Abstract
We previously generated a transgenic mouse model for acute promyelocytic leukemia (APL) by expressing the promyelocytic leukemia (PML)–retinoic acid receptor (RARα) cDNA in early myeloid cells. This fusion protein causes a myeloproliferative disease in 100% of animals, but only 15–20% of the animals develop acute leukemia after a long latency period (6–13 months). PML-RARα is therefore necessary, but not sufficient, for APL development. The coexpression of a reciprocal form of the fusion, RARα-PML, increased the likelihood of APL development (55–60%), but did not shorten latency. Together, these results suggested that additional genetic events are required for the development of APL. We therefore evaluated the splenic tumor cells from 18 transgenic mice with APL for evidence of secondary genetic events, by using spectral karyotyping analysis. Interstitial or terminal deletions of the distal region of one copy of chromosome 2 [del(2)] were found in 1/5 tumors expressing PML-RARα, but in 11/13 tumors expressing both PML-RARα and RARα-PML (P < 0.05). Leukemic cells that contained a deletion on chromosome 2 often contained additional chromosomal gains (especially of 15), chromosomal losses (especially of 11 or X/Y), or were tetraploid (P ≤ 0.001). These changes did not commonly occur in nontransgenic littermates, nor in aged transgenic mice that did not develop APL. These results suggest that expression of RARα-PML increases the likelihood of chromosome 2 deletions in APL cells. Deletion 2 appears to predispose APL cells to further chromosomal instability, which may lead to the acquisition of additional changes that provide an advantage to the transformed cells.
Acute promyelocytic leukemia (APL) is characterized by the accumulation of promyelocytes in the bone marrow and peripheral blood and often is accompanied by disseminated intravascular coagulation (reviewed in refs. 1 and 2). The major genetic abnormality associated with APL is a balanced translocation involving chromosomes 15 and 17 [t(15;17)(q22;q11.2–12)], fusing the promyelocytic leukemia (PML) gene with the retinoic acid receptor (RARα) gene (reviewed in refs. 1 and 2). We and others have demonstrated that expression of PML-RARα (PR) in early myeloid cells causes a myeloproliferative disease in 100% of animals and leads to the development of APL in 15–20% of animals after a latent period of 6–13 months (3–7). PML-RARα expression is detected in virtually 100% of t(15;17) APL patients, and it appears to determine the characteristic leukemic phenotype in transgenic mouse models. However, more than 80% of human APL patients also express the reciprocal fusion RARα-PML (RP) in their leukemic cells (8–11). Expression of the RARα-PML cDNA in early myeloid cells does not alter myeloid development, but expression of both PR and RP cDNAs leads to a higher fraction of mice that develop APL (55–60%) (12); the latency of APL development was not altered by coexpression of RP. These data suggested that expression of the reciprocal RP fusion gene plays an important role in the development of APL in transgenic mice, but the persistent long latency suggested that additional genetic changes are required for APL progression.
Both primary and secondary genetic lesions can be found in many hematologic malignancies (13, 14). Primary genetic changes are usually specific and are required for development of the transformed phenotype. Secondary changes are usually less specific in nature and may occur in many different kinds of cancer (e.g., chromosomal gains or losses, loss of function of p53 or Rb, etc.). In acute leukemias, the primary alteration is often a translocation that creates a novel oncoprotein or an abnormally expressed protein. Secondary changes may result from genomic instability caused by the primary event; these secondary changes may provide an advantage for the transformed cell, because they are frequently found in a majority of tumor cells from a given patient. In newly diagnosed patients with APL and t(15;17), approximately 30–45% have leukemic cells with secondary abnormalities (10, 15–17). The most common secondary event is trisomy of chromosome 8, occurring in 15–40% of t(15;17) patients, but a variety of additional chromosomal abnormalities also have been described (10, 14–17). The high frequency of these secondary events has led to the hypothesis that PML-RARα expression somehow may cause genomic instability, similar to that described for the expression of bcr-abl in patients with chronic myelogenous leukemia (18). These secondary changes in APL are not associated with poor prognosis, nor do they predict response to therapy with AtRA (10) or Ara-C (17). Therefore, their significance is currently unknown.
In this report, we demonstrate that 11/13 APL tumors derived from mice expressing both PR and RP acquire a common deletion of the distal segment of one copy of chromosome 2, in contrast to 1/5 tumors expressing PR only. Tumor cells with chromosome 2 deletions had a much greater likelihood of acquiring additional chromosomal abnormalities (P < 0.005), including chromosomal gains, losses, and/or tetraploidy. Expression of the RP fusion therefore increases the likelihood that chromosome 2 deletions will occur, which in turn increases the likelihood of additional chromosomal abnormalities. These progressive genetic abnormalities may contribute to the progression of APL in transgenic mice, and perhaps also in humans with APL.
Materials and Methods
Mice.
Transgenic PR and RP founders were generated as described (3, 12) on a (C3H × Bl/6) F1 background. Tumors in this study were taken from leukemic animals from three separate intercross colonies. Nontransgenic littermate controls and nonleukemic transgenic mice were killed after 109–156 days of life. Spleen and liver sections were analyzed for evidence of myeloid leukemia by histopathology, as described (12). Blood was obtained at the time of death and analyzed with a Coulter counter for complete blood counts and automated differentials. Bone marrow was harvested from these animals for spectral karyotyping (SKY) analysis and morphologic analysis. Transgenic nonleukemic animals also were evaluated after 520 days, by using the same tests.
Cryopreservation of APL Tumors.
Leukemic transgenic animals were killed and spleen cells were cryopreserved as described (12). Cells were always frozen on the day of harvest and were cultured only for chromosome preparation.
SKY Analysis.
Chromosomes were prepared from APL spleen cells and normal bone marrow cells after culture for 24–48 h. Cells were arrested in metaphase by incubation with colcemide for 2–3 h before chromosome preparation. Chromosomes were obtained by standard hypotonic KCl and methanol/acetic acid fixation procedures.
