Summary
The COP9 signalosome (CSN) is an evolutionarily conserved macromolecular complex that interacts with cullin-RING E3 ligases (CRLs) and regulates their activity by hydrolyzing cullin-Nedd8 conjugates. The CSN sequesters inactive CRL4Ddb2, which rapidly dissociates from the CSN upon DNA damage. Here we systematically define the protein interaction network of the mammalian CSN through mass spectrometric interrogation of the CSN subunits Csn1, Csn3, Csn4, Csn5, Csn6 and Csn7a. Notably, we identified a subset of CRL complexes that stably interact with the CSN and thus might similarly be activated by dissociation from the CSN in response to specific cues. In addition, we detected several new proteins in the CRL-CSN interactome, including Dda1, which we characterized as a chromatin-associated core subunit of multiple CRL4 proteins. Cells depleted of Dda1 spontaneously accumulated double-stranded DNA breaks in a similar way to Cul4A-, Cul4B- or Wdr23-depleted cells, indicating that Dda1 interacts physically and functionally with CRL4 complexes. This analysis identifies new components of the CRL family of E3 ligases and elaborates new connections between the CRL and CSN complexes.
Keywords: Ubiquitin-dependent proteolysis, Cullin-RING E3 ligases, Neddylation, Deneddylation, Dda1
Introduction
Multisubunit cullin-RING type ubiquitin ligases constitute the most prominent family of ubiquitin ligases, in which the cullin protein functions as an assembly platform (Petroski and Deshaies, 2005). Cullins recruit the RING finger protein Rbx1 (also known as Roc1 and Hrt1) through their conserved C-terminus and the substrate recognition module through their N-terminal domains. The human genome encodes at least six cullins including Cul1, Cul2, Cul3, Cul4A, Cul4B and Cul5 (Kipreos et al., 1996), which bind distinct substrate recognition modules. The SCF complexes use the Bric-a-brac, Tramtrack, Broad-complex (BTB)-fold adaptor Skp1 to connect Cul1 to a myriad of F-box proteins, which recognize their substrates mostly through WD40- or leucine-rich repeats (LRR). Likewise, the cullin-RING E3 ligases (CRLs) CRL2 and CRL5 use the BTB-fold adaptor ElonginC to connect Cul2 and Cul5 to BC-VHL box and BC-SOCS box proteins, respectively. In CRL3, a single polypeptide containing a BTB domain and a substrate-binding interface merges the function of Skp1-F-box or ElonginC-BC box heterodimers (Pintard et al., 2004). Finally, heterodimers composed of the large Ddb1 protein, which does not harbor a BTB-fold but three seven-bladed β-propellers, and a member of the DCAF family (Ddb1-Cul4-associated factor), function as the substrate recognition modules of CRL4s (Angers et al., 2006; Jin et al., 2006). The small subunit Dda1 may also be part of some CRL4s (Jin et al., 2006), but its molecular function and regulation is still poorly understood.
Despite the fact that they display distinct molecular compositions, all CRLs are regulated by the covalent linkage of the ubiquitin-like protein Nedd8 to a conserved C-terminal lysine residue of the cullins (Pan et al., 2004). Neddylation is essential for viability in all tested species, except budding yeast. Nedd8 activates CRLs by promoting a drastic conformational change of the C-terminal part of the cullin that frees Rbx1 and allows it to adopt multiple orientations that stimulate substrate ubiquitination in vitro (Duda et al., 2008).
The COP9 signalosome (CSN) complex physically interacts with CRLs and counteracts neddylation by hydrolyzing cullin-Nedd8 conjugates (Lyapina et al., 2001; Schwechheimer et al., 2001). Several lines of evidence indicate that the main function of the CSN is to inactivate CRLs by deneddylating cullins, at least in part by preventing the autocatalytic instability of their substrate-specific adaptors and subunits (Wee et al., 2005; Wu et al., 2005).
Although significant progress has been made in understanding how specific cues regulate substrate modification and in turn CRL binding, much less is known about how substrate binding is coordinated with cullin neddylation and deneddylation. Interestingly, several recent reports indicate that the availability of the adaptor bound to its substrate may promote CRL assembly and activation through cullin neddylation. For example, the availability of the F-box protein Skp2 along with its substrate p27, triggers assembly of the SCFSkp2 ubiquitin ligase and its activation through neddylation of the Cul1 subunit in vitro (Bornstein et al., 2006). Likewise, cullin mutants unable to bind substrate recognition modules exhibit reduced neddylation (Chew and Hagen, 2007). However, not all CRLs appear to be regulated by this mechanism, as substrate recruitment does not stimulate neddylation of Keap1-associated Cul3 (Chew and Hagen, 2007). Interestingly, specific cues may trigger dissociation of fully assembled but inactive CRLs from the CSN, and in turn their activation through cullin neddylation. For example, inactive CRL4Ddb2 ubiquitin ligase is tightly bound to the CSN but is rapidly activated upon ultraviolet (UV) irradiation by dissociation from the CSN and subsequent neddylation (Groisman et al., 2003). These observations raise the possibility that, in a similar way to the CRL4Ddb2 complex, some fully assembled CRLs might be sequestered and kept inactive by the CSN in the absence of specific cues.
In this study, we sought to identify all pre-assembled CRLs that are tightly bound to the CSN in mammalian cells. We stably expressed and purified each FLAG-tagged CSN subunit from HEK 293T cells, and systematically identified CSN-associated polypeptides by sensitive tandem mass spectrometry (LC-MS-MS). Interestingly, we not only identified a total of fifteen associated CRLs but also found new components such as Dda1 as core subunits of multiple CRL4 complexes. Our subsequent functional analysis indicates that Dda1 acts as a positive regulator of several CRL4s.
Results
The proteomic interaction network of the mammalian COP9 signalosome
To systematically map the protein interaction network of the mammalian COP9 signalosome, we generated cell lines (HEK 293T) that stably expressed CSN subunits (Csn1, 3, 4, 5, 6 and 7a) fused to three repeats of the FLAG epitope (Fig. 1A), and analyzed anti-FLAG immunoprecipitates by sensitive LC-MS-MS. Although fibroblasts expressing FLAG-Csn2 exist (Huang et al., 2005), we were unable to obtain a cell line stably expressing FLAG-Csn2, thus Csn2 and 8 are the only subunits missing in our analysis.
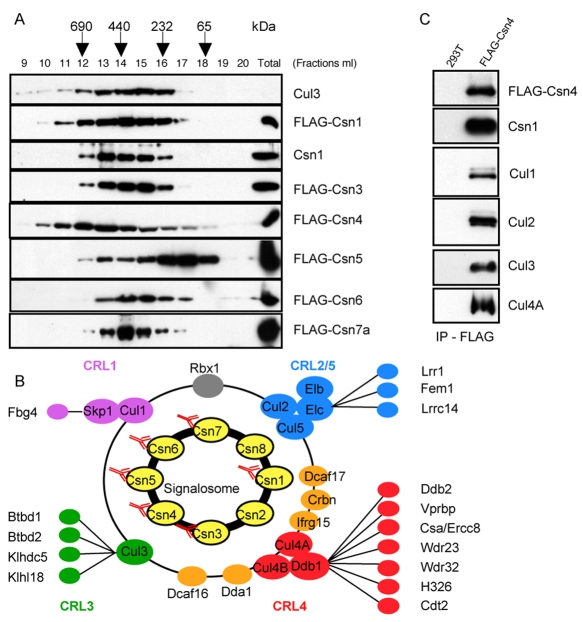
Fig. 1.
The protein interaction network of the mammalian CSN. (A) Protein extracts prepared from HEK 293T cells stably expressing the indicated FLAG-tagged CSN subunits were fractionated on a Superose 6 column (bed volume: 24 ml) and 1-ml fractions were collected and analyzed by SDS-PAGE with specific FLAG, Csn1 and Cul3 antibodies. (B) CSN-interacting CRL subunits are highlighted in purple (SCF), blue (CRL2 and CRL5), green (CRL3) and red (CRL4). Subunits highlighted in orange have been previously found associated with CRL4s. (C) CSN-cullin interactions observed by LC-MS-MS were confirmed by co-immunoprecipitation experiments combined with western blot identification.
