Abstract
Aims
The aim of this study was to evaluate the effect of hormone replacement therapy (HRT) on coronary vasomotor function in post-menopausal women (PM) with medically treated cardiovascular risk factors (RFs) in a cross-sectional and a longitudinal follow-up (FU) study.
Methods and results
Myocardial blood flow (MBF) response to cold pressor testing (CPT) and during pharmacologically induced hyperaemia was measured with positron emission tomography in pre-menopausal women (CON), in PM with HRT and without HRT, and repeated in PM after a mean FU of 24 ± 14 months. When compared with CON at baseline, the endothelium-related change in MBF (ΔMBF) to CPT progressively declined in PM with HRT and without HRT (0.35 ± 0.23 vs. 0.24 ± 0.20 and 0.16 ± 0.12 mL/g/min; P = 0.171 and P = 0.021). In PM without HRT and in those with HRT at baseline but with discontinuation of HRT during FU, the endothelium-related ΔMBF to CPT was significantly less at FU than at baseline (0.05 ± 0.19 vs. 0.16 ± 0.12 and −0.03 ± 0.14 vs. 0.25 ± 0.18 mL/g/min; P = 0.023 and P = 0.001), whereas no significant change was observed in PM with HRT (0.19 ± 0.22 vs. 0.23 ± 0.22 mL/g/min; P = 0.453). Impaired hyperaemic MBFs when compared with CON were not significantly altered from those at baseline exam.
Conclusion
Long-term administration of oestrogen may contribute to maintain endothelium-dependent coronary function in PM with medically treated cardiovascular RFs.
Keywords: Cardiovascular disease prevention, Coronary circulation, Oestrogen, Endothelium, Post-menopausal women, Positron emission tomography
Introduction
Women with early menopause are at increased risk of developing cardiovascular disease.1 The exact mechanisms underlying the increased risk in developing cardiovascular disease in post-menopausal women (PM) remain poorly understood. Post-menopausal women commonly present higher total cholesterol, LDL cholesterol, triglycerides, and lower HDL levels as well as increases in body weight and arterial hypertension.1 The latter changes in cardiovascular risk profile, which may accompany the menopause, have been suggested as underlying mechanism for the increased manifestation of cardiovascular disease.2 Deprivation of endogenous atheroprotective oestrogen can be assumed as another important cause for the increased risk for cardiovascular disease in PM.2 Observational studies1,3 have demonstrated a lower cardiovascular event rate in PM, who were on hormone replacement therapy (HRT), than in those without HRT. Results of the use of HRT with oestrogen to improve the cardiovascular clinical outcome in PM, however, are rather conflicting.1,4,5 The latter disconsonant findings may be reconciled by the emerging ‘timing hypothesis’ for HRT, which favours the concept, that beneficial effects of HRT in preventing the coronary atherosclerotic process may only manifest, when HRT is commenced in younger PM (50–59 years old) or within 10 years after menopause.2,4 The latter concept, however, has also been challenged by an observational cohort study in PM.6 As it was observed, overall there was no association between HRT and the occurrence of myocardial infarction, whereas an increased risk of myocardial infarction was noted in a subgroup of younger women between 51 and 54 years of age.6
The normal function of the vascular endothelium exerts numerous nitric-oxide mediated antiatherosclerotic and antithrombotic effects, while a dysfunctional endothelium predisposes to the development of coronary artery disease (CAD) and future cardiovascular events.7 Previous investigations from our institution8 have demonstrated that long-term HRT restored endothelial function of the coronary arteriolar vessels in PM without coronary risk factors (RFs), while it was not observed in those PM with RFs. Given that medical treatment of coronary RFs, i.e. with statins or angiotensin-converting enzyme inhibitors, commonly improves endothelium-dependent vasomotor dysfunction,7 it remains uncertain whether HRT in PM with medically treated RFs might still exert an additional beneficial effect on coronary vasomotor function. Accordingly, we aimed to evaluate the effect of long-term HRT on vasomotor function of the coronary microcirculation in PM with medically treated RFs in an observational cross-sectional and a longitudinal follow-up (FU) study.
Methods
Study design
In a prospective, observational cross-sectional baseline and longitudinal FU study, vasomotor function of the coronary microcirculation was assessed by measuring myocardial blood flow (MBF) with positron emission tomography (PET) at rest, and its responses to sympathetic stimulation with cold pressor testing (CPT), and during pharmacologic vasodilation in 48 PM (Table 1). Post-menopausal women and 12 healthy pre-menopausal women had been recruited by flyers and newspaper advertisement. Among 53 PM with medically treated cardiovascular RFs who underwent PET baseline examination, 48 repeated the PET exam for the observational FU study. Thus, there were five dropouts in the FU exams and these were not included in the final analysis. For the cross-sectional baseline analysis, PET-measured MBFs of 12 healthy pre-menopausal women served as reference to define the range of normal coronary vasomotor function, and they were compared with both groups of PM with and without HRT (Table 1). For the longitudinal observational FU study, MBF measurements were repeated in all PM after an FU period of at least 12 months (median FU: 22 months and inter-quartile range, IQR: 14–28 months) (Table 2). All vasoactive medications such calcium channel blockers, ACE inhibitors, long-acting nitrates, or β-adrenergic blockers were withheld for at least 48 h prior to the PET studies. PM were followed-up prospectively by the status of HRT at baseline. In the group with HRT, however, eight women discontinued HRT during the observational FU as they became aware of the concomitant minor risk for breast cancer. In addition, another four women decided to drop HRT during the FU due to the controversy about a possible increased risk for cardiovascular events owing to HRT. Consequently, PM were finally subgrouped according to the HRT status both at baseline and FU: Group 1 (n = 18) on HRT at both baseline and FU, Group 2 without HRT (n = 18) at baseline and FU, and Group 3 on HRT at baseline but not at FU (n = 12) (Table 2). The study was approved by the UCLA Institutional Review Board, and each participant signed the approved informed consent form.
Table 1.