SKY analysis was performed according to original procedure (19) with minor modifications. The mouse chromosome probe mixture (Applied Spectral Imaging, Carlsbad, CA) labeled with spectrum orange, Texas red, Cy5, spectrum green, and Cy5.5 was denatured and hybridized on denatured target slides. Visualization for biotin- and digoxigenin-labeled DNAs of the probe mixture were carried out by using avidin-Cy5 (Amersham Pharmacia) and antidigoxigenin-Cy5 antibody (Sigma). An interferogram for each metaphase was generated by using SD200 Spectracube (Applied Spectral Imaging) mounted on a Zeiss Axioscope II fluorescent microscope, equipped with a custom-made optical filter (Chroma Technology, Brattleboro, VT). Spectral information, upon recovery by Fourier transformation, was used to produce multicolor digital image with red, green, and blue colors assigned to certain ranges of recorded spectrum. Further analysis and classification were performed in sky view 1.5 karyotyping software (Applied Spectral Imaging) by using a Windows NT Workstation.
Flow Cytometric Analysis.
Bone marrow and spleen cells were isolated from mice dying with APL, and also from (C3H × Bl/6) F1 and wild-type littermate animals, to characterize the percentage of cells expressing Gr-1 and/or CD34. Murine antibodies for Gr-1, CD34, and their isotype controls were purchased from PharMingen. One microgram of each antibody was used to stain 1 × 106 normal or APL cells, following the manufacturer's instructions. Cells were analyzed by using a Becton Dickenson FACScan and cell quest analysis software.
Morphologic Analysis.
Normal spleen and bone marrow and APL cells were affixed to slides by using a Shandon Cytospin apparatus and stained with Wright-Giemsa stain (Sigma) per the manufacturer's protocol. All stained slides were evaluated at ×1,000 magnification, and cells were scored morphologically as either early myeloid (blast, promyelocyte, myelocyte), late myeloid (metamyelocyte, band, neutrophil), or as another cell type (i.e., lymphocyte, eosinophil, red cell precursor). Photography of stained slides was performed with an Optronics digital camera and assembled with Adobe photoshop software.
Results
Frequent Deletions of Chromosome 2 in APL Cells Derived from PR/RP Mice.
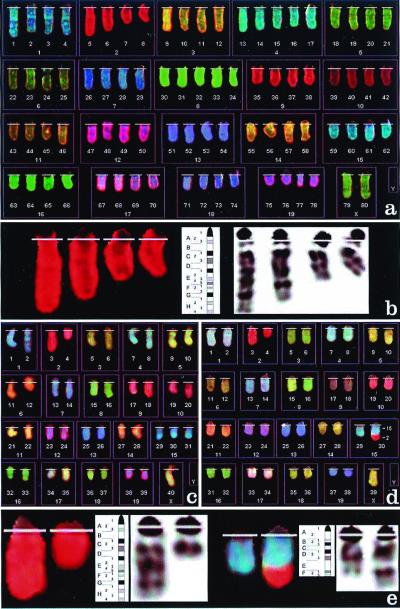
We analyzed tumor cells from 18 freshly harvested or cryopreserved tumor samples by using SKY and G banding. As shown in Table 1, 12/18 APL samples contained either an interstitial [del(2)(E2H1)] or terminal [del(2)(Dter)] deletion in one copy of chromosome 2 (Fig. 1). Eleven of 13 tumors expressing PR and RP contained cells with deletion 2, compared with 1/5 tumors expressing PR only (P < 0.05). Additional painting with a chromosome 2-specific probe was performed on all 12 tumors containing del(2), to more accurately assess the frequency of this deletion. As shown in Table 1, 9/12 tumors displayed del(2) in 100% of metaphases examined (with an average of 27 metaphases examined per tumor). In the only PR tumor with del(2) (12705), two cellular populations were identified: one was diploid, and 0/19 cells in this population displayed del(2). The other was tetraploid, with 16/16 cells displaying del(2) in two of four copies of chromosome 2. In the remaining three tumors, del(2) was present in 12/29, 21/28, or 19/34 metaphases examined. In the tumor derived from mouse 10826, the deletion of chromosome 2 was accompanied by an unbalanced translocation t(2;15) (G-ter;E), as shown in Fig. 1d. The size of the translocated fragment was smaller than the missing part of chromosome 2 (Fig. 1e), suggesting that band F (and possibly the adjacent proximal subband E5) was lost.
Table 1.