Importantly, immunopurification of FLAG-Csn1, 3, 4 and 6 led to the identification of a common set of associated proteins, thus defining the CSN interaction network (Fig. 1B; supplementary material Table S3). By contrast, immunopurification of FLAG-Csn5 or FLAG-Csn7a was rather poorly efficient in pulling down this set of associated proteins. In both cases these results are expected because Csn5 also exists as a monomeric form (Tomoda et al., 2002) and thus a large fraction of FLAG-Csn5 was not incorporated into to the CSN complex as revealed by gel filtration experiments (Fig. 1A), and recombinant FLAG-Csn7a subunit competes for incorporation into the CSN complex not only with endogenous Csn7a but also with the Csn7b subunit. Indeed, Csn7b was not recovered in the FLAG-Csn7a immunoprecipitate (supplementary material Table S3), indicating that the incorporation of Csn7a and Csn7b into the CSN complex is mutually exclusive.
Although the COP9 signalosome has been shown previously to interact with a myriad of factors (Wei and Deng, 2003), our systematic proteomic analysis of the FLAG-tagged COP9 signalosome exclusively identified subunits of CRLs [SCF (purple), CRL2 and CRL5 (blue), CRL3 (green), and CRL4 (red and orange)]. The presence of Cul1, Cul2, Cul3, Cul4A and Cul4B in FLAG-Csn4 immunoprecipitates was confirmed by immunoblotting with specific antibodies (Fig. 1C). Conversely, immunoprecipitations with Cul3 antibodies specifically recovered CSN subunits (Fig. 2A and supplementary material Table S5) demonstrating that cullins physically interact with the CSN. In addition to cullin proteins, we readily identified Rbx1, and the linkers Skp1, ElonginC, ElonginB and Ddb1, which bridge the interaction of cullins with their specific substrate adaptors (Fig. 1B; supplementary material Table S3). Besides these core CRL subunits, we systematically found the same set of substrate recruitment factors including one F-box (Fbg4-Fbx17), three BC-box (Lrr1, Lrrc14 and Fem1), four BTB (Btbd1, Btbd2, Klhdc5 and Klhl18), and several DCAF (Ddb1- and Cul4-associated factor) proteins including Csa-Ercc8 and Ddb2, which have been shown previously to specifically rearrange from the CSN in response to DNA damage (Groisman et al., 2003). Conversely mass spectrometry analysis of FLAG-Lrrc14 and FLAG-Lrr1 immunoprecipitates identified Cul2, ElonginC, ElonginB, Rbx1 and every CSN subunit (Fig. 2G; supplementary material Table S4). Therefore, among the 16 BC-box proteins that were recently identified in FLAG-ElonginB pulldowns (Mahrour et al., 2008), only Lrrc14, Lrr1 and Fem1 stably associated with the CSN. Similarly, only four BTB proteins (Btbd1, Btbd2, Klhldc5 and Klhl18) were systematically identified in the CSN immunoprecipitates (Fig. 1B), whereas at least five additional BTB-substrate adaptors (Klhl9, Klhl13, Kctd10, Klhl22 and Kbtbd6) were recovered in endogenous Cul3 immunoprecipitates (Fig. 2A and supplementary material Table S5). Indeed, the FLAG-Csn4 readily co-immunoprecipitated with the endogenous Btbd1-Btbd2 complex but not with Klhl9-Klhl13, which preferentially immunoprecipitated neddylated Cul3 (Fig. 2B). Likewise, several DCAF proteins were also found to be stably associated with the CSN by MS-MS analysis (Fig. 1B; Fig. 2D), supporting the notion that the CSN may sequester many pre-assembled CRL4 complexes. Accordingly, co-immunoprecipitation experiments with HA-tagged versions of Ddb2, Wdr23 and H326 confirmed the presence of Csn2 and Csn3 (Fig. 2E) and Csn subunits were recovered in endogenous Vprbp immunoprecipitates (data not shown) (McCall et al., 2008). Taken together, these results suggest that the CSN stably associates with a specific subset of CRLs in vivo (Fig. 2F).
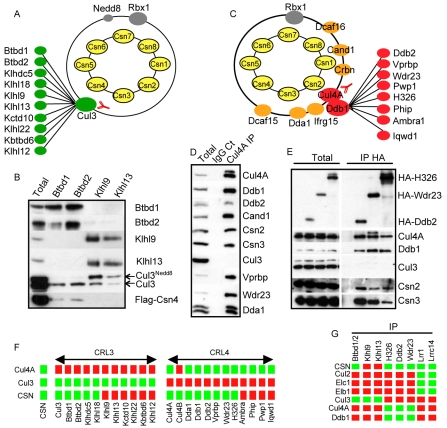
Fig. 2.
The CSN stably interacts with a limited subset of CRLs. (A) Endogenous Cul3 immunocomplexes were affinity-purified with specific antibodies, and associated proteins were analyzed by LC-MS-MS (left panel). (B) Klhl9 and 13, and Btbd1 and 2 were immunoprecipitated from 293T cells expressing FLAG-Csn4, separated by SDS-PAGE and immunoblotted with specific antibodies against the indicated proteins. The arrows mark neddylated (Cul3Nedd8) and unneddylated Cul3. (C) Endogenous Cul4A immunocomplexes were separated by SDS-PAGE and analyzed by tandem mass spectrometry. (D) Cul4A immunocomplexes were separated by SDS-PAGE and blotted with specific antibodies directed against Cul4A, Ddb1, Ddb2, Cand1, Csn2, Csn3, Cul3, Vprbp, Wdr23 and Dda1. (E) HA-tagged DCAF proteins H326, Wdr23 and Ddb2 were expressed in HeLa cells and immunoprecipitated. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with specific antibodies directed against the HA peptide, Cul4A, Cul4B, Ddb1, Cul3, Csn2 and Csn3. (F) Summary of the LC-MS-MS analysis of FLAG-CSN, Cul3 and Cul4A immunoprecipitates. Green squares indicate presence in the immunoprecipitate and red indicate absence. Cul3 and CRL4 core subunits (Cul4A, Cul4B and Ddb1) as well as BTB and DCAF adaptors are presented. (G) Summary of the LC-MS-MS analysis of FLAG-Lrr1, -Lrrc14 immunoprecipitates and co-immunoprecipitation analysis of Btbd1 and 2, Klhl9 and 13 and HA-H326, -Ddb2 and -Wdr23 immunoprecipitates. Green squares indicate presence in the immunoprecipitate and red indicate absence.
The CSN-interacting protein Dda1 is an unstable nuclear core subunit of multiple Cul4 ubiquitin ligases
In addition to substrate-recruitment factors, we identified several proteins that did not contain any recognizable domain (Fig. 1B, highlighted in orange). Some of these factors were recovered at low abundance, or were primarily detected when cells were treated with the proteasome inhibitor MG132, among them Dda1. Dda1 is a small evolutionarily conserved protein that has been shown previously to interact with Ddb1; however, its role and regulation remain elusive (Jin et al., 2006).
Consistent with our mass spectrometry data, FLAG-Dda1 strongly accumulated in cells treated with MG132, and FLAG-Dda1 was degraded with a half-life of approximately 1 hour (Fig. 3A). Interestingly, single-cell analysis using indirect immunofluorescence revealed that HeLa cells depleted for Cul4A by RNAi exhibited high levels of FLAG-Dda1 compared with control cells (Fig. 3B). Moreover, siRNA-mediated depletion of Cul4A and Cul4B was accompanied by accumulation of endogenous Dda1 (Fig. 3C), suggesting that Dda1 is degraded by Cul4 complexes, most likely through an autocatalytic mechanism.
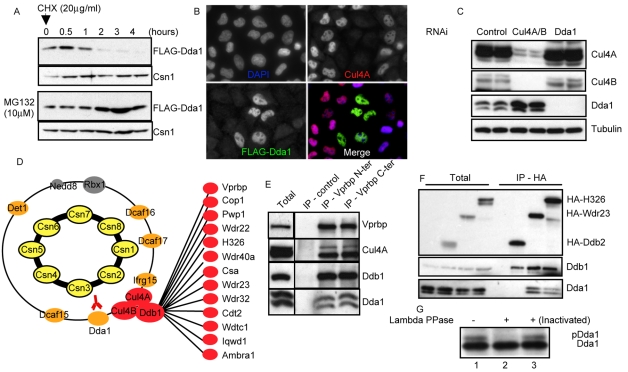
Fig. 3.
