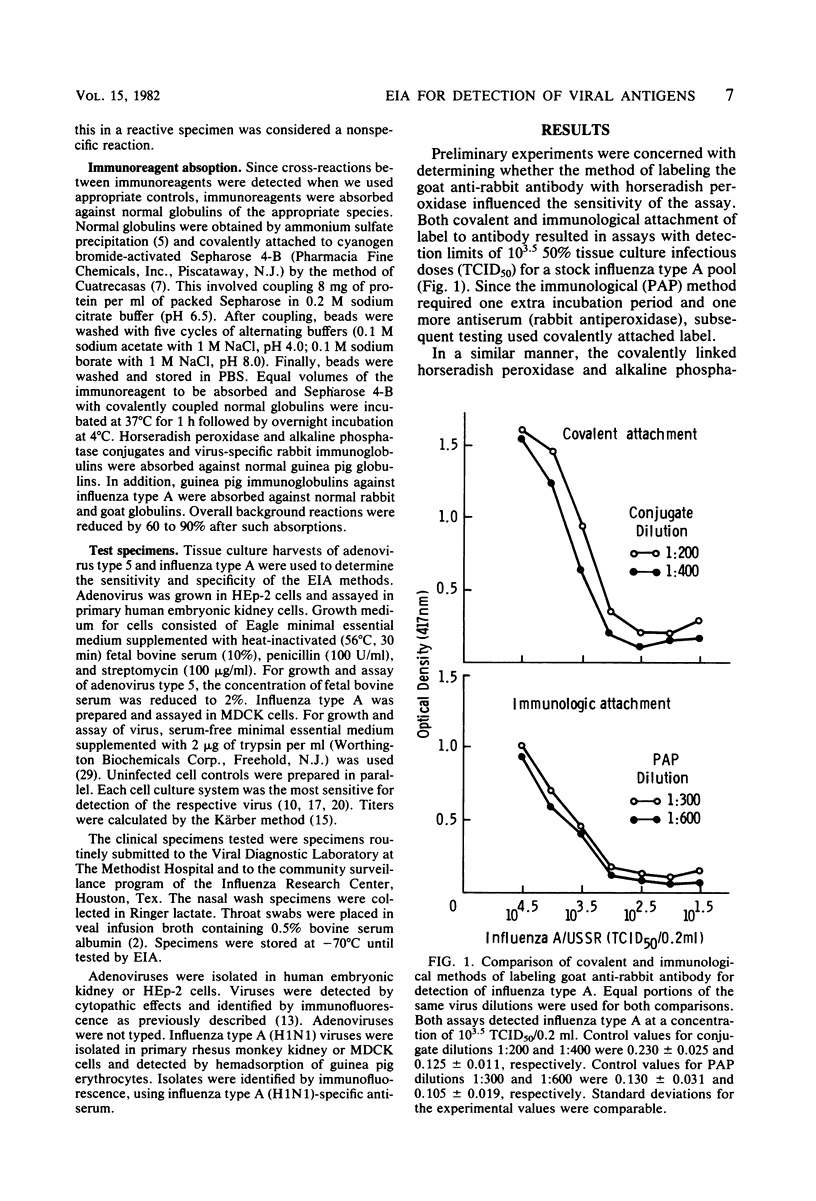
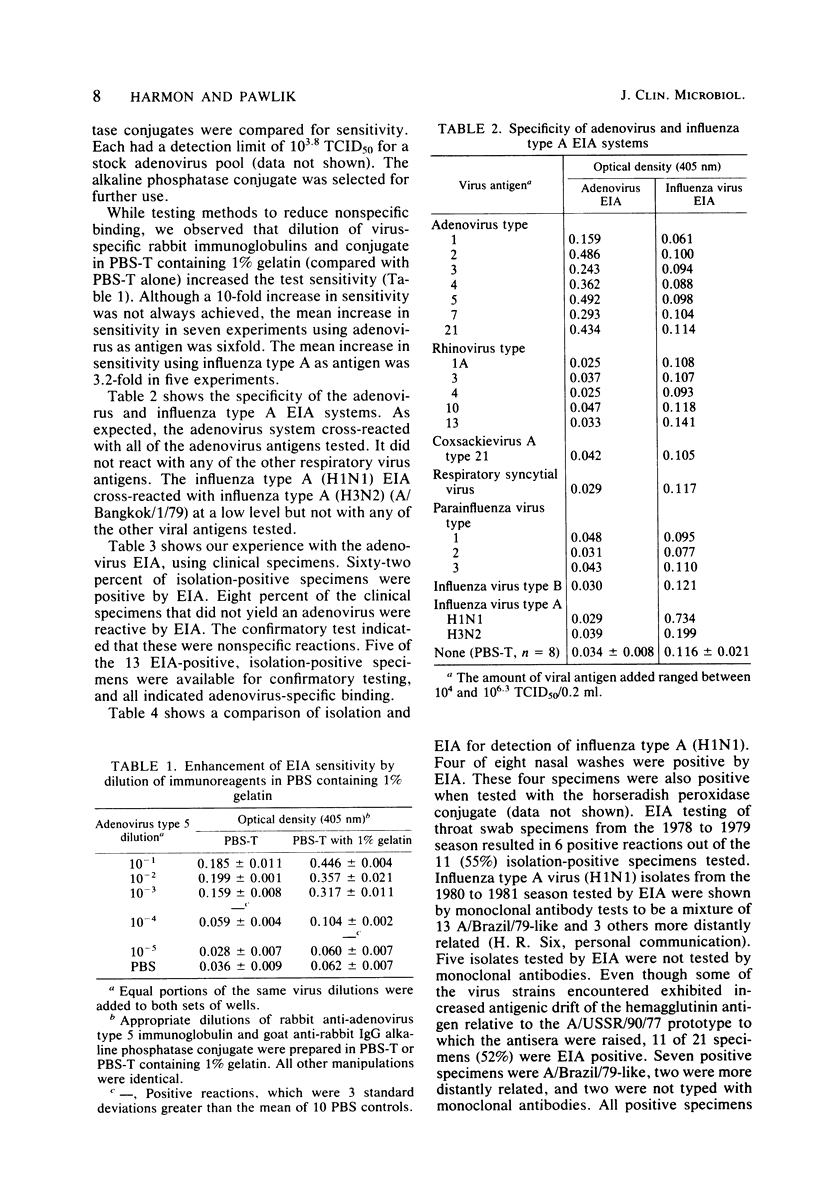
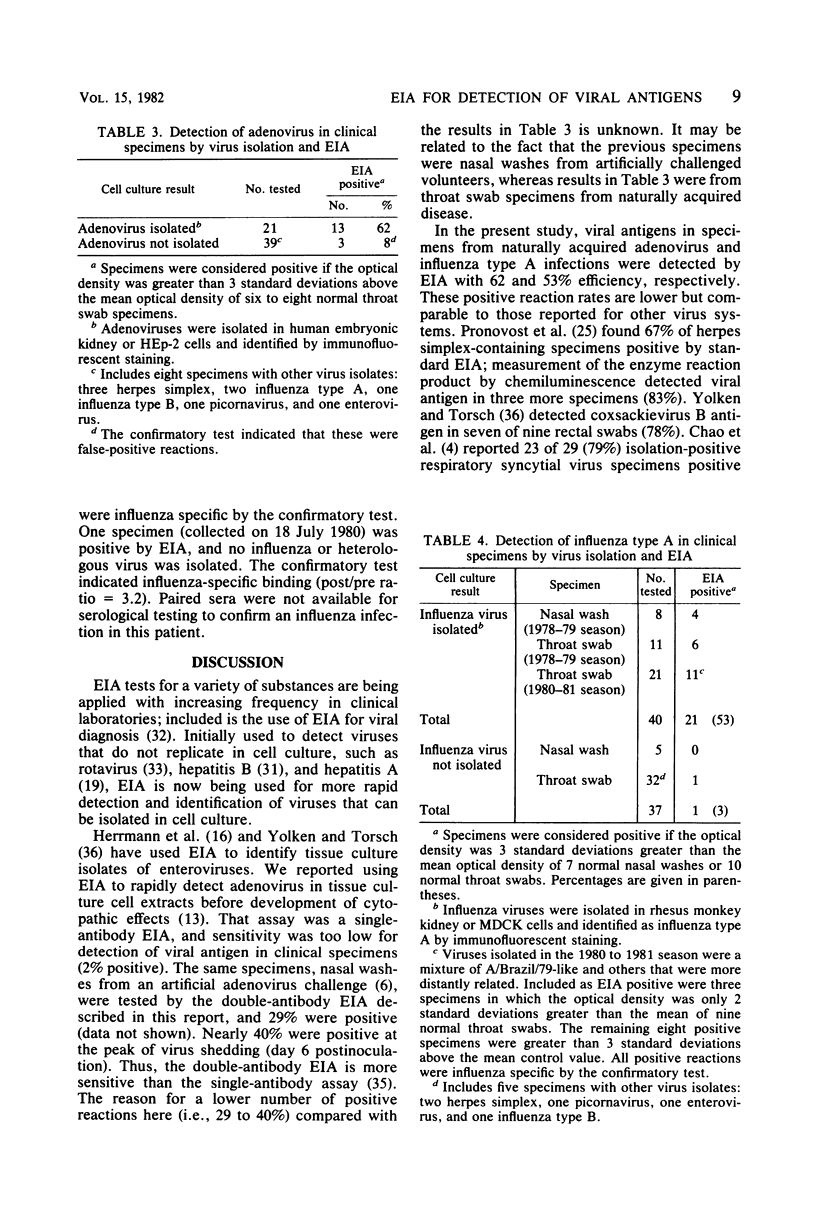
Detection of viral antigens in specimens without prior cultivation in cell culture provides the most rapid method for specific viral diagnosis. A solid-phase, double-antibody enzyme immunoassay was developed for this purpose and tested with clinical specimens containing influenza type A and adenovirus. Polystyrene microtiter wells were the solid phase and were coated with virus-specific guinea pig immunoglobulins. Specimens were added, and bound viral antigens were detected by addition of virus-specific rabbit immunoglobulins followed by enzyme-labeled goat antirabbit immunoglobulin G. Two methods of labeling goat anti-rabbit immunoglobulin G with horseradish peroxidase were investigated: covalent attachment and a noncovalent, immunological binding of antibody to enzyme, the peroxidase-antiperoxidase method. Both methods of labeling resulted in assays that could detect 10(3.5) 50% tissue culture infectious doses of influenza type A and 10(3.8) 50% tissue culture infectious doses of adenovirus. Equal sensitivity was noted with alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G. An increase in sensitivity of three- to sixfold was achieved when virus-specific rabbit immunoglobulins and conjugate were diluted in 1% gelatin. The solid-phase, double-antibody enzyme immunoassay detected influenza type A and adenovirus in isolation-positive clinical specimens with 53% (21/40) and 62% (13/21) efficiency, respectively. The solid-phase, double-antibody enzyme immunoassay has considerable potential as a practical and rapid method for detection of respiratory viral antigens in nasal wash and throat swab specimens. For optimal value, however, greater sensitivity than was provided by the present methods is desirable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour H. M. Development of an enzyme immunoassay for human placental lactogen using labelled antibodies. J Immunol Methods. 1976;11(1):15–23. doi: 10.1016/0022-1759(76)90014-4. [DOI] [PubMed] [Google Scholar]
