Abstract
Heterologous expression allows the production of plant proteins in an organism which is simpler than the natural source. This technology is widely used for large-scale purification of plant proteins from microorganisms for biochemical and biophysical analyses. Additionally expression in well-defined model organisms provides insights into the functions of proteins in complex pathways. The present review gives an overview of recombinant plant protein production methods using bacteria, yeast, insect cells, and Xenopus laevis oocytes and discusses the advantages of each system for functional studies and protein characterization.
1. Introduction
Heterologous expression involves identification of genes and transfer of the corresponding DNA fragments to hosts other than the original source for synthesis of the encoded proteins. Protein isolation, especially from plant sources, can be costly, cumbersome and lengthy, and heterologous expression provides a convenient alternative. This methodology allows large-scale production of plant proteins in microorganisms to study their biochemical and biophysical features. Foreign hosts may also provide a simpler system for studies on functions of proteins and for elucidation of their roles in complex mechanisms such as metabolic reactions and membrane transport. Recombinant plant proteins and peptides produced by heterologous expression are also used in industrial applications. Examples are provided by the synthesis of a medicinal peptide from ginseng as potential drug against diabetes [1] or production of plant lectins [2] in both cases in yeast.
The present review covers the recent literature on plant gene expression in bacteria, yeast, insect cells and Xenopus oocytes and presents the comparative advantages and disadvantages of each system. It also provides a survey of recent examples of application of heterologous expression technology to plant proteins. A comprehensive list of plant proteins expressed heterologously is given in Table 1. Factors influencing the choice of hosts, including the stability and folding characteristics of the protein, requirement for posttranslational modifications, efficiency of the expression system, as well as simplicity and cost are discussed in the following sections.
Table 1.
Heterologous expression of plant proteins grouped according to the host cells.
| Protein expressed | Plant | Reference |
|---|---|---|
| Escherichia coli | ||
| Lipase B (PalB) | Pseudozyma antarctica | [18] |
| Oxalate oxidase | Hordeum vulgare, Triticum aestivum | [21] |
| Osmotin-like cryoprotective protein | Solanum dulcamara | [22] |
| Thaumatin-like protein (ATLP3) | Arabidopsis thaliana | [23] |
| Osmotin-like protein (SnOLP) | Solanum nigrum | [24] |
| RHG1-LRR domain | Glycine max | [25] |
| Chloroplast transglutaminase (TGZ) | Zea mays | [26] |
| FatB thioesterase | Madhuca butyracea | [27] |
| Glutamatecysteine ligase (GCL) | Arabidopsis thaliana | [28] |
| DELLA proteins | Arabidopsis thaliana, Malus domestica | [29] |
| K+ transporters; KAT1, AKT2-3, AtKUP1/AtKT1/AtPOT1, AtKUP2/AtKT2/AtPOT2, AtHKT1 | Arabidopsis thaliana | [30–32] |
| K+ transporters, EcHKT1, EcHKT2 | Eucalyptus globulus | [33] |
| ATP/ADP transporter | Arabidopsis thaliana | [34] |
| HAK K+ transporters, CnHAK1,CnHAK2 | Cymodocea nodosa | [35] |
| Peptide transporter family member, AgDCAT1 | Alnus glutinosa | [36] |
| Type 1 MT, dMT | Triticum durum | [37] |
| Type 1 and Type 2 MTs | Vicia faba | [38] |
| MT1, MT2, and MT3 | Arabidopsis thaliana | [39] |
| Type 3 MT3-A | Elaeis guineensis | [40] |
| Type 2 MT, QsMT | Quercus suber | [41] |
| Soybean seed ferritin | Glycine max | [125] |
| Saccharomyces cerevisiae | ||
| H+-amino acid symporter and K+ channel, KATl | Arabidopsis thaliana | [47] |
| Phosphate transporters; AtPT1 and AtPT2 | Arabidopsis thaliana | [48] |
| K+transporter, HvHAKI | Hordeum vulgare | [49] |
| K+ transporters, AtKT1, and AtKT2, AtKUP1 | Arabidopsis thaliana | [50, 51] |
| K+ transporter, HKT1 | Triticum aestivum | [52, 53] |
| Sulfate transporters, LeST1-1 and LeST1-2 | Lycopersicon esculentum | [54] |
| Copper transporters, (COPT1–5) | Arabidopsis thaliana | [55] |
| Peptide transporter, AtPTR1 | Arabidopsis thaliana | [56] |
| K+/H+ antiporter, AtChx17 | Arabidopsis thaliana | [57] |
| Hexose transporters, VvHT4 and VvHT5 | Vitis vinifera | [58] |
| Plasma membrane-localized H+/inositol symporter, AtINT2 | Arabidopsis thaliana | [59] |
| High affinity GABAtransporter, AtGAT1 | Arabidopsis thaliana | [60] |
| Tonoplast Intrinsic Proteins, AtTIP2;1 and AtTIP2;3 | Arabidopsis thaliana | [61] |
| Sorbitol transporters, PmPLT1 and PmPLT2 | Plantago major | [62] |
| Pichia pastoris | ||
| Nitrate reductase | Spinacia oleracea, Zea mays | [69] |
| Invertase | Ipomoea batatas | [70] |
| α1,6-galactosyltransferase | Trigonella foenum-graecum | [71] |
| α1,6-xylosyltransferase | Arabidopsis thaliana | [72] |
| Glycosyltransferases | Arabidopsis thaliana Bos taurus, Drosophila melanogaster, Caenorhabditis elegans, Leucopersicon esculentum | [73] |
| β-D-fructofuranosidase | Oryza sativa | [74] |
| Apyrase | Solanum tuberosum | [75] |
| Oxalate oxidases, HvOXO, TaOXO | Hordeum vulgare, Triticum aestivum | [76, 77] |
| Lectin | Canavalia brasiliensis, Nicotiana tabacum | [2, 79] |
| Low-affinity cation transporter (LCT1) | Triticum aestivum | [80] |
| 2S albumin storage proteins (AL1 and AL3) | Glycine max | [126] |
| Baculovirus-mediated insect cell | ||
| Patatin | Solanum tuberosum | [81] |
| Reductase isoforms, AR1 and AR2 | Arabidopsis thaliana | [82] |
| Peroxisomal short-chain acyl-CoA oxidase A | Arabidopsis thaliana | [83] |
| Cyclin-dependent kinase A (CDKA) | Arabidopsis thaliana | [84] |
| NADH-cytochrome (Cyt) b5 reductase | Arabidopsis thaliana | [85] |
| Geranylgeranyltransferase-I (GGT-I) | Arabidopsis thaliana | [86] |
| Acyl-CoA synthetase | Arabidopsis thaliana | [87] |
| Homogentisate phytyltransferase | Arabidopsis thaliana | [88] |
| (+)-Abscisic Acid 8’-Hydroxylase | Arabidopsis thaliana | [89] |
| β1,2-xylosyltransferase | Arabidopsis thaliana | [90] |
| Ethylene-inducing xylanase | Nicotiana tabacum | [91] |
| ADP-glucose pyrophoshorylase (AGPase) | Hordeum vulgare | [92] |
| K+ channels, AKT1, KAT1, and KCO1 | Arabidopsis thaliana | [93–95] |
| K+ channels KST1, SKT1, and KST1 | Solanum tuberosum | [96, 97] |
| Transporter AUX1 | Arabidopsis thaliana | [98] |
| β-phaseolin polypeptides | Phaseolus vulgaris | [127] |
| Ac-specific ORFa protein, | Zea mays | [128] |
| Cysteine protease papain | Carica papaya | [129] |
| Mitochondrial protein URF13 | Zea mays | [130] |
| LAT52 protein | Lycopersicon esculentum | [131] |
| Auxin-binding protein (ABP1) | Zea mays, Nicotiana tabacum | [132, 133] |
| Calreticulin and auxin binding protein | Zea mays | [134] |
| Cinnamate 4-Hydroxylase | Arabidopsis thaliana | [135] |
| Cryptochrome-1 | Arabidopsis thaliana | [136] |
| Phototropin 2 | Arabidopsis thaliana | [137] |
| Histidinol dehydrogenase | Brassica oleracea | [138] |
| Putative soluble epoxide hydrolase (sEH) | Solanum tuberosum | [139] |
| lmidazoleglycerolphosphate dehydratase | Arabidopsis thaliana | [140] |
| Phytone synthase, Phytoene desaturase | Narcissus pseudonarcissus | [141, 142] |
| 4-coumarate:coenzyme A ligase (4Cl) | Populus trichocarpa, Populusdeltoides | [143] |
| Xenopus laevis oocytes | ||
| Na+ − K+cotransporter HKT1 | Arabidopsis thaliana | [39] |
| AgDCAT1 nodule-specific transporter | Alnus glutinosa | [43] |
| AtNAR2.1/AtNRT2 Nitrate Transport System | Arabidopsis thaliana | [102] |
| HKT Constructs, AtHKT1_HKT1 chimeras | Triticum aestivum, Arabidopsis thaliana | [103] |