Dda1 is an unstable core subunit of multiple CRL4s. (A) HEK 293T cells stably expressing FLAG-Dda1 were treated with MG132 or for control DMSO. After 3 hours, protein translation was inhibited with cycloheximide (CHX) and samples were collected at various times (in hours) after CHX addition. Protein extracts were analyzed by SDS-PAGE with specific FLAG and Csn1 antibodies. (B) HeLa cells incubated with oligonucleotides targeting Cul4A were transfected with a plasmid expressing FLAG-Dda1. Cells were fixed and immunostained with anti-Cul4A (upper right panel) and anti-FLAG antibodies (bottom left panel). DAPI was used to counterstain DNA (upper left panel), and merged images are presented in the bottom right panel. (C) Protein extracts prepared from cells incubated with control oligonucleotides (lanes 1 and 2), or oligonucleotides targeting Dda1 (lanes 3 and 4) or Cul4 paralogs (lanes 5 and 6) were separated by SDS-PAGE. Cul4A and B, Cdt1, and Dda1 levels were assessed by immunoblotting using specific antibodies. Tubulin was used as a loading control. (D) LC-MS-MS analysis of anti-FLAG-Dda1 immunoprecipitates. Note that FLAG-Dda1 co-purifies with all COP9 signalosome (subunits highlighted in yellow) and many CRL4 subunits (colored in red and orange). (E) Vprbp was immunoprecipitated with control antibodies or antibodies directed against the N- or C-terminal part of the protein. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with specific antibodies specific for Vprbp, Cul4A, Ddb1 and Dda1. (F) HA-tagged DCAF proteins H326, Wdr23 and Ddb2 were expressed in HeLa cells and immunoprecipitated. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with specific antibodies directed against the HA peptide, Ddb1 and Dda1. (G) Dda1 immunoprecipitates were treated with lambda phosphatase (lane 2) or an inactivated phosphatase (lane 3) and analyzed by western blotting using specific Dda1 antibodies.
To investigate whether Dda1 associates with Ddb1 in assembled Cul4 ubiquitin ligases, we analyzed FLAG-Dda1 immunoprecipitates by LC-MS-MS. As shown in Fig. 3D and supplementary material Table S6, the CSN, as well as all known core subunits of CRL4 complexes specifically co-purified with FLAG-Dda1. Moreover, thirteen DCAF proteins including Vprbp, Cop1, Pwp1, Wdr22, H326, Wdr40A, Ercc8-Csa, Wdr23, Wdr32, Cdt2, Wdtc1, Iqwd1 and Ambra1 (Loc55626) were specifically recovered in the immunoprecipitates. Reciprocal immunopurifications using two distinct anti-Vprbp antibodies led to the co-precipitation of Dda1 (Fig. 3E). Likewise, Dda1 co-purified with HA-tagged versions of Wdr23 and H326, confirming that DCAF proteins are part of the Dda1 protein interaction network (Fig. 3F). Interestingly, Dda1 was phosphorylated in vivo and both forms of Dda1 specifically interacted with multiple CRL4DCAF complexes (Fig. 3E,F,G). Surprisingly, Dda1 purifications did not contain the DCAF Ddb2 (Fig. 3D) and conversely, Dda1 was not recovered in the HA-Ddb2 immunoprecipitates containing the CSN and core CRL4 subunits (Fig. 2E; Fig. 3F; and data not shown). Together, these data indicate that Dda1 is a core subunit of many but not all CRL4 complexes.
The localization of Dda1 is cell cycle regulated
To investigate the molecular function of Dda1, we determined its expression levels during the cell division cycle using a double thymidine block-release synchronization protocol (Sumara et al., 2007). Dda1 was detected throughout the cell cycle with lower levels of expression during mitosis (Fig. 4A). Indirect immunofluorescence experiments confirmed that Dda1 levels were reduced in mitotic cells, whereas Cul4A levels remained unchanged (data not shown). Dda1 was predominantly nuclear during interphase and resisted extraction procedures that solubilize nucleoplasmic proteins (Fig. 4B), suggesting that, like Cul4A, Dda1 may be associated with chromatin. Indeed, biochemical purification of chromatin from S-phase-arrested cells confirmed that significant fractions of Dda1, Ddb1 and Cul4A were found in chromatin preparations and could be solubilized by nuclease treatment (Fig. 4C). Interestingly, immunofluorescence analysis revealed that in contrast to Cul4A, Dda1 staining was strongly reduced on chromosomes aligned on the metaphase plate or on segregating chromosomes during anaphase, and Dda1 was only recruited to chromatin during telophase when the chromosomes start to decondense (Fig. 4C,D). However, the total amount of Dda1 co-precipitating with Cul4A was comparable in cells arrested in S-phase or mitosis (Fig. 4F), suggesting that Dda1 only dissociates from a small fraction of Cul4 complexes during mitosis.
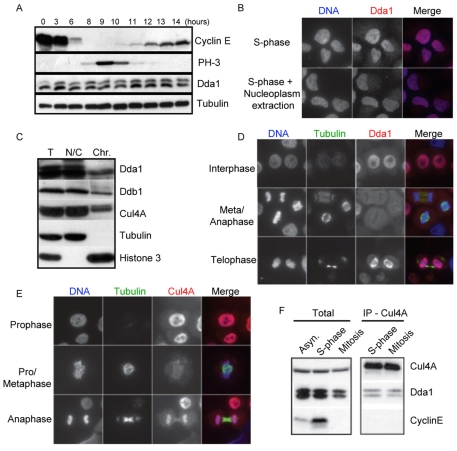
Fig. 4.
Dda1 associates with the chromatin in a cell cycle-dependent manner. (A) Cells were arrested in S-phase using a double thymidine block and samples were collected at various times after release. Protein extracts were separated by SDS-PAGE and immunoblotted with antibodies directed against Dda1 and the indicated cell cycle markers (Cyclin E and PH3). Tubulin was used as a loading control. (B) The localization of Dda1 was analyzed by immunofluorescence in interphase cells pre-extracted (left panels) or not (right panels) with buffers that remove nucleoplasmatic proteins. DAPI staining was used to visualize the DNA. Merge images are shown in the right row. (C) Total lysates (T) were prepared from aphidicolin-arrested S-phase cells and separated into a nucleo-cytoplasmic (NC) and a chromatin-bound fraction (Chr), solubilized after digestion with micrococcal nuclease. Fractions were separated by SDS-PAGE and immunoblotted with antibodies directed against Dda1, Ddb1, Cul4A, tubulin and histone H3. (D,E) Dda1 and Cul4A localize to the nucleus but Dda1 is excluded from chromatin in mitosis. The localization of Dda1 (panel D) and Cul4A (panel E) was analyzed by immunofluorescence at different stages of mitosis. Antibodies against tubulin visualize the mitotic spindle, and the DNA was stained with DAPI (left row). Merge images are presented in the right row. Note that Cul4A remains associated with chromosomes throughout mitosis, whereas Dda1 staining is diffuse in metaphase and anaphase cells. (F) Cul4A immunoprecipitates from extracts prepared from cells arrested in S-phase or mitosis with aphidicolin or nocodazole, respectively, were analyzed by immunoblotting for the presence of Cul4A, Ddb1 and Dda1 as indicated.