Cross-sectional investigation: clinical characteristics, myocardial blood flow, and haemodynamics during baseline positron emission
| Young women | PM with HRT | P-value | PM without HRT | P-value | |
|---|---|---|---|---|---|
| Numbers (n) | 12 | 30 | — | 18 | — |
| Age (years) | 22 ± 4 | 59 ± 7 | 0.0001 | 59 ± 8 | 0.0001 |
| BMI (kg/m2) | 21 ± 1.9 | 27 ± 4.9 | 0.0001 | 30 ± 3.9 | 0.0001 |
| Years of menopause | — | 13 ± 6 | — | 11 ± 6 | — |
| Duration of HRT (years) | — | 8 ± 5 | — | — | — |
| Risk factors | |||||
| Hypertension, n | 0 | 9 (30%) | — | 8 (44%) | — |
| Smoking, n | 0 | 6 (20%) | — | 3 (17%) | — |
| Hypercholesterolaemia, n | 0 | 14 (47%) | — | 9 (50%) | — |
| Obesity, n | 0 | 7 (23%) | — | 9 (50%) | — |
| Family history of CAD, n | 0 | 11 (37%) | — | 4 (22%) | — |
| Diabetes mellitus, n | 0 | 1 (3%) | — | 3 (17%) | — |
| Fasting plasma concentrations | |||||
| Oestrone (ng/dL) | 6.04 ± 3.52 | 9.23 ± 7.81 | 0.186 | 3.02 ± 1.59 | 0.029 |
| Oestradiol (ng/dL) | 117 ± 104 | 34.31 ± 16.60 | 0.0001 | 17.80 ± 6.70 | 0.001 |
| Lipid status | |||||
| Cholesterol (mg/dL) | 146 ± 17 | 207 ± 38 | 0.0001 | 211 ± 57 | 0.001 |
| LDL (mg/dL) | 76 ± 17 | 116 ± 39 | 0.0001 | 135 ± 27 | 0.0001 |
| HDL (mg/dL) | 59 ± 8 | 61 ± 15 | 0.021 | 51 ± 8 | 0.0001 |
| TG (mg/dL) | 58 ± 27 | 156 ± 68 | 0.0001 | 155 ± 107 | 0.007 |
| Glucose (mg/dL) | 83 ± 16 | 85 ± 18 | 0.766 | 95 ± 23 | 0.156 |
| MBF (mL/g/min) | |||||
| MBF at rest | 0.66 ± 0.14 | 0.68 ± 0.15 | 0.687 | 0.73 ± 0.18 | 0.213 |
| MBF during CPT | 1.00 ± 0.24 | 0.92 ± 0.30 | 0.334 | 0.89 ± 0.17 | 0.191 |
| ΔMBF to CPT from rest | 0.35 ± 0.23 | 0.24 ± 0.20 | 0.171 | 0.16 ± 0.12 | 0.021 |
| NMBF during CPT | 1.02 ± 0.20 | 0.86 ± 0.27 | 0.055 | 0.79 ± 0.15 | 0.004 |
| MBF during hyperaemia | 2.47 ± 0.51 | 1.66 ± 0.29 | 0.0001 | 1.92 ± 0.41 | 0.008 |
| MFR | 3.74 ± 0.61 | 2.56 ± 0.62 | 0.0001 | 2.55 ± 0.41 | 0.0001 |
| Haemodynamics | |||||
| Rest-HR (b.p.m.) | 62 ± 10 | 66 ± 10 | 0.295 | 67 ± 9 | 0.172 |
| CPT-HR (b.p.m.) | 81 ± 17 | 70 ± 10 | 0.017 | 73 ± 8 | 0.103 |
| Hyperaemia-HR (b.p.m.) | 101 ± 13 | 89 ± 14 | 0.020 | 88 ± 10 | 0.015 |
| Rest-SBP (mmHg) | 101 ± 11 | 126 ± 19 | 0.0001 | 132 ± 17 | 0.0001 |
| CPT-SBP (mmHg) | 124 ± 14 | 154 ± 25 | 0.0001 | 157 ± 26 | 0.0001 |
| Hyperaemia-SBP (mmHg) | 107 ± 12 | 127 ± 19 | 0.003 | 129 ± 20 | 0.001 |
| Rest RPP | 6275 ± 1094 | 8309 ± 1899 | 0.0001 | 8883 ± 1717 | 0.0001 |
| ΔRPP to CPT | 3748 ± 1969 | 2504 ± 1351 | 0.063 | 2588 ± 1859 | 0.120 |
Numbers, n (%); PM, post-menopausal women; HRT, hormone replacement therapy; CAD, coronary artery disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; CPT, cold pressor test; MBF, myocardial blood flow; MFR, myocardial flow reserve; NMBF, normalized MBF; HR, heart rate; SBP, systolic blood pressure; RPP, rate-pressure product (HR × SBP). P-values vs. young women (t-test for independent samples).
Table 2.