Chromosomal abnormalities detected by SKY splenic tumor cells derived from animals dying with APL
| Animal | PR | RP | Sex | Age | Stage | DEL 2* | Location | Chr no. | Gains
|
Losses
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 15 | Other | 11 | X | Y | Random | |||||||||
| 13123 | + | − | M | 104 | 3 | 0/8 | – | 29–40 | 0/8 | 0/8 | 0/8 | 2/8 | 3/8 | 1/8 | 2/8 |
| 10760 | + | − | M | 144 | 3 | 0/7 | – | 33–40 | 0/7 | 0/7 | 1/7 | 3/7 | 0/7 | 0/7 | 2/7 |
| 11309 | + | − | M | 397 | 3 | 0/5 | – | 39–40 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 3/5 |
| 12070 | + | − | M | 315 | 2 | 0/6 | – | 38–40 | 0/6 | 0/6 | 0/6 | 1/5 | 0/6 | 0/6 | 3/6 |
| 12705a | + | − | F | 199 | 2 | 0/19 | – | 38–40 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | N/A | 1/5 |
| 12705b | + | − | F | 199 | 2 | 16/16 | 2E2-2H1 | 73–78 | 2/5 | 2/5 | 1/5 | 3/5 | 5/5 | N/A | 5/5 |
| 13095 | + | + | M | 162 | 3 | 0/6 | – | 36–40 | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 3/6 |
| 13080 | + | + | M | 163 | 2 | 0/6 | – | 35–40 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 5/6 |
| 13107 | + | + | M | 113 | 2 | 12/29 | 2E2-2H1 | 37–40 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 2/7 | 1/7 |
| 10822 | + | + | M | 143 | 3 | 31/31 | 2D-ter | 35–40 | 0/6 | 0/6 | 2/6 | 0/6 | 0/6 | 3/6 | 2/6 |
| 10759 | + | + | M | 170 | 3 | 25/25 | 2E2-2H1 | 58–80 | 1/7 | 1/7 | 3/7 | 2/7 | 1/7 | 7/7 | 6/7 |
| 11903 | + | + | M | 180 | 2 | 21/28 | 2E2-2H1 | 32–40 | 0/7 | 2/7 | 2/7 | 1/7 | 1/7 | 1/7 | 3/7 |
| 11025 | + | + | M | 180 | 3 | 19/34 | 2E2-2H1 | 37–40 | 0/9 | 1/9 | 0/9 | 0/9 | 1/9 | 3/9 | 2/9 |
| 11908 | + | + | M | 182 | 2 | 28/28 | 2D-ter | 39–80 | 0/6 | 2/6 | 1/6 | 0/6 | 0/6 | 5/6 | 3/6 |
| 10552 | + | + | M | 194 | 1 | 25/25 | 2E2-2H1 | 35–42 | 4/6 | 5/6 | 0/6 | 0/6 | 1/6 | 5/6 | 3/6 |
| 10292 | + | + | M | 206 | 2 | 31/31 | 2D-ter | 58–77 | 1/8 | 0/8 | 0/8 | 4/8 | 1/8 | 8/8 | 8/8 |
| 10299 | + | + | M | 232 | 2 | 29/29 | 2E2-2H1 | 32–42 | 0/8 | 3/8 | 8/8 | 2/8 | 4/8 | 0/8 | 6/8 |
| 10826 | + | + | M | 415 | 2 | 22/22 | 2E2-2H1† | 32–80 | 0/5 | 0/5 | 0/5 | 3/5 | 1/5 | 5/5 | 4/5 |
| 10836 | + | + | M | 469 | 1 | 26/26 | 2D-ter | 51–73 | 0/6 | 3/6 | 1/6 | 6/6 | 1/6 | 6/6 | 6/6 |
PR indicates that mice contain the hCG-PML-RARα transgene, and RP indicates that mice have the hCG-RARα-PML transgene. Male (M) and female (F) mice were analyzed. Predominantly male animals were used to generate the APL tumor bank for an independent immunology-based study. Age indicates the age (in days) at the time of death from APL. Stage was defined using the ratio of Gr-1l0/−/CD34+ cells to the total CD34+ cells in the splenic tumors. Ratios of 0.0–0.125 were designated stage 1, 0.125–0.25 were stage 2, and >0.25 were stage 3. A higher stage therefore indicates that the tumor is less differentiated. N/A = not applicable. Chr, chromosome.
For all chromosomal abnormalities tested, the number of mitoses with the abnormality is shown over the total number of mitoses examined. For tumors containing del 2 on the initial screen, a large number of extra mitoses were evaluated specifically for del 2 status.
A complete description of the deletion of chromosome 2 is provided in Fig. 1 B and E.
Figure 1.
(a) Spectral karyotype of a tetraploid cell from mouse 10759, containing an interstitial deletion of chromosome 2 as the only structural abnormality. (b) Localization of the chromosome 2 interstitial deletion from a. The comparison of chromosome's inverted 4′,6-diamidino-2-phenylindole G-banding pattern and the G-banding ideogram for mouse chromosome 2 allows the identification of the deletion as del(2)(E2H1). (c) Spectral karyotype of a pseudodiploid tumor cell from mouse 11908, containing a deletion of chromosome 2 del(2)(Dter), an extra chromosome 15, and loss of the Y chromosome. (d) Spectral karyotype of a hypodiploid cell from mouse 10826, containing a translocation 2;15 as the only structural alteration, and loss of a sex chromosome. (e) Localization of the breakpoints of the deleted chromosome 2 and derivative 15 from d. Chromosome 2 (Left) is deleted at band 2D (as in c), and chromosome 15 (Right) at band 15E. The translocated fragment from chromosome 2 appears to span bands 2G through the terminus. Therefore, the abnormality is defined as translocation t(2;15) (G-ter;E) and most likely involves loss of chromosome 2 material containing bands 2E and 2F.
Because our transgenic animals were derived on a mixed C3H × C57BL/6 background, we wanted to determine whether the deletions of chromosome 2 were occurring randomly, or whether they were restricted to one strain or the other. We therefore determined the origins of chromosome 2 in each tumor by using PCR to amplify allele-specific products, defined by at least 10 microsatellite-length polymorphisms (Research Genetics, Huntsville, AL). Of the six APL samples without deletion of chromosome 2, chromosome 2 was Bl/6×Bl/6 in three, C3H×C3H in two, and Bl/6×C3H in one. Tumors that contained cells with del(2) were analyzed for the origin of the remaining chromosome 2. In two samples, the residual chromosome was derived from Bl/6, and in four others, the residual chromosome was derived from C3H. For the six remaining tumors, both C3H and Bl/6-derived genetic material was detected (probably from residual normal cells in the sample), so the origin of the residual chromosome 2 could not be determined.
Additional Chromosomal Gains and Losses in APL Cells Containing del(2).
Chromosome 2 deletions were highly associated with additional abnormalities involving chromosome copy number (P < 0.005). We calculated the frequency of nonrandom changes based on a minimum of two cells from a tumor displaying the same abnormality. In the six tumors without del(2), no nonrandom chromosomal gains were noted, and none of the tumors was tetraploid. In contrast, del(2) tumors were associated with chromosomal copy-number gains (P < 0.001), losses (P < 0.005), and tetraploidy (P < 0.001). Six of 12 del(2)-containing tumors also exhibited a specific gain of chromosome 15 (P = 0.001), and three of these six also exhibited gain of chromosome 6 in some cells (Table 1). In addition, 6/12 tumors with del(2) displayed near tetraploidy, which was not seen in the absence of del(2) (P < 0.001).
