Abstract
Steroid hormones act in brain and throughout the body to regulate a variety of functions, including development, reproduction, stress and behavior. Many of these effects of steroid hormones are mediated by their respective receptors, which are members of the steroid/nuclear receptor superfamily of transcriptional activators. A variety of studies in cell lines reveal that nuclear receptor coregulators are critical in modulating steroid receptor-dependent transcription. Thus, in addition to the availability of the hormone and the expression of its receptor, nuclear receptor coregulators are essential for efficient steroid-dependent transactivation of genes. This review will highlight the importance of nuclear receptor coregulators in modulating steroid-dependent gene expression in brain and the regulation of behavior.
Keywords: steroid receptor coactivator-1 (SRC-1), steroid hormones, estrogen receptor, progestin receptor, androgen receptor, brain development, sex behavior, sexual differentiation, acetylation, methylation
Introduction
Steroid hormones have profound effects on homeostasis, development, reproduction and behavior. Many of the biological effects of steroid hormones are mediated through their respective receptors, which are members of the steroid/nuclear receptor superfamily of transcriptional activators [1; 2]. Receptors for the gonadal steroids, estrogens (ER), progestins (PR) and androgens (AR), can act in a classic, genomic mechanism by interacting directly with DNA to regulate gene transcription [1; 2]. Nuclear receptor coregulators enhance (coactivators) or repress (corepressors) the transcriptional activity of these steroid receptors, as well as other nuclear receptors [3; 4]. In brain, while ER, PR and AR can also function independent of ligand on DNA or at the membrane to rapidly activate cytoplasmic signaling pathways [5; 6; 7; 8; 9; 10], these receptors elicit many changes in behavior and physiology by acting through a classic, genomic mechanism of action. Approximately 300 coactivators and corepressors have been identified to date [11; 12], indicating the growing complexity these coregulators in receptor action. This review will focus on the function of some of these important nuclear receptor coregulators in genomic mechanisms of steroid action in brain and behavior.
Classic Genomic Mechanism of Steroid Action
Steroid receptors, including ER, PR and AR, have a modular domain structure consisting of an amino-terminal region (N-domain), a central DNA binding domain (DBD) and a carboxy-terminal ligand binding domain (LBD) [1; 2]. In general, steroid receptors have two transcriptional activation domains: one in the amino terminal (AF-1) and one in the carboxyl terminal LBD (AF-2) [13]. Intracellular ER exist in two forms, α and β, which are transcribed from different genes [14; 15]. These subtypes differ in their abilities to bind different ligands [16], distribution in brain [17; 18; 19; 20], and functions in brain and behavior [21; 22; 23; 24]. In addition, cell culture experiments indicate that ERα is a stronger transcriptional activator than ERβ, due to differences in the AF-1 region [25]. In most species, PR are expressed in two forms; the full-length PR-B and the truncated PR-A, which are encoded by the same gene but are regulated by different promoters [26]. Under certain conditions, in vitro studies indicate that human PR-B is a stronger transcriptional activator than PR-A [27; 28; 29], due to an additional AF domain in the N-terminus of PR-B [30]. These two PR isoforms appear to have distinct functions in reproductive behavior and physiology [31; 32].
In the absence of hormone, steroid receptors are complexed with several chaperone molecules, including heat shock proteins (hsp). These interactions are requisite for proper protein folding and assembly of stable receptor-hsp heterocomplexes that are competent to bind ligand [33]. Upon binding hormone, steroid receptors undergo a conformational change that causes dissociation of these hsp and allow receptors to dimerize [34]. Activated receptors bind directly to specific steroid response elements (SREs) and SRE-like sequences in the promoter regions of target genes [1; 2]. Binding of receptors to DNA increases or decreases gene transcription by altering the rate of recruitment of general transcription factors and influencing the recruitment of RNA polymerase II to the initiation site [35; 36]. Thus, in brain it is thought that steroids can act via their respective receptors to alter neuronal gene transcription, resulting in profound changes in behavior and physiology [37; 38].
General Mechanisms of Molecular Action of Nuclear Receptor Coregulators
Nuclear receptor coregulators are required for efficient transcriptional regulation by nuclear receptors [3; 4] (Figure 1). Nuclear receptor coactivators dramatically enhance the transcriptional activity of nuclear receptors, including ER and PR [3; 4]. In vitro studies using antibodies against nuclear receptor coactivators indicate that recruitment of coactivators is rate-limiting in steroid receptor-mediated gene transcription [3; 39]. In further support for nuclear receptor coactivator-dependent facilitation of transcription in vitro, squelching, or the repression of the transcriptional activity of one steroid receptor by another, is reversed by the addition of coactivators [40]. Thus, a critical component of efficient steroid receptor transcription is the recruitment of nuclear receptor coactivators, which dramatically enhance transcriptional activity. Under most conditions, steroid receptors interact with coactivators in the presence of an agonist, but not in the absence of ligand or in the presence of an antagonist or a selective receptor modulator [40; 41; 42; 43] but c.f. with findings revealing coactivator interactions with antagonist bound receptors [44; 45; 46]. While nuclear receptor coactivators usually interact with the C-terminal AF-2 of the receptor [41; 44; 47; 48; 49; 50], there are coactivators that associate with the N-terminus of the receptor [51]. Nuclear receptor coactivators influence receptor transcription through a variety of mechanisms, including acetylation, methylation, phosphorylation, chromatin remodeling and mRNA splicing [3; 52]. The importance of these coregulators in a variety of human diseases, including cancer and some neurological disorders, is becoming more apparent [53].
Figure 1. Simplified models of coactivator and corepressor matrices.
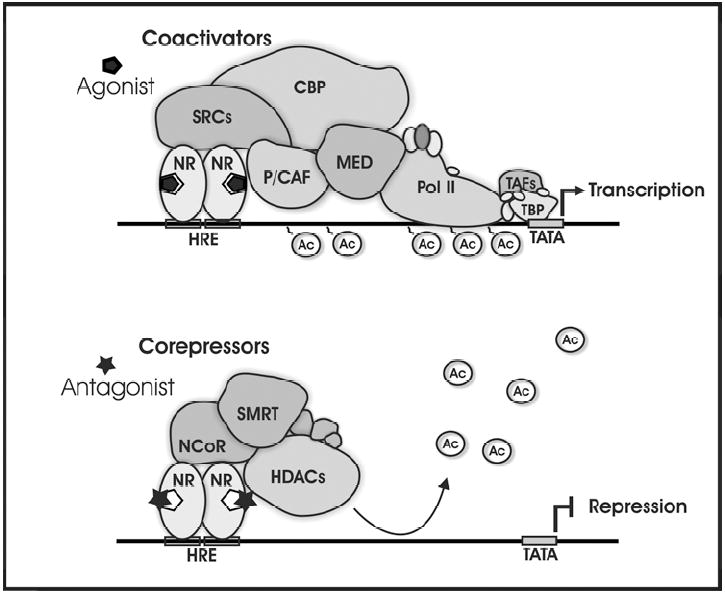
Top panel shows how agonist bound nuclear receptors associate with coactivators and other proteins to regulate gene transcription. Coactivators can increase gene transcription via the acetylation of histones and through the recruitment and stabilization of the transcriptional complex. Bottom panel shows how antagonist bound nuclear receptors can associate with corepressors and additional proteins to repress gene transcription. Corepressors can repress gene expression by causing the deacetylation of histones via the recruitment of HDACs to the genome. HRE, hormone response element; NR, nuclear receptor; SRCs, p160 steroid receptor coactivator family; CBP, CREB-binding protein; P/CAF, p300/CBP-associated factor; MED, mediator complex; HDACs, histone deacetylases; Ac, acetyl groups; Pol II, RNA Polymerase II; TBP, TATA-binding protein; TAFs, TBP-associated factors; TATA, TATA box.
Nuclear receptor coactivators of steroid receptors
The p160 family
Steroid receptor coactivator-1 (SRC-1/NcoA-1) was one of the first coactivators found to interact with hormone-bound steroid receptors [40]. SRC-1 is a member of a larger family of p160 proteins that includes SRC-2 (also known as GRIP1, TIF2 and NCoA-2) [49; 54] and SRC-3 (AIB1, TRAM-1, p/CIP, ACTR, RAC3) [55; 56]. The SRC family of coactivators physically interacts with steroid receptors, including ER, PR and GR, in a ligand-dependent manner [3; 4; 40]. The SRCs physically associate with agonist-bound receptors through multiple LXXLL motifs (L, leucine; X, any amino acid) that make up nuclear receptor (NR) boxes [57]. In vitro experiments reveal that depletion of SRC-1 in cultured cells by micro-injection of antibodies to SRC-1 prevents receptor-dependent transcription, suggesting that SRC-1 is important for transcriptional activity of steroid receptors [39]. In cell culture, hormone induced transactivation of PR is reduced by coexpression of ERα, presumably due to squelching or sequestering of shared coactivators [40]. This squelching can be reversed by over-expression of SRC-1, suggesting that coactivators are a limiting factor necessary for full transcriptional activation of receptors [40]. In further support, over-expression of SRC-1 relieves thyroid hormone receptor inhibition of ERα-mediated transcription of the preproenkephalin gene in transient transfection assays in CV-1 cells [58].
The SRC family of coactivators appear to act as a platform for the recruitment of other coactivators (Figure 1), including CREB binding protein (CBP) and p300/CBP associated factor (p/CAF), that possess histone acetyltransferase activity and aid in chromatin remodeling [59; 60]. In addition, SRC-1 itself is known to posses endogenous histone acetyltransferase activity [61]. The p160 coactivators contain two activation domains, AD1 and AD2, in the C-terminal region. AD1 mediates interactions with CBP [62], while AD2 allows binding of other proteins, including the protein arginine methyltransferase CARM1 [63].
While much is known about the molecular mechanisms of nuclear receptor coactivators from a variety of in vitro studies [3; 64], we are just beginning to understand their role in hormone action in vivo. SRC-1 knockout mice, while fertile, have decreased responsiveness in progesterone target tissues [65], partial resistance to thyroid hormone [66] and delayed development of cerebellar Purkinje cells [67]. However, it should be noted that SRC-2 is up-regulated in steroid sensitive tissues, including brain and testes, suggesting that increased expression of this coactivator compensates for the loss of SRC-1 [65]. In addition, SRC-1 is critical in maintaining energy balance by regulating both energy intake and expenditure [68]. As discussed below, SRC-1 has profound effects in brain on hormone-dependent development, gene expression and behavior. It is interesting to note that SRC-1 exists in at least two isoforms, SRC-1a and the truncated SRC-1e [69; 70], which appear to have different functions [69; 71].
As is the case with SRC-1, SRC-2 enhances transcriptional activity of a variety of nuclear receptors, including ER, PR and AR [49; 54]. The mid-region of the SRC-2 protein, which mediates interactions with steroid receptors, has relatively low homology with SRC-1, suggesting functional differences between these two proteins [49; 54]. SRC-2 knock-out mice reveal that this coactivator is important in fertility and ductal branching in mammary gland [72; 73; 74]. Generation of mice in which SRC-2 is ablated specifically in cell types that express PR (PRCre/+SRC-2flox/flox) have allowed the investigated of the function of this coactivator in progestin action [74]. Disruption of SRC-2 expression in uterine PR-positive cells led to an early block in embryo implantation. Furthermore, removal of SRC-1 in PRCre/+SRC-2flox/flox uteri caused a block in decidualization, suggesting that both SRC-1 and SRC-2 are required for complete PR-dependent decidualization. In addition, SRC-2 is important for PR action in mammary gland as demonstrated by the lack of significant branching and alveolar morphogenesis in the PRCre/+SRC-2flox/flox mammary gland [74; 75]. Microarray analysis of uteri from SRC-2 null mice reveal that SRC-2 is involved in the ability of progesterone to repress specific genes involved in a variety of functions, including cell cycle and immunity [76].
SRC-3/AIB1, which is amplified in human breast tumors [55], coactivates a variety of nuclear receptors, including ER and PR [39; 55; 77]. Phosphorylation of SRC-3 is thought to be important for its ability to coactivate specific steroid receptors and has been associated with its oncogenic properties [78]. Female SRC-3 null mice, while fertile, have delayed puberty, longer estrous cycles, ovulate fewer eggs and have impaired mammary gland development [79; 80]. Recent studies in SRC-3 null mice reveal that this coactivator is critical for normal PR-dependent mammary gland development and function [80]. Using chromatin immunoprecipitation (ChIP) assays, gonadotropin-releasing hormone (GnRH) stimulated more efficient recruitment of SRC-3 by PR, on the progestin response element of a luciferase reporter gene or the gonadotropin α subunit gene promoter, than progesterone [81]. These findings suggest that phosphorylation of PR and its interaction with SRC-3 and binding to DNA may play an important role in the possible ligand-independent activation of PR by GnRHs [81].
