Abstract
Pathogenesis in the Rpe65−/− mouse model of Leber's congenital amaurosis (LCA) is characterized by a slow and progressive degeneration of the rod photoreceptors. On the opposite, cones degenerate rapidly at early ages. Retinal degeneration in Rpe65 −/− mice, showing a null mutation in the gene encoding the retinal pigment epithelium 65-kDa protein (Rpe65), was previously reported to depend on continuous activation of a residual transduction cascade by unliganded opsin. However, the mechanisms of apoptotic signals triggered by abnormal phototransduction remain elusive. We previously reported that activation of a Bcl-2-dependent pathway was associated with apoptosis of rod photoreceptors in Rpe65−/− mice during the course of the disease. In this study we first assessed whether activation of Bcl-2-mediated apoptotic pathway was dependent on constitutive activation of the visual cascade through opsin apoprotein. We then challenged the direct role of pro-apoptotic Bax protein in triggering apoptosis of rod and cone photoreceptors.
Quantitative PCR analysis showed that increased expression of pro-apoptotic Bax and decreased level of anti-apoptotic Bcl-2 were restored in Rpe65−/−/Gnat1−/− mice lacking the Gnat1 gene encoding rod transducin. Moreover, photoreceptor apoptosis was prevented as assessed by TUNEL assay. These data indicate that abnormal activity of opsin apoprotein induces retinal cell apoptosis through the Bcl-2-mediated pathway. Following immunohistological and real-time PCR analyses, we further observed that decreased expression of rod genes in Rpe65-deficient mice was rescued in Rpe65−/−/Bax−/− mice. Histological and TUNEL studies confirmed that rod cell demise and apoptosis in diseased Rpe65−/− mice were dependent on Bax-induced pathway. Surprisingly, early loss of cones was not prevented in Rpe65−/−/Bax−/− mice, indicating that pro-apoptotic Bax was not involved in the pathogenesis of cone cell death in Rpe65-deficient mice.
This is the first report, to our knowledge, that a single genetic mutation can trigger two independent apoptotic pathways in rod and cone photoreceptors in Rpe65-dependent LCA disease. These results highlight the necessity to investigate and understand the specific death signaling pathways committed in rods and cones to develop effective therapeutic approaches to treat RP diseases.
Introduction
Mutations in the gene encoding the protein RPE65 are associated with several forms of inherited retinal dystrophies, such as autosomal recessive retinitis pigmentosa (RP) [1] and autosomal recessive childhood-onset severe retinal dystrophy [2] which are characterized by profound visual deficiency, night blindness and reduced or non detectable electroretinogram (ERG). LCA consists in a severe form of early-onset autosomal recessive RP and is due in 6 to 15% of the cases to mutations in the Rpe65 gene [3]. RPE65, abundantly expressed in the RPE, is the retinoid isomerase responsible to generate 11-cis-retinal necessary for light-induced phototransduction mediated by the visual pigments rod and cone opsins [4]–[9]. In the absence of the RPE65 protein, rod opsin apoprotein triggers light-independent, constitutive activation of the visual cascade resulting in photoreceptor cell loss [10]. Human RPE65-LCA is characterized as a rod-cone dystrophy with severe retinal defect in the first decades of life. The clinical outcome of this disease has mainly been attributed to rod and cone loss, although the molecular pathways leading to retinal cell death still remain to be identified [11], [12].
Rpe65-deficient mice are a murine model of LCA. These mice exhibit changes in retinal morphology, function, and biochemistry resembling the alterations seen in human LCA patients, including severely depressed light- and dark-adapted ERG responses [4], [13]. In the absence of RPE65, rod photoreceptors display a slow and progressive degeneration [4] dependent on continuous activation of residual transduction cascade by unliganded opsin [10]. Of note is also the early disorganization and loss of photoreceptor outer segments (OS) [4]. The remaining visual capacity is attributed to small amount of 9-cis-retinal forming the photosensitive isorhodopsin in the rods [14]. The residual ERG recording, first evaluated as cone response, actually corresponded to rod function mimicking cone function by responding under normally cone-isolating lighting conditions [9]. Recent studies showed that the decreased expression of cone-specific opsins and transducin correlated with cone degeneration at early ages in Rpe65-deficient retinas [15]–[17]. These data indicate that Rpe65 deficiency affects more severely cones than rods in mice.
Several studies highlighted that many of the molecular pathways involved in ocular diseases rely on mitochondria-dependent apoptotic pathway involving proteins of the Bcl-2 family [18]. Bcl-2 members, known as key regulatory proteins in apoptotic events, can promote either cell survival or cell death. Indeed, the relative amount or equilibrium between pro- and anti-apoptotic members is crucial to sensitize cell fate towards survival or apoptosis. The anti-apoptotic action of Bcl-2 acts through binding and inhibiting pro-apoptotic effector proteins Bax and Bak. The latter promote apoptosis by altering mitochondrial functions and activating the release of downstream apoptogenic factors [19]. Anti- and pro-apoptotic Bcl-2 members are thought to play a role in the pathogenesis of several retinal disorders. While expression of Bax is upregulated following ischemia-induced retinal injury in rat [20], concomitant decreased Bcl-2 and increased Bax protein levels have been reported after elevated intraocular pressure in murine glaucoma model [21]. Following experimental retinal detachment, photoreceptor cell death is abolished in Bax−/− mice, suggesting a critical role for Bax-mediated apoptosis [22]. Similarly, retinas of double knock-out Bax−/−/Bak−/− mice have been shown to be resistant to light-induced photoreceptor degeneration [23]. On the opposite, Bax deficiency is described to be insufficient to protect photoreceptors from death in rd mouse, a fast degenerative RP model characterized by a homozygous mutation in the Pde6b gene encoding rod-specific cGMP phosphodiesterase [24].
We previously observed that anti- and pro-apoptotic genes of the Bcl-2 family were differentially regulated during the development of LCA in the Rpe65−/− mouse model [25]. Moreover, we reported that activation and translocation of pro-apoptotic Bax to mitochondria was associated with apoptosis of rod photoreceptors as the disease progressed [26]. In this study, we first assessed whether triggering of Bcl-2-related apoptotic pathway was mediated by constitutive phototransduction signaling. We further challenged whether disruption of pro-apoptotic Bax was sufficient to prevent rod and cone photoreceptor cell death.
Results
Phototransduction-dependent Apoptosis of Photoreceptors Is Mediated by Activation of the Bcl-2 Apoptotic Pathway In Rpe65-deficient Mice
Our previous work [25], [26], showing that activation of the Bcl-2-related signaling pathway was associated with retinal degeneration in Rpe65-deficient mice, led us to investigate whether this apoptotic pathway was triggered by light-independent, constitutive activity of the phototransduction cascade.
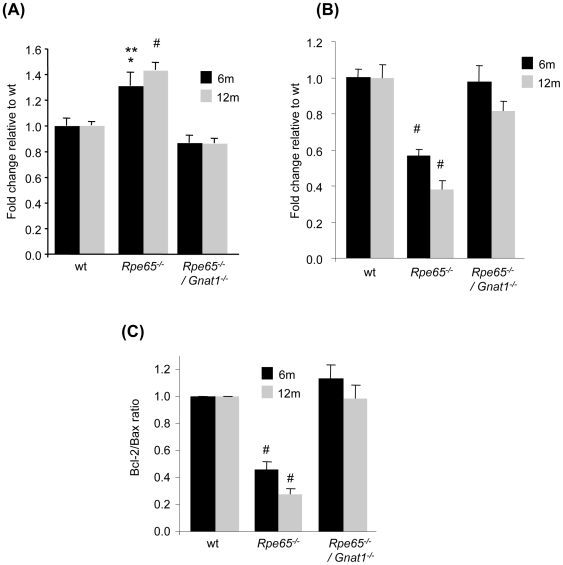
Regulation of Bcl-2 and Bax gene expression was assessed by real-time PCR in retinas of wt, Rpe65−/− and Rpe65−/−/Gnat1−/− mice at 6 and 12 months of age. As depicted in figure 1A, whereas Bax expression was increased by 1.5-fold in 6 and 12 month-old Rpe65-deficient mice, transcriptional upregulation of the pro-apoptotic gene was abolished in Rpe65−/−/Gnat1−/− mice in which phototransduction signaling was blocked in the absence of functional rod transducin. Similarly, the strong decrease in Bcl-2 mRNA in Rpe65−/− retinas as the disease progresses, as reflected by a 40% reduction at 6 months and a 60% reduction at 12 months, was prevented in Rpe65−/−/Gnat1−/− retinas (Figure 1B). This resulted in rescue of the impaired balance between anti- and pro-apoptotic expressed genes observed in Rpe65-deficient mice, with Bcl-2/Bax ratio of 0.46±0.06 and 0.28±0.05 at 6 and 12 months, respectively (Figure 1C). This is in close correlation with our previous observation showing decreased ratio of Bcl-2 toward Bax at the protein level in Rpe65−/− mice during the course of the disease [26].
