Abstract
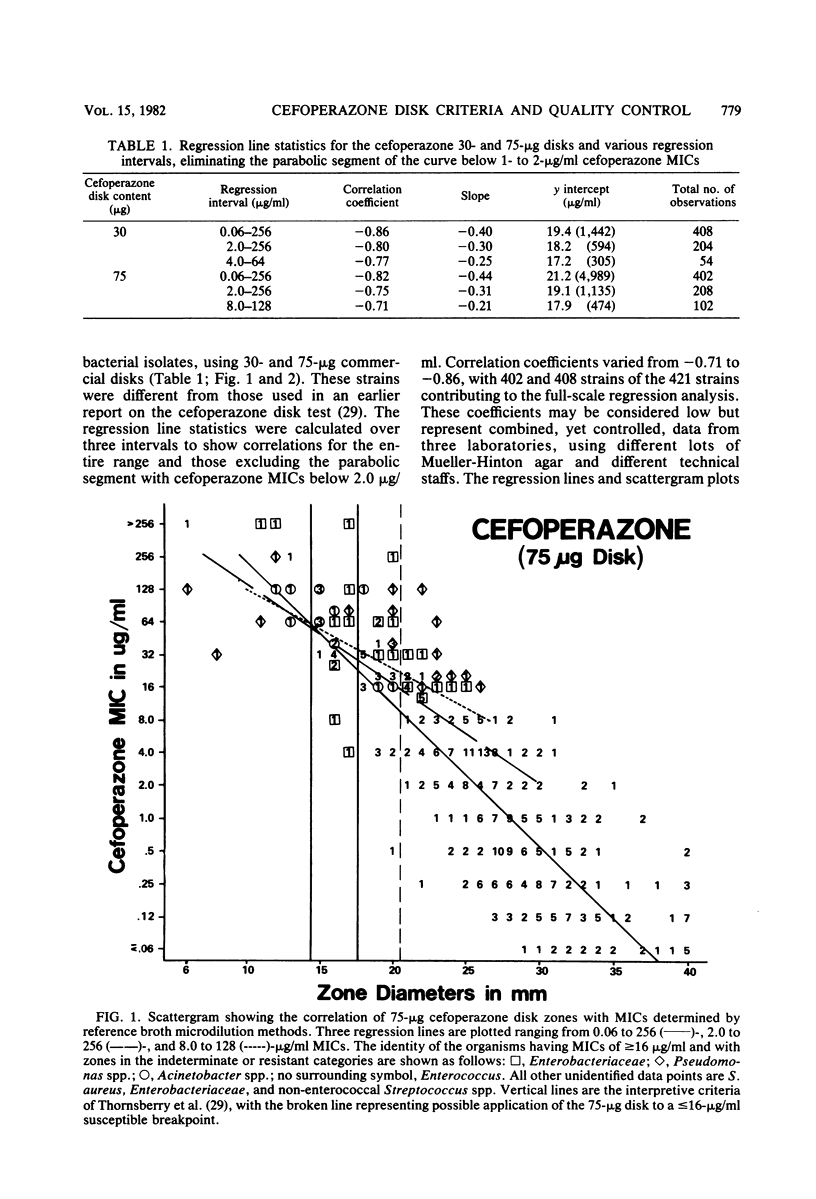
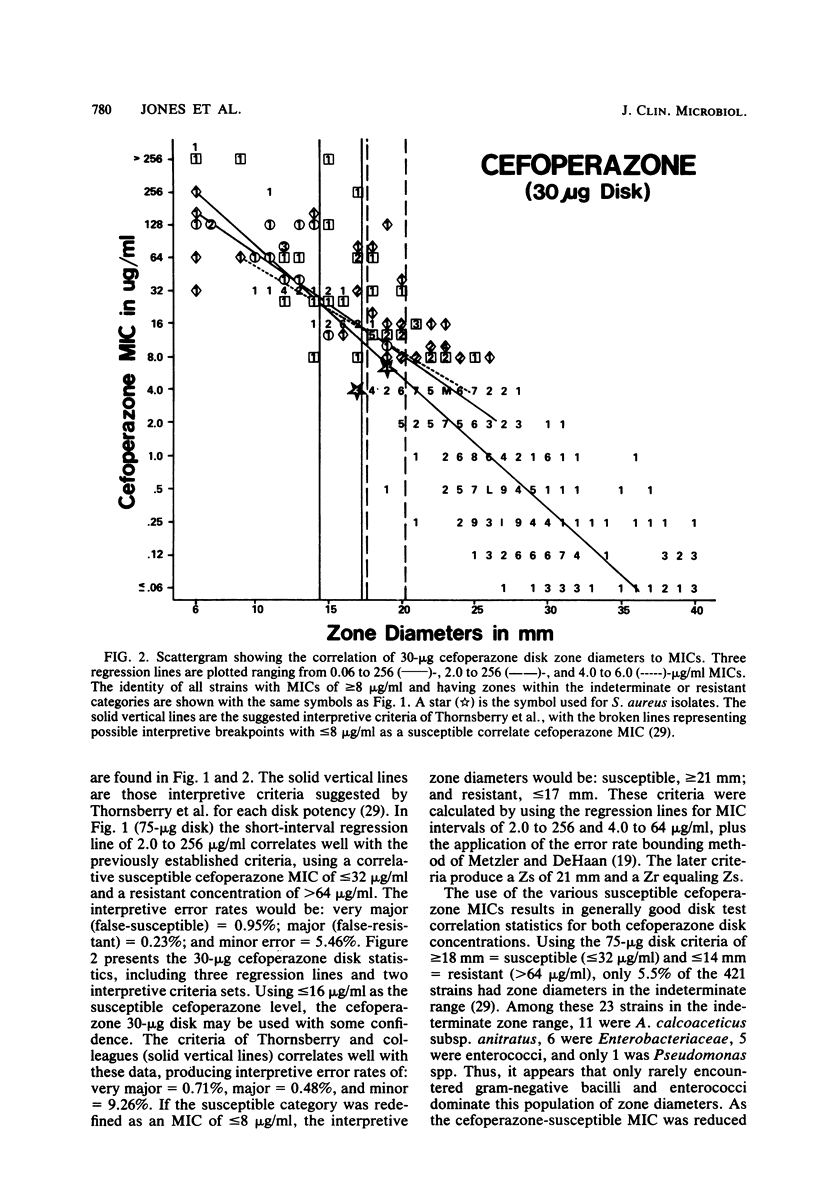
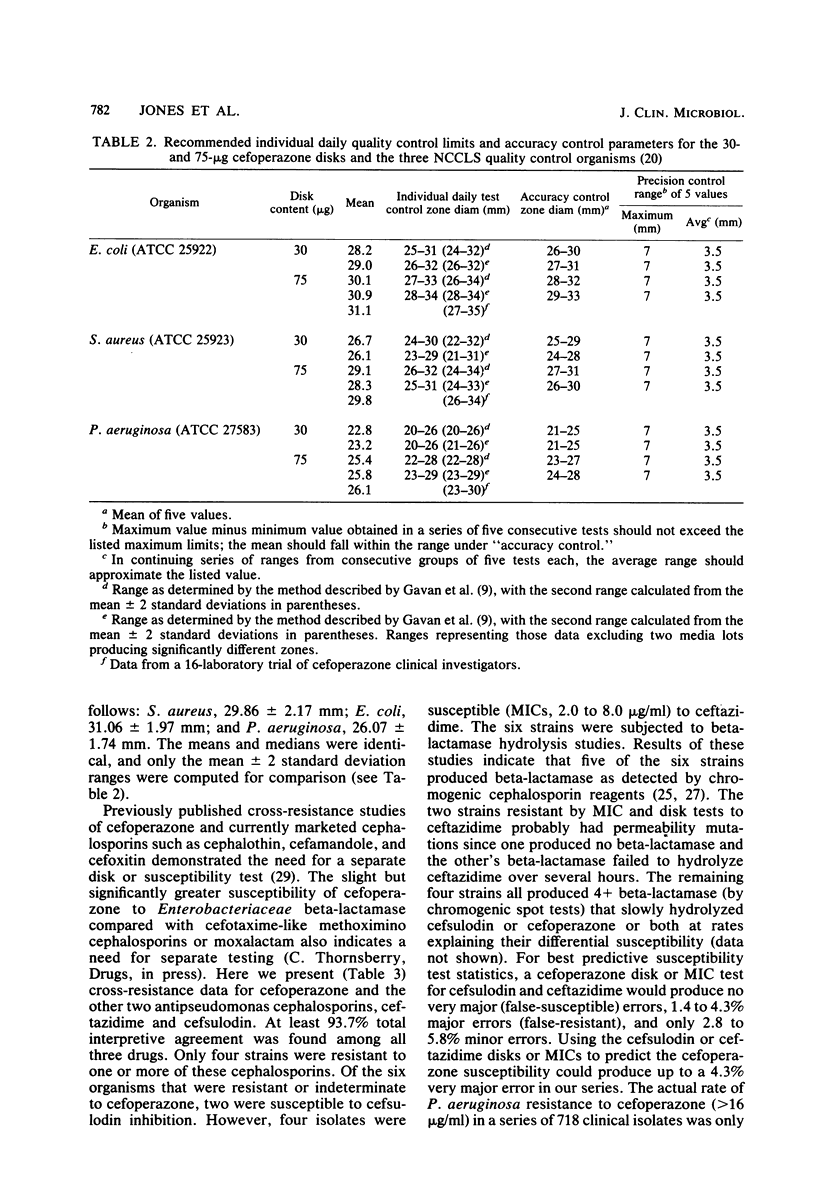
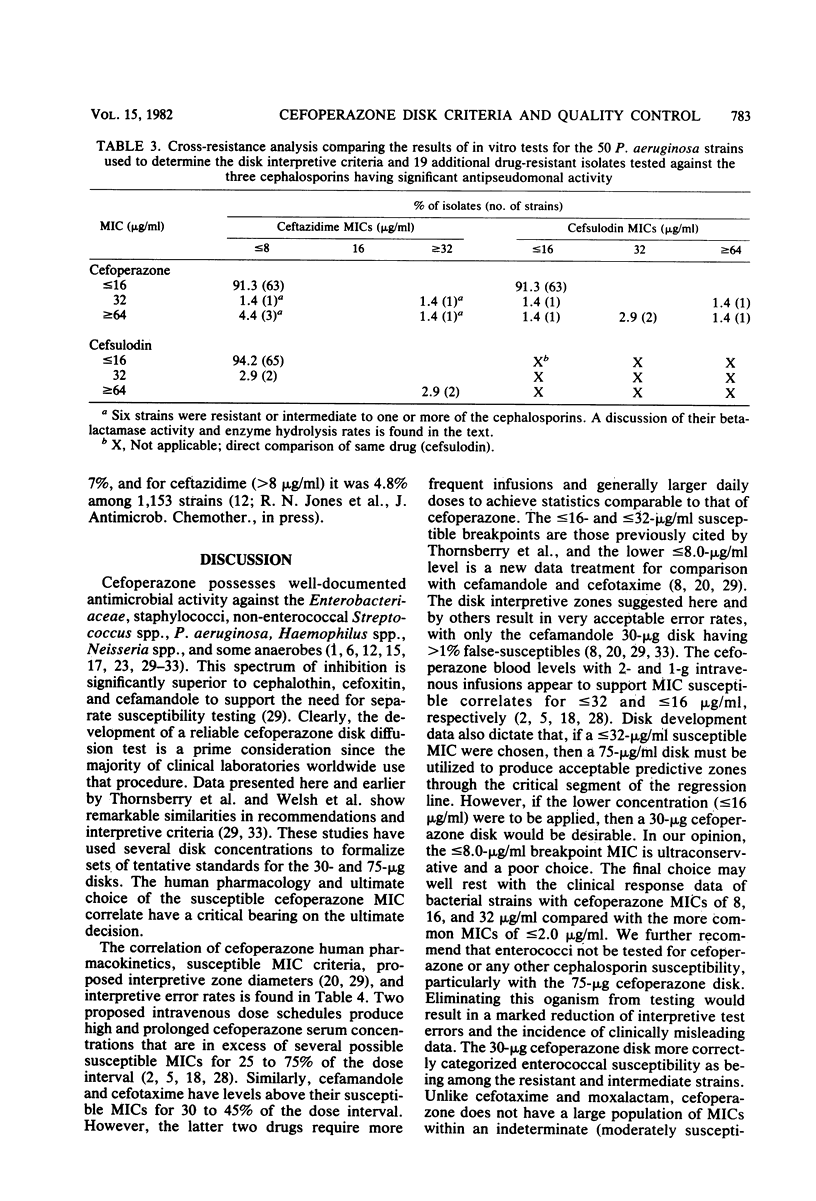
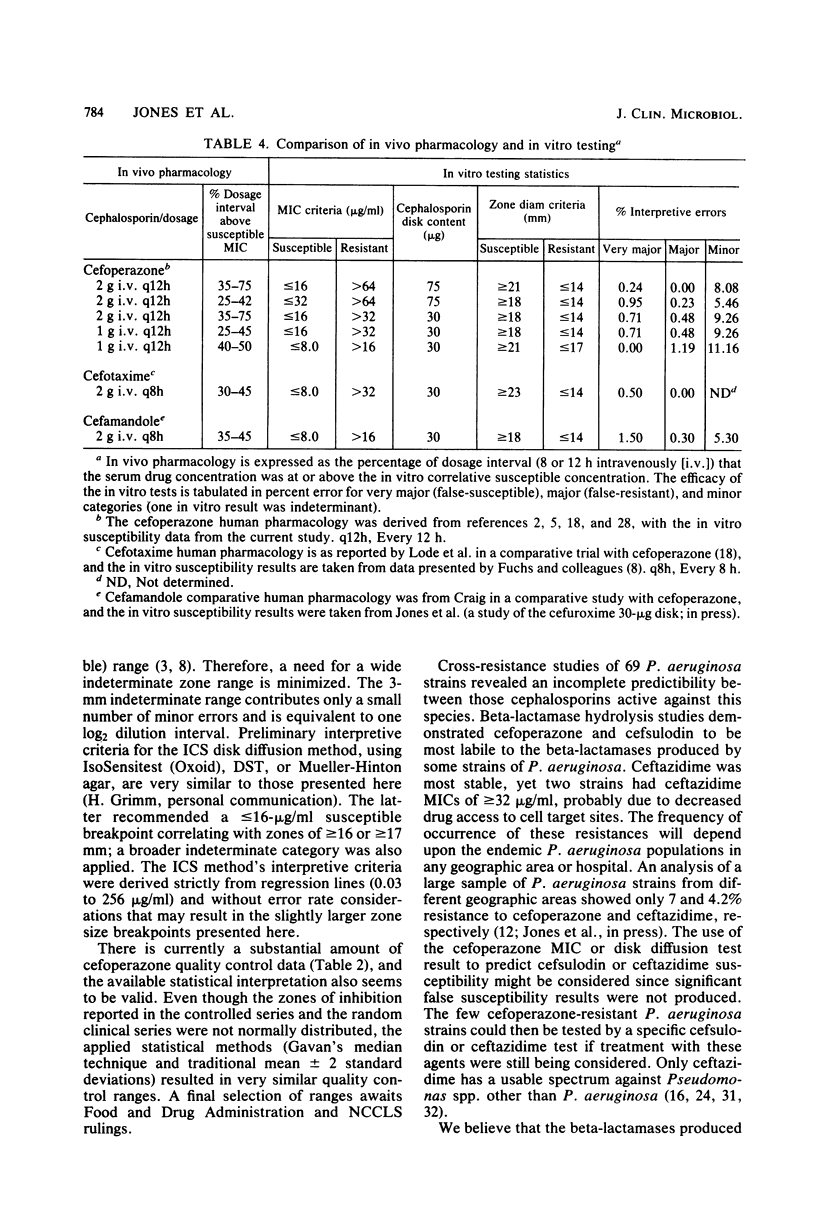
Cefoperazone disk diffusion test and minimum inhibitory concentration comparison studies were performed on 421 recent bacterial isolates, using 30- and 75-micrograms commercially prepared disks. Acceptable correlation coefficients (-0.82 to -0.86) and very major (false-susceptible) interpretive error rates (less than 1%) were obtained with both disk concentrations. The interpretive criteria for both disks were identical. Using the preferred 75-micrograms disk, the Thornsberry et al. criteria (J. Clin. Microbiol. 15:769-776, 1982) of greater than or equal to 18 mm = susceptible (less than or equal to 32 micrograms/ml) and less than or equal to 14 mm = resistant (greater than 64 micrograms/ml) resulted in only 5.5% of strains having indeterminate-range zone diameters; the 30-micrograms disk had 6.9% of strains with indeterminate zone diameters. The 75-micrograms disk, excluding the testing of enterococci, minimized the very major and other interpretive errors to less than 5%. Larger zone diameters will contribute few technical problems with either disk concentration. Data from 1,320 zone diameters submitted for each quality control strain indicated no significant (P greater than 0.05) difference between disks made by the three major manufacturers, and