- Baxter B. D., Couch R. B., Greenberg S. B., Kasel J. A. Maintenance of viability and comparison of identification methods for influenza and other respiratory viruses of humans. J Clin Microbiol. 1977 Jul;6(1):19–22. doi: 10.1128/jcm.6.1.19-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Yolken R. H., Rennard S. I., Dolin R., Murphy B. R., Straus S. E. New enzyme immunoassays for measurement of influenza A/Victoria/3/75 virus in nasal washes. Lancet. 1980 Apr 19;1(8173):851–853. doi: 10.1016/s0140-6736(80)91356-2. [DOI] [PubMed] [Google Scholar]
- Chao R. K., Fishaut M., Schwartzman J. D., McIntosh K. Detection of respiratory syncytial virus in nasal secretions from infants by enzyme-linked immunosorbent assay. J Infect Dis. 1979 Apr;139(4):483–486. doi: 10.1093/infdis/139.4.483. [DOI] [PubMed] [Google Scholar]
- Couch R. B., Kasel J. A., Perreira H. G., Haase A. T., Knight V. Induction of immunity in man by crystalline adenovirus type 5 capsid antigens. Proc Soc Exp Biol Med. 1973 Sep;143(4):905–910. doi: 10.3181/00379727-143-37438. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Daisy J. A., Lief F. S., Friedman H. M. Rapid diagnosis of influenza A infection by direct immunofluorescence of nasopharyngeal aspirates in adults. J Clin Microbiol. 1979 Jun;9(6):688–692. doi: 10.1128/jcm.9.6.688-692.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Frank A. L., Couch R. B., Griffis C. A., Baxter B. D. Comparison of different tissue cultures for isolation and quantitation of influenza and parainfluenza viruses. J Clin Microbiol. 1979 Jul;10(1):32–36. doi: 10.1128/jcm.10.1.32-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R. E., Middleton P. J. Comparison of immunofluorescence and isolation techniques in the diagnosis of respiratory viral infections of children. Infect Immun. 1974 Jul;10(1):92–101. doi: 10.1128/iai.10.1.92-101.