Abstract
Objective
To correlate the pathologic findings of temporal artery biopsies in patients clinically defined as positive, presumed, or negative for giant cell arteritis (GCA).
Design
Retrospective case series.
Participants and Controls
Patients evaluated for giant cell arteritis.
Methods
Temporal artery biopsies examined between 1989 and 2007 were studied. Clinical information and residual tissue for immunohistochemical staining was identified in 107 patients. Clinical information was used in order to make a diagnosis of “positive”, “presumed”, or “negative” GCA. The biopsies were reviewed in a masked fashion and classified as “positive”, “indeterminate”, or “negative” based on published, classic pathologic diagnosis (CPD) criteria. All biopsies were immunostained for CD3 and CD68 and graded as “negative”, “mildly” (+), “moderately” (++), or “markedly” (+++) positive. Clinical and pathologic results were correlated and a modified pathologic diagnosis classification (MPD) scheme was developed. The modified scheme was compared in a masked fashion with the final clinical diagnosis and positive and negative predictive values (PVs) were calculated.
Main Outcome Measures
Pathologic diagnosis and final clinical diagnosis.
Results
Using the MPD classification, there were 25%, 16% and 61% positive, indeterminate, and negative biopsies, respectively. There was excellent correlation between the modified pathologic criteria and final clinical diagnosis (correlation coefficient 0.997, p value <0.0001, kappa 0.81). The positive predictive values (PVs) for CPD and MPD were 85% and 96%, respectively. The negative PVs for CPD and MPD were 64% and 61%, respectively. Positive and negative biopsies strongly correlated with clinical diagnoses of positive and negative for GCA, respectively whereas indeterminate cases moderately correlated with presumed GCA. The diagnosis did not change from the original biopsy in 11 patients who had a second biopsy. Immunostaining for CD 68 was helpful in several indeterminate cases.
Conclusions
We recommend using the modified histologic classification of temporal artery biopsies. There are indeterminate cases that cannot be further defined using current pathologic classification criteria. A second biopsy has very limited value. Immunostaining for CD68 stain may be helpful in indeterminate cases, although the diagnosis in these cases is based on clinical judgement.
Giant cell arteritis (GCA) is a primary inflammatory vasculopathy that affects large and medium-sized arteries as a result of an abnormal adaptive immune response.1–3 Advanced age is the single most important risk factor for the development of GCA. Consequently, GCA has gained increasing importance as life expectancy lengthens. Clinical manifestations of GCA include a broad spectrum of signs and symptoms such as headache, visual changes including blindness, jaw claudication, polymyalgia rheumatica, and elevated erythrocyte sedimentation rate and C-reactive protein.4 The current treatment for GCA is systemic corticosteroid therapy, which controls inflammation and prevents ischemic complications.
Temporal artery biopsy (TAB) is the standard procedure for diagnosing GCA. Typical lesions of GCA consist of T lymphocytes, macrophages, epithelioid histiocytes, and multinucleated giant cells that infiltrate all layers of the arterial wall (intima, media, and adventitia).5–7 Additional findings in a positive biopsy include intimal hyperplasia and fragmentation of the inner elastic lamina. Pathologic changes may be interpreted as indeterminate or consistent with old, healed GCA. These indeterminate changes may be difficult to distinguish from other disease entities such as scarring in arteriosclerotic lesions. Interpretation may be particularly difficult in instances of GCA in the setting of arteriosclerosis, which often occurs in elderly patients.
In this study, we correlated the pathologic diagnoses of positive, indeterminate, or negative TABs with the final clinical diagnoses of positive, presumed, or negative GCA. We developed a modified histopathologic diagnosis scheme for temporal artery biopsies and also evaluated the utility of immunohistochemical staining of the biopsies for evaluation of GCA.
Methods
Patients and Specimens
After Emory University Institutional Review Board (IRB) approval was granted, the pathology reports of a total of 407 archived TABs examined by a single ophthalmic pathologist (HEG) in the L.F. Montgomery Ophthalmic Pathology Laboratory, Emory University, over a 19-year period between 1989 and 2007, were retrieved. Of those cases, we identified a total of 107 patients who had detailed pre- and post-biopsy clinical information and tissue in the residual paraffin block.