Dda1 is a positive regulator of CRL4s
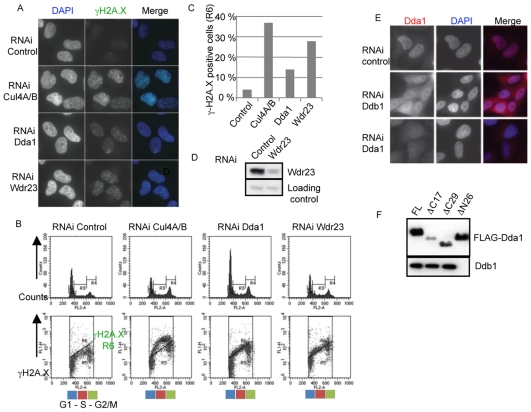
Cul4-type E3 ligases are involved in controlling DNA metabolism such as DNA replication and repair (O'Connell and Harper, 2007). Indeed, cells depleted of Cul4A and Cul4B spontaneously accumulate double-stranded DNA breaks that can be visualized by staining phosphorylated H2A.X foci using appropriate antibodies (Fig. 5A). Importantly, Dda1-depleted cells similarly accumulate spontaneous DNA breaks, though to a lesser extent compared with Cul4A- and Cul4B-depleted controls. Quantification by FACS analysis showed that almost 20% and 40% of Dda1 and Cul4A-B-depleted cells accumulated phosphorylated H2A.X foci, respectively (Fig. 5B,C). Likewise, we found that inactivation of the uncharacterized Dda1-interacting DCAF protein, Wdr23, specifically resulted in an accumulation of DNA breaks (Fig. 5C). Analysis of cell cycle repartition revealed that these cells were primarily in S-phase (data not shown), suggesting that the observed double-stranded breaks may occur during DNA replication leading in turn to the activation of the S-phase checkpoint. As double-stranded breaks have also been observed in cells depleted for Vprbp (Hrecka et al., 2007), these observations indicate that Dda1 may act as a positive regulator of multiple Cul4 ubiquitin ligases during DNA replication in vivo. The effect of Dda1 in suppressing the formation of double-stranded DNA breaks likely depends on Ddb1, as Dda1 partially accumulated in the cytoplasm in Ddb1-depleted cells (Fig. 5E). Likewise, a truncated version of Dda1(ΔN26), which failed to bind Ddb1 in co-immunoprecipitation assays (Fig. 5F), also localized to the cytoplasm (data not shown), indicating that nuclear accumulation of Dda1 requires its interaction with Ddb1. However, consistent with previous findings (Pick et al., 2007), we did not detect any effect resulting from Dda1 inactivation on UV-induced degradation of Cdt1 by the CRL4Cdt2 ligase (data not shown), indicating that Dda1 is not an essential regulator of all Cul4-based E3 ligases.
Fig. 5.
Dda1 functionally interacts with a subset of CRL4s. (A) Cul4A- and B-, Dda1- and Wdr23-depleted cells accumulate γH2A.X foci. Cells were immunostained with anti-phospho H2A.X antibodies and DNA was counterstained with DAPI. (B,C) Quantification of the number of γH2A.X-positive cells by FACS analysis in control cells or cells depleted of Cul4A and B, Dda1 and Wdr23. Cells were fixed with ethanol, stained with an γH2A.X antibody and analyzed by FACS. The number of γH2A.X-positive cells (R6 area) was plotted. (D) Immunoblotting of Wdr23 in extracts prepared from control and Wdr23 siRNA-depleted HeLa cells. (E) Ddb1 is required for nuclear accumulation of Dda1. Endogenous Dda1 was localized by indirect immunofluorescence (left column) in control cells (upper panel), or cells treated with Ddb1 (middle panel) or Dda1 siRNA (bottom panel). DNA was counterstained with DAPI (middle column), and merged images are presented in the right panels. (F) Dda1 uses its N-terminal domain to bind Ddb1. Full-length and truncated versions of FLAG-Dda1 that remove the first 26 amino acids (ΔN26), or the last 17 and 29 amino acids, respectively (ΔC17 and ΔC29), were transiently expressed in HEK 293T and immunoprecipitated with M2-agarose. The immunoprecipitates were separated by SDS-PAGE and blotted with specific Ddb1 (upper panel) and FLAG antibodies (lower panel).
Discussion
We report a comprehensive interaction network of the mammalian CSN, an evolutionarily conserved macromolecular complex that regulates CRLs by promoting deneddylation of cullin subunits.
Interestingly, we found fifteen CRLs that are stably associated and sequestered by the CSN but may dissociate in response to specific cues, in a similar way to the CRL4Ddb2 complex upon DNA damage (Groisman et al., 2003). In addition, we identified several poorly characterized proteins, including the evolutionarily conserved protein Dda1. We show that Dda1 is an unstable subunit of multiple CRL4 complexes, which associates with chromatin in a cell cycle-dependent manner. Our results suggest that Dda1 is a chromatin-associated CRL4 subunit that may specifically regulate a subset of Cul4-based E3 ligases involved in DNA replication and repair.
Dda1 is a chromatin-associated subunit of multiple CRL4 ligases involved in faithful DNA replication
We identified the conserved protein Dda1 as a specific subunit of many Cul4-type complexes. In particular, a dozen distinct CRL4 complexes including CRL4Cdt2, CRL4Wdr23 and CRL4Vprbp were specifically recovered in FLAG-Dda1 immunocomplexes together with all subunits of the COP9 signalosome, suggesting that Dda1 is a core subunit of multiple CRL4s. Dda1 uses its N-terminal part to bind Ddb1, the peculiar CRL4 adaptor, which contains three repeats of the seven-bladed β propeller called BPA, BPB and BPC, which are all engaged in protein interaction. Ddb1 binds Dda1 through its BPA domain, and it binds the amino-terminal domain of Cul4 and the DCAFs through its BPB and BPC domains, respectively (Pick et al., 2007; Jin et al., 2006). Although the precise molecular mechanisms by which Dda1 regulates CRL4 function remain unclear, several lines of evidence indicate that Dda1 may contribute to the spatiotemporal regulation of chromatin-bound CRL4. Indeed, Dda1 is recruited to chromatin via Ddb1, and is required to prevent the accumulation of DNA damage during S-phase. Several CRL4 complexes control chromatin-associated processes such as silencing of gene expression, DNA replication and repair (O'Connell and Harper, 2007). For example, Cul4 co-purifies with chromatin-bound complexes involved in RNAi-mediated mechanisms (Hong et al., 2005). Moreover, the CRL4Cdt2 complex is required to degrade the replication licensing factor Cdt1 on chromatin after S-phase entry or upon DNA damage, thereby restricting DNA replication to once per cell cycle or preventing DNA replication in conditions of damaged DNA (Jin et al., 2006). Ddb1 and Cul4A were also implicated in S-phase-dependent genomic instability and nucleotide excision repair (Higa and Zhang, 2007), and indeed, the CRL4Wdr23 ligase is also required to prevent the formation of double-stranded breaks during S-phase. These functions are reminiscent of the yeast cullin Rtt101p, which regulates progression of replication forks through nucleosome-dense chromosomal regions or sites of DNA damage (Luke et al., 2006). In the absence of Rtt101p function, replication forks often collapse, resulting in the formation of double-stranded breaks. Based on these data, we speculate that Dda1 may be necessary to activate a subset of Cul4 ligases during DNA replication, perhaps by stabilizing the association of Ddb1 with chromatin-associated DCAFs. Interestingly, in contrast to Cul4A, Dda1 is cell cycle regulated and specifically excluded from chromatin during mitosis. It is thus conceivable that this chromatin exclusion prevents the activity of Dda1-dependent Cul4 ligases during chromosome segregation.
The CSN stably binds a subset of CRLs predominantly involved in DNA-metabolism
Bioinformatic sequence analysis indicates that the human genome codes for over 600 potential substrate-specific adaptors of cullin-based E3 ligases that are characterized by specific motifs such as the F-box, BC box, BTB domain or WDXR motifs (Willems et al., 2004). However, despite this complexity, we systematically recovered only 15 substrate adaptors in CSN purifications, which belong to all four major cullin subfamilies and include Fbg4, Lrr1, Fem1, Lrrc14, Btbd1, Btbd2, Klhdc5, Klhl18, Ddb2, Vprbp, Csa, Wdr23, Wdr32, H326 and Cdt2. Likewise, a SILAC-based mass spectrometry approach similarly identified cullins 1, 2, 3, 4A and 4B, Rbx1, Ddb1 as well as the adaptors Vprbp, Klhdc5 and Ddb2 associated with Csn5 complexes (Fang et al., 2008). It is unlikely that this limited set can simply be explained by differences in expression levels, as several other substrate-specific adaptors are readily detected in cell lysates and/or in immunoprecipitation experiments with specific cullin antibodies. For example, Klhl9 and Klhl13 readily immunoprecipitate Cul3 but failed to associate with the CSN. Likewise, several DCAFs were present in Cul4A or Dda1-immunoprecipitates, but were not found in CSN immunopurifications. Interestingly, Klhl9 and Klhl13 precipitated a significant fraction of neddylated Cul3, whereas predominantly unneddylated Cul3 was bound to the Btbd1 and Btbd2 adaptors. Finally, we have performed reciprocal immunopurifications and confirmed that the CRL2 adaptors Lrr1 and Lrrc14, the CRL3 adaptors Btbd1 and Btbd2, and the CRL4 adaptors Ddb2, Wdr23, and H326 are indeed part of the CSN interaction network. For example, LC-MS-MS analysis of FLAG-Lrr1 immunoprecipitates identified CRL2 components and every CSN subunit (Csn1, 2, 3, 4, 5, 6, 7a, 7b, 8). Likewise, Cdt2 has been shown to co-precipitate each CSN subunits in Schizosaccharomyces pombe (Liu et al., 2005), and more recently, CSN subunits were readily identified in Vprbp immunoprecipitates from human cells (McCall et al., 2008). Taken together, these data suggest that the CSN stably binds a small but specific subset of substrate adaptors.