| HKT1 superfamily of K+/Na+ transporters | Eucalyptus camaldulensis | [104] |
| Ammonium transporter, LeAMT1 | Lycopersicon esculentum | [105] |
| Ammonium transporter, AtAMT1;2 | Arabidopsis thaliana | [106] |
| Sucrose transporters, AtSUC2, AtSUC9, LjSUT4 | Arabidopsis thaliana, Lotus japonicus | [107–109] |
| Al-activated malate transporter, BnALMT1,BnALMT2, ALMT1 | Brassica napus, Triticum aestivum | [110, 111] |
| Polyol transporters, AtPLT5, PmPLT1 | Arabidopsis thaliana, Plantago major | [112, 113] |
| Inositol transporter2, AtINT2, AtINT4 | Arabidopsis thaliana | [114, 115] |
| Amino acid transporter, AtCAT6, | Arabidopsis thaliana | [116] |
| Cation–Cl- cotransporter, CCC | Arabidopsis thaliana | [117] |
| Anion-selective transporter, ZmALMT1 | Zea mays | [118] |
| K+channel, SIRK | Vitis vinifera | [144] |
| K+ channel, KZM1 | Zea mays | [145] |
| K+ channel, ZMK1 | Zea mays | [146] |
| K+ channels, SKT1 and LKT1 | Solanum tuberesum, Lycopersicon esculentum | [147] |
| AKT2-KAT2 subunitits | Arabidopsis thaliana | [148] |
| K + channel, KAT1 | Arabidopsis thaliana | [149] |
| Cyclic nucleotide-gated ion channels AtCNGC2, AtCNGC1, -2 | Arabidopsis thaliana, Nicotiana tobacum | [150, 151] |
| Putative transporter (GmN70) | Glycine max | [152] |
| Al-activated malate transporter, TaALMT1 | Triticum aestivum | [153] |
| High affinity γ-aminobutyric acid transporter, AtGAT1 | Arabidopsis thaliana | [154] |
| Aquaporins, ZmPIP1a, ZmPIP1b, ZmPIP2a, PIP1, ZmPIP2;1 | Zea mays | [124, 155, 156] |
| Aquaporin, PIP1 | Lycopersicon esculentum | [157] |
| Aquaporin, PIP2 | Juglans regia | [158] |
| Tonoplast intrinsic protein, AtTIP2;1 | Arabidopsis thaliana | [159, 160] |
| Aquaporin, McTIP1;2 | Mesembryanthemum crystallinum | [161] |
| Aquaporin, HvPIP1;6 | Hordeum vulgare | [162] |
| Tonoplast intrinsic protein, PgTIP1 | Panax ginseng | [163] |
| Nodulin 26 intrinsic protein, AtNIP2;1 | Arabidopsis thaliana | [164] |
| PIP-1-type; NtPIP1;1, NtAQP1; PIP-2-type; NtPIP2;1 | Nicotiana tabacum | [165] |
| CjMDR1, ATP-binding cassette protein | Coptis japonica | [166] |
| GlpF-like intrinsic protein (GIP1;1), | Physcomitrella patens | [167] |
| Metal tolerance protein1, AtMTP1 | Arabidopsis thaliana | [159] |
| AtTPK4 tandem-pore K+channel | Arabidopsis thaliana | [168] |
| FRD3, multidrug and toxin efflux (MATE) | Arabidopsis thaliana | [169] |
2. Principal Components of Heterologous Expression
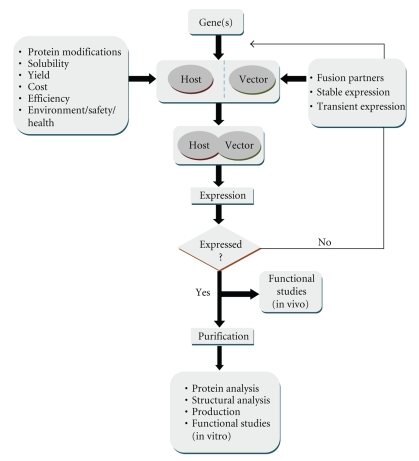
Basic principles of heterologous cloning and expression are summarized in Figure 1. Major parameters that affect choices at different stages are also indicated. The choice of the expression system and vector is a critical step in this procedure and, as indicated, advantages and disadvantages of several factors have to be considered. Expression systems are selected depending on whether the purpose of study is production of large quantities of protein or investigation of functional features of the cloned protein. The physicochemical properties of the investigated protein also play a role in this choice. A general review of frequently used expression systems is provided by Yin et al. [3].
Figure 1.
Flow chart for heterologous expression.
A comprehensive survey of commercially available expression vectors has recently been published [4]. The most commonly used vectors are fusion systems that link additional amino acid sequences (tags) to the protein through a recognition site for a specific protease. Tags may consist of a short peptide sequence or a full protein which can be cleaved from the protein when desired. Presence of tag sequences facilitates solubility, purification, quantification, identification, localization, and assaying of the expressed protein. Frequently used fusion partners include glutathione-S-transferease (GST), his-tag (poly-histidines), maltose binding protein (MBP), thioredoxin (TrxA), FLAG epitope-tag, c-Myc epitope-tag, disulfide isomerase I (DsbA), polyarginine-tag (Arg-tag), calmodulin-binding peptide, cellulose-binding domain, poly-histidine affinity tag (HAT-tag), N-utilizing substance-A (NusA), S-tag, streptavidin-binding peptide (SBP-tag), strep-tag, fluorescent proteins (e.g., green fluorescent protein (GFP)) and ubiquitin [4]. MBP and NusA are specifically used to increase the solubility. MBP is considered to be much more effective for enhancing solubility than GST and thioredoxin [5]. The major disadvantages of fusion protein systems are the requirement of expensive proteases for cleavage from the recombinant protein and the low yield of cleavage reactions [6].
Depending on the host system, vectors for transient or stable expression can be chosen as indicated below.
3. Expression Hosts
3.1. Prokaryotic Expression Systems
3.1.1. Escherichia Coli
Escherichia coli (E. coli) is the first and most extensively used prokaryotic expression system for heterologous protein production [7]. It remains generally the first choice due to its simplicity, rapid growth rate, and relatively low cost. Almost all commercially available inducible cloning vectors are compatible with E. coli and extensive biochemical and genetic information is available.
One of the disadvantages of using E. coli as an expression host arises from its inability to perform post-translational modifications, which are often required for correct folding and functional activity of the recombinant protein. This applies particularly to some membrane proteins and enzymes [3]. Another disadvantage is that E. coli is generally not suitable for proteins which contain many disulfide bonds or require glycosylation, proline cis/trans isomerization, disulfide isomerization, lipidation, sulphation, or phosphorylation [8]. Some eukaryotic proteins that retain their full biological activity in the nonglycosylated form have, however, been produced in E. coli. The unglycosylated human growth hormone (hGH) binding protein secreted from E.coli retains the same binding affinity and specificity as the wild-type hGH binding protein suggesting that recombinant protein is properly folded and glycosylation is not required for binding [9].
Production of proteins that are stabilized by disulfide bonds in E.coli often results in proteolytic degradation or misfolding and formation of inclusion bodies [6]. One strategy developed to improve this situation is to target these proteins to the periplasm where the nonreducing environment allows formation of disulfide bonds [10, 11]. In addition, the E.coli periplasm contains chaperone-like disulfide-binding proteins (DsbA, DsbB, DsbC, and DsbD), folding catalysts, and peptidyl-prolyl isomerases (SurA, RotA, FklB, and FkpA) that support disulfide bond formation and are important for correct folding of periplasmic proteins [12–14]. Disulfide bond formation is achieved via fusion to DsbA or DsbC [15, 16] and periplasmic secretion results in the functional production of a variety of recombinant proteins [17]. In a recent study, the rescue of unstable lipase B from Pseudozyma antarctica (PalB), with periplasmic folding factors was demonstrated [18]. Another strategy involves the use of the trxB gor double mutant lacking thioredoxin reductase and glutathione reductase genes [19, 20]. This double mutant was used for heterologous expression of barley oxalate oxidase (HvOXO) in E.coli [21]. The gene for an osmotin-like cryoprotective protein from Solanum dulcamara was expressed in E.coli and directed to periplasmic localization using an expression vector containing the pelB signal sequence [22]. This resulted in high concentrations of soluble protein with cryoprotective activity, whereas expression in the bacterial cytoplasm only yielded large amounts of insoluble and aggregated protein.