consistent results were obtained within each laboratory with numerous lots of Mueller-Hinton agar (except for one manufacturer). Individual daily test and accuracy quality control ranges were calculated from clinical investigator laboratory data at 16 hospitals based on mean zone sizes and from an additional 8 laboratories with both mean and median calculations. The quality control data were nearly identical, and ranges calculated by the two methods were very similar. Susceptibility tests of Pseudomonas aeruginosa indicate that the cefoperazone disk or minimum inhibitory concentration test would accurately predict P. aeruginosa susceptibility test results for other pseudomonas-active cephalosporins (cefsulodin and ceftazidime), thus producing no very major interpretive errors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Thornsberry C., Jones R. N. In vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (LY127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Apr;17(4):757–761. doi: 10.1128/aac.17.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balant L., Dayer P., Rudhardt M., Allaz A. F., Fabre J. Cefoperazone: pharmacokinetics in humans with normal and impaired renal function and pharmacokinetics in rats. Clin Ther. 1980;3(Spec Issue):50–59. [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Gerlach E. H. Tentative interpretive standards for disk susceptibility tests with moxalactam (LY127935). Antimicrob Agents Chemother. 1980 Nov;18(5):716–721. doi: 10.1128/aac.18.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Craig W. A. Single-dose pharmacokinetics of cefoperazone following intravenous administration. Clin Ther. 1980;3(Spec Issue):46–49. [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findell C. M., Sherris J. C. Susceptibility of Enterobacter to cefamandole: evidence for a high mutation rate to resistance. Antimicrob Agents Chemother. 1976 Jun;9(6):970–974. doi: 10.1128/aac.9.6.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Thornsberry C., Jones R. N., Gavan T. L., Gerlach E. H., Sommers H. M. Cefotaxime: in vitro activity and tentative interpretive standards for disk susceptibility testing. Antimicrob Agents Chemother. 1980 Jul;18(1):88–93. doi: 10.1128/aac.18.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L., Fuchs P. C., Gerlach E. H., Matsen J. M., Reller L. B., Thornsberry C., Thrupp L. D. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981 Jul;14(1):67–72. doi: 10.1128/jcm.14.1.67-72.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymès R., Lutz A., Schrinner E. Experimental evaluation of HR756, a new cephalosporin derivative: pre-clinical study. Infection. 1977;5(4):259–260. doi: 10.1007/BF01640793. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C., Wilson H. W. In vitro antimicrobial activity evaluation of cefodizime (HR221), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981 Dec;20(6):760–768. doi: 10.1128/aac.20.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Gavan T. L., Sommers H. M., Gerlach E. H. Cefoperazone (T-1551), a new semisynthetic cephalosporin: comparison with cephalothin and gentamicin. Antimicrob Agents Chemother. 1980 Apr;17(4):743–749. doi: 10.1128/aac.17.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Sommers H. M., Gavan T. L., Barry A. L., Gerlach E. H. Moxalactam (LY127935), a new semisynthetic 1-oxa-beta-lactam antibiotic with remarkable antimicrobial activity: in vitro comparison with cefamandole and tobramycin. Antimicrob Agents Chemother. 1980 Apr;17(4):750–756. doi: 10.1128/aac.17.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura T., Matsumoto Y., Okada N., Mine Y., Nishida M., Goto S., Kuwahara S. Ceftizoxime (FK 749), a new parenteral cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1979 Nov;16(5):540–548. doi: 10.1128/aac.16.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H. Microbiological studies on cefoperazone. Clin Ther. 1980;3(Spec Issue):24–33. [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. In vitro antibacterial activity and susceptibility of cefsulodin, an antipseudomonal cephalosporin, to beta-lactamases. Antimicrob Agents Chemother. 1980 Feb;17(2):165–169. doi: 10.1128/aac.17.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H., Nishida M., Goto S., Kuwahara S. Antibacterial activity of ceftizoxime (FK 749), a new cephalosporin, against cephalosporin-resistant bacteria, and its stability to beta-lactamase. Antimicrob Agents Chemother. 