What could be the molecular function of this association with the CSN complex? Interestingly, Ddb2 stably interacts with the CSN but rapidly dissociates in response to DNA damage, in particular during global genome repair (Groisman et al., 2003). Released CRL4Ddb2 complexes bind damaged chromatin, and process DNA lesions in part through ubiquitinylation of the repair protein XPC (Xeroderma pigmentosum group C protein). Post-recovery repair involves re-association of the CSN with the CRL4Ddb2 complex and its inactivation through deneddylation. Thus, in this case, the CSN sequesters and thereby inactivates preassembled CRL4Ddb2 complexes, while allowing rapid and regulated release in case of need. The mechanism that controls the regulated association of the CRL4Ddb2 ligase with the CSN complex is still unclear but intriguingly a recent large-scale analysis identified several CSN subunits as substrates of the DNA damage-induced kinases ATM (ataxia telangiectasia mutated) and ATR (ataxia telangiectasia-Rad3-related), including Csn1, Csn3 and Csn7a (Matsuoka et al., 2007). It is thus conceivable that phosphorylation of CSN subunits may regulate their interaction with specific CRLs. In particular, ATM-ATR-dependent phosphorylation of CSN subunits may release the CRL4Ddb2 ubiquitin ligase in response to UV irradiation and promote its subsequent activation through cullin neddylation. In principle, all CRLs identified in our systematic proteomic analysis are candidates for this type of regulation. Strikingly, most of them are functionally linked to DNA metabolism and are likely to be regulated by the chromatin state and the ATM-ATR DNA damage pathway, in particular CRL3Btbd1/2, CRL4Cdt2, CRL4Ddb2, CRL4Vprbp and CRL4Wdr23. For example, CRL3Btbd1/2 has been implicated in the degradation of topoisomerase (Zhang et al., 2004), CRL4Vprbp has been shown recently to control DNA replication (McCall et al., 2008), and CRL4Cdt2 regulates DNA replication by triggering the rapid proteolysis of the replication-licensing factor Cdt1 (Jin et al., 2006).
During DNA replication and DNA damage, Cdt1 interacts with PCNA, which acts as a landing pad for the CRL4Cdt2 ligase. Interestingly, Cdt2 has been also identified as a target of ATM-ATR, which may thus generally coordinate CRL4 activation in response to specific chromatin states. The ATM-ATR kinases may also play a prominent role in the regulation of the CRL4Vprbp ligase. Vprbp (Vpr binding protein, also called DCAF1) was identified several years ago as a partner of the HIV protein Vpr, which blocks cell cycle progression by activating the ATM-ATR pathway and hijacking the CRL4Vprbp ligase to target unknown cellular factor(s) for degradation (Roshal et al., 2003). Vpr directly interacts with the CSN subunit Csn6 (Mahalingam et al., 1998) and appears to stimulate CRL4Vprbp activity by elevating Cul4 neddylation (Hrecka et al., 2007). Vpr may thus promote dissociation of CRL4Vprbp from the CSN through an ATM-ATR signaling cascade. Based on these observations we propose that the CSN reversibly sequesters a specific subset of CRLs, which may be rapidly released in response to appropriate cues. This mechanism ensures a rapid and coordinated response by simultaneously regulating various enzymes by a single mechanism. For example, UV irradiation may coordinate the release of several E3 ligases including CRL4Ddb2 and CRL4Cdt2 complexes on the one hand to prevent replication of damaged DNA and on the other hand to initiate DNA repair. Further experiments are now required to test this attractive model. In particular, it will be crucial to identify the specific signals and pathways that trigger the regulated release of the stably CSN-associated CRLs, thus determining their cellular function and targets.
Materials and Methods
DNA recombinant work
Standard procedures were used for Gateway cloning (Invitrogen) and DNA manipulations (Sambrook et al., 1989). To construct the pMT3989 and pMT4149 mammalian gateway vectors, the cassette EcoRV-(FLAG)3X-Attb1-Ccdb-Ccmr-Attb2-EcoRV was PCR amplified and cloned into pBlueScript (pBKS) vector. After verification by direct sequencing, the cassette was subcloned into the pMX-pie vector (gift from T. Pawson) (EcoRV site) or into the pCMV5 (SmaI site) to generate pMT3989 (Luke-Glaser et al., 2007) and pMT4149, respectively. Csn1, Csn5, Csn7a cDNA and the various mutant versions of Dda1 were PCR amplified and cloned into the entry vector pDONR201 (Invitrogen). Csn3, Csn4, Cdt2, Lrrc14 and Dda1 cDNA directly cloned into pDONR223 were obtained from Open Biosystems. Full-length Lrr1 entry clone was purchased from GeneCopoeia cat. No. T2299. The cDNAs of Ddb2, H326 and Wdr23 (ImaGenes, Berlin, Germany) were amplified and extended with the restriction site pairs BamHI-XhoI by PCR, cloned into pcDNA3.1(+)-HA(n-term) and verified by direct sequencing. The plasmids used in this study are listed in the supplementary Table S1.
HeLa and HEK 293T cell culture and stable cell line selection
HeLa and Human Embryonic Kidney (HEK) 293T cells were grown in Dulbecco's modified Eagle's high-glucose medium (DMEM, high glucose) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and antibiotic-antimycotic (Gibco) or with 10% FBS (PAA) and 1 unit/ml penicillin, 1 μg/ml streptomycin, 0.25 μg/ml amphotericin B (Gibco). Transient transfections were carried out for 24 hours in 10-cm dishes with 12 μg of DNA or in 6-cm dishes using 3 μg of DNA using FuGENE (Roche) (HA-Wd40IPs, FLAG-Dda1 overexpression in Cul4A-RNAi cells). For western blotting, equal amount of cells were harvested and directly resuspended in Laemmli buffer. The lysates were separated by SDS-PAGE, transferred onto a PVDF membrane and immunoblotted with the indicated antibodies. Stable HEK 293T cell lines were generated as described (Luke-Glaser et al., 2007).
Cell cycle and FACS analysis, and drug treatment
Cells were synchronized in G1 phase of the cell cycle using a double-thymidine block release as previously described (Sumara et al., 2007). Drug treatments were applied for 12 hours using the following concentrations: 2 μM nocodazole and 2 μg/ml aphidicolin prepared in 0.1% DMSO.
For flow cytometry analysis to assay the cell cycle position of γ-H2A.X-positive cells, cells were fixed in cold 70% ethanol, incubated with PBS containing 0.25% Triton X-100 for 15 minutes on ice, blocked for 1 hour with PBS containing 0.01% Triton X-100 and 5% fetal calf serum (FCS) and stained with mouse antibody to γ-H2A.X (1:500) for 1 hour at room temperature, followed by 30 minutes of incubation with conjugated anti-mouse IgG (Alexa Fluor 488 at 1:500). Cells were resuspended in FACS solution containing 38 mM Na3 citrate (pH 7.5), 50 μg/ml propidium iodide (PI) and 20 μg/ml ribonuclease (RNase), stained for 30 minutes at 37°C and analyzed with a FACSCalibur flow cytometer (BD Biosciences) using CellQuest software.
RNA interference knockdown
RNA interference experiments were performed either using Dharmacon's siGENOME SMARTpool siRNAs or single siRNA duplexes (Microsynth, Balgach, Switzerland) to deplete endogenous levels of Ddb1, Dda1, Cul4A, Cul4B and Cul3 in HeLa cells. The siRNAs used are listed in supplementary information (supplementary material Table S2).
HeLa cells were transfected with 50 nM siRNAs using Oligofectamine (Invitrogen) for 48 hours or 72 hours. For simultaneous knockdown and overexpression of genes, 0.4 μg DNA, 200 nM siRNAs and 2.0 μl Lipofectamine 2000 were used in 12-well dishes for 48 hours. Equal amounts of cells were harvested and directly resuspended in Laemmli buffer. HEK 293 cells were transfected with 50 nM final concentration of siRNA using DharmaFECT1. Whole-cell lysates were quantified using the BioRad DC protein assay (BioRad) and equal amounts of lysate were separated by SDS-PAGE.