Other coactivators of steroid receptors
CREB binding protein (CBP)
While CBP was initially discovered to be a transcriptional activator of cAMP-response element binding protein (CREB) [82; 83], it is also now known to function as an integrator of nuclear receptors with other cell signaling pathways, including CREB and AP-1 [60; 83; 84]. As is the case with the p160 family, CBP is important in ligand-dependent transcriptional activity of nuclear receptors, including ER and PR [85]. While p300 is closely related to CBP, genetic knockout mice for CBP and p300 exhibit different phenotypes, suggesting a functional distinction of these coactivators [86]. Interestingly, mutation of the CBP gene causes Rubinstein-Taybi syndrome, which results in severe mental retardation and a variety of physiological deformities in humans [87]. In mice, mutations of CBP lead to similar physical deformities as well as impaired memory [88]. A variety of in vitro studies indicate that SRC-1 and CBP act synergistically to enhance ER and PR transcriptional activity and function [85; 89; 90; 91]. In support of this concept, SRC-1 physically interacts with CBP and recruits it to the coactivator complex to form a ternary complex at target gene promoters [60; 85]. For ligand-bound PR to induce transcription of target genes, SRC-1 must be recruited to the receptor dimer complex first, followed by CBP [90]. Deletion of either the CBP/p300 binding site, or the C-terminal region containing the PR binding site, of SRC-1 dramatically reduces PR transactivation [90].
E6-associated protein (E6-AP)
E6-AP was originally identified as an E3 ubiquitin ligase [92; 93] and is best known for its role in Angelman Syndrome. Nawaz et al. [94] demonstrated that E6-AP acts also as a coactivator for steroid receptors, including PR, ER, AR, GR, as well as RARα and TR. The binding of E6-AP to nuclear receptors is hormone-dependent and the over-expression of this coactivator can reverse the squelching between ER and PR. E6-AP contains an intrinsic activation function independent of the ligase activity and only the loss of ligase activity, and not the coactivator function, is responsible for the symptoms of Angelman Syndrome. It should be noted that the ubiquitin ligase activity and the coactivator function are both necessary during the transcription process. Although the exact identity of the ubiquitin ligase(s) involved in the proteolysis of the steroid receptors is currently controversial, some results suggest that the stability of ERα and AR is directly linked to the expression levels of E6-AP [95; 96]. Moreover, E6-AP is required for the degradation of the coactivator SRC-3 [97] and may also target itself [98]. E6-AP is expressed in most brain regions, including the hippocampus, cerebellum, cortex and thalamus [99]. It is also expressed in peripheral tissues and has been shown to be important in various aspects of reproduction, including the hormonal induction of breast and prostate growth, sperm production and ovulation [100]. Electron microscopic studies in various cell lines found that E6-AP is present in both cytoplasm and nucleus, in agreement with the ubiquitin ligase and nuclear receptor coactivator activities [101].
Estrogen receptor associated protein 140 (ERAP140)
ERAP140 interacts in a ligand-depend manner with, and enhances the transcriptional activity of, ERα, ERβ, TR, PPARγ and RARα. Even in the absence of the classical LXXLL motifs common for most coactivators, ERAP140 interact with ERα through a binding site similar to that of other coactivators [102]. Contrary to the wide distribution of many nuclear receptor coregulators, high levels of expression of ERAP140 is mainly restricted to brain tissue and kidney, with very low levels in mammary gland, lungs and testis and undetected in liver or intestine. In situ hybridization studies suggest an exclusive neuronal localization, in areas such as cortex, hippocampus, thalamus, hypothalamus and cerebellum [102].
Steroid receptor RNA activator (SRA)
SRA is a distinctive coactivator in that it functions as an RNA transcript to enhance transcriptional activity of steroid receptors, including PR, ER, GR and AR [103; 104]. While liganded ER reduced PR transcriptional activation, addition of SRA reversed this squelching effect of ER [104]. Treatment of cells with SRC-1 and SRA antisense, greatly reduced activity of ERα or PR [103; 104]. Antisense to either SRA or SRC-1 alone had a less dramatic effect on ERα activity, suggesting SRA association with SRC-1 [103]. In further support, SRA co-purified with SRC-1, indicating that SRA exists in a ribonucleoprotein complex containing SRC-1 [104]. Expression of SRA is tissue specific, with Northern blot analyses revealing that SRA mRNA expressed at high levels in liver, skeletal muscle and heart, and at lower levels in brain and placenta [104]. Finally, over-expression of SRA in transgenic mice reveals a role for SRA in estrogen-induced expression of PR in mammary gland [105].
Ribosomal large subunit protein 7 (RPL7)
Similar to E6-AP, RPL7 (also known as L7/SPA) was not identified as a coregulatory protein at first, but as a cytoplasmic and nuclear protein involved in translational regulation [106]. It was only later that a dual role of RPL7 in both transcription and translation was described [107; 108; 109]. RPL7 increases the transcriptional activity of ER and PR only when bound to the selective ER modulator (SERM), tamoxifen, or the selective PR modulator (SPRM), RU486, respectively [110]. In contrast, estradiol, or the full estrogen receptor antagonist ICI 164,384, does not elicit binding of RPL7 to ER [110]. RPL7 is expressed in estrogen-sensitive regions of reproductive organs, including the uterus [111; 112]. In zebra finch brain, RPL7 is expressed at high levels in the song system of the adult male [113]. Furthermore, RPL7 mRNA concentration is higher in male telencephalon the day of hatch (P1) and in adult compared to female [113]. Western blot analyses suggest that the expression of three different functional peptides of RPL7, the full length peptide (44kDa), the ribosomal functional unit (27kDa) and the coactivator functioning peptide (31kDa), is sex and age dependent in zebra finches [113]. While the role of RPL7 in the modulation of ER in brain remains unknown, it is possible that the sexually differentiated expression of RPL7 in brain is involved in modulating retinoic acid or vitamin D action, both of which are important in brain development [114; 115]. Although this has not been directly studied in brain, another mechanism by which RPL7 functions to modulate vitamin D receptor is by interfering with the binding of the receptor to its response element [116]. When vitamin D receptor binds hormone, it can form a heterodimer with retinoid X receptor (RXR). This dimer can then recognize a specific vitamin D response element and enhance transcription of target genes. In the presence of RPL7, the heterodimer is no longer able to enhance transcription due to repression of dimer binding to the response element by RPL7. Depletion of RPL7 by antibodies restored binding of the heterodimer on the response element [116]. Thus, RPL7 can coactivate antagonist-bound steroid receptors and repress ligand bound VDR in the presence of RXR. Therefore, because the actions of RPL7 are context-dependent, the term “selective coactivator” has been used to describe this coregulator and others [117]. In future studies it will be important to determine the biological function of RPL7 in brain.
Finally, there are a variety of other coactivators, including TRAP220 [118], PGC-1 [119], chromatin high mobility group proteins 1 and 2 [120] and TIP60 [121], that are known to interact with ER and PR. Thus, there is much more to be learned about the function of coactivators in steroid receptor action. In addition to the study of individual nuclear receptor coactivators, it should be noted that coregulators are usually present in preformed complexes, or “coregulatorsomes”, ready to be recruited when needed [59]. Therefore, it is important to determine the different coregulators involved in specific physiological responses and define whether these coactivators act together, in parallel, or independently from each other, in the modulation of nuclear receptor action.
Nuclear Receptor Corepressors
Overview of nuclear receptor corepressors in gene silencing
While much is being discovered about the functional roles of steroid receptor coactivators in modulating steroid receptor function at the genome, less is known about the role of corepressors, especially in brain. However, the recent expansion in investigating the epigenetic suppression of gene transcription will allow for a better understanding of how these repressor molecules also regulate gene transcription. Whether or not the diversity of directional coding exists with corepressors, as it does with coactivators, is yet to be determined. That is, it is becoming clear that coactivator molecules can be modified by phosphorylation, methylation, ubiquitination, sumoylation, and acetylation, which can determine the functional role of the transcriptional complex [3; 64; 122]. While the major function of nuclear receptor coactivators is to increase gene transcription, corepressors generally suppress or silence gene transcription (Figure 1). The basic model of gene silencing occurs following methylation of DNA at CpG sites by a particular DNA methyltransferase (DNMT) [123; 124]. While methylation alone can interfere with protein binding, it is the binding of methyl-CpG binding proteins (MBDs), which subsequently recruit corepressor and histone deacetylase (HDAC) repressor complexes, that mainly result in gene repression. Some notable repressor complexes that form on DNA to repress gene transcription are the Sin3, NuRD, CoREST, and the NCoR/SMRT repressor complexes [125]. These multi-protein complexes are associated with relatively unique combinations of HDACs and transcriptional regulating factors.
While the components of corepressor complexes are still being identified, there are some shared and some unique molecules that make up these different corepressor complexes [125]. For example, the Sin3 and NuRD complexes both contain HDAC1, HDAC2, RbAp46, and RbAp48; however, the Sin3 complex also contains Sin3a, SAP18, and SAP30; whereas, the NuRD complex contains MBD3, MTA-2, and Mi-2. The CoREST complex contains HDAC1, HDAC2, CoREST, SHARP and Sin3. Lastly, the SMRT/NCoR complex contains HDAC3, SMRT, and NCoR. It is likely that these different combinations contribute to different functional consequences on the genome. While NCoR and SMRT can function within the same complex, they can act independent of each other to regulate steroid receptor function and gene transcription. The recruitment of corepressor complexes to DNA can occur via interacting with steroid receptors directly or by interacting with the MBDs bound to methylated CpG sites. Thus, there exists the potential for specification of function by which complexes are formed on DNA. Further specification is obtained by MBDs exhibiting distinct associations with particular corepressors complexes. For example, MeCP2 associates with the Sin3 corepressor complex, whereas, MBD2 is associated with the NuRD complex. The general mechanism by which corepressor complexes suppress gene expression is by deacetylating the histones via the HDACs in the complex [125]. The removal of acetyl groups on the histones restores a positive charge on the histone tails, allowing them to become tightly associated with DNA, condensing DNA, and ultimately suppressing gene transcription. Thus, corepressors oppose the biological properties of coactivators, which lead to the acetylation of histones to increase gene transcription. The first corepressors of nuclear receptors to be identified were NCoR (nuclear receptor corepressor) [126] and SMRT (silencing mediator of retinoid and thyroid hormone receptors) [127].
Nuclear Receptor Corepressor (NCoR)
NCoR was one of the first corepressors of nuclear receptors to be discovered and was identified via its interaction with thyroid receptors [126]. Unliganded thyroid hormone receptors bind DNA and suppress gene transcription. It was hypothesized that additional proteins are bound to unliganded thyroid receptors on DNA that are responsible for gene repression. This led to the discovery of a 270 kDa protein bound to unliganded thyroid receptors which was termed NCoR [126]. While the functional role of NCoR is still being elucidated, its presence appears to be critical for survival as global deletion of NCoR is embryonic lethal [128]. Interestingly, this suggests that the loss of NCoR function cannot be compensated by another corepressor, in contrast to the loss of SRC-1 which can be partially compensated by increased SRC-2 [65]. While NCoR is known to repress the activity of thyroid hormone [129], it is believed that NCoR may be a non-selective repressor of nuclear receptor function. However, the mechanism by which NCoR suppresses gene transcription seems to have some selectivity with which HDAC it associates with on DNA. In particular, NCoR appears to assemble with HDAC3 and SMRT [130]. NCoR has also been associated with the Sin3 corepressor complex via its interaction with Sap30. The transcriptional repression by NCoR appears to occur mainly via its associations with Sin3 and HDACs [131; 132; 133]. Interestingly, while NCoR and SMRT co-immunoprecipitate with many of the same molecules, the methyl CpG binding protein, Kaiso, appears to associate only with NCoR [134]. Due to NCoR’s association with particular methyl-binding proteins, such as MeCP2 and Kaiso, it has been suggested that NCoR plays a larger role in methyl-directed gene repression. As NCoR forms distinct corepressor complexes, it is likely to be involved in the regulation of a small subset of genes.