Figure 1. Constitutive Opsin Apoprotein Signaling Activity Triggers Bcl-2-related Apoptotic Pathway in Rpe65-deficient Mice.
Real-time PCR analysis in 6 month-old (6 m, black square) and 12 month-old (12 m, grey square) wt, Rpe65−/− and Rpe65−/−/Gnat1−/− retinas showing that (A) increased transcriptional expression of pro-apoptotic Bax and (B) reduced level of anti-apoptotic Bcl-2 mRNA observed in Rpe65-deficient retinas were rescued in Rpe65−/−/Gnat1−/− retinas lacking functional rod transducin. (C) Restoration of impaired equilibrium between normalized levels of Bcl-2 toward Bax members of the Bcl-2 family of proteins, expressed as Bcl-2/Bax ratio. Data are the mean±SE of six independent experiments. * p<0.05 by ANOVA test for Rpe65−/− versus wt ; ** p<0.001 by ANOVA test for Rpe65−/− versus Rpe65−/−/Gnat1−/−; # p<0.001 by ANOVA test for Rpe65−/− versus wt and Rpe65−/−/Gnat1−/−.
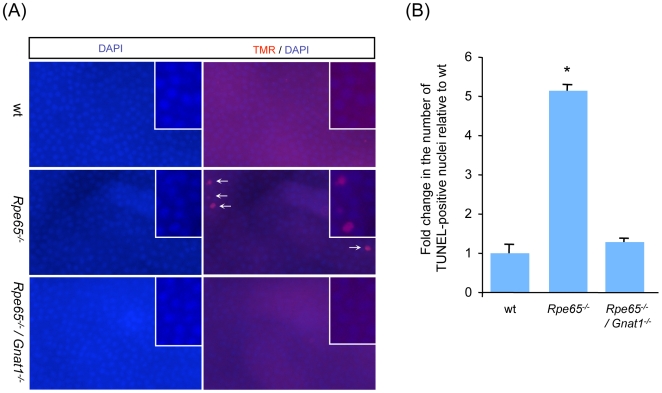
To address whether inhibition of Bcl-2-related apoptotic pathway was sufficient to prevent apoptosis of photoreceptors in diseased retinas, retinal cell apoptosis was investigated by TUNEL assay in retina flatmounts in 6 month-old wt, Rpe65−/− and Rpe65−/−/Gnat1−/− mice (Figure 2A). When counting fluorescent-labelled TUNEL-positive nuclei, a 5-fold increase in the number of apoptotic photoreceptors was observed in Rpe65−/− retinas as compared with wt retinas (5.14±0.14 in Rpe65−/− vs 1.0±0.22 in wt), whereas cell death was prevented in Rpe65−/−/Gnat1−/− retinas (1.29±0.08) (Figure 2B).
Figure 2. Bcl-2-related Apoptosis of Photoreceptors Is Prevented in Phototransduction-deficient Rpe65−/−/Gnat1−/− Mice.
(A) TUNEL assay was performed in 6 month-old wt, Rpe65−/− and Rpe65−/−/Gnat1−/− retina flatmounts (400×) labeled with TMR nucleotides to detect red fluorescence staining of apoptotic photoreceptor nuclei. Counterstaining with DAPI was performed to localize faced up photoreceptors. (B) Increase in the number of apoptotic cells relative to wt retinas was measured by counting TUNEL-positive nuclei. Double knock-out mice deficient for rod transducin were protected from phototransduction-induced apoptosis as compared in Rpe65-deficient mice. Data are the mean±SE of three independent experiments. * p<0.001 by ANOVA test for Rpe65−/− versus wt and Rpe65−/−/Gnat1−/−
Altogether, these results demonstrate that abnormal, unliganded opsin signaling activity induces photoreceptor apoptosis in Rpe65-mediated LCA disease triggered by Bcl-2-related apoptotic pathway.
Disruption of Bax in Rpe65-deficient Mice
To assess the role of Bax as a main pro-death effector of photoreceptor apoptosis in the Rpe65−/− mouse model of LCA, we generated Rpe65−/−/Bax−/− mutant mice both deficient for Rpe65 and Bax.
Analysis of genomic DNA in multiplex PCR reaction using specific primers hybridizing to wild-type and mutant alleles allowed to assess the genotype of the wt, Rpe65−/−, Bax−/− and Rpe65−/−/Bax−/− mouse strains (Figure S1A). To confirm that Bax transcript was disrupted in Bax−/− mice, RT-PCR was performed on total RNA isolated from mouse retinas. No Bax-specific RT-PCR product was seen in either Bax−/− or Rpe65−/−/Bax−/− mice as compared with wt and Rpe65−/− mice, whereas control Gapdh transcript was amplified in all retina samples (Figure S1B).
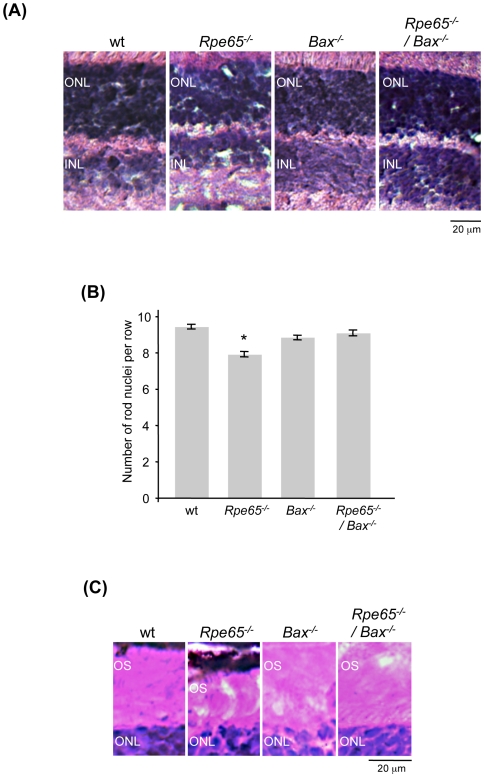
We performed histological analysis at 2 months of age to examine the retinal morphology of Bax−/− mice on a Rpe65-null genetic background. As shown in previous studies, we confirmed following morphological examination (Figure 3A) and counting of rod nuclei (Figure 3B) that no change in outer nuclear layer (ONL) thickness, containing the photoreceptor nuclei, was obvious in Bax-deficient retinas as compared to wt retinas. A slight decrease in ONL thickness was only observed in Rpe65−/− mice. It was previously reported that the increased number of cells in the inner nuclear layer (INL) and ganglion cell layer (GCL) by adulthood in Bax-deficient mice correlated with a corresponding decrease in physiological apoptosis during retinal development and not to ongoing proliferation as assessed by BrdU incorporation [22], [24], [27]–[28]. We also observed that the secondary neuronal layers were increased in Bax-deficient retinas, further confirming the genotype of Rpe65−/−/Bax−/− mice (Figure 3A). Disorganization and shortening of outer segments are early markers of retinal degeneration in Rpe65 −/− retinas [4]. While the length of OS was slightly decreased in Rpe65−/− retinas, this shortening was not observed in Rpe65-deficient retinas lacking Bax, suggesting that early in the disease OS morphology is preserved in the absence of Bax (Figure 3C).