Clinical Classification
The clinical diagnosis was based in part on the American Association of Rheumatology criteria for the diagnosis of GCA5, and the results of detailed ophthalmic evaluations. Patients were classified into three categories. The GCA group included patients with definite clinical GCA as having more than two of the following features: 1) typical clinical history, 2) elevated ESR and/or C-Reactive protein, 3) ocular findings highly suggestive of GCA such as anterior or posterior ischemic optic neuropathy, retinal and/or choroidal ischemia, and 4) involvement of extraocular arteries. Since all patients who underwent a TAB had clinical symptoms or signs suggesting GCA, all patients with a positive TAB were classified as definite clinical GCA, as is typical in clinical practice. A neuroophthalmologist (VB) evaluated the clinical information on all the remaining patients that had been collated by another investigator (KL) and classified the cases as presumed GCA or no GCA. The presumed GCA group included patients for whom we could not definitely rule-out GCA, and who had more than two of the following factors: 1) minimally suspicious clinical history, 2) elevated ESR and/or CRP, 3) previously treated as GCA or response to empirical corticosteroid, and 4) withdrawal corticosteroid treatment was impossible. The no GCA group included patients for whom there was another explanation for their clinical history or laboratory findings.
Histopathologic Classification
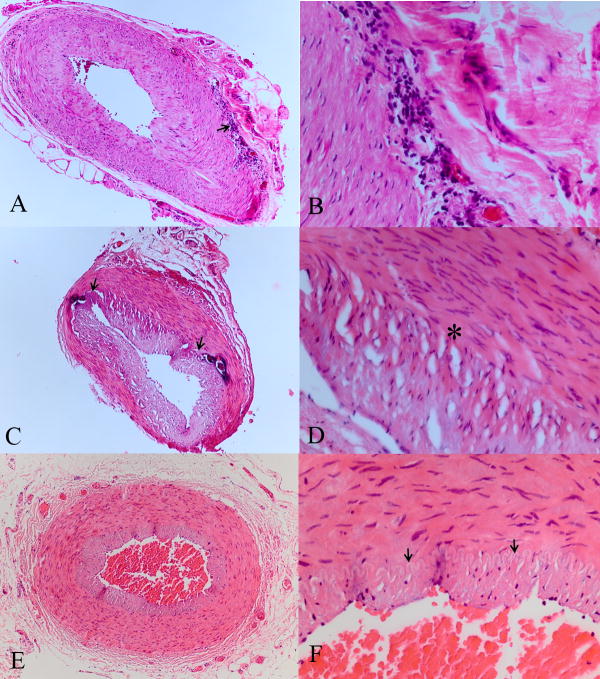
The biopsies had been submitted in 10% formalin and were grossly examined for length and color. They were all embedded on end, routinely processed, serially step sectioned at 25μm intervals, 5μm thick sections, 20 sections per slide, with >90% of the paraffin block sectioned. The sections were all stained with hematoxylin and eosin and periodic acid Schiff (PAS). A pathologist masked to the patients’ clinical status reviewed all the biopsies for the presence of GCA and classified them as positive, indeterminate, or negative, using previously published (classic) criteria.6–8 The classic pathological diagnosis (CPD) criteria defines positive as intimal hyperplasia with associated chronic inflammation of the full thickness wall of the artery (including histocytes and lymphocytes), primarily centered around the inner elastic lamina; indeterminate as intimal hyperplasia and a 360 degree concentric scar around the inner elastic lamina and/or full thickness scar in the wall of the artery; and negative as without the aforementioned features (Figure 1). The biopsies in all cases were classified by an ophthalmic pathologist (HEG) using the CPD criteria and masked to the clinical information.
Figure 1.