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon M. W., Drake S., Kasel J. A. Detection of adenovirus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979 Mar;9(3):342–346. doi: 10.1128/jcm.9.3.342-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Yolken R. H., Krokan H., Hsu I. C. Ultrasensitive enzymatic radioimmunoassay: application to detection of cholera toxin and rotavirus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5336–5339. doi: 10.1073/pnas.76.10.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Hendry R. M., Collins M. F. Factors involved in enzyme-linked immunoassay of viruses and evaluation of the method for identification of enteroviruses. J Clin Microbiol. 1979 Aug;10(2):210–217. doi: 10.1128/jcm.10.2.210-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C. Rapid diagnosis of human influenza infection from nasal smears by means of fluorescein-labeled antibody. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):883–887. doi: 10.3181/00379727-92-22642. [DOI] [PubMed] [Google Scholar]
- Mathiesen L. R., Feinstone S. M., Wong D. C., Skinhoej P., Purcell R. H. Enzyme-linked immunosorbent assay for detection of hepatitis A antigen in stool and antibody to hepatitis A antigen in sera: comparison with solid-phase radioimmunoassay, immune electron microscopy, and immune adherence hemagglutination assay. J Clin Microbiol. 1978 Feb;7(2):184–193. doi: 10.1128/jcm.7.2.184-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H., Bryant J. D., Torrence A. E., Wright P. F. Canine kidney cell line for isolation of respiratory viruses. J Clin Microbiol. 1979 Feb;9(2):175–179. doi: 10.1128/jcm.9.2.175-179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich L., Ray C. G. Comparison of direct immunofluorescent staining of clinical specimens for respiratory virus antigens with conventional isolation techniques. J Clin Microbiol. 1980 Sep;12(3):391–394. doi: 10.1128/jcm.12.3.391-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Petrali J. P., Hinton D. M., Moriarty G. C., Sternberger L. A. The unlabeled antibody enzyme method of immunocytochemistry. Quantitative comparison of sensitivities with and without peroxidase-antiperoxidase complex. J Histochem Cytochem. 1974 Aug;22(8):782–801. doi: 10.1177/22.8.782. [DOI] [PubMed] [Google Scholar]
- Pronovost A. D., Baumgarten A., Hsiung G. D. Sensitive chemiluminescent enzyme-linked immunosorbent assay for quantification of human immunoglobulin G and detection of herpes simplex virus. J Clin Microbiol. 1981 Jan;13(1):97–101. doi: 10.1128/jcm.13.1.97-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Arstila P. P., Salmi A. A. Detection of respiratory syncytial, parainfluenza type 2, and adenovirus antigens by radioimmunoassay and enzyme immunoassay on nasopharyngeal specimens from children with acute respiratory disease. J Clin Microbiol. 1981 Feb;13(2):258–265. doi: 10.1128/jcm.13.2.258-265.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- Wolters G., Kuijpers L., Kacaki J., Schuurs A. Solid-phase enzyme-immunoassay for detection of hepatitis B surface antigen. J Clin Pathol. 1976 Oct;29(10):873–879. doi: 10.1136/jcp.29.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme-linked immunosorbent assay (ELISA): a practical tool for rapid diagnosis of viruses and other infectious agents. Yale J Biol Med. 1980 Jan-Feb;53(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Comparison of seven enzyme immunoassay systems for measurement of cytomegalovirus. J Clin Microbiol. 1980 Jun;11(6):546–551. doi: 10.1128/jcm.11.6.546-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Enzyme-linked fluorescence assay: Ultrasensitive solid-phase assay for detection of human rotavirus. J Clin Microbiol. 1979 Sep;10(3):317–321. doi: 10.1128/jcm.10.3.317-321.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Torsch V. Enzyme-linked immunosorbent assay for the detection and identification of coxsackie B antigen in tissue cultures and clinical specimens. J Med Virol. 1980;6(1):45–52. doi: 10.1002/jmv.1890060107. [DOI] [PubMed] [Google Scholar]