Figure 3. Histological Analysis of Rpe65−/−/Bax−/− Retinas at 2 Months of Age.
(A) Examination of retinal morphology (600×) and (B) Counting rows of rod nuclei within the ONL of Bax-deficient retinas showed no increase in outer nuclear layer thickness as compared with wt retinas. A slight decrease in ONL thickness was only shown in Rpe65−/− retinas. * p<0.001 by ANOVA test for Rpe65−/− versus wt, Bax−/− and Rpe65−/−/Bax−/−. (C) Early shortening of OS length at the onset of retinal degeneration in Rpe65−/− mice was not observed in Rpe65−/−/Bax−/− mice. ONL, outer nuclear layer; INL, inner nuclear layer; OS, outer segment.
Rod Photoreceptors Are Protected from Apoptosis in Rpe65-deficient LCA Mice Lacking Pro-apoptotic Bax
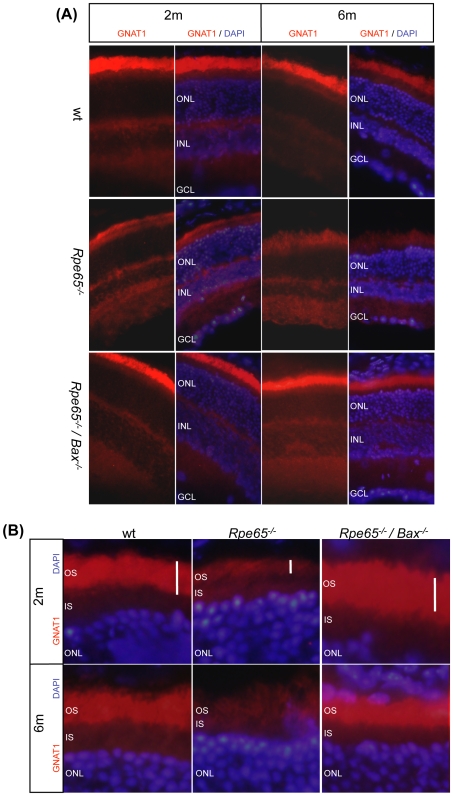
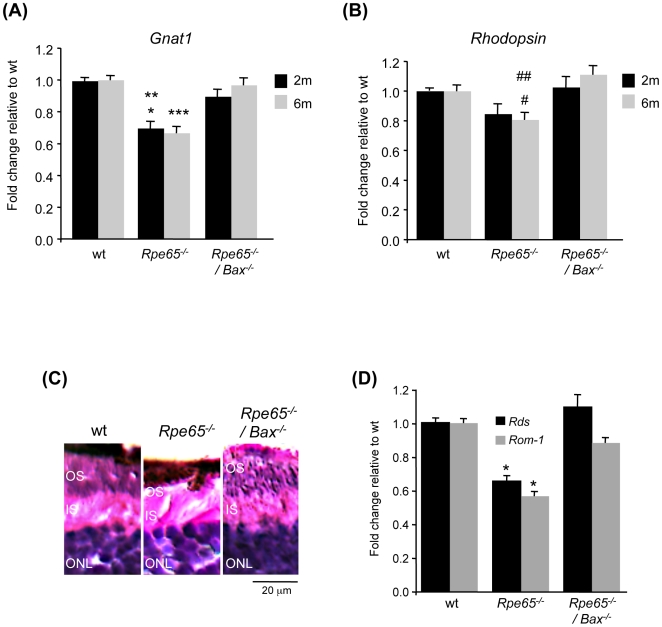
To further investigate the role of Bax in retinal degeneration, we performed immunohistological analysis of rod transducin (Gnat1) expression in Rpe65− /− mice during the course of the disease at 2 and 6 months of age. Counterstaining with DAPI was performed to identify the retinal cell layers. Immunohistological staining with gnat1 antibody showed localized expression of transducin in rod OS (Figure 4). Decreased expression of rod transducin (Figure 4A) observed in Rpe65− /− mice at both ages was rescued in Rpe65−/−/Bax−/− mice, as compared with wt animals. Restored levels of rod-specific markers transducin (Figure 5A) and rhodopsin (Figure 5B) were further confirmed by real-time PCR analysis. Furthermore, impaired OS structure in Rpe65−/− mice, already obvious by 2 months of age (Figures 3C and 4B), was highly disrupted and reduced as the disease progresses by 6 months of age, as shown in immunohistological (Figure 4B) and histological (Figure 5C) analyses. This was correlated with decreased expression of the OS markers Rds and Rom-1 (Figure 5D). However, OS morphological defects as well as decreased mRNA levels of the OS-specific structural proteins were rescued in diseased retinas lacking pro-apoptotic Bax.
Figure 4. Immunohistological Analysis of Rod Transducin Expression during the Course of the Disease.
(A) Immunohistological staining (600×) with gnat1 antibody (GNAT1 panels) showing expression of transducin in rod OS at 2 (2 m) and 6 (6 m) months of age. Counterstaining with DAPI was performed to identify the retinal cell layers (GNAT1/DAPI panels). While the amount of transducin was decreased in Rpe65−/− retinas at both ages, as compared with wt retinas, expression of the protein was restored in Rpe65−/−/Bax−/− retinas. (B) Higher magnification of immunostained retina sections showing that OS shortening in Rpe65-deficient retinas was fully preserved at both ages in Rpe65−/− retinas lacking Bax. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; IS, inner segment; OS, outer segment.
Figure 5. Decreased Expression of Rod Photoreceptor Markers Was Prevented in Rpe65−/−/Bax−/− Mice.
(A) rod transducin and (B) rhodopsin mRNA levels were assessed by quantitative PCR in 2 month-old (2 m, black square) and 6 month-old (6 m, grey square) wt, Rpe65−/− and Rpe65−/−/Bax−/− mice. Decreased expression of rod-specific transcripts in Rpe65-deficient retinas was restored in Rpe65−/−/Bax−/− retinas at both ages. Data are the mean±SE of three (6 m) to four (2 m) independent experiments. * p<0.001 by ANOVA test for Rpe65−/− versus wt; ** p<0.01 by ANOVA test for Rpe65−/− versus Rpe65−/−/Bax−/−; *** p<0.001 by ANOVA test for Rpe65−/− versus wt and Rpe65−/−/Bax−/−; # p<0.05 by ANOVA test for Rpe65−/− versus wt; ## p<0.001 by ANOVA test for Rpe65−/− versus Rpe65−/−/Bax−/−. (C) Histological analysis (600×) showing almost complete disrupted rod OS in Rpe65−/− retinas at 6 months, as compared to preserved OS structure in wt and Rpe65−/−/Bax−/− retinas. (D) Quantitative PCR analysis of expressed Rds and Rom-1 OS marker genes in 6 month-old mice. Data are the mean±SE of three independent experiments. * p<0.001 by ANOVA test for Rpe65−/− versus wt and Rpe65−/−/Bax−/−. ONL, outer nuclear layer; IS, inner segment; OS, outer segment.