Classic Pathologic Diagnosis (CPD) criteria. A&B. CPD positive includes intimal hyperplasia and chronic inflammation in all layers of the artery wall, including in many cases giant cells (arrow). C&D. CPD indeterminate includes intimal hyperplasia and a concentric scar centered for 3600 around the inner elastic lamina (arrows, between brackets) and/or a full thickness scar in the wall of the artery. There is also fibroplasia of the intima present (*) E&F., CPD negative shows a normal intima, media and adventia. The lumen is patent, there is no intimal hyperplasia, and the inner elastic lamina is intact (arrows). Note the intimal hyperplasia and narrowing of the lumen in A–D compared with E and F. (hematoxylin and eosin, A, C, E 25X; B, D, F 100X)
Clinicopathologic Correlation
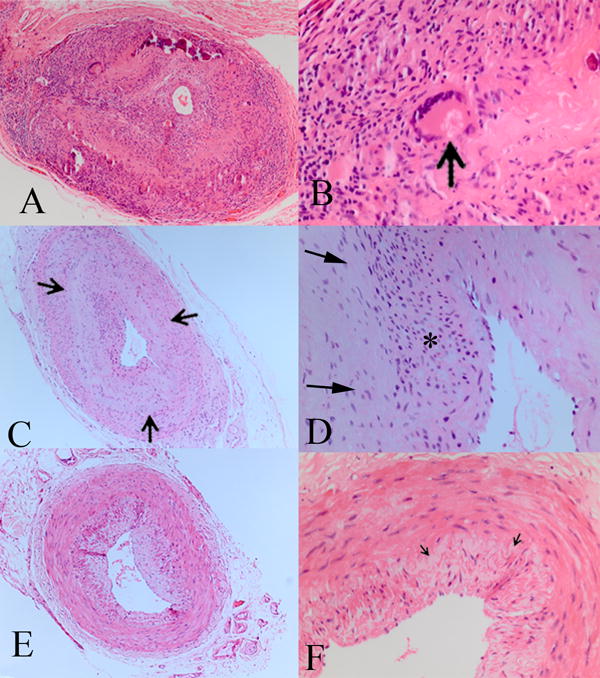
Cases in which the pathologic and clinical diagnoses were positive/positive, indeterminate/presumed and negative/negative were considered to be concordant. Discrepancies between the pathologic and clinical diagnoses were identified. A modified pathologic diagnosis (MPD) classification was developed after review of the pathology of the discrepant cases. At least one of the findings for the MPD classification of positive for GCA were present in all cases with a flinal clinical diagnosis of GCA and were less stringent than the CPD classification. Although intimal hyperplasia and focal breaks in the inner elastic layer may be present in arteriosclerosis, concentric scarring with delicate fibroplasia around the inner elastic layer is not a histologic feature described in arterioslcerosis or aging. Additionally, inflammation in the wall of the artery is not typical in arteriosclerosis. The MPD findings included for positive: intimal hyperplasia and chronic inflammation in at least one layer of the artery wall and/or vasa vasora; indeterminate: intimal hyperplasia and a concentric scar anywhere around the inner elastic lamina; negative: an absence of the above features (Figure 2). All cases were reclassified using the MPD classification and again compared with the final clinical diagnosis in a double-blind masked fashion.
Figure 2.
Modified Pathologic Diagnosis (MPD) criteria. A&B. MPD positive includes the same features as for CPD. Additionally, focal chronic inflammation in any layer of the artery wall (arrow), including surrounding vasa vasora, are included. C&D. MPD indeterminate includes the same features as for CPD. Additionally, a focal concentric scar around the inner elastic lamina for any number of clock hours (between arrows) with loss of the inner elastic lamina (*) are included. E&F. MPD negative is the same as for CPD negative. The inner elastic lamina (arrows) is intact. (hematoxylin and eosin, A, C, E 25X; B, D, F 100X)
Immunohistochemistry
The residual, formalin fixed, paraffin embedded tissue was sectioned (5μ) and mounted on non-adhesive positively charged glass slides (Kimble/Kontes, Vineland, NJ). The sections were dried, de-waxed and re-hydrated two times with 100% xylene, two times with 100% ethanol, one time with 95% ethanol, and one time with tap water for 10 minutes each. Antigenic epitopes were recovered by steaming the slides in target retrieval solution (DakoCytomation, Inc. Carpinteria, CA) for 30 minutes, cooled down to room temperature, and the retrieval solution was slowly replaced with tap water.
