Abstract
Francisella tularensis
is a Category A biothreat agent for which there is no approved vaccine and the correlates of protection are not well understood. In particular, the relationship between the humoral and cellular immune response to F. tularensis, and the relative importance of each in protection, is controversial. Yet, understanding this relationship will be crucial to the development of an effective vaccine against this organism. We demonstrate, for the first time, a differential requirement for humoral versus cellular immunity in vaccine-induced protection against F. tularensis infection, and that the requirement for Ab observed in some protection studies, may be overcome through the induction of enhanced cellular immunity. Specifically, following intranasal/mucosal immunization of mice with inactivated F. tularensis organisms (iFt) plus Cholera Toxin B subunit (CTB), we observe increased production of IgG2a/2c versus IgG1 Ab, as well as IFN-γ, indicating induction of a Th1 response. In addition, the requirement for F. tularensis-specific IgA Ab production, observed in studies following immunization with iFt alone, is eliminated. Thus, these data indicate that enhanced Th1 responses can supersede the requirement for anti-F. tularensis-specific IgA. This observation also has important ramifications for vaccine development against this organism.
Keywords: Cholera Toxin B, Vaccine, F. tularensis, Mucosal Immunity, Protection
Introduction
Elucidating the immune mechanisms involved in protection against microbial pathogens is key to successful vaccine development. In fact, there is a pressing need for development of vaccines against mucosal pathogens. In these studies, we examine the immunological requirements for protection against the intracellular bacterium, Francisella tularensis. The two subspecies of F. tularensis: tularensis (biovar A) and holarctica (biovar B), are the causative agents of pneumonic tularemia (1). The subspecies (biovar A) is the most virulent, as inhalation of fewer than 25 organisms can cause fulminate disease in humans (2). The disease appears abruptly 3 to 5 days after exposure, at which point, it can progress to severe pneumonia, respiratory failure, and even death (3). In addition, F. tularensis has been designated a Category A biothreat agent, due not only to its virulence, but also due to the potential for it to be developed as a bioterrorism agent, which can be dispersed within heavily populated areas (3).
While it is currently believed that cellular immunity plays the key role in protection against F. tularensis infection (4–7), the precise role played by F. tularensis-specific Ab is controversial. In regard to cellular immunity, protection studies in mice utilizing either attenuated or inactivated F. tularensis organisms as immunogens have demonstrated the requirement for IFN-γ and/or induction of robust cellular immune responses to these immunogens, as indicated by increased IFN-γ, IL-2, and/or IL-12 production, evidence of a Th1-type response (7–10). In regard to humoral immunity, immunization with F. tularensis LPS generated Ab-dependent protection against intradermal and intraperitoneal challenges with F. tularensis subspecies holarctica (biovar B), but not against the more virulent biovar A strain (11–15). More specifically, passive immunization of naive mice with sera from LPS-immunized mice protected recipient mice against F. tularensis live vaccine strain (LVS) challenge, highlighting the importance of Ab in this instance. Furthermore, depletion of CD4+ and CD8+ T cells from immunized mice did not affect protection significantly (12). However, more recent studies utilizing outer membrane proteins from F. tularensis (OMP), administered i.n., provided partial protection against the highly virulent biovar A strain, SchuS4, and appeared to involve the generation of both anti- OMP-specific Ab, as well as IL-2 and TNF-α (15). As a result, it was suggested that the mechanism of protection in this case is complex, and that cellular and humoral immunity, both correlates of protection in this instance, likely play a role in protection against F. tularensis infection. In addition, a more significant role for Ab in generating protection against F. tularensis challenge has been supported in other recent studies (9, 10). However, despite evidence favoring a role for Ab in protection against F. tularensis infection, the role of specific Ab isotypes is unclear. While F. tularensis-specific IgM Ab is generated in response to vaccination with F. tularensis LPS and F. tularensis infection, F. tularensis-specific IgG Ab is also generated in these cases (14, 16–18). In addition, there is no direct evidence IgM alone can be protective against virulent F. tularensis. In fact, it is generally believed that the generation of immunogen-specific IgG is preferable in most vaccines, in part due to its high affinity, its ability to enhance opsinophagocytosis, and its ability to fix complement (19).
Importantly, recent studies from this laboratory have demonstrated that inactivated F. tularensis (iFt) can induce protection against subsequent challenge with F. tularensis biovar B, as well as biovar A, when targeted to Fc receptors, and that this protection requires both humoral and cellular immunity (10). Furthermore, these observations are consistent with those using OMP as immunogen (15). Thus, we sought to further enhance these protective responses, and generate a more effective vaccine, by utilizing a well-established mucosal adjuvant, CTB (20–27). CTB is a potent mucosal adjuvant, in particular for the induction of protective Ab. In addition, it lacks the toxicity of cholera toxin itself, due to the absence of the toxic A subunit (22–25, 28). In addition, CTB also enhances cellular immunity, although the precise impact on Th1 versus Th2 responses can vary significantly. For example, i.n. and oral administration of CTB tends to drive Th2-like responses (29–31), while transcutaneous and intravaginal routes tend to elicit Th1 responses (32, 33). However, not only does the route of immunization influence the ability of CTB to stimulate cellular immunity, but also the type of Ag used (34). Thus, we considered the possibility CTB may enhance both the humoral and cellular responses to iFt when administered i.n..
In fact, when iFt is administered i.n. with CTB, it enhances both cellular (Th1) and humoral immune responses, while also enhancing protection against both biovar A and B strains of F. tularensis. More significantly, we demonstrate for the first time, a differential requirement for protection in the presence and absence of CTB. Specifically, the requirement for anti-F. tularensis-specific IgA, demonstrated in previous protection studies following immunization with iFt alone (10), is eliminated when immunizing with iFt plus CTB. In contrast, the requirement for IFN-γ remains. Furthermore, iFt plus CTB concomitantly enhances the production of IgG2a/IgG2c and IFN-γ, characteristics of a Th1 response. Whether IgA is a Th1 or Th2 dominant isotype is unclear, although there is some evidence that IgA is produced in the mucosa under the influence of IL-4, a Th2 cytokine (35). Thus, a shift towards a Th1 response induced by CTB could potentially reduce the production of IgA in response to iFt. However, this was not observed. Alternatively, the increased level of the cellular immune response induced by CTB could simply overcome the need for Ab-mediated protection. Most importantly, these data demonstrate for the first time, a correlation between the level of Th1 response induced and the requirement for IgA, in vaccine-induced protection against F. tularensis challenge. This observation not only has significant ramifications for vaccine development, but may also help to resolve ongoing disagreement regarding the role of Ab in protection against F. tularensis infection.
Materials and Methods
Mice
BALB/c and C57BL/6 mice were procured from Taconic Farms (Germantown, NY). IgA−/− mice with a C57BL/6×129 background were provided by Dr. Dennis Metzger (Albany Medical College). The B6.129S7-Ifngtm1Ts/J (IFN-γ−/−) and the B10.129S2(B6)-IgH-6tm1Cgm/J (B cell-deficient) mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed in the Animal Resources Facility at Albany MedicalCollege. Mice were provided with ad lib water and food during the course of each experiment. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee.
Bacterial strains
F. tularensis LVS organisms were provided by Dr. Mats Forsman (Swedish Defense Research Agency, Umea, Sweden). F. tularensis SchuS4 organisms, originally isolated from a human case of tularemia (36), were obtained from the U.S. Army Medical Research Institute for Infectious Diseases (Frederick, MD). All experiments using SchuS4 were conducted within a Centers for Disease Control-certified ABSL-3/BSL-3 facility at Albany Medical College. F. tularensis organisms were grown in Mueller Hinton Broth (MHB) (Fisher Scientific, Pittsburgh, PA) at 37°C to a density of approximately 0.5–1 × 109 CFU/ml. Live F. tularensis preparations were stored in liquid nitrogen at −80°C, in PBS.
Immunogen
Inactivated GFP-expressing F. tularensis LVS (iFt) was used as immunogen. F. tularensis LVS organisms were grown in MHB at 37°C to a density of approximately 0.5–1 × 109 CFU/ml. To inactivate F. tularensis LVS, 1 × 1010 CFU/ml of live bacteria were incubated in 1 ml of sterile PBS (Mediatech, Inc, Herndon, VA) containing 2% paraformaldehyde (Sigma, St. Louis, MO) for two hours at room temperature. Fixed bacteria (iFt) were then washed with sterile PBS three times. Inactivation was verified by culturing a 100μl sample (1 × 109 iFt organisms) on chocolate agar plates (Fisher Scientific, Pittsburgh, PA) for seven days. The iFt preparations were stored at −20°C in PBS.