Longitudinal follow-up study: clinical characteristics, myocardial blood flow, and haemodynamics during positron emission tomography at baseline and follow-up
| Group 1 |
Group 2 |
Group 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | FU | P-value | Baseline | FU | P-value | Baseline | FU | P-value | |
| Numbers (n) | 18 | 18 | — | 18 | 18 | — | 12 | 12 | — |
| Age (years) | 57 ± 6 | 59 ± 7 | 0.0001 | 59 ± 8 | 61 ± 6 | 0.0001 | 61 ± 8 | 63 ± 7 | 0.0001 |
| BMI (kg/m2) | 27 ± 5.5 | 27 ± 6.2 | 0.610 | 30 ± 3.9 | 30 ± 4.3 | 0.744 | 26 ± 3.85 | 26 ± 2.95 | 0.835 |
| Risk factors | |||||||||
| Hypertension, n | 6 (33%) | — | — | 8 (44%) | — | — | 3 (25%) | — | — |
| Smoking, n | 3 (17%) | — | — | 3 (17%) | — | — | 3 (25%) | — | — |
| Hypercholesterolaemia, n | 10 (56%) | — | — | 9 (50%) | — | — | 4 (33%) | — | — |
| Obesity, n | 5 (28%) | — | — | 9 (50%) | — | — | 2 (17%) | — | — |
| Family history of CAD, n | 7 (39%) | — | — | 4 (22%) | — | — | 4 (33%) | — | — |
| Diabetes mellitus, n | 0 (0%) | — | — | 3 (17%) | — | — | 1 (8%) | — | — |
| Fasting plasma concentrations | |||||||||
| Oestrone (ng/dL) | 10.66 ± 8.48 | 7.69 ± 5.36 | 0.095 | 3.02 ± 1.59 | 3.24 ± 1.90 | 0.930 | 7.16 ± 6.55 | 1.61 ± 1.17 | 0.025 |
| Oestradiol (ng/dL) | 37.14 ± 17.58 | 29.80 ± 14.44 | 0.125 | 17.80 ± 6.70 | 19.25 ± 6.54 | 0.238 | 31.55 ± 16.09 | 15.55 ± 0.93 | 0.019 |
| Lipid status | |||||||||
| Cholesterol (mg/dL) | 212 ± 48 | 213 ± 40 | 0.980 | 211 ± 57 | 214 ± 32 | 0.946 | 201 ± 19 | 204 ± 36 | 0.787 |
| LDL (mg/dL) | 123 ± 47 | 128 ± 35 | 0.505 | 135 ± 27 | 136 ± 26 | 0.313 | 108 ± 17 | 116 ± 33 | 0.432 |
| HDL (mg/dL) | 60 ± 17 | 56 ± 12 | 0.507 | 51 ± 8 | 51 ± 8 | 0.935 | 63 ± 11 | 64 ± 12 | 0.451 |
| TG (mg/dL) | 158 ± 76 | 142 ± 78 | 0.437 | 155 ± 107 | 127 ± 55 | 0.170 | 152 ± 59 | 119 ± 52 | 0.402 |
| Glucose (mg/dL) | 85 ± 9 | 84 ± 7 | 0.978 | 95 ± 23 | 92 ± 18 | 0.309 | 85 ± 25 | 87 ± 8 | 0.062 |
| MBF (mL/g/min) | |||||||||
| Rest | 0.68 ± 0.16 | 0.73 ± 0.21 | 0.286 | 0.73 ± 0.18 | 0.78 ± 0.16 | 0.779 | 0.67 ± 0.14 | 0.70 ± 0.12 | 0.429 |
| CPT | 0.91 ± 0.32 | 0.92 ± 0.26 | 0.935 | 0.89 ± 0.17 | 0.83 ± 0.16 | 0.171 | 0.92 ± 0.29 | 0.67 ± 0.12 | 0.006 |
| ΔMBF to CPT | 0.23 ± 0.22 | 0.19 ± 0.22 | 0.453 | 0.16 ± 0.12 | 0.05 ± 0.19 | 0.023 | 0.25 ± 0.18 | -0.03 ± 0.14 | 0.001 |
| Hyperaemia | 1.69 ± 0.28 | 1.68 ± 0.40 | 0.616 | 1.92 ± 0.41 | 1.98 ± 0.44 | 0.146 | 1.62 ± 0.32 | 1.58 ± 0.32 | 0.332 |
| MFR | 2.62 ± 0.76 | 2.49 ± 0.98 | 0.655 | 2.55 ± 0.41 | 2.60 ± 0.62 | 0.779 | 2.46 ± 0.34 | 2.30 ± 0.56 | 0.426 |
| Haemodynamics | |||||||||
| Rest-HR (b.p.m.) | 65 ± 9 | 65 ± 11 | 0.958 | 67 ± 9 | 65 ± 8 | 0.176 | 67 ± 11 | 65 ± 12 | 0.463 |
| CPT-HR (b.p.m.) | 70 ± 10 | 71 ± 11 | 0.473 | 73 ± 8 | 70 ± 8 | 0.264 | 71 ± 11 | 71 ± 15 | 0.815 |
| Hyperaemia-HR (b.p.m.) | 88 ± 12 | 92 ± 12 | 0.280 | 88 ± 10 | 93 ± 13 | 0.202 | 89 ± 16 | 93 ± 14 | 0.042 |
| Rest-SBP (mmHg) | 125 ± 19 | 125 ± 16 | 0.960 | 132 ± 17 | 130 ± 21 | 0.571 | 128 ± 21 | 127 ± 12 | 0.876 |
| CPT-SBP (mmHg) | 148 ± 23 | 152 ± 17 | 0.247 | 157 ± 26 | 155 ± 18 | 0.645 | 163 ± 26 | 166 ± 19 | 0.610 |
| Hyperaemia-SBP (mmHg) | 121 ± 15 | 119 ± 13 | 0.706 | 129 ± 20 | 129 ± 19 | 0.910 | 135 ± 21 | 127 ± 17 | 0.015 |
| Rest-RPP | 8148 ± 1777 | 8175 ± 2017 | 0.929 | 8883 ± 1717 | 8450 ± 1661 | 0.078 | 8551 ± 2126 | 8313 ± 1827 | 0.533 |
| ΔRPP to CPT | 2149 ± 1132 | 2654 ± 1099 | 0.082 | 2588 ± 1859 | 2428 ± 1028 | 0.743 | 3039 ± 1523 | 3494 ± 1180 | 0.131 |
P-values vs. corresponding baseline in each group (t-test for paired samples). Group 1: post-menopausal women (PM) with hormone replacement therapy (HRT) at baseline and follow-up (FU); group 2: PM without HRT at baseline and FU; group 3: PM with HRT at baseline but not at FU. CAD, coronary artery disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride. CPT, cold pressor test; MBF, myocardial blood flow; MFR; myocardial flow reserve; HR, heart rate; RPP, rate-pressure product (HR x SBP).
Study population
All study participants underwent an initial screening visit before baseline PET study that included a physical examination, electrocardiogram (ECG) blood pressure measurements, and routine blood chemistry. Furthermore, study applicants were recruited, if they were without a history of variant angina, clinically manifest cardiovascular, or any other disease. All were normal on physical examination and had normal resting ECGs. Women on HRT were recruited for study purpose, if they had cessation of menses for ≥1 year and used HRT for ≥6 months (Group 1 and Group 3). In addition, women not on HRT qualified for the study purpose if their serum oestradiol levels were <20 pg/mL, oestrone <4 ng/dL, and if menses had ceased for ≥1 year (Group 2). At baseline and FU study, in Group 1, seven women were taking orally oestrogen alone and 11 were taking oestrogen plus a progesterone [10 received medroxyprogesterone acetate (MPA) and 1 received micronized progesterone] at baseline and FU. In Group 3, nine women were taking orally oestrogen alone and three were taking oestrogen plus a progesterone (three received MPA and none received micronized progesterone) at baseline but, as indicated above, not any more at FU. Hormone replacement therapy regimen in PM was installed previously by the treating gynaecologist before study participants were recruited for the study. Further, concomitant preventive medical therapy for RFs (Table 3) was taken continuously by PM (no gaps in the medication >7 days). In the current study population, long-term use of HRT was defined as at least 3 years of HRT before the baseline PET exam (median period: 6 years, IQR 5–9). According to the observational study protocol, the HRT regimens were not altered throughout the FU period.