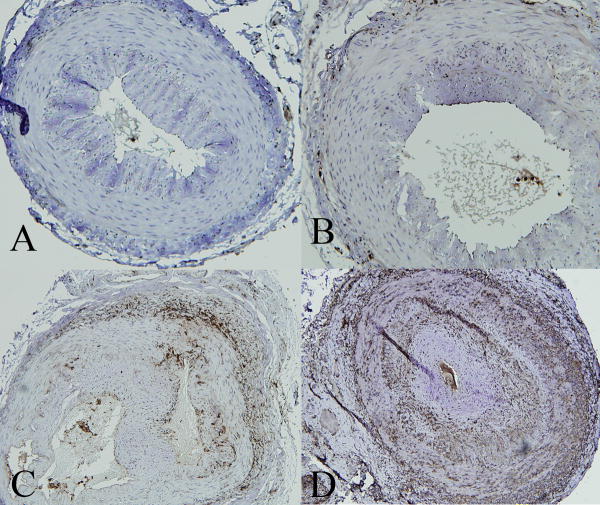
Most staining procedures were at room temperature unless specifically indicated. The slides were incubated with 5% normal goat serum for 15 minutes in a humidity chamber to block non-specific binding. Primary antibody (monoclonal mouse anti-human CD3 or CD68; DakoCytomation, Inc. Carpinteria, CA), was applied at a dilution of 1:50 in 1% normal goat serum and incubated overnight incubation at 4°C, followed by washing 5 times with 0.01M phosphate buffered saline (PBS), pH 7.2, for one minute each time. Then, secondary antibody (polyclonal goat anti-mouse biotinylated immunoglobulin; DakoCytomation, Inc., at a dilution of 1:300 in 1% normal goat serum) was applied and incubated for 30 minutes followed by washing 3 times with PBS. The tissue sections were then covered with ABC complexes (Vector Laboratories, Burlingame, CA) for 30 minutes followed by washing with PBS 3 times and addition of DAB (Vector Laboratories, Burlingame, CA) for 4 minutes followed by one time of washing with tap water. The slides were counterstained with hematoxylin for 1 minute, dried with 95% ethanol (1X), two times with 100% ethanol, and two times with 100% xylene for one minute each. They were then mounted in oil medium and coverslipped. The staining reaction was graded as 0 (no staining), + (up to 1/3 of the circumference of at least one layer containing cells that stain), ++ (up to 2/3 of the circumference of at least one layer containing cells that stain), or +++ (the complete circumference of at least one layer containing cells that stain). The grading scheme is illustrated in Figure 3.
Figure 3.
The grading system defined in this study for immunohistochemistry staining in the temporal artery sections. A.CD68−; B. CD68+; C. CD68++; D. CD68+++ (peroxidase anti-peroxidase, 10X)
Statistical Analysis
Four tested modalities were compared with the final clinical diagnosis in this study: classic pathologic diagnosis (CPD), modified pathological diagnosis (MPD), immunostaining for CD3 and immunostaining for CD68. A Chi square test was used to determine statistical significance between the final clinical diagnosis and the CPD or MPD classification. For correlation, dichotomous two by two tables were constructed in which the clinical diagnoses of GCA and presumed GCA were considered to be positive, a pathologic diagnosis of GCA or indeterminate was considered to be positive, and any immunostaining was considered to be positive. The remaining interpretations were considered negative. The degree of concordance between the tested modality and final clinical diagnosis was studied using the Kappa coefficient. Excellent concordance was defined as Kappa coefficient between 0.81 and 1, good concordance was defined as Kappa coefficient between 0.61 and 0.8, and moderate concordance was defined as Kappa coefficient between 0.41–0.60. Concordance was considered low if the Kappa coefficient was 0.40 or less. Positive and negative predictive values (PVs) were determined by dividing positive or negative CPD or MPD cases by positive or negative final clinical diagnosis cases, respectively.
Results
The patients were clinically classified into three categories: GCA (26 patients); no GCA (69 patients); and presumed GCA (12 patients) (Table 1). All temporal artery biopsies were at least 1 centimeter in length. Eleven patients had bilateral biopsies. One of the eleven was re-biopsied because the first biopsy didn’t contain an artery. The histopathologic classification of the second biopsy was exactly the same using both the CPD and MPD classification for the remaining ten patients; therefore, the histopatholgic classification remained unchanged for all patients with bilateral temporal artery biopsies. Of 52 patients where a history of systemic corticosteroid use prior to the biopsy was obtained, 30, 15 and 7 patients had corticosteroid use for less than one week, between one and two weeks, and between two weeks and one year prior to the biopsy, respectively. We found that although the use of corticosteroids tended to decrease the inflammatory cell infiltrate in the biopsy, it appeared to have no effect on concentric scars surrounding the inner elastic lamina.