Antibodies
Antibodies directed against the following polypeptides were used in this study: anti-FLAG (Sigma), anti-Cul2 [Rockland Immunochemicals Inc. (Chemicon)], anti-Cul3 (Sumara et al., 2007), anti-Cul4A (this study), anti-Vprbp (this study), anti-Csn1 (Bethyl), anti-Csn2 (Biomol), anti-Csn3 (Abcam), anti-Btbd1 and anti-Btbd2 (this study), anti-Ddb1 (Bethyl), anti-Dda1 (Hrecka et al., 2007), anti-Tubulin (Sigma), anti-HA (Covance), anti-Klhl9/Klhl13 (Sumara et al., 2007), anti-Wdr23 (this study), anti-Ddb2 (Santa Cruz), anti-Cand1 (Santa Cruz), anti-Cyclin E (Santa Cruz), anti-PH3 (Upstate), anti-γ-H2A.X (Upstate). Secondary antibodies coupled to peroxidase were purchased from Amersham or from Sigma. Gel filtration experiments were performed as described (Luke-Glaser et al., 2007).
Immunoprecipitations, protein extracts, immunoblotting, and chromatin purification
Standard procedures were used (Sambrook et al., 1989). For immunoprecipitation experiments, cellular extracts were prepared in extraction buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM CHAPS, 20 mM beta-glycerophosphate, 10% glycerol, 0.5 mM DTT, 1 tablet complete protease inhibitor cocktail 50 ml). Affinity-purified antibodies were coupled to Affiprep protein A beads (Biorad) in a ratio of 1 mg antibodies to 1 ml beads. The antibody beads were rotated end-over-end in HeLa cell extracts for 2 hours at 4°C. A ratio of 10 μl beads to 1–2 mg of protein in the extract was used. The beads were washed several times with extraction buffer and eluted using 100 mM glycine, pH 2.3. The eluates were neutralized using 1.5 M Tris pH 9.2, and 4× Laemmli buffer. For dephosphorylation assays, 250 μg of total protein were treated with 400 Units of active or heat-inactivated (1 hour at 65°C) lambda-phosphatase (NEB) for 1 hour at 30°C. The reaction was stopped by addition of 4× Laemmli buffer.
For chromatin purification, cell pellets were resuspended in lysis buffer (100 mM KCl, 1 mM MgCl2, 0.1 mM CaCl2, 10 mM Hepes, pH 7.7, 50 μM sucrose, 0.25% Triton X-100) and lysed using a syringe. Lysates were layered over lysis buffer containing 1 M sucrose and centrifuged at 8000 g for 30 minutes at 4°C. The supernatant was collected (cytosol-nucleoplasm) and the pellet was digested with micrococcal nuclease at 37°C for 1 hour in lysis buffer containing 5 mM CaCl2 and 350 mM KCl. After centrifugation for 10 minutes at 16,100 g, the supernatant was collected as solubilized chromatin.
Immunostaining
Cells were washed three times with warm PBS and fixed with paraformaldehyde (PFA) 4% for 10 minutes at room temperature. Where indicated, cells were pre-extracted by incubation with ice-cold pre-extraction buffer (300 mM sucrose, 3 mM MgCl2, 1 mM EDTA, 50 mM NaCl, 25 mM HEPES, pH 7.5, 0.5% Triton X-100) for 5 minutes and washed with PBS before fixation. In some cases cells were spun down onto a glass slide using a cytospin centrifuge as previously described (Sumara et al., 2007). PFA was washed away with PBS, cells were permeabilized in PBS-NP40 0.5% for 5 minutes at room temperature, washed three times with PBS-0.01% Triton X-100 (PBST) and incubated for 1 hour at room temperature in the blocking solution (5% filtered FBS in PBST) additionally containing 0.1 μg/ml DAPI. The antibodies were diluted to 1 μg/ml in blocking solution and added for 1 hour. After three washing steps with PBST, cells were incubated for 30 minutes with the secondary antibody and then washed again three times with PBST. Finally, cells were mounted in mounting medium Immu-Mount (Thermo) and visualized on the epifluorescence microscopes, Zeiss Life Cell Station or Zeiss Axioplan 2. The secondary antibodies coupled to the fluorophores Alexa 488 and Alexa 543 were purchased from Molecular Probes.
LC-MS-MS analysis of FLAG-CSN complexes
Immunopurification
(HEK) 293T stable cell lines were grown in five 15-cm dishes containing 30 ml of selection medium (see above). For proteasome inhibition, the cells were incubated for 3 hours with the proteasome inhibitor MG132 (10 μM) or its vehicle DMSO. Cells were placed on ice, washed twice with ice-cold Dulbecco's phosphate buffered saline, pH 7.4 (Gibco) and scraped into 2 ml of lysis buffer (CLB3: 0.1% NP-40, 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 5 mM NaF, 10% glycerol supplemented with 1 mM DTT, 1 μg/μl leupeptin-pepstatin A, 10 μg/μl aprotinin, 100 μg/μl PMSF and 0.2 mM NaV03) and nutated for 30 minutes at 4°C. Detergent-insoluble material was removed by centrifugation, twice for 20 minutes each at ∼30,000 g at 4°C. Protein concentration of the clarified extract was determined using the BioRad DC protein assay. Extracts were then incubated with 60 μl of anti-FLAG-M2 agarose beads (Sigma) for 5 hours at 4°C with end-over-end rotation. Beads were washed three times with CLB3 wash buffer (0.01% NP-40, 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 5 mM NaF, 10% glycerol supplemented with 1 mM DTT, 1 μg/μl leupeptin-pepstatin A, 10 μg/μl aprotinin, 100 μg/μl PMSF and 0.2 mM NaVO3) and once with wash buffer 2 (0.01% NP-40, 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 5 mM NaF, supplemented with 1 mM DTT, 1 μg/μl leupeptin-pepstatin A, 10 μg/μl aprotinin, 100 μg/μl PMSF and 0.2 mM NaVO3) and once with Tris-buffered saline (TBS: 50 mM Tris-Cl, pH 7.5, 100 mM NaCl) containing protease inhibitors. Bound proteins were eluted twice by incubating beads for 15 minutes at 4°C in 50 μl of 50 mM phosphoric acid, pH 2.8. Eluates were then combined and centrifuged once more for 5 minutes at 800 g at 4°C to remove excess IgG in eluates.
On-column protein digestion and LC-MS-MS analysis
Sample preparation
Samples were digested on-column as described previously overnight at room temperature (Luke-Glaser et al., 2007). Each sample was then dried under vacuum and reconstituted in 10 μl of 0.1% (v/v) formic acid 2.5 % (v/v) acetonitrile in preparation for nano-LC-MS-MS.
Gel-free samples were analyzed on a 2-hour gradient. The micro-LC solvent gradient program was as follows: 0% buffer B (0-12 minutes), 0-5% buffer B (12-16 minutes), 5-35% (16-70 minutes) buffer B, 35-65% buffer B (70-85 minutes), 65-100% buffer B (85-95 minutes), followed by 100% buffer B for 18 minutes and back to 0% buffer B for 7 minutes. For all measurements, 8 μl of sample was injected using an Agilent μ-WPS auto sampler at 3 μl/minute. The flow rate across the column was reduced to approximately 100-200 nl/minute using a vented column arrangement (Le Bihan et al., 2003; Licklider et al., 2002).
Prior to each 2-hour micro-LC-MS-MS analysis, a column-pre-column wash and conditioning step consisting of one gradient of 0-100% buffer B over 20 minutes followed by an isocratic conditioning step at 0% buffer B over 40 minutes was performed.
The ESI voltage was set at 1.7 kV and the interface temperature set at 175°C for all measurements. The instrument transmission was optimized using a standard peptide, angiotensinogen (1-14) DRVYIHPFHLVIHN (American Peptide) by monitoring the 4+, 3+ and 2+ charge state at, respectively, 440.7, 587.3 and 880.5 amu. The MS acquisition settings were as follows: a single centroid MS reference scan (400-2000 m/z) was first performed, followed by five data-dependent MS-MS scans of the five most intense ions also in centroid mode. The MS-MS precursor ion data selected were excluded for 120 seconds and the exclusion list was set at 250 for a mass window of –0.5 to 1.6 amu. The maximum fill time, number of microscans and target value were 50 ms, 1 and 4 × 104 ions for the IT reference scan.