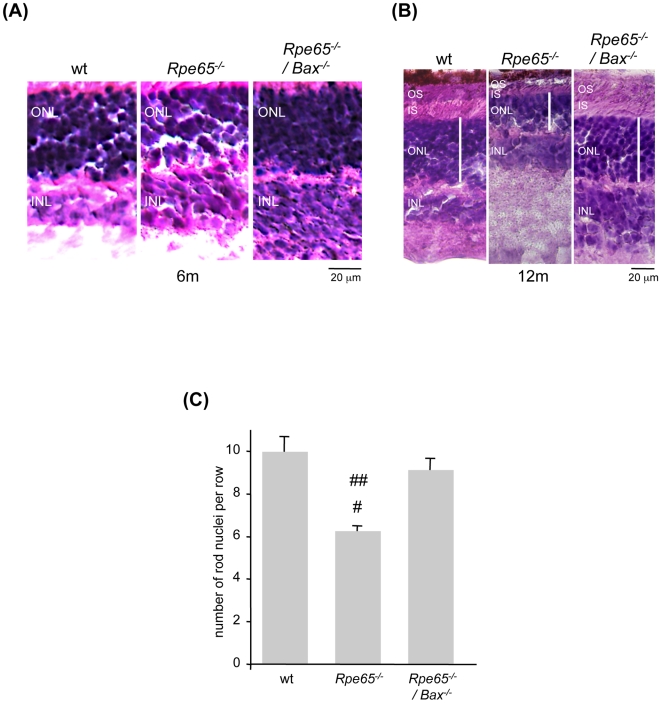
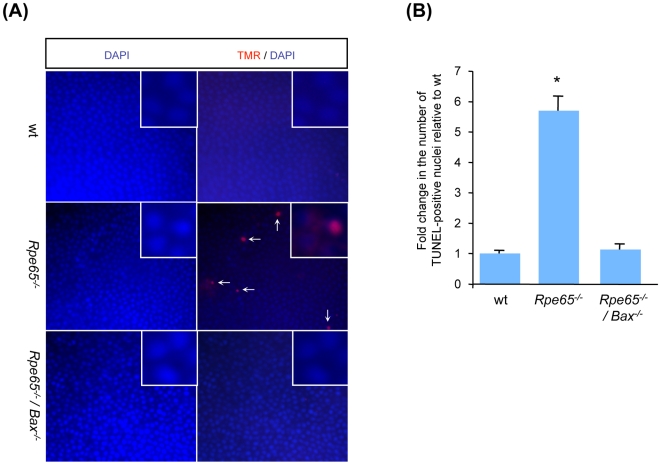
To more accurately assess whether pro-apoptotic Bax is sufficient to trigger retinal cell death, loss of rod photoreceptor nuclei was investigated. Retinal degeneration was assessed by histological analysis (Figure 6A) and counting rows of the surviving photoreceptor nuclei in the ONL at 6 months of age (Figure 6C). The number of rod nuclei was reduced by about 30 to 40% in Rpe65−/− retinas, which is in accordance with previous observations [4], [10], [29], whereas ONL thickness was preserved in double knock-out retinas as compared with wt retinas. More importantly, histological analysis at 12 months of age not only showed a delayed retinal defect but also an efficient, long term protection of ONL and OS integrity in the absence of functional Bax (Figure 6B). To confirm that photoreceptor apoptosis was prevented in diseased retinas lacking pro-apoptotic Bax, TUNEL analysis was performed on 6 month-old retina flatmounts (Figure 7A). While the number of TUNEL-positive apoptotic rods was increased 6-fold in Rpe65−/− retinas (5.7±0.46) as compared with wt retinas (1.0±0.08), apoptosis of rod photoreceptors was prevented in Rpe65−/−/Bax−/− retinas (1.13±0.17) (Figure 7B). Furthermore, prevention of photoreceptor cell death in Bax-deficient diseased retinas may not be explained by re-expression of Bcl-2 since transcriptional expression of the anti-apoptotic member was not restored (Figure S2).
Figure 6. Disruption of Pro-apoptotic Bax Was Sufficient to Prevent Rod Photoreceptor Defect and Loss in Rpe65-deficient Animals.
(A) Examination of retinal morphology (600×) and (C) counting rows of rod nuclei at 6 months (6 m) of age showed that whereas ONL thickness was reduced in Rpe65−/− retinas, loss of photoreceptor nuclei was prevented in Rpe65-deficient retinas lacking Bax. The data presented are the mean±SE of four independent experiments. # p<0.01 by ANOVA test for Rpe65−/− versus wt; ## p<0.05 by ANOVA test for Rpe65−/− versus Rpe65−/−/Bax−/−. (B) Histological analysis at 12 months (12 m) of age showing long-term protection of ONL and OS integrity in the absence of pro-apoptotic Bax in Rpe65−/−/Bax−/− mice. ONL, outer nuclear layer; INL, inner nuclear layer; IS, inner segment; OS, outer segment.
Figure 7. Bax-dependent Rod Photoreceptor Apoptosis Was Abolished in Rpe65−/−/Bax−/− Mice.
(A) TUNEL assay was performed in 6 month-old wt, Rpe65−/− and Rpe65−/−/Bax−/− retina flatmounts (400×) labeled with TMR nucleotides to detect red fluorescence staining of apoptotic rod nuclei. Counterstaining with DAPI was performed to localize faced up photoreceptor cells. (B) Increase in the number of apoptotic TUNEL-positive cells in Rpe65−/− retinas, relative to wt retinas, was abolished in Rpe65−/−/Bax−/− retinas. Data are the mean±SE of three independent experiments. * p<0.001 by ANOVA test for Rpe65−/− versus wt and Rpe65−/−/Bax−/−.
Altogether, these results indicate that rod photoreceptor degeneration is triggered by a Bax-induced apoptotic pathway in Rpe65-dependent LCA disease.
Early Degeneration of Cone Photoreceptors in Rpe65-deficient Mice Did Not Rely on Activation of Bax-mediated Apoptotic Pathway
In the Rpe65−/− mouse model of LCA, cone photoreceptors degenerate much more rapidly than rods [9], [15]–[16]. We thus addressed the question whether the early apoptotic events involved in cone cell death were also dependent on activation of pro-apoptotic Bax.
