Abstract
Background
In acromegaly, expert surgery is curative in only about 60% of patients. Postoperative radiation therapy is associated with a high incidence of hypopituitarism and its effect on growth hormone (GH) production is slow, so that adjuvant medical treatment becomes of importance in the management of many patients.
Objective
To delineate the role of lanreotide in the treatment of acromegaly.
Methods
Search of Medline, Embase, and Web of Science databases for clinical studies of lanreotide in acromegaly.
Results
Treatment with lanreotide slow release and lanreotide Autogel® normalized GH and insulin-like growth factor-I (IGF-I) concentrations in about 50% of patients. The efficacy of 120 mg lanreotide Autogel® on GH and IGF-I levels was comparable with that of 20 mg octreotide LAR. There were no differences in improvement of cardiac function, decrease in pancreatic β-cell function, or occurrence of side effects, including cholelithiasis, between octreotide LAR and lanreotide Autogel®. When postoperative treatment with somatostatin analogs does not result in normalization of serum IGF-I and GH levels after noncurative surgery, pegvisomant alone or in combination with somatostatin analogs can control these levels in a substantial number of patients.
Keywords: acromegaly, lanreotide, somatostatin analog, growth hormone, pegvisomant
Introduction
Growth hormone (GH), a polypeptide consisting of 191 amino acids and which is secreted by the pituitary gland, has a multitude of effects. The most obvious effect is the stimulation of growth in prepubertal and pubertal children. In childhood, lack of this hormone leads to dwarfism and excessive secretion results in gigantism. Growth hormone has profound metabolic effects by stimulating protein anabolism and lipolysis. Other effects include stimulation of bone turnover, leading to a net increase in bone volume, muscle growth, insulin antagonism, renal sodium retention, and immuno modulation. Most of the effects of GH are indirectly mediated via insulin-like growth factor-I (IGF-I). IGF-I is a peptide synthesized and secreted as a result of GH-signaling, which acts locally in an autocrine or paracrine manner, or systematically as a hormone when secreted by the liver (Le Roith et al 2001). The liver secretes about 70% of the total circulating IGF-I in mice (Sjogren et al 1999).
Excessive secretion of GH leads to acromegaly, a disfiguring and debilitating condition causing severe co-morbidity and premature death (Wright et al 1970; Ezzat et al 1994; Melmed 2006; Ben-Shlomo and Melmed 2008).
The purpose of this review is to establish the role of lanreotide, particularly lanreotide Autogel®, in the management of acromegaly based on published data. It is appropriate, however, to outline first the clinical features of acromegaly and to discuss therapeutic approaches in its management.
Acromegaly
Acromegaly is a rare disease, caused by a GH-secreting adenoma and in even more seldom instances (about 1%) due to excessive GHRH secretion, usually by a carcinoid tumor of the lung or gastrointestinal tract (Biermasz et al 2007). The incidence of acromegaly is about 3–4 per 1 million per year and the prevalence is 60–70 per 1 million, without geographical or sex differences (Alexander et al 1980 Bengtsson et al 1988; Ritchie et al 1990; Mestron et al 2004). Clinical features of acromegaly include acral enlargement, prognatism, jaw malocclusion, arthropathy, carpal tunnel syndrome, hyperhydrosis, sleep apnea, and visceromegaly (Colao et al 2004; Melmed 2006). Acromegaly is also associated with increased cardiovascular morbidity and mortality. Active disease leads to a specific form of cardiomyopathy which involves myocardium, conduction system, and heart valves. Clinical manifestations include arrhythmias, valvular regurgitation, concentric left ventricular hypertrophy, and left ventricular systolic and diastolic dysfunction (Clayton 2003; Colao et al 2004; Pereira et al 2004). The incidence of hypertension and of decreased glucose tolerance is also increased. This is also true for the incidence of colon polyps and colon carcinoma (Orme et al 1998; Renehan and Shalet 2002). It is controversial, however, whether the relative risk of cancer is increased in patients with acromegaly compared with that of the general population (Jenkins and Besser 2001; Melmed 2001; Loeper and Ezzat 2008).
Local tumoral symptoms include chronic headache, visual field defects, and rarely cranial nerve palsies. Hypopituitarism is mostly associated with large tumors with a generally low incidence in patients with acromegaly varying from 3% to 10 % (Greenman et al 1995).
The increased standardized mortality rate (SMR) decreased from 3-fold in older series to 1.3-fold in series with predominantly primary transsphenoidal surgery (Swaeringen et al 1998; Holdaway et al 2004; Kauppinen et al 2005; Holdaway 2007; Dekkers et al 2008). Reported risk factors include diabetes mellitus, cardiomyopathy, sleep apnea, and cerebrovascular events and in some, but not all, studies also pituitary irradiation (Ayuk et al 2004; Biermasz et al 2004a; Kauppinen et al 2005). The decrease in mortality observed in acromegaly is likely to be due to the introduction of effective therapies such as transsphenoidal surgery in the 1970s and to postoperative radiotherapy, leading to normalization of GH and IGF-I concentrations in a substantial number of patients. The effective treatment of systemic co-morbidities also plays a role in the observed decrease in mortality. Only few patients using adjuvant somatostatin analogs are included in mortality series and it is of note that at present no mortality data exist for primary medical treatment including pegvisomant treatment. Most studies have suggested that a lower GH, for example below 2.5 μg/L, is associated with improved and even normal survival. In some but not all studies normal IGF-I was also associated with improved mortality (Ayuk et al 2004; Biermasz et al 2004a; Holdaway et al 2004; Kauppinen et al 2005). Discrepancies between studies may be explained by a single GH or IGF-I measurement being used in most studies, which is hardly representative for disease status in the entire follow-up period; by the unavailability of IGF-I in a substantial number of patients; and by GH and IGF-I assay differences. In addition, individual mortality studies consist of relatively small numbers of patients with large confidence intervals including 1.0, limiting statistical power.
In acromegaly detailed studies of spontaneous GH secretion have demonstrated increased pulsatility (increased pulse frequency), amplified burst mass, and increased basal secretion, associated with decreased regularity (Barkan et al 1989; Ho et al 1994; van den Berg et al 1994). Biochemical criteria of active disease and remission are the (mean) GH level, glucose-suppressed GH concentration, and the IGF-I level (Giustina et al 2000). GH assays differ in specificity, sensitivity, and GH standard, and therefore individual clinical endocrine laboratories should establish normal ranges of gender- and age-related GH and IGF-I values and ideally corrected for fat mass or a fat mass-derived parameter (Gullu et al 2004; Bidlingmaier and Strasburger 2007). Circulating IGF-I reflects GH secretion rate and serum concentrations of IGF-I are elevated in all patients with active disease (Melmed 2006). IGF-I concentrations decrease with advancing age. In addition, gender, sex hormone status, the use of oral estrogens, thyroxin, and body composition can all influence IGF-I concentrations (Clemmons 2007).
Treatment of acromegaly
As discussed above, epidemiological studies have clearly demonstrated that controlling GH and IGF-I secretion is the most significant determinant of restoring survival in patients with acromegaly The main goal of treatment of acromegaly is therefore to achieve GH levels of less than 1 μg/L after a glucose load, to normalize age- and gender-matched IGF-I levels, to ablate or reduce tumor mass and prevent its recurrence, and to alleviate significant co-morbidities, especially cardiovascular, pulmonary, and metabolic disturbances (Melmed et al 2002). The currently available treatment modalities for acromegaly are selective transsphenoidal adenomectomy, radiotherapy, medical treatment, or combinations thereof.
Transsphenoidal surgery
This oldest treatment modality was developed a century ago by the Austrian neurosurgeon Schloffer (Schloffer 1907). It is generally performed via the transnasal, transsphenoidal route and is associated with low morbidity and mortality. In recent years most neurosurgeons have adopted the endoscope in lieu of the surgical microscope, which has obvious advantages for the patient and also leads to better visualization of the operating field. Other variants of surgical techniques are neuronavigating and real-time intraoperative MRI scanning, aimed at visualization of tiny tumor remnants after resection of the adenoma (Fahlbusch et al 2005; Thomale et al 2005). GH secretion pattern is restored when the adenoma is completely removed (van den Berg et al 1998). Surgical cure is highest in patients with a microadenoma (diameter less than 10 mm) varying from 80% to more than 90% in the hands of experienced neurosurgeons. However, complete tumor removal becomes more difficult with increasing size of the tumor and expansion into the neighboring delicate structures, and the cure rate of large macroadenoma drops to only 20%–40% of cases (Freda et al 1998; Biermasz et al 2000a; Kaltsas et al 2001; Kreutzer et al 2001; Shimon et al 2001; Beauregard et al 2003; De et al 2003; Nomikos et al 2005; Lüdecke and Abe 2006). The obvious advantage of successful surgery is the rapid normalization of GH secretion and decrease in IGF-I levels, while the complication of (partial) hypopituitarism is generally below 10% (Nomikos et al 2005; Lüdecke and Abe 2006). Second surgical procedures are generally safe but less successful than primary surgery (Long et al 1996). The experience of the neurosurgeon is critical for a high cure rate (Ahmed et al 1999; Bates et al 2008).
Radiotherapy
Conventional radiotherapy is administered by a linear accelerator (4–8 MeV) with a total dose of 40–45 Gy, fractionated in at least 20 sessions. A rotational field, 2 opposing fields, or a 3-field technique are used. A mean GH decrease of about 50% is observed in the first 2 years after irradiation and after 5 years a 75% decline is reported (Biermasz et al 2000b; Wass et al 2003). Whether the GH level normalizes post irradiation mainly depends on pre-irradiation serum GH concentration and the time interval between radiotherapy and the measurement of GH and IGF-1 levels. Post-irradiation remission rates are, however, largely affected by the extent of surgical debulking prior to radiotherapy. Other than the slow onset of GH control another drawback is the increasing incidence of hypopituitarism varying from 50%–85% after a follow-up of 10 years or longer (Minniti et al 2005; Biermasz et al 2006).
Barkan and colleagues were the first to question the efficacy of radiotherapy in normalizing serum IGF-I concentrations, with many studies addressing the effects of conventional pituitary irradiation on IGF-I and strict GH criteria being reported thereafter (Barkan et al 1997). A few reports supported an apparent lack of efficacy of pituitary irradiation (Thalassinos et al 1998; Cozzi et al 2001), whereas others reported normalization of IGF-I in 44%–79% of patients after 5–15 years of follow-up (Ciccarelli et al 1993; Barrande et al 2000; Powell et al 2000; Epaminonda et al 2001; Minniti et al 2005).
Another radiation technique is radiosurgery, which is the precise, stereotactic delivery of a single high radiation dose to a defined target with a steep dose gradient at the tumor margin (Mahmoud Ahmed et al 2001; Castinetti et al 2005; Roberts et al 2007). This form of radiotherapy is performed using a gamma knife with up to 200 60Co sources, a Linac-based system, or proton beams (Marcou and Plowman 2000; Brada et al 2004; Sheehan et al 2005). The perceived advantage of this form of irradiation is that only one session is required. There is otherwise no convincing evidence as yet that radio-surgery is superior to conventional irradiation in terms of GH control, time needed to reach clinically acceptable GH levels, and incidence of hypopituitarism (Landolt et al 1998; Attanasio et al 2003a; Biermasz et al 2006).
Disadvantages of pituitary irradiation other than the development of hypopituitarism include decreased quality of life (QoL), the development of secondary tumors, cerebrovascular disease, and increased mortality. In one cross-sectional study, decreased health-related QoL was described in acromegalic patients in long-term remission (Biermasz et al 2004b). These data were confirmed by another QoL analysis of treated acromegalic patients (Rowles et al 2005). A significant predictor of poor QoL was radiotherapy, but the pathophysiologic mechanism remains unclear. Increased mortality due to cerebrovascular disease was observed in two of the studies (Ayuk et al 2004; Kauppinen et al 2005) but not in the other three (Bates et al 1993; Ahmed et al 1999; Biermasz et al 2004a).The effect of radiotherapy on mortality is thus as yet to be established. The likelihood of secondary tumor formation after pituitary irradiation is very low (Brada et al 1992).
Medical treatment
The three most important drugs used for medical treatment of acromegaly are dopamine agonists, somatostatin analogs, and GH-receptor modulating chemicals.