Materials
Acetonitrile and water used for LC-MS-MS analysis or sample preparation were of HPLC quality (Fisher, Fairlawn, NJ). Formic acid was Suprapure 98-100%, (Merck, Darmstadt, Germany) and trifluoroacetic acid 99% purity sequencing grade (Aldrich, Milwaukee, WI). All other chemicals used in the preparation of sample were of reagent grade or better (Sigma, St Louis, MO), unless specified. Sequencing grade modified porcine trypsin was purchased from Promega (Madison, WI). All protein and peptide standards were of >95% purity from Sigma and American Peptides (SunnyVale, CA).
HPLC and mass spectrometry
Micro-HPLC-MS-MS analyses were performed using an on-line system consisting of a micro-pump Agilent 1100 binary HPLC system (Palo Alto, CA) coupled to a LTQ instrument (ThermoQuest Corp, San Jose, CA). The LTQ was controlled through Xcalibur 2.0 SR2 and LTQ MS2.2. Capillary Picotip columns (10 cm × 360 μm OD × 75 μm ID) with a 15 μm tip opening and fitted with a borosilicate frit were purchased from New Objective (Cambridge, MA) and fused silica tubing was purchased from Innovaquartz (Phoenix, AZ). Column and pre-columns were packed as previously described (Le Bihan et al., 2003).
All connector fittings were purchased from Upchurch Scientific (Oak Harbor, WA) or Valco (Houston, TX). The reverse-phase bulk material used in this study was 5-μm Pursuit C18 obtained from Varian (Palo Alto, CA). Buffer A was 97.5% H2O, 2.5% acetonitrile, 0.1% formic acid, and buffer B was 90% acetonitrile, 10% H2O, 0.025% trifluoroacetic acid, 0.1% formic acid. For the analysis of FLAG-Lrr1 immunoprecipitates micro-HPLC-MS-MS analyses were performed using an on-line system consisting of a micro-pump Agilent 1200 binary HPLC system (Palo Alto, CA) coupled to a hybrid LTQ-Orbitrap XL instrument (ThermoQuest Corp, San Jose, CA). The mass spectrometer was controlled through Xcalibur 2.0.7.
Data processing
Mascot Generic Format (MGF) input files were generated with the EXTRACT_MSN tool (Bioworks 3.3, ThermoQuest Corp, San Jose, CA), and merged with the precursor grouping option disabled and spectra containing less than ten fragment data points were discarded. MS-MS data were searched using MASCOT Version 2.1 (Matrix Science Ltd, UK) against a human subset of IPI Uni-Prot database (EBI) (May 2005). All basic Mascot searches were performed using a maximum missed-cut value of 1, variable methionine oxidation modification (+16 Da), and protein N-term acetylation modification (+42 Da), fixed cysteine carbamidomethylation modification (+57 Da) a precursor mass tolerance of 2 amu and a MS-MS tolerance of 0.8 amu.
Identified peptides and proteins were re-evaluated using Scaffold files (Proteome software, Portland, OR), which combine the Peptide Prophet (Keller et al., 2002) and Protein Prophet algorithms (Nesvizhskii et al., 2003). A threshold of 90% and greater for peptide probability was set and for protein identifications a probability greater than 95% with at least two peptides was set as an acceptance criterion.
LC-MS-MS analysis of endogenous Cul3 and Cul4 protein complexes
Immunopurification
For immunoprecipitation experiments, antibody-coupled beads (see above) were incubated with total HeLa cell extracts (Cil Biotech, Belgium) for 2 hours at 4°C. The immunoprecipitated hCul3 and hCul4A complexes were washed several times with extractions buffer containing 500 mM and 150 mM NaCl.
Limited electrophoresis
After removal of the supernatant, beads were eluted by boiling in 50 μl electrophoresis buffer. The supernatant was loaded on an 8% polyacrylamide gel and proteins were separated on a distance of 18 mm. Prestained molecular weight markers were run in parallel to ensure that proteins up to 250 kDa had entered the separating gel at time of stop. After a quick fixing and Coomassie-staining steps (15 minutes), every gel lane was cut into slices corresponding to molecular weight regions.
Digestion, HPLC and mass spectrometry
Bands or gel regions were excised from gels as cubes of 2.0-mm length. Proteins were manually in-gel digested with trypsin according to a described protocol (Shevchenko et al., 1996). Tryptic peptides were recovered in the supernatant of the digestion, concentrated by evaporation to 15 μl and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS-MS) on a SCIEX QSTAR Pulsar i (Concord, Ontario, Canada) hybrid quadrupole-time of flight instrument equipped with a nanoelectrospray source and interfaced to an LC-Packings Ultimate (Amsterdam, Holland) HPLC system. Separation was performed on a PepMap (LC-Packings, Amsterdam) reversed-phase capillary C18 (75 μm ID × 15 cm) column at a flow rate of 200 nl/minute along a 52-minute gradient of acetonitrile (0-40%). The Analyst QS 1.1 instrument controlling software was used to perform peak detection and automatically selecting sequentially eluting peptides for collision-induced fragmentation (CID). The two most intense ions in the mass range 400-1200 with charge state 2+ to 4+ were selected for analysis after a 1-second survey scan. CID spectra were accumulated for 3 seconds for every precursor. Analyzed ions were excluded for 180 seconds from further analysis (the tolerance window for exclusion was 0.075 amu).
CID data generation
Collections of tandem mass spectra for database searching were generated from the Analyst files with the script Mascot.dll version 1.6b4 (Matrix Science, London). The tool was set to try to determine precursor charge state from the survey scan, after centroiding peaks at 50% height and merging data points within a distance of 0.1 amu. Charge state information thus determined was used whenever available, while all the default charge states 2+, 3+ and 4+ were written when charge state could not be auto determined. CID spectra from the same precursor were added and averaged to yield one spectrum if they fell within a mass window of 1.0 amu and a time window of 10 measurement cycles (maximum of 2.5 minutes). Spectra containing fewer than ten peaks before treatment were discarded. Accepted CID spectra were processed as follows: peaks below 0.5% of the base peak were removed; remaining peaks were not smoothed but were centroided at 50% height with a merge distance of 2.0 amu. Collections of spectra were written as flat text files in Mascot Generic Format (mgf) and pooled for every gel lane to perform one database search per sample.
Database searching
Tandem mass spectra were extracted by Analyst 1.1 using the Mascot.dll version. Charge state deconvolution and deisotoping were performed. All MS-MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.1.0). Mascot was set up to search the UNIPROT database (selected for Homo sapiens, release 7.0 of 7 February 2006, with 71855 entries after taxonomy filter) assuming the digestion enzyme trypsin with a maximum of one missed cleavage. Both the parent and fragment ion mass tolerances used were 0.30 Da. The iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification. Deamidation of asparagine and glutamine, oxidation of methionine and N-acetylation of the protein N-terminus were specified in Mascot as variable modifications.
Criteria for protein identification
Scaffold (version Scaffold-01_05_06, Proteome Software, Portland, OR) was used to validate MS-MS-based peptide and protein identifications. The thresholds defined previously for the validation of CSN interacting partners were used.
