Abstract
An assay for detecting human immunoglobulin M (IgM) antibodies to Japanese encephalitis (JE) virus was developed by using the antibody capture solid-phase radioimmunoassay approach (JE IgM ACRIAAA). Heavy-chain-specific goat antihuman IgM was first bound to the wells of a polyvinyl microtiter plate, and successive steps involved sequential binding of test sample IgM, acetone-extracted mouse brain JE antigen, and (125)I-labeled flavivirus hyperimmune human IgG. Among 20 patients hospitalized in Bangkok with clinical diagnoses of acute encephalitis, and with acute flavivirus infections proven by hemagglutination inhibition (HAI) serology, 16 had detectable (positive/negative [P/N] ratio, greater than 3.0) JE IgM ACRIA antibodies in the acute-phase serum specimen, and 19 had such antibodies in the convalescent-phase serum specimen. Convalescent patient sera regularly had higher P/N values than the corresponding acute-phase sera (mean +/- 1 standard deviation = 13.0 +/- 9.3 with acute-phase sera and 25.8 +/- 19.6 with convalescent-phase sera). JE virus-infected patients with HAI serological responses indicative of a primary flavivirus infection had higher JE IgM ACRI P/N responses than did those patients whose serological response indicated past exposure to other flaviviruses. None of 70 serum specimens from healthy Thai adults and children with serum JE HAI antibodies had detectable JE IgM ACRIA activity (P/N ratios all less than or equal to 3.0). Biological false-positives with low P/N ratios (range, 3 to 15) were found in sera from patients with acute or recent infections with flaviviruses other than JE virus but could be differentiated by the fact that these sera gave higher P/N ratios with homologous antigens than with JE virus. False-positive reactions with low P/N ratios (range, 3 to 6) due to serum rheumatoid factor activity were differentiated by testing with control antigen. The JE IgM ACRIA technique permits a rapid, accurate diagnosis of acute JE virus infections in both patients with and those without previous exposure to other flaviviruses.