Table 3.
Continuous concomitant cardiovascular medication
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Platelet inhibitor | 4 (22%) | 5 (28%) | 4 (33%) |
| Beta-blocker | 3 (17%) | 3 (17%) | 1 (8%) |
| Ca-CB | 2 (11%) | 2 (11%) | 0 (0%) |
| ACE-I | 2 (11%) | 3 (17%) | 2 (17%) |
| Diuretic | 3 (17%) | 3 (17%) | 2 (17%) |
| Statin | 8 (44%) | 6 (33%) | 6 (50%) |
| Anti-diabetics | 0 (0%) | 0 (0%) | 1 (8%) |
Numbers, n; ACI, angiotensin-converting enzyme inhibitor; Ca-CB, calcium channel blocker.
Quantification of myocardial blood flow with positron emission tomography
Myocardial perfusion was determined with 13N-ammonia and positron emission tomography (PET; ECAT EXACT HR+; CTI/Siemens, Knoxville, TN, USA).9 The relative distribution of MBF was evaluated visually on reoriented short- and long-axis myocardial slices and semi-quantitatively on the corresponding polar map from the last static 15 min transaxial image. All study participants revealed normal homogeneous 13N-ammonia tracer uptake at rest and during vasomotor stress. Further, MBF was quantified in mL/g/min with 13N-ammonia serial image acquisition (12 frames of 10 s each, 2 frames of 30 s, 1 frame of 60 s, and 1 frame of 900 s) by PET as described previously.9 Measurements were performed first at rest, then during CPT (reflecting predominantly endothelium-dependent vasomotion) and during pharmacologically induced hyperaemia with standard infusion of adenosine or dipyridamole (140 µg/kg/min) (reflecting predominantly endothelium-independent vasomotion).7 Regional MBF values from the three major coronary artery territories on the polar map were averaged to yield mean MBF in mL/g/min. Heart rate, blood pressure, and a 12-lead ECG were recorded continuously during each MBF measurement. Hyperaemic MBFs in PM were grouped into MBF ≤1.45 and >1.45 mL/g/min according to the flow values deviating outside ± 2SD limits of normal values obtained in pre-menopausal women in the current study. In addition, we also defined a definitively normal hyperaemic flow response in PM ≥1.98 mL/g/min based on own previous investigations in a healthy group of pre- and post-menopausal women.7
Statistical analysis
Data are presented as mean ± SD for quantitative and absolute frequencies for qualitative variables. For comparison of differences, appropriate t tests for independent or paired samples were used (Statistical Analysis Software Institute, Cary, NC, USA). A comparison of CPT-induced ΔMBF and pharmacologically induced hyperaemic MBFs between the different groups was performed by one-way analysis of variance (ANOVA), followed by Scheffe’s multiple comparison test. No formal procedure was applied to account for multiple testing. Multivariable analysis was performed by means of a generalized linear model adjusting for the following a priori selected predictors of the CPT-induced ΔMBF and pharmacologically induced hyperaemic MBFs: age, body mass index (BMI), systolic blood pressure (SBP), oestrone, oestradiol, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and glucose. All test procedures were two-tailed, and P < 0.05 was considered statistically significant. Based on a standard deviation of 0.18 of ΔMBF to vasomotor stress, and a minimum clinically relevant difference in ΔMBF of 0.20 in a normal and abnormal flow response,9 α= 0.05, and a power (1 − β) of 0.8, the number of patients necessary for the cross-sectional baseline analysis was calculated to be 25 and 10 individuals for long-term studies.
Results
Cross-sectional study part at baseline
Clinical characteristics
Healthy young pre-menopausal women (controls, CON) had a significantly lower BMI than PM (Table 1). Serum oestrone levels statistically did not differ significantly between CON and PM with HRT, but were significantly lower in PM without HRT. Serum oestradiol levels were lower and total cholesterol, LDL cholesterol, and triglyceride levels were higher in PM with or without HRT than in CON. In PM without HRT, HDL cholesterol was statistically significantly lower and total cholesterol, LDL cholesterol, and triglyceride higher than in CON. Conversely, in PM with HRT, total cholesterol, LDL cholesterol, and glucose tended to be lower than in PM without HRT. In addition, HDL cholesterol levels were statistically significantly higher in PM with HRT when compared with the group without HRT (P = 0.005). The years of menopause were statistically non-significant between PM with and without HRT (P = 0.093) (Table 1).
Endothelium-related myocardial blood flow responses to cold pressor testing
Table 1 lists haemodynamic and MBF responses to vasomotor stress in all three groups at baseline. At rest, heart rate was similar among the groups, whereas blood pressures were statistically significantly higher in both groups of PM than in CON. Accordingly, rate-pressure product (RPP) at rest was also higher in PM than in CON but similar between both groups of PM. Myocardial blood flows at rest tended to be lower in CON than in PM. The group comparison of MBF at rest in controls was statistically not significantly different from PM (P = 0.373 by ANOVA).
In all three groups, sympathetic stimulation with CPT significantly increased heart rate and SBP from rest (Table 1). The responses of heart rate and SBP to sympathetic stimulation by CPT were higher in CON than in the groups of PM with or without HRT. The corresponding change of the RPP from rest during CPT (denoted as ΔRPP to CPT), however, was statistically non-significantly higher in CON than in both groups of PM, while it was similar between the two groups of PM (Table 1). As given in Table 1, increases in MBF from rest to CPT (ΔMBF to CPT) were statistically non-significantly higher in CON than in PM with HRT but attenuated in PM without HRT (Table 1, Figure 1A). Since the haemodynamic response to CPT differed between CON and PM, we normalized the MBF response to CPT to the RPP, and thus the myocardial workload (Table 1). The normalized MBF (NMBF) during CPT tended to be higher in CON than in PM with HRT, although this difference did not reach statistical significance (P = 0.055) (Table 1). Compared with CON, the NMBF during CPT was statistically significantly less in PM without HRT. The NMBF during CPT, however, statistically did not differ significantly between PM with HRT and PM without HRT. The group comparison of ΔMBF to CPT and of NMBF during CPT in CON was statistically significantly different from PM with and without HRT (P < 0.037 and P < 0.036 by ANOVA, respectively). In addition, in order to identify determinants of ΔMBF to CPT, a univariate and multivariable analysis was performed. As demonstrated in Table 4, only age was statistically significantly associated with the ΔMBF to CPT in PM (Table 4). Further, by multivariable analysis, oestrone and oestradiol were the only independent predictors of the endothelium-related ΔMBF to CPT (Table 4), indicating direct effects of oestrogens on coronary endothelial function.