Supplementary Material
We thank J. Skowronski (Cold Sping Harbor Laboratory, NY) for providing the anti-Dda1 antibody, D. Dewar (Samuel Lunenfeld Research Institute, Toronto, Canada) and C. Rupp (ETH Zurich, Switzerland) for excellent technical assistance, the LMC-RISC staff for their help with light microscopy, and members of the L.P., M.T. and M.P. laboratories for fruitful discussions. M.H.O. is a member of the Molecular Life Science (MLS) Zurich graduate program, and was supported by a fellowship from the Bonizzi-Theler Stiftung. T.L.B. was funded by CSBE (Centre for Integrative and Systems Biology funded by BBSRC and EPSRC). L.P. was funded by an ATIP grant from the Centre National de la Recherche Scientifique, and grants from the FRM, ARC and the city of Paris. Work in the laboratories of M.P. and M.T. was supported by grants from the Swiss National Science Foundation (SNF), Oncosuisse and the ETHZ, and by grants from the Canadian Institutes of Health Research and the National Cancer Institute of Canada, respectively. M.T. was also supported by a Canada Research Chair, a Howard Hughes Medical Institute International Research Scholar Award, a Royal Society Wolfson Research Merit Award and the Scottish Universities Life Sciences Alliance. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/7/1035/DC1
References
- Angers, S., Li, T., Yi, X., MacCoss, M. J., Moon, R. T. and Zheng, N. (2006). Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443, 590-593. [DOI] [PubMed] [Google Scholar]
- Bornstein, G., Ganoth, D. and Hershko, A. (2006). Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc. Natl. Acad. Sci. USA 103, 11515-11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, E. H. and Hagen, T. (2007). Substrate-mediated regulation of cullin neddylation. J. Biol. Chem. 282, 17032-17040. [DOI] [PubMed] [Google Scholar]
- Duda, D. M., Borg, L. A., Scott, D. C., Hunt, H. W., Hammel, M. and Schulman, B. A. (2008). Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134, 995-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, L., Wang, X., Yamoah, K., Chen, P. L., Pan, Z. Q. and Huang, L. (2008). Characterization of the human COP9 signalosome complex using affinity purification and mass spectrometry. J. Proteome Res. 7, 4914-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman, R., Polanowska, J., Kuraoka, I., Sawada, J., Saijo, M., Drapkin, R., Kisselev, A. F., Tanaka, K. and Nakatani, Y. (2003). The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113, 357-367. [DOI] [PubMed] [Google Scholar]
- Higa, L. A. and Zhang, H. (2007). Stealing the spotlight: CUL4-DDB1 ubiquitin ligase docks WD40-repeat proteins to destroy. Cell Div. 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, E. J., Villen, J., Gerace, E. L., Gygi, S. P. and Moazed, D. (2005). A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol. 2, 106-111. [DOI] [PubMed] [Google Scholar]
- Hrecka, K., Gierszewska, M., Srivastava, S., Kozaczkiewicz, L., Swanson, S. K., Florens, L., Washburn, M. P. and Skowronski, J. (2007). Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc. Natl. Acad. Sci. USA 104, 11778-11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Hetfeld, B. K., Seifert, U., Kahne, T., Kloetzel, P. M., Naumann, M., Bech-Otschir, D. and Dubiel, W. (2005). Consequences of COP9 signalosome and 26S proteasome interaction. FEBS J. 272, 3909-3917. [DOI] [PubMed] [Google Scholar]
- Jin, J., Arias, E. E., Chen, J., Harper, J. W. and Walter, J. C. (2006). A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell 23, 709-721. [DOI] [PubMed] [Google Scholar]
- Keller, A., Nesvizhskii, A. I., Kolker, E. and Aebersold, R. (2002). Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383-5392. [DOI] [PubMed] [Google Scholar]
- Kipreos, E. T., Lander, L. E., Wing, J. P., He, W. W. and Hedgecock, E. M. (1996). cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell 85, 829-839. [DOI] [PubMed] [Google Scholar]
- Le Bihan, T., Duewel, H. S. and Figeys, D. (2003). On-line strong cation exchange micro-HPLC-ESI-MS/MS for protein identification and process optimization. J. Am. Soc. Mass Spectrom. 14, 719-727. [DOI] [PubMed] [Google Scholar]
- Licklider, L. J., Thoreen, C. C., Peng, J. and Gygi, S. P. (2002). Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column. Anal. Chem. 74, 3076-3083. [DOI] [PubMed] [Google Scholar]
- Liu, C., Poitelea, M., Watson, A., Yoshida, S. H., Shimoda, C., Holmberg, C., Nielsen, O. and Carr, A. M. (2005). Transactivation of Schizosaccharomyces pombe cdt2+ stimulates a Pcu4-Ddb1-CSN ubiquitin ligase. EMBO J. 24, 3940-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke-Glaser, S., Roy, M., Larsen, B., Le Bihan, T., Metalnikov, P., Tyers, M., Peter, M. and Pintard, L. (2007). CIF-1, a shared subunit of the COP9/signalosome and eukaryotic initiation factor 3 complexes, regulates MEL-26 levels in the Caenorhabditis elegans embryo. Mol. Cell. Biol. 27, 4526-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke, B., Versini, G., Jaquenoud, M., Zaidi, I. W., Kurz, T., Pintard, L., Pasero, P. and Peter, M. (2006). The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol. 16, 786-792. [DOI] [PubMed] [Google Scholar]
- Lyapina, S., Cope, G., Shevchenko, A., Serino, G., Tsuge, T., Zhou, C., Wolf, D. A., Wei, N. and Deshaies, R. J. (2001). Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science 292, 1382-1385. [DOI] [PubMed] [Google Scholar]
- Mahalingam, S., Ayyavoo, V., Patel, M., Kieber-Emmons, T., Kao, G. D., Muschel, R. J. and Weiner, D. B. (1998). HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc. Natl. Acad. Sci. USA 95, 3419-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrour, N., Redwine, W. B., Florens, L., Swanson, S. K., Martin-Brown, S., Bradford, W. D., Staehling-Hampton, K., Washburn, M. P., Conaway, R. C. and Conaway, J. W. (2008). Characterization of Cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases. J. Biol. Chem. 283, 8005-8013. [DOI] [PubMed] [Google Scholar]
- Matsuoka, S., Ballif, B. A., Smogorzewska, A., McDonald, E. R., 3rd, Hurov, K. E., Luo, J., Bakalarski, C. E., Zhao, Z., Solimini, N., Lerenthal, Y. et al. (2007). ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160-1166. [DOI] [PubMed] [Google Scholar]
- McCall, C. M., Miliani de Marval, P. L., Chastain, P. D. n., Jackson, S. C., He, Y. J., Kotake, Y., Cook, J. G. and Xiong, Y. (2008). HIV-1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol. Cell. Biol. 28, 5621-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii, A. I., Keller, A., Kolker, E. and Aebersold, R. (2003). A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646-4658. [DOI] [PubMed] [Google Scholar]
- O'Connell, B. C. and Harper, J. W. (2007). Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr. Opin. Cell Biol. 19, 206-214. [DOI] [PubMed] [Google Scholar]
- Pan, Z. Q., Kentsis, A., Dias, D. C., Yamoah, K. and Wu, K. (2004). Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23, 1985-1997. [DOI] [PubMed] [Google Scholar]
- Petroski, M. D. and Deshaies, R. J. (2005). Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell. Biol. 6, 9-20. [DOI] [PubMed] [Google Scholar]
- Pick, E., Lau, O. S., Tsuge, T., Menon, S., Tong, Y., Dohmae, N., Plafker, S. M., Deng, X. W. and Wei, N. (2007). Mammalian DET1 regulates Cul4A activity and forms stable complexes with E2 ubiquitin-conjugating enzymes. Mol. Cell. Biol. 27, 4708-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintard, L., Willems, A. and Peter, M. (2004). Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family. EMBO J. 23, 1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshal, M., Kim, B., Zhu, Y., Nghiem, P. and Planelles, V. (2003). Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J. Biol. Chem. 278, 25879-25886. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fristch, E. F. and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schwechheimer, C., Serino, G., Callis, J., Crosby, W. L., Lyapina, S., Deshaies, R. J., Gray, W. M., Estelle, M. and Deng, X. W. (2001). Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science 292, 1379-1382. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O. and Mann, M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- Sumara, I., Quadroni, M., Frei, C., Olma, M. H., Sumara, G., Ricci, R. and Peter, M. (2007). A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev. Cell 12, 887-900. [DOI] [PubMed] [Google Scholar]
- Tomoda, K., Kubota, Y., Arata, Y., Mori, S., Maeda, M., Tanaka, T., Yoshida, M., Yoneda-Kato, N. and Kato, J. Y. (2002). The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J. Biol. Chem. 277, 2302-2310. [DOI] [PubMed] [Google Scholar]
- Wee, S., Geyer, R. K., Toda, T. and Wolf, D. A. (2005). CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nat. Cell Biol. 7, 387-391. [DOI] [PubMed] [Google Scholar]
- Wei, N. and Deng, X. W. (2003). The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 19, 261-286. [DOI] [PubMed] [Google Scholar]
- Willems, A. R., Schwab, M. and Tyers, M. (2004). A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 1695, 133-170. [DOI] [PubMed] [Google Scholar]
- Wu, J. T., Lin, H. C., Hu, Y. C. and Chien, C. T. (2005). Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat. Cell Biol. 7, 1014-1020. [DOI] [PubMed] [Google Scholar]
- Zhang, H. F., Tomida, A., Koshimizu, R., Ogiso, Y., Lei, S. and Tsuruo, T. (2004). Cullin 3 promotes proteasomal degradation of the topoisomerase I-DNA covalent complex. Cancer Res. 64, 1114-1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.