Table 1.
Clinical and demographic data of 107 patients
| Final Diagnosis(number) | Age in years(range/mean) | Sex(F/M) | Biopsy(B/A/S/N) | Outcome(I/S/D/N*) |
|---|---|---|---|---|
| GCA (26) | 65–90/77 | 18/8 | 3/15/2/6 | 5/4/2/15 |
| Presumed GCA (12) | 50–82/71 | 8/4 | 1/9/0/2 | 1/0/0/11 |
| No GCA (69) | 38–89/71 | 49/20 | 2/28/2/37 | 4/5/1/59 |
Note: F=female; M=male, B=biopsy before corticosteroid use; A=biopsy after corticosteroid use; S=biopsy was conducted at the same day of corticosteroid treatment; N=not documented when the biopsy was performed versus corticosteroid treatment;. I=improvement; S=stable; D=deterioration; N* =no documentation of outcome; GCA=giant cell arteritis
Of the 26 patients with clinically positive GCA, 22, 3 and 1 had positive, indeterminate, and negative biopsies, respectively, using CPD criteria, and 25, 1 and 0 had positive, indeterminate and negative biopsies, respectively, using MPD criteria (Table 2). Of 12 patients with clinically presumed GCA, 0, 7 and 5 had positive, indeterminate, and negative biopsies, respectively, using CPD criteria and 0, 9 and 3 had positive, indeterminate, and negative biopsies, respectively, using MPD criteria. Of the 69 patients with clinically no GCA, 0, 6 and 63 had positive, indeterminate, and negative biopsies, respectively, using CPD criteria and 0, 7 and 62 had positive, indeterminate, and negative biopsies using MPD criteria. The correlation coefficient between the final clinical diagnosis and CPD was 0.996, p<0.001, kappa statistic 0.76, and between the final clinical diagnosis and MPD was 0.997, p<0.001, kappa statistic 0.81. The positive PVs for CPD and MPD were 85% and 96%, respectively. The negative PVs for PCD and MPD were 64% and 61%, respectively. Using the MPD, there was 96%, 90% and 75% concordance between positive/GCA, negative/no GCA, and indeterminate/presumed GCA, respectively.
Table 2.
Clinical and pathologic diagnosis of 107 patients with temporal artery biopsies
| Pathologic Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|
| GCA | Indeterminate | Negative | Total | ||||
| Clinical Diagnosis | CPD | MPD | CPD | MPD | CPD | MPD | |
| GCA | 22 | 25 | 3 | 1 | 1 | 0 | 26 |
| Presumed -GCA | 0 | 0 | 7 | 9 | 5 | 3 | 12 |
| NO GCA | 0 | 0 | 6 | 7 | 63 | 62 | 69 |
| Total | 22 | 25 | 16 | 17 | 69 | 65 | 107 |
GCA=giant cell arteritis; CPD=Classic Pathologic Diagnosis Criteria; MPD=Modified Pathologic Diagnosis Criteria
Results of CD3 and CD68 staining and grading are shown in Table 3. The correlation coefficient between the clinical diagnosis and any CD3 staining was 0.994 and between the clinical diagnosis and any CD68 staining was 0.856. Of the two patients who had indeterminate TABs reclassified after the clinical classification of positive (Figure 2), both biopsies were CD3−, one was CD68+ and the other was CD68++, indicating that CD 68 staining may be useful in indeterminate cases.
Table 3.