Differences in the sub-cellular localization of NCoR have been observed and appear critical for normal cell development and function. For example, activation and cytoplasmic localization of NCoR allows neural stem cells to differentiate into astrocytes [135]. This would suggest that NCoR functions during development as a key factor inhibiting neural differentiation into glia. It also supports the concept that sub-cellular localization of coregulator proteins can dictate not only gene transcription, but also cellular differentiation. The differential sub-cellular localization of NCoR can also be associated with abnormal cell function, such as the increased cytoplasmic localization following abnormal IKKalpha activation in colorectal cancer cells [133]. Interestingly, the presence of NCoR within a cell can also dictate whether the SERM tamoxifen can be agonistic or antagonistic on ER function [136]. If NCoR is present, than tamoxifen blocks ER function. However, in the absence of NCoR, SRC-1 is recruited to the complex resulting in a tamoxifen-induced increase in ER activity [136]. Therefore the presence and sub-cellular localization of NCoR, as well as the availability of coactivators, can be critical for normal cell function.
Silencing Mediator of Retinoid and Thyroid Hormone Receptors (SMRT)
While SMRT represses thyroid hormone receptor function and interacts with NCoR [127], it is also known to modulate ER activity in a ligand-dependent and cell-type specific manner. Some studies in MCF-7 cells suggest that SMRT may be involved in the suppressive actions of raloxifene on ER activity. For example, while estradiol binding to ER causes recruitment of coactivators and increased expression of IGF-1 and c-Myc, raloxifene binding to ER causes the recruitment of NCoR, SMRT, and HDAC2 [137]. Interestingly, while depletion of NCoR in MCF-7 cells can increase estradiol-induced ER activity; in the same study, depletion of SMRT led to a reduction of estradiol-induced ER activity [138]. These findings suggest that while SMRT is involved in gene silencing of thyroid hormone receptors, its presence appears to be important for full ER activity within a cell. It is likely that this enhancement of ER activity is occurring through a direct interaction with ER or coactivators, rather than through the formation of the SMRT/NCoR repressor complex. These data indicate that the biological role of SMRT is functionally different than NCoR, even though they can appear within the same compressor complex. It is likely that the functional role of SMRT and NCoR in either enhancing, or suppressing, gene transcription is dependent upon the sub-cellular localization and expression of coactivators and corepressors within a cell. Therefore, it seems to be important to determine the cellular diversity of coactivators and corepressors in order to predict a biological outcome on DNA.
Nuclear Receptor Coregulator Function in Brain and Behavior
Nuclear receptor coactivators
Neuroanatomical expression and regulation of coactivators
While much is known about the molecular mechanisms of nuclear receptor coactivators from a variety of cell culture studies [3; 4], we are just beginning to understand their role in hormone action in brain. SRC-1 mRNA and protein are expressed at high levels in the cortex, hypothalamus, hippocampus and cerebellum of rodents [67; 139; 140; 141; 142; 143; 144; 145] and birds [146; 147]. SRC-2 is also expressed at high levels in the hypothalamus and hippocampus [67; 148; 149], while SRC-3 is mainly expressed in the hippocampus [67; 149]. Male and female guinea pig fetuses have high expression of SRC-1 mRNA and protein in the limbic system, which decrease in the hippocampus with advancing gestation [150]. In contrast, SRC-1 mRNA levels increase in the guinea pig dentate gyrus near birth [150]. In addition to the p160s, other nuclear receptor coactivators, including CBP, TRAP220 and ERAP140, are expressed at high levels in steroid-sensitive brain regions [102; 144; 151; 152; 153]
In order for coactivators to function with steroid receptors, they must be expressed in the same cells. Indeed, SRC-1 is expressed in the majority of estradiol-induced PR cells in reproductively-relevant brain regions, including the VMN, medial preoptic area and arcuate nucleus [154]. In addition, the majority of estradiol-induced PR cells in these same brain regions coexpress CBP [154]. Given that virtually all estradiol-induced PR cells in the hypothalamus contain ERα [155; 156], these findings suggest that these specialized cells represent functional sites of interaction between ovarian steroid receptors and coactivators (SRC-1 and CBP) in brain [154]. These findings provide neuroanatomical evidence that nuclear receptor coactivators are integral in mediating steroid hormone action in reproductively-relevant brain regions. It is important to note that not all ER and PR cells express SRC-1 [145; 154], suggesting that the receptors in these cell populations use other coactivators. Thus, different populations of ER cells, as defined by the coactivators they express, could have different responses (i.e. activation of different target genes) to ligand.
Because coactivators play a major role in the transcriptional activity of nuclear receptors, it has been suggested that different nuclear receptors present in the same cell could compete for the same coregulator protein [157]. The decrease of one coactivator would increase the competition between different hormonal systems and thus reduce the response of one or both of the hormone systems. For example, it seems that the thyroid-mediated decrease of estrogen-induced sexual behavior is controlled at the transcriptional level though the competition by TR and ER for SRC-1. The coactivator would thus be a limiting factor in hormone-mediated gene expression [158; 159; 160]. Therefore, it is important to evaluate the potential regulation of coactivator concentrations by hormones and other factors.
A limited number of studies have focused on the potential regulation of coactivator expression in brain. Misiti and colleagues [139] were the first to suggest a sex difference in coactivator expression, with the concentration of SRC-1 mRNA higher in the pituitary gland in adult male rats compared to females. This difference was later confirmed at the protein level [161]. Sex differences in SRC-1 expression were also observed in the song control nuclei HVC and Area X in canaries [147] and in the preoptic are/hypothalamus region in Japanese quail [146; 162]. While a sex difference in SRC-1 expression was not detected in the hypothalamus of adult rats [161], likely candidates to explain these sex differences in adult birds are 17β-estradiol and testosterone. 17β-estradiol treatment reduces the levels of SRC-1 in rat pituitary and in vitro [139], while treatment with this same hormone reverses the down-regulation of SRC-1 expression in the rat ventromedial hypothalamus caused by ovariectomy [163]. In the hypothalamus of cycling female rats, SRC-1 levels were lowest during diestrus, and highest at proestrus and estrus [164]. Interestingly, the endocrine disruptor 4-methylbenzylidene camphor (4-MBC), which has estrogenic activity and also interferes with the thyroid axis, alters SRC-1 expression in brain [165]. Pre-and post-natal exposure of 4-MBC increased SRC-1 mRNA in the VMH and MPOA of female rats [165], which could further accentuate the estrogenic effect of this disruptor and alter other nuclear receptor signaling pathways.
In castrated male Japanese quail, testosterone treatment increases the level of SRC-1 mRNA and protein in the preoptic area region/hypothalamus [162]. However, testosterone had no effect on SRC-1 expression in the male hamster medial preoptic region, BNST, arcuate nucleus and amygdala [166]; in the male rat hypothalamus [148] or in the telencephalon of male zebra finches [167]. While anabolic androgenic steroids did not alter SRC-1 expression in the hypothalamus of male rats, testosterone decreased SRC-2 protein in this brain region [148]. Finally, thyroid hormone decreases expression of SRC-1 in neonatal mouse cerebellum [168] and rat cortex and dentate gyrus [169].
In addition to gonadal steroids, there is evidence that glucocorticoids regulate SRC-1 [170]. Stress and the associated increase in glucocorticoids were shown to reduce SRC-1 mRNA in the brain, as well as in the stomach, heart, liver and kidney [170]. More specifically, a reduction of SRC-1 expression in the hypothalami of both male and female rats is found after acute restraint stress [161]. Interestingly, acute stress also decreased SRC-1 in the frontal cortex in male rats, but increased its concentration in the male pituitary and the female hippocampus [161]. While chronic exposure of adrenalectomized male rats to relatively high levels of corticosterone did not affect SRC-1 mRNA levels in the hippocampus, it did lead to a down-regulation of the SRC-1 splice variant, SRC-1e, in the anterior pituitary [70]. In addition, stress blocks the testosterone-induced increase in SRC-1 expression in brains of castrated male quail [162]. Taken together, these studies suggest that coactivators are important in modulating the glucocorticoid-mediated stress response.
In addition to steroids and stress, it appears there is photoperiodic regulation of SRC-1 expression in brain. SRC-1 concentration fluctuates through the day in Japanese quail in the hippocampus, hindbrain and optic lobes, independently of sex, testosterone treatment and stress [162]. In male Siberian hamsters, short days reduced SRC-1 expression in the posteromedial bed nucleus of the stria terminalis and posterodorsal medial amygdala [166]. This photoperiodic regulation of SRC-1 may modulate androgen regulation of seasonal reproduction. Both the Japanese quail and Siberian hamster show an important seasonal cycle, with high levels of gonadal steroids during reproduction in the spring and low to undetectable levels in the winter when reproduction is suppressed [171; 172]. Superimposed on these seasonal changes are the fluctuations of steroid release during the day with high levels early in the morning and low levels during the night [173]. It may be that the central nervous system responds to changes in circulating levels of steroids by altering the concentration of available coactivators.
While coactivators are regulated at the transcriptional level, numerous studies indicate that post-translational modifications of coactivators play a major role in modulating the properties of these important proteins. The total concentration of the coactivator, as measured at the mRNA or protein level, is sometimes not sufficient to determine whether the coactivator will function with a specific nuclear receptor. Numerous studies have shown that coactivators such as the SRCs, CBP and peroxisomal proliferator-activated receptor γ coactivator-1alpha (PGC-1alpha) are modified by phosphorylation, methylation, acetylation and sumoylation [174; 175; 176; 177; 178]. For example, phosphorylation of SRCs is essential for optimal activity of the coactivator, notably through the regulation of protein-protein interactions. Phosphorylation of SRC-1 by cAMP enhances both ligand-dependent and -independent activity of PR [178]. In addition, phosphorylation is important for the physical and functional interaction of SRC-1 [178] and SRC-3 [78] with CBP. This phosphorylation of the SRCs is induced by multiple kinases, including MAPK and IκB kinases, which are involved in a variety of cellular signaling pathways [78; 179]. Post-translational modifications also seem to be involved in defining the specificity of coregulators for a given nuclear receptor. Because nuclear receptor coactivators interact with a broad range of transcription factors, it has been suggested that coactivators could distinguish one downstream class of transcription factors from another, so as to prevent global gene activation [57]. Post-translational modifications of the coactivators, such as phosphorylation, seem to be involved in the specificity of these interactions with various transcription factors. Androgens or estrogens induce phosphorylation at 6 specific amino acids of SRC-3, all of which are required for the transactivation of target genes by ER [78]. But the phosphorylation of only 5 specific sites on SRC-3 is induced by TNFα-stimulated signaling pathway and is required for transactivation of NF-κB [78]. In addition to influencing protein-protein interactions between the coactivator and a specific nuclear receptor or another coactivator, post-translational modifications are involved in sub-cellular distribution [180; 181; 182], stabilizing the protein [182; 183] and regulation of enzymatic activity [184; 185]. Finally, studies suggest that degradation of coactivators is an important aspect of their regulation. Treatment with a 26S proteasome inhibitor (MG132) increases SRC-1 expression in the hypothalamus, preoptic area and hippocampus [186]. In addition, this same treatment up-regulated PR and ERβ in the same brain regions, while ERα was increased in the POA only [186]. These findings suggest that degradation of important components of steroid action, the receptors and coactivators, are regulated in a brain region-specific manner.
The study of coregulator expression and posttranslational modifications has most often focused on one or a few specific coregulators. However, it is important to note that coactivators are not assembled into static and stable complexes, but rather exist in dynamic complexes that are rapidly dissociating and recruiting other coregulatory proteins. In addition, these coregulators are constantly undergoing a variety of posttranslational modifications that may further alter their function [187] and also see www.nursa.org/10.1621/datasets.01001 for datasets). Relatively little is know about the control of the expression of the proteins in these complexes in a brain region of interest or in defined cell types. All together, cellular differences in coactivator ratio, as well as their unique posttranslational modifications, are likely to be involved in cell-specific responses to hormone in a promoter and ligand-dependent manner [188; 189].