Some of the plant proteins accumulated in insoluble inclusion bodies in E.coli can be solubilized and refolded to restore activity after purification from the host. Examples include Arabidopsis thaumatin-like protein (ATLP3) which was purified from inclusion bodies and the refolded form displayed activity against some pathogenic fungi [23]. To validate the potential antifungal activity of Solanum nigrum osmotin-like protein (SnOLP) was overexpressed in E.coli and the recombinant protein was refolded using reduced:oxidized gluthatione redox buffer and its in vitro activity was demonstrated [24]. The soybean RHG1-LRR domain protein was solubilized from inclusion bodies using urea and refolded by removing the urea in the presence of arginine and reduced/oxidized glutathione [25].
Many plant enzymes are expressed in insoluble inclusion bodies but it is still possible to obtain high yields of active forms for structural studies [26]. The mature polypeptide of FatB thioesterase from the developing seed tissues of Madhuca butyracea was characterized by heterologous expression in E.coli [27]. The functionality of the MbFatB in the heterologous system was revealed by the altered growth behavior and cell morphology of the bacteria due to the changes in the fatty acid profile. The maize chloroplast transglutaminase (TGZ) [26] and glutamatecysteine ligase (GCL) [28] were efficiently overexpressed in E.coli. Recently, DELLA proteins from both Arabidopsis and Malus domestica, which are involved in regulation of plant growth in response to phytohormonal signals, were isolated and expressed in E. coli [29].
Examples of functional expression of plant proteins in E.coli are provided mostly by studies on membrane proteins. A mutant with very low K+ uptake was used as host for studies on the K+ transporters AKT2 [30], AtKUP1-2 [31], AtHKT1 [32] from Arabidopsis and EcHKT1 and EcHKT2 from Eucalyptus camaldulensis [33]. In another example, E.coli C43 strain, which is suitable for expression of membrane proteins was used for functional characterization of chloroplast ATP/ADP transporter from Arabidopsis [34]. The seagrass HAK K+ transporters, CnHAK1 and CnHAK2 were also overexpressed in E.coli and it was found that CnHAK1, but not CnHAK2, mediated very rapid K+ or Rb+ influxes [35]. Using a dicarboxylate uptake-deficient E.coli mutant, a peptide transporter, AgDCAT1 from alder, was shown to be a dicarboxylate, including malate, succinate, fumarate, and oxaloacetate, transporter [36].
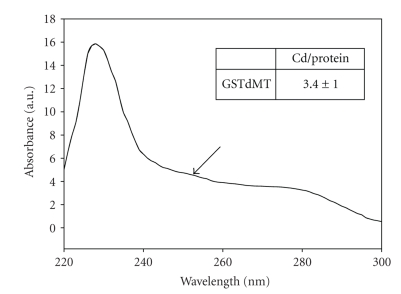
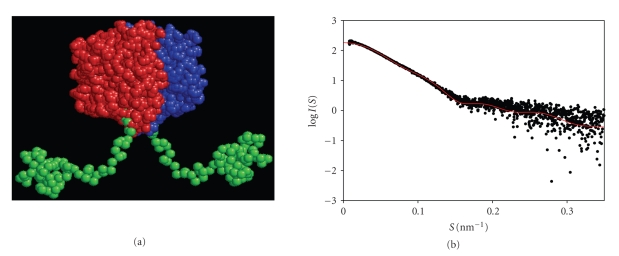
E.coli has also been used for expression of small plant proteins with a fusion partner. Metallothioneins (MTs), which are difficult to purify from natural sources because of their small molecular weight (7 kD), unusual amino acid sequences containing a large number of cysteins and their proteolytic susceptibility belong to this class. Several MTs including a Cd2+ binding Type 1 durum wheat metallothionein (dMT) [37], fava bean Type 1 and Type 2 MTs [38], Arabidopsis MT1, MT2 and MT3 proteins [39], Type 3 MT3-A from the oil palm [40], Type 2 MT, QsMT from Quercus suber [41] have been produced in E.coli mainly for structural analyses. Since the fusion constructs of durum MT with GST (GSTdMT) can be purified in well-defined oligomeric states they are used as model systems for studies on metal-binding and for structural analyses. Figure 2 illustrates that Cd-binding to GSTdMT can be detected by UV-visible spectroscopy. The metal content of GSTdMT was shown to be the same as that expected from dMT alone. An example of the shape models generated from X-ray solution scattering data for GSTdMT is shown in Figure 3, together with the fit to experimental data. The models support a fold for dMT similar to that expected for the free molecule [42, 43]. These results are in agreement with earlier work suggesting independent folding of GST and its fusion components [44] and indicate that recombinant fusion complexes are useful as model systems for structural studies.
Figure 2.
UV-visible absorption spectrum of GSTdMT at 2.7 mg/mL concentration in 20 mM HEPES buffer at pH 8.0. The charge transfer band between 240 and 260 nm due to Cd-S interaction is indicated by the arrow. The Cd/protein ratio is given in the inset.
Figure 3.
A: a low resolution shape model for GSTdMT. The GST dimer (red and blue) is located at the center from which the dMT molecules extend (green). B: the scattering curve expected from the model (–) agrees well with the experimental data (…). I(S) is the scattering intensity and S the scattering vector given by S = 4πsinθ/λ, where 2θ is the scattering angle and λ= 1.5 Å is the wavelength of X-rays. The model and the expected scattering pattern were calculated using the programs in the ATSAS package (EMBL Hamburg Outstation).
3.2. Eukaryotic Expression Systems
Eukaryotic expression systems offer the possibility of posttranslational modifications and are often used for investigations of protein function. Processing reactions such as O-and N-linked glycosylation, tyrosine, serine, and threonine phosphorylation, addition of fatty acid chains, processing of signal sequences, disulfide bond formation, and correct folding can all be readily performed in eukaryotic hosts. The most commonly used eukaryotic systems are yeast, insect, mammalian, and plant cells.
3.2.1. Yeast
As a single cell eukaryotic organism, yeast has molecular, genetic, and biochemical characteristics which are similar to those of higher eukaryotes, and is useful for heterelogous protein production. Yeast cells can grow rapidly with high cell densities, and are easy to manipulate and yeast cultures are cost effective. The two most commonly used organisms are Saccharomyces cerevisiae (S. cerevisiae) and Pichia pastoris (P. pastoris) [7].
Saccharomyces Cerevisiae —
Baker's yeast, S. cerevisiae, is widely used as a host organism for heterologous expression of proteins. Its genetics and physiology are well documented and proteins are posttranslationally modified through the mechanisms similar to those found in plants. The limitations of this host system are low yields, cell stress due to the presence of the foreign gene and hyperglycosylation of secreted foreign proteins. Lack of a strong inducible promoter can be circumvented using P. pastoris [45].
Earlier work on heterelogous expression for screening of plant cDNA libraries by complementation in S. cerevisiae null mutants was reviewed by Frommer and Ninnemann [7]. The S. cerevisiae mutants provide a convenient system for functional and kinetic studies of transporters [46]. The electrophysiological properties of membrane transporters, H+-amino acid symporter and K+ channel, KAT1 [47] and phosphate transporters; AtPT1 and AtPT2 of Arabidopsis were characterized using S. cerevisiae [48]. Recently, functional expression of transporters such as an HvHAKI from barley [49], AtKT1 and AtKT2 [50], and AtKUP1 from Arabidopsis [51] also utilized S. cerevisiae mutants. Another K+ transporter characterized in this system is HKT1 from wheat [52, 53]. Kinetic uptake analyses of tomato sulfate transporters, LeST1-1 and LeST1-2 were carried out using the S. cerevisiae sulfate transporter mutant [54]. The five members of the copper transporter family COPT1–5 from Arabidopsis were characterized using a copper transport null mutant [55]. A peptide transporter AtPTR1 gene from Arabidopsis was isolated and complemented in a peptide transport-deficient mutant [56]. A putative K+/H+ antiporter, AtChx17 was heterologously expressed and characterized in an S. cerevisiae kha1 deletion mutant [57]. To test their functional activity, the grapevine hexose transporters VvHT3, VvHT4, and VvHT5 were expressed in the S. cerevisiae mutant EBY.VW4000, which is deficient in glucose transport due to concurrent knock-out of 20 endogenous transporter genes [58]. Growth-based complementation assays were used to demonstrate function of the transporters but resulted in inadequate rates of glucose uptake. A more sensitive assay based on direct measurement of radioactively labelled glucose uptake revealed that this mutant expressing VvHT4 and VvHT5 accumulated labelled glucose at higher rates than yeast transformed with the empty vector, demonstrating the functionality of the glucose transporters. Although VvHT3:GFP (green fluorescent protein) fusion protein was targeted to the plasma membrane in plant cells, VvHT3 was found not to be functional in the yeast system [58].Yeast expression studies were, in several instances, complemented by studies in other organisms to verify functional and kinetic properties of recombinant proteins. The plasma membrane-localized H+/inositol symporter AtINT2 of Arabidopsis was studied by expression in an inositol uptake/inositol biosynthesis double mutant in S. cerevisiae and in Xenopus oocytes [59]. In this study, the amount of AtINT2 protein in yeast plasma membrane was sufficient for complementation, but not for functional and kinetic analyses. In oocytes, however, it was possible to show that AtINT2 mediated the symport of H+ [59]. Expression and functional characterization of Arabidopsis AtGAT1 in S. cerevisiae and Xenopus oocytes revealed that AtGAT1 mediates H+-dependent, high affinity transport of high affinity γ-aminobutyric acid (GABA) and GABA-related compounds. Properties of this protein could be examined in more detail in Xenopus oocytes [60]. Heterologous expression of AtTIP2;1 and AtTIP2;3 from Arabidopsis in both ammonium uptake-defective yeast and oocytes indicated that these TIPs transport both ammonium and methyl-ammonium in addition to water and urea [61]. The kinetic characteristics of the sorbitol transporters, PmPLT1, and PmPLT2 from common plantain (Plantago major) were investigated by functional expression in S. cerevisiae and in Xenopus oocytess. In the yeast system, both proteins were characterized as low-affinity and low-specificity polyol symporters. These data were confirmed in the Xenopus system, where PmPLT1 was analyzed in detail and characterized as an H+ symporter [62].