Immunization and challenge experiments
Mice were divided into groups consisting of 6–8 mice/group, 8–12 weeks of age. Each mouse was anesthetized by i.p. injection of 20% ketamine plus 5% xylazine, and subsequently administered i.n. either 20μl of PBS (vehicle), 5μg of CTB (Sigma, St. Louis, MO) in 20μl PBS, 2 × 107 iFt organisms in 20μl PBS, or 2 × 107 iFt organisms combined with 5μg of CTB in 20μl PBS. Prior to immunization, mice were bled and serum was isolated and tested for the presence of F. tularensis-specific Ab. Unless otherwise indicated, mice were immunized on day 0 and boosted on day 21. Immunized mice were then challenged on day 35 i.n. with 2–8 × 104 CFU of live F. tularensis LVS organisms, or approximately 20 CFU of F. tularensis SchuS4 organisms, and subsequently monitored for survival for at least 21 days.
Quantification of bacterial burden
Following immunization and challenge mice were euthanized at various time intervals and tissues such as lung, liver and spleen, were collected aseptically in PBS containing a protease inhibitor mixture (1 tablet in 10 ml sterile PBS) (Roche Diagnostics, Indianapolis, IN), and subjected to mechanical homogenization using a MiniBeadBeater-8 (BioSpec Products Inc, Bartlesville, OK) using 1mm. zirconia/silica beads. This is a recently established protocol, which has been successfully utilized in similar studies that involve bacterial quantification in tissues (37–39). Tissue homogenates were then diluted 10-fold in sterile saline, and 10 μl of each dilution was spotted onto Chocolate agar plates in duplicate and incubated at 37°C for two days. The number of colonies on the plates were counted and expressed as log10 CFU per ml for the respective tissue. Tissue homogenate was also spun at 14,000 × g for 20 min, and the clarified homogenate was stored at −20°C for cytokine analysis.
Cytokine measurement
Mice were divided into groups consisting of 6 mice each and were immunized i.n. on day 0 and 21. Mice received either: 20μl of PBS (vehicle), 5μg of CTB in 20μl PBS, 2 × 107 iFt organisms in 20μl PBS, or 2 × 107 iFt organisms combined with 5μg of CTB in 20μl PBS. For “in vivo” cytokine measurements mice were bled sub-mandibularly two days post-immunization boost (day 23) and the sera was obtained. For “in vitro” cytokine measurements, on day 35 post-primary immunization spleen cells were harvested from each mouse and incubated in either presence of fixed F. tularensis LVS organisms in RPMI-1640 (Mediatech, Inc, Herndon, VA) plus 10% Fetal Bovine Serum (FBS, Hyclone, Logan, Utah) or media alone. More specifically, 4 × 106 spleen cells/ml were incubated with 4 × 106 CFU/ml fixed F. tularensis LVS organisms in a total volume of 1 ml RPMI/10%FBS per well, and cultured for 3, 5 and 7 days at 37°C. Supernatants were then harvested at each time point and kept at 20°C until they were analyzed. Mouse Th1/Th2 cytometric bead array (CBA) kits(BD Biosciences-BD Pharmingen, San Diego, CA) were used for measurement of multiple cytokines in the sera and in the collected spleen cell supernatants. Data were acquired on a FACSArray Instrument (BD ImmunocytometrySystems; BDIS, San Jose, CA) and analyzed using CBA software version 1.0.1 (BDIS).
Histological evaluation
Following immunization and challenge mice were euthanized at various time intervals and tissues including lung, liver and spleen, were collected aseptically in 10% neutral-buffered formalin (Richard Allan Scientific, Kalamazoo, MI) and embedded in paraffin (Richard Allan Scientific, Kalamazoo, MI). Paraffin-embedded sections (3 μm) were then stained with H&E (Richard Allan Scientific, Kalamazoo, MI), and the disease severity then characterized by Dr. Kurkure, a veterinary pathologist. His conclusions were based upon cellular infiltration, thickening of alveolar septa, and airway passage congestion.
Measurement of F. tularensis-specific Ab production
Anti-F. tularensis Ab production in response to immunization with iFt was measured by ELISA. Briefly, ELISA plates (Nalge Nunc International, Rochester, NY) were coated with 100 μl of live F. tularensis LVS [5 × 107 CFU/ml) in carbonate buffer (4.3 g/l Sodium Bicarbonate (Sigma, St. Louis, MO) and 5.3 g/l Sodium Carbonate (Sigma, St. Louis, MO) at pH 9.4] for 2 hr at 37°C. The plates were washed with PBS containing 0.05% Tween 20 (Fisher Scientific, NJ) and blocked for 2 hr with 200 μl of PBS containing 10% BSA (Baxter Healthcare Corporation, Glendale, CA). Serial two-fold dilutions of sera (starting with 1:50) and BAL fluids (starting with 1:1) were added to the plates (100 μl/well) and incubated for 90 min at 37°C. After washing, biotin conjugated goat anti-mouse Ab specific for IgG, IgG1, IgG2a, IgG2c or IgA (Caltag, Burlingame, CA) were added and incubated for 1 h at 37°C. Plates were washed and then 100 μl/well of streptavidin conjugated to HRP (Caltag, Burlingame, CA) were added. The plates were incubated for 20 min followed by wash and addition of 100 μl of TMB peroxidase substrate (KPL, Gaithersburg, MD). All samples were read simultaneously using a kinetic microplate reader (Molecular Devices, CA). Therefore, acid stop solution was not added, and the samples were read at 650nm as instructed by the manufacturer when acid stop is not utilized. BSA-coated wells were used as specificity controls for F. tularensis LVS.
Statistical analysis
To compare survival curves, a regular Mann-Whitney two sample rank test was used. Statistical data for bacterial burden and cytokine analysiswere generated using ANOVA on day 7, which is the peak of infection. Ab titers were assessed by using the unpaired two-tailed t test comparing the log values of each experimental group. GraphPad Prism 4 analysis software was utilized.
Results
Use of CTB as a mucosal adjuvant increases protection against lethal F. tularensis LVS challenge when using iFt as immunogen
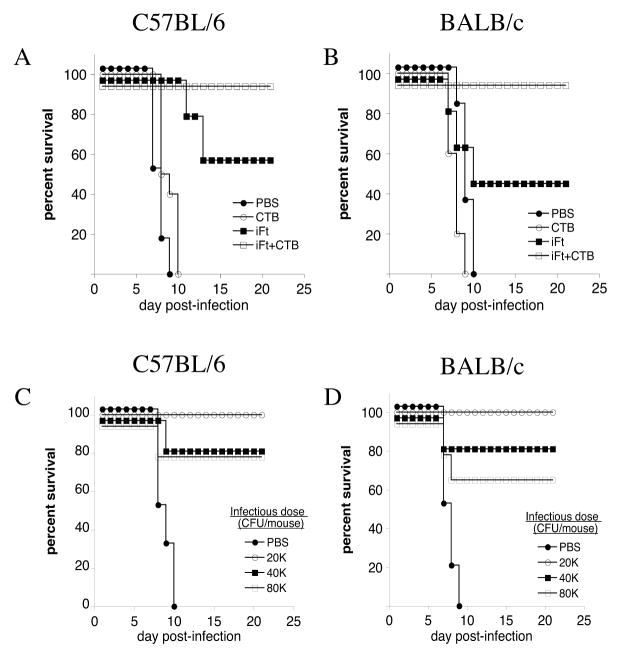
Previous studies from our laboratory demonstrated that immunization with iFt provided only partial protection against lethal F. tularensis LVS challenge (10). Thus, we sought to improve this level of protection by using CTB as adjuvant. In the current study, co-administration of iFt with 5μg of CTB i.n., followed by a booster immunization 21 days later, provided 100% protection against lethal F. tularensis LVS challenge in both C57BL/6 (Fig. 1A) and BALB/c mice (Fig. 1B), while iFt alone continued to provide only partial protection. Furthermore, CTB in the absence of iFt provided no protection (Figs. 1A and B). Protective responses were also titratable, in that the protective effect of co-administering CTB with iFt could be reduced with increased challenge doses of F. tularensis LVS (Figs. 1C and D).
Figure 1. iFt Plus CTB Enhances iFt-induced Protection Against i.n. Challenge With F. tularensis LVS.