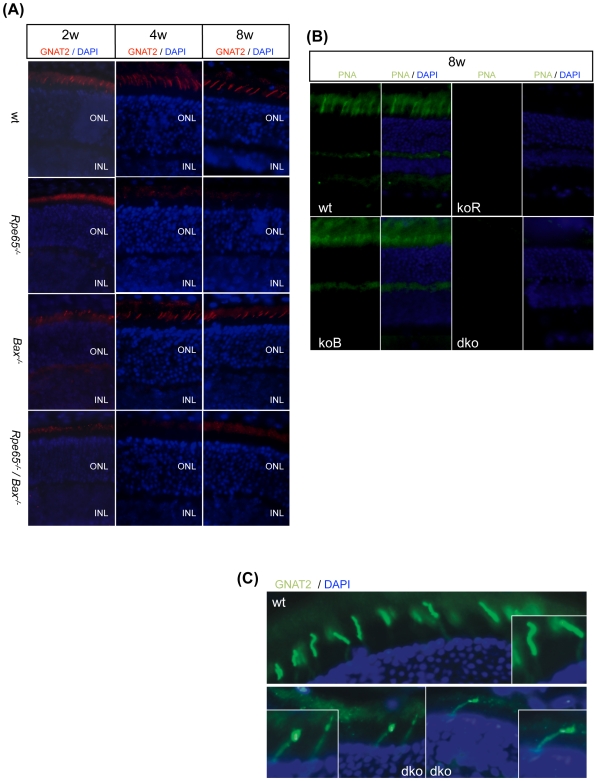
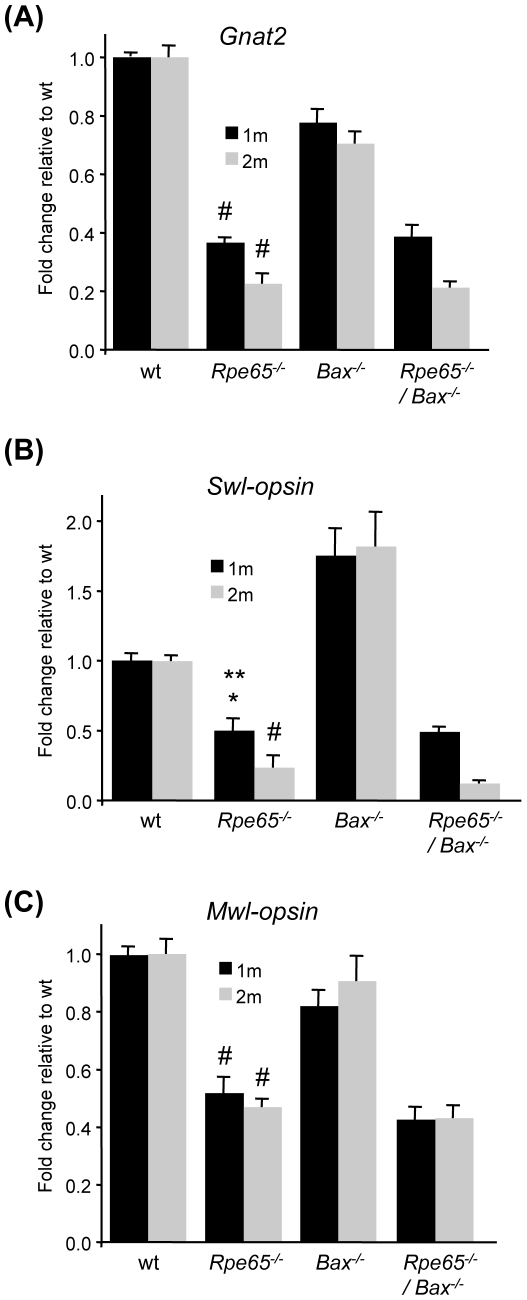
By immunostaining analysis, early and fast reduction in cone transducin (Gnat2) expression was observed between 2 and 8 weeks of age in Rpe65−/− retinas as compared to wt and Bax−/− retinas (Figure 8A). However, in contrary to our observation in rod photoreceptors, decreased level of cone transducin was not restored in Rpe65−/−/Bax−/− mice lacking pro-apoptotic Bax (Figure 8A). Specific staining of cone extracellular matrix with FITC-labelled PNA further showed that, by 2 months of age, cones were lost both in Rpe65−/− and Rpe65−/−/Bax−/− retinas (Figure 8B). Moreover, cone transducin failed to properly traffic to the OS in the few surviving cones at later times in peripheral retina in Rpe65−/−/Bax−/− mice (Figure 8C). This observation correlates with previous reports describing impaired localization of cone opsins in synaptic pedicle and IS in residual cones almost exclusively found at the periphery of Rpe65−/− retinas [16], [17]. Confirmation of the loss of cone-specific markers in Rpe65−/−/Bax−/− mice, similarly to what was observed in Rpe65−/− mice, was demonstrated by quantitative PCR study of cone transducin (Gnat2) and cone opsins (short wavelength (Swl) and middle wavelength (Mwl)-opsins) transcriptional expression at 1 and 2 months of age (Figure 9). Decreased expression of Gnat2 and Swl-opsin was more pronounced at 2 months of age in Rpe65-deficient retinas as well as in Rpe65−/−/Bax−/− retinas.
Figure 8. Early Degeneration of Cone Photoreceptors Was Not Prevented in Rpe65−/−/Bax−/− Mice.
(A) Immunohistological staining (600×) with gnat2 antibody showing that early lost expression of cone transducin, as early as 2 weeks of age, was not prevented in Rpe65−/−/Bax−/− retinas as compared to Rpe65−/− retinas (2–8 w, 2–8 weeks; ONL, outer nuclear layer; INL, inner nuclear layer). (B) FITC-labelled PNA staining (600×) in 8 week-old (8 w) Rpe65-deficient retinas confirmed that degenerating cones were not rescued in the absence of Bax. (C) Immunohistological analysis demonstrating that cone transducin failed to properly traffic to the OS in the few surviving cones still present at the retinal periphery at 6 months of age in Rpe65−/−/Bax−/− mice as compared to wt mice. Counterstaining with DAPI allowed for the identification of photoreceptor cell nuclei. koR, Rpe65−/−; koB, Bax−/−; dko, Rpe65−/−/Bax−/−.
Figure 9. Loss of Cones in Rpe65−/−/Bax−/− Mice Was Confirmed by qPCR Analysis of Cone-specific Markers.
Strong decrease in transcriptional expression of (A) Gnat2, (B) Swl-opsin and (C) Mwl-opsin at 1 (1 m) and 2 months (2 m) of age in Rpe65−/− and Rpe65−/−/Bax−/− retinas indicated that loss of cones was not rescued in the absence of pro-apoptotic Bax. Data are the mean±SE of four independent experiments. # p<0.001 by ANOVA test for Rpe65−/− versus wt and Bax−/−; * p<0.05 by ANOVA test for Rpe65−/− versus wt; ** p<0.001 by ANOVA test for Rpe65−/− versus Bax−/−.
Altogether, these results demonstrate that Bax-induced signaling pathway is not critical for mislocalization of the cone-specific phototransduction components and not sufficient to induce early degeneration of cones in Rpe65-deficient LCA mice.
Discussion
In the current study, we reported in the Rpe65−/− mouse model of LCA that photoreceptor apoptosis induced by continuous transducin-mediated signaling is triggered by the Bcl-2-mediated apoptotic pathway. Indeed, we observed that altered regulation of pro-and anti-apoptotic members of the Bcl-2 family was prevented in Rpe65−/−/Gnat1−/− mice lacking functional transducin. Moreover, the restored equilibrium between Bax and Bcl-2 was associated with the survival of the photoreceptors in diseased retinas. Several lines of evidence show that mutations activating persistent visual transduction can cause congenital stationary night blindness and retinal degeneration. Deficiency in the visual cycle, during vitamin A deprivation in diet [30], [31] or in Lrat−/− mice [32], also leads to over-stimulation of the transduction cascade through unliganded opsin. As for the described mutations in RPE65, affected patients with mutations in the gene encoding the lecithin retinol acyl transferase (LRAT) develop severe early-onset retinal dystrophy [33]. Other defects in visual transduction, such as constitutively active mutant opsins [34]–[36], mutations in cyclic guanosine monophosphate-gated channel [37] or in retinal guanylate cyclase [38], [39], are associated with photoreceptor dystrophies for which continuous activation of the visual cascade is the most likely explanation for the outcome of the disease. Genetically engineered null mutations in arrestin [40] and rhodopsin kinase [41], prolonging light-activated rhodopsin signaling, also show similar physiological and cellular dysfunctions leading to retinal degeneration. Inhibition of transducin activity in these mice prevents continuous, moderate light-induced damage, indicating that rod photoreceptor apoptosis is triggered by prolonged light-activated transduction cascade in these mice [42].
The Bcl-2-related apoptotic pathway is activated and participates in the pathogenesis of several RP diseases [18] as well as in glaucoma [21], [43], retinal ischemia [20], [44], [45] and retinal detachment [22]. In this study, we further challenged the direct role of Bax in retinal cell death associated with RPE65 defect by disrupting the pro-apoptotic gene in the Rpe65−/− mice. Absence of the pro-apoptotic protein was sufficient to rescue the phenotype of the disease. Indeed, the altered OS structure and shortening as well as the reduced expression of rod-specific genes were rescued in Rpe65−/−/Bax−/− retinas. ONL thinning and apoptosis of rod photoreceptors were also prevented in the absence of functional Bax. More important, disruption of the pro-apoptotic protein provided a long term protection and preservation of the OS and ONL, still effective in older mice at 12 months of age. From our results, it is tempting to speculate that the proteins of the Bcl-2 family may trigger a common apoptotic pathway in a subset of inherited retinal defects resulting from excessive visual transduction. Future studies will be needed to address the impact of the Bcl-2-related pathway in other inherited mutations leading to constitutive phototransduction-dependent retinal degeneration.
The nature of cone dysfunction in Rpe65 deficiency still remains to be elucidated and thus prompted us to assess whether, similarly to our observation in rods, Bax-induced apoptosis was the cause of early cone loss in Rpe65-deficient mice. Early and major cone degeneration was recently reported in LCA patients with RPE65 mutations [11], indicating that the murine disease phenotype is very similar to that of the human counterpart. We observed in this study that degeneration of the cones, reflected by the loss of cone-specific markers and the disruption of cone extracellular matrix, was not prevented in Rpe65 −/− lacking the pro-apoptotic protein. Moreover, the few surviving cones observed at the peripheral retina in Rpe65−/−/Bax−/− mice showed mislocalized cone transducin. Impaired localization of cone opsins within the synaptic pedicle, cell body and inner segments, was previously reported in Rpe65−/− mice [16], [17]. This was similarly observed in Lrat−/− [17], retinal guanylate cyclase GC1−/− [46], [47] and cone cyclic nucleotide-gated channel Gnga3−/− [48] mice, suggesting that some genetic defects in proteins of the visual cascade may converge at a common degenerative pathway causing cone cell death. Treatment with exogenous 11-cis-retinal restored correct OS targeting of cone phototransduction components in Rpe65-deficient mice. This was associated with increased cone response and survival, suggesting that visual chromophore may act as a chaperone to improve sorting and correct trafficking of these proteins [16]–[17], [49]. These results indicate that cone loss may be attributed, at least partially, to impaired protein folding and deficient targeting triggering altered photoreceptor physiology and intracellular death cascade. It has been similarly observed that rod opsin mutations resulting in protein misfolding and impaired sorting may lead to retinal degeneration (reviewed in [50]). Protein misfolding elicits an adaptive endoplasmic reticulum-stress response and enhances protein degradation by the ubiquitin-proteasome system. If endoplasmic reticulum stress is maintained, proteasome-mediated protein degradation can culminate in apoptotic cell death involving caspase activation and mitochondrial signaling. In case the normal proteolytic machinery becomes saturated, protein aggregation in cytosol may activate other cytotoxic pathways including impaired cytoskeletal network–induced apoptosis as well as autophagic cell death [51]–[53]. Abnormal sorting of cone-specific proteins may further induce cell death through different molecular mechanisms involving impaired vesicular trafficking, interference with synaptic transmission and metabolic stress due to continuous degradation of mis-sorted proteins. Further studies in pure cone mouse models, the Rpe65−/−/Nrl−/− or Rpe65−/−/Rho−/− mice, will be necessary to identify the intracellular apoptotic pathways involved in the demise of the cones.
In summary, this is the first report showing that phototransduction-dependent apoptosis of rod photoreceptors in Rpe65−/− mice is triggered by the Bcl-2-related apoptotic pathway. The observations that Bax-induced apoptosis is responsible for progressive loss of rods, while early degeneration of cones is not mediated by pro-apoptotic Bax, indicate that two independent apoptotic pathways are activated in rods and cones in Rpe65-deficient LCA disease. This highlights the importance to decipher the specific death signaling pathways committed in rods and cones and understand their relative contribution to retinal defect toward the development of complementary and effective therapeutic treatments.
Materials and Methods
Mouse lines and genotyping
These studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research and were approved by the Veterinary service of the State of Valais (Switzerland). Wild-type C57BL/6 mice (wt) were purchased from Charles River Laboratories (Les Oncins, France). Rpe65−/− mice are on a C57BL/6 genetic background (from Dr T.M. Redmond, National Institutes of Health, Bethesda, USA) [4] and Gnat1−/− (from Dr. J. Lem, Tufts-New England Medical Center, Boston, USA) are on a BALB/c genetic background [54]. Rpe65−/−/Gnat1−/− double knockout mice were generated by crossbreeding Rpe65−/− mice with Gnat1−/− mice. Genotyping of the mice was determined by PCR analysis with genomic DNA isolated from tail tissue, as described [4], [54]. Animals were kept in a 12-h light/12-h dark cycle with unlimited access to food and water.
Generation of Rpe65−/−/Bax−/− double knock-out mice
Bax−/− mice are homozygous for the Baxtm1Sjk mutation (Charles River Laboratories). The targeted disruption of the Bax gene was performed in a 129-derived RW-G ES cell line and has been backcrossed 8 generations to C57BL/6. Bax−/− progeny have varying coat color, are poorly pigmented and have pink eye because the coat color loci tyrosinase (Tyr) and pink-eyed dilution (p) are linked to the Bcl2-associated X protein (Bax) gene. The strain is maintained through heterozygous matings since females are poor breeders and males are infertile. Heterozygous mice for Bax (+/−) and homozygous mice for Rpe65 (−/−) were first crossbred to obtain Bax+/−/Rpe65+/− double heterozygous F1 animals. Double heterozygous F1 males and females were mated to generate Bax+/−/Rpe65−/− F2 mice both heterozygous for Bax (+/−) and homozygous for Rpe65 (−/−). F2 mice were then mated to obtain Bax−/−/Rpe65−/− double knock-out F3 animals used in this study. Bax-specific genotyping was performed by PCR analysis with genomic DNA isolated from tail tissue, as recommended by The Jackson Laboratory (http:// jaxmice.jax.org).
Tissue isolation and RNA preparation
Age-matched animals were killed by cervical dislocation. Retinas from each mouse strain were dissected under a microscope to exclude extra-retinal tissues, and were quickly isolated in RNAlater (Ambion, Huntingdon, United Kingdom) before being transferred in TRIzol (Invitrogen, Basel, Switzerland) and stored at −80°C until RNA extraction. Total RNA was extracted according to manufacturer's instructions and the amount of total RNA was determined by Ribogreen assay (Invitrogen).
RT-PCR analysis
One µg of total RNA in a 20-µl reaction was used for cDNA synthesis using oligo (dT)18 according to the manufacturer's procedure (AffinityScript™ Reverse Transcriptase; Agilent, Basel, Switzerland). The equivalent of 50 ng original total RNA was used for PCR using the 2× Master Mix (Qiagen, Basel, Switzerland) and 1 µM forward and reverse primer pairs. PCR was performed with the following cycling conditions: 35 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec. The primers used for detection of mouse Bax transcript were the following: forward primer 5′-CCAGGATGCGTCCACCAAGA-3′ and reverse primer 5′-GGTGAGGACTCCAGCCACAA-3′ (Eurogentec, Seraing, Belgium).
Real-time PCR analysis
The equivalent of 50 ng original total RNA was used for PCR amplification using the 2× brilliant SYBR Green QPCR Master Mix (Agilent) with either 125 nM (Gapdh, Gnat1, Gnat2, Crx), 250 nM (Bax, Swl opsin, Mwl opsin, Rhodopsin, Rom-1, Rds) or 500 nM (Bcl-2) forward and reverse primer pairs, designed to span an intron of the target gene. Real-time PCR was performed in triplicate in a Mx3000PTM system (Agilent) with the following cycling conditions: 40 cycles of denaturation at 95°C for 30 sec, annealing either at 55°C (all genes except Bcl-2) or 60°C (Bcl-2) for 30 sec, and extension at 72°C for 30 sec. Quantitative values were obtained by the cycle number (Ct value) reflecting the point at which fluorescence starts to increase above background at a fixed threshold level. Values obtained for the target genes were normalized with the housekeeping gene Gapdh. Gapdh-normalized values for Bax−/− and Rpe65−/−/Bax−/− retinal RNA were additionally normalized with the photoreceptor marker gene Crx to compensate for the increased thickness of the whole retina due to increased cell number present in the INL and GCL of these mouse strains. For primer sequences, see Table S1.
Histological analysis
Eyes were fixed in 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS) for 45 min, followed by cryoprotection in 30% sucrose/PBS. Ten µm-embedded frozen sections were stained with hematoxylin and eosine for light microscopy histology examination. For each individual eye (n = 4), four retina sections of various depths were stained for histology. For each retina section, a count of rows of photoreceptors in the ONL was performed from two different areas in the central retina on each side of the optic nerve, across both the superior and inferior hemispheres. In each area, five adjacent rows of nuclei from three individual fields were counted and the resulting numbers from each individual retina were averaged.
Immunohistochemistry and peanut agglutinin (PNA) staining
Eyes were fixed in 4% PFA/PBS for 45 min, followed by cryoprotection in 30% sucrose/PBS. Ten µm-embedded frozen sections were further processed for immunohistochemistry. Briefly, frozen retina sections were blocked in PBS with 2% normal goat serum (Sigma, Buchs, Switzerland) and 0.2% Triton X-100 (Sigma) for 1 h at room temperature (RT) and incubated with primary antibodies in the blocking buffer overnight at 4°C. Sections were blocked again in blocking buffer for 30 min at RT before to be incubated with fluorochrome-conjugated secondary antibody for 1 h at RT. Incubation with secondary antibody alone was used as a negative control. Species and dilutions of the antibodies used were as follows: rabbit anti-gnat1 (1∶1'000; Calbiochem, San Diego, USA), rabbit anti-gnat2 (1∶500; Santa Cruz Biotechnology, Santa Cruz, USA) and Alexa Fluor 594 goat anti-rabbit IgG (1∶1'000; Invitrogen) or Alexa Fluor 488 goat anti-rabbit IgG (1∶500; Invitrogen). For PNA staining, retina sections were incubated at RT with fluorescein-congugated PNA (Sigma) used at 20 µg/ml for 75 min. Following 3 washes in PBS, sections were mounted in Citifluor AF1 (Citifluor Ltd, London, United Kingdom). Tissue sections were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen) to identify retinal cell layers.
Terminal dUTP Nick End-Labeling (TUNEL) of fragmented DNA
DNA strand breaks in retinal cell nuclei were detected by TUNEL assay on retina flatmounts. Enucleated eyes were fixed in 4% PFA/PBS for 5 min at RT, followed by dissection of the retina in PBS, after removal of cornea and lens, and additional fixation of the retina with photoreceptors faced up in 4% PFA/PBS for 60 min at RT. Each retina was then transferred on (3-Aminopropyl)triethoxysilane (APTES; Sigma)-treated slide and flattened into 4 quadrants by making incisions each 90° apart, from the ora serrata and stopping short of the optic nerve opening. Retina flatmounts on microscope slides were stored at −20°C until used. Before TUNEL staining, retinas were rehydrated in PBS for a few seconds and fixed in 4% PFA/PBS for 10 min at RT. The tissue was then dehydrated 2 minutes in each graded alcohol (2 times 95%, 2 times 100%), and defatted in xylene overnight to allow for better penetration across the outer limiting membrane of the retina. The following day, retina flatmounts were rehydrated in alcohol (2 times 100%, once 95%, once 80%) and in PBS, then permeabilized with 0.3% Triton X-100 for 15 min at RT, and finally digested with proteinase K (20 µg/ml; Roche, Rotkreuz, Switzerland) for 2 h at 37°C before TUNEL staining with terminal deoxynucleotidyl transferase (TdT) and TMR nucleotides (Roche) according to manufacturer's instructions. Retina flatmounts were further counterstained with DAPI (Invitrogen) to identify photoreceptor nuclei, followed by three washes in PBS, before to be mounted in Citifluor AF1 (Citifluor). For each retina flatmount, apoptotic cells were counted in at least three to four areas of each 4 quadrants and the resulting numbers from each retina flatmount (n = 3–5) were averaged.
Imaging
Images were viewed under a fluorescence microscope equipped with a digital camera (Olympus BX61; Olympus, Lausanne, Switzerland) using appropriate filters.
Statistical analysis
All results were expressed as means±SE of the indicated number of experiments. Statistical significance was calculated with the ANOVA test followed by Bonferroni post test adjustment.
Supporting Information
Supplemental table S1. Nucleotide sequences of primers used in real-time PCR.
(0.04 MB PDF)
Disruption of Bax in Rpe65-deficient Mice (A) PCR screening from genomic DNA showed specific amplification of the corresponding wild-type (wt) and mutant (mut) alleles from wt, Rpe65−/−, Bax−/− and Rpe65−/−/Bax−/− mouse genotypes. Genomic DNA from heterozygous Bax mice (Bax+/−) was used as control of PCR amplification of both alleles in a single reaction. (B) RT-PCR analysis confirmed disruption of Bax transcript in Bax−/− and Rpe65−/−/Bax−/− retinas, while Bax-specific RT-PCR product of the expected size (394-bp amplicon spanning exons 3 to 6) was observed in wt and Rpe65−/− retinas. Gapdh transcript amplification (287-bp spanning exons 3 to 5) was perfomed as control. Sample without cDNA template (dH2O) was used as a control of PCR specificity. MW, DNA ladder in base pairs.
(3.33 MB TIF)
Downregulated Expression of Anti-apoptotic Bcl-2 Was Not Restored in Rpe65-deficient Mice Lacking Bax Quantitative PCR analysis of Bcl-2 mRNA expression in 6 month-old mice showing that decreased expression in Rpe65−/− retinas was not restored in Rpe65−/−/Bax−/− retinas, as compared with wt retinas. Data are the mean±SE of three independent experiments. * p<0.001 by ANOVA test for Rpe65−/− and Rpe65−/−/Bax−/− versus wt.
(0.79 MB TIF)
Acknowledgments
We thank Sylviane Métrailler for excellent technical assistance and Drs Nathalie Allaman-Pillet, Pascal Escher and Raphaël Roduit for critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by The Swiss National Science Foundation (SNSF) Grant 3100A0-118336. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, et al. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci USA. 1998;95(6):3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17(2):194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 3.El Matri L, Ambresin A, Schorderet DF, Kawasaki A, Seeliger MW, et al. Phenotype of three consanguineous Tunisian families with early-onset retinal degeneration caused by an R91W homozygous mutation in the RPE65 gene. Graefe's Arch Clin Exp Ophthalmol. 2006;244(9):1104–1112. doi: 10.1007/s00417-005-0096-2. [DOI] [PubMed] [Google Scholar]
- 4.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 5.Gollapalli D, Maiti P, Rando A. RPE65 operates in the vertebrate visual cycle by stereospecifically binding all-trans-retinyl esters. Biochemistry. 2003;42(40):11824–11830. doi: 10.1021/bi035227w. [DOI] [PubMed] [Google Scholar]
- 6.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, et al. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem. 2004;279(1):635–643. doi: 10.1074/jbc.M310042200. [DOI] [PubMed] [Google Scholar]
- 7.Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004;117(6):761–771. doi: 10.1016/j.cell.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeliger M, Grimm C, Stahlberg F, Friedburg C, Jaissle G, et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29(1):70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff M, Wang Z, Chung H, Redmond T, Fain G, et al. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35(2):158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA. 2007;104(38):15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Windsor EAM, et al. Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2008;49(10):4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvin J, Fishman G, Stone E, Koenekoop R. Clinical phenotypes in carriers of Leber congenital amaurosis mutations. Ophthalmology. 2005;112(2):349–356. doi: 10.1016/j.ophtha.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Fan J, Rohrer B, Moiseyev G, Ma J-X, Crouch RK. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci USA. 2003;100(23):13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, et al. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46(4):1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 16.Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, et al. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46(10):3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Fan J, Li S, Karan S, Rohrer B, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28(15):4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cottet S, Schorderet DF. Mechanisms of apoptosis in retinitis pigmentosa. Curr Mol Med. 2009;9:375–383. doi: 10.2174/156652409787847155. [DOI] [PubMed] [Google Scholar]