The major disadvantages of using S. cerevisiae mutants in transporter studies are the hyperpolarization of the membrane, mislocalization of membrane proteins and recruitment of non-K+-transporters into K+-transporters [63].
Pichia Pastoris —
P. pastoris, methylotrophic yeast, is considered a valuable tool for high yield heterologous expression of various proteins. The possibility of obtaining posttranslational modifications, high level expression of foreign proteins in either intracellular or extracellular forms, simplicity of genetic manipulations, and availability of various P. pastoris strains and vectors make this expression system highly popular [64]. Molecular manipulations such as gene targeting, high frequency DNA transformation, and cloning for functional complementation are similar to those in S. cerevisiae [64]. Tightly regulated promoters, easy integration of heterologous DNA into the host chromosome and the capacity to generate more posttranslational modifications make P. pastoris the preferred system compared to S. cerevisiae.
The wide use of P. pastoris expression system for recombinant plant proteins can be seen from recent reviews [64, 65]. P. pastoris is particularly well suited for studying plant enzymes since glycosylation of the foreign proteins is expected to be closer to that in plants [66, 67] and glycosylated proteins have shorter glycosyl chains in P. pastoris than in S. cerevisiae [68]. This expression system has the potential to produce high levels of recombinant proteins [67], up to 400 mg/L of culture [69]. Several plant enzymes have been produced in Pichia. Two examples are cytosolic expression of nitrate reductase from spinach and corn at high levels needed for detailed biochemical studies [69] and expression of a sweet potato invertase in milligram quantities [70]. Enzymatic activity of the membrane-bound α1,6-galactosyltransferase was shown through overexpression in P. pastoris [71]. The hypothesis that α-xylosyltransferase is involved in xyloglucan biosynthesis was tested by overexpressing the corresponding genes and identifying the gene product that displayed activity [72]. P. pastoris has been used for production of a number of glycosyltransferases involved in the biosynthesis of N- and O-linked oligosaccharides [73]. To confirm that Osβfruct3 from rice encoded a vacuolar type β-D-fructofuranosidase, the Osβfruct3 cDNA was expressed in this host [74]. A recombinant potato apyrase was expressed and purified in the hyperglycosylated form at 1 mg/L protein concentration [75]. The catalytically active barley oxalate oxidase, HvOXO was produced with a yield of 50 mg/L culture and biochemically characterized [76].
High-level expression of wheat germin/oxalate oxidase was achieved in P. pastoris as an α-mating factor signal peptide fusion to increase secretion of the protein of interest into the culture medium. Approximately 1 g (4 × 104 U) of TaOXO was produced in 5 L fermentation cultures following 8 days of methanol induction, demonstrating the possibility of large-scale production of oxalate oxidase for biotechnological applications. Glycosylation of the recombinant protein was evidenced by mass spectrometry [77]. Another application using P. pastoris is the expression of the α-subunit of heterotrimeric G-proteins, GPA1, from Arabidopsis. Several attempts had previously failed to produce this protein in E. coli, whereas in the yeast system the protein could be expressed with a his6-tag and purified by affinity chromatography with a yield up to 20 mg from 700 mL culture [78].
Several allergens including, Cyn d 1 from Bermuda grass, Bla g 4 from German cockroach, Amb a 6 from Ambrosia artemisiifolia, and Ole e 1 from Olea europaea have also been produced in P. pastoris (see list in 64).
This system was also used for the expression of a number of plant lectins such as Canavalia brasiliensis lectin (ConBr) [2] and the Nicotiana tabacum lectin [79]. In a recent study, the low-affinity cation transporter (LCT1) from wheat was also expressed and functionally characterized using P. pastoris [80].
3.2.2. Insect Cells
Baculoviruses have been used for the synthesis of a wide variety of eukaryotic recombinant proteins in insect cells. In this expression system one of the nonessential viral genes is replaced with the target protein through homologous recombination. The resulting recombinant baculovirus is used to infect cultured insect cells and the heterologous genes can be expressed under the control of the extremely strong pPolh, polyhedron promoter in the late phase of infection.
The most common baculovirus used for expression studies is Autographa californica multiple capsid nucleopolyhedrovirus (AcMNPV) and the most frequently used host insects are Spodoptera frugiperda and Trichoplusia ni. This expression system produces high levels of recombinant proteins which are soluble, post-translationally modified, biologically active, and functional [81]. The virus is not pathogenic to vertebrates or plants. The main drawback of this system over the bacterial and yeast systems lies in the noncontinuous expression of the heterologous gene; every round of protein production needs reinfection [3].
This heterologous expression system is mainly used to investigate enzymatic mechanisms in plants. The most recent examples include the Arabidopsis reductase isoforms, AR1 and AR2 [82], peroxisomal short-chain acyl-CoA oxidase A [83], cyclin-dependent kinase A [84], NADH-cytochrome b5 reductase [85], geranylgeranyltransferase-I [86], acyl-CoA synthetase [87], homogentisate phytyltransferase [88], (+)-abscisic acid 8’-hydroxylase [89], β1,2-xylosyltransferase [90], tobacco ethylene-inducing xylanase [91], and barley ADP-glucose pyrophoshorylase [92]. The overall yield of heterelogous proteins obtained with this system is usually lower than with P. pastoris.
Baculovirus-infected insect cells have been used as an alternative system to Xenopus oocytes for expression and characterization of plant channel proteins. Several channel proteins which were not functional in oocytes could be characterized in baculovirus-infected insect cells such as the K+ channel proteins AKT1 [93], KAT1 [94], KCO1 [95] from Arabidopsis, and KST1 [96] and SKT1 [97] from potato.
To investigate the interaction between AUX1 and its transport substrate indole-3-acetic acid (IAA) from Arabidopsis, an epitope-tagged version of AUX1 was expressed at high levels in a baculovirus expression system and suitable membrane fragments were prepared from baculovirus-infected insect cells for direct measurement of IAA binding to AUX1. AUX1-IAA interactions were determined using a radio-ligand binding assay to confirm that AUX1 was able to bind IAA with an affinity (Kd) of 2.6 mM, comparable with estimates of the Km for IAA transport [98].
The main disadvantages of using baculovirus-infected insect cells are difficulties in constructing the expression vectors, requirements for more complex laboratory facilities and skills, and the short expression periods after infection.
3.2.3. Xenopus Laevis Oocytes
The oocytes of the South African clawed frog, Xenopus laevis, are also used for heterelogous expression of eukaryotic genes. The mRNA for the target protein, introduced by microinjection into the cytoplasm, is translated and the protein is posttranslationally modified by the oocyte [99]. Direct injections of DNA into the nucleus are also possible, but the manipulations are difficult as the nucleus can easily be damaged in the process.
Investigations on membrane transport proteins can be readily performed on oocytes where techniques for electrophysiological measurements are well established. Although, a high proportion of cells express the foreign gene after injection variations in the quality of oocytes and in the ability of individual cells to produce the heterelogous protein can cause problems. Oocytes are not suitable for preparing large quantities of proteins and the short expression period often leads to technical difficulties. The system can also not be sustained over long periods of time and is not suitable for stable expression [99, 100].
Xenopus oocytes have, however, provided a powerful heterologous expression system for animal as well as plant genes. The possibility of using Xenopus oocytes as heterologous expression systems for the identification of plant transporters was first demonstrated by the expression of the H+/glucose transporter STP1 from Arabidopsis [101]. It has, since, been mainly used for production of transporters including potassium channels, H+/hexose cotransporters, aquaporins, and chloride channels [99]. In addition, functional expression of a nitrate transporter [102], a K+/Na+ transporter [39, 103, 104], ammonium transporters [105, 106], sucrose transporters [107–109], Al-activated malate transporters [110, 111], polyol transporters [112, 113], inositol transporters [114, 115], an amino acid transporter [116], a cation–Cl-cotransporter [117], and an anion-selective transporter [118] in Xenopus oocytes were investigated. Cases where channel proteins expressed in oocytes were not functional have also been reported. These include the K+ channels AKT1 from Arabidopsis [93, 119, 120], TaAKT1 from wheat [121], DKT1 from carrot [122], and OsAKT1 from rice [123]. The causes for the lack of function of these recombinant proteins are not clear.