C57BL/6 (A) and BALB/c (B) mice were divided into four groups and immunized i.n. with 20 μl of either PBS, 5μg of CTB in 20 μl PBS, 2 × 107 iFt organisms in 20 μl PBS, or 2 × 107 iFt organisms with 5μg of CTB in 20 μl PBS. Mice were immunized on day 0 and boosted on day 21. Mice were then challenged on day 35 i.n. with 2 × 104 CFU (LD50 X 4) (A and B) or 2–8 × 104 CFU (C and D) of live F. tularensis LVS, and subsequently monitored for 21 days for survival. Survival curves are presented. The survival data are representative of four individual experiments (a total of 6 mice/group in each experiment).
Use of CTB as an adjuvant reduces bacterial burden in the tissues of immunized mice
Following our observation of increased protection against F. tularensis LVS challenge when co-administering CTB with iFt, we measured the bacterial burden in the tissues of immunized then challenged animals. Indeed the use of CTB as an adjuvant significantly reduced bacterial burden in the lung (Fig. 2A), liver (Fig. 2B) and spleen (Fig. 2C) of immunized mice on day 7 post-infection versus that of mice immunized with iFt alone. This time point was chosen based on our previous studies showing day 7 to be the peak of F. tularensis LVS infection (10).
Figure 2. Bacterial Burden is Reduced in Mice Immunized With iFt Plus CTB as Compared to Mice Immunized With iFt Alone.
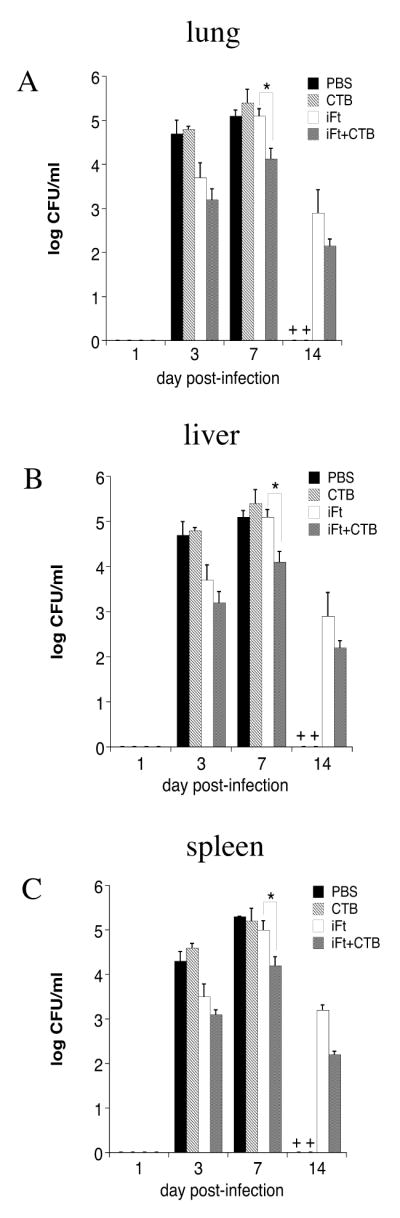
BALB/c mice were immunized as described in Figure 1. Bacterial burden studies were then conducted as described in Materials and Methods. Briefly, lung (A), liver (B), and spleen (C) were harvested at 1, 3, 7, and 14 days post-challenge and bacterial counts were carried out. Each point represents the average of three mice sacrificed per time point + SD. On day 7 iFt plus CTB-immunized mice had significantly lower bacterial burden in tissues than iFt-treated mice (p<0.001). On day 14, all mice immunized with PBS or CTB alone succumbed to the infection, as indicated by a cross (+) on the x-axis. The data is representative of two experiments.
Levels of pro-inflammatory cytokines are reduced when CTB is used as an adjuvant
Severe tissue pathology during systemic and often lethal bacterial infection with F. tularensis has been previously demonstrated (10). Pro-inflammatory cytokines play a central role in triggering and driving these tissue destructive responses. Thus, we chose to examine the generation of pro-inflammatory cytokines. In this study we demonstrate that when CTB is used as an adjuvant and co-administered with iFt, a significant reduction of pro-inflammatory cytokines in the tissues of immunized and subsequently infected mice is observed at 7 days post-infection. This is likely due to the fact that immunization with iFt plus CTB enables the mice to respond more rapidly and control infection, resulting in lower bacterial counts, and thus lower levels of inflammation and pro-inflammatory cytokines by day 7. Indeed, immunized mice had reduced levels of IFN-γ (Fig. 3A), TNF-α (Fig. 3B) and MCP1 (Fig. 3C) in their lungs compared to mice immunized with iFt alone. Measurement of these cytokines in the spleen and liver of immunized mice showed analogous differences. We also examined non pro-inflammatory cytokines including IL-4, IL-5, and IL-2. However, the levels of these cytokines were similar among all immunized groups as well as unvaccinated animals (data not shown).
Figure 3. Levels of Pro-Inflammatory Cytokines Are Reduced in The Tissues of iFt Plus CTB-Immunized Mice.
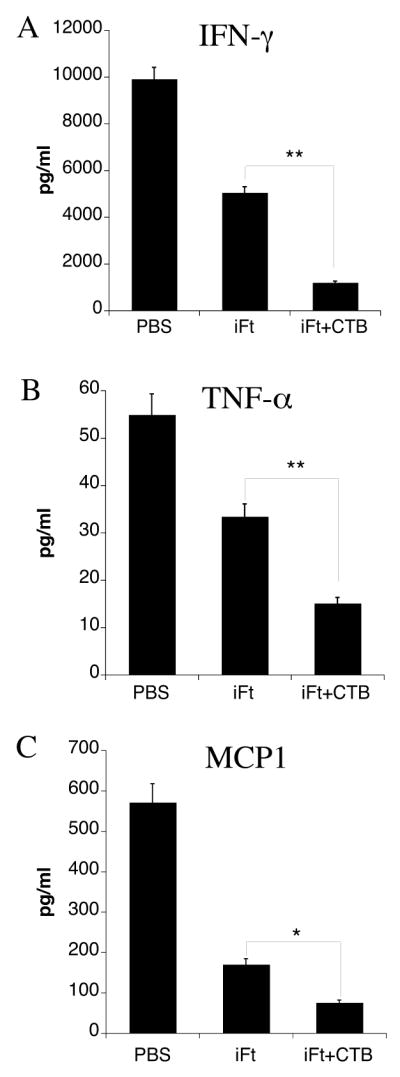
BALB/c mice were immunized and challenged as indicated in Figure 1. On day 7 post-challenge the lung, spleen and liver of mice were harvested and the cytokine levels were measured in the tissue homogenates by CBA. The levels of IFN-γ (A), TNF-α (B) and MCP1 (C) in the lungs of immunized mice are presented. The levels of these cytokines were significantly lower in mice immunized with iFt plus CTB (*<0.05, **<0.01). The data are a representative of two experiments.
Immunization of mice with iFt plus CTB reduces tissue pathology
To determine if reduced levels of pro-inflammatory cytokines observed following immunization with iFt plus CTB would be reflected in the tissue pathology of F. tularensis LVS infected mice, tissues from immunized mice were harvested on day 7 post-F. tularensis LVS infection, sectioned and stained with H&E. Control (PBS) mice demonstrated severe pathology in the lungs with extended tissue necrosis, thickened alveolar septa, alveolar congestion, and extensive cellular infiltrates compared to normal naive mice (Figs. 4B and A, respectively). In contrast, mice immunized with iFt plus CTB showed minimal tissue destruction on day 7 post-infection (Fig. 4D), most likely due to the reduced bacterial loads observed in these mice as a consequence of a more rapid and effective immune response primed by immunization with iFt plus CTB. Pathology was also reduced in mice immunized with iFt alone (Fig. 4C), however, not to the extent of their iFt plus CTB-immunized counterparts. Histopathology in the liver and spleen of immunized mice showed similar results (data not shown).
Figure 4. Inflammation is Reduced in The Lungs, Liver, and Spleen of iFt Plus CTB-Immunized Mice as Compared to Mice Immunized With iFt Alone.
BALB/c mice were immunized and challenged as indicated in Figure 1. Tissues were then harvested 7 days post-challenge, fixed with paraformaldehyde, paraffin embedded, sectioned, and stained with H&E. In this figure we show lung samples of a normal un-immunized and un-challenged mouse (A), a PBS-immunized and challenged mouse (B), a mouse immunized with iFt alone and challenged (C) and a mouse immunized with iFt plus CTB and challenged (D). All samples are at 20X magnification. Reduced tissue pathology was observed in the iFt plus CTB-immunized mice.