- 19.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneda K, Kashii S, Kurosawa T, Kaneko S, Akaike A, et al. Apoptotic DNA fragmentation and upregulation of Bax induced by transient ischemia of the rat retina. Brain Res. 1999;815(1):11–20. doi: 10.1016/s0006-8993(98)01074-9. [DOI] [PubMed] [Google Scholar]
- 21.Ji J, Chang P, Pennesi ME, Yang Z, Zhang J, et al. Effects of elevated intraocular pressure on mouse retinal ganglion cells. Vision Res. 2005;45(2):169–179. doi: 10.1016/j.visres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Bula D, Arroyo JG, Chen DF. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Invest Ophthalmol Vis Sci. 2004;45(2):648–654. doi: 10.1167/iovs.03-0827. [DOI] [PubMed] [Google Scholar]
- 23.Hahn P, Lindsten T, Lyubarsky A, Ying GS, Pugh JEN, et al. Deficiency of Bax and Bak protects photoreceptors from light damage in vivo. Cell Death Differ. 2004;11(11):1192–1197. doi: 10.1038/sj.cdd.4401486. [DOI] [PubMed] [Google Scholar]
- 24.Mosinger Ogilvie J, Deckwerth TL, Knudson CM, Korsmeyer SJ. Suppression of developmental retinal cell death but not of photoreceptor degeneration in Bax-deficient mice. Invest Ophthalmol Vis Sci. 1998;39(9):1713–1720. [PubMed] [Google Scholar]
- 25.Cottet S, Michaut L, Boisset G, Schlecht U, Gehring W, et al. Biological characterization of gene response in Rpe65−/− mouse model of Leber's congenital amaurosis during progression of the disease. FASEB J. 2006;20(12):2036–2049. doi: 10.1096/fj.06-6211com. [DOI] [PubMed] [Google Scholar]
- 26.Cottet S, Schorderet DF. Triggering of Bcl-2-related pathway is associated with apoptosis of photoreceptors in Rpe65 (−/−) mouse model of Leber's Congenital Amaurosis. Apoptosis. 2008;13(3):329–342. doi: 10.1007/s10495-008-0180-2. [DOI] [PubMed] [Google Scholar]
- 27.Péquignot M, Provost A, Sallé S, Taupin P, Sainton K, et al. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003;228(2):231–238. doi: 10.1002/dvdy.10376. [DOI] [PubMed] [Google Scholar]
- 28.Hahn P, Lindsten T, Ying GS, Bennett J, Milam AH, et al. Proapoptotic bcl-2 family members, Bax and Bak, are essential for developmental photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2003;44(8):3598–3605. doi: 10.1167/iovs.02-1113. [DOI] [PubMed] [Google Scholar]
- 29.Lai C-M, Yu M, Brankov M, Barnett N, Zhou X, et al. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet Vaccines Ther. 2004;2(1):3–3. doi: 10.1186/1479-0556-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proc Natl Acad Sci USA. 1958;44(7):648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowling JE, Wald G. The biological function of vitamin A acid. Proc Natl Acad Sci USA. 1960;46(5):587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65−/− and Lrat−/− mice: comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008;49(6):2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson D, Li Y, McHenry C, Carlson T, Ding X, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001;28(2):123–124. doi: 10.1038/88828. [DOI] [PubMed] [Google Scholar]
- 34.Rao VR, Cohen GB, Oprian DD. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994;367(6464):639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- 35.Sieving PA, Fowler ML, Bush RA, Machida S, Calvert PD, et al. Constitutive “light” adaptation in rods from G90D rhodopsin: a mechanism for human congenital nightblindness without rod cell loss. J Neurosci. 2001;21(15):5449–5460. doi: 10.1523/JNEUROSCI.21-15-05449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin S, Cornwall MC, Oprian D. Opsin activation as a cause of congenital night blindness. Nat Neurosci. 2003;6(7):731–735. doi: 10.1038/nn1070. [DOI] [PubMed] [Google Scholar]
- 37.Dryja T, Finn J, Peng Y, McGee T, Berson E, et al. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92(22):10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelsell R, Gregory-Evans K, Payne A, Perrault I, Kaplan J, et al. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum Mol Genet. 1998;7(7):1179–1184. doi: 10.1093/hmg/7.7.1179. [DOI] [PubMed] [Google Scholar]
- 39.Semple-Rowland SL, Lee NR, Van Hooser JP, Palczewski K, Baehr W. A null mutation in the photoreceptor guanylate cyclase gene causes the retinal degeneration chicken phenotype. Proc Natl Acad Sci USA. 1998;95(3):1271–1276. doi: 10.1073/pnas.95.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Dodd R, Makino C, Simon M, Baylor D, et al. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389(6650):505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- 41.Chen CK, Burns ME, Spencer M, Niemi GA, Chen J, et al. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci USA. 1999;96(7):3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao W, Wenzel A, Obin M, Chen C-K, Brill E, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32(2):254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- 43.Libby RT, Li Y, Savinova OV, Barter J, Smith RS, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PloS Genet. 2005;1(1):17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Rosenbaum DM, Shaikh AR, Li Q, Rosenbaum PS, et al. Ischemic preconditioning attenuates apoptotic cell death in the rat retina. Invest Ophthalmol Vis Sci. 2002;43(9):3059–3066. [PubMed] [Google Scholar]
- 45.Zhang Y, Cho C-H, Atchaneeyasakul L-o, McFarland T, Appukuttan B, et al. Activation of the mitochondrial apoptotic pathway in a rat model of central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2005;46(6):2133–2139. doi: 10.1167/iovs.04-1235. [DOI] [PubMed] [Google Scholar]
- 46.Baehr W, Karan S, Maeda T, Luo D-G, Li S, et al. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282(12):8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008;48(3):442–452. doi: 10.1016/j.visres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalakis S, Geiger H, Haverkamp S, Hofmann F, Gerstner A, et al. Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci. 2005;46(4):1516–1524. doi: 10.1167/iovs.04-1503. [DOI] [PubMed] [Google Scholar]
- 49.Jin M, Li S, Nusinowitz S, Lloyd M, Hu J, et al. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J Neurosci. 2009;29(5):1486–1495. doi: 10.1523/JNEUROSCI.3882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendes H, van der Spuy J, Chapple J, Cheetham M. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005;11(4):177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277(37):34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- 52.Saliba RS, Munro PMG, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci. 2002;115(Pt 14):2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 53.Ravikumar B, Vacher C, Berger Z, Davies J, Luo S, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 54.Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci USA. 2000;97(25):13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table S1. Nucleotide sequences of primers used in real-time PCR.
(0.04 MB PDF)
Disruption of Bax in Rpe65-deficient Mice (A) PCR screening from genomic DNA showed specific amplification of the corresponding wild-type (wt) and mutant (mut) alleles from wt, Rpe65−/−, Bax−/− and Rpe65−/−/Bax−/− mouse genotypes. Genomic DNA from heterozygous Bax mice (Bax+/−) was used as control of PCR amplification of both alleles in a single reaction. (B) RT-PCR analysis confirmed disruption of Bax transcript in Bax−/− and Rpe65−/−/Bax−/− retinas, while Bax-specific RT-PCR product of the expected size (394-bp amplicon spanning exons 3 to 6) was observed in wt and Rpe65−/− retinas. Gapdh transcript amplification (287-bp spanning exons 3 to 5) was perfomed as control. Sample without cDNA template (dH2O) was used as a control of PCR specificity. MW, DNA ladder in base pairs.
(3.33 MB TIF)
Downregulated Expression of Anti-apoptotic Bcl-2 Was Not Restored in Rpe65-deficient Mice Lacking Bax Quantitative PCR analysis of Bcl-2 mRNA expression in 6 month-old mice showing that decreased expression in Rpe65−/− retinas was not restored in Rpe65−/−/Bax−/− retinas, as compared with wt retinas. Data are the mean±SE of three independent experiments. * p<0.001 by ANOVA test for Rpe65−/− and Rpe65−/−/Bax−/− versus wt.
(0.79 MB TIF)