Hormone-dependent brain development
Steroid hormones act within the developing brain to have lasting organizational effects on adult sexually dimorphic brain physiology and behavior. On the day of birth, testosterone secretion from the testis results in masculinization (increased adult male sexual behavior) and defeminization (decreased adult female sexual behavior) of adult reproductive behavior. In the brain, testosterone is converted into dihydrotestosterone and estradiol, which then bind to AR and ER, respectively. To determine if nuclear receptor coactivators are necessary for the organizing actions of steroid hormones in developing brain, antisense oligonucleotides were targeted at SRC-1 mRNA within the hypothalamus during the first few days of life and their impact on steroid-mediated differentiation the sexually dimorphic nucleus (SDN) of the preoptic area and adult sexual behavior was assessed [143]. It is well known that testosterone exposure, via its aromatization and binding to ERs, increases the size of the female SDN [190; 191]. It was found that testosterone-treated females receiving hypothalamic SRC-1 antisense infusions during development had a smaller SDN size compared to testosterone-treated control females, suggesting that interfering with SRC-1 disrupts differentiation of the SDN [143]. To test if SRC-1 was important in mediating the long-term steroid-mediated differentiation of adult sexual behavior, males, testosterone-treated females, and control females were infused with either SRC-1 antisense, scrambled controls or vehicle into the hypothalamus during the first few days of neonatal life and sexual behavior was examined in adulthood. As neonatal testosterone exposure defeminizes adult sexual behavior, it was confirmed that that males and testosterone-treated females receiving control infusions exhibited lower levels of adult female sexual behavior contrasted to control females [143]. However, males and testosterone-treated females infused with SRC-1 antisense during the neonatal period exhibited high levels of adult female sexual behavior that were comparable to control females, suggesting that SRC-1 is critical in mediating the defeminizing actions of testosterone on adult female sexual behavior. Interestingly, there were no differences between rats treated with SRC-1 antisense or controls in the number of mounts directed toward an estrous female or in the latency to mount an estrous female. These findings suggest that reducing SRC-1 had no long-term effect on the organization of male sexual behavior. Therefore, SRC-1 appears crucial for testosterone-mediated defeminization of female sexual behavior, but not for testosterone-mediated masculinization of male sexual behavior. In addition, SRC-1 appears to have a role in development of other brain regions. SRC-1 null mice have delayed development of cerebellar Purkinje cell precursors at an early embryonic stage, delayed maturation of Purkinje cells after birth, and impaired motor function in adulthood [67]. These findings suggest that SRC-1 is important in Purkinje cell development and normal motor function [67].
The divergent outcome of SRC-1 in the development of sexual behavior appears to be the same for the role of CBP in steroid-mediated sexual differentiation of adult sexual behavior. In a similar follow-up study, the role of CBP in sexual differentiation of adult sexual behavior was examined. It was hypothesized that reducing postnatal CBP levels within the hypothalamus would block the defeminizing actions of testosterone on adult female sexual behavior in female rats [192]. As predicted, testosterone-treated female rats infused with CBP antisense neonatally exhibited higher levels of female sexual behavior in adulthood contrasted to scrambled controls. This indicates that reducing CBP expression interfered with testosterone’s ability to defeminize or reduce female sexual behavior. Comparable to SRC-1 antisense infusions, reducing CBP expression did not interfere with the masculinization of male sexual behavior. Although these data do not rule out a possible role for CBP and SRC-1 in modulating the development of some components of male sexual behavior, they do suggest that some coactivators, such as CBP and SRC-1, may be more important in processes mediating defeminization than those mediating masculinization of sexual behavior. It is possible that both CBP and SRC-1 need to be reduced in order to influence masculinization. Indeed, as discussed below, simultaneous reduction of both CBP and SRC-1 was required to interfere with steroid-induced sexual behavior in adult female rats [144].
Hormone-dependent gene expression in brain
The function of nuclear receptor coactivators in hormone-dependent gene expression in brain and behavior in adult rodents has also been investigated [144; 149]. Estradiol-induction of PR gene expression in the VMN is important for hormone-dependent female sexual behavior [193]. Therefore, studies have addressed if SRC-1 and CBP are critical in modulating ER-mediated transactivation of the PR gene in the VMN. Infusions of antisense oligodeoxynucleotides to SRC-1 and CBP mRNA into one side of the VMN of adult female rats reduced the expression of ER-mediated activation of PR gene expression compared to the contralateral control ODN-treated VMN [144]. These findings are supported by previous in vitro studies indicating that SRC-1 and CBP function together to modulate ER activity [85]. In further support of SRC-1 and CBP/p300 functioning together in brain, neurons in the rat hippocampus and dentate gyrus coexpress SRC-1 and p300 [145]. Another study in brain supports these findings and extend them to include a role of SRC-2 in ER-mediated induction of PR in the VMN [149]. This same study found that SRC-3 is not involved in estradiol induction of PR. Finally, the p160 coactivators function in glucocorticoid receptor (GR) action in glial cells [194] and in GR-mediated repression of the corticotropin-releasing hormone gene [71]. Taken together, these findings indicate that nuclear receptor coactivator action in brain is essential for full steroid receptor transcriptional activity.
Steroid-dependent behaviors
Given that nuclear receptor coactivators are critical for hormone-dependent gene expression in brain, it was hypothesized that coactivators act in brain to modulate the expression of hormone-dependent behaviors [144]. Female rats treated with antisense to both SRC-1 and CBP mRNA into the VMN displayed reduced levels of hormone-dependent female sexual receptivity compared to scrambled treated controls [144]. Another study supported these findings with SRC-1 and extended them to include a role for SRC-2 in hormone-dependent lordosis [149]. Moreover, the effects of these nuclear receptor coactivators on specific ER- and PR-dependent aspects of female sexual behavior have been investigated [195]. There are two modes of hormone regulated female reproductive behavior in rats: estrogen-mediated (elicited by estradiol alone) and progesterone-facilitated (requires estradiol priming followed by progesterone) [38]. To test the hypothesis that nuclear receptor coactivators function in brain to modulate ER-mediated aspects of female reproductive behavior, animals were injected with estradiol only [195]. Antisense to SRC-1 and CBP infused into the VMN of animals treated with estradiol alone decreased lordosis intensity, suggesting that these coactivators modulate ER-mediated aspects of female sexual behavior. Proceptive behaviors by the female, which serve to solicit interaction by the male, are PR-dependent and include ear-wiggling and hopping and darting [196; 197; 198; 199; 200; 201]. Infusion of antisense to SRC-1 and CBP mRNA into the VMN around the time of progesterone administration reduced PR-dependent ear-wiggling and hopping and darting (Figure 2) [195]. Thus, it appears that nuclear receptor coactivators function in brain to modulate PR and ER action in brain and influence specific aspects of hormone-dependent sexual behaviors in rodents.
Figure 2. Nuclear receptor coactivators modulate PR action in brain.
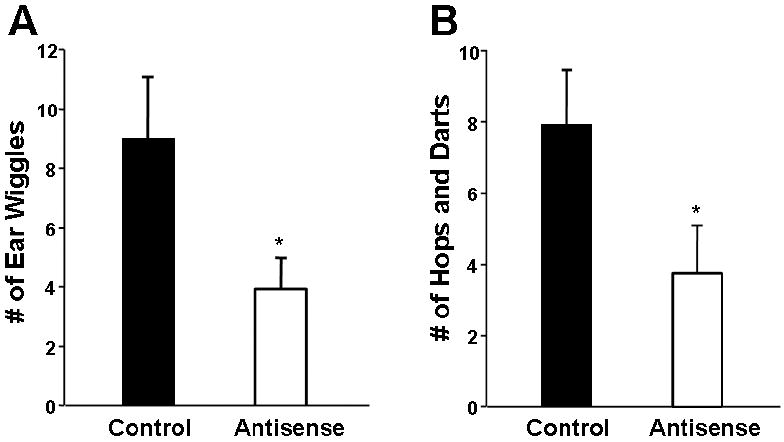
Female rats were ovariectomized, primed with estradiol and progesterone, and exposed to male rats. Infusions of antisense to both SRC-1 and CBP mRNA in the ventromedial nucleus of the hypothalamus of female rats, decreased PR-dependent A) ear wiggling and B) hops and darts compared to scrambled control-treated animals [195]. *p < 0.05; one-tailed t test
Receptor-coactivator interactions
Recently, a proteomics-based approach has been used to study the interactions of steroid receptors with coactivators from rat brain. To test the hypotheses that SRC-1 from brain physically associates with ER and PR subtypes in a ligand-dependent manner, pull-down assays with brain tissue from female rats were developed [202]. SRC-1 from hypothalamus or hippocampus interacted with ERα and ERβ when bound to estradiol (Figure 3A), which was confirmed by mass spectrometry [202]. SRC-1 may function with ERα in the hypothalamus to mediate expression of female sexual behavior [21; 22; 23; 203], and with both ER subtypes in the hippocampus to differentially modulate estrogen’s effects on cognition [24; 204] and stress [24; 205]. Very little to no association of SRC-1 from brain was detected with ERα or ERβ in the absence of ligand or in the presence of the selective ER modulator (SERM) tamoxifen (Figure 3A). These findings suggest tamoxifen is functioning as an antagonist to prevent receptor-coactivator interactions, and are consistent with a variety of studies using cell lines demonstrating that estradiol facilitates, while antagonists prevent, SRC-1 association with ER [206; 207; 208]. In contrast to these findings using brain tissue, cell culture studies suggest that both ERα and ERβ can recruit coactivators to AF-1 in the absence of ligand under certain phosphorylation conditions [209; 210]. In the absence of ligand, little to no interactions between receptor and SRC-1 from brain were detected, and therefore it will be important to investigate whether physiologically-relevant events that modulate ligand-independent activation impact on receptor-coactivator interactions in brain.
Figure 3. SRC-1 from rat brain associates with ERα and ERβ in a ligand-dependent and receptor isoform-specific manner.
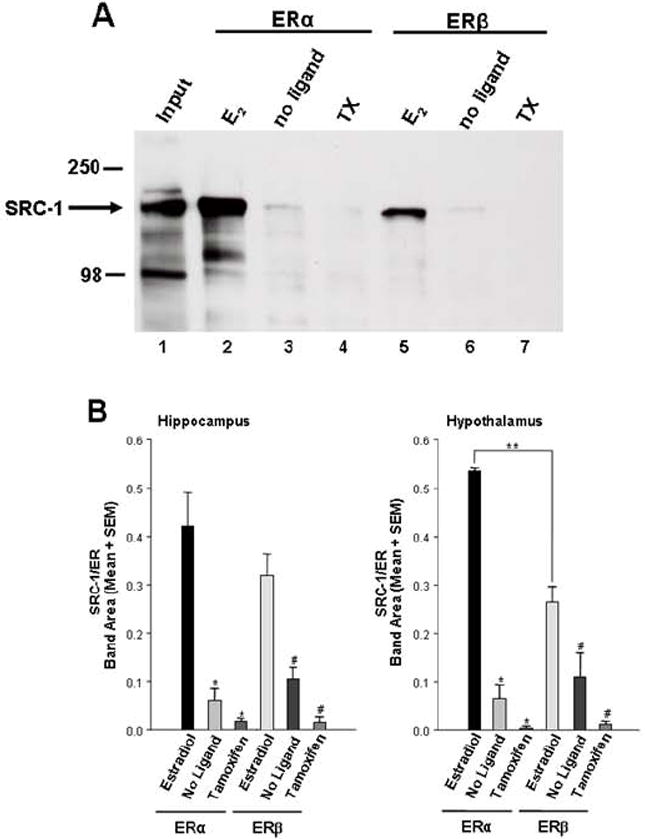
A) SRC-1 from the hypothalamus associates with ERα and ERβ in the presence of estradiol (lanes 2 and 5), but not in the absence of ligand (lanes 3 and 6), or in the presence of the SERM, tamoxifen (lanes 4 and 7). Input (1% of total) of SRC-1 from hypothalamic extract is shown in Lane 1.
B) In the presence of estradiol, both ERα and ERβ interacted with hippocampal SRC-1, but little to no interactions were detected in the absence of ligand or when receptors were bound to Tamoxifen. Hypothalamic SRC-1 interacted more strongly with ERα than ERβ in the presence of estradiol * p < 0.0001, significantly different from ERα + estradiol. # p < 0.01, significantly different from ERβ + estradiol. ** p < 0.05, t-test, n = 4-5 per treatment group. From [202], Copyright 2008, The Endocrine Society.