F. tularensis-specific IgA responses are enhanced when using CTB as adjuvant
The ability of CTB to enhance Ab responses is well established. In addition, studies by this laboratory and others have indicated that Ab, including IgA, can play a role in controlling F. tularensis infection, in particular when iFt is used as the protective immunogen (9, 10). For this reason we examined the ability of CTB to enhance F. tularensis-specific IgA responses in the BAL fluid and sera of immunized mice. BAL fluid and blood was collected from mice 10–12 days post-booster immunization. Indeed, we found that the levels of F. tularensis LVS-specific IgA were significantly elevated in BAL fluid and sera of iFt plus CTB-immunized mice as compared to mice immunized with iFt alone (Figs. 5A and B). This was the case for both BALB/c and C57BL/6 mice (Fig. 5C).
Figure 5. Immunization With iFt Plus CTB Enhances Production of F. tularensis-Specific IgA.
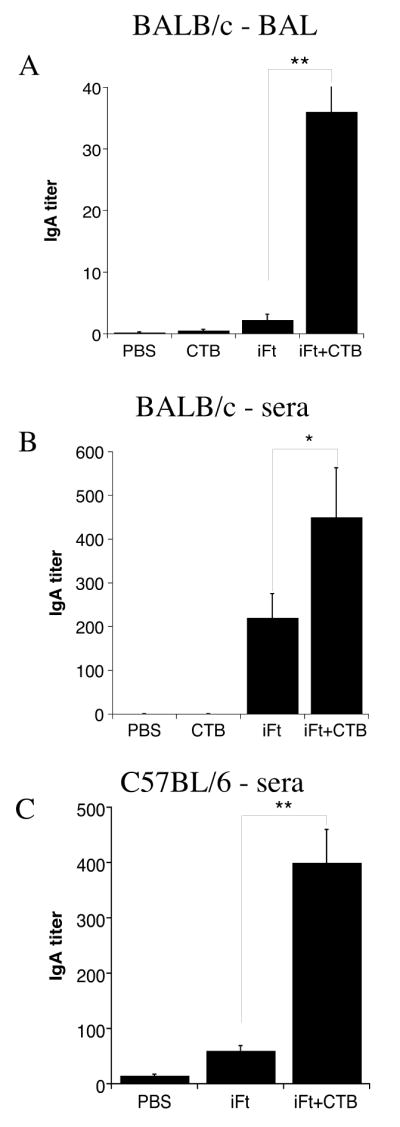
BALB/c (A and B) and C57BL/6 (C) mice were divided into four and three groups respectively (5 mice/group) and immunized i.n. with 20μl of either PBS, 5μg of CTB in 20 μl PBS (only BALB/c), 2 × 107 iFt in 20 μl PBS, or 2 × 107 iFt plus 5μg of CTB in 20 μl PBS. Mice were immunized on day 0 and boosted on day 21 as in challenge studies in Figure 1. BAL fluid or sera was then collected at 35 days and tested for the presence of anti-F. tularensis IgA Ab by ELISA, as described in Materials and Methods. Ab data represent the mean of 4–5 mice + SD. The levels of IgA were significantly higher in the BAL fluid and sera of iFt plus CTB-immunized mice versus mice immunized with iFt alone (p<0.01 and p<0.05, respectively in BALB/c mice and p<0.01 in C57BL/6). These results are representative of two experiments.
F. tularensis-specific IgA is not required for protection in iFt plus CTB-immunized mice
Consistent with our previously published observations (10), we observed that immunization with iFt provides partial protection against F. tularensis LVS challenge (Fig. 6A). In addition, the role of IgA in mucosal immunity has been widely established (40–42). In this regard, we also demonstrated the partial protection generated by iFt is IgA dependant (10). To demonstrate that iFt plus CTB-induced protection was similarly IgA dependent, we conducted immunization experiments using IgA-deficient mice (Fig. 6B). Mice were immunized with iFt plus CTB, iFt alone, or PBS, and subsequently infected with a lethal challenge of F. tularensis LVS. However, in contrast to our previous studies, in which IgA-deficient mice immunized with iFt alone did not survive (10), 100% protection was observed when iFt was co-administered with CTB in IgA-deficient mice (Fig. 6B).
Figure 6. IgA is Not Required For Protection Induced by iFt Plus CTB Immunization.
IgA-deficient mice were divided into three groups, immunized, and challenged with F. tularensis LVS as described in Figure 1. Survival was monitored for at least 21 days post-infection. IgA-deficient mice (B), as well as control (C57BL/6) mice (A), were protected against F. tularensis LVS challenge when immunized with iFt plus CTB. These data are representative of three separate experiments.
F. tularensis-specific Ab is not required for protection in iFt plus CTB-immunized mice
It remained possible that CTB-enhanced F. tularensis-specific IgG production could compensate for the lack of IgA, and thus humoral immunity could still play a critical role in the observed iFt plus CTB-induced protection we observed. To investigate this possibility, B cell-deficient mice were immunized with either: iFt plus CTB, iFt, or PBS, and boosted 21 days later. On day 35 the mice were challenged with a lethal dose of F. tularensis LVS and their survival was monitored. While iFt alone protected 60% of normal mice (Fig. 7A), 90% of B cell deficient mouse died when immunized with iFt alone. In contrast, 100% of B cell-deficient mice immunized with iFt plus CTB were protected (Fig. 7B), indicating that Ab are not required for protection when immunizing with iFt plus CTB.
Figure 7. B Cells Are Not Required For Protection Induced by iFt Plus CTB Immunization.
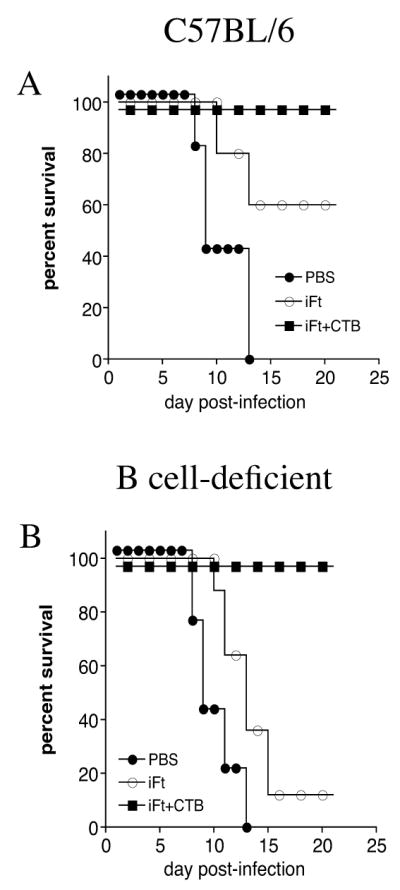
C57BL/6 (A) and B cell-deficient (B) mice were divided into three groups, immunized and infected with F. tularensis LVS as described in Figure 1. Their survival was monitored for at least 21 days post-infection. B cell-deficient mice were protected against F. tularensis LVS challenge when immunized with iFt plus CTB (B).
IFN-γ is required for iFt plus CTB-induced protection
Having demonstrated Ab is not necessary for iFt plus CTB-induced protection, we sought to determine if cell-mediated immunity was required. Numerous studies support the idea that cell- mediated immunity (in particular IFN-γ) can protect against F. tularensis infection. Thus, we immunized IFN-γ-deficient mice, with either: iFt plus CTB, iFt, or PBS. Two weeks after the booster immunization, the mice were infected with a lethal dose of F. tularensis LVS. In fact, the use of CTB as a mucosal adjuvant with iFt did not overcome the previously demonstrated requirement for IFN-γ, demonstrated when using iFt alone as immunogen (10). Specifically, no protection was observed in IFN-γ-deficient mice whether or not CTB was added to iFt (Fig. 8B). This is also despite the fact that IFN-γ-deficient mice immunized with iFt plus CTB exhibited a three-fold increase in F. tularensis-specific IgA levels as compared to iFt-immunized mice (Fig. 8C).
Figure 8. IFN-γ is Required For iFt Plus CTB-Induced Protection.