Dopamine agonists
Bromocriptine, a dopamine agonist, effectively reduces GH secretion in only a minority of GH-secreting adenoma (Jaffe and Barkan 1992). Cabergoline, a more potent dopamine agonist with prolonged duration of action, was reported to normalize GH in 35% and IGF-I in 44% of 46 patients with a purely GH-secreting adenoma when given at a dose of 1–1.75 mg/week (Abs et al 1998). The efficacy of cabergoline was somewhat better in tumors co-secreting prolactin. Quinagolide, another dopamine agonist, was reported to normalize IGF-I in 28% of patients (Freda 2003). Most endocrinologists use long-acting dopamine agonists as adjunct therapy in patients who fail to normalize GH secretion with octreotide monotherapy. The combination therapy normalizes serum IGF-I concentrations in 30%–40% of patients, irrespective of the prolactin concentration (Cozzi et al 2004). Side effects of cabergoline are rare although there has been recent concern about cardiac valve hypertrophy, as observed in patients with Parkinson’s disease. Whereas the dose in Parkinson’s disease is generally much higher than that used for endocrine indications (Schade et al 2007), patients with acromegaly generally require long-term medical treatment for GH control. The use of dopaminergic drugs other than cabergoline is probably safer in acromegaly.
Somatostatin analogs
Somatostatin was isolated in 1973 from the hypothalamus and subsequently synthesized (Brazeau et al 1973). The hormone is processed from a large pre-prohormone into 2 cyclic peptides, consisting of 14 or 28 amino acids. The short form, SS14, is predominantly present in the brain, whereas SS28 is widely distributed in peripheral organs. Somatostatin acts as neuromodulator and neurotransmitter in the brain and as a neurohormone in the regulation of GH and thyroid-stimulating hormone secretion. In addition, somatostatin inhibits tumoral adrenocorticotropic hormone secretion in Cushing’s disease (van der Hoek et al 2004). Somatostatin acts as neurotransmitter in the extensive myo-enteric plexus, and as hormone in a paracrine and autocrine fashion. Via specific receptors, somatostatin exerts many inhibitory effects on gut and pancreatic hormones, including gastrin, insulin, glucagon, vasoactive intestinal peptide, motilin, and gastric inhibitory polypeptide. Other effects of somatostatin include inhibition of gastric emptying, pancreatic enzymes and bicarbonate secretion, gastrointestinal blood flow and bile flow (Brazeau et al 1973; Reichlin 1983; Patel 1999). Somatostatin acts via a G-protein-coupled receptor, of which 5 subtypes have been cloned and characterized (Lamberts et al 1996). After binding of somatostatin to its receptor, the activities of adenyl cyclase and of calcium channels are inhibited, whereas phosphotyrosine phosphatase activity and mitogen-activated protein kinases activity are stimulated. The first two processes are involved in the inhibition of secretory processes, and the latter two may play a role in cell proliferation, eg, activation of the SST3 receptor may induce apoptosis (Danilla et al 2001; Bevan 2005). Analogs of somatostatin differ in binding properties to different receptor subtypes (Lamberts et al 1996). Many benign and malign tumors express one or more somatostatin receptors. Receptor distribution and density and homogeneity of receptor expression within the tumor determine whether a particular analog can be effectively used therapeutically (Krantic et al 2004; Olias et al 2004).
GH-secreting pituitary adenomas express predominantly SST2 and SST5 receptors. The current clinically used analogs, octreotide and lanreotide, inhibit GH secretion via the somatostatin receptor subtypes 2 and 5 (Hofland and Lamberts 2003). The plasma half-life of these analogs is about 20 times longer than that of native somatostatin, which is less than 3 minutes (Lamberts et al 1996). Although the most important effect of somatostatin analogs is the inhibition of GH secretion by the adenoma leading to a subsequent decrease in circulating liver-derived IGF-I, part of the peripheral effects of these analogs is caused by the direct inhibition of IGF-I gene transcription after binding to the somatostatin receptor (Serri et al 1992; Murray et al 2004). The magnitude of this latter effect in various organs is not exactly known.
GH receptor antagonists
Pegvisomant is an engineered GH analog that antagonizes GH at the receptor site, and thus prevents endogenous GH activation of its receptor and subsequent downstream signaling. In short-term studies, the lowest dose (10 mg/day) normalized IGF-I in 38% of the patients and 20 mg normalized IGF-I in 82% of patients (Trainer et al 2000; van der Lely et al 2001). In a minority of patients (2 out of 112 and 7 out of 229 patients, respectively) adenoma size increased during a relatively short treatment period (van der Lely et al 2001; Schreiber et al 2007). Careful documentation of tumor size before starting pegvisomant treatment is therefore compulsory and long-term monitoring is advisable. A small number of patients (2 out of 167 cases) developed abnormalities in liver function tests, necessitating withdrawal of the drug, although increased liver enzyme levels, ie, more than 3 times the upper level of normal, was observed in 5.5% of 229 patients, normalizing spontaneously in 3.1% on continuing treatment (van der Lely et al 2001; Schreiber et al 2007). About 40% of patients develop minor abnormalities in liver function tests on combined treatment with somatostatin analogs, which do not requiring stopping of the drug and which usually resolve spontaneously (Feenstra et al 2005).
Pharmokinetics of lanreotide
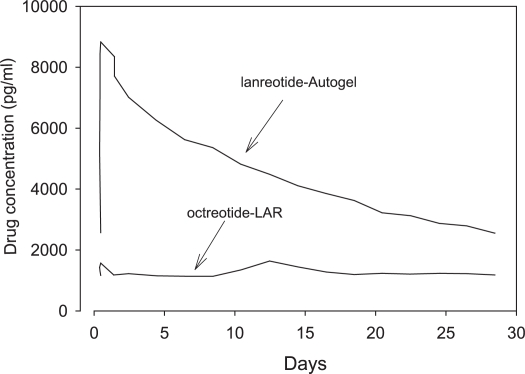
The first pharmaceutical available form of lanreotide (BIM 23014) was relatively short-acting, requiring multiple dosing, 3 times a day, or subcutaneous infusion. This was nevertheless a major advance in the treatment of many patients who had already undergone unsuccessful surgery and pituitary irradiation and for whom there were no other treatment options (Figure 1). In healthy subjects, maximal serum concentrations of lanreotide were reached after 30 min and the serum half-life was 90 min, 30 times greater than that of native somatostatin (Kuhn et al 1994; Antonijoan et al 2004; Table 1). Subsequently, a long-acting form of lanreotide was developed by incorporating the drug into polyactide – poly-glycolide microspheres, so that the half-life was considerably prolonged, and the injection interval could be extended to 7–14 days (Heron et al 1993). The lanreotide release pattern from the long-acting form is biphasic, ie, an early release during 2 days from the drug adsorbed onto the surface of the microspheres, followed by sustained release for about 1 week, starting at day 4, as a result of enzymatic breakdown of the microspheres, followed again by an exponential decrease in drug release. It was subsequently discovered that lanreotide had the unique property of self aggregation under favorable conditions, leading to a stable structure of highly organized nanotubules (Valery et al 2003, 2008). This formulation of the drug was named lanreotide Autogel® and has a long half-life after subcutaneous injection determined by pseudo-first order kinetics. Maximal serum concentrations are reached after 1–2 days (see Table 1) in healthy subjects and the serum half-life amounts to 25.5 days (Antonijoan et al 2004; Astruc et al 2005). In acromegalic patients maximal values are reached after 3.8–7.7 days under steady state conditions, depending on the dose administered (Table 2). Simulated steady state pharmacokinetic profiles of long-acting octreotide and lanreotide Autogel® differ significantly (Astruc et al 2005; Bronstein et al 2005). During long-acting octreotide treatment, serum concentrations of the drug are more or less stable, whereas the characteristic first-order kinetics of lanreotide Autogel® is superimposed on levels just before the next administration (see Figure 2; Astruc et al 2005). The pharmacokinetic differences therefore indicate that octreotide LAR can be better tailored to therapeutic levels, whereas serum levels of lanreotide must be (too) high for part of the interval between injections in order to be effective in the period before the next administration. The possible clinical consequence(s) of these different pharmacokinetic profiles can be resolved only in long-term studies in which lanreotide Autogel® is compared with octreotide or drugs with a similar pharmacokinetic profile.
Figure 1.
Amino acid structure of somatostatin-14, octreotide. and lanreotide.
Table 1.
Pharmacokinetic analysis of a single subcutaneous dose of short-acting lanreotide and lanreotide Autogel® in healthy subjects
| Short-acting lanreotidea n = 24 | lanreotide Autogela n = 24 | lanreotide Autogelb n = 10 | lanreotide Autogelb n = 10 | |
|---|---|---|---|---|
| Dose | 7 μg/kg | 60 mg | 90 mg | 120 mg |
| Cmax | 7.98 ng/mL | 5.71 ng/mL | 6.7 ng/mL | |
| Tmax | 0.43 h | 0.38 day | 2.4 day | 1.1 day |
| Half-life | 1.74 h | 22 days | 25.5 day | |
| AUC | 16.51 ng.mL−1.h | 79.48 ng.mL−1.day | 116 ng.mL−1.day | 133 ng.mL−1.day |
| MRT | 1.95 h | 31.97 days |
Abbreviations: AUC, area under the curve; MRT, mean residence time.
Table 2.
Pharmacokinetics of lanreotide Autogel® during steady state conditions in patients with acromegaly
| Dose | 60 mg | 90 mg | 120 mg |
|---|---|---|---|
| Tmax (days) | 85 | 84 | 85 |
| Cmean (ng/mL) | 2.46 | 3.04 | 4.52 |
| Cmin (ng/mL) | 1,82 | 2.51 | 3.76 |
| Cmax (ng/mL) | 3.82 | 5.69 | 7.69 |
| AUC (ng.mL−1.day | 68.8 | 85.1 | 127 |
From data of Bronstein et al (2005).
Abbreviation: AUC, area under the curve.
Figure 2.
Pharmacokinetic profiles of lanreotide Autogel® (90 mg) and octreotide-LAR (20 mg) at steady state. The lines represent mean values of 10 simulated profiles. From data of Astruc et al (2005).
Efficacy of lanreotide
The first studies with lanreotide were performed using lanreotide Slow Release (lanreotide SR). The drug was first available in vials containing 30 mg, to be injected at 2-weekly intervals. The interval was shortened, however, to 7–10 days when GH was insufficiently suppressed. The drug later also became available in vials containing 60 mg of lanreotide so that the injection interval could be extended to 4 weeks, similar to that of the long established octreotide LAR. Studies using lanreotide SR 30 mg and lanreotide SR 60 mg are summarized in Table 3. Most patients had undergone pituitary surgery and many were irradiated, either as primary treatment (a minority) or as adjuvant treatment after noncurative surgery. In addition, in almost all studies patients had been treated with octreotide. Normal mean GH concentration, as defined by the authors (generally below 2.5 μg/L) was achieved in 23%–93% of the cases treated with lanreotide SR 30 mg, and in 25%–65% of the cases treated with lanreotide 60 mg. Normal values of IGF-I were obtained in 23%–68% of patients on lanreotide SR 30 mg, and 35%–62% of these on lanreotide SR 60 mg. The weighted means of normalization of GH and of IGF-I were 54% and 49%, respectively, during treatment with 30 mg lanreotide, whereas during treatment with lanreotide SR 60 mg these values were 60% and 58%.
Table 3.