Evaluation of chromosomal losses in the APL samples was more difficult, because random losses were noted in a small fraction of cells derived from normal animals and also in nonleukemic transgenic animals (see below). Four losses occurred in at least six of the 12 tumors with del(2): loss of 11 (6/12), loss of 12 (6/12), loss of 17 (6/12), and loss of X/Y (11/12). However, loss of 12 and loss of 17 were observed at a similar frequency in the bone marrow cells of nontransgenic and nonleukemic animals, and therefore these losses were not leukemia-specific. Loss of 11 was detected in 52/123 metaphases examined in leukemic samples, compared with 2/66 metaphases from nonleukemic animals (P < 0.001). Loss of X/Y was seen in 59/123 leukemia cell metaphases, compared with 6/66 nonleukemic cells (P < 0.001).
Deletion 2 appeared to occur in tumors before additional secondary changes occurred. For example, in tumor 10552, which expresses both PR and RP, del(2) was detected in all metaphases examined (Table 2). One of these metaphases contained no additional genetic abnormalities, and another contained gain of 15 only. In the remainder of the cells, the cells have gained 15 and also six. This finding suggests that in this tumor, del(2) occurred first, and that gains of 15 and then six provided a survival advantage or proliferative advantage to the transformed cells. A second clear example of this phenomenon is provided by tumor 12705 (Table 2). In the spleen cells derived from this animal, two dominant leukemic clones were clearly present. One had not acquired del(2), and no nonrandom chromosomal abnormalities were detected, other than loss of one copy of chromosome 11 in one cell. In the other clone, del(2) was present in 16/16 cells examined. All of these cells were also tetraploid, and two of the four copies of chromosome 2 were deleted at the same position, suggesting that tetraploidy developed after del(2). A large variety of additional chromosomal gains and losses were present in individual cells from this tumor, suggesting that genomic instability followed the acquisition of del(2).
Table 2.
Chromosomal abnormalities detected by SKY in the individual metaphases of two informative APML samples
| Animal | Mitosis number | Sex chr | Chr no. | DEL (2) | No. of Chr 2 | No. of DEL 2 | DEL 2 LOC | Chr gained | Chr lost |
|---|---|---|---|---|---|---|---|---|---|
| 10522 | 1 | 0 | 2n = 36 | + | 2 | 1 | 2E2-2H1 | 0 | 8, 12, X, Y |
| 2 | XY | 2n = 42 | + | 2 | 1 | 2E2-2H1 | 6, 15 | 0 | |
| 3 | X | 2n = 40 | + | 2 | 1 | 2E2-2H1 | 6, 15 | 12, Y | |
| 4 | X | 2n = 35 | + | 2 | 1 | 2E2-2H1 | 15 | 3, 5, 9, 11, 12, Y | |
| 5 | X | 2n = 40 | + | 2 | 1 | 2E2-2H1 | 6, 15 | 4, Y | |
| 6 | X | 2n = 41 | + | 2 | 1 | 2E2-2H1 | 6, 15 | Y | |
| 12705 | 1 | XX | 4n = 76 | + | 3 | 1 | 2E2-2H1 | 6 | 2, 14, X, X |
| 2 | XX | 4n = 75 | + | 4 | 2 | 2E2-2H1 | 6 | 3, 10, 11, 14, X, X | |
| 3 | XX | 4n = 78 | + | 4 | 2 | 2E2-2H1 | 17 | 14, X, X | |
| 4 | 0 | 4n = 74 | + | 4 | 2 | 2E2-2H1 | 15 | 9, 11, 13, X, X, X, X | |
| 5 | XX | 4n = 73 | + | 4 | 2 | 2E2-2H1 | 15 | 1, 3, 11, 14, 16, 17, X, X | |
| 6 | XX | 2n = 39 | − | 2 | – | – | 0 | 11 | |
| 7 | XX | 2n = 38 | − | 2 | – | – | 0 | 12, 17 | |
| 8 | XX | 2n = 40 | − | 2 | – | – | 0 | 0 | |
| 9 | XX | 2n = 38 | − | 2 | – | – | 0 | 5, 19 | |
| 10 | XX | 2n = 40 | − | 2 | – | – | 0 | 0 |
Chr, chromosome.
Occasional Random Chromosomal Losses in the Bone Marrow Cells of Wild-Type and Nonleukemic Transgenic Animals.
Bone marrow cells of nontransgenic or RP expressing, littermate-matched animals (109–156 days old) were analyzed with SKY and G banding and found to have normal chromosome numbers (Table 3). Random chromosomal losses may have been caused by artifacts intrinsic to the chromosomal preparation process. In age-matched PR/RP littermates (at high risk for APL development), similar random losses were seen in a small fraction of bone marrow cells. Two 520-day-old PR/RP nonleukemic animals were evaluated. These animals displayed the myeloproliferative phenotype (>85% of bone marrow cells were of myeloid origin in both animals), but their bone marrow cells contained no nonrandom chromosomal abnormalities.
Table 3.