Evaluation of the clinical diagnosis and immunohistochemical staining
| Clinical Dx | CD3 | CD68 | Total |
|---|---|---|---|
| GCA | 26 | ||
| 0 | 3 | 0 | |
| + | 4 | 4 | |
| ++ | 1 | 5 | |
| +++ | 18 | 17 | |
| Presumed-GCA | 12 | ||
| 0 | 5 | 2 | |
| + | 7 | 6 | |
| ++ | 0 | 4 | |
| +++ | 0 | 0 | |
| NO GCA | 69 | ||
| 0 | 60 | 41 | |
| + | 9 | 22 | |
| ++ | 0 | 6 | |
| +++ | 0 | 0 | |
| Total | 107 |
GCA=giant cell arteritis; CD3=cluster of differentiation protein 3, a T lymphocyte marker; CD68=cluster of differentiation 68, a macrophage marker
Discussion
Of the 107 patients in this study undergoing temporal artery biopsy to exclude GCA, the final diagnosis of GCA, presumed GCA and no GCA was 26 (24%), 12 (11%) and 69 (65%), respectively. We evaluated and refined the prevailing diagnostic modalities for GCA and designated them as modified pathological diagnostic criteria. The classic pathological diagnosis criteria for GCA are the presence of intimal hyperplasia, fragmentation of inner elastic lamina, and focal or transmural diffuse inflammatory cell infiltrates including lymphocytes, epithelioid histiocytes, and sometimes multinucleated giant cells.6–8 We refined the classic pathological diagnostic criteria to include the following: focal areas of intimal hyperplasia, focal areas of fragmentation of inner elastic lamina, focal chronic inflammatory cell infiltrates involving any layer in the temporal artery biopsy specimen (especially around the inner elastic membrane, or involving accompanying vessels, such as vasa vasorum), or focal concentric scars around the inner elastic lamina. We observed excellent correlation between the modified pathologic criteria and final clinical diagnosis (kappa 0.81), which was slightly better than the classic pathologic diagnosis criteria (kappa 0.76).
Although prior treatment with systemic corticosteroids appeared to decrease the inflammatory infiltrate in the wall of the artery, the concentric scarring around the inner elastic lamina appeared not to be affected. This finding is similar to what has been reported by Font and Prabhakaran.7 The difference between our study and that study is that the final clinical diagnosis in the previous study was the same as the biopsy diagnosis7 whereas ours was a correlative study comparing pathologic findings and the final clinical diagnosis made by two expert neuroophthalmologists (VB, NN) and a rheumatologist who is a leading authority on giant cell arteritis (CW). We feel that our study more accurately represents clinical practice. Patients with definitely positive biopsies are considered to have GCA and with definitely negative biopsies are considered not to have GCA. However, there are a group of patients who are adequately biopsied with indeterminate results where the final diagnosis is based on clinical judgement. Our retrospective, double masked study shows that there is excellent correlation between the clinical and pathologic diagnosis in the positive and negative cases whereas there is moderate correlation for indeterminate cases. These findings support the concept that one cannot make a diagnosis based on the biopsy alone in these indeterminate cases as has been implied.7 The use of other modalities, such as duplex ultrasonography for the diagnosis of GCA, are controversial and we did not evaluate them in our study.
Most patients with the clinical diagnosis of GCA had strong immunostaining of their biopsy for CD3 and D68. Most patients with the clinical diagnosis of no GCA had no immunostaining of their biopsy for CD3 and CD68. Any staining for CD3 correlated with clinically diagnosed (correlation coefficient, 0.994), although this correlation was no better than examination using either the CPD or MPD without immunostaining. A few patients with clinically diagnosed GCA had no immunostaining of their biopsy for CD68 and a few patients with clinically diagnosed no GCA had weak staining of their biopsy for CD3 and CD68. CD 68 staining was + and ++ in the two presumed GCA cases classified as indeterminate using CPD criteria that were re-classified as GCA using MPD criteria, whereas both of those cases had CD3− biopsies. This indicates that although in general, immunostaining does not improve diagnostic accuracy, immunostaining for CD68 may be of value when choosing between indeterminate and positive in difficult temporal artery biopsy specimens.