Efficacy of lanreotide slow release (SR) on serum GH and IGF-I concentrations in acromegaly
| Reference | Patient no | Previous surgery and/or radiotherapy | Previous medication (number) | Used medication | Duration (months) | GH normal | IGF normal | Comments |
|---|---|---|---|---|---|---|---|---|
| Heron 1993 | 14 | 14 | none | Lanreotide SR 30 mg/14 d | 6 | 93% | 64% | IGF-I < 350 ng/mL, GH < 5 μg/L |
| Johnson 1994 | 8 | 5 | none | Lanreotide SR 30 mg/14 d | 6 | 50% | 38% | No SDS for IGF-I,GH < 10 mU/L |
| Marek 1994 | 13 | 13 | none | Lanreotide SR 30 mg/14 d | 9–19 | 23 | 23% | IGF-I < 270 μg/L, GH < 5 μg/L |
| al-Maskari 1996 | 10 | 10 | octreotide (7) | Lanreotide SR 30 mg/10–14 d | 6 | 60% | 50% | No SDS for IGF-I,GH < 5 mU/L |
| Caron 1997 | 22 | 7 | octreotide (21) | Lanreotide SR 30 mg/10–14 d | 6–36 | 27.2% | 64% | IGF <300 ng/mL for all subjects |
| Giusti 1997 | 57 | octreotide SR (37) | Lanreotide SR 30 mg/10–14 d | 6 | 54% | 35% | Normal IGF < mean + 3SD; normal GH < 5 μg/L | |
| Morange 1994 | 19 | 13 | octreotide (14) | Lanreotide SR 30 mg/10–14 d | 6 | 68% | 68% | IGF-I not age-adjusted |
| Suliman 1999 | 30 | 21 | octreotide (7) | Lanreotide SR 30 mg/10–14 d | 12 | 78% | 35% | IGF-I not age-adjusted |
| Colao 1999 | 45 | 25 | octreotide (45) | Lanreotide SR 30 mg/10–14 d | 6 | 58% | 58% | Age-adjusted IGF-I |
| Baldelli 2000 | 118 | 95 | octreotide (95) | Lanreotide SR 30 mg/10–14 d | 24 | 61% | 52% | Age-adjusted IGF-I |
| Verhelst 2000 | 66 | 37 | octreotide (55) | Lanreotide SR 30 mg/7–14 d | 12 | 45% | 44% | Age-adjusted IGF-I |
| Chanson 2000b | 58 | 58 | octreotide (38) | Lanreotide SR 30 mg/10–4 d | 12 | 41% | 41% | Selected from 116 patients |
| Cannavo 2000 | 18 | 10 | octreotide (16) | Lanreotide SR 30 mg/10–30 d | 6 | 50% | 44% | IGF-I not age-adjusted |
| Cozzi 2000 | 10 | none | none | Lanreotide 60 mg/28–21 d | 6 | 25% | 62% | IGF-I not age-adjusted |
| Ambrosio 2002 | 20 | 13 | lanreotide SR 30 mg/octreotide (15) | Lanreotide 60 mg/28–21 d | 8 | 65% | 35% | Age-adjusted IGF-I |
| Attanasio 2003b | 92 | 62 | octreotide or lanreotide SR (40) | Lanreotide 60 mg/28/21/14 d | 24 | 63% | 65% | Age-adjusted IGF-I |
Comparative studies of efficacy between octreotide and lanreotide are summarized in Table 4. Short-acting octreotide, mostly given 3 times a day, had a similar GH-suppressive effect as lanreotide SR. Normal GH was obtained in 52% and 49% of a total of 218 patients, but normalized IGF-I was more frequently found in patients treated with lanreotide SR 30 mg/10–14 days, ie, 49% versus 64%. The efficacy of octreotide LAR was slightly higher than that of lanreotide SR: normalized GH and IGF-I were obtained in 64% and 62% of 155 patients treated with octreotide LAR versus 52% and 50%, respectively, in the same patients during treatment with lanreotide SR. A limitation of all these studies, with one exception, is that they were not randomized. The overall better efficacy of octreotide LAR compared with lanreotide SR agrees with findings from a recent meta-analysis (Freda et al 2005).
Table 4.
Comparison of efficacy of octreotide versus lanreotide SR in acromegaly
| Reference | Number of patients | Octreotide
|
Lanreotide
|
||||
|---|---|---|---|---|---|---|---|
| GH normalized | IGF-I normalized | Octrotide dose | GH normalized | IGF-I normalized | Lanreotide dose | ||
| Morange 1994 | 19 | 16/19 | 16/19 | 0.15–0.6 mg/d | 8/19 | 13/19 | 30 mg/10–14 d |
| Caron 1997 | 21 | 12/21 | 17/21 | 0.1–0.6 mg/d | 6/22 | 14/22 | 30 mg/10–14 d |
| Colao 1999 | 45 | 23/45 | 23/45 | 0.15–0.6 mg/d | 26/45 | 26/45 | 30 mg/10–14 d |
| Razzore 1999 | 38 | 18/38 | 19/38 | 0.15–0.6 mg/d | 9/38 | 16/38 | 30 mg/10–14 d; 60 mg/14 d |
| Baldelli 2000 | 95 | 45/95 | 32/95 | 0.1–0.6 mg/d | 58/95 | 72/95 | 30 mg/10–14 d |
| Kendall-Taylor 2000 | 5 | 4/5 | 5/5 | LAR 20– 30 mg/4 w | 4/5 | 5/5 | 30 mg/10–14 d |
| Turner 1999 | 10 | 8/10 | 7/10 | LAR 20–30 mg/4 w | 7/9 | 5/9 | 30 mg/7–10 d |
| Cozzi 1999 | 12 | 4/10 | 5/10 | LAR 10–30 mg/4 w | 1/10 | 4/10 | 30 mg/7–21 d |
| Chanson 2000b | 125 | 68% | 65% | LAR 20–30 mg/4 w | 54% | 48% | 30 mg/10–14 d |
| Amato 2002§ | 20 | 50% | 50% | LAR 10–30 mg/4 w | 58% | 67% | 30 mg/7–10 d |
§Randomized study
Lanreotide Autogel® was introduced about 8 years ago, and the first report in the English literature was published in 2002. Clinical efficacy studies are summarized in Table 5. Most of the patients who took part in these studies had undergone pituitary surgery, often with adjuvant irradiation, and almost all patients were on octreotide or lanreotide SR treatment, while a minority also used dopaminergic drugs. The results of these studies should therefore be considered critically, as a selection bias cannot be excluded. Normal GH, defined as a concentration below 2.5 μg/L in fasting single blood samples or as the mean of serial samples was observed in 38%–80% of cases and normal age-related IGF-I was recorded in 39%–80% of patients on lanreotide Autogel®. In these studies the weighted mean for GH normalization was 58% and for IGF-I 48% in a population of 370 patients. The results mentioned above refer to measurements at the end of the study when dose titration of lanreotide Autogel® was fully effective. Indeed, most of the patients ended receiving the highest dose of 120 mg. These results do not differ from data obtained in patients on lanreotide SR (see above). Part of these studies compared the efficacy of octreotide LAR and lanreotide Autogel®. A drawback of these studies is that with the exception of one study none were randomized (Andries et al 2008). An open-label, uncontrolled, single-group assignment study on the effects of lanreotide Autogel® in 27 previously untreated patients with acromegaly was recently completed (ClinicalTrial.gov NCT00627796). Although the study is rather small it will contribute further data on IGF-I control and tumor reduction.
Table 5.
Clinical studies with lanreotide Autogel® in acromegaly
| Reference | Study design | Patient no. ITT/PPP | Previous treatment PS/RT/MT | Dosing lanreotide Autogel | Duration (months) | Normal GH (< 2.5 μg/L) | Normal age-adjusted IGF-I | Previous medication |
|---|---|---|---|---|---|---|---|---|
| Caron 2002 | open label MC | 144/107 | 83/49/107 | 60/90/120 mg | 3 | 56% | 48% | LSR 30 mg/7–14 d |
| Alexopoulou 2004 | open label MC | 25/25 | 13/5/25 | 60/90/120 mg | 6 | 48% | 52% | oLAR 20–40 mg/4 w |
| Ashwell 2004 | open label MC | 12/10 | 7/5/10 | 60/90/120 mg | 7 | 80% | 80% | oLAR 20 mg/4 w |
| Caron 2004 | extension study | 131/130 | 99/57/131 | 60/90/120 mg | 12 | 68% | 50% | Lan-Autogel |
| Gutt 2005 | open label | 11 | 10/0/11 | 60/90/120 mg | 16 | ? | 54% | LSR 30 mg/7–14 d |
| Caron 2006 | extension study | 14/14 | 9/5/14 | 60/90/120 mg | 36 | 77% | 54% | LSR 30 mg/10–14 d |
| Lucas 2006 | open label MC | 99/93 | 76/53/99 | 120 mg/4–8 w | 3 | 54% | 55% | LSR 30 mg/7–14 d |
| Ronchi 2007 | open label MC | 23/21 | ?/0/23 | 60/90/120 mg | 9 | 56% | 39% | oLAR 10–30 mg/4 w |
| Andries 2008 | randomized cross-over | 12/10 | 7/3/11 | Fixed dose 60/90/120 mg | 12 | 42%a | 50% | oLAR 10–30 mg/4 w |
| Chanson 2008 | open label MC | 63/57 | 37/12/49 | Fixed phase and dose titration | 12 | 38% | 43% | oLAR, lanreotide SR, |
anormal GH concentration <0.38 μg/L.
Abbreviations: ITT, intention-to-treat; PPP, patients per protocol; PS, pituitary surgery; RT, radiotherapy; MT, previous medical treatment; LRS, lanreotide slow release; oLAR, octreotide LAR; MC, multicenter.
In a 3-month study in 107 patients, the normalization rate for GH was 48% during lanreotide SR and 56% during lanreotide Autogel® therapy, whereas a normal IGF-I was obtained in respectively 45% and 48% of cases (Caron et al 2002). In an extension phase of this study to 12 months, normalized GH frequency increased from 49% to 68% in 130 patients; these figures were 44% and 50% for IGF-I (Caron et al 2004). Fourteen patients of these studies were treated for 3 years with lanreotide Autogel®. In these patients the frequency of normal GH increased from 36% to 77% and that for IGF-I from 36% to 54% (Caron et al 2006). Finally, the Spanish multicenter study extended the Autogel® injection interval to 8 weeks in patients who were controlled by 2-weekly injections with lanreotide SR. The overall GH control increased from 46% to 54% (Lucas et al 2006). The studies comparing the efficacy between octreotide LAR and lanreotide are shown in Table 6. Only the small study by Andries was properly designed, and showed equal efficacies of both drugs in terms of normalization of GH. Nevertheless, this study demonstrated a better GH-suppressive effect of octreotide on absolute GH concentrations than lanreotide. In contrast, the suppressive effect on IGF-I was similar. There was no difference in GH suppressive effect in a small study in 7 patients in whom the 24 h GH secretion was precisely measured with a 10 min blood sampling protocol. (Van Thiel et al 2004). From the data presented above and despite limitations in design, it would appear that lanreotide Autogel® and octreotide LAR are equipotent in normalizing GH and IGF-I concentrations. Although patients require generally the highest lanreotide dose, most patients on octreotide LAR had safe GH and normal IGF-I levels on the 20 mg dose. For the practicing endocrinologist the message is that patients on octreotide LAR 20–30 mg need 120 mg lanreotide Autogel® and somatostatin-sensitive patients on octreotide LAR 10 mg require mostly 90 mg of the Autogel® formulation. Lanreotide Autogel® is registered under the trade name Somatuline Autogel® in the majority of countries, as Somatuline Depot Injection® in the US, and as Ipstyl Autogel® in a few European countries.
Table 6.
Efficacy of lanreotide Autogel® compared with octreotide LAR and lanreotide SR in acromegaly
| Reference | Duration (months) | Patient no ITT/PPP | Octreotide LAR dose | Lanreotide Autogel dose | Normal GH oLAR | Normal GH lanreotide Autogel | Normal IGF-I oLAR | Normal IGF-I lanreotide Autogel |
|---|---|---|---|---|---|---|---|---|
| Alexopoulou 2004 | 6 | 25/25 | 20–40 mg/4 w | 60/90/120 mg | 64% | 48% | 52% | 52% |
| Ronchi 2007 | 9 | 23/21 | 10–30 mg/4 w | 60/90/120 mg | 40% | 56% | 35% | 39% |
| Andries 2008 | 12 | 12/10 | 10–30 mg/4 w | Fixed dose 60/90/120 mg | 50% | 50%a | 50% | 60% |
anormal GH concentration <0.38 μg/L.
Abbreviations: ITT, intention-to-treat; PPP, patients per protocol; oLAR, octreotide LAR.
Side effects
The most frequent side effects of lanreotide are diarrhea, abdominal pain, and nausea. These symptoms start mostly shortly after an injection, decrease subsequently, and tend to decrease in severity on continuing treatment. Table 7 lists the side effects mentioned in the clinical studies with the 2 long-acting formulations of lanreotide. For the SR formulation the gastrointestinal side effects were observed in 48% of the patients and for the Autogel® formulation in 52%. The most serious complication of somatostatin analogs is cholelithiasis. The prevalence of somatostatin analog-induced gallstones varies geographically and may be influenced by dietary, environmental, and racial factors. The formation of gallstones involves the inhibition of gallbladder emptying and intestinal motility, inhibition of the secretion of pro-kinetic peptides, including cholecystokinin, and increased intestinal and biliary production of deoxycholic acid, all of which promote the nucleation of cholesterol crystals and their aggregation into stones (Dowling et al 1992). We analyzed the occurrence of new cholelithiasis in patients who were already on somatostatin analog treatment, a condition thus not quite comparable to drug-naïve patients in terms of risk of developing gallstones. The incidence of new gallstones was 6% for lanreotide SR and 8.7% for lanreotide Autogel®. These figures are smaller than generally cited in literature, but many patients had cholelithiasis caused by previous treatment.
Table 7.
Side effects during treatment with lanreotide SR and lanreotide Autogel® in acromegaly
| Author | Number of patients | Number of naive patients | Current treatment | GI side effects | New cholelithiasis | Fasting glucose | Tumor size decrease |
|---|---|---|---|---|---|---|---|
| Heron 1993 | 14 | 0 | LSR 30 mg | 9 | 2 | nc | |
| Morange 1994 | 19 | 0 | LSR 30 mg | 3 | 2 | nc | |
| Johnson 1994 | 8 | 3 | LSR 30 mg | 5 | 1 | nd | nd |
| Marek 1994 | 13 | 0 | LSR 30 mg | 13 | 1 | nc | 5/13 (>20%) |
| Giusti 1996 | 57 | 0 | LSR 30 mg | 22 | 2 | nc | |
| al-Maskiri 1996 | 10 | 0 | LSR 30 mg | 10 | 1 | nc | |
| Caron 1997 | 22 | 0 | LSR 30 mg | 13 | 4 | nc | |
| Suliman 1999 | 30 | 7 | LSR 30 mg | 26 | 2 | nc | 1/7 |
| Colao 1999 | 45 | 0 | LSR 30 mg | 12 | 1 | nm | |
| Chanson 2000a | 58 | 0 | LSR 30 mg | 40 | 6 | nc | |
| Baldelli 2000 | 118 | 23a | LSR 30 mg | 64 | 4 | nm. | 5/23 (>20%) |
| Cozzi 2000 | 21 | 8 | LSR 60 mg | nd | 0 | nc | 5/13 |
| Cannavo 2000 | 22 | 0 | LSR 30 mg | few | 2 | nm. | |
| Verhelst 2000 | 66 | 3 | LSR 30 mg | 41 | 2 | nc | |
| Ambrosio 2002 | 20 | 0 | LSR 60 mg | 10 | 0 | nc | 0/4 |
| Attanasio 2003b | 92 | 22 | LSR 60 mg | 8 | 10 | nc | 11/22 |
| Caron 2004 | 130 | 0 | LAUT | 58 | 12 | nd | |
| Ashwell 2004 | 12 | 0 | LAUT | 0 | nd | nd | |
| Alexopoulou 2004 | 25 | 0 | LAUT | 8 | 0 | nc | |
| Ronchi 2007 | 23 | 0 | LAUT | nd | 1 | nc | |
| Chanson 2008 | 63 | nd | LAUT | 53 | 8 | increase in 4 |
Decrease of tumor size is given only for patients who had no previous radiotherapy or somatostatin analog treatment.
aSix patients had been treated with bromocriptin.
Abbreviations: nd, no data available; nc, no significant change of glucose concentrations; nm, not mentioned; LSR, lanreotide slow release; LAUT, lanreotide Autogel.
Other side effects were local pain after injection and rarely (less than 1%) the development of nodules at the injection site. However, local infiltration signs did not decrease the efficacy of the drug. Other uncommon side effects included sinus bradycardia, asthenia, headache, pruritus, decreased libido, increased serum bilirubin, fatigue, constipation, and hair loss.
Influence of lanreotide Autogel® on clinical manifestations
Some studies have investigated specific aspects of lanreotide action in acromegaly. These include detailed studies on glucose and insulin metabolism, effects on cardiac function, tumor growth, quality of life, and predictors of clinical response. These reports are briefly summarized below.
Insulin and glucose homeostasis
GH is important in regulating glucose tolerance and insulin sensitivity. GH counteracts the effects of insulin by inhibiting the phosphorylation of the insulin receptor. Moreover, GH also inhibits the phosphorylation of one of the proximate molecules of the insulin signaling cascade, insulin receptor substrate-1 in response to insulin (Kuhn et al 1992). In acromegaly, several studies have shown that increased GH induces insulin resistance (Kasayama et al 2000). However, GH also potentiates insulin release which is reflected in the high prevalence of high insulin levels both at rest and after glucose challenge (Cerasi and Luft 1964). Indeed, many untreated patients exhibit decreased glucose tolerance and more detailed studies have shown reduced insulin-stimulated glucose disposal in muscle and impaired non-oxidative glucose metabolism (Sonksen et al 1967; Wass et al 1980; Hansen et al 1986; Foss et al 1991; Koop et al 1994). Effects of somatostatin analogs on glucose homeostasis are the resultant of delayed intestinal absorption of carbohydrates, inhibition of insulin release and increased insulin sensitivity via diminished GH secretion. Results from studies with lanreotide do not differ essentially from earlier data obtained with octreotide. The acute effects of subcutaneously infused lanreotide were studied in healthy subjects. Oral glucose tolerance worsened during the first day of administration, but was restored on day 7 while drug administration continued (Kuhn et al 1992). In a study in 27 patients the homeostasis model assessment (HOMA) index improved, but not the quantitative insulin check index (QUICKI) index (Ronchi et al 2003). In a cross-sectional study with 51 acromegalic patients of whom 18 were on lanreotide Autogel® the pancreatic β-cell function deteriorated but insulin resistance remained unchanged (Steffin et al 2006). The most precise study used the euglycemic hyperinsulinemic clamp. Twenty-four patients were studied at baseline and after 6 months treatment with either octreotide LAR or with lanreotide SR. Hemoglobin A1c (HbA1c) increased significantly. In patients with a normal glucose tolerance at baseline the glucose concentration at 120 min increased, together with decreased and delayed insulin response. Insulin sensitivity increased in all 12 clamped patients. The investigators could not demonstrate differences between octreotide and lanreotide, ie, the effects on GH, IGF-I, and insulin were all similar (Baldelli et al 2003). The effects of other pharmacologic therapies currently used for the treatment of acromegaly on glucose metabolism and insulin resistance were recently reviewed (Pereira et al 2005). In most studies, not specifically focused on insulin and glucose metabolism, fasting glucose concentrations and/or HbA1c levels did not change significantly when the GH-suppressive medication was changed to lanreotide or when the period of lanreotide administration was compared with the period without GH-suppressive medication.
Cardiac effects
Acromegaly is associated with increased cardiac morbidity and mortality. Recognized cardiac manifestations include chronic cardiac failure due to systolic dysfunction (cardiomyopathy) or isolated diastolic dysfunction (Colao et al 2004; Pereira et al 2004). In addition, our group documented the increased prevalence of regurgitant valvular heart disease in acromegaly (Pereira et al 2004). An important question is whether effective GH-suppressive medication can improve cardiac function. One of the first studies reported on 13 patients treated with lanreotide. In this study there was a parallel decrease in GH and IGF-I and in left ventricular mass index; these data were confirmed in another study (Baldelli et al 1999; Hradec et al 1999). Octreotide was used in most studies on cardiac function, because this drug was the earliest available for clinical studies (Maison et al 2007). These studies indicate that effective GH-suppressive medication improves morphological and functional hemodynamic parameters, although medical therapy does not normalize all parameters. These observations concur with results of another study, which compared outcome in long-term surgically cured patients with medically controlled patients and which showed better results in the first group (van Thiel et al 2005), suggesting that GH-suppressive therapy in its present form is unable to fully correct cardiac dysfunction. The impact of this finding on long-term mortality in acromegaly is unknown.
Tumor growth
The anti-tumoral effects of somatostatin analogs are linked to the activation of the subtype receptors SSTR1, SSRT2, SSTR4, and SSTR5, which all induce cell cycle arrest. Apoptosis is associated with SSTR3 and possibly also with SSTR2 signaling (Danilla et al 2001; Bevan 2005). GH secreting adenomas express different somatostatin receptors, as shown for example by a recent study in which 77% expressed SSTR2, 69% SSTR1 and SSTR3, and 60% SSTR5. In the same study, lanreotide inhibited cell proliferation in vitro in 10 out of 13 adenomas (Florio et al 2003). Lanreotide also stimulates apoptosis as was found in surgically removed GH secreting adenomas to 8.7 ± 2.6% in tumors compared with less than 3.5 % in controls (Wasko et al 2003). The clinical response in terms of GH control and tumor size reduction correlates with the expression of somatostatin receptor subtype 2a (Fougner et al 2008; Taboada et al 2008) Preoperative treatment with lanreotide SR for 1–3 months in 104 acromegalic patients led to tumor size reduction in 66%, with a mean decrease of 152 mm3. A decrease in adenoma size of more than 20% was found in 29% of the patients (Lucas et al 2003). Other studies in which the decrease in adenoma size could be evaluated are listed in Table 7. In the meta-analysis of 14 clinical studies using somatostatin analogs as primary treatment, 36.6% of the patients exhibited a significant reduction in tumor size, with a weighted mean of 19.4% (Melmed et al 2005). Factors (not necessarily predictors) associated with tumor shrinkage after primary therapy with somatostatin analogs were post-treatment IGF-I, the age of the patient and the percentage GH decrease (Colao et al 2006a), and essentially confirming previously reported findings (Lucas et al 2003). In another meta-analysis of 44 trials, tumor shrinkage was related to the choice of the somatostatin analog. Octreotide LAR appeared to be more potent than lanreotide SR, with an odds ratio of 9.4 (Freda et al 2005). Preliminary data on biochemical remission of acromegaly after somatostatin analogs withdrawal suggest that some well-responsive patients might be cured, but long-term follow up is clearly needed (Ronchi et al 2008).
Quality of life
QoL remains impaired in acromegaly even after successful pituitary surgery due to persisting joint-related complaints (Biermasz et al 2005a). An early open study on the effect of lanreotide SR on QoL suggested a positive effect of treatment (Sonino et al 1999). However, in another study comprising 52 acromegalic patients no differences could be shown between lanreotide-controlled and noncontrolled patients using the AcroQoL, a questionnaire specifically developed for acromegaly. Interestingly, in the controlled group, surgically cured patients were much better off than patients controlled with lanreotide (Hua et al 2006). This observation underscores subtle differences between restoration of normal physiology and effective GH-suppressive medication, as found in intensive GH sampling studies in acromegalic cohorts (Biermasz et al 2004c). Finally, in a study of 93 patients with acromegaly control of GH and IGF-I had a positive impact on the subscale appearance, but overall QoL was severely impaired (Matta et al 2008).
Predictors of clinical response
A priori conditions for a favorable clinical response to somatostatin analog therapy are the density and distribution of SSTR2a receptors in the adenoma (Lamberts et al 1996). It is controversial whether a single acute octreotide test can predict the clinical response during long-term treatment. In this respect 3 studies reported positive results (Biermasz et al 2005b; Gilbert et al 2005; Karavitaki et al 2005), whereas 3 others concluded that the test was not useful (Colao et al 1996; de Herder et al 2005; Prokajac et al 2005). The absolute height of pretreatment GH levels is obviously another important factor for the efficacy of treatment, and indeed several studies have demonstrated that tumor debulking procedures improved the clinical outcome of medical therapy (Colao et al 2006b; Karavitaki et al 2007).
Primary pharmacologic treatment
Patients with a high chance of curative surgery should be offered this treatment. However, primary medical treatment should be considered in patients with a high surgical risk, patients with large invasive tumors and obviously in those who refuse surgery. Dose escalation with short-acting octreotide resulted in a better outcome in patients treated with octreotide as primary medication than those who received this drug as adjuvant medication after surgery (Newman et al 1995). Given as primary treatment, octreotide LAR controlled GH secretion in 57%, IGF-I in 45%, and caused tumor reduction of more than 50% in 44% out of 99 patients (Colao et al 2006a). This group and Cozzi and colleagues also found that dose escalating resulted in an even better outcome (Cozzi et al 2003; Colao et al 2007). Limitations of these studies are that they are not randomized to primary surgery and that no data are available on long-term effects on survival.
Primary medical treatment may also be aimed at improvement of surgical outcome. Most of the studies addressing this issue had an open label design. Three studies reported beneficial effect on outcome (Barkan et al 1988; Colao et al 1997; Stevenaert and Beckers 1996), whereas three others did not (Biermasz et al 1999; Kristof et al 1999; Abe and Lüdecke 2001). Therefore, a conclusive statement cannot be made on this issue.
Failures of medical therapy
As outlined above, somatostatin analog treatment will not control clinical symptoms and biochemical parameters in all acromegalic patients, and about half of them will still have raised IGF-I and/or GH levels. An increase in the injection frequency of lanreotide Autogel® to once every 2–3 weeks is generally not successful (Abrams et al 2007). Another, less expensive approach is to combine treatment with dopaminergic agonists (Freda 2003; Cozzi et al 2004). More effective is combined treatment with pegvisomant as demonstrated by a single center open labeled study. Long-term efficacy of combined treatment was demonstrated in 32 patients who all normalized IGF-I with pegvisomant in a dose of 40–160 mg given once weekly (24 patients) or twice weekly (Neggers et al 2007). Two large multicenter studies are respectively ongoing and complete, in which weekly administered pegvisomant is combined with lanreotide Autogel® in patients not controlled during treatment with 120 mg lanreotide Autogel® (ClinicalTrials.gov, NCT 00383708) and daily pegvisomant injections with ocreotide LAR (ClinicalTrials. gov, NCT 0068029). Preliminary results of the latter study suggest equal efficacy in the two randomized parallel treatment groups towards serum IGF-I normalization, but with a higher incidence of side effects in the combined treatment group (Harris et al 2007). Considering the number of patients included, these studies will most likely answer questions about the efficacy of combined somatostatin analog and GH-receptor blockage in the treatment of acromegaly. However, both studies did not exclude previous surgery or radiation therapy, so that any conclusions drawn from these studies may not be applicable to primary medical treatment.
Due the favorable receptor binding profile, SOM230 (pasireotide) is likely to be a powerful somatostatin analog, which might be used in therapy-resistant cases to the registered somatostatin analogs (van der Hoek et al 2005; Ben-Shlomo and Melmed 2007). Clinical Phase II studies in acromegaly are now being carried out in the US with both the short-acting form as well as the slow-release formulation (ClinTrial.gov NCT000088582, NCT00171730, and NCT00600886). Other somatostatin agonists currently developed were recently reviewed (Roelfsema et al 2006). Potential interesting drugs are chimeric somatostatin analogs. This class of drugs combines dopamine and somatostatin structural elements and retains affinity for specific somatostatin and dopamine receptor subtypes. These new drugs can not only suppress GH (and other pituitary hormones) better than currently clinically used drugs, but may also have much stronger antiproliferative actions, at least in vitro (Ferone et al 2007; Zatelli et al 2007).
Summary and future perspectives
Lanreotide Autogel® is an exceptional pharmaceutical achievement, based on the unique property of self-aggregation of lanreotide. The formulation is delivered in prefilled syringes and can be easily injected without medical supervision by the patient or partner after proper training (Bevan et al 2008), whereas octreotide LAR requires qualified personnel for administration.
Lanreotide SR 30 mg/7–14 days can control serum GH in 59% and IGF-I concentrations in 49% of patients, while the results of the 60 mg formulation/4 weeks are 60% and 58%, respectively. Lanreotide Autogel® controls GH in 58% and IGF-I in 48% of patients. Compared with octreotide LAR the efficacy of lanreotide SR is less, although the differences are not large (Freda et al 2005). No large scale data are available for lanreotide Autogel®, a latecomer in this therapeutic field, for making a reasonable comparison with octreotide LAR.
The present formulations of somatostatin analogs can be classified as a second generation of effective GH-suppressive drugs, but these agents are clearly not adequate for all patients, depending on tumor somatostatin receptor status. New somatostatin analogs include SOM230, which is currently being used in several trials in the US, and the potentially very powerful chimeric drugs developed by Ipsen SA. The latter drugs, if successful in phase II–IV studies, will probably take another 5–10 years before becoming available for clinical use by endocrinologists. At present patients not controlled by somatostatin analogs should be treated with adjuvant pegvisomant, either as daily injections, as recommended by Pfizer, or as once-weekly or 2-weekly injections in a titrated dose, which data in the literature have suggested as sufficient (Feenstra et al 2005; Jørgensen et al 2005; Harris et al 2007; Neggers et al 2007). It is to be expected that other GH receptor blocking agents will become available in the future, which might not have the potential drawbacks of pegvisomant (Roelfsema et al 2006).
Footnotes
Disclosures
The authors have no conflict of interest and have received no payment in the preparation of this manuscript.
References
- Abe T, Lüdecke DK. Effects of preoperative octreotide treatment on different subtypes of 90 GH-secreting pituitary adenomas and outcome in one surgical centre. Eur J Endocrinol. 2001;145:137–45. doi: 10.1530/eje.0.1450137. [DOI] [PubMed] [Google Scholar]
- Abrams P, Alexopoulou O, Abs R, et al. Optimalization and cost management of lanreotide-Autogel therapy in acromegaly. Eur J Endocrinol. 2007;157:571–7. doi: 10.1530/EJE-07-0366. [DOI] [PubMed] [Google Scholar]
- Abs R, Verhelst J, Maiter D, et al. Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab. 1998;83:374–8. doi: 10.1210/jcem.83.2.4556. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Elsheikh M, Stratton IM, et al. Outcome of transsphenoidal surgery for acromegaly and its relationship to surgical experience. Clin Endocrinol (Oxf) 1999;50:561–7. doi: 10.1046/j.1365-2265.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- Alexander L, Appleton D, Hall R, et al. Epidemiology of acromegaly in the Newcastle region. Clin Endocrinol (Oxf) 1980;12:71–9. doi: 10.1111/j.1365-2265.1980.tb03135.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulou O, Abrams P, Verhelst J, et al. Efficacy and tolerability of lanreotide Autogel therapy in acromegalic patients previously treated with octreotide LAR. Eur J Endocrinol. 2004;151:317–24. doi: 10.1530/eje.0.1510317. [DOI] [PubMed] [Google Scholar]
- al-Maskari M, Gebbie J, Kendall-Taylor P. The effect of a new slow-release, long-acting somatostatin analogue, lanreotide, in acromegaly. Clin Endocrinol (Oxf) 1996;45:415–21. doi: 10.1046/j.1365-2265.1996.8270836.x. [DOI] [PubMed] [Google Scholar]
- Amato G, Mazziotti G, Rotondi M, et al. Long-term effects of lanreotide SR and octreotide LAR on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin Endocrinol (Oxf) 2002;56:65–71. doi: 10.1046/j.0300-0664.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- Ambrosio MR, Franceschetti P, Bondanelli M, et al. Efficacy and safety of the new 60-mg formulation of the long-acting somatostatin analog lanreotide in the treatment of acromegaly. Metabolism. 2002;51:387–93. doi: 10.1053/meta.2002.30526. [DOI] [PubMed] [Google Scholar]
- Andries M, Glintborg D, Kvistborg A, et al. A 12-month randomized crossover study on the effects of lanreotide Autogel and octreotide long-acting repeatable on GH and IGF-l in patients with acromegaly. Clin Endocrinol (Oxf) 2008;68:473–80. doi: 10.1111/j.1365-2265.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- Antonijoan RM, Barbanoj MJ, Cordero JA, et al. Pharmacokinetics of a new Autogel formulation of the somatostatin analogue lanreotide after a single subcutaneous dose in healthy volunteers. J Pharm Pharmacol. 2004;56:471–6. doi: 10.1211/0022357023123. [DOI] [PubMed] [Google Scholar]
- Ashwell SG, Bevan JS, Edwards OM, et al. The efficacy and safety of lanreotide Autogel in patients with acromegaly previously treated with octreotide LAR. Eur J Endocrinol. 2004;150:473–80. doi: 10.1530/eje.0.1500473. [DOI] [PubMed] [Google Scholar]
- Astruc B, Marbach P, Bouterfa H, et al. Long-acting octreotide and prolonged-release lanreotide formulations have different pharmacokinetic profiles. J Clin Pharmacol. 2005;45:836–44. doi: 10.1177/0091270005277936. [DOI] [PubMed] [Google Scholar]
- Attanasio R, Baldelli R, Pivonello R, et al. Lanreotide 60 mg, a new long-acting formulation: effectiveness in the chronic treatment of acromegaly. J Clin Endocrinol Metab. 2003b;88:5258–65. doi: 10.1210/jc.2003-030266. [DOI] [PubMed] [Google Scholar]
- Attanasio R, Epaminonda P, Motti E, et al. Gamma-knife radio-surgery in acromegaly: A 4-year follow-up study. J Clin Endocrinol Metab. 2003a;88:3105–12. doi: 10.1210/jc.2002-021663. [DOI] [PubMed] [Google Scholar]
- Ayuk J, Clayton RN, Holder G, et al. Growth hormone and pituitary radiotherapy, but not serum insulin-like growth factor I concentrations predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab. 2004;89:1613–17. doi: 10.1210/jc.2003-031584. [DOI] [PubMed] [Google Scholar]
- Baldelli R, Battista C, Leonetti F, et al. Glucose homeostasis in acromegaly: effects of long-acting somatostatin analogues treatment. Clin Endocrinol (Oxf) 2003;59:492–9. doi: 10.1046/j.1365-2265.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- Baldelli R, Colao A, Razzore P, et al. Two-year follow-up of acromegalic patients treated with slow release lanreotide (30 mg) J Clin Endocrinol Metab. 2000;85:4099–103. doi: 10.1210/jcem.85.11.6948. [DOI] [PubMed] [Google Scholar]
- Baldelli R, Ferretti E, Jaffrain-Rea ML, et al. Cardiac effects of slow-release lanreotide, a slow-release somatostatin analog, in acromegalic patients. J Clin Endocrinol Metab. 1999;84:527–32. doi: 10.1210/jcem.84.2.5467. [DOI] [PubMed] [Google Scholar]
- Barkan AL, Halasz I, Dornfeld KJ, et al. Pituitary irradiation is ineffective in normalizing plasma insulin-like growth factor I in patients with acromegaly. J Clin Endocrinol Metab. 1997;82:3187–91. doi: 10.1210/jcem.82.10.4249. [DOI] [PubMed] [Google Scholar]
- Barkan AL, Lloyd RV, Chandler WF, et al. Preoperative treatment of acromegaly with long-acting somatostatin analog SMS 201–995: shrinkage of invasive pituitary macroadenomas and improved surgical remission rate. J Clin Endocrinol Metab. 1988;76:1040–8. doi: 10.1210/jcem-67-5-1040. [DOI] [PubMed] [Google Scholar]
- Barkan AL, Stred SE, Reno K, et al. Increased growth hormone pulse frequency in acromegaly. J Clin Endocrinol Metab. 1989;69:1225–33. doi: 10.1210/jcem-69-6-1225. [DOI] [PubMed] [Google Scholar]
- Barrande G, Pittino-Lungo M, Coste J, et al. Hormonal and metabolic effects of radiotherapy in acromegaly: long-term results in 128 patients followed in a single center. J Clin Endocrinol Metab. 2000;85:3779–85. doi: 10.1210/jcem.85.10.6870. [DOI] [PubMed] [Google Scholar]
- Bates AS, Van’tHoff W, Jones JM, et al. An audit of outcome of treatment in acromegaly. Q J Med. 1993;86:293–9. [PubMed] [Google Scholar]
- Bates PR, Carson MN, Trainer PJ, et al. Wide variation in surgical outcomes for acromegaly in the UK. Clin Endocrinol (Oxf) 2008;68:136–42. doi: 10.1111/j.1365-2265.2007.03012.x. [DOI] [PubMed] [Google Scholar]
- Beauregard C, Truong U, Hardy J, Serri O. Long-term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol (Oxf) 2003;58:86–91. doi: 10.1046/j.1365-2265.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson BA, Eden S, Ernest I, et al. Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med. Scand. 1988;223:327–35. doi: 10.1111/j.0954-6820.1988.tb15881.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo A, Melmed S. Pasireotide – a somatostatin analog for the potential treatment of acromegaly, neuroendocrine tumors and Cushing’s disease. Drugs. 2007;10:885–95. [PubMed] [Google Scholar]
- Ben-Shlomo A, Melmed S. Acromegaly. Endocrinol Metab N Am. 2008;27:101–22. doi: 10.1016/j.ecl.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan JS. Clinical review: The antitumoral effects of somatostatin analog therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:1856–63. doi: 10.1210/jc.2004-1093. [DOI] [PubMed] [Google Scholar]
- Bevan JS, Newell-Price J, Wass JAH, et al. Home administration of lanreotide Autogel by patients with acromegaly, or their partners, is safe and effective. Clin Endocrinol (Oxford) 2008;68:343–349. doi: 10.1111/j.1365-2265.2007.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier M, Strasburger CJ. Growth hormone assays: current methodologies and their limitations. Pituitary. 2007;10:115–19. doi: 10.1007/s11102-007-0030-1. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Dekker FW, Pereira AM, et al. Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor-I measurements. J Clin Endocrinol Metab. 2004a;89:2789–96. doi: 10.1210/jc.2003-032041. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Pereira AM, Frolich M, et al. Octreotide represses secretory-burst mass and non-pulsatile secretion but does not restore event frequency of orderly GH secretion in acromegaly. Am J Physiol Endocrinol Metab. 2004c;286:E25–E30. doi: 10.1152/ajpendo.00230.2003. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Pereira AM, Neelis KJ, et al. The role of radiotherapy in the management of acromegaly. Expert Rev Endocrinol Metab. 2006;1:449–60. doi: 10.1586/17446651.1.3.449. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Pereira AM, Smit JW, et al. Morbidity after long-term remission for acromegaly: persisting joint-related complaints cause reduced quality of life. J Clin Endocrinol Metab. 2005a;90:2731–9. doi: 10.1210/jc.2004-2297. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Pereira AM, Smit JW, et al. Intravenous octreotide test predicts the long term outcome of treatment with octreotide-long-acting repeatable in active acromegaly. Growth Horm IGF Res. 2005b;15:200–6. doi: 10.1016/j.ghir.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Smit JW, Pereira AM, et al. Acromegaly caused by growth hormone-releasing hormone-producing tumors: long-term observational studies in three patients. Pituitary. 2007;10:237–49. doi: 10.1007/s11102-007-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermasz NR, van Dulken H, Roelfsema F. Direct postoperative and follow-up results of transsphenoidal surgery in 19 acromegalic patients pretreated with octreotide compared to those in untreated matched controls. J Clin Endocrinol Metab. 1999;84:3551–5. doi: 10.1210/jcem.84.10.6027. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, van Dulken H, Roelfsema F. Ten-year follow-up results of transsphenoidal microsurgery in acromegaly. J Clin Endocrinol Metab. 2000a;85:4596–602. doi: 10.1210/jcem.85.12.7042. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, van Dulken H, Roelfsema F. Postoperative radiotherapy in acromegaly is effective in reducing GH concentration to safe levels. Clin Endocrinol (Oxf) 2000b;53:321–7. doi: 10.1046/j.1365-2265.2000.01095.x. [DOI] [PubMed] [Google Scholar]
- Biermasz NR, van Thiel SW, Pereira AM, et al. Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab. 2004b;89:5369–76. doi: 10.1210/jc.2004-0669. [DOI] [PubMed] [Google Scholar]
- Brada M, Ajithkumar TV, Minniti G, et al. Radiosurgery for pituitary adenomas. Clin Endocrinol (Oxf) 2004;61:531–43. doi: 10.1111/j.1365-2265.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- Brada M, Ford D, Ashley S, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992;304:1343–6. doi: 10.1136/bmj.304.6838.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–9. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Bronstein M, Musolino N, Jallad R, et al. Pharmacokinetic profile of lanreotide Autogel in patients with acromegaly after four deep subcutaneous injections of 60, 90 or 120 mg every 28 days. Clin Endocrinol (Oxf) 2005;63:514–19. doi: 10.1111/j.1365-2265.2005.02372.x. [DOI] [PubMed] [Google Scholar]
- Cannavo S, Squadrito S, Curto L, et al. Results of a two-year treatment with slow release lanreotide in acromegaly. Horm Metab Res. 2000;32:224–9. doi: 10.1055/s-2007-978625. [DOI] [PubMed] [Google Scholar]
- Caron P, Beckers A, Cullen DR, et al. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocrinol Metab. 2002;87:99–104. doi: 10.1210/jcem.87.1.8153. [DOI] [PubMed] [Google Scholar]
- Caron P, Bex M, Cullen DR, et al. One-year follow-up of patients with acromegaly treated with fixed or titrated doses of lanreotide Autogel. Clin Endocrinol (Oxf) 2004;60:734–40. doi: 10.1111/j.1365-2265.2004.02045.x. [DOI] [PubMed] [Google Scholar]
- Caron P, Cogne M, Raingeard I, et al. Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clin Endocrinol (Oxf) 2006;64:209–14. doi: 10.1111/j.1365-2265.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- Caron P, Morange-Ramos I, Cogne M, et al. Three year follow-up of acromegalic patients treated with intramuscular slow-release lanreotide. J Clin Endocrinol Metab. 1997;82:18–22. doi: 10.1210/jcem.82.1.3714. [DOI] [PubMed] [Google Scholar]
- Castinetti F, Taieb D, Kuhn JM, et al. Outcome of gamma knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90:4483–8. doi: 10.1210/jc.2005-0311. [DOI] [PubMed] [Google Scholar]
- Cerasi E, Luft R. Insulin response to glucose loading in acromegaly. Lancet. 1964;284:769–71. doi: 10.1016/s0140-6736(64)90556-2. [DOI] [PubMed] [Google Scholar]
- Chanson P, Boerlin V, Ajzenberg C, et al. Comparison of octreotide acetate LAR and lanreotide SR in patients with acromegaly. Clin Endocrinol (Oxf) 2000b;53:577–86. doi: 10.1046/j.1365-2265.2000.01134.x. [DOI] [PubMed] [Google Scholar]
- Chanson P, Borson-Chazot F, Kuhn JM, et al. 2008Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly Clin Endocrinol (Oxf)doi: 10.1111/j.1365–2265.2008.03208.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson P, Leselbaum A, Blumberg J, et al. Efficacy and tolerability of the long-acting somatostatin analog lanreotide in acromegaly. A 12-month multicenter study of 58 acromegalic patients. French Multicenter Study Group on Lanreotide in Acromegaly. Pituitary. 2000a;2:269–76. doi: 10.1023/a:1009961116472. [DOI] [PubMed] [Google Scholar]
- Ciccarelli E, Valetto MR, Vassario E, et al. Hormonal and radiological effects of megavoltage radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Endocrinol Invest. 1993;16:565–72. doi: 10.1007/BF03347671. [DOI] [PubMed] [Google Scholar]
- Clayton RN. Cardiovascular function in acromegaly. Endocr Rev. 2003;24:272–7. doi: 10.1210/er.2003-0009. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. IGF-I assays: current assay methodologies and their limitations. Pituitary. 2007;10:121–8. doi: 10.1007/s11102-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Colao A, Attanasio R, Pivonello R, et al. Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2006b;91:85–92. doi: 10.1210/jc.2005-1208. [DOI] [PubMed] [Google Scholar]
- Colao A, Ferone D, Cappabianca, et al. Effect of octreotide pretreatment on surgical outcome in acromegaly. J Clin Endocrinol Metab. 1997;82:3308–14. doi: 10.1210/jcem.82.10.4283. [DOI] [PubMed] [Google Scholar]
- Colao A, Ferone D, Lastoria S, et al. Prediction of efficacy of octreotide therapy in patients with acromegaly. J Clin Endocrinol Metab. 1996;81:2356–62. doi: 10.1210/jcem.81.6.8964877. [DOI] [PubMed] [Google Scholar]
- Colao A, Ferone D, Marzullo P, et al. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–52. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- Colao A, Marzullo P, Ferone D, et al. Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly. J Endocrinol Invest. 1999;22:40–7. doi: 10.1007/BF03345477. [DOI] [PubMed] [Google Scholar]
- Colao A, Pivonello R, Auriemma RS, et al. Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly : a prospective study in 99 patients. J Clin Endocrinol Metab. 2006a;91:2112–18. doi: 10.1210/jc.2005-2110. [DOI] [PubMed] [Google Scholar]
- Colao A, Pivonello R, Aurriemma RS, et al. Beneficial effect of dose escalation of octreotide-LAR as first-line therapy in patients with acromegaly. Eur J Endocrinol. 2007;157:579–87. doi: 10.1530/EJE-07-0383. [DOI] [PubMed] [Google Scholar]
- Cozzi R, Attanasio R, Montini M, et al. Four-year treatment with octreotide-LAR in 110 acromegalic patients: the predictive value of short-term results by ROC analysis. J Clin Endocrinol Metab. 2003;88:3090–8. doi: 10.1210/jc.2003-030110. [DOI] [PubMed] [Google Scholar]
- Cozzi R, Attanasio R, Lodrini S, et al. Cabergoline addition to depot somatostatin analogues in resistant acromegalic patients: efficacy and lack of predictive value of prolactin status. Clin Endocrinol (Oxf) 2004;61:209–15. doi: 10.1111/j.1365-2265.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- Cozzi R, Barausse M, Ashnaghi D, et al. Failure of radiotherapy in acromegaly. Eur J Endocrinol. 2001;145:717–26. doi: 10.1530/eje.0.1450717. [DOI] [PubMed] [Google Scholar]
- Cozzi R, Barausse M, Sberna M, et al. Lanreotide 60 mg, a longer-acting somatostatin analog: tumor shrinkage and hormonal normalization in acromegaly. Pituitary. 2000;3:231–8. doi: 10.1023/a:1012832230598. [DOI] [PubMed] [Google Scholar]
- Cozzi R, Dallabonzana D, Attanasio R, et al. A comparison between octreotide-LAR and lanreotide-SR in the chronic treatment of acromegaly. Eur J Endocrinol. 1999;141:267–71. doi: 10.1530/eje.0.1410267. [DOI] [PubMed] [Google Scholar]
- Danila DC, Haidar JN, Zhang X, et al. Somatostatin receptor-specific analogs: effects on cell proliferation and growth hormone secretion in human somatotroph tumors. J Clin Endocrinol Metab. 2001;86:2976–81. doi: 10.1210/jcem.86.7.7620. [DOI] [PubMed] [Google Scholar]
- De P, Rees DA, Davies N, et al. Transsphenoidal surgery for acromegaly in Wales: results based on stringent criteria of remission. J Clin Endocrinol Metab. 2003;88:3567–72. doi: 10.1210/jc.2002-021822. [DOI] [PubMed] [Google Scholar]
- De Herder WW, Taal HR, Uitterlinden P, et al. Limited predictive value of an acute test with subcutaneous octreotide for long-term IGF-I normalization with Sandostatin-LAR in acromegaly. Eur J Endocrinol. 2005;153:67–71. doi: 10.1530/eje.1.01935. [DOI] [PubMed] [Google Scholar]
- Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2008;93:61–7. doi: 10.1210/jc.2007-1191. [DOI] [PubMed] [Google Scholar]
- Dowling RH, Hussaini SH, Murphy GM, Besser GM, Wass JAH. Gallstones during octreotide therapy. Metabolism. 1992;41(Suppl 2):22–33. doi: 10.1016/0026-0495(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Epaminonda R, Porretti S, Cappiello V, et al. Efficacy of radiotherapy in normalizing serum IGF-I, acid labile subunit (ALS) and IGFBP-3 levels in acromegaly. Clin Endocrinol (Oxf) 2001;55:183–9. doi: 10.1046/j.1365-2265.2001.01294.x. [DOI] [PubMed] [Google Scholar]
- Ezzat S, Forster MJ, Berchtold P, et al. Acromegaly. Clinical and biochemical features in 500 patients. Medicine (Baltimore) 1994;73:233–40. [PubMed] [Google Scholar]
- Fahlbusch R, Keller B, Ganslandt O, et al. Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol. 2005;153:239–48. doi: 10.1530/eje.1.01970. [DOI] [PubMed] [Google Scholar]
- Feenstra J, de Herder WW, ten Have SMTH, et al. Combined therapy with somatostatin analogues and weekly pegvisomant in active acromegaly. Lancet. 2005;365:63011–15. doi: 10.1016/S0140-6736(05)63011-5. [DOI] [PubMed] [Google Scholar]
- Ferone D, Saveanu A, Culler MD, et al. Novel chimeric somatostatin analogs: facts and perspectives. Eur J Endocrinol. 2007;156:S23–S8. doi: 10.1530/eje.1.02356. [DOI] [PubMed] [Google Scholar]
- Florio T, Thellung S, Corsaro A, et al. Characterization of the intra-cellular mechanisms mediating somatostatin and lanreotide inhibition of DNA synthesis and growth hormone release from dispersed human GH-secreting pituitary adenoma cells in vitro. Clin Endocrinol (Oxf) 2003;59:115–28. doi: 10.1046/j.1365-2265.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- Foss MC, Saad MJ, Paccola GM, et al. Peripheral glucose metabolism in acromegaly. J Clin Endocrinol Metab. 1991;72:1048–53. doi: 10.1210/jcem-72-5-1048. [DOI] [PubMed] [Google Scholar]
- Fougner SL, Borota OC, Berg JP, et al. The clinical response to somatostatin analogues in acromegaly correlates to the somatostatin receptor subtype 2a protein expression of the adenoma. Clin Endocrinol (Oxf) 2008;68:458–65. doi: 10.1111/j.1365-2265.2007.03065.x. [DOI] [PubMed] [Google Scholar]
- Freda PU. How effective are current therapies for acromegaly? Growth Horm IGF Res. 2003;13(Suppl A):S144–S51. doi: 10.1016/s1096-6374(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Freda PU, Katznelson L, van der Lely AJ, et al. Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2005;90:4465–73. doi: 10.1210/jc.2005-0260. [DOI] [PubMed] [Google Scholar]
- Freda PU, Wardlaw SL, Post KD, et al. Long-term endocrinological follow-up evaluation in 115 patients who underwent transsphenoidal surgery for acromegaly. J Neurosurg. 1998;89:353–8. doi: 10.3171/jns.1998.89.3.0353. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Miell JP, Chambers, et al. The nadir growth hormone after an octreotide test dose predicts the long-term efficacy of somatostatin analogue therapy in acromegaly. Clin Endocrinol (Oxf) 2005;620:742–7. doi: 10.1111/j.1365-2265.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- Giusti M, Ciccarelli E, Dallabonzana D, et al. Clinical results of long-term slow-release lanreotide treatment of acromegaly. Eur J Clin Invest. 1997;27:277–84. doi: 10.1046/j.1365-2362.1997.1190659.x. [DOI] [PubMed] [Google Scholar]
- Giustina A, Barkan A, Casanueva FF, et al. Consensus. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526–9. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- Greenman Y, Tordjman K, Kisch E, et al. Relative sparing of anterior pituitary function in patients with growth hormone-secreting macroadenomas: comparison with nonfunctioning macroadenomas. J Clin Endocrinol Metab. 1995;80:1577–83. doi: 10.1210/jcem.80.5.7745003. [DOI] [PubMed] [Google Scholar]
- Gullu S, Keles H, Delibasi T, et al. Remission criteria for the follow-up of patients with acromegaly. Eur J Endocrinol. 2004;150:465–71. doi: 10.1530/eje.0.1500465. [DOI] [PubMed] [Google Scholar]
- Gutt B, Bidlingmaier M, Kretschmar K, et al. Four-year follow-up of acromegalic patients treated with the new long-acting formulation of Lanreotide (Lanreotide Autogel) Exp Clin Endocrinol Diabetes. 2005;113:139–44. doi: 10.1055/s-2005-837520. [DOI] [PubMed] [Google Scholar]
- Hansen I, Isalikian E, Beaufrere B, et al. Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. Am J Physiol. 1986;250:E269–E73. doi: 10.1152/ajpendo.1986.250.3.E269. [DOI] [PubMed] [Google Scholar]
- Harris PE, D’Souza G, Good AJ, et al. Treatm ent with pegvisomant alone compared to combination therapy with pegvisomant/octreotide LAR in acromegaly. Abstract OR 53–3. The Endocrine Society Annual Meeting; Toronto, Ontario, Canada. June 2–5, 2007.2007. [Google Scholar]
- Heron I, Thomas F, Dero M, et al. Pharmacokinetics and efficacy of a long-acting formulation of the new somatostatin analog BIM 23014 in patients with acromegaly. J Clin Endocrinol Metab. 1993;76:721–7. doi: 10.1210/jcem.76.3.8095269. [DOI] [PubMed] [Google Scholar]
- Ho KY, Weisberger AJ. Characterization of 24-hour growth hormone secretion in acromegaly: implications for diagnosis and therapy. Clin Endocrinol (Oxf) 1994;41:75–83. doi: 10.1111/j.1365-2265.1994.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Hofland LJ, Lamberts SWJ. The Pathophysiological Consequences of Somatostatin Receptor Internalization and Resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- Holdaway IM. Excess mortality in acromegaly. Horm Res. 2007;(Suppl 5):166–72. doi: 10.1159/000110617. [DOI] [PubMed] [Google Scholar]
- Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–74. doi: 10.1210/jc.2003-031199. [DOI] [PubMed] [Google Scholar]
- Hradec J, Krai J, Janota T, et al. Regression of acromegalic left ventricular hypertrophy after lanreotide (a slow-release somatostatin analog) Am J Cardiol. 1999;83:1506–9. doi: 10.1016/s0002-9149(99)00135-6. [DOI] [PubMed] [Google Scholar]
- Hua SC, Yan YH, Chang TC. Associations of remission status and lanreotide treatment with quality of life in patients with treated acromegaly. Eur J Endocrinol. 2006;155:831–7. doi: 10.1530/eje.1.02292. [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Barkan AL. Treatment of acromegaly with dopamine agonists. Endocrinol Metab Clin North Am. 1992;21:713–35. [PubMed] [Google Scholar]
- Jenkins PJ, Besser M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab. 2001;86:2935–41. doi: 10.1210/jcem.86.7.7634. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Chowdrey HS, Thomas F, et al. Pharmacokinetics and efficacy of the long-acting somatostatin analogue somatuline in acromegaly. Eur J Endocrinol. 1994;130:229–34. doi: 10.1530/eje.0.1300229. [DOI] [PubMed] [Google Scholar]
- Jørgensen JOL, Feldt-Rasmusen U, Frystyk J, et al. Cotreatment of acromegaly with a somatostatin analog and a growth hormone receptor antagonist. J Clin Endocrinol Metab. 2005;90:5627–31. doi: 10.1210/jc.2005-0531. [DOI] [PubMed] [Google Scholar]
- Kaltsas GA, Isidori AM, Florakis D, et al. Predictors of outcome of surgical treatment in acromegaly and the value of the mean growth hormone day curve in assessing postoperative disease activity. J Clin Endocrinol Metab. 2001;86:1645–52. doi: 10.1210/jcem.86.4.7398. [DOI] [PubMed] [Google Scholar]
- Karavitaki N, Botusan I, Radian S, et al. The value of an acute octreotide suppression test in predicting long-term responses to depot somatostatin analogues in patients with active acromegaly. Clin Endocrinol (Oxf) 2005;62:282–8. doi: 10.1111/j.1365-2265.2004.02191.x. [DOI] [PubMed] [Google Scholar]
- Karavitaki N, Turner HE, Adams CB, et al. 2007Surgical debulking of pituitary macroadenomas causing acromegaly improves control by lanreotide Clin Endocrinol (Oxf)doi: 10.1111/j.1365–2265.2007.03139.x [DOI] [PubMed] [Google Scholar]
- Kasayama S, Otsuki M, Tagaki M, et al. Impaired beta-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clin Endocrinol (Oxf) 2000;52:549–55. doi: 10.1046/j.1365-2265.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen-Makelin R, Sane T, Reunanen A, et al. A nationwide survey of mortality in acromegaly. J Clin Endocrinol Metab. 2005;90:4081–6. doi: 10.1210/jc.2004-1381. [DOI] [PubMed] [Google Scholar]
- Kendall-Taylor P, Miller M, Gebbie J, et al. Long-acting octreotide LAR compared with lanreotide SR in the treatment of acromegaly. Pituitary. 2000;3:61–5. doi: 10.1023/a:1009997506216. [DOI] [PubMed] [Google Scholar]
- Krantic S, Goddard I, Saveanu A, et al. Novel modalities of somatostatin actions. Eur J Endocrinol. 2004;151:643–55. doi: 10.1530/eje.0.1510643. [DOI] [PubMed] [Google Scholar]
- Kreutzer J, Vance ML, Lopes MB, et al. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86:4072–7. doi: 10.1210/jcem.86.9.7819. [DOI] [PubMed] [Google Scholar]
- Koop BL, Harris AG, Ezzat S. Effect of octreotide on glucose tolerance in acromegaly. Eur J Endocrinol. 1994;130:581–6. doi: 10.1530/eje.0.1300581. [DOI] [PubMed] [Google Scholar]
- Kristof RA, Stoffel-Wagner B, Klingmuller D, et al. Does octreotide treatment improve the surgical results of macroadenomas in acromegaly? A randomized study. Acta Neurochirg. 1999;141:399–405. doi: 10.1007/s007010050316. [DOI] [PubMed] [Google Scholar]
- Kuhn JM, Basin C, Mollard M, et al. Effects of the new somatostatin analogue (BIM 23014) on blood glucose homeostasis in normal men. Eur J Clin Invest. 1992;22:793–9. doi: 10.1111/j.1365-2362.1992.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Kuhn JM, Legrand A, Ruiz JM, et al. Pharmacokinetic and pharmacodynamic properties of a long-acting formulation of the new somatostatin analogue, lanreotide, in normal healthy volunteers. Br J Clin Pharmacol. 1994;38:213–19. doi: 10.1111/j.1365-2125.1994.tb04344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts SW, Van der Lely AJ, De Herder WW, et al. Octreotide. N Engl J Med. 1996;334:246–54. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- Landolt AM, Haller D, Lomax N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg. 1998;88:1002–8. doi: 10.3171/jns.1998.88.6.1002. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, et al. The somatomedin hypothesis. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Loeper S, Ezzat S. Acromegaly: Re-thinking the cancer risk. Rev Endocr Metab Disord. 2008;9:41–58. doi: 10.1007/s11154-007-9063-z. [DOI] [PubMed] [Google Scholar]
- Long H, Beauregard H, Somma M, et al. Surgical outcome after repeated transsphenoidal surgery in acromegaly. J Neurosurg. 1996;85:239–47. doi: 10.3171/jns.1996.85.2.0239. [DOI] [PubMed] [Google Scholar]
- Lucas T, Astorga R, Catala M, et al. Preoperative lanreotide treatment for GH-secreting pituitary adenomas: effect on tumour volume and predictive factors of significant tumour shrinkage. Clin Endocrinol (Oxf) 2003;58:471–81. doi: 10.1046/j.1365-2265.2003.01741.x. [DOI] [PubMed] [Google Scholar]
- Lucas T, Astorga R, Spanish-Portuguese Multicentre Autogel Study Group on Acromegaly Efficacy of lanreotide Autogel administered every 4–8 weeks in patients with acromegaly previously responsive to lanreotide microparticles 30 mg: a phase III trial. Clin Endocrinol (Oxf) 2006;65:320–6. doi: 10.1111/j.1365-2265.2006.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke DK, Abe T. Transsphenoidal microsurgery for newly diagnosed acromegaly: a personal view after more than 1000 operations. Neuroendocrinology. 2006;83:230–9. doi: 10.1159/000095533. [DOI] [PubMed] [Google Scholar]
- Mahmoud-Ahmed AS, Suh JH, Mayberg MR. Gamma knife radio-surgery in the management of patients with acromegaly: a review. Pituitary. 2001;4:223–30. doi: 10.1023/a:1020794329975. [DOI] [PubMed] [Google Scholar]
- Maison P, Tropeano A-I, Macquin-Mavier I, et al. Impact of somatostatin analogs on the heart in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2007;92:1743–7. doi: 10.1210/jc.2006-2547. [DOI] [PubMed] [Google Scholar]
- Marcou Y, Plowman PN. Stereotactic radiosurgery for pituitary adenomas. Trends Endocrinol Metab. 2000;11:132–7. doi: 10.1016/s1043-2760(00)00242-3. [DOI] [PubMed] [Google Scholar]
- Marek J, Hana V, Krsek M, et al. Long-term treatment of acromegaly with the slow-release somatostatin analogue lanreotide. Eur J Endocrinol. 1994;131:20–26. doi: 10.1530/eje.0.1310020. [DOI] [PubMed] [Google Scholar]
- Matta MP, Couture E, Cazals L, et al. Impaired quality of life of patients with acromegaly: control of GH/IGF-I excess improves psychological subscale appearance. Eur J Endocrinol. 2008;158:305–10. doi: 10.1530/EJE-07-0697. [DOI] [PubMed] [Google Scholar]
- Melmed S. Acromegaly and cancer: not a problem? J Clin Endocrinol Metab. 2001;86:2929–34. doi: 10.1210/jcem.86.7.7635. [DOI] [PubMed] [Google Scholar]
- Melmed S. Acromegaly. N Engl J Med. 2006;355:2558–73. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- Melmed S, Casanueva FF, Cavagni T, et al. Consensus. Guidelines for acromegaly management. J Clin Endocrinol Metab. 2002;87:4054–8. doi: 10.1210/jc.2002-011841. [DOI] [PubMed] [Google Scholar]
- Melmed S, Sternberg R, Cool D, et al. Clinical review: A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:4405–10. doi: 10.1210/jc.2004-2466. [DOI] [PubMed] [Google Scholar]
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA) Eur J Endocrinol. 2004;151:439–46. doi: 10.1530/eje.0.1510439. [DOI] [PubMed] [Google Scholar]
- Minniti G, Jaffrain-Rea ML, Osti M, et al. The long-term efficacy of conventional radiotherapy in patients with GH-secreting pituitary adenomas. Clin Endocrinol (Oxf) 2005;62:210–16. doi: 10.1111/j.1365-2265.2005.02199.x. [DOI] [PubMed] [Google Scholar]
- Morange I, De Boisvilliers F, Chanson P, et al. Slow release lanreotide treatment in acromegalic patients previously normalized by octreotide. J Clin Endocrinol Metab. 1994;79:145–51. doi: 10.1210/jcem.79.1.8027218. [DOI] [PubMed] [Google Scholar]
- Murray RD, Kim K, Ren S-G. Central and peripheral actions of soma-tostatin on growth hormone-IGF-I axis. J Clin Invest. 2004;114:349–56. doi: 10.1172/JCI19933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers JCM, van Aken MO, Janssen JAM, et al. Long-term efficacy and safety of combined treatment of somatostatin analogs and pegvisomant in acromegaly. J Clin Endocrinol Metab. 2007;92:4598–601. doi: 10.1210/jc.2007-1234. [DOI] [PubMed] [Google Scholar]
- Newman CB, Melmed S, Snyder PJ, et al. Safety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients-a clinical research center study. J Clin Endocrinol Metab. 1995;80:2768–75. doi: 10.1210/jcem.80.9.7673422. [DOI] [PubMed] [Google Scholar]
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol. 2005;152:379–87. doi: 10.1530/eje.1.01863. [DOI] [PubMed] [Google Scholar]
- Olias G, Viollet C, Kusserow H, et al. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–91. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- Orme S, McNally RJ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. J Clin Endocrinol Metab. 1998;83:2730–4. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- Pereira AM, Biermaz NR, Roelfsema F, et al. Pharmacologic therapies for acromegaly. Treat Endocrinol. 2005;4:43–53. doi: 10.2165/00024677-200504010-00005. [DOI] [PubMed] [Google Scholar]
- Pereira AM, Van Thiel SW, Lindner JR, et al. Increased prevalence of regurgitant valvular heart disease in acromegaly. J Clin Endocrinol Metab. 2004;89:71–5. doi: 10.1210/jc.2003-030849. [DOI] [PubMed] [Google Scholar]
- Pokrajac A, Claridge AG, Abdoul Shakoor SK, et al. The octreotide test dose is not a reliable predictor of the subsequent response to somatostatin analogue therapy in patients with acromegaly. Eur J Endocrinol. 2006;154:267–74. doi: 10.1530/eje.1.02073. [DOI] [PubMed] [Google Scholar]
- Powell JS, Wardlaw SL, Post KD, et al. Outcome of radiotherapy for acromegaly using normalization of insulin-like growth factor I to define cure. J Clin Endocrinol Metab. 2000;85:2068–71. doi: 10.1210/jcem.85.5.6586. [DOI] [PubMed] [Google Scholar]
- Razzore P, Colao A, Baldelli R, et al. Comparison of six months therapy with octreotide versus lanreotide in acromegalic patients: a retrospective study. Clin Endocrinol (Oxf) 1999;51:159–64. doi: 10.1046/j.1365-2265.1999.00812.x. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Shalet SM. Acromegaly and colorectal cancer: risk assessment should be based on population-based studies. J Clin Endocrinol Metab. 2002;87:1909. doi: 10.1210/jcem.87.4.8369. [DOI] [PubMed] [Google Scholar]
- Ritchie CM, Atkinson AB, Kennedy AL, et al. Ascertainment and natural history of treated acromegaly in Northern Ireland. Ulster Med J. 1990;59:55–62. [PMC free article] [PubMed] [Google Scholar]
- Roberts BK, Ouyang DL, Shivanand PL, et al. Efficacy and safety of cyberknife radiosurgery for acromegaly. Pituitary. 2007;10:19–25. doi: 10.1007/s11102-007-0004-3. [DOI] [PubMed] [Google Scholar]
- Roelfsema F, Biermasz NR, Pereira AM, et al. Nanomedicines in the treatment of acromegaly: focus on pegvisomant. Int J Nanomedicine. 2006;1:385–98. doi: 10.2147/nano.2006.1.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchi CL, Boschetti M, Degli Uberti EC, et al. Efficacy of a slow-release formulation of lanreotide (Autogel) 120 mg in patients with acromegaly previously treated with octreotide long acting release (LAR): an open, multicentre longitudinal study. Clin Endocrinol (Oxf) 2007;67:512–19. doi: 10.1111/j.1365-2265.2007.02917.x. [DOI] [PubMed] [Google Scholar]
- Ronchi CL, Orsi E, Giavoli C, et al. Evaluation of insulin resistance in acromegalic patients before and after treatment with somatostatin analogues. J Endocrinol Invest. 2003;26:533–8. doi: 10.1007/BF03345216. [DOI] [PubMed] [Google Scholar]
- Ronchi CL, Rizzo E, Lania AG, et al. Preliminary data on biochemical remission of acromegaly after somatostatin analogs withdrawal. Eur J Endocrinol. 2008;158:19–25. doi: 10.1530/EJE-07-0488. [DOI] [PubMed] [Google Scholar]
- Rowles SV, Prieto L, Badia X, et al. Quality of life (QOL) in patients with acromegaly is severely impaired: use of a novel measure of QOL: acromegaly quality of life questionnaire. J Clin Endocrinol Metab. 2005;90:3337–41. doi: 10.1210/jc.2004-1565. [DOI] [PubMed] [Google Scholar]
- Schade R, Andersohn F, Suisse S, et al. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356:29–38. doi: 10.1056/NEJMoa062222. [DOI] [PubMed] [Google Scholar]
- Schloffer H. Erfolgreiche Operation eines Hypophysentumors auf nasalem Wege. Wien Klin Wochenschr. 1907;20:621–4. [Google Scholar]
- Schreiber I, Buchfelder M, Droste M, et al. Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol. 2007;156:75–82. doi: 10.1530/eje.1.02312. [DOI] [PubMed] [Google Scholar]
- Serri O, Brazeau P, Kachra Z, et al. Octreotide inhibits insulin-like growth factor-I hepatic gene expression in hypophysectomized rat: evidence for a direct and indirect mechanism of action. Endocrinology. 1992;130:1816–21. doi: 10.1210/endo.130.4.1547711. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Niranjan A, Sheehan JM, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and the role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678–91. doi: 10.3171/jns.2005.102.4.0678. [DOI] [PubMed] [Google Scholar]
- Shimon I, Cohen ZR, Ram Z, et al. Transsphenoidal surgery for acromegaly: endocrinological follow-up of 98 patients. Neurosurgery. 2001;48:1239–43. doi: 10.1097/00006123-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principle source of IGF-I in blood but it is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–92. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonino N, Scarpa E, Paoletta A, et al. Slow-release lanreotide treatment in acromegaly: effects on quality of life. Psychother Psychosom. 1999;68:165–7. doi: 10.1159/000012326. [DOI] [PubMed] [Google Scholar]
- Sonksen PH, Greenwood FC, Ellis JP, et al. Changes of carbohydrate tolerance in acromegaly with progress of the disease and in response to treatment. J Clin Endocrinol Metab. 1967;27:1418–30. doi: 10.1210/jcem-27-10-1418. [DOI] [PubMed] [Google Scholar]
- Steffin B, Gutt B, Bidlingmaier M, et al. Effects of the long-acting somatostatin analogue Lanreotide Autogel on glucose tolerance and insulin resistance in acromegaly. Eur J Endocrinol. 2006;155:73–8. doi: 10.1530/eje.1.02185. [DOI] [PubMed] [Google Scholar]
- Stevenaert A, Beckers A. Presurgical octreotide treatment in acromegaly. Metabolism. 1996;45(Suppl 1):72–4. doi: 10.1016/s0026-0495(96)90088-8. [DOI] [PubMed] [Google Scholar]
- Suliman M, Jenkins R, Ross R, et al. Long-term treatment of acromegaly with the somatostatin analogue SR-lanreotide. J Endocrinol Invest. 1999;22:409–18. doi: 10.1007/BF03343583. [DOI] [PubMed] [Google Scholar]
- Swaeringen B, Barker FG, Katznelson L, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83:3419–26. doi: 10.1210/jcem.83.10.5222. [DOI] [PubMed] [Google Scholar]
- Taboada GF, Luque RM, Neto LV, et al. Quantitative analysis of somatostatin receptor subtypes (1–5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur J Endocrinol. 2008;158:295–303. doi: 10.1530/EJE-07-0562. [DOI] [PubMed] [Google Scholar]
- Thalassinos NC, Tsagarakis S, Ioannides G, et al. Megavoltage pituitary irradiation lowers, but seldom leads to safe GH levels in acromegaly : a long-term follow-up study. Eur J Endocrinol. 1998;138:160–3. doi: 10.1530/eje.0.1380160. [DOI] [PubMed] [Google Scholar]
- Thomale UW, Stover JF, Unterberg AW. The use of neuronavigation in transnasal transsphenoidal pituitary surgery. Zentralbl Neurochir. 2005;66:126–32. doi: 10.1055/s-2005-836602. [DOI] [PubMed] [Google Scholar]
- Trainer PJ, Drake WM, Katznelson L, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342:1171–7. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- Turner HE, Vadivale A, Keenan J, Wass JA. A comparison of lanreotide and octreotide LAR for treatment of acromegaly. Clin Endocrinol (Oxf) 1999;51:275–280. doi: 10.1046/j.1365-2265.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Valéry C, Paternostre M, Robert B, et al. Biomimetic organization: Octapeptide self-assembly into nanotubes of viral capsid-like dimension. Proc Natl Acad Sci USA. 2003;100:10258–62. doi: 10.1073/pnas.1730609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valéry C, Pouget E, Pandit A, et al. Molecular origin of the self-assembly of lanreotide into nanotubes: a mutational approach. Biophys J. 2008;94:1782–95. doi: 10.1529/biophysj.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg G, Frolich M, Veldhuis JD, et al. Growth hormone secretion in recently operated acromegalic patients. J Clin Endocrinol Metab. 1994;79:1706–15. doi: 10.1210/jcem.79.6.7989479. [DOI] [PubMed] [Google Scholar]
- Van den Berg G, Pincus SM, Frolich M, et al. Reduced disorderliness of growth hormone release in biochemically inactive acromegaly after pituitary surgery. Eur J Endocrinol. 1998;138:164–9. doi: 10.1530/eje.0.1380164. [DOI] [PubMed] [Google Scholar]
- Van der Hoek J, Lamberts SW, Hofland LJ. The role of somatostatin analogs in Cushing’s disease. Pituitary. 2004;7:257–64. doi: 10.1007/s11102-005-1404-x. [DOI] [PubMed] [Google Scholar]
- Van der Hoek J, van der Lely AJ, Feelders RA, et al. The somatostatin analogue SOM230, compared with octreotide, induces differential effects in several metabolic pathways in acromegalic patients. Clin Endocrinol (Oxf) 2005;63:176–84. doi: 10.1111/j.1365-2265.2005.02322.x. [DOI] [PubMed] [Google Scholar]
- Van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–9. doi: 10.1016/s0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- Van Thiel SW, Bax JJ, Biermasz NR, et al. Persistent diastolic dysfunction despite successful long-term octreotide treatment in acromegaly. Eur J Endocrinol. 2005;153:231–8. doi: 10.1530/eje.1.01955. [DOI] [PubMed] [Google Scholar]
- Van Thiel SW, Romijn JA, Biermasz NR, et al. Octreotide long-acting repeatable and lanreotide Autogel are equally effective in controlling growth hormone secretion in acromegalic patients. Eur J Endocrinol. 2004;150:489–95. doi: 10.1530/eje.0.1500489. [DOI] [PubMed] [Google Scholar]
- Verhelst JA, Pedroncelli AM, Abs R, et al. Slow-release lanreotide in the treatment of acromegaly: a study in 66 patients. Eur J Endocrinol. 2000;143:577–84. doi: 10.1530/eje.0.1430577. [DOI] [PubMed] [Google Scholar]
- Wasko R, Jankowska A, Kotwicka M, et al. Effects of treatment with somatostatin analogues on the occurrence of apoptosis in somatotropinomas. Neuro Endocrinol Lett. 2003;24:334–8. [PubMed] [Google Scholar]
- Wass JA. Radiotherapy in acromegaly: a protagonist’s viewpoint. Clin Endocrinol (Oxf) 2003;58:128–31. doi: 10.1046/j.1365-2265.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- Wass JA, Cudworth AG, Bottazzo GF, et al. An assessment of glucose intolerance in acromegaly and its response to medical treatment. Clin Endocrinol (Oxf) 1980;12:53–59. doi: 10.1111/j.1365-2265.1980.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Wright AD, Hill DM, Lowy C, et al. Mortality in acromegaly. Q J Med. 1970;39:1–16. [PubMed] [Google Scholar]
- Zatelli MC, Ambrosio MR, Bondanelli M, et al. Control of pituitary adenoma cell proliferation by somatostatin analogs, dopamine agonists and novel chimeric compounds. Eur J Endocrinol. 2007;156:S29–S35. doi: 10.1530/eje.1.02352. [DOI] [PubMed] [Google Scholar]