Chromosomal abnormalities detected by SKY in the bone marrow cells of nontransgenic animals or nonleukemic transgenic animals
| Animal | PR | RP | Age | Stage | DEL 2 | Location | Chr no. | Gains
|
Losses
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 15 | Other | 11 | X/Y | Random | ||||||||
| 13113 | − | − | 109 | N/A | 0/7 | N/A | 37–40 | 0/7 | 0/7 | 0/7 | 0/7 | 1/7 | 2/7 |
| 13104 | − | + | 156 | N/A | 0/7 | N/A | 34–40 | 0/7 | 0/7 | 0/7 | 1/7 | 0/7 | 3/7 |
| 13105 | − | + | 156 | N/A | 0/6 | N/A | 31–40 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 |
| 13106 | + | + | 113 | N/A | 0/8 | N/A | 35–40 | 0/8 | 0/8 | 0/8 | 1/8 | 1/8 | 7/8 |
| 13101 | + | + | 156 | N/A | 0/6 | N/A | 27–40 | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 2/6 |
| 13102 | + | + | 156 | N/A | 0/5 | N/A | 38–40 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 2/5 |
| 13103 | + | + | 156 | N/A | 0/7 | N/A | 29–40 | 0/7 | 0/7 | 0/7 | 0/7 | 1/7 | 2/7 |
| 11414 | + | + | 520 | N/A | 0/10 | N/A | 32–40 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 7/10 |
| 11415 | + | + | 520 | N/A | 0/10 | N/A | 19–40 | 0/10 | 0/10 | 0/10 | 0/10 | 2/10 | 7/10 |
N/A indicates not applicable. Chr, chromosome.
Staging of APL.
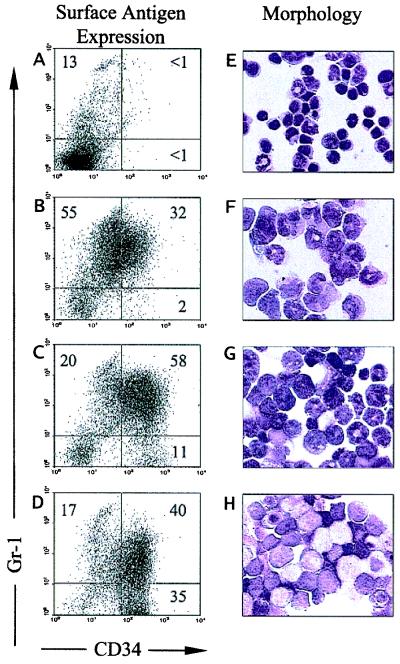
The APL tumors were classified based on the expression of the myeloid lineage marker Gr-1 and the primitive stem cell marker CD34 (Fig. 2). Recently, several groups have reported CD34 expression on human APL cells (20, 21), although earlier studies suggested that APL cells did not express CD34 (22). APL tumor cells derived from PR or PR/RP transgenic animals contain a large abnormal population of spleen cells that express the surface antigen CD34 (12). Normal spleens contain <1–2% CD34+ cells (Fig. 2A). A significant subset of tumor cells also express the late myeloid antigen Gr-1, either alone or in conjunction with CD34. The most primitive cells in the tumor populations are Gr-1lo/−/CD34+. To compare the relative level of differentiation of the tumors used in this study, we calculated the ratio of Gr-1lo/−/CD34+ cells to total CD34+ cells. Tumors with a ratio of 0–0.125 were highly differentiated (Fig. 2B, stage score 1), tumors with a ratio of 0.125–0.25 were intermediate (Fig. 2C, stage score 2), and tumors with ratios of 0.25 or greater were the most immature (Fig. 2D, stage score 3). These findings were confirmed morphologically (Fig. 2).
Figure 2.
Staging of APL tumors. (A--D) Flow cytometric analysis of spleen cells from a wild-type mouse (A) or spleen cells from mice dying with APL (B–D) for expression of Gr-1 and CD34. (E–H) Wright-Giemsa stains of the same splenic cells shown in A–D. Samples are as follows: (A and E) wild-type spleen; (B and F) a highly differentiated stage 1 tumor from mouse 10836; (C and G) an intermediate stage 2 APL tumor from mouse 13080; (D and H) an immature stage 3 APL tumor from mouse 11309.
The Age at Death from APL and Tumor Stage Do Not Correspond with Chromosomal Abnormalities.
To determine whether the presence of secondary cytogenetic abnormalities influences the age of onset of disease, we compared the age (in days) of animals at the time of leukemic death with the cytogenetic findings. No correlation was noted between any cytogenetic finding and the age of death from APL [for example, the average age of death was 199 ± 75 days for animals without del(2), and 223 ± 102 days for animals with del(2) APL]. We also correlated APL stage (as defined above) with cytogenetic abnormalities. No chromosomal abnormalities were associated with an undifferentiated phenotype. The average stage score was 2.57 ± 0.5 for tumors without del(2) vs. 2.08 ± 0.6 for tumors with del(2). Finally, complete blood counts from six mice with APL and del(2) were compared with six APL mice without del(2). There was no correlation between total white blood counts, hemoglobin levels, or platelet counts and the presence of del(2) (data not shown).
Discussion
In this report, we examined the tumor cells of transgenic mice dying from APL for evidence of secondary genetic changes. Mice that expressed only PML-RARα in early myeloid cells usually progressed to acute leukemia without the acquisition of secondary chromosomal abnormalities. However, in transgenic mice that expressed both PML-RARα and RARα-PML, 11 of 13 animals acquired a nonrandom deletion of the distal part of chromosome 2, a region that is syntenic with human chromosome 20, and part of human chromosome 15 (23). The leukemic cells that contained del(2) frequently contained additional abnormalities associated with the leukemia, such as specific chromosomal gains, losses, or near tetraploidy. These results therefore revealed that expression of the RARα-PML fusion protein in early myeloid cells is strongly associated with the development of del(2) in animals that develop APL, and that del(2) is further associated with additional chromosomal changes that usually involve alterations in chromosome number. These secondary chromosomal changes may provide an advantage to the transformed cells by mechanisms that are not yet understood.