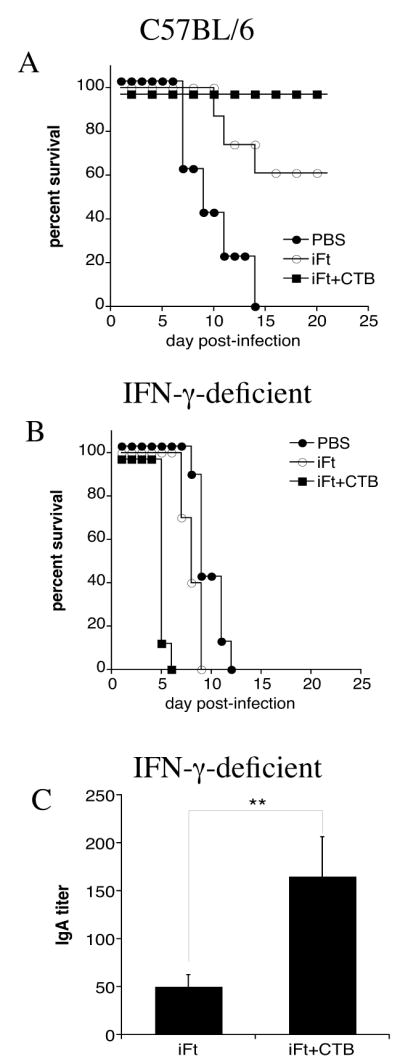
C57BL/6 (A) and IFN-γ-deficient (B) mice were divided into three groups, immunized and infected with F. tularensis LVS as described in Figure 1. Survival was monitored for at least 21 days post-infection. IFN-γ-deficient mice were not protected against F. tularensis LVS challenge when immunized with iFt plus CTB (B). Sera from IFN-γ-deficient mice were obtained prior to the F. tularensis LVS challenge and the F. tularensis-specific IgA levels were measured by ELISA. The mice immunized with iFt plus CTB exhibited a three-fold increase in IgA levels versus iFt-immunized mice (C). These data are representative of two separate experiments.
iFt plus CTB induces a Th1-type response against F. tularensis
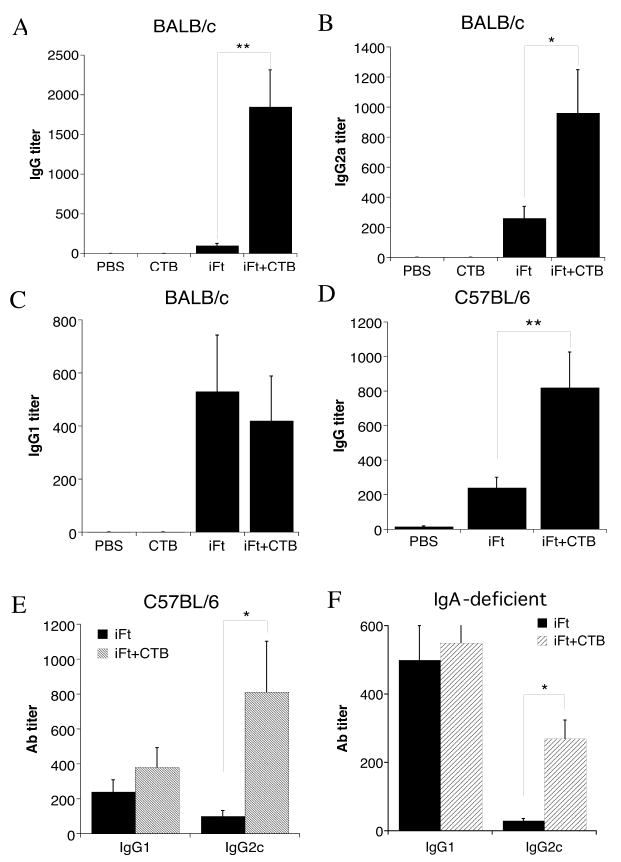
We next sought to determine if CTB enhances the Th1-type response, thereby explaining its protective activity. First, we focused on the specific IgG responses. Sera from mice immunized with PBS, CTB, iFt alone, or iFt plus CTB were obtained from mice 10–12 days post-boost. F. tularensis-specific total IgG, IgG2a (BALB/c), IgG2c (C57BL/6), and IgG1 Ab titers were measured by ELISA. Use of iFt plus CTB-enhanced total IgG levels in both BALB/c and C57BL/6 mice (Figs. 9A and D, respectively). Also co-administering iFt plus CTB significantly increased the levels of IgG2a (BALB/c) and IgG2c (C57BL/6) in the sera of these mice, compared to mice immunized with iFt alone (Figs. 9B and E, respectively). On the other hand, the levels of IgG1 in the sera of these mice were comparable between iFt plus CTB and iFt-immunized groups (Figs. 9C and E, respectively). This subclass Ab profile is indicative of the induction of a Th1-type response. Control (PBS) mice and mice immunized with CTB alone induced no significant F. tularensis-specific Ab responses (Figs. 9A, B and C). Furthermore, the levels of IgG2c were also increased in the sera of IgA-deficient mice immunized with iFt plus CTB (Fig. 9F).
Figure 9. Enhanced Production of Th1-Type IgG Isotypes in The Sera of Mice Immunized With iFt Plus CTB.
BALB/c (A–C), C57BL/6 (D,E) and IgA-deficient mice (F) were divided into four or three groups (4–5 mice/group), respectively, and immunized i.n. with 20μl of either PBS, 5μg of CTB in 20 μl PBS (only BALB/c), 2 × 107 iFt in 20 μl PBS, or 2 × 107 iFt plus 5μg of CTB in 20 μl PBS. Mice were immunized on day 0 and boosted on day 21 as in challenge studies in Figure 1. Serum was then collected at 35 days and tested for the presence of anti-F. tularensis IgG2a/2c and IgG1 sub-classes by ELISA, as described in Materials and Methods. Ab data represent the mean of 4–5 mice + SD. The levels of IgG2a/2c were significantly higher (p<0.05) in BALB/c (A–C), C57BL/6 (D and E) and IgA-deficient (F) mice immunized with iFt plus CTB compared to mice immunized with iFt alone. No significant difference was detected in the case of IgG1 between the same groups of mice. These data are representative of two different experiments.
To provide additional evidence for the induction of a Th1-type response by iFt plus CTB, the cytokine profile in the sera of iFt plus CTB-immunized mice, as well as that secreted by splenocytes from these mice “in vitro” was analyzed by CBA and compared to that of PBS and iFt-immunized mice. In the first instance, immunized mice were bled two days following booster immunization (day 23). Alternatively, in a separate experiment, but under the same immunization conditions, the spleens from immunized mice were harvested on day 35 post-primary immunization and cultured in complete media in the presence or absence of iFt. On days 3, 5 and 7 supernatants were collected and analyzed by CBA. CBA analysis showed significantly elevated levels of IFN-γ in the sera of mice immunized with iFt plus CTB compared to the iFt alone (Fig. 10A). Also, levels of IL-4, a Th2-type cytokine, were similarly low in both groups (Fig. 10A). The cytokine profile present in the supernatants of the cultured splenocytes derived from iFt plus CTB-immunized mice also supported the induction of a Th1-type response, in that IFN-γ production was enhanced by splenocytes obtained from iFt plus CTB-immunized mice (Fig. 10B). Other cytokines tested included IL-2, IL-5, TNF-α and MCP1 with no significant differences among these experimental groups being observed (data not shown).
Figure 10. Enhanced Th1-Type Cytokine Production in Response to Immunization With iFt Plus CTB.
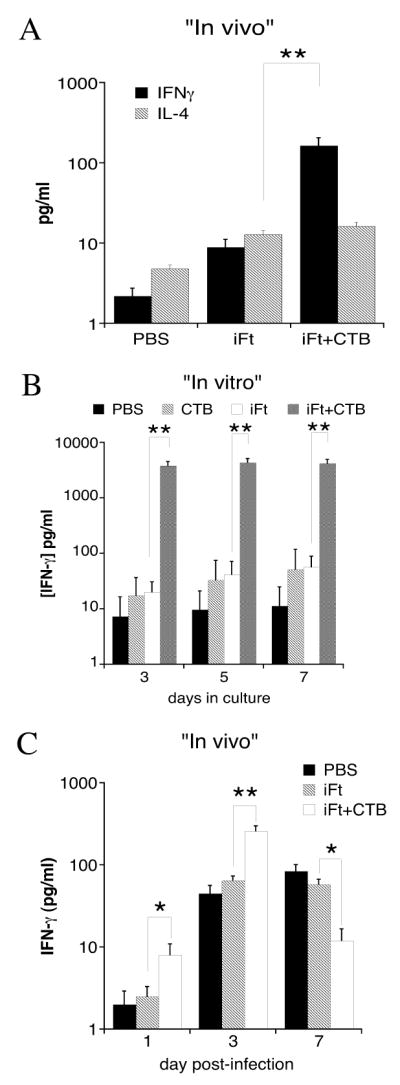
Immunizations were done as indicated in Figure 1. At 23 days post-primary immunization sera were obtained from mice and their cytokine content measured by CBA (A). In a different experiment, at 35 days post-primary immunization, spleen cells were harvested from each mouse and incubated in presence or absence of iFt as described in Materials and Methods. Supernatants were then harvested and Th1 and Th2 cytokines were measured by CBA (B). These data are representative of two experiments. Each bar represents the average of 4–6 individual mice ± SD. Both “in vivo” (A) and “in vitro” (B) results showed a significant increase of IFN-γ when mice were immunized with iFt plus CTB versus iFt alone (p<0.01). In an additional experiment (C), C57BL/6 mice were immunized and challenged on day 35 post-primary immunization as indicated in Figure 1. On days 1, 3, and 7 post-challenge the lungs were harvested and the IFN-γ levels were measured in the tissue homogenate by CBA. The levels of IFN-γ in the lungs of immunized mice are presented. The levels of IFN-γ were significantly higher at days 1 and 3 post-challenge in mice immunized with iFt plus CTB versus iFt alone, but significantly lower at day 7 in mice immunized with iFt plus CTB versus iFt alone (*<0.05, **<0.01).