In the same studies discussed above [202], SRC-1 from the hippocampus appears to interact equally with ERα and ERβ (Figure 3B). In contrast, SRC-1 obtained from hypothalamic extracts interacted more with ERα than with ERβ (Figure 3B). The different functions of the ER subtypes in brain (discussed above) may be explained in part by the lower transcriptional activity of ERβ observed in particular cell lines [25]. These differences in transcriptional abilities between ERα and ERβ may be attributed to differential recruitment of coactivators, or differences in the ability of the same coactivator to facilitate transcription of the ER subtypes [211]. While some studies using recombinant SRC-1 [211] are consistent with the findings that SRC-1 from brain interacts more with ERα than with ERβ, other studies suggest that SRC-1 associates equally with each ER subtype [212; 213]. While these later findings are consistent with the results using SRC-1 from hippocampus, it was found that SRC-1 from hypothalamus interacted more with ERα than with ERβ. These data suggest that ERα is a more efficient transcriptional activator of SRC-1 dependent signaling pathways in the hypothalamus than ERβ [202]. In support, previous findings indicate that SRC-1 function in the hypothalamus is important for maximal expression of ER-mediated female sexual behavior [195], which appears to be ERα-dependent [21; 214]. In addition, SRC-1 from brain interacts more with PR-B than with PR-A [202]. These differential interactions of SRC-1 from hypothalamus or hippocampus with the ER and PR subtypes suggest that these brain regions have distinct expression patterns of coregulators involved in these important protein-protein interactions. In addition, it is possible that SRC-1 undergoes differential phosphorylation in these two brain regions, leading to distinct patterns of interaction with receptors. Future experiments will need to apply mass spectrometry analysis to determine if, in a brain region specific manner, different coregulators are present in the receptor-coactivator complex and/or if SRC-1 undergoes differential phosphorylation. Finally, these findings suggest the importance of using biologically-relevant tissue, in contrast to the use of cell lines alone, in investigating receptor-coactivator interactions. It may be that other coregulators and proteins that are present in tissue (e.g. brain) are important for appropriate SRC-1 interactions with receptor. Understanding how nuclear receptor coactivators function with various steroid receptors, and their subtypes, is critical to understanding how hormones act in different brain regions to profoundly influence physiology and behavior. Ultimately, investigation of these receptor-coactivator interactions using brain tissue may allow the identification of novel coregulators involved in the steroid receptor complex in brain.
The function of coactivators has also been studied in hormone action in bird brain. To test whether SRC-1 was involved in steroid-dependent male sexual behavior in Japanese quail, locked nucleic acid-derived antisense oligodeoxynucleotides were used to down-regulate the expression of SRC-1 in the medial preoptic nucleus, a key center in the control of male sexual behavior [215]. Reducing the expression of SRC-1 in this region decreased the testosterone-induced mounts and cloacal contact movement (similar to intromission and ejaculation in rat) and the number of struts, a pre- and post-ejaculatory display (Figures 4A & B). It should be noted that the expression of the copulatory behavior is ER-dependent, while struts are known to be mediated by AR [216; 217]. The behavioral inhibition induced by the blockade of SRC-1 expression was paralleled in the medial preoptic nucleus by major neuroanatomical and neurochemical modifications [218]. The volume of the nucleus defined by Nissl staining and by aromatase-immunoreactive material was reduced in SRC-1 depleted males compared to controls (Figure 4C). The number of vasotocine fibers, another marker of testosterone action [219], was also reduced [218]. The confirmation of a significant role of SRC-1 for steroid-dependent behavior and physiology was obtained from birds in which SRC-1 was up-regulated. This up-regulation of SRC-1 protein in the medial preoptic region was observed forty-eight hours after the termination of the antisense treatment, probably resulting from a rebound of SRC-1 expression (see [218] for further explanation). This up-regulation of SRC-1 expression in the medial preoptic area was associated with a pronounced increase in copulatory behavior and a parallel increase in the volume of the median preoptic nucleus [218]. Taken together, these data strongly support that idea that SRC-1 is an important modulator of estrogen and androgen-dependent gene transcription and behavior in quail brain. Surprisingly, further investigations of the role of SRC-1 on aromatase expression and activity suggested a dissociation between the effect of testosterone on male sexual behavior and the neurochemical plasticity of the median preoptic nucleus. Indeed, while sexual behavior was significantly reduced after inhibition of SRC-1 expression, no change in the preoptic area volume or aromatase activity was observed [162]. The behavioral inhibition is likely to have resulted from an effect of the SRC-1 depletion on other neurochemical systems, such as the peptidergic system, involved in the control of male sexual behavior [220; 221]. The extent to which SRC-1 protein expression was down-regulated was smaller in these last studies [162] compared to the first experiment [218]. These studies reveal important information about the relative threshold of effects of steroids (e.g. testosterone) on related traits, such as male sexual behavior and aromatase expression.
Figure 4. Down-regulation of SRC-1 affects testosterone-dependent male sexual behavior and medial preoptic area morphology in Japanese quail.
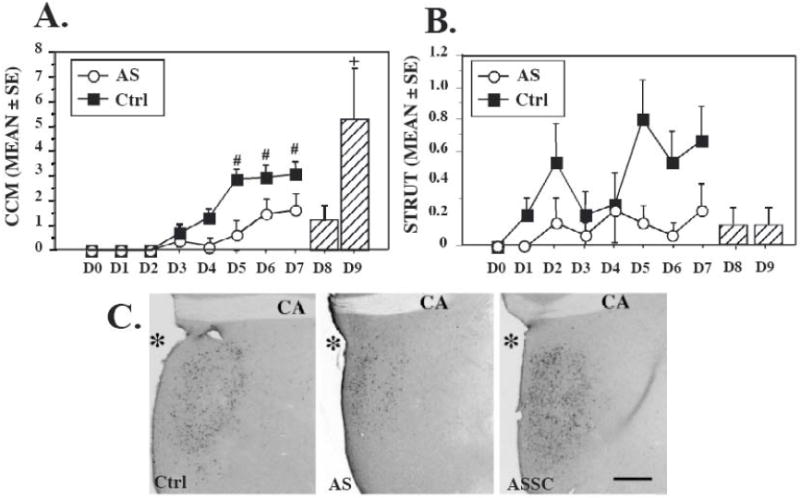
Inhibitory effects of SRC-1 down-regulation on the frequency of two testosterone–dependent male sexual behaviors, A) cloacal contact movements (CCM, estrogen-dependent) and B) struts, a pre-and post-copulatory display (androgen-dependent). Frequency of behavior was recorded each day from D0 to D7. Average (±SE) are shown for controls (Ctrl) and antisense-injected (AS) subjects. Mixed-two way ANOVA revealed a significant main effect of antisense injections on the frequencies of both cloacal contact movements and struts. Post hoc Tukey’s tests: # p<0.01versus control on the corresponding day. Bar graph on the right illustrates the behavioral recovery after cessation of the antisense treatment in AS treated birds for which antisense injections were replaced on day 8 and 9 by control injections and behavioral testing was continued for two additional days (D8-D9). This group is referred as ASSC. + p<0.05 versus AS at D7. It should be noted that only male copulatory behavior per se was restored and even increased compared to control group, while the frequency of struts remained low. C. Effects of manipulations of SRC-1 expression on the testosterone-dependent increase of the preoptic median nucleus (POM) volume identified in sections stained for aromatase. The figure illustrates the POM in a control (left), AS (middle), and ASSC (right) subject. CA, Anterior commissure; *, third ventricle; Scale bar, 200 μm. [218]
Nuclear receptor corepressor function in brain and behavior
Corepressor expression and regulation in brain
Few studies have examined the expression and regulation of corepressor expression in brain. One particular study found that both NCoR and SMRT were expressed ubiquitously throughout the brain with some small anatomical differences in expression, such as higher levels of SMRT in the paraventricular nucleus and the substantia nigra [222]. The regulation of corepressor expression appears to be complex. Some early studies reported no influence of estradiol or thyroid hormone on NCoR and SMRT mRNA expression in male or female hypothalami [223]. Furthermore, another study examined the developmental profile of NCoR and SMRT and the potential impact of thyroid hormone on their expression within the cerebellum. It was found that NCoR, but not SMRT, expression increased over neonatal development within this area and this increase appeared to be independent of thyroid hormone [141]. A later study found that NCoR, as well as SRC-1, mRNA was decreased in the cortex and dentate gyrus, but not the CA3 region, in hypothyroid-induced rats [169]. Therefore, it appears that thyroid hormone may regulate corepressor expression in a brain region-specific manner. Interestingly, some of the same processes (i.e. hormones and 26S proteasome) that regulate ER and PR expression appear to regulate corepressors, such as SMRT, in adult female rat brain [186]. Indeed, recently it was reported that estradiol leads to down-regulation of NCoR, but not SMRT, expression in MCF-7 cells [224]. This down-regulation of NCoR by estradiol is likely to influence the expression of numerous downstream targets and provides a mechanism by which estradiol can have a broad genomic impact. It was also found recently that the levels of SMRT were lowest at metestrus within the preoptic area of adult female rat brain (NCoR expression was not examined in this study) [164]. These findings suggest that some other hormone, besides estradiol, could regulate SMRT expression in adult female rat brain.
Corepressors and brain development
While the functional roles of corepressors in brain are currently unclear, mounting evidence indicates that NCoR, as well as other corepressors, play a major role in cellular differentiation during early brain development [225]. As mentioned above, NCoR appears to be critical for the suppression of glia differentiation and essential in maintaining neural stem cells in an undifferentiated multi-potent state in developing brain [135]. In support, mice containing a disruption of NCoR have a 70% increase in cells expressing glia fibrillary acidic protein (GFAP), a marker of astroglia differentiation. As sex differences in glia differentiation exist in developing brain, it will be interesting to determine if sex differences in NCoR expression underlie these sex differences [226]. The sub-cellular localization of NCoR within the brain has also been correlated with abnormal brain function, such as the increased cytoplasmic localization found in individuals with Huntington’s Disease [227]. In drosophila, NCoR physically interacts with MeCP2 [228], the apparent cause of Rett syndrome [229]. NCoR also appears to associate with DISC1 (disrupted-in-schizophrenia) [230], a potential key factor in schizophrenia [231; 232]. NCoR also interacts directly with CBP, suggesting that it can modify coactivator function [233]. Therefore, while NCoR is of key interest in investigating neurodevelopmental and behavioral disorders that are also sexually dimorphic, its function in modulating steroid receptor activity in developing or adult brain remains relatively unexplored.
Summary and future directions
The mechanisms by which steroids act in a region-specific, and cell type-specific, manner is a fundamental issue in steroid hormone action in brain. Recent investigations indicate that, in addition to the bioavailability of hormone and receptor levels, nuclear receptor coregulators are critical molecules in modulating steroid receptor-mediated transcription. Studies from cell lines have revealed much about the molecular mechanisms of action of these coactivators and corepressors. Furthermore, work in brain, as well as other steroid-sensitive tissues, indicates that nuclear receptor coregulators are critical in the fine-tuning of steroid-responsiveness within individual cells. In order to understand how hormones function in the brain to regulate complex behaviors, it will be critical to understand the recruitment of different coactivator and corepressor complexes to the promoter, which is likely to be cell- and tissue-specific. Finally the distinction between coactivator and corepressor is becoming less clear. For example, some in vitro studies now suggest that SRC-1 can act as a repressor of ligand-activated TR [234] and GR [71; 235; 236]. It is increasingly apparent that the cellular environment and the complexity of the coregulator complex are important parameters in the modulation of steroid action in the brain and behavior.
Acknowledgments
Studies contributed by the author’s laboratory were supported by grants from National Science Foundation IBN 0080818 and National Institutes of Health R01 DK61935 (MJT) and R01 MH072956 (APA) and a Fonds National de la Recherche Scientifique-FRS postdoctoral fellowship (TDC).