Figure 1.

(A) Change in myocardial blood flow (ΔMBF) from rest in response to cold pressor testing (CPT) in young women (controls) and in post-menopausal women (PM) with and without hormone replacement therapy (HRT) at baseline study. (B) Hyperaemic MBF during pharmacologic vasodilation in young women (controls) and in PM with and without HRT at baseline study.
Table 4.
Univariate and multivariable analysis in post-menopausal women implying cardiovascular risk factors
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Coefficient β (95% CI) | P-value | Coefficient β (95% CI) | P-value | |
| Endothelium-related ΔMBF to CPT | ||||
| Age (years) | 0.324 (0.001–0.016) | 0.025 | 0.253 (–0.005–0.015) | 0.317 |
| BMI (kg/m2) | −0.146 (−0.017–0.006) | 0.321 | 0.326 (−0.004–0.024) | 0.160 |
| SBP (mmHg) | 0.182 (−0.001–0.005) | 0.216 | −0.250 (−0.005–0.002) | 0.353 |
| Oestrone (ng/dL) | 0.066 (−0.005–0.007) | 0.677 | −1.157 (−0.046–0.000) | 0.047 |
| Oestradiol (ng/dL) | 0.189 (−0.001–0.006) | 0.238 | 1.479 (0.003–0.022) | 0.013 |
| Total cholesterol (mg/dL) | −0.205 (−0.002–0.000) | 0.176 | −1.599 (−0.028–0.015) | 0.547 |
| LDL cholesterol (mg/dL) | −0.244 (−0.003–0.000) | 0.115 | 1.886 (−0.014–0.030) | 0.472 |
| HDL cholesterol (mg/dL) | 0.100 (−0.003–0.006) | 0.517 | 1.049 (−0.012–0.031) | 0.373 |
| Triglycerides (mg/dL) | −0.031 (−0.001–0.001) | 0.843 | 0.574 (−0.003–0.005) | 0.591 |
| Glucose (mg/dL) | 0.081 (−0.002–0.003) | 0.604 | 0.312 (−0.001–0.005) | 0.144 |
| Hyperaemic MBF during pharmacologic vasodilation | ||||
| Age (years) | 0.151 (−0.008–0.023) | 0.328 | −0.381 (−0.050–0.012) | 0.218 |
| BMI (kg/m2) | −0.087 (−0.029–0.017) | 0.576 | −0.382 (−0.077–0.016) | 0.180 |
| SBP (mmHg) | 0.281 (0.000–0.011) | 0.065 | 0.398 (−0.002–0.017) | 0.135 |
| Oestrone (ng/dL) | −0.135 (−0.029–0.012) | 0.418 | −0.199 (−0.083–0.062) | 0.761 |
| Oestradiol (ng/dL) | −0.166 (−0.011–0.004) | 0.327 | −0.083 (−0.032–0.028) | 0.900 |
| Total cholesterol (mg/dL) | 0.226 (−0.001–0.004) | 0.156 | 0.944 (−0.057–0.077) | 0.764 |
| LDL cholesterol (mg/dL) | −0.010 (−0.003–0.003) | 0.953 | −1.079 (−0.079–0.056) | 0.728 |
| HDL cholesterol (mg/dL) | 0.037 (−0.007–0.009) | 0.821 | −0.318 (−0.072–0.058) | 0.819 |
| Triglycerides (mg/dL) | 0.193 (−0.001–0.002) | 0.240 | −0.268 (−0.014–0.011) | 0.823 |
| Glucose (mg/dL) | 0.008 (−0.006–0.006) | 0.963 | −0.028 (−0.010–0.009) | 0.912 |
P-values by analysis of variance; MBF, myocardial blood flow; BMI, body mass index; SBP, systolic blood pressure; LDL cholesterol, low-density lipoprotein cholesterol; HDL cholesterol, high-density lipoprotein cholesterol.
Total hyperaemic vasodilator capacity
During pharmacologic vasodilation, the heart rate was statistically significantly higher in CON than in PM with and without HRT, whereas the SBP was significantly higher in both groups of PM (Table 1). Overall, there was a definitively abnormal hyperaemic MBF response of ≤1.45 mL/g/min in 21% (10/48) of PM, whereas 29% (14/48) of PM had normal hyperaemic flow increases of ≥1.98 mL/g/min. It follows then that 50% (24/48) of PM had borderline hyperaemic MBFs of >1.45 and <1.98 mL/g/min. Groupwise, hyperaemic MBFs were statistically significantly higher in CON than in PM with and without HRT (Table 1 and Figure 1B). Moreover, they were statistically significantly lower in PM with HRT compared with PM without HRT (P = 0.039). When the hyperaemic MBFs were related to the mean arterial blood pressure, in order to account for differences in coronary driving pressure, the resulting estimates of coronary vascular resistance (CVR; mean arterial blood pressure/MBF) were statistically significantly higher in PM with and without HRT than in CON (56 ± 15 and 50 ± 13 vs. 32 ± 7 mmHg/mL/min/g, P < 0.0001, respectively), but did not differ significantly between the both groups of PM. The group comparison of MBF and CVR during pharmacologic vasodilation in CON was statistically significant when compared with both groups of PM (P < 0.0001 by ANOVA, respectively). Finally, on univariate and multivariable analysis, no predictors of hyperaemic MBF in response to pharmacologic vasodilation in PM were observed (Table 3).