Despite the above observations, IFN-γ levels in the lungs of iFt plus CTB-immunized mice are actually lower than non-immunized mice 7 days post-infection (Fig. 3), raising what would seem to be a paradox regarding the role of IFN-γ in protection. Thus, we sought to determine if there was in fact an early burst of IFN-γ production shortly (1–3 days) post-challenge, followed by the observed reduction in IFN-γ levels observed in Figure 3. In fact, consistent with our observations in Figure 3 and Figures 10A and 10B, iFt plus CTB-immunized animals had significantly higher IFN-γ levels in the first three days after challenge with F. tularensis LVS as compared to PBS and iFt-immunized mice (Fig. 10C), followed by a significant reduction in IFN-γ levels in the iFt plus CTB-immunized group versus PBS and iFt-immunized groups 7 days post-infection (Fig. 10C).
Immunization with iFt plus CTB induces partial protection against the highly virulent F. tularensis SchuS4 strain
To assess the potential efficiency of CTB as a mucosal adjuvant against the more virulent strain of F. tularensis, SchuS4, we immunized C57BL/6 mice with either: PBS (control), CTB alone, iFt alone, or iFt plus CTB. All mice were subjected to two immunization boosters on days 14 and 28 post-initial administration. Two weeks after the final boost (day 42) all mice were infected with 23 CFUs of SchuS4. All the mice from the PBS, CTB alone, and iFt alone immunized groups succumbed to the infection within 10 days post-SchuS4 challenge. In the iFt plus CTB group, 50% protection against SchuS4 was observed (Fig. 11). This result indicates that the use of CTB as adjuvant also represents a viable adjuvant approach for use against the highly virulent Biovar A strains of F. tularensis.
Figure 11. Protection Against Mucosal F. tularensis SchuS4 Challenge is Also Enhanced in Mice Immunized With iFt Plus CTB.
C57BL/6 mice were divided into four groups (8 mice/group) and immunized i.n. with 20μl of PBS, 5μg of CTB in 20 μl PBS, 2 × 107 iFt in 20 μl PBS, or 2 × 107 iFt with 5μg of CTB in 20 μl PBS. Mice subsequently received an immunization boost on days 14 and 28. Mice were then challenged on day 42 i.n. with 23 CFU (approximately 10–20 × LD50) of live F. tularensis SchuS4, and subsequently monitored for survival. The figure is representative of data from three different experiments.
Discussion
Until recently, it was widely accepted that cellular immunity, in particular the production of IFN-γ and the development of a Th1-type T cell response, was the critical response in generating protection against F. tularensis infection (14, 43–45). However, more recent studies (9, 15, 46, 47), including studies from this laboratory (10), have indicated Ab may also be vital to the generation of protection against this infectious organism. Importantly, understanding the relationship between the humoral and cellular immune response to F. tularensis, and the relative importance of each in protection, is crucial to the development of an effective vaccine against this pathogen.
Given recent studies suggesting humoral, as well as cellular immunity may be critical for protection against F. tularensis infection, we sought to test the efficacy of a mucosal adjuvant, CTB, which is known for its ability to stimulate humoral immunity (24, 48–51). In addition, while the ability of CTB to stimulate Th1-type cellular immunity is less clear, evidence suggests such responses can also occur under appropriate circumstances (32, 33, 52).
In fact, when iFt plus CTB was administered i.n., enhanced protection against subsequent challenge with both F. tularensis biovar A and biovar B organisms was observed (Figs. 1 and 11). Survival of immunized mice was correlated with reduced bacterial burden (Fig. 2) and a subsequent reduction of pro-inflammatory cytokines (Fig. 3). The protection also correlated with enhanced F. tularensis-specific IgG and IgA Ab in the sera and BAL fluid of iFt plus CTB-immunized mice, as compared to iFt-immunized recipients (Figs. 9 and 5, respectively). In regard to enhanced IgG production, the levels of IgG2a/IgG2c versus IgG1 were also significantly increased (Fig. 9), suggesting the promotion of a Th1-type response. This conclusion was also supported by an observed increase in the production of IFN-γ, both in vitro and in vivo, by mice immunized with iFt plus CTB, as opposed to mice immunized with iFt only (Fig. 10). The CTB-mediated enhancement of the Th1-type response is of particular significance, since this has not been demonstrated previously following i.n. immunization with CTB-adjuvanted vaccine, while prior studies have demonstrated enhanced Th1-type responses correlate with protection against F. tularensis infection (53–55). Thus, this observation is consistent with the overall belief that the Th1-type response is key to protection against F. tularensis infection. Indeed, mice lacking IFN-γ were not protected following immunization with either: iFt alone or iFt plus CTB (Fig. 8). Also consistent with the belief IFN-γ plays a key role in CTB-induced protection, iFt plus CTB-immunized mice mounted a more rapid IFN-γ response in the first 3 days post-infection with F. tularensis (Fig. 10C). The subsequent control of infection likely results in the significant reduction of IFN-γ levels in the iFt plus CTB-immunized animals as observed by day 7 post-infection (Figs. 3).
It is also important to note, that in previous studies using iFt as a protective immunogen, a requirement for both IFN-γ and IgA was observed (9, 10), suggesting a critical role for IgA in the protective response when using iFt alone as immunogen. Thus, we fully expected this also to be the case in the presence of CTB. In fact, despite studies by others suggesting a Th1-type response may suppress IgA production (35), enhanced F. tularensis-specific IgA production was observed in the presence of iFt plus CTB as compared to iFt alone. Surprisingly however, F. tularensis-specific IgA was not required for protection, as IgA-deficient mice challenged with F. tularensis LVS survived when immunized with iFt plus CTB, but not when immunized with iFt alone (Fig. 6). In fact, this is the first instance in which a differential role for Ab in vaccine-induced protection against F. tularensis infection has been observed. The significance of this observation is further strengthened by recent studies, which have re-examined the role of Ab, and questioned the belief that the Th1-type T cell response plays the primary role in protection against F. tularensis infection (8–10, 39, 47, 56).
In fact, IgA is known to play a central role in immune defense, controlling the spread of mucosal pathogens. Indeed, in some models of F. tularensis infection, IgA has been shown to play a key role in protection against challenge (10, 39). This was also evident in our current study, in that IgA-deficient mice vaccinated with iFt in the absence of CTB were not protected, whereas partial protection was observed in wildtype mice (Fig. 6). Somewhat surprisingly however, this requirement was overcome when mice were immunized with iFt plus CTB, in that IgA-deficient mice were protected (Fig. 6). The latter also correlated with an enhanced Th1-type response (Figs. 9 and 10), which was required, in that immunization with iFt plus CTB still failed to protect IFN-γ-deficient mice, even in the presence of enhanced production of F. tularensis- specific IgA (Fig. 8C). Furthermore, any requirement for Ab in general was eliminated by CTB, in that 100% of B cell-deficient mice immunized with iFt plus CTB and subsequently challenged with F. tularensis LVS, survived, while 90% of the same strain immunized with iFt alone did not. These results suggest that the requirement for IgA previously observed using iFt alone as immunogen, can be overcome in the presence of a sufficiently high Th1-type T cell response, potentially explaining seeming contradictions between studies which question the role of humoral versus cell-mediated immunity in protection against infection with this pathogen.
Although we did not observe full protection (approximately 50%) against SchuS4 challenge under the specific conditions of vaccination we utilized, we do anticipate this can be improved upon with further optimization of the vaccine regimen, specifically, through enhancement of the cellular immune response via alterations in the dose of immunogen and/or CTB, timing and/or number of immunizations, or use of additional adjuvants in combination with CTB. It should also be noted that 50% protection is particularly significant in this case, given the fact C57BL//6 mice and a challenge dose of 23 CFU (approximately 20 X LD50) were used. Specifically, the C57BL/6 mouse strain is particularly difficult to protect against SchuS4 challenge (as opposed to BALB/c mice for example) (57, 58), and as little as 1 CFU administered i.n. is fatal without vaccination. To further enhance the cellular response to F. tularensis, it may also be possible to combine other adjuvant strategies with CTB, such as the use of Fc receptor-targeted immunogens (10) or IL-12 (59). In fact, additional studies are currently under way utilizing these approaches.