Several studies have used expression of a wild type and its mutant forms in Xenopus oocytes to confirm the in vivo functions of plant proteins, especially transporters and plasma membrane intrinsic proteins (PIPs or aquaporins). To demonstrate whether or not the plant K+ channels form multimers, the wild type and a mutant were coexpressed in Xenopus oocytes [120]. Coexpression of tomato ammonium transporter (LeAMT1;1) and its mutant in Xenopus oocytes inhibited ammonium transport, suggesting homooligomerization [105]. In another study, the role of phosphorylation in the water channel activity of wild-type and mutant ZmPIP2;1 was studied in Xenopus oocytes [124].
In recent studies, the Xenopus oocyte expression system was used to investigate structure-function relationships. In one example, differences in the function of two cation transporters, wheat HKT1 and Arabidopsis AtHKT1, were investigated using a series of AtHKT1/HKT1 chimeras with point mutations [103].
4. Conclusions
Heterologous expression of plant genes in other host organisms has two main applications: (1) overexpression of the encoded protein, for biochemical and biophysical characterization and (2) expression of foreign genes for determination of the function of the encoded protein by complementing in a mutant host. Overexpression of recombinant proteins is usually carried out with a cleavable tag to simplify purification in large quantities. In contrast, complementation studies are carried out in null mutants to restore a missing activity in vivo.
Decisions on which expression vectors to use and the choice of the expression host depend on the particular application. In general E.coli is the first choice as host because of its simplicity, availability of expression vectors, cost effectiveness, and availability of extensive genetic information on this host. Alternative expression systems are used only if the recombinant protein is inactive due to lack of essential posttranslational modifications and when detailed studies on the recombinant protein function are planned. Yeast systems have the advantage of ease of manipulation and short generation time. S. cerevisiae has been extensively used for functional complementation, biochemical, and electrophysiolagical characterization of plant membrane and transporter proteins. P. pastoris is the preferred host for overexpression of several plant enzymes. Baculovirus-mediated insect cell expression offers the possibility for detailed investigations of plant enzymes and transporters. The oocyte from Xenopus laevis is often used for monitoring activity and biochemical and electrophysiological characterization of plant plasma membrane transporter and pump proteins.
Heterologous expression is a powerful tool for functional and biochemical analyses of genes and gene families isolated from various organisms. It is particularly important for plants where the whole genome sequence is not available. This system will also provide denovo analysis. Its limitations, however, should be kept in mind, especially when interpreting the results in terms of the native structure and function of proteins. Major problems arise from misfolding and mislocalization of recombinant proteins in foreign hosts. Strategies developed to avoid misfolding of recombinant proteins include expression in periplasmic space, expression with a tag, and utilization of different hosts. Mislocalization, on the other hand, may occur because the recombinant protein may take over the function of the missing host protein [170]. Conclusions on function need to be tested in alternative hosts and eventually in the plant itself.
References
- 1.Yan Y, Chen J, Li J. Overexpression of a small medicinal peptide from ginseng in the yeast Pichia pastoris . Protein Expression and Purification. 2003;29(2):161–166. doi: 10.1016/s1046-5928(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 2.Bezerra WM, Carvalho CP, Moreira RA, Grangeiro TB. Establishment of a heterologous system for the expression of Canavalia brasiliensis lectin: a model for the study of protein splicing. Genetics and Molecular Research. 2006;5(1):216–223. [PubMed] [Google Scholar]
- 3.Yin J, Li G, Ren X, Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. Journal of Biotechnology. 2007;127(3):335–347. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Applied Microbiology and Biotechnology. 2003;60(5):523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- 5.Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Science. 1999;8(8):1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baneyx F. Recombinant protein expression in Escherichia coli . Current Opinion in Biotechnology. 1999;10(5):411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 7.Frommer WB, Ninnemann O. Heterologous expression of genes in bacterial, fungal, animal, and plant cells. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:419–444. [Google Scholar]
- 8.Lueking A, Holz C, Gotthold C, Lehrach H, Cahill D. A system for dual protein expression in Pichia pastoris and Escherichia coli . Protein Expression and Purification. 2000;20(3):372–378. doi: 10.1006/prep.2000.1317. [DOI] [PubMed] [Google Scholar]
- 9.Fuh G, Mulkerrin MG, Bass S, et al. The human growth hormone receptor. Secretion from Escherichia coli and disulfide bonding pattern of the extracellular binding domain. The Journal of Biological Chemistry. 1990;265(6):3111–3115. [PubMed] [Google Scholar]
- 10.Wülfing C, Plückthun A. Protein folding in the periplasm of Escherichia coli . Molecular Microbiology. 1994;12(5):685–692. doi: 10.1111/j.1365-2958.1994.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 11.Georgiou G, Segatori L. Preparative expression of secreted proteins in bacteria: status report and future prospects. Current Opinion in Biotechnology. 2005;16(5):538–545. doi: 10.1016/j.copbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Shokri A, Sandén AM, Larsson G. Cell and process design for targeting of recombinant protein into the culture medium of Escherichia coli . Applied Microbiology and Biotechnology. 2003;60(6):654–664. doi: 10.1007/s00253-002-1156-8. [DOI] [PubMed] [Google Scholar]
- 13.Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli . Nature Biotechnology. 2004;22(11):1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- 14.Choi JH, Lee SY. Secretory and extracellular production of recombinant proteins using Escherichia coli . Applied Microbiology and Biotechnology. 2004;64(5):625–635. doi: 10.1007/s00253-004-1559-9. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa Y, Yanagi H, Yura T. Overproduction of bacterial protein disulfide isomerase (DsbC) and its modulator (DsbD) markedly enhances periplasmic production of human nerve growth factor in Escherichia coli . The Journal of Biological Chemistry. 2001;276(17):14393–14399. doi: 10.1074/jbc.M100132200. [DOI] [PubMed] [Google Scholar]
- 16.Sahdev S, Khattar SK, Saini KS. Production of active eukaryotic proteins through bacterial expression systems: a review of the existing biotechnology strategies. Molecular and Cellular Biochemistry. 2008;307(1-2):249–264. doi: 10.1007/s11010-007-9603-6. [DOI] [PubMed] [Google Scholar]
- 17.Mergulhão FJM, Summers DK, Monteiro GA. Recombinant protein secretion in Escherichia coli . Biotechnology Advances. 2005;23(3):177–202. doi: 10.1016/j.biotechadv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Lewis D, Chou CP. Effect of folding factors in rescuing unstable heterologous lipase B to enhance its overexpression in the periplasm of Escherichia coli . Applied Microbiology and Biotechnology. 2008;79(6):1035–1044. doi: 10.1007/s00253-008-1514-2. [DOI] [PubMed] [Google Scholar]
- 19.Bessette PH, Åslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prinz WA, Åslund F, Holmgren A, Beckwith J. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. The Journal of Biological Chemistry. 1997;272(25):15661–15667. doi: 10.1074/jbc.272.25.15661. [DOI] [PubMed] [Google Scholar]
- 21.Cassland P, Larsson S, Nilvebrant N-O, Jönsson LJ. Heterologous expression of barley and wheat oxalate oxidase in an E. coli trxB gor double mutant. Journal of Biotechnology. 2004;109(1-2):53–62. doi: 10.1016/j.jbiotec.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Newton SS, Duman JG. An osmotin-like cryoprotective protein from the bittersweet nightshade Solanum dulcamara . Plant Molecular Biology. 2000;44(5):581–589. doi: 10.1023/a:1026599028063. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Reddy ASN. Cloning and expression of a PR5-like protein from Arabidopsis: inhibition of fungal growth by bacterially expressed protein. Plant Molecular Biology. 1997;34(6):949–959. doi: 10.1023/a:1005893119263. [DOI] [PubMed] [Google Scholar]