Abbreviations
- AF
activation function domain
- AR
androgen receptor
- ARC
arcuate nucleus
- AS
antisense oligodeoxynucleotides
- CBP
CREB binding protein
- DNMT
DNA methyltransferase
- E6-AP
E6-associated protein
- EB
estradiol benzoate
- ER
estrogen receptor
- ERAP140
estrogen receptor associated protein 140
- GnRH
gonadotropins-releasing hormone
- GR
glucocorticoid receptor
- GST
glutathione S-transferase tag
- Hipp
hippocampus
- HDAC
histone deacetylase
- hsp
heat shock protein
- Hyp
hypothalamus
- IHC
immunohistochemistry
- IP
immunoprecipitation
- IR
immunoreactivity
- MCG
midbrain central gray
- MPOA
medial preoptic area
- NCoR
nuclear receptor corepressor
- ODN
oligodeoxynucleotides
- p/CAF
p300/CBP associated factor
- PR
progestin receptor
- R5020
a progestin agonist
- RARα
retinoic acid receptor alpha
- RPL7
ribosomal large subunit protein 7
- RXR
retinoid X receptor
- SDN
sexually dimorphic nucleus
- SERM
selective estrogen receptor modulator (e.g. tamoxifen)
- SMRT
Silencing Mediator of Retinoid and Thyroid Hormone Receptors
- SPRM
selective progestin receptor modulator (e.g. RU486)
- SRA
steroid receptor RNA activator
- SRC-1/2/3
steroid receptor coactivator-1/2/3
- SRE
steroid response element
- TR
thyroid hormone receptor
- TRAP220
thyroid hormone receptor associating protein 220
- VMH
ventromedial hypothalamus
- VMN
ventromedial nucleus of the VMH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual Review of Biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 4.O’Malley BW. Molecular biology. Little molecules with big goals. Science. 2006;313:1749–1750. doi: 10.1126/science.1132509. [DOI] [PubMed] [Google Scholar]
- 5.Mani S. Mini Review: Progestin Receptor Subtypes in the Brain: The known and the Unknown. Endocrinology. 2008 doi: 10.1210/en.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- 7.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: An emerging mechanism of estrogen action in brain. Molecular Neurobiology. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in Neuroendocrinology. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Frontiers in Neuroendocrinology. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Malley BW. Coregulators: from whence came these “master genes”. Mol Endocrinol. 2007;21:1009–1013. doi: 10.1210/me.2007-0012. [DOI] [PubMed] [Google Scholar]
- 12.Lanz RB, Lonard DM, O’Malley B W. Nuclear receptor coregulators in human diseases. In: Kumar R, O’Malley B W, editors. Nuclear receptor coregulators and human diseases. World Scientific Books; 2008. pp. 1–133. [Google Scholar]
- 13.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent non-acidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 14.Jensen EV, Suzuki T, Kawasima T, Stumpf WE, Jungblut PW, de Sombre ER. A two-step mechanism for the interaction of estradiol with rat uterus. Proceedings of the National Academy of Sciences USA. 1968;59:632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 17.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: Effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- 19.Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–80. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 20.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 22.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene- deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584–90. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- 26.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO Journal. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Molecular Endocrinology. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 28.Tung L, Kamel Mohamed M, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Molecular Endocrinology. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- 29.Giangrande PH, Pollio G, McDonnell DP. Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. Journal of Biological Chemistry. 1997;272:32889–32900. doi: 10.1074/jbc.272.52.32889. [DOI] [PubMed] [Google Scholar]
- 30.Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Molecular Endocrinology. 1994;8:1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- 31.Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM. Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Molecular Endocrinology. 2006;20:1322–1332. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- 32.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- 33.Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- 34.DeMarzo A, Beck CA, Oñate SA, Edwards DP. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proceedings of the National Academy of Sciences USA. 1991;88:72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein-Hitpass L, Tsai SY, Weigel NL, Allan GF, Riley D, Rodriguez R, Schrader WT, Tsai MJ, O’Malley BW. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990;60:247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- 36.Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaff D. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. J Endocrinol. 2005;184:447–53. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- 38.Blaustein JD, Mani SK. Feminine sexual behavior from neuroendocrine and molecular neurobiological perspectives. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Neurobiology. Springer; New York: 2006. pp. 95–150. [Google Scholar]
- 39.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 40.Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 41.McInerney EM, Tsai MJ, O’Malley BW, Katzenellenbogen BS. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proceedings of the National Academy of Sciences USA. 1996;93:10069–10073. doi: 10.1073/pnas.93.19.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanenbaum DM, Wang Y, Williams SP, Sigler PB. Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci U S A. 1998;95:5998–6003. doi: 10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 44.Oñate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O’Malley BW. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. Journal of Biological Chemistry. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 45.Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Molecular Endocrinology. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 46.Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-Binding Protein (CBP) with estrogen receptor- à: Regulation by phosphorylation sites in the A/B region depends on other receptor domains. Molecular Endocrinology. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- 47.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 48.Robyr D, Wolffe AP, Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol Endocrinol. 2000;14:329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- 49.Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO Journal. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi Y, Kitamoto T, Masuhiro Y, Watanabe M, Kase T, Metzger D, Yanagisawa J, Kato S. p300 Mediates Functional Synergism between AF-1 and AF-2 of Estrogen Receptor alpha and beta by Interacting Directly with the N-terminal A/B Domains. Journal of Biological Chemistry %19. 2000;275:15645–15651. doi: 10.1074/jbc.M000042200. [DOI] [PubMed] [Google Scholar]
- 51.Wardell SE, Boonyaratanakornkit V, Adelman J, Aronheim A, Edwards DP. Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator. Molecular and Cellular Biology. 2002;22:5451–5466. doi: 10.1128/MCB.22.15.5451-5466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lonard DM, O’Malley BW. Expanding functional diversity of the coactivators. Trends Biochem Sci. 2005;30:126–132. doi: 10.1016/j.tibs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Lonard DM, Lanz RB, O’Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–87. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- 54.Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Molecular and Cellular Biology. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 56.Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. Journal of Biological Chemistry. 1998;273:27645–27653. doi: 10.1074/jbc.273.42.27645. [DOI] [PubMed] [Google Scholar]
- 57.Wu RC, Smith CL, O’Malley BW. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr Rev. 2005;26:393–399. doi: 10.1210/er.2004-0018. [DOI] [PubMed] [Google Scholar]
- 58.Vasudevan N, Zhu YS, Daniel S, Koibuchi N, Chin WW, Pfaff D. Crosstalk between oestrogen receptors and thyroid hormone receptor isoforms results in differential regulation of the preproenkephalin gene. J Neuroendocrinol. 2001;13:779–90. doi: 10.1046/j.1365-2826.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- 59.McKenna NJ, Nawaz Z, Tsai SY, Tsai MJ, O’Malley BW. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proceedings of the National Academy of Sciences USA. 1998;95:11697–11702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 61.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–197. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 62.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 63.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 64.Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, Qiu Y, Demayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 66.Weiss RE, Xu J, Ning G, Pohlenz J, O’Malley BW, Refetoff S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J. 1999;18:1900–1904. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishihara E, Yoshida-Kimoya H, Chan C, Liao L, Davis RL, O’Malley BW, Xu J. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. The Journal of Neuroscience. 2003;23:213–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Qi C, Krones A, Woodring P, Zhu X, Reddy JK, Evans RM, Rosenfeld MG, Hunter T. Critical roles of the p160 transcriptional coactivators p/CIP and SRC-1 in energy balance. Cell Metab. 2006;3:111–22. doi: 10.1016/j.cmet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Kalkhoven E, Valentine JE, Heery DM, Parker MG. Isoforms of steroid receptor co-activator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J. 1998;17:232–243. doi: 10.1093/emboj/17.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meijer OC, Kalkhoven E, van der LS, Steenbergen PJ, Houtman SH, Dijkmans TF, Pearce D, de Kloet ER. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology. 2005;146:1438–1448. doi: 10.1210/en.2004-0411. [DOI] [PubMed] [Google Scholar]
- 71.van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–32. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- 72.Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Molecular and Cellular Biology. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee A, Amato P, Allred DC, DeMayo FJ, Lydon JP. Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nucl Recept Signal. 2007;5:e011. doi: 10.1621/nrs.05011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandez-Valdivia R, Mukherjee A, Amato P, Allred DC, Nguyen J, DeMayo FJ, Lydon JP. Progesterone-action in the murine uterus and mammary gland requires steroid receptor coactivator 2: relevance to the human. Front Biosci. 2007;12:3640–7. doi: 10.2741/2340. [DOI] [PubMed] [Google Scholar]
- 75.Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O’Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006;26:6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O’Malley B W, Demayo FJ. The p160 steroid receptor coactivator-2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–50. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- 77.Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci U S A. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Molecular Cell. 2004;15:937–949. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 79.Xu J, Liao L, Ning G, Yoshida-Kimoya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/cip/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proceedings of the National Academy of Sciences USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han SJ, Demayo FJ, Xu J, Tsai SY, Tsai MJ, O’Malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- 81.An BS, Selva DM, Hammond GL, Rivero-Muller A, Rahman N, Leung PC. Steroid receptor coactivator-3 is required for progesterone receptor trans-activation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J Biol Chem. 2006;281:20817–24. doi: 10.1074/jbc.M600743200. [DOI] [PubMed] [Google Scholar]
- 82.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 83.Kwok RPS, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SGE, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–229. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 84.Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 85.Smith CL, Oñate SA, Tsai MJ, O’Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proceedings of the National Academy of Sciences USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–8. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 87.Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van OGJ, Goodman RH, Peters DJ. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 88.Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- 89.Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Molecular Endocrinology. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- 90.Liu Z, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc Natl Acad Sci U S A. 2001;98:12426–12431. doi: 10.1073/pnas.231474798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Y, Klein-Hitpass L, Bagchi MK. E1A-mediated repression of progesterone receptor-dependent transactivation involves inhibition of the assembly of a multisubunit coactivation complex. Mol Cell Biol. 2000;20:2138–46. doi: 10.1128/mcb.20.6.2138-2146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with E6 oncoprotein of human paillomavirus types 16 ort 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huibregtse JM, Scheffner M, Howley M. cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of thehuman papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O’Malley BW. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z. Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology. 2005;146:1707–1712. doi: 10.1210/en.2004-1198. [DOI] [PubMed] [Google Scholar]