In regard to the use of CTB as a mucosal adjuvant against F. tularensis, additional evaluation will be required. However, the observation that iFt plus CTB can enhance IgA, IgG, and Th1-type T cell responses, as well as protection against F. tularensis SchuS4 challenge, is promising. In fact, CTB has been used successfully with various infectious disease models, both bacterial and viral. For example, co-administration of CTB with Mycobacterium bovis-bacillus Calmette-Guerin (BCG) was shown to enhance delayed-type hypersensitivity reactions to purified protein derivative (PPD) (60). Also mucosal administration of CTB with Streptococcus pneumoniae surface Ags, such as PsaA and Rib, reduced bacterial colonization in the nasal passages and lungs of the immunized animals (61, 62). Comparable results were observed in an influenza study in which i.n. administration of the subunit vaccine with CTB, induced influenza virus-specific IgG and IgA Ab in the nasal passages and sera of immunized mice, and reduced viral load following subsequent influenza infection (63). Furthermore, in studies similar to our own, Cholera Toxin (CT) induced CD4+ T cell-mediated immunity to S. pneumoniae infection, which was also Ab independent (64–66). Promising results have also been observed in human studies. In a study carried out by Bergquist et al., (1997), intranasal administration of CTB to human subjects induced systemic and local Ab responses in the upper respiratory tract and the vagina (49). Despite these promising results however, some safety concerns do exist. Observed side effects from the use of CTB include: excessive nasal secretions, “bloody nose”, headaches, and migraines (67). In addition, CTB has affinity for GM1 ganglioside receptors in the brain, although studies also suggest potential problems associated with this can be avoided through appropriate dosing (68). In fact, numerous human trails have indicated minimal side effects in subjects following immunization with CTB (69, 70).
In summary, these are the first studies to demonstrate a differential requirement for Ab in vaccine-induced protection against F. tularensis infection, dependent on the immunization strategy utilized. These studies are particularly timely, given recent disagreement regarding the importance of Ab versus cellular immunity in generating protection against this infection, and suggest that the requirement for Ab observed may be overcome when the level of cellular immunity generated is sufficient. Furthermore, we demonstrate for the first time, the ability of CTB to induce a Th1-type immune response when administered i.n. with an inactivated immunogen.
Acknowledgments
These studies were funded by grants from the National Institutes of Health (P01 AI056320 and R21 AI065476)
We wish to thank the Immunology Core at AMC as well as the Microbiology/BSL3 Core at AMC for significant contributions to this work.
Abbreviations in this paper
- iFt
Inactivated F. tularensis Live Vaccine Strain
- MO
Macrophages
- DC
Dendritic Cells
- LVS
F. tularensis Live Vaccine Strain
- i.n
Intranasal
- CTB
Cholera Toxin B
- BAL
Bronchial Alveolar Lavage
Footnotes
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in the author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 3.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 4.Claflin JL, Larson CL. Infection-immunity in tularemia: specificity of cellular immunity. Infect Immun. 1972;5:311–318. doi: 10.1128/iai.5.3.311-318.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostiala AA, McGregor DD, Logie PS. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975;28:855–869. [PMC free article] [PubMed] [Google Scholar]
- 6.Surcel HM. Diversity of Francisella tularensis antigens recognized by human T lymphocytes. Infect Immun. 1990;58:2664–2668. doi: 10.1128/iai.58.8.2664-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KuoLee R, Zhao X, Austin J, Harris G, Conlan JW, Chen W. Mouse model of oral infection with virulent type A Francisella tularensis. Infect Immun. 2007;75:1651–1660. doi: 10.1128/IAI.01834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pammit MA, Raulie EK, Lauriano CM, Klose KE, Arulanandam BP. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun. 2006;74:2063–2071. doi: 10.1128/IAI.74.4.2063-2071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 10.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, Metzger DW, Gosselin EJ. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulop M, Manchee R, Titball R. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine. 1995;13:1220–1225. doi: 10.1016/0264-410x(95)00062-6. [DOI] [PubMed] [Google Scholar]
- 12.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 13.Conlan JW, Shen H, Webb A, Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20:3465–3471. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 14.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntley JF, Conley PG, Rasko DA, Hagman KE, Apicella MA, Norgard MV. Native outer membrane proteins protect mice against pulmonary challenge with virulent type A Francisella tularensis. Infect Immun. 2008;76:3664–3671. doi: 10.1128/IAI.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waag DM, Galloway A, Sandstrom G, Bolt CR, England MJ, Nelson GO, Williams JC. Cell-mediated and humoral immune responses induced by scarification vaccination of human volunteers with a new lot of the live vaccine strain of Francisella tularensis. J Clin Microbiol. 1992;30:2256–2264. doi: 10.1128/jcm.30.9.2256-2264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koskela P. Humoral immunity induced by a live Francisella tularensis vaccine. Complement fixing antibodies determined by an enzyme-linked immunosorbent assay (CF-ELISA) Vaccine. 1985;3:389–391. doi: 10.1016/0264-410x(85)90129-x. [DOI] [PubMed] [Google Scholar]
- 18.Koskela P, Salminen A. Humoral immunity against Francisella tularensis after natural infection. J Clin Microbiol. 1985;22:973–979. doi: 10.1128/jcm.22.6.973-979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30(Suppl 3):S266–270. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren J, Lycke N, Czerkinsky C. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine. 1993;11:1179–1184. doi: 10.1016/0264-410x(93)90039-z. [DOI] [PubMed] [Google Scholar]
- 21.Tochikubo K, Isaka M, Yasuda Y, Kozuka S, Matano K, Miura Y, Taniguchi T. Recombinant cholera toxin B subunit acts as an adjuvant for the mucosal and systemic responses of mice to mucosally co-administered bovine serum albumin. Vaccine. 1998;16:150–155. doi: 10.1016/s0264-410x(97)00194-1. [DOI] [PubMed] [Google Scholar]
- 22.Wu HY, Russell MW. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1998;16:286–292. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 23.Isaka M, Yasuda Y, Kozuka S, Taniguchi T, Matano K, Maeyama J, Komiya T, Ohkuma K, Goto N, Tochikubo K. Induction of systemic and mucosal antibody responses in mice immunized intranasally with aluminium-non-adsorbed diphtheria toxoid together with recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1999;18:743–751. doi: 10.1016/s0264-410x(99)00258-3. [DOI] [PubMed] [Google Scholar]
- 24.Isaka M, Yasuda Y, Taniguchi T, Kozuka S, Matano K, Maeyama J, Morokuma K, Ohkuma K, Goto N, Tochikubo K. Mucosal and systemic antibody responses against an acellular pertussis vaccine in mice after intranasal co-administration with recombinant cholera toxin B subunit as an adjuvant. Vaccine. 2003;21:1165–1173. doi: 10.1016/s0264-410x(02)00516-9. [DOI] [PubMed] [Google Scholar]
- 25.Isaka M, Komiya T, Takahashi M, Yasuda Y, Taniguchi T, Zhao Y, Matano K, Matsui H, Maeyama J, Morokuma K, Ohkuma K, Goto N, Tochikubo K. Recombinant cholera toxin B subunit (rCTB) as a mucosal adjuvant enhances induction of diphtheria and tetanus antitoxin antibodies in mice by intranasal administration with diphtheria-pertussis-tetanus (DPT) combination vaccine. Vaccine. 2004;22:3061–3068. doi: 10.1016/j.vaccine.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Kim JK, Seo SB, Lee HJ. Intranasal vaccination with peptides and cholera toxin subunit B as adjuvant to enhance mucosal and systemic immunity to respiratory syncytial virus. Arch Pharm Res. 2007;30:366–371. doi: 10.1007/BF02977620. [DOI] [PubMed] [Google Scholar]
- 27.Isaka M, Zhao Y, Nobusawa E, Nakajima S, Nakajima K, Yasuda Y, Matsui H, Hasegawa T, Maeyama J, Morokuma K, Ohkuma K, Tochikubo K. Protective effect of nasal immunization of influenza virus hemagglutinin with recombinant cholera toxin B subunit as a mucosal adjuvant in mice. Microbiol Immunol. 2008;52:55–63. doi: 10.1111/j.1348-0421.2008.00010.x. [DOI] [PubMed] [Google Scholar]