- 24.de A Campos M, Silva MS, Magalhães CP, et al. Expression in Escherichia coli, purification, refolding and antifungal activity of an osmotin from Solanum nigrum . Microbial Cell Factories. 2008;7:7–17. doi: 10.1186/1475-2859-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afzal AJ, Lightfoot DA. Soybean disease resistance protein RHG1-LRR domain expressed, purified and refolded from Escherichia coli inclusion bodies: preparation for a functional analysis. Protein Expression and Purification. 2007;53(2):346–355. doi: 10.1016/j.pep.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Carvajal-Vallejos PK, Campos A, Fuentes-Prior P, et al. Purification and in vitro refolding of maize chloroplast transglutaminase over-expressed in Escherichia coli . Biotechnology Letters. 2007;29(8):1255–1262. doi: 10.1007/s10529-007-9377-7. [DOI] [PubMed] [Google Scholar]
- 27.Jha JK, Maiti MK, Bhattacharjee A, Basu A, Sen PC, Sen SK. Cloning and functional expression of an acyl-ACP thioesterase FatB type from Diploknema (Madhuca) butyracea seeds in Escherichia coli . Plant Physiology and Biochemistry. 2006;44(11-12):645–655. doi: 10.1016/j.plaphy.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Jez JM, Cahoon RE, Chen S. Arabidopsis thaliana glutamate-cysteine ligase. Functional properties, kinetic mechanism, and regulation of activity. The Journal of Biological Chemistry. 2004;279(32):33463–33470. doi: 10.1074/jbc.M405127200. [DOI] [PubMed] [Google Scholar]
- 29.Sun X, Frearson N, Kirk C, et al. An E. coli expression system optimized for DELLA proteins. Protein Expression and Purification. 2008;58(1):168–174. doi: 10.1016/j.pep.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Uozumi N, Nakamura T, Schroeder JI, Muto S. Determination of transmembrane topology of an inward-rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli . Proceedings of the National Academy of Sciences of the United States of America. 1998;95(17):9773–9778. doi: 10.1073/pnas.95.17.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. The Plant Cell. 1998;10(1):51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uozumi N, Kim EJ, Rubio F, et al. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae . Plant Physiology. 2000;122(4):1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairbairn DJ, Liu W, Schachtman DP, Gomez-Gallego S, Day SR, Teasdale RD. Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis . Plant Molecular Biology. 2000;43(4):515–525. doi: 10.1023/a:1006496402463. [DOI] [PubMed] [Google Scholar]
- 34.Tjaden J, Schwöppe C, Möhlmann T, Quick PW, Neuhaus HE. Expression of a plastidic ATP/ADP transporter gene in Escherichia coli leads to a functional adenine nucleotide transport system in the bacterial cytoplasmic membrane. The Journal of Biological Chemistry. 1998;273(16):9630–9636. doi: 10.1074/jbc.273.16.9630. [DOI] [PubMed] [Google Scholar]
- 35.Garciadeblas B, Benito B, Rodríguez-Navarro A. Molecular cloning and functional expression in bacteria of the potassium transporters CnHAK1 and CnHAK2 of the seagrass Cymodocea nodosa . Plant Molecular Biology. 2002;50(4-5):623–633. doi: 10.1023/a:1019951023362. [DOI] [PubMed] [Google Scholar]
- 36.Jeong J, Suh S, Guan C, et al. A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family. Plant Physiology. 2004;134(3):969–978. doi: 10.1104/pp.103.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilecen K, Ozturk UH, Duru AD, et al. Triticum durum metallothionein: isolation of the gene and structural characterization of the protein using solution scattering and molecular modeling. The Journal of Biological Chemistry. 2005;280(14):13701–13711. doi: 10.1074/jbc.M412984200. [DOI] [PubMed] [Google Scholar]
- 38.Foley RC, Liang ZM, Singh KB. Analysis of type 1 metallothionein cDNAs in Vicia faba . Plant Molecular Biology. 1997;33(4):583–591. doi: 10.1023/a:1005790927581. [DOI] [PubMed] [Google Scholar]
- 39.Murphy A, Zhou J, Goldsbrough PB, Taiz L. Purification and immunological identification of metallothioneins 1 and 2 from Arabidopsis thaliana . Plant Physiology. 1997;113(4):1293–1301. doi: 10.1104/pp.113.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdullah SNA, Cheah SC, Murphy DJ. Isolation and characterisation of two divergent type 3 metallothioneins from oil palm, Elaeis guineensis . Plant Physiology and Biochemistry. 2002;40(3):255–263. [Google Scholar]
- 41.Mir G, Domènech J, Huguet G, et al. A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. Journal of Experimental Botany. 2004;55(408):2483–2493. doi: 10.1093/jxb/erh254. [DOI] [PubMed] [Google Scholar]
- 42.Dede F, Dinler G, Sayers Z. 3D Macromolecular structure analyses: applications in plant proteins. In: Proceedings of the NATO Advanced Research Workshop; 2006; Springer; pp. 135–146. [Google Scholar]
- 43.Yesilirmak F. Biophysical and functional characterization of wheat metallothionein at molecular level. Istanbul, Turkey: Sabanci University; 2008. Ph.D. thesis. [Google Scholar]
- 44.Zhan Y, Song X, Zhou GW. Structural analysis of regulatory protein domains using GST-fusion proteins. Gene. 2001;281(1-2):1–9. doi: 10.1016/s0378-1119(01)00797-1. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt M, Hoffman DR. Expression systems for production of recombinant allergens. International Archives of Allergy and Immunology. 2002;128(4):264–270. doi: 10.1159/000063865. [DOI] [PubMed] [Google Scholar]
- 46.Dreyer I, Horeau C, Lemaillet G, et al. Identification and characterization of plant transporters using heterologous expression systems. Journal of Experimental Botany. 1999;50:1073–1087. [Google Scholar]
- 47.Bertl A, Anderson JA, Slayman CL, Gaber RF. Use of Saccharomyces cerevisiae for patch-clamp analysis of heterologous membrane proteins: characterization of Kat1, an inward-rectifying K+ channel from Arabidopsis thaliana, and comparison with endogeneous yeast channels and carriers. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(7):2701–2705. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muchhal US, Pardo JM, Raghothama KG. Phosphate transporters from the higher plant Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America. 1996;93(19):10519–10523. doi: 10.1073/pnas.93.19.10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. The Plant Cell. 1997;9(12):2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quintero FJ, Blatt MR. A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Letters. 1997;415(2):206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 51.Fu H-H, Luan S. AtKUP1: a dual-affinity K+ transporter from arabidopsis. The Plant Cell. 1998;10(1):63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370(6491):655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 53.Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270(5242):1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 54.Howarth JR, Fourcroy P, Davidian J-C, Smith FW, Hawkesford MJ. Cloning of two contrasting high-affinity sulfate transporters from tomato induced by low sulfate and infection by the vascular pathogen Verticillium dahliae . Planta. 2003;218(1):58–64. doi: 10.1007/s00425-003-1085-5. [DOI] [PubMed] [Google Scholar]
- 55.Sancenón V, Puig S, Mira H, Thiele DJ, Peñarrubia L. Identification of a copper transporter family in Arabidopsis thaliana . Plant Molecular Biology. 2003;51(4):577–587. doi: 10.1023/a:1022345507112. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich D, Hammes U, Thor K, et al. AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis . The Plant Journal. 2004;40(4):488–499. doi: 10.1111/j.1365-313X.2004.02224.x. [DOI] [PubMed] [Google Scholar]
- 57.Maresova L, Sychrova H. Arabidopsis thaliana CHX17 gene complements the kha1 deletion phenotypes in Saccharomyces cerevisiae . Yeast. 2006;23(16):1167–1171. doi: 10.1002/yea.1424. [DOI] [PubMed] [Google Scholar]
- 58.Hayes MA, Davies C, Dry IB. Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. Journal of Experimental Botany. 2007;58(8):1985–1997. doi: 10.1093/jxb/erm061. [DOI] [PubMed] [Google Scholar]
- 59.Schneider S, Schneidereit A, Udvardi P, et al. Arabidopsis Inositol Transporter2 mediates H+ symport of different inositol epimers and derivatives across the plasma membrane. Plant Physiology. 2007;145(4):1395–1407. doi: 10.1104/pp.107.109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer A, Eskandari S, Grallath S, Rentsch D. AtGAT1, a high affinity transporter for γ-aminobutyric acid in Arabidopsis thaliana . The Journal of Biological Chemistry. 2006;281(11):7197–7204. doi: 10.1074/jbc.M510766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loqué D, Ludewig U, Yuan L, von Wirén N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiology. 2005;137(2):671–680. doi: 10.1104/pp.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramsperger-Gleixner M, Geiger D, Hedrich R, Sauer N. Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells. Plant Physiology. 2004;134(1):147–160. doi: 10.1104/pp.103.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madrid R, Gómez MJ, Ramos J, Rodríguez-Navarro A. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. The Journal of Biological Chemistry. 1998;273(24):14838–14844. doi: 10.1074/jbc.273.24.14838. [DOI] [PubMed] [Google Scholar]