Most human patients with APL (M3) have the t(15;17)(q22;q11.2–12) translocation. Virtually 100% of the patients express PML-RARα, and at least 80% express the reciprocal RARα-PML (8–11). However, 30–45% of newly diagnosed t(15;17) APL patients contain cells with additional secondary chromosomal abnormalities (10, 15–17). The most common of these is trisomy 8, which occurs in 15–40% of APL tumors in most series examined (10, 15–17). Trisomy 8 frequently is detected in chronic and acute myeloid leukemias and several solid tumors, as a primary or secondary abnormality (reviewed in ref. 10). The mechanism(s) by which the extra copy of chromosome 8 contributes to leukemogenesis is unknown. However, the minimal essential region of 8 that provides an advantage to transformed cells is found on the long arm (8q); part of the chromosome 8q syntenic region is located on mouse chromosome 15, and the remainder is found on mouse chromosomes 3 and 4 (23). Additional secondary chromosomal abnormalities [such as del(17p) and del(7q)] have been identified in APL cells, but none are known to occur as frequently as trisomy 8 (10, 15–17).
In our study, del(2) occurred more frequently in tumors expressing both transgenes. The strong correlation between RP expression and del(2) suggest that the two could be functionally associated. However, this association occurs only in the context of APL driven by PML-RARα; RARα-PML expression alone does not alter myeloid development, cause leukemia, or independently cause del(2) or genomic instability (ref. 12 and Table 3). We previously have shown that mice that express PR and RP have a greater likelihood of developing APL, and that the tumors that develop in these animals are more biologically aggressive (12). The mechanism of this phenomenon is unknown. However, recent studies have shown that the carboxyl-terminal region of PML (a region that is present in the RARα-PML fusion protein) is capable of interacting with the Rb protein and repressing its function (24). It is not clear to us at this time how suppression of Rb function could predispose to del(2), or whether these events are even related.
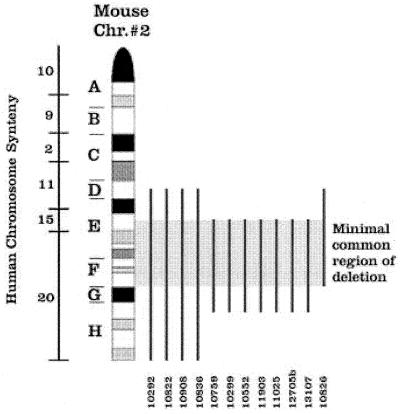
The most common secondary genetic alteration noted in this series is the acquisition of a deletion of the distal part of chromosome 2, a region that is syntenic with parts of human chromosomes 11 and 15, and all of human chromosome 20 (Fig. 3). Seven tumors had interstitial deletion involving bands 2(E2H1), four had a terminal deletion 2(Dter), and one tumor had a translocation t(2;15)(G-ter;E) rearrangement, resulting in the loss of the region 2(E1-F3). It is possible that del(2)(Dter) is also an interstitial deletion that retains a small part of the distal region of chromosome 2, including the telomere. Regardless, the minimal common region of deletion for all 12 of the del(2)-positive tumors is confined to region 2E-2F, which is mostly syntenic to human 20p, but also to parts of human 15q13–23. Although the human PML gene resides on 15q22, the mouse PML gene has been mapped to chromosome 9 by using somatic cell hybrids (25); therefore, the del(2) described here does not appear to cause haploinsufficiency for PML. Although deletions of 15q have not been described in APL patients, del(15q) has been detected in a small number of patients with AML and lymphoid malignancies (26); in addition, a tumor suppressor locus has been mapped to 15q21.1 in a sporadic form of colorectal cancer (27). Similarly, del(20p) has not been described in APL patients. Small interstitial deletions of the long arm of chromosome 20 (20q− syndrome) have been detected in patients with myeloproliferative or myelodysplastic syndromes (28–34), but the minimal commonly deleted region is found at 20q11.2–12 (33, 34). The mouse region syntenic to human 20q11.2–12 is deleted in the four tumors with del(2)(Dter), but it is not in the seven tumors with del(2)(E2H1) or the tumor with t(2;15)(G-ter;E). The minimal common region of del(2) is currently very large (≈20 cM); the analysis of additional deletions [or of the breakpoint region in the t(2;15) containing tumor] may provide important clues regarding the relevant loci. Del(2) occurred on only one half of chromosomes 2 in every tumor studied, similar to the 5q− and 20q− syndromes, where the deletion always occurs in only one copy of the chromosome (28–34). Therefore, the phenotype in these syndromes may be associated with haploinsufficiency of the critical gene(s) [which has recently been described for a familial AML syndrome (35)], or the second allele of the critical gene(s) is mutated in a fashion that cannot be detected by gross chromosomal analyses.
Figure 3.
Summary of chromosome 2 deletions. Regions of synteny between mouse chromosome 2 and human chromosomes are shown. The deleted portion of chromosome 2 is designated by the vertical line for each APL tumor. In the tumor derived from mouse 10826, the distal part of chromosome 2 (G-ter) is translocated to chromosome 15. The minimal common region of deletion for all 12 tumors therefore is confined within the region 2E2-F3.
Patients with APL or chronic myelogenous leukemia frequently acquire secondary chromosomal abnormalities during leukemia progression, which has led to the hypothesis that the fusion proteins responsible for these diseases might directly cause genomic instability (17, 18, 36). The PML gene has been shown to interact with the product of a gene associated with Bloom's syndrome (37), which is associated with genomic instability, sister chromatid exchanges, and an increased incidence of cancer (38). However, we did not detect nonrandom chromosomal changes in 520-day-old nonleukemic mice expressing both PR and RP, which suggests that these transgenes do not directly cause genomic instability.