- 28.Jackson RJ, Fujihashi K, Xu-Amano J, Kiyono H, Elson CO, McGhee JR. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee JR. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 30.Toida N, Hajishengallis G, Wu HY, Russell MW. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to the cholera toxin B subunit elicits T-helper-cell responses in gut-associated lymphoid tissues. Infect Immun. 1997;65:909–915. doi: 10.1128/iai.65.3.909-915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun MC, He J, Wu CY, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anjuere F, George-Chandy A, Audant F, Rousseau D, Holmgren J, Czerkinsky C. Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses. J Immunol. 2003;170:1586–1592. doi: 10.4049/jimmunol.170.3.1586. [DOI] [PubMed] [Google Scholar]
- 33.Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuere F. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J Immunol. 2006;176:2749–2757. doi: 10.4049/jimmunol.176.5.2749. [DOI] [PubMed] [Google Scholar]
- 34.Holmgren J, Harandi AM, Czerkinsky C. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev Vaccines. 2003;2:205–217. doi: 10.1586/14760584.2.2.205. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Vancott JL, Okahashi N, Marinaro M, Kiyono H, Fujihashi K, Jackson RJ, Chatfield SN, Bluethmann H, McGhee JR. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- 36.Eigelsbach HT, Braun W, Herring RD. Studies on the variation of Bacterium tularense. J Bacteriol. 1951;61:557–569. doi: 10.1128/jb.61.5.557-569.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakshi CS, Malik M, Mahawar M, Kirimanjeswara GS, Hazlett KR, Palmer LE, Furie MB, Singh R, Melendez JA, Sellati TJ, Metzger DW. An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain. Vaccine. 2008;26:5276–5288. doi: 10.1016/j.vaccine.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik M, Bakshi CS, McCabe K, Catlett SV, Shah A, Singh R, Jackson PL, Gaggar A, Metzger DW, Melendez JA, Blalock JE, Sellati TJ. Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J Immunol. 2007;178:1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- 39.Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect Immun. 2007;75:2152–2162. doi: 10.1128/IAI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamm ME, Robinson JK, Kaetzel CS. Transport of IgA immune complexes across epithelial membranes: new concepts in mucosal immunity. Adv Exp Med Biol. 1992;327:91–94. doi: 10.1007/978-1-4615-3410-5_11. [DOI] [PubMed] [Google Scholar]
- 41.Lamm ME, Nedrud JG, Kaetzel CS, Mazanec MB. IgA and mucosal defense. APMIS. 1995;103:241–246. doi: 10.1111/j.1699-0463.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 42.Lamm ME. Current concepts in mucosal immunity. IV. How epithelial transport of IgA antibodies relates to host defense. Am J Physiol. 1998;274:G614–617. doi: 10.1152/ajpgi.1998.274.4.g614. [DOI] [PubMed] [Google Scholar]
- 43.Green SJ, Nacy CA, Schreiber RD, Granger DL, Crawford RM, Meltzer MS, Fortier AH. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from L-arginine and protection against Francisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect Immun. 1993;61:689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wayne Conlan J, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005;23:2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 46.Stenmark S, Lindgren H, Tarnvik A, Sjostedt A. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb Pathog. 2003;35:73–80. doi: 10.1016/s0882-4010(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 47.Lavine CL, Clinton SR, Angelova-Fischer I, Marion TN, Bina XR, Bina JE, Whitt MA, Miller MA. Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol. 2007;37:3007–3020. doi: 10.1002/eji.200737620. [DOI] [PubMed] [Google Scholar]
- 48.Bergquist C, Lagergard T, Lindblad M, Holmgren J. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–2025. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergquist C, Johansson EL, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isaka M, Yasuda Y, Kozuka S, Taniguchi T, Miura Y, Matano K, Goto N, Tochikubo K. Intranasal or subcutaneous co-administration of recombinant cholera toxin B subunit stimulates only a slight or no level of the specific IgE response in mice to tetanus toxoid. Vaccine. 1999;17:944–948. doi: 10.1016/s0264-410x(98)00280-1. [DOI] [PubMed] [Google Scholar]
- 51.Park SJ, Chun SK, Kim PH. Intraperitoneal delivery of cholera toxin B subunit enhances systemic and mucosal antibody responses. Mol Cells. 2003;16:106–112. [PubMed] [Google Scholar]
- 52.Bublin M, Hoflehner E, Wagner B, Radauer C, Wagner S, Hufnagl K, Allwardt D, Kundi M, Scheiner O, Wiedermann U, Breiteneder H. Use of a genetic cholera toxin B subunit/allergen fusion molecule as mucosal delivery system with immunosuppressive activity against Th2 immune responses. Vaccine. 2007;25:8395–8404. doi: 10.1016/j.vaccine.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 55.Sjostedt A, North RJ, Conlan JW. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology. 1996;142(Pt 6):1369–1374. doi: 10.1099/13500872-142-6-1369. [DOI] [PubMed] [Google Scholar]
- 56.Lu Z, Roche MI, Hui JH, Unal B, Felgner PL, Gulati S, Madico G, Sharon J. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol Lett. 2007;112:92–103. doi: 10.1016/j.imlet.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green M, Choules G, Rogers D, Titball RW. Efficacy of the live attenuated Francisella tularensis vaccine (LVS) in a murine model of disease. Vaccine. 2005;23:2680–2686. doi: 10.1016/j.vaccine.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Shen H, Webb A, KuoLee R, Conlan JW. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine. 2003;21:3690–3700. doi: 10.1016/s0264-410x(03)00386-4. [DOI] [PubMed] [Google Scholar]
- 59.Arulanandam BP, O’Toole M, Metzger DW. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J Infect Dis. 1999;180:940–949. doi: 10.1086/314996. [DOI] [PubMed] [Google Scholar]
- 60.Maeyama J, Isaka M, Yasuda Y, Matano K, Morokuma K, Ohkuma K, Tochikubo K, Yamamoto S, Goto N. Effects of recombinant cholera toxin b subunit (rCTB) on cellular immune responses: enhancement of delayed-type hypersensitivity following intranasal co-administration of Mycobacterium bovis-BCG with rCTB. Microbiol Immunol. 2004;48:457–463. doi: 10.1111/j.1348-0421.2004.tb03536.x. [DOI] [PubMed] [Google Scholar]
- 61.Pimenta FC, Miyaji EN, Areas AP, Oliveira ML, de Andrade AL, Ho PL, Hollingshead SK, Leite LC. Intranasal immunization with the cholera toxin B subunit-pneumococcal surface antigen A fusion protein induces protection against colonization with Streptococcus pneumoniae and has negligible impact on the nasopharyngeal and oral microbiota of mice. Infect Immun. 2006;74:4939–4944. doi: 10.1128/IAI.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larsson C, Holmgren J, Lindahl G, Bergquist C. Intranasal immunization of mice with group B streptococcal protein rib and cholera toxin B subunit confers protection against lethal infection. Infect Immun. 2004;72:1184–1187. doi: 10.1128/IAI.72.2.1184-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Pacheco S, Acuna CL, Switzer KC, Wang Y, Gilmore X, Harriman GR, Mbawuike IN. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology. 2002;105:286–294. doi: 10.1046/j.0019-2805.2001.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102:4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basset A, Thompson CM, Hollingshead SK, Briles DE, Ades EW, Lipsitch M, Malley R. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect Immun. 2007;75:5460–5464. doi: 10.1128/IAI.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trzcinski K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun. 2008;76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scerpella EG, Sanchez JL, Mathewson IJ, Torres-Cordero JV, Sadoff JC, Svennerholm AM, DuPont HL, Taylor DN, Ericsson CD. Safety, Immunogenicity, and Protective Efficacy of the Whole-Cell/Recombinant B Subunit (WC/rBS) Oral Cholera Vaccine Against Travelers’ Diarrhea. J Travel Med. 1995;2:22–27. doi: 10.1111/j.1708-8305.1995.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 68.Hagiwara Y, Iwasaki T, Asanuma H, Sato Y, Sata T, Aizawa C, Kurata T, Tamura S. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile enterotoxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine. 2001;19:1652–1660. doi: 10.1016/s0264-410x(00)00412-6. [DOI] [PubMed] [Google Scholar]
- 69.Qadri F, Wenneras C, Ahmed F, Asaduzzaman M, Saha D, Albert MJ, Sack RB, Svennerholm A. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine. 2000;18:2704–2712. doi: 10.1016/s0264-410x(00)00056-6. [DOI] [PubMed] [Google Scholar]
- 70.Jertborn M, Svennerholm AM, Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1992;10:130–132. doi: 10.1016/0264-410x(92)90030-n. [DOI] [PubMed] [Google Scholar]