- 96.Khan OY, Fu G, Ismail A, Srinivasan S, Cao X, Tu Y, Lu S, Nawaz Z. Multifunction steroid receptor coactivator, E6-associated protein, is involved in the development of the prostate gland. Mol Endocrinol. 2006;20:544–559. doi: 10.1210/me.2005-0110. [DOI] [PubMed] [Google Scholar]
- 97.Mani A, Oh AS, Bowden ET, Lahusen T, Lorick KL, Weissman AM, Schlegel R, Wellstein A, Riegel AT. E6AP mediates regulated proteosomal degradation of the nulcear receptor coactivator amplified in breast cancer 1 in imortalized cells. Cancer Res. 2006;66:8680–8686. doi: 10.1158/0008-5472.CAN-06-0557. [DOI] [PubMed] [Google Scholar]
- 98.Nuber U, Schwarz SE, Scheffner M. The ubiquitin-protein ligase E6-associated protein (E6-AP) serves as its own substrate. Eur J Biochem. 1998;254:643–649. doi: 10.1046/j.1432-1327.1998.2540643.x. [DOI] [PubMed] [Google Scholar]
- 99.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 100.Smith CL, DeVera DG, Lamb DJ, Nawaz Z, Jiang YH, Beaudet AL, O’Malley BW. Genetic ablation of the steroid receptor coactivator-ubiquitin ligase, E6-AP, results in tissue-selective steroid hormone resistance and defects in reproduction. Mol Cell Biol. 2002;22:525–535. doi: 10.1128/MCB.22.2.525-535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vaeteewoottacharn K, Chamutpong S, Ponglikitmongkol M, Angeletti PC. Differential localization of HPV16 E6 splice products with E6-associated protein. Virol J. 2005;16:50. doi: 10.1186/1743-422X-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shao W, Halachmi S, Brown M. ERAP140, a conserved tissue-specific nuclear receptor coactivator. Molecular and Cellular Biology. 2002;22:3358–3372. doi: 10.1128/MCB.22.10.3358-3372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cavarretta ITR, Mukopadhyay R, Lonard DM, Cowsert LM, Bennet CF, O’Malley B, Smith CL. Reduction of coactivator expression by antisense oligodeoxynucleotides inhibits ERa transcriptional activity and MCF-7 proliferation. Molecular Endocrinology. 2002;16(2):253–269. doi: 10.1210/mend.16.2.0770. [DOI] [PubMed] [Google Scholar]
- 104.Lanz RB, McKenna NJ, Oñate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O’Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 105.Lanz RB, Chua SS, Barron N, Soder BM, DeMayo F, O’Malley BW. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Molecular and Cellular Biology. 2003;23:7163–7176. doi: 10.1128/MCB.23.20.7163-7176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neumann F, Hemmerich P, von Mikecz A, Peter H H, Krawinkel U. Human ribosomal protein L7 inhibits cell-free translation in reticulocyte lysates and affects the expression of nuclear proteins upon transfection in Jurkat T-lymphoma. Nucleic Acids Res. 1995;23:195–202. doi: 10.1093/nar/23.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meyuhas O, Klein A. The mouse ribosomal protein L7 gene. Its primary structure and functional analysis of the promoter region. J Biol Chem. 1990;265:11465–11473. [PubMed] [Google Scholar]
- 108.Hemmerich P, von Mikecz A, Neumann F, Sözeri O, Wolff-Vorbeck G, Zoebelein R, Krawinkel U. Structural and functional properties of ribosomal protein L7 from humans and rodents. Nucleic Acid Res. 1993;21:223–231. doi: 10.1093/nar/21.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.von Mikecz A, Neu E, Krawinkel U, Hemmerich P. Human ribosomal protein L7 carries two nucleic acid-binding domains with distinct specificities. Biochem Biophys Res Commun. 1999;258:530–536. doi: 10.1006/bbrc.1999.0682. [DOI] [PubMed] [Google Scholar]
- 110.Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Molecular Endocrinology. 1997;11:693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- 111.Nephew KP, Ray S, Hlaing M, Ahluwalia A, Wu SD, Long X, Hyder SM, Bigsby RM. Expression of estrogen receptor coactivators in the rat uterus. Biology of Reproduction. 2000;63:361–367. doi: 10.1095/biolreprod63.2.361. [DOI] [PubMed] [Google Scholar]
- 112.Hlaing M, Nam K, Lou J, Pope WF, Nephew KP. Evidence for expression of estrogen receptor cofactor messenger ribonucleic acid in the ovary and uterus of domesticated animals (sheep, cow, and pig) Life Sci. 2001;68:1427–1438. doi: 10.1016/s0024-3205(01)00937-7. [DOI] [PubMed] [Google Scholar]
- 113.Duncan KA, Carruth LL. The sexually dimorphic expression of L7/SPA, an estrogen receptor coactivator, in zebra finch telencephalon. Dev Neurobiol. 2007 doi: 10.1002/dneu.20539. [DOI] [PubMed] [Google Scholar]
- 114.Maden M. Retinoid signaling of the central nervous system. Nat Rev Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- 115.McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-Implications for brain development. J Steroid Biochem Mol Biol. 2004;89(90):557–560. doi: 10.1016/j.jsbmb.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 116.Berghöfer-Hochheimer Y, Zurek C, Wölfl S, Hemmerich P, Munder T. L7 protein is a coregulator of vitamin D receptor-retinoid X receptor-mediated transactivation. J Cell Biochem. 1998;69:1–12. doi: 10.1002/(sici)1097-4644(19980401)69:1<1::aid-jcb1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 117.O’Malley BW, McKenna NJ. Editorial: Coactivators and Corepressors: What’s in a Name? Mol Endocrinol. 2008;22:2213–4. doi: 10.1210/me.2008-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ito M, Yuan CX, Malik S, Gu W, Fondell JD, Yamamura S, Fu ZY, Zhang X, Qin J, Roeder RG. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol Cell. 1999;3:361–70. doi: 10.1016/s1097-2765(00)80463-3. [DOI] [PubMed] [Google Scholar]
- 119.Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP. Modulation of Estrogen Receptor-alpha Transcriptional Activity by the Coactivator PGC-1. Journal of Biological Chemistry. 2000;275:16302–16308. doi: 10.1074/jbc.M001364200. [DOI] [PubMed] [Google Scholar]
- 120.Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Molecular and Cellular Biology. 1998;18:4471–4487. doi: 10.1128/mcb.18.8.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brady ME, Ozanne DM, Gaughan L, Waite I, Cook S, Neal DE, Robson CN. Tip60 is a nuclear hormone receptor coactivator. J Biol Chem. 1999;274:17599–604. doi: 10.1074/jbc.274.25.17599. [DOI] [PubMed] [Google Scholar]
- 122.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 123.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–93. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 124.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends in Biochemical Sciences. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Cunliffe VT. Eloquent silence: developmental functions of Class I histone deacetylases. Curr Opin Genet Dev. 2008;18:404–10. doi: 10.1016/j.gde.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 127.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 128.Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, McEvilly RJ, Kurokawa R, Kumar V, Liu F, Seto E, Hedrick SM, Mandel G, Glass CK, Rose DW, Rosenfeld MG. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–763. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 129.Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci U S A. 2008;105:19544–19549. doi: 10.1073/pnas.0804604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karagianni P, Wong J. HDAC3: taking the SMRT-N-CoRrect road to repression. Oncogene. 2007;26:5439–5449. doi: 10.1038/sj.onc.1210612. [DOI] [PubMed] [Google Scholar]
- 131.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 132.Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 133.Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 134.Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell. 2003;12:723–34. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 135.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419:934–939. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- 136.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2381. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 137.Sasaki H, Hayakawa J, Terai Y, Kanemura M, Tanabe-Kimura A, Kamegai H, Seino-Noda H, Ezoe S, Matsumura I, Kanakura Y, Sakata M, Tasaka K, Ohmichi M. Difference between genomic actions of estrogen versus raloxifene in human ovarian cancer cell lines. Oncogene. 2008;27:2737–2745. doi: 10.1038/sj.onc.1210926. [DOI] [PubMed] [Google Scholar]
- 138.Peterson TJ, Karmakar S, Pace MC, Gao T, Smith CL. The silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) corepressor is required for full estrogen receptor alpha transcriptional activity. Mol Cell Biol. 2007;27:5933–48. doi: 10.1128/MCB.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Misiti S, Schomburg L, Yen PM, Chin WW. Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology. 1998;139:2493–2500. doi: 10.1210/endo.139.5.5971. [DOI] [PubMed] [Google Scholar]
- 140.Shearman LP, Zylka MJ, Reppert SM, Weaver DR. Expression of basic helix-loop-helix/PAS genes in the mouse suprachiasmatic nucleus. Neuroscience. 1999;89:387–397. doi: 10.1016/s0306-4522(98)00325-x. [DOI] [PubMed] [Google Scholar]
- 141.Martinez de Arrieta C, Koibuchi N, Chin WW. Coactivator and corepressor gene expression in rat cerebellum during postnatal development and the effect of altered thyroid status. Endocrinology. 2000;141:1693–1698. doi: 10.1210/endo.141.5.7467. [DOI] [PubMed] [Google Scholar]
- 142.Meijer OC, Steenbergen PJ, de Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- 143.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 mediates the development of sex specific brain morphology and behavior. Proceedings of the National Academy of Sciences USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 145.Ogawa H, Nishi M, Kawata M. Localization of nuclear coactivators p300 and steroid receptor coactivator 1 in the rat hippocampus. Brain Research. 2001;890:197–202. doi: 10.1016/s0006-8993(00)03158-9. [DOI] [PubMed] [Google Scholar]
- 146.Charlier TD, Lakaye B, Ball GF, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology. 2002;76:297–315. doi: 10.1159/000066624. [DOI] [PubMed] [Google Scholar]
- 147.Charlier TD, Balthazart J, Ball GF. Sex differences in the distribution of the steroid coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Research. 2003;959:263–274. doi: 10.1016/s0006-8993(02)03758-7. [DOI] [PubMed] [Google Scholar]
- 148.McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figuiera HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiology and Behavior. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O’Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Molecular Endocrinology. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 150.Setiawan E, Owen D, McCabe L, Kostaki A, Andrews MH, Matthews SG. Glucocorticoids do not alter developmental expression of hippocampal or pituitary steroid receptor coactivator-1 and -2 in the late gestation fetal guinea pig. Endocrinology. 2004;145:3796–3803. doi: 10.1210/en.2003-1723. [DOI] [PubMed] [Google Scholar]
- 151.Stromberg H, Svensson SP, Hermanson O. Distribution of CREB-binding protein immunoreactivity in the adult rat brain. Brain Res. 1999;818:510–514. doi: 10.1016/s0006-8993(98)01219-0. [DOI] [PubMed] [Google Scholar]
- 152.Auger CJ, Bentley GE, Auger AP, Ramamurthy M, Ball GF. Expression of cAMP response element binding protein-binding protein in the song control system and hypothalamus of adult European starlings (Sturnus vulgaris) Journal of Neuroendocrinology. 2002;14:805–813. doi: 10.1046/j.1365-2826.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- 153.Galeeva A, Treuter E, Tuohimaa P, Pelto-Huikko M. Comparative distribution of the mammalian mediator subunit thyroid hormone receptor-associated protein (TRAP220) mRNA in developing and adult rodent brain. European Journal of Neuroscience. 2002;16:671–683. doi: 10.1046/j.1460-9568.2002.02115.x. [DOI] [PubMed] [Google Scholar]
- 154.Tetel MJ, Siegal NK, Murphy SD. Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors. J Neuroendocrinol. 2007;19:262–71. doi: 10.1111/j.1365-2826.2007.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- 156.Warembourg M, Jolivet A, Milgrom E. Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- 157.Meyer ME, Gronemeyer H, Bocquel B, Bocquel MT, Tasset D, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57:433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- 158.Dellovade TL, Kia HK, Zhu YS, Pfaff DW. Thyroid hormone coadministration inhibits the estrogen-stimulated elevation of preproenkephalin mRNA in female rat hypothalamic neurons. Neuroendocrinology. 1999;70:168–174. doi: 10.1159/000054473. [DOI] [PubMed] [Google Scholar]
- 159.Dellovade TL, Zhu YS, Krey L, Pfaff DW. Thyroid hormone and estrogen interact to regulate behavior. Proceedings of the National Academy of Sciences USA. 1996;93:12581–12586. doi: 10.1073/pnas.93.22.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vasudevan N, Ogawa S, Pfaff D. Estrogen and thyroid hormone receptor interactions; physiological flexibility by molecular specificity. Physiol Rev. 2002;82:923–944. doi: 10.1152/physrev.00014.2002. [DOI] [PubMed] [Google Scholar]
- 161.Bousios S, Karandrea D, Kittas C, Kitraki E. Effects of gender and stress on the regulation of steroid receptor coactivator-1 expression in the rat brain and pituitary. The Journal of Steroid Biocheminstry and Molecular Biology. 2001;78:401–407. doi: 10.1016/s0960-0760(01)00123-6. [DOI] [PubMed] [Google Scholar]
- 162.Charlier TD, Ball GF, Balthazart J. Plasticity in the expression of the steroid receptor coactivator-1 in the Japanese quail brain: Effect of sex, testosterone, stress and time of the day. Neuroscience. 2006;172:333–343. doi: 10.1016/j.neuroscience.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 163.Mitev YA, Wolf SS, Almeida OF, Patchev VK. Developmental expression profiles and distinct regional estrogen responsiveness suggest a novel role for the steroid receptor coactivator SRC-l as a discriminative amplifier of estrogen signaling in the rat brain. The FASEB Journal. 2003;17:518–519. doi: 10.1096/fj.02-0513fje. [DOI] [PubMed] [Google Scholar]