Because the transgenes in this study were randomly integrated, it is possible that genetic alterations at the transgene insertion sites could contribute to the observed phenotypes; however, the same phenotypes were observed in three independent crosses of PR and RP, in which the insertion sites of the transgenes differed. In addition, because our mice were made in a C3H × Bl/6 background, it was difficult to know whether some of the secondary genetic changes were related to strain. We performed an extensive characterization of the origin of chromosome 2 in each tumor and did not detect an association between strain and del(2). In tumors where del(2) was not present, we did not detect homozygosity of chromosome 2 from a single strain (which would have suggested that one of the strains contained a germ-line mutation of a tumor suppressor on chromosome 2). Similarly, in tumors with del(2), we did not find that the remaining chromosome 2 was always derived from a single strain (which again would imply that the remaining chromosome contained a germ-line mutation in a tumor suppressor, and that the deletion on the other chromosome had caused loss of heterozygosity). Moreover, Kogan and Bishop and their colleagues recently have evaluated their mouse model of APL for secondary genetic changes, by using comparative genomic hybridization (personal communication). In their model, the same bcr-1-derived PML-RARα cDNA is driven by the MRP-8 promoter that is expressed in both early and late myeloid cells; their model was produced in FVB/N mice (4). Those investigators also identified APL tumors that contained loss of genetic information on chromosome 2, gain of 15, and loss of the sex chromosomes (as well as other chromosomal gains and losses). Therefore, some similar nonrandom changes were found in APL cells derived from a different PR transgenic model, making it unlikely that the changes reported here are caused by transgenic integration sites, transgene expression patterns, or strain-dependent factors.
In summary, our results have suggested that expression of RARα-PML, in conjunction with PML-RARα, may somehow facilitate the development of secondary genetic events that provide an advantage for cells expressing these transgenes. The loss of material from chromosome 2 was strongly associated with the development of additional genetic abnormalities, including a gain of chromosome 15 [containing a region syntenic with part of human 8q (23)], the loss of chromosome 11 [resulting in haplo-insufficiency for p53 (39), which may be a progression factor for AML (40) and chronic myelogenous leukemia (41–43)], and abnormalities in chromosome number. Additionally, expression of PML-RARα and RARα-PML may further inhibit p53 activation and cell senescence, because intact PML plays a critical role in p53 regulation (44). The similarities between the secondary genetic changes observed in humans and mice suggest that similar mechanisms may contribute to APL progression in both species.
Acknowledgments
We thank Drs. Timothy A. Graubert and Philip Miller for their help with data presentation and statistical studies. We acknowledge Nancy Reidelberger for providing outstanding administrative and secretarial skills. We thank Pam Goda and Kelly Schrimpf for their husbandry of the animals in our transgenic mouse facility. J.L.P. was supported by National Institutes of Health Training Grant T32 HL07088. P.W. was supported by National Institutes of Health Grant KO8 HL03991, and T.J.L. was supported by National Institutes of Health Grant CA49712 and the Buder Foundation.
Abbreviations
- APL
acute promyelocytic leukemia
- PML
promyelocytic leukemia
- RARα
retinoic acid receptor
- PR
PML-RARα
- RP
RARα-PML
- SKY
spectral karyotyping
References
- 1.Lo Coco F, Diverio D, Falini B, Biondi A, Nervi C, Pelicci P G. Blood. 1999;94:12–22. [PubMed] [Google Scholar]
- 2.Melnick A, Licht J D. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 3.Grisolano J L, Wesselschmidt R L, Pelicci P G, Ley T J. Blood. 1997;89:376–387. [PubMed] [Google Scholar]
- 4.Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci P G, Atwater S, Bishop J M. Proc Natl Acad Sci USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L-Z, Tribioli C, Rivi R, Peruzzi D, Pelicci P G, Soares V, Cattoretti G, Pandolfi P P. Proc Natl Acad Sci USA. 1997;94:5302–5307. doi: 10.1073/pnas.94.10.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L-Z, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi P P. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G-X, Zhu X-H, Men X-Q, Wang L, Huang Q-H, Jin X L, Xiong S-M, Zhu J, Guo W-M, Chen J-Q, et al. Proc Natl Acad Sci USA. 1999;96:6318–6323. doi: 10.1073/pnas.96.11.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcalay M, Zangrilli D, Fagioli M, Pandolfi P P, Mencarelli A, Lo Coco F, Biondi A, Grignani F, Pelicci P G. Proc Natl Acad Sci USA. 1992;89:4840–4844. doi: 10.1073/pnas.89.11.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow J, Goddard A D, Gibbons B, Katz F, Swirsky D, Fioretos T, Dube I, Winfield D A, Kingston J, Hagemeijer A, et al. Br J Haematol. 1992;82:529–540. doi: 10.1111/j.1365-2141.1992.tb06463.x. [DOI] [PubMed] [Google Scholar]
- 10.Grimwade D, Howe K, Langabeer S, Davies L, Oliver F, Walker H, Swirsky D, Wheatley K, Goldstone A, Burnett A, Solomon E. Br J Haematol. 1996;94:557–573. [PubMed] [Google Scholar]