- 64.Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris . FEMS Microbiology Reviews. 2000;24(1):45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 65.Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22(4):249–270. doi: 10.1002/yea.1208. [DOI] [PubMed] [Google Scholar]
- 66.Grinna LS, Tschopp JF. Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast, Pichia pastoris . Yeast. 1989;5(2):107–115. doi: 10.1002/yea.320050206. [DOI] [PubMed] [Google Scholar]
- 67.Cregg JM, Vedvick TS, Raschke WC. Recent advances in the expression of foreign genes in Pichia pastoris . Nature Biotechnology. 1993;11(8):905–910. doi: 10.1038/nbt0893-905. [DOI] [PubMed] [Google Scholar]
- 68.Bretthauer RK, Castellino FJ. Glycosylation of Pichia pastoris-derived proteins. Biotechnology and Applied Biochemistry. 1999;30(3):193–200. [PubMed] [Google Scholar]
- 69.Mertens JA, Shiraishi N, Campbell WH. Recombinant expression of molybdenum reductase fragments of plant nitrate reductase at high levels in Pichia pastoris . Plant Physiology. 2000;123(2):743–756. doi: 10.1104/pp.123.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W-C, Wang A-Y, Wang L-T, Sung H-Y. Expression and characterization of sweet potato invertase in Pichia pastoris . Journal of Agricultural and Food Chemistry. 2003;51(5):1494–1499. doi: 10.1021/jf026032i. [DOI] [PubMed] [Google Scholar]
- 71.Edwards ME, Dickson CA, Chengappa S, Sidebottom C, Gidley MJ, Reid JSG. Molecular characterisation of a membrane-bound galactosyltransferase of plant cell wall matrix polysaccharide biosynthesis. The Plant Journal. 1999;19(6):691–697. doi: 10.1046/j.1365-313x.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 72.Faik A, Price NJ, Raikhel NV, Keegstra K. An Arabidopsis gene encoding an α-xylosyltransferase involved in xyloglucan biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7797–7802. doi: 10.1073/pnas.102644799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bencúrová M, Rendić D, Fabini G, Kopecky E-M, Altmann F, Wilson IBH. Expression of eukaryotic glycosyltransferases in the yeast Pichia pastoris . Biochimie. 2003;85(3-4):413–422. doi: 10.1016/s0300-9084(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 74.Fu R-H, Wang A-Y, Wang Y-C, Sung H-Y. A cDNA encoding vacuolar type β-D-fructofuranosidase (Osβfruct3) of rice and its expression in Pichia pastoris . Biotechnology Letters. 2003;25(18):1525–1530. doi: 10.1023/a:1025434600474. [DOI] [PubMed] [Google Scholar]
- 75.Nourizad N, Ehn M, Gharizadeh B, Hober S, Nyrén P. Methylotrophic yeast Pichia pastoris as a host for production of ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum) Protein Expression and Purification. 2003;27(2):229–237. doi: 10.1016/s1046-5928(02)00605-8. [DOI] [PubMed] [Google Scholar]
- 76.Whittaker MM, Whittaker JW. Characterization of recombinant barley oxalate oxidase expressed by Pichia pastoris . Journal of Biological Inorganic Chemistry. 2002;7(1-2):136–145. doi: 10.1007/s007750100281. [DOI] [PubMed] [Google Scholar]
- 77.Pan H-Y, Whittaker MM, Bouveret R, Berna A, Bernier F, Whittaker JW. Characterization of wheat germin (oxalate oxidase) expressed by Pichia pastoris . Biochemical and Biophysical Research Communications. 2007;356(4):925–929. doi: 10.1016/j.bbrc.2007.03.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaplan B, Tunca S, Sayers Z. Expression of A. thaliana G protein alpha subunit in P. pastoris . FEBS Journal. 2005;272(supplement 1):1–11. [Google Scholar]
- 79.Lannoo N, Vervecken W, Proost P, Rougé P, Van Damme EJM. Expression of the nucleocytoplasmic tobacco lectin in the yeast Pichia pastoris . Protein Expression and Purification. 2007;53(2):275–282. doi: 10.1016/j.pep.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Diatloff E, Forde BG, Roberts SK. Expression and transport characterisation of the wheat low-affinity cation transporter (LCT1) in the methylotrophic yeast Pichia pastoris . Biochemical and Biophysical Research Communications. 2006;344(3):807–813. doi: 10.1016/j.bbrc.2006.03.212. [DOI] [PubMed] [Google Scholar]
- 81.Andrews DL, Beames B, Summers MD, Park WD. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochemical Journal. 1988;252(1):199–206. doi: 10.1042/bj2520199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizutani M, Ohta D. Two isoforms of NAPDH: cytochrome p450 reductase in Arabidopsis thaliana gene structure, heterologous expression in insect cells, and differential regulation. Plant Physiology. 1998;116(1):357–367. doi: 10.1104/pp.116.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashi H, De Bellis L, Ciurli A, Kondo M, Hayashi M, Nishimura M. A novel Acyl-CoA oxidase that can oxidize short-chain Acyl-CoA in plant peroxisomes. The Journal of Biological Chemistry. 1999;274(18):12715–12721. doi: 10.1074/jbc.274.18.12715. [DOI] [PubMed] [Google Scholar]
- 84.Harashima H, Shinmyo A, Sekine M. Phosphorylation of threonine 161 in plant cyclin-dependent kinase A is required for cell division by activation of its associated kinase. The Plant Journal. 2007;52(3):435–448. doi: 10.1111/j.1365-313X.2007.03247.x. [DOI] [PubMed] [Google Scholar]
- 85.Fukuchi-Mizutani M, Mizutani M, Tanaka Y, Kusumi T, Ohta D. Microsomal electron transfer in higher plants: cloning and heterologous expression of NADH-cytochrome b5 reductase from Arabidopsis . Plant Physiology. 1999;119(1):353–361. doi: 10.1104/pp.119.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caldelari D, Sternberg H, Rodríguez-Concepción M, Gruissem W, Yalovsky S. Efficient prenylation by a plant geranylgeranyltransferase-I requires a functional Caal box motif and a proximal polybasic domain. Plant Physiology. 2001;126(4):1416–1429. doi: 10.1104/pp.126.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayashi H, De Bellis L, Hayashi Y, et al. Molecular characterization of an Arabidopsis acyl-coenzyme a synthetase localized on glyoxysomal membranes. Plant Physiology. 2002;130(4):2019–2026. doi: 10.1104/pp.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savidge B, Weiss JD, Wong Y-HH, et al. Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis . Plant Physiology. 2002;129(1):321–332. doi: 10.1104/pp.010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saito S, Hirai N, Matsumoto C, et al. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiology. 2004;134(4):1439–1449. doi: 10.1104/pp.103.037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pagny S, Bouissonnie F, Sarkar M, et al. Structural requirements for Arabidopsis β1, 2-xylosyltransferase activity and targeting to the Golgi. The Plant Journal. 2003;33(1):189–203. doi: 10.1046/j.0960-7412.2002.01604.x. [DOI] [PubMed] [Google Scholar]
- 91.Furman-Matarasso N, Cohen E, Du Q, Chejanovsky N, Hanania U, Avni A. A point mutation in the ethylene-inducing xylanase elicitor inhibits the β-1-4-endoxylanase activity but not the elicitation activity. Plant Physiology. 1999;121(2):345–351. doi: 10.1104/pp.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doan DNP, Rudi H, Olsen O-A. The allosterically unregulated isoform of ADP-glucose pyrophosphorylase from barley endosperm is the most likely source of ADP-glucose incorporated into endosperm starch. Plant Physiology. 1999;121(3):965–975. doi: 10.1104/pp.121.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaymard F, Cerutti M, Horeau C, et al. The baculovirus/insect cell system as an alternative to Xenopus oocytes. First characterization of the AKT1 K+ channel from Arabidopsis thaliana . The Journal of Biological Chemistry. 1996;271(37):22863–22870. doi: 10.1074/jbc.271.37.22863. [DOI] [PubMed] [Google Scholar]
- 94.Marten I, Gaymard F, Lemaillet G, Thibaud J-B, Sentenac H, Hedrich R. Functional expression of the plant K+ channel KAT1 in insect cells. FEBS Letters. 1996;380(3):229–232. doi: 10.1016/0014-5793(96)00042-7. [DOI] [PubMed] [Google Scholar]
- 95.Czempinski K, Zimmermann S, Ehrhardt T, Müller-Röber B. New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+ dependency. The EMBO Journal. 1997;16(10):2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ehrhardt T, Zimmermann S, Müller-Röber B. Association of plant K+ (in) channels is mediated by conserved and does not affect subunit assembly. FEBS Letters. 1997;409(2):166–170. doi: 10.1016/s0014-5793(97)00502-4. [DOI] [PubMed] [Google Scholar]
- 97.Zimmermann S, Talke I, Ehrhardt T, Nast G, Müller-Röber B. Characterization of SKT1, an inwardly rectifying potassium channel from potato, by heterologous in insect cells. Plant Physiology. 1998;116(3):879–890. doi: 10.1104/pp.116.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carrier DJ, Bakar NTA, Swarup R, et al. The binding of auxin to the Arabidopsis auxin influx transporter AUX1. Plant Physiology. 2008;148(1):529–535. doi: 10.1104/pp.108.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller AJ, Zhou JJ. Xenopus oocytes as an expression system for plant transporters. Biochimica et Biophysica Acta. 2000;1465(1-2):343–358. doi: 10.1016/s0005-2736(00)00148-6. [DOI] [PubMed] [Google Scholar]