- 164.Camacho-Arroyo I, Neri-Gomez T, Gonzalez-Arenas A, Guerra-Araiza C. Changes in the content of steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid hormone receptors in the rat brain during the estrous cycle. The Journal of Steroid Biocheminstry and Molecular Biology. 2005;94:267–272. doi: 10.1016/j.jsbmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 165.Maerkel K, Durrer S, Henseler M, Schlumpf M, Lichtensteiger W. Sexually dimorphic gene regulation in brain as a target for endocrine disrupters: developmental exposure of rats to 4-methylbenzylidene camphor. Toxicol Appl Pharmacol. 2007;218:152–65. doi: 10.1016/j.taap.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 166.Tetel MJ, Ungar TC, Hassan B, Bittman EL. Photoperiodic regulation of androgen receptor and steroid receptor coactivator-1 in Siberian hamster brain. Molecular Brain Research. 2004;131:79–87. doi: 10.1016/j.molbrainres.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duncan KA, Clancy AN, Carruth LL. Society of Neuroscience. Washington D.C.: 2008. Hormonal regulation of estrogen receptor coactivators in adult male zebra finches; pp. 474–8. [Google Scholar]
- 168.Ramos HE, Weiss RE. Regulation of nuclear coactivator and corepressor expression in mouse cerebellum by thyroid hormone. Thyroid. 2006;16:211–6. doi: 10.1089/thy.2006.16.211. [DOI] [PubMed] [Google Scholar]
- 169.Iannacone EA, Yan AW, Gauger KJ, Dowling ALS, Zoeller RT. Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Molecular and Cellular Endocrinology. 2002;186:49–59. doi: 10.1016/s0303-7207(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 170.Kurihara I, Shibata H, Suzuki T, Ando T, Kobayashi S, Hayashi M, saito I, Saruta T. Expression and regulation of nuclear receptor coactivators in glucocorticoid action. Mol Cell Endocrinol. 2002;189:181–189. doi: 10.1016/s0303-7207(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 171.Pendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin BS, editors. Hormones, brain and behavior. Elsevier; 2002. pp. 93–156. [Google Scholar]
- 172.Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 173.Kriegsfeld LJ, LeSauter J, Hamada T, Pitts SM, Silver R. Circadian Rhythms in the endocrine system. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin BS, editors. Hormones, Brain and Behavior. Elsevier; 2002. pp. 33–91. [Google Scholar]
- 174.Chevillard-Briet M, Trouche D, Vandel L. Control of CBP co-activating activity by arginine methylation. EMBO. 2002;21:5457–5466. doi: 10.1093/emboj/cdf548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chauchereau A, Amazit L, Quesne M, Guiochon-Mantel A, Milgrom E. Sumoylation of the progesterone receptor and of the coactivator SRC-1. J Biol Chem. 2003 doi: 10.1074/jbc.M207148200. [DOI] [PubMed] [Google Scholar]
- 176.Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. Regulation of hormone-induced histone hyperactetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 177.Kotaja N, Karvonen U, Janne OA, O’Malley BW. The nuclear receptor interacting domain of GRIP1 is modulated by covalent attachment of SUMO-1. J Biol Chem. 2002;277:30283–30288. doi: 10.1074/jbc.M204768200. [DOI] [PubMed] [Google Scholar]
- 178.Rowan BG, Weigel NL, O’Malley BW. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. Journal of Biological Chemistry. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 179.Wu RC, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai M-J, O’Malley BW. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappaB kinase. Mol Cell Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Amazit L, Alj Y, Tyagi RK, Chauchereau A, Loosfelt H, Pichon C, Pantel J, Foulon-Guinchard E, Leclerc P, Milgrom E, Guiochon-Mantel A. Subcellular localization and mechanisms of nucleocytoplasmic trafficking of steroid receptor coactivator-1. J Biol Chem. 2003;278:32195–32203. doi: 10.1074/jbc.M300730200. [DOI] [PubMed] [Google Scholar]
- 181.Chen J, Halappanavar S, Th’ng JP, Li Q. Ubiquitin-dependent distribution of the transcriptional coactivator p300 in cytoplasmic inclusion bodies. Epigenetics. 2007;2:92–99. doi: 10.4161/epi.2.2.4326. [DOI] [PubMed] [Google Scholar]
- 182.Hoang T, F IS, Cook C, Børud B, Bakke M, Lien EA, Mellgren G. cAMP-dependent protein kinase regulates ubiquitin-proteasome-mediated degradation and subcellular localization of the nuclear receptor coactivator GRIP1. J Biol Chem. 2004;279:49120–49130. doi: 10.1074/jbc.M409746200. [DOI] [PubMed] [Google Scholar]
- 183.Wu RC, Feng Q, Lonard DM, O’Malley BW. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell. 2007;15:1125–1140. doi: 10.1016/j.cell.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 184.Higashimoto K, Kuhn P, Desai D, Cheng X, Xu W. Phosphorylation-mediated inactivation of coactivator-associated arginine methyltransferase 1. Proc Natl Acad Sci USA. 2007;104:12318–12323. doi: 10.1073/pnas.0610792104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yi P, Wu RC, Sandquist J, Wong J, Tsai SY, Tsai MJ, Means AR, O’Malley BW. Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1) Mol Cell Biol. 2005;25:9687–99. doi: 10.1128/MCB.25.21.9687-9699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Villamar-Cruz O, Manjarrez-Marmolejo J, Alvarado R, Camacho-Arroyo I. Regulation of the content of progesterone and estrogen receptors, and their cofactors SRC-1 and SMRT by the 26S proteasome in the rat brain during the estrous cycle. Brain Res Bull. 2006;69:276–281. doi: 10.1016/j.brainresbull.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 187.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 188.Zhang H, Yi X, Sun X, Yin N, Shi B, Wu H, Wang D, Wu G, Shang Y. Differential gene regulation by the SRC family of coactivators. Genes and Development. 2004;18:1753–1765. doi: 10.1101/gad.1194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Auboeuf D, Dowhan DH, Kang YK, Larkin K, Lee JW, Berget SM, O’Malley BW. Differential recruitment of nuclear receptor coactivators may determine alternative RNA splice site choice in target genes. Proceedings of the National Academy of Sciences USA. 2004;101:2270–2274. doi: 10.1073/pnas.0308133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- 191.McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 192.Auger AP, Perrot-Sinai TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 194.Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. Mol Endocrinol. 2006;20:254–267. doi: 10.1210/me.2005-0061. [DOI] [PubMed] [Google Scholar]
- 195.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Hormones and Behavior. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horms Behav. 1971;2:287–297. [Google Scholar]
- 197.Whalen RE. Estrogen-progesterone induction of mating in female rats. Horm Behav. 1974;5:157–162. doi: 10.1016/0018-506x(74)90040-3. [DOI] [PubMed] [Google Scholar]
- 198.Tennent BJ, Smith ER, Davidson JM. The effects of estrogen and progesterone on female rat proceptive behavior. Horms Behav. 1980;14:65–75. doi: 10.1016/0018-506x(80)90016-1. [DOI] [PubMed] [Google Scholar]
- 199.Edwards DA, Pfeifle JK. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol Behav. 1983;30:437–443. doi: 10.1016/0031-9384(83)90150-6. [DOI] [PubMed] [Google Scholar]
- 200.Erskine MS. Solicitation behavior in the estrous female rat: A review. Horms Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 201.Ogawa S, Olazabal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Molenda-Figueira HA, Murphy SD, Shea KL, Siegal NK, Zhao Y, Chadwick JG, Denner LA, Tetel MJ. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinology. 2008;149:5272–9. doi: 10.1210/en.2008-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–83. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 204.Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–64. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 205.Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–45. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- 206.Margeat E, Poujol N, Boulahtouf A, Chen Y, Muller JD, Gratton E, Cavailles V, Royer CA. The human estrogen receptor alpha dimer binds a single SRC-1 coactivator molecule with an affinity dictated by agonist structure. Journal of Molecular Biology. 2001;306:433–442. doi: 10.1006/jmbi.2000.4418. [DOI] [PubMed] [Google Scholar]
- 207.Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Molecular Endocrinology. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 208.Yi P, Driscoll MD, Huang J, Bhagat S, Hilf R, Bambara RA, Muyan M. The effects of estrogen-responsive element- and ligand-induced structural changes on the recruitment of cofactors and transcriptional responses by ER alpha and ER beta. Molecular Endocrinology. 2002;16:674–693. doi: 10.1210/mend.16.4.0810. [DOI] [PubMed] [Google Scholar]
- 209.Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-alpha: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- 210.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 211.Wong C, Komm B, Cheskis BJ. Structure-Function evaluation of ER alpha and beta interplay with SRC family coactivators. ER selective ligands. Biochemistry. 2001;40:6756–6765. doi: 10.1021/bi010379h. [DOI] [PubMed] [Google Scholar]
- 212.Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, Riggs BL, Spelsberg TC. Mutual antagonism of estrogen receptors alpha and beta and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol. 2003;176:349–357. doi: 10.1677/joe.0.1760349. [DOI] [PubMed] [Google Scholar]
- 213.Cowley SM, Parker MG. A comparison of transcriptional activation by ER alpha and ER beta. J Steroid Biochem Mol Biol. 1999;69:165–75. doi: 10.1016/s0960-0760(99)00055-2. [DOI] [PubMed] [Google Scholar]
- 214.Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 215.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pnieswski EE. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiol Behav. 1980;24:441–446. doi: 10.1016/0031-9384(80)90233-4. [DOI] [PubMed] [Google Scholar]
- 217.Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- 218.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. Journal of Neuroscience. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Panzica G, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid -induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- 220.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 221.Argiolas A. Neuropeptides and sexual behavior. Neurosci Biobehav Rev. 1999;23:1127–1142. doi: 10.1016/s0149-7634(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 222.van der Laan S, Lachize SB, Schouten TG, Vreugdenhil E, de Kloet ER, Meijer OC. Neuroanatomical distribution and colocalisation of nuclear receptor corepressor (N-CoR) and silencing mediator of retinoid and thyroid receptors (SMRT) in rat brain. Brain Res. 2005;1059:113–21. doi: 10.1016/j.brainres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 223.Misiti S, Koibuchi N, Bei M, Farsetti A, Chin WW. Expression of steroid receptor coactivator-1 mRNA in the developing mouse embryo: a possible role in olfactory epithelium development. Endocrinology. 1999;140:1957–1960. doi: 10.1210/endo.140.4.6782. [DOI] [PubMed] [Google Scholar]
- 224.Frasor J, Danes JM, Funk CC, Katzenellenbogen BS. Estrogen down-regulation of the corepressor N-CoR: mechanism and implications for estrogen derepression of N-CoR-regulated genes. Proc Natl Acad Sci U S A. 2005;102:13153–13157. doi: 10.1073/pnas.0502782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nishihara E, O’Malley BW, Xu J. Nuclear receptor coregulators are new players in nervous system development and function. Molecular Neurobiology. 2004;30:307–325. doi: 10.1385/MN:30:3:307. [DOI] [PubMed] [Google Scholar]
- 226.Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- 227.Boutell JM, Thomas P, Neal JW, Weston VJ, Duce J, Harper PS, Jones AL. Aberrant interactions of transcriptional repressor proteins with the Huntington’s disease gene product, huntingtin. Hum Mol Genet. 1999;8:1647–1655. doi: 10.1093/hmg/8.9.1647. [DOI] [PubMed] [Google Scholar]
- 228.Cukier HN, Perez AM, Collins AL, Zhou Z, Zoghbi HY, Botas J. Genetic modifiers of MeCP2 function in Drosophila. PLoS Genet. 2008;4:e1000179. doi: 10.1371/journal.pgen.1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 230.Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K, Shimoda M, Toda H, Sawamura-Yamamoto T, Makuch LA, Hayashi A, Ishizuka K, Cascella NG, Kamiya A, Ishida N, Tomoda T, Hai T, Furukubo-Tokunaga K, Sawa A. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry. 2008;13:1138–48. 1069. doi: 10.1038/mp.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mackie S, Millar JK, Porteous DJ. Role of DISC1 in neural development and schizophrenia. Curr Opin Neurobiol. 2007;17:95–102. doi: 10.1016/j.conb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 232.Muir WJ, Pickard BS, Blackwood DH. Disrupted-in-Schizophrenia-1. Curr Psychiatry Rep. 2008;10:140–7. doi: 10.1007/s11920-008-0025-2. [DOI] [PubMed] [Google Scholar]
- 233.Cowger JJ, Torchia J. Direct association between the CREB-binding protein (CBP) and nuclear receptor corepressor (N-CoR) Biochemistry. 2006;45:13150–13162. doi: 10.1021/bi060562g. [DOI] [PubMed] [Google Scholar]
- 234.Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu J, O’Malley BW, Weiss RE, Refetoff S. Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T(3)-regulated transcription of genes in vivo. Endocrinology. 2002;143(4):1346–1352. doi: 10.1210/endo.143.4.8730. [DOI] [PubMed] [Google Scholar]
- 235.Li GY, Heaton JH, Gelehrter TD. Role of steroid receptor coactivators in glucocorticoid and transforming growth factor b regulation of plasminogen activator inhibitor gene expression. Mol Endocrinol. 2006;20:1025–1034. doi: 10.1210/me.2005-0145. [DOI] [PubMed] [Google Scholar]
- 236.Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proceedings of the National Academy of Sciences USA. 2002;99:16701–16706. doi: 10.1073/pnas.262671599. [DOI] [PMC free article] [PubMed] [Google Scholar]