- 11.Li Y-P, Andersen J, Zelent A, Rao S, Paietta E, Tallman M S, Wiernik P H, Gallagher R E. Blood. 1997;90:306–312. [PubMed] [Google Scholar]
- 12.Pollock J L, Westervelt P, Kurichety A K, Pelicci P G, Grisolano J L, Ley T J. Proc Natl Acad Sci USA. 1999;96:15103–15108. doi: 10.1073/pnas.96.26.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heim S, Mitelman F. In: Cancer Cytogenetics. Heims S, Mitelman F, editors. New York: Wiley; 1995. pp. 19–32. [Google Scholar]
- 14.Johansson B, Mertens F, Mitelman F. Genes Chromosomes Cancer. 1996;16:155–163. doi: 10.1002/(SICI)1098-2264(199607)16:3<155::AID-GCC1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Johansson B, Mertens F, Mitelman F. Leukemia. 1994;8:953–962. [PubMed] [Google Scholar]
- 16.Berger R, Le Coniat M, Derré J, Vecchione D, Jonveau P. Genes Chromosomes Cancer. 1991;3:332–337. doi: 10.1002/gcc.2870030503. [DOI] [PubMed] [Google Scholar]
- 17.Slack J L, Arthur D C, Lawrence D, Mrózek K, Mayer R J, Davey F R, Tantravahi R, Pettenati M J, Bigner S, Carroll A J, et al. J Clin Oncol. 1997;15:1786–1795. doi: 10.1200/JCO.1997.15.5.1786. [DOI] [PubMed] [Google Scholar]
- 18.Laneuville P, Sun G, Timm M, Vekemans M. Blood. 1992;80:1788–1797. [PubMed] [Google Scholar]
- 19.Schröck E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith M A, Ning Y, Ledbetter D H, Bar-Am I, Soenksen D, et al. Science. 1996;273:494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 20.Edwards R H, Wasik M A, Finan J, Rodriguez R, Moore J, Kamoun M, Rennert H, Bird J, Nowell P C, Salhany K E. Am J Clin Pathol. 1999;112:819–827. doi: 10.1093/ajcp/112.6.819. [DOI] [PubMed] [Google Scholar]
- 21.Orfao A, Chillón M C, Bortoluci A M, López-Berges M C, García-Sanz R, Gonzalez M, Tabernero M D, García-Marcos M A, Rasillo A I, Hernández-Rivas J, San Miguel J F S. Haematologica. 1999;84:405–412. [PubMed] [Google Scholar]
- 22.Sanz M A, Sempere A. Bailliere's Clin Haematol. 1996;9:35–55. doi: 10.1016/s0950-3536(96)80036-9. [DOI] [PubMed] [Google Scholar]
- 23.DeBry R W, Seldin M F. Genomics. 1996;33:337–351. doi: 10.1006/geno.1996.0209. [DOI] [PubMed] [Google Scholar]
- 24.Alcalay M, Tomassoni L, Colombo E, Stoldt S, Grignani F, Fagioli M, Szekely L, Helin K, Pelicci P G. Mol Cell Biol. 1998;18:1084–1093. doi: 10.1128/mcb.18.2.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goddard A D, Yuan J Q, Fairbairn L, Dexter M, Borrow J, Kozak C, Solomon E. Mamm Genome. 1995;6:732–737. doi: 10.1007/BF00354296. [DOI] [PubMed] [Google Scholar]
- 26.Yahata N, Ohyashiki K, Iwase O, Kimura Y, Kodama A, Fukutake K, Toyama K. Leukemia Res. 1998;22:845–847. doi: 10.1016/s0145-2126(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 27.Park W S, Park J Y, Oh R R, Yoo N J, Lee S H, Shin M S, Lee H K, Han S, Yoon S K, Kin S K, et al. Cancer Res. 2000;60:70–73. [PubMed] [Google Scholar]
- 28.Heim S, Mitelman F. In: Cancer Cytogenetics. Heim S, Mitelman F, editors. New York: Wiley; 1995. pp. 69–140. [Google Scholar]
- 29.Nacheva E, Holloway T, Carter N, Grace C, White N, Green A R. Cancer Genet Cytogenet. 1995;80:87–94. doi: 10.1016/0165-4608(94)00150-a. [DOI] [PubMed] [Google Scholar]
- 30.Kurtin P J, Dewald G W, Shields D J, Hanson C A. J Clin Pathol. 1996;106:680–688. doi: 10.1093/ajcp/106.5.680. [DOI] [PubMed] [Google Scholar]
- 31.Roulston D, Espinosa R, III, Stoffel M, Bell G I, Le Beau M M. Blood. 1993;82:3424–3429. [PubMed] [Google Scholar]
- 32.Asimakopoulos F A, Green A R. Br J Haematol. 1996;95:219–226. doi: 10.1046/j.1365-2141.1996.d01-1896.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang P W, Iannantuoni K, Davis E M, Espinosa R, III, Stoffel M, Le Beau M M. Genes Chromosome Cancer. 1998;21:75–81. [PubMed] [Google Scholar]
- 34.Bench A J, Aldred M A, Humphray S J, Champion K M, Gilbert J G R, Asimakopoulos F A, Deloukas P, Gwilliam R, Bentley D R, Green A R. Genomics. 1998;49:351–362. doi: 10.1006/geno.1998.5231. [DOI] [PubMed] [Google Scholar]
- 35.Song W-J, Sullivan M G, Legare R D, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende I C, Haworth C, Hock R, et al. Nat Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein R. Semin Hematol. 1988;25:20–34. [PubMed] [Google Scholar]
- 37.Zhong S, Hu P, Ye T-Z, Stan R, Ellis N A, Pandolfi P P. Oncogene. 1999;18:7941–7947. doi: 10.1038/sj.onc.1203367. [DOI] [PubMed] [Google Scholar]
- 38.Vessey C J, Norbury C J, Hickson I D. Progr Nucleic Acid Res Mol Biol. 2000;63:189–221. doi: 10.1016/s0079-6603(08)60723-0. [DOI] [PubMed] [Google Scholar]
- 39.Munke M, Francke U. J Mol Evol. 1987;25:134–140. doi: 10.1007/BF02101755. [DOI] [PubMed] [Google Scholar]
- 40.Kurosawa M, Okabe M, Kunieda Y, Asaka M. Ann Hematol. 1995;71:83–87. doi: 10.1007/BF01699251. [DOI] [PubMed] [Google Scholar]
- 41.Kelman Z, Prokocimer M, Peller S, Kahn Y, Rechavi G, Manor Y, Cohen A, Rotter V. Blood. 1989;74:2318–2324. [PubMed] [Google Scholar]
- 42.Mashal R, Shtalrid M, Talpaz M, Kantarjian H, Smith L, Beran M, Cork A, Trujillo J, Gutterman J, Deisseroth A. Blood. 1990;75:180–189. [PubMed] [Google Scholar]
- 43.Stuppia L, Calabrese G, Peila R, Guanciali-Franchi P, Morizio E, Spadano A, Palka G. Cancer Genet Cytogenet. 1997;98:28–35. doi: 10.1016/s0165-4608(96)00413-x. [DOI] [PubMed] [Google Scholar]
- 44.Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, Higashimoto Y, Apella E, Minucci S, Pandolfi P P, Pelicci P G. Nature (London) 2000;13:207–210. doi: 10.1038/35018127. [DOI] [PubMed] [Google Scholar]