1979 Nov;16(5):549–553. doi: 10.1128/aac.16.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Kemmerich B., Koeppe P., Belmega D., Jendroschek H. Comparative pharmacokinetics of cefoperazone and cefotaxime. Clin Ther. 1980;3(Spec Issue):80–88. [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Fu K. P., Aswapokee P. Antibacterial activity of a new 1-oxa cephalosporin compared with that of other beta-lactam compounds. Antimicrob Agents Chemother. 1979 Aug;16(2):141–149. doi: 10.1128/aac.16.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Shimizu K. Cefoperazone: absorption, excretion, distribution, and metabolism. Clin Ther. 1980;3(Spec Issue):60–79. [PubMed] [Google Scholar]
- Thornsberry C., Barry A. L., Jones R. N., Baker C. N., Badal R. E. Tentative interpretive standards for agar disk diffusion antimicrobial susceptibility testing of cefoperazone. J Clin Microbiol. 1982 May;15(5):769–776. doi: 10.1128/jcm.15.5.769-776.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Nagatomo H. SCE-129, antipseudomonal cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1978 Feb;13(2):137–145. doi: 10.1128/aac.13.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. GR-20263: a new aminothiazolyl cephalosporin with high activity against Pseudomonas and Enterobacteriaceae. Antimicrob Agents Chemother. 1980 May;17(5):807–812. doi: 10.1128/aac.17.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]