- 100.Sigel E. The Xenopus oocyte: system for the study of functional expression and modulation of proteins. Molecular Nutrition and Food Research. 2005;49(3):228–234. doi: 10.1002/mnfr.200400104. [DOI] [PubMed] [Google Scholar]
- 101.Boorer KJ, Forde BG, Leigh RA, Miller AJ. Functional expression of a plant plasma membrane transporter in Xenopus oocytes. FEBS Letters. 1992;302(2):166–168. doi: 10.1016/0014-5793(92)80431-f. [DOI] [PubMed] [Google Scholar]
- 102.Orsel M, Chopin F, Leleu O, et al. Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis. Physiology and protein-protein interaction. Plant Physiology. 2006;142(3):1304–1317. doi: 10.1104/pp.106.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu W, Fairbairn DJ, Reid RJ, Schachtman DP. Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability. Plant Physiology. 2001;127(1):283–294. doi: 10.1104/pp.127.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mäser P, Hosoo Y, Goshima S, et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6428–6433. doi: 10.1073/pnas.082123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ludewig U, Wilken S, Wu B, et al. Homo- and heterooligomerization of ammonium transporter-1 NH4 + uniporters. The Journal of Biological Chemistry. 2003;278(46):45603–45610. doi: 10.1074/jbc.M307424200. [DOI] [PubMed] [Google Scholar]
- 106.Neuhäuser B, Dynowski M, Mayer M, Ludewig U. Regulation of NH4 + transport by essential cross talk between AMT monomers through the carboxyl tails. Plant Physiology. 2007;143(4):1651–1659. doi: 10.1104/pp.106.094243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chandran D, Reinders A, Ward JM. Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. The Journal of Biological Chemistry. 2003;278(45):44320–44325. doi: 10.1074/jbc.M308490200. [DOI] [PubMed] [Google Scholar]
- 108.Sivitz AB, Reinders A, Johnson ME, et al. Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiology. 2007;143(1):188–198. doi: 10.1104/pp.106.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reinders A, Sivitz AB, Starker CG, Gantt JS, Ward JM. Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus . Plant Molecular Biology. 2008;68(3):289–299. doi: 10.1007/s11103-008-9370-0. [DOI] [PubMed] [Google Scholar]
- 110.Sasaki T, Yamamoto Y, Ezaki B, et al. A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal. 2004;37(5):645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 111.Ligaba A, Katsuhara M, Ryan PR, Shibasaka M, Matsumoto H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiology. 2006;142(3):1294–1303. doi: 10.1104/pp.106.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klepek Y-S, Geiger D, Stadler R, et al. Arabidopsis POLYOL TRANSPORTERS, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. The Plant Cell. 2005;17(1):204–218. doi: 10.1105/tpc.104.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramsperger-Gleixner M, Geiger D, Hedrich R, Sauer N. Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells. Plant Physiology. 2004;134(1):147–160. doi: 10.1104/pp.103.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schneider S, Schneidereit A, Konrad KR, et al. Arabidopsis INOSITOL TRANSPORTER4 mediates high-affinity H+ symport of myoinositol across the plasma membrane. Plant Physiology. 2006;141(2):567–577. doi: 10.1104/pp.106.077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider S, Schneidereit A, Udvardi P, et al. Arabidopsis INOSITOL TRANSPORTER2 mediates H+ symport of different inositol epimers and derivatives across the plasma membrane. Plant Physiology. 2007;145(4):1395–1407. doi: 10.1104/pp.107.109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hammes UZ, Nielsen E, Honaas LA, Taylor CG, Schachtman DP. AtCAT6, a sink-tissue-localized transporter for essential amino acids in Arabidopsis . The Plant Journal. 2006;48(3):414–426. doi: 10.1111/j.1365-313X.2006.02880.x. [DOI] [PubMed] [Google Scholar]
- 117.Colmenero-Flores JM, Martínez G, Gamba G, et al. Identification and functional characterization of cation-chloride cotransporters in plants. The Plant Journal. 2007;50(2):278–292. doi: 10.1111/j.1365-313X.2007.03048.x. [DOI] [PubMed] [Google Scholar]
- 118.Piňeros MA, Cançado GMA, Maron LG, Lyi SM, Menossi M, Kochian LV. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: the case of ZmALMT1—an anion-selective transporter. The Plant Journal. 2008;53(2):352–367. doi: 10.1111/j.1365-313X.2007.03344.x. [DOI] [PubMed] [Google Scholar]
- 119.Sentenac H, Bonneaud N, Minet M, et al. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256(5057):663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 120.Dreyer I, Antunes S, Hoshi T, et al. Plant K+ channel α-subunits assemble indiscriminately. Biophysical Journal. 1997;72(5):2143–2150. doi: 10.1016/S0006-3495(97)78857-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI. Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiology. 2000;122(4):1387–1397. doi: 10.1104/pp.122.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Formentin E, Varotto S, Costa A, et al. DKT1, a novel K+ channel from carrot, forms functional heteromeric channels with KDC1. FEBS Letters. 2004;573(1–3):61–67. doi: 10.1016/j.febslet.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 123.Fuchs I, Stölzle S, Ivashikina N, Hedrich R. Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta. 2005;221(2):212–221. doi: 10.1007/s00425-004-1437-9. [DOI] [PubMed] [Google Scholar]
- 124.Van Wilder V, Miecielica U, Degand H, Derua R, Waelkens E, Chaumont F. Maize plasma membrane aquaporins belonging to the PIP1 and PIP2 subgroups are in vivo phosphorylated. Plant and Cell Physiology. 2008;49(9):1364–1377. doi: 10.1093/pcp/pcn112. [DOI] [PubMed] [Google Scholar]
- 125.Dong X, Tang B, Li J, Xu Q, Fang S, Hua Z. Expression and purification of intact and functional soybean (Glycine max) seed ferritin complex in Escherichia coli . Journal of Microbiology and Biotechnology. 2008;18(2):299–307. [PubMed] [Google Scholar]
- 126.Lin J, Fido R, Shewry P, Archer DB, Alcocer MJC. The expression and processing of two recombinant 2S albumins from soybean (Glycine max) in the yeast Pichia pastoris . Biochimica et Biophysica Acta. 2004;1698(2):203–212. doi: 10.1016/j.bbapap.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Bustos MM, Luckow VA, Griffing LR, Summers MD, Hall TC. Expression, glycosylation and secretion of phaseolin in a baculovirus system. Plant Molecular Biology. 1988;10(6):475–488. doi: 10.1007/BF00033603. [DOI] [PubMed] [Google Scholar]
- 128.Kunze R, Starlinger P. The putative transposase of transposable element Ac from Zea mays L. interacts with subterminal sequences of Ac. The EMBO Journal. 1989;8(11):3177–3185. doi: 10.1002/j.1460-2075.1989.tb08476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vernet T, Tessier DC, Richardson C, et al. Secretion of functional papain precursor from insect cells. Requirement for N-glycosylation of the pro-region. The Journal of Biological Chemistry. 1990;265(27):16661–16666. [PubMed] [Google Scholar]
- 130.Korth KL, Levings CS., III Baculovirus expression of the maize mitochondrial protein URF13 confers insecticidal activity in cell cultures and larvae. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3388–3392. doi: 10.1073/pnas.90.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muschietti J, Dircks L, Vancanneyt G, McCormick S. LAT52 protein is essential for tomato pollen development: pollen expressing antisense LAT52 RNA hydrates and germinates abnormally and cannot achieve fertilization. The Plant Journal. 1994;6(3):321–328. doi: 10.1046/j.1365-313x.1994.06030321.x. [DOI] [PubMed] [Google Scholar]
- 132.MacDonald H, Henderson J, Napier RM, Venis MA, Hawes C, Lazarus CM. Authentic processing and targeting of active maize auxin-binding protein in the baculovirus expression system. Plant Physiology. 1994;105(4):1049–1057. doi: 10.1104/pp.105.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bauly JM, Sealy IM, Macdonald H, et al. Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiology. 2000;124(3):1229–1238. doi: 10.1104/pp.124.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meller Harel HY, Fontaine V, Chen H, Jones IM, Millner PA. Display of a maize cDNA library on baculovirus infected insect cells. BMC Biotechnology. 2008;8:64–69. doi: 10.1186/1472-6750-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mizutani M, Ohta D, Sato R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiology. 1997;113(3):755–763. doi: 10.1104/pp.113.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouly J-P, Giovani B, Djamei A, et al. Novel ATP-binding and autophosