Abstract
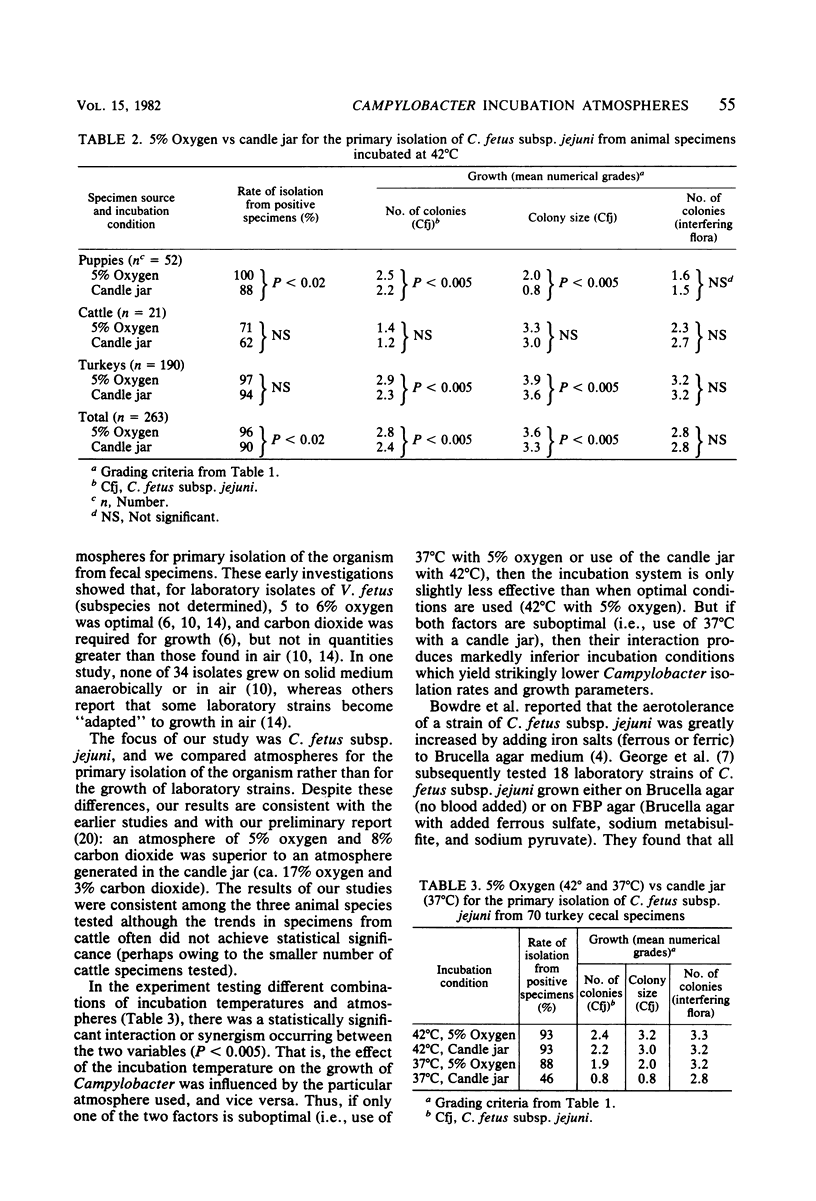
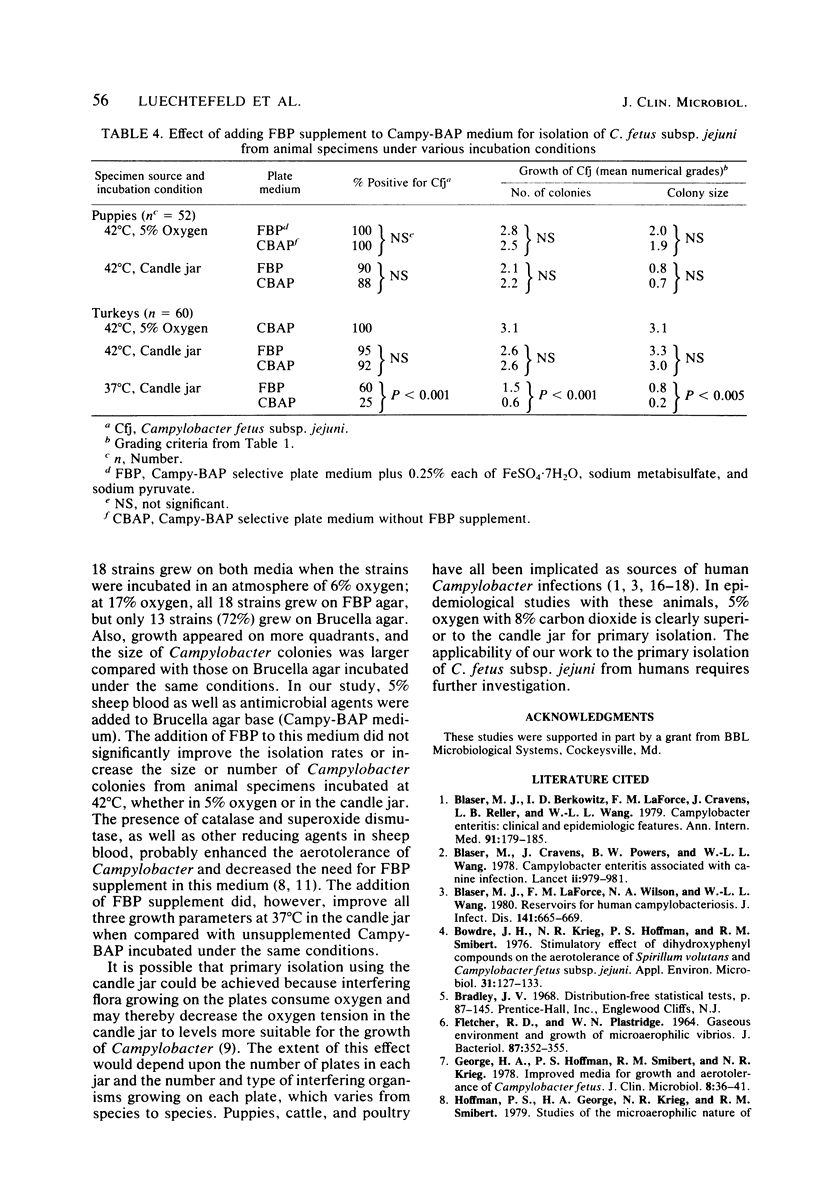
An atmosphere with reduced oxygen tension is required for the primary isolation of Campylobacter fetus subsp. jejuni. Therefore, we compared use of the conventional atmosphere of 5% oxygen and 8% carbon dioxide with use of a candle jar (17% oxygen and 3% carbon dioxide) for primary isolation of C. fetus subsp. jejuni from 263 positive canine, cattle, and turkey fecal or cecal specimens. At an incubation temperature of 42 degrees C, the atmosphere with 5% oxygen resulted in more Campylobacter colonies per plate (P less than 0.005) and consistently larger Campylobacter colonies (P less than 0.005) than did the candle jar, whereas the growth of interfering flora was similar. Overall, 96% of the 263 specimens were positive for C. fetus subsp. jejuni with 5% oxygen, and 90% were positive with the candle jar (P less than 0.02). More striking differences in isolation rates were seen when both the temperature and the atmosphere were varied: 5% oxygen at 42 degrees C enabled recovery of 93% of the isolates from 70 positive specimens, versus 46% recovery with the candle jar at 37 degrees C. Results with 5% oxygen at 37 degrees C were intermediate. The addition of FBP supplement (0.25% each of ferrous sulfate, sodium metabisulfite, and sodium pyruvate) to Campy-BAP selective medium made no improvement over unsupplemented medium at 42 degrees C (whether in 5% oxygen or in the candle jar), but there was significant improvement over unsupplemented medium when both media were incubated at 37 degrees in the candle jar.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Berkowitz I. D., LaForce F. M., Cravens J., Reller L. B., Wang W. L. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979 Aug;91(2):179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., LaForce F. M., Wilson N. A., Wang W. L. Reservoirs for human campylobacteriosis. J Infect Dis. 1980 May;141(5):665–669. doi: 10.1093/infdis/141.5.665. [DOI] [PubMed] [Google Scholar]
- Blaser M., Cravens J., Powers B. W., Wang W. L. Campylobacter enteritis associated with canine infection. Lancet. 1978 Nov 4;2(8097):979–981. doi: 10.1016/s0140-6736(78)92541-2. [DOI] [PubMed] [Google Scholar]
- Bowdre J. H., Krieg N. R., Hoffman P. S., Smibert R. M. Stimulatory effect of dihydroxyphenyl compounds on the aerotolerance of Spirillum volutans and Campylobacter fetus subspecies jejuni. Appl Environ Microbiol. 1976 Jan;31(1):127–133. doi: 10.1128/aem.31.1.127-133.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLETCHER R. D., PLASTRIDGE W. N. GASEOUS ENVIRONMENT AND GROWTH OF MICROAEROPHILIC VIBRIOS. J Bacteriol. 1964 Feb;87:352–355. doi: 10.1128/jb.87.2.352-355.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Hoffman P. S., Smibert R. M., Krieg N. R. Improved media for growth and aerotolerance of Campylobacter fetus. J Clin Microbiol. 1978 Jul;8(1):36–41. doi: 10.1128/jcm.8.1.36-41.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- KIGGINS E. M., PLASTRIDGE W. N. Effect of gaseous environment on growth and catalase content of Vibrio fetus cultures of bovine origin. J Bacteriol. 1956 Sep;72(3):397–400. doi: 10.1128/jb.72.3.397-400.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINGSCOTE B. Use of catalase in the culture of Vibrio fetus. Can J Microbiol. 1961 Dec;7:951–952. doi: 10.1139/m61-120. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Fleming P. C. Application of the Fortner principle to isolation of Campylobacter from stools. J Clin Microbiol. 1979 Aug;10(2):245–247. doi: 10.1128/jcm.10.2.245-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luechtefeld N. W., Wang W. L. Campylobacter fetus subsp. jejuni in a turkey processing plant. J Clin Microbiol. 1981 Feb;13(2):266–268. doi: 10.1128/jcm.13.2.266-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F., Bruin-Mosch C. W. Carriage of Campylobacter jejuni in healthy and diarrheic animals. Am J Vet Res. 1981 Jan;42(1):164–165. [PubMed] [Google Scholar]
- REICH C. V., MORSE E. V., WILSON J. B. Gaseous requirements for growth of Vibrio fetus. Am J Vet Res. 1956 Jan;17(62):140–143. [PubMed] [Google Scholar]
- Richardson N. J., Koornhof H. J. Campylobacter infections in Soweto. S Afr Med J. 1979 Jan 20;55(3):73–74. [PubMed] [Google Scholar]
- Robinson D. A., Edgar W. J., Gibson G. L., Matchett A. A., Robertson L. Campylobacter enteritis associated with consumption of unpasteurised milk. Br Med J. 1979 May 5;1(6172):1171–1173. doi: 10.1136/bmj.1.6172.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert R. M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]