Full text
PDF


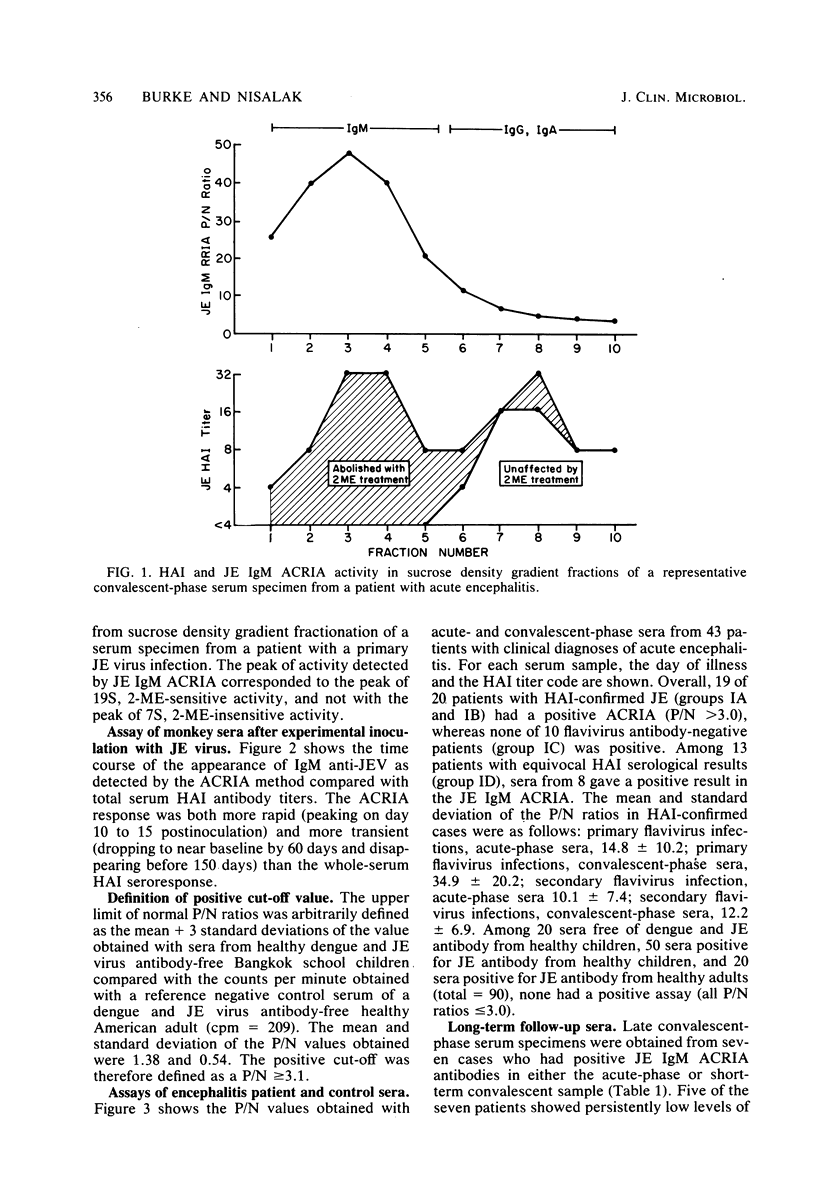
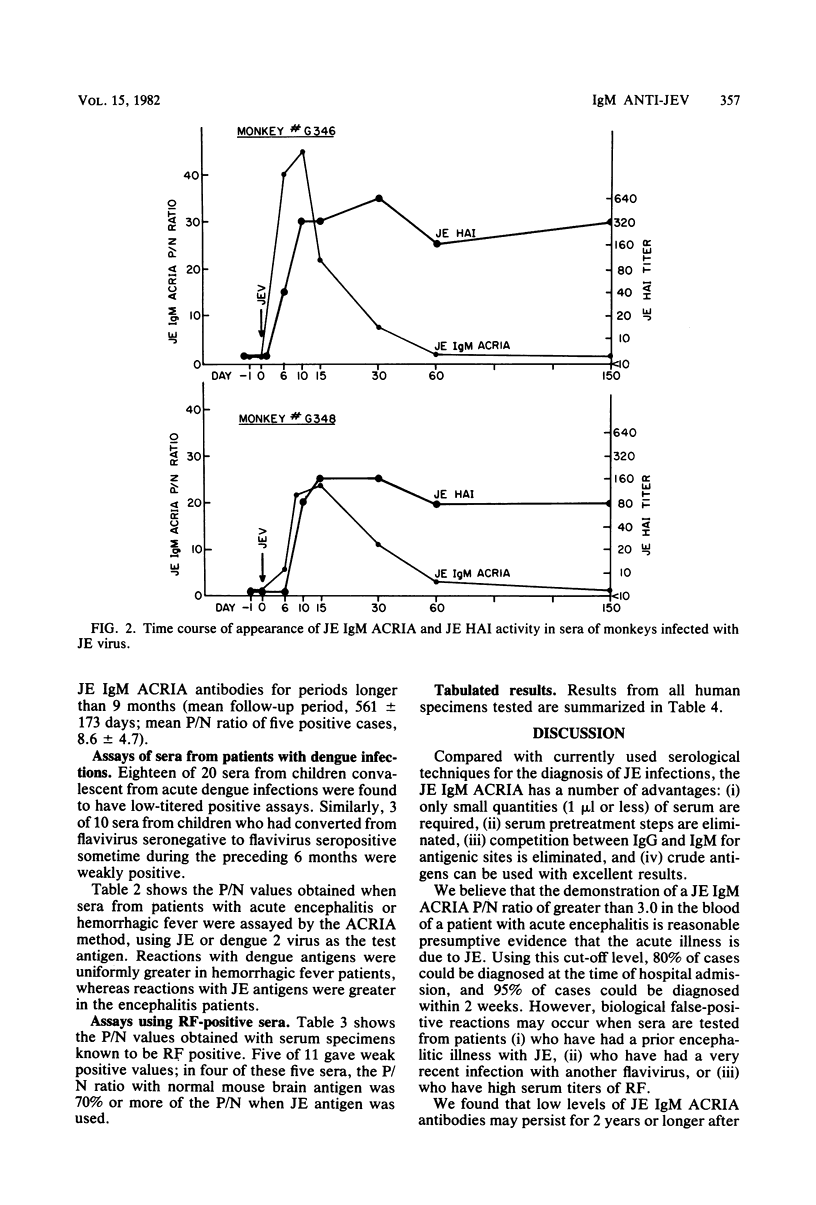
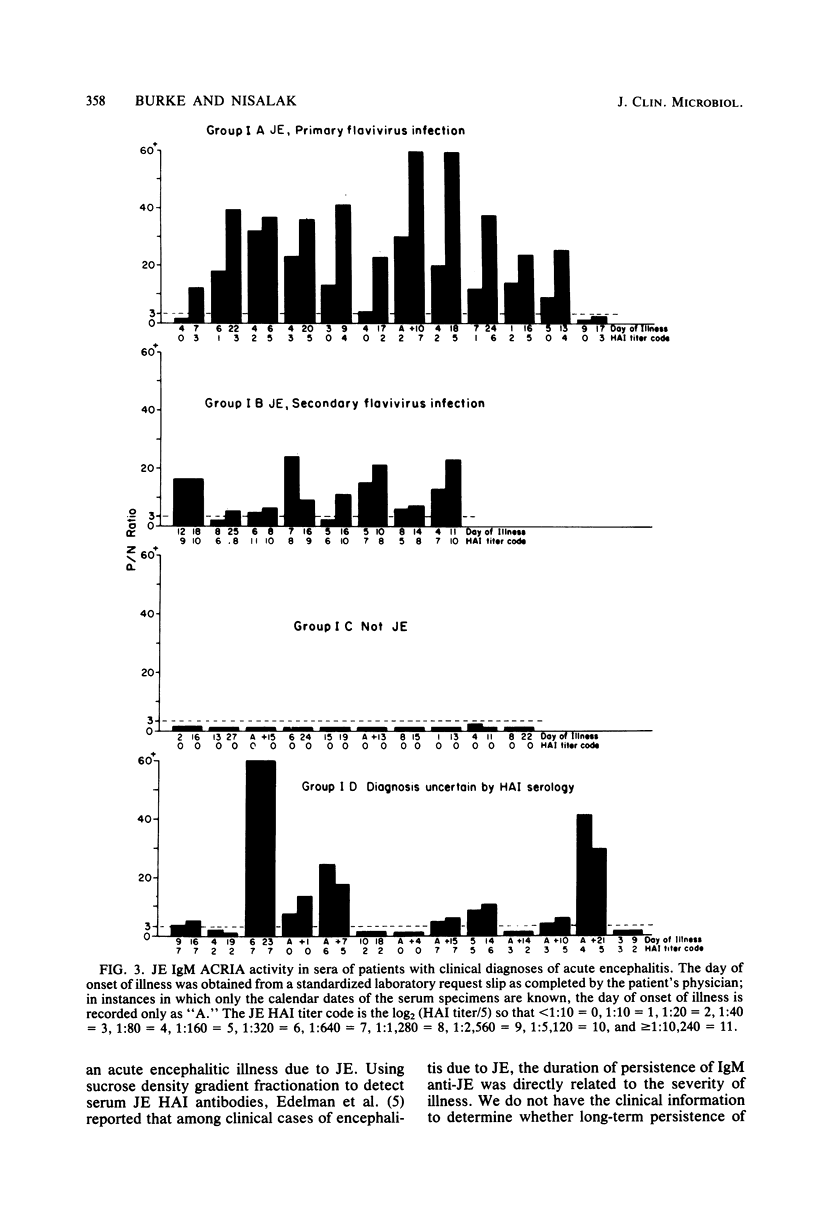

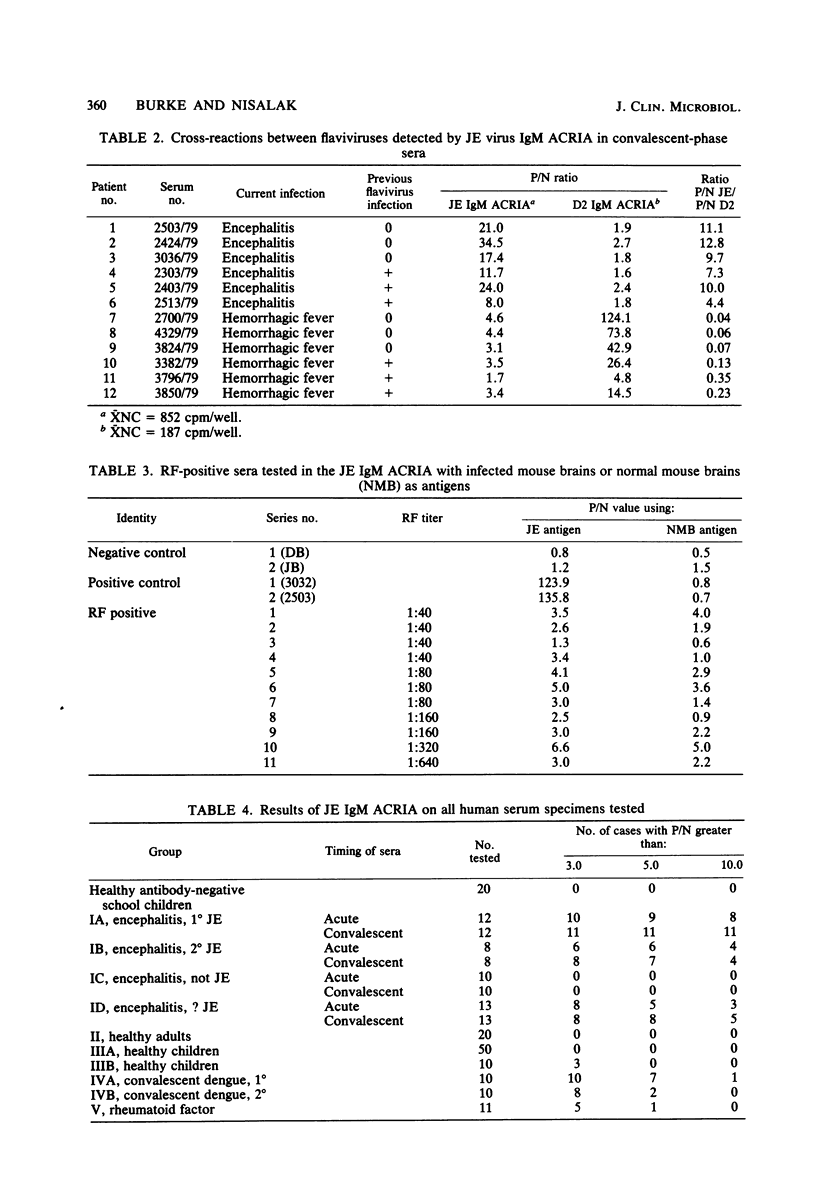

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Duermeyer W., Wielaard F., van der Veen J. A new principle for the detection of specific IgM antibodies applied in an ELISA for hepatitis A. J Med Virol. 1979;4(1):25–32. doi: 10.1002/jmv.1890040104. [DOI] [PubMed] [Google Scholar]
- Edelman R., Nisalak A., Pariyanonda A., Udomsakdi S., Johnsen D. O. Immunoglobulin response and viremia in dengue-vaccinated gibbons repeatedly challenged with Japanese encephalitis virus. Am J Epidemiol. 1973 Mar;97(3):208–218. doi: 10.1093/oxfordjournals.aje.a121501. [DOI] [PubMed] [Google Scholar]
- Edelman R., Pariyanonda A. Human immunoglobulin M antibody in the sero-diagnosis of Japanese encephalitis virus infections. Am J Epidemiol. 1973 Jul;98(1):29–38. doi: 10.1093/oxfordjournals.aje.a121529. [DOI] [PubMed] [Google Scholar]
- Edelman R., Schneider R. J., Vejjajiva A., Pornpibul R., Voodhikul P. Persistence of virus-specific IgM and clinical recovery after Japanese encephalitis. Am J Trop Med Hyg. 1976 Sep;25(5):733–738. doi: 10.4269/ajtmh.1976.25.733. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ishii K., Matsunaga Y., Kono R. Immunoglobulins produced in response to Japanese encephalitis virus infections of man. J Immunol. 1968 Oct;101(4):770–775. [PubMed] [Google Scholar]
- Kuberski T. T., Rosen L. A simple technique for the detection of dengue antigen in mosquitoes by immunofluorescence. Am J Trop Med Hyg. 1977 May;26(3):533–537. doi: 10.4269/ajtmh.1977.26.533. [DOI] [PubMed] [Google Scholar]
- Maiolini R., Masseyeff R. A sandwich method of enzymoimmunoassay. I. Application to rat and human alpha-fetoprotein. J Immunol Methods. 1975 Sep;8(3):223–234. doi: 10.1016/0022-1759(75)90115-5. [DOI] [PubMed] [Google Scholar]
- THEILER M., CASALS J. The serological reactions in yellow fever. Am J Trop Med Hyg. 1958 Nov;7(6):585–594. doi: 10.4269/ajtmh.1958.7.585. [DOI] [PubMed] [Google Scholar]