The immunostains suggested that the presence of macrophages is more sensitive to identify subtle inflammation than accumulation of T cells. Macrophage populations in the vascular lesions are functionally distinct and further characterization of infiltrating macrophages may provide useful information.9 Many of the biopsies were collected after the patients had been started on corticosteroids. While corticosteroid therapy may not eradicate entirely the vasculitis infiltrates10,11, it is possible that steroids are more effective in suppressing the recruitment and survival of T cells than that of macrophages, causing selective suppression of intrawall T cells. In model systems inducing vasculitis in non-inflamed human arteries dendritic cells are required to initiate the process whereas macrophages appear to not provide T cell stimulatory functions.12,13
Compared with the final clinical diagnosis, the positive predictive value improved using MPD (96%) versus CPD (85%) criteria, although the negative predictive value was slightly worse (61% versus 64%). We believe that the diagnostic schemes are comparable. The MPD provided excellent correlation with the final clinical diagnosis (kappa=0.81) and is slightly less restrictive than the CPD. It appears that while fewer positive biopsies are missed by using the MPD, there are more negatives that are upgraded into the indeterminate category. Although there is good to excellent correlation between the pathologic diagnosis and final clinical diagnosis, there are cases that exist in which it may be impossible to determine if the patient does or does not have GCA. Many of these cases fall into the indeterminate pathology category. This is a weakness of the study, as we considered the indeterminate biopsies and presumed clinical GCA diagnoses to be concordant for evaluation of statistical significance. We believe that this is an acceptable assumption, as in both instances, the pathologist and clinician cannot determine with certainty if the patient does or does not have GCA. For correlation and kappa statistic determination, we considered presumed GCA and indeterminate biopsies to be positive, as in most instances, these patients are treated for GCA. The clinical diagnosis of presumed GCA is a diagnosis of exclusion, and it is likely that some patients in this clinical category do not have GCA. At this time, it is impossible to further separate patients in this category. Treatment decisions should be based on the totality of the clinical situation.
Using the MPD classification, there were 23%, 15% and 61% positive, indeterminate, and negative biopsies, respectively. This information may be used as a benchmark for the practicing ophthalmologist when considering his or her own positive, negative and indeterminate temporal artery biopsy rates. Using the MPD, there was excellent correlation between positive biopsy/GCA and negative biopsy/no GCA, with 96% and 90% concordance, respectively, whereas there was moderate (75%) concordance between indeterminate/presumed GCA. Based on the results of our study, we advise obtaining serial step cross-sections of temporal artery biopsies through the entire length of the specimen, evaluating the biopsy using MPD criteria, and using CD68 immunostaining for equivocal (indeterminate) cases. If the first biopsy is examined properly using the criteria in our study, there is virtually no diagnostic yield in doing a second biopsy. The ophthalmologist, neurologist, rheumatologist and pathologist should confer, especially regarding indeterminate cases.
Acknowledgments
Supported in part by NIH P30 EY 06360, NIH R01 EY11916, and an unrestricted grant from Research to Prevent Blindness (RBP), Inc. Dr. Grossniklaus is an RBP Senior Scientific Investigator. Dr Newman is a Lew Wasserman Merit Award Recipient.
Footnotes
None of the authors have any financial interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–9. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–15. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Ma-Krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmun Rev. 2004;3:46–53. doi: 10.1016/S1568-9972(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 4.Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92–101. doi: 10.1001/jama.287.1.92. [DOI] [PubMed] [Google Scholar]
- 5.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. Cytokines in giant-cell arteritis. Clev Clin J Med. 2002;69(suppl):SII91–4. doi: 10.3949/ccjm.69.suppl_2.sii91. [DOI] [PubMed] [Google Scholar]
- 7.Font RL, Prabhakaran VC. Histological parameters helpful in recognising steroid-treated temporal arteritis: an analysis of 35 cases. Br J Ophthalmol. 2007;91:204–9. doi: 10.1136/bjo.2006.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonnell PJ, Moore GW, Miller NR, et al. Temporal arteritis: a clinicopathologic study. Ophthalmology. 1986;93:518–30. doi: 10.1016/s0161-6420(86)33706-0. [DOI] [PubMed] [Google Scholar]
- 9.Weyand CM, Wagner AD, Björnsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98:1642–9. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brack A, Rittner HL, Younge BR, et al. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest. 1997;99:2842–50. doi: 10.1172/JCI119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achkar AA, Hunder GG, Gabriel SE. Effect of previous corticosteroid treatment on temporal artery biopsy results [letter] Ann Intern Med. 1998;128:410. doi: 10.7326/0003-4819-128-5-199803010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Ma-Krupa W, Jeon MS, Spoerl S, et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–83. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han JW, Shimada K, Ma-Krupa W, et al. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–53. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]