Longitudinal study part
Clinical characteristics
At the FU, oestrone and oestradiol levels in Groups 1 and 2 had statistically not changed significantly from baseline (Table 2). In Group 3, however, serum oestrone and oestradiol levels declined from baseline to FU, so that they were statistically significantly lower at FU than in Group 1, while comparable to Group 2. In Group 1, there was no statistically significant change in lipid profile and plasma glucose levels during FU. Further, no statistically significant differences in lipid profile and plasma glucose levels were observed between baseline and FU in Groups 2 and 3. In Group 1, PM on HRT were followed-up over a period of a median of 22 months (IQR: 14–25). In Group 2, PM who were not on HRT at baseline and FU, the median study period was 16 months (IQR: 14–25). Further in Group 3, PM on HRT at baseline but not at FU had discontinued HRT after a median of 15 months from baseline (IQR: 11–20), while the median observational study period to FU was 28 months (IQR: 23–41). Concomitant cardiovascular medications are listed in Table 3. The major indication of medical treatment was hypercholesterolaemia (14 in PM with HRT and 9 in PM without HRT). Six PM had borderline hypercholesterlaemia not treated with statins. All cardiovascular medications had been maintained throughout the FU period. Although treatment of cardiovascular RFs differed between groups, the treatment in each group was not altered between baseline and FU study.
Myocardial blood flow responses to cold pressor test and total hyperaemic vasodilator capacity
In the FU groups, resting heart rate and SBP as well as corresponding RPPs statistically did not differ significantly in repeat assessments (Table 2). In addition, statistically there was no significant change in resting MBF from baseline to FU. Cold induced a statistically significant increase in heart rate and SBP were comparable at baseline and FU. Accordingly, the RPP, and thus cardiac work, was similar at baseline and FU (Table 2). The ΔMBF in response to CPT at FU was similar to those at baseline in Group 1, but was statistically significantly diminished in Group 2 at FU (Table 2, Figure 2A). Importantly, the CPT-induced ΔMBF in Group 3 was statistically significantly less at FU after discontinuation of HRT than at baseline (Figure 2). The group comparison of the ΔMBF to CPT from rest in Group 1 after the FU period was statistically significantly different from Groups 2 and 3 (P < 0.0001 by ANOVA). In order to determine whether changes of the MBF response to CPT from baseline to FU are different between groups, we compared the change in MBF from rest to CPT (ΔMBF) between baseline and FU defined as ΔMBF difference (ΔMBF at baseline − ΔMBF at FU). This ΔMBF difference was statistically not significantly different between Groups 1 and 2 (−0.04 ± 0.22 vs. −0.11 ± 0.19 mL/g/min; P = 0.310), but was statistically significantly reduced in Group 3 when compared with Group 1 (−0.04 ± 0.22 vs. −0.28 ± 0.22 mL/g/min; P = 0.009). Moreover, the ΔMBF difference was statistically significantly greater in Group 3 than in Group 2 (−0.28 ± 0.22 vs. −0.11 ± 0.19 mL/g/min; P = 0.043). The group comparison of the difference in ΔMBF to CPT in Group 1 was statistically significantly different from groups and 3 (P = 0.018 by ANOVA). Further, hyperaemic MBFs were similar at baseline and FU for all three groups (Table 2, Figure 2B). Accordingly, estimates of the CVR during pharmacologic vasodilation statistically did not change significantly in repeat assessments for the three groups (Group 1: 53 ± 13 vs. 54 ± 15 mmHg/mL/min/g, Group 2: 50 ± 13 vs. 46 ± 12 mmHg/mL/min/g, and Group 3: 60 ± 16 vs. 58 ± 12 mmHg/mL/min/g).
Figure 2.
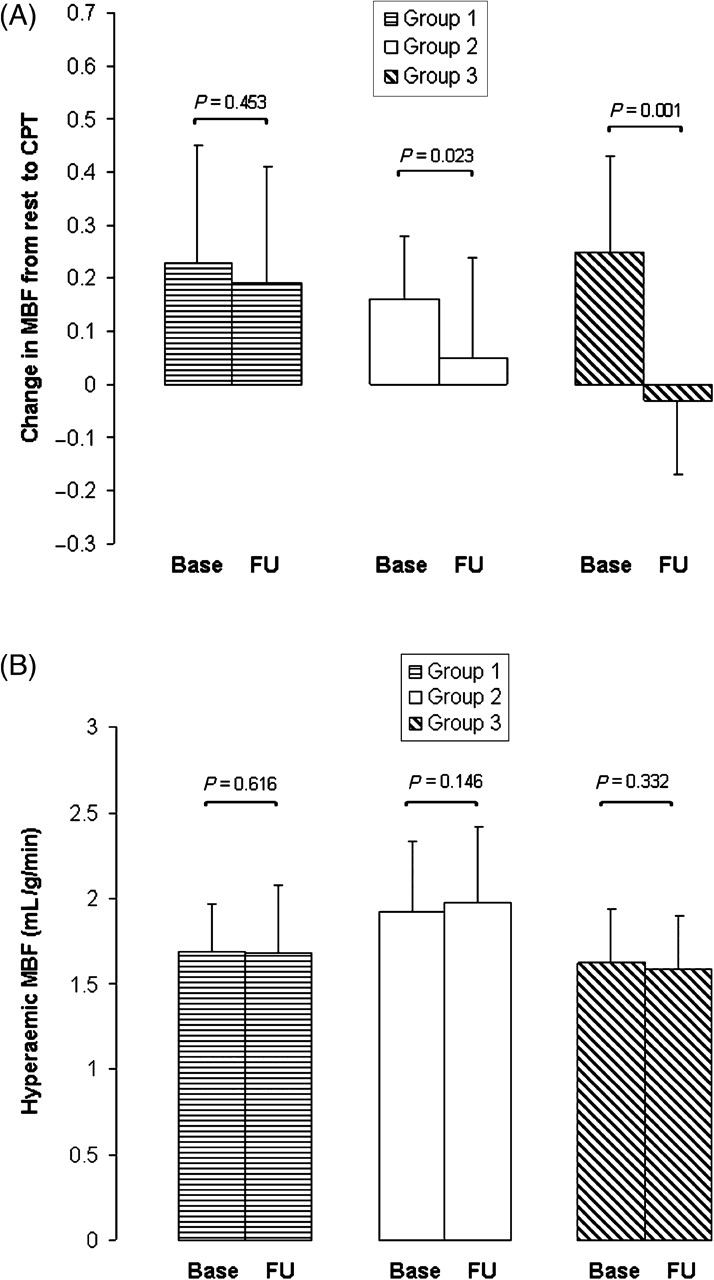
(A) The endothelium-related change in myocardial blood flow (ΔMBF) from rest to cold pressor testing (CPT) at baseline and follow-up (FU) in Groups 1–3. (B) Hyperaemic MBF during pharmacologic vasodilation in Groups 1–3 of post-menopausal women at baseline and at FU.
Discussion
The new finding of the current prospective observational investigation is that HRT with oestrogen alone or in concert with a progesterone in PM, in addition to standard management of traditional cardiovascular RFs, may contribute to maintain the functional integrity of vascular endothelium of the coronary microcirculation. These observations agree with the results of previous investigations in PM without coronary RFs8 but extend the latter observations to PM with medically treated cardiovascular RFs.
Metabolic profile and coronary circulatory function
At the baseline exam, total cholesterol, LDL cholesterol, and triglyceride concentrations were within normal range in PM but higher than in healthy young women, whereas HDL cholesterol levels were lower. Further, it was observed that in PM, who had been on HRT in the past, total cholesterol, LDL cholesterol, and glucose tended to be lower, whereas HDL cholesterol was significantly higher than in those PM without HRT. As the treatment with statins was comparable between both groups, the differences in lipoprotein fraction could be related, at least in part, to the beneficial actions of HRT on lipids.10 The lipid profile and/or still undetermined factors in the current population of PM, however, may account for the observed impairment of the total coronary vasodilator capacity in PM. Surprisingly, on multivariate analysis, we did not find predictors of impaired hyperaemic coronary flows in PM possibly due to the concurrent effects of HRT, medical treatment of cardiovascular RFs, and/or undetermined factors on the arterial wall. Of particular relevance, oestrogen plasma levels did not predict hyperaemic MBF increases in these PM with or without HRT. The latter observations may suggest that alterations in hyperaemic MBF increases, as indicated by previous investigations,8 appear to be independent of the HRT status. Other clinical investigations in the assessment of coronary vasomotor function in PM8,11–13 have indicated alterations in lipid profile rather than reduced circulating oestrogen levels to account for the altered hyperaemic coronary flow response. Notably, current observations may also suggest that even statin-induced cholesterol lowering associated with beneficial effects on lipids and vasomotor function may not necessarily lead to a normalization of diminished hyperaemic coronary flow in PM. This may give rise to the consideration that, apart from traditional coronary RFs, undetermined factors such as insulin resistance and/or a complex interplay of RFs in the arterial wall may cause an impairment in hyperaemic flows or predominantly vascular smooth muscle cell function of the coronary arteriolar vessels, which merits further investigations. Our results differ from other investigations.11,14 For example, in PM with angina pectoris symptoms, oestrogen therapy tended to increase the total coronary vasodilatory capacity owing to dipyridamole stimulation.11 More recently again, the effects of 17β-oestradiol combined with the progestin drospirenone on hyperaemic MBFs in symptomatic PM were studied. Post-menopausal women did not have type 2 diabetes mellitus or hyperlipidaemia, while some were smokers and others were medically treated for arterial hypertension.14 In a double-blind randomized study protocol, however, a 6-week FU with 17β-oestradiol and drospirenone did lead to a mild but statistically significant improvement in hyperaemic MBFs and the corresponding myocardial flow reserve when compared with a placebo group.14 The reason for the contradictory observations of a beneficial effect of HRT on hyperaemic MBFs in PM8,12,14 remains uncertain but is likely multifactorial. Differences in the clinical characteristics of PM studied,8,12,14 differences in the duration of the post-menopause, differences in the state of altered vascular smooth muscle cell and/or endothelial function, differences in structural alterations in the myocardium, and/or differences in the use of progestins combined with oestrogens10 are likely to account for the observed contrasting effects of HRT on hyperaemic MBFs.
In the assessment of coronary vasomotor function, MBF responses to sympathetic stimulation with CPT have been shown to provide specific information on the endothelium-dependent vasomotion of the coronary microcirculation.7 In the current study, the cross-sectional comparison of MBF responses to vasomotor stress at baseline yielded comparable endothelium-related flow increases to cold exposure between PM with HRT and healthy young women, whereas the endothelium-related flow responses were significantly diminished in PM without HRT. The latter observations may indeed suggest that HRT therapy beneficially affects endothelial function of the coronary microcirculation in PM, even when RFs were treated by medical intervention. As endothelial dysfunction in PM is considered as an independent predictor of subsequent cardiovascular events,7 coronary microcirculatory dysfunction in PM may emerge as a specific target for HRT to improve the cardiovascular outcome.15 Confounding effects of the lipid profile, medical treatment of RFs, and/or undetermined factors, however, are likely to have also altered endothelial function of the coronary arteriolar vessels apart from HRT. On multivariate analysis, however, oestrogen plasma levels were indeed independent predictors of the endothelium-related MBF response to CPT. With regard to the effects of HRT on coronary vasomotor function, current observations provide direct evidence that oestrogen concentrations, in addition to standard management of traditional RFs, may exert beneficial effects on endothelium-related microvascular function. Further support of this comes from the observational longitudinal FU investigations in the current study population. At FU, the endothelium-related MBF response to CPT in PM with treated RFs and with HRT had remained unchanged, but had further declined in those PM without HRT. Notably, while at baseline, endothelium-related flow increases to CPT were maintained in PM with HRT when compared with young women, the flow response to CPT had only declined in those PM who had not pursuit HRT during the FU. In addition, the endothelium-related MBF response to CPT in PM who had discontinued HRT was worse than in those PM who had never been on HRT. The underlying mechanism for the ‘rebound’ phenomenon on coronary vascular function remains uncertain but it is possible that discontinuation of HRT may have conferred an enhanced adverse effect on the coronary endothelium similar to the one that has been described for statin withdrawal.16
As ∼40–50% of PM were on HRT with oestrogen plus a progesterone, however, it is equally possible that progestins had affected endothelium vasomotor function. Unlike as in subhuman primates,17 progestin application in PM did not negate the beneficial effect of oestrogen on endothelial function of the coronary and peripheral circulation.8 The reason for this discrepant observation is unclear, but is likely to be related to differences in metabolites of progestational substances comprising MPA specific to subhuman primates,8 which might account for adverse effects on endothelial function.
Since functional abnormalities of the coronary circulation in PM, associated with pro-atherosclerotic and pro-thrombotic effects,7 may reflect an important mechanistic link between coronary vasomotor dysfunction and cardiovascular outcome, the beneficial effect of long-term HRT on endothelium-dependent coronary microvascular function, as observed in the current study and by other investigations,8,18 may agree with improved cardiovascular outcome of HRT in younger PM from the overall WHI-CEE trial analysis,4 and, as reported recently, when HRT was initiated between the ages of 50 and 59 years or within 10 years after menopause.19 Post-menopausal women in the current study were mostly between 50 and 59 years of age and, thus, relatively young. Thus, the observations of the beneficial effect of HRT on endothelium-dependent coronary microvascular function may indeed accord with the ‘timing hypothesis’ for HRT. According to the latter, a benefit of HRT in preventing the CAD process may manifest when HRT is started before advanced stages of atherosclerosis are present.2 And indeed, a beneficial effect of HRT on flow-mediated brachial artery function was predominantly observed in PM in their 50s, which appeared also to be affected by the time period since menopause.18
Limitations
There are limitations worthy to be considered in interpreting the current study data. The current study did not assess the molecular mechanism underlying the beneficial effect of HRT on endothelium-dependent vasomotion of the coronary microcirculation. Future experimental studies may further elucidate the role of various components of HRT in determining the mechanism of beneficial effects on coronary endothelial function. Also, because all women were asymptomatic and had normal PET perfusion imaging at rest and hyperaemic stress, arguing against the presence of flow-limiting epicardial lesions, the use of coronary angiography and intravascular ultrasound was not deemed ethically justified and, thus, it was not performed. This again means that we do not know whether PM had none or early stages of subclinical CAD, which might have affected the vasomotor response to HRT. Finally, in view of the relatively small sample size of the study population and the observational study design with its inherent limitations such as a possible selection bias, the lack of randomization of study participants to HRT at baseline inclusion, the presence of confounding cardiovascular RFs in concert with their medical treatment, and the absence of an objective criteria to continue or interrupt HRT, the current study does certainly not permit definite conclusions but may contribute to stimulate further large-scale, randomized clinical investigations of the effects of HRT on vasomotor function in PM and its clinical outcome.
Conclusions
Long-term administration of oestrogen may exert beneficial effects on endothelium-dependent vasomotion of the coronary microcirculation in PM with treated cardiovascular RFs and without clinical CAD.
Funding
This work was supported by Research Grant HL 33177, National Heart, Lung and Blood Institute (NIH), Bethesda, MD, USA.
Conflict of interest: none declared.
Acknowledgements
The authors thank Larry Pang, Richard Eugene, and Jennie Kusnadi for assisting in the PET studies, Nagichettiar Satyamurthy and staff for 13N-ammonia, and Victoria Bender and Mary Smith for administrative assistance.
References
- 1.Stramba-Badiale M, Fox KM, Priori SG, Collins P, Daly C, Graham I, Jonsson B, Schenck-Gustafsson K, Tendera M. Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J. 2006;27:994–1005. doi: 10.1093/eurheartj/ehi819. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 3.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 4.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 5.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard O. Hormone therapy and risk of myocardial infarction: a national register study. Eur Heart J. 2008;29:2660–2668. doi: 10.1093/eurheartj/ehn408. [DOI] [PubMed] [Google Scholar]
- 7.Schindler TH, Zhang XL, Vincenti G, Mhiri L, Lerch R, Schelbert HR. Role of PET in the evaluation and understanding of coronary physiology. J Nucl Cardiol. 2007;14:589–603. doi: 10.1016/j.nuclcard.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campisi R, Nathan L, Pampaloni MH, Schoder H, Sayre JW, Chaudhuri G, Schelbert HR. Noninvasive assessment of coronary microcirculatory function in postmenopausal women and effects of short-term and long-term estrogen administration. Circulation. 2002;105:425–430. doi: 10.1161/hc0402.102860. [DOI] [PubMed] [Google Scholar]
- 9.Schindler TH, Zhang XL, Prior JO, Cadenas J, Dahlbom M, Sayre J, Schelbert HR. Assessment of intra- and interobserver reproducibility of rest and cold pressor test-stimulated myocardial blood flow with (13)N-ammonia and PET. Eur J Nucl Med Mol Imaging. 2007;34:1178–1188. doi: 10.1007/s00259-007-0378-5. [DOI] [PubMed] [Google Scholar]
- 10.Koh KK, Han SH, Shin MS, Ahn JY, Lee Y, Shin EK. Significant differential effects of lower doses of hormone therapy or tibolone on markers of cardiovascular disease in post-menopausal women: a randomized, double-blind, crossover study. Eur Heart J. 2005;26:1362–1368. doi: 10.1093/eurheartj/ehi311. [DOI] [PubMed] [Google Scholar]
- 11.Duvernoy CS, Rattenhuber J, Seifert-Klauss V, Bengel F, Meyer C, Schwaiger M. Myocardial blood flow and flow reserve in response to short-term cyclical hormone replacement therapy in postmenopausal women. J Gend Specif Med. 2001;4:21–27, 47. [PubMed] [Google Scholar]
- 12.Duvernoy C, Martin J, Briesmiester K, Bargardi A, Muzik O, Mosca L. Myocardial blood flow and flow reserve in response to hormone therapy in postmenopausal women with risk factors for coronary disease. J Clin Endocrinol Metab. 2004;89:2783–2788. doi: 10.1210/jc.2003-031674. [DOI] [PubMed] [Google Scholar]
- 13.Peterson LR, Eyster D, Davila-Roman VG, Stephens AL, Schechtman KB, Herrero P, Gropler RJ. Short-term oral estrogen replacement therapy does not augment endothelium-independent myocardial perfusion in postmenopausal women. Am Heart J. 2001;142:641–647. doi: 10.1067/mhj.2001.118111. [DOI] [PubMed] [Google Scholar]
- 14.Knuuti J, Kalliokoski R, Janatuinen T, Hannukainen J, Kalliokoski KK, Koskenvuo J, Lundt S. Effect of estradiol-drospirenone hormone treatment on myocardial perfusion reserve in postmenopausal women with angina pectoris. Am J Cardiol. 2007;99:1648–1652. doi: 10.1016/j.amjcard.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 16.Laufs U, Endres M, Custodis F, Gertz K, Nickenig G, Liao JK, Bohm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 17.Clarkson TB, Anthony MS, Williams JK, Honore EK, Cline JM. The potential of soybean phytoestrogens for postmenopausal hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:365–368. doi: 10.3181/00379727-217-44246. [DOI] [PubMed] [Google Scholar]
- 18.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol. 2008;28:348–352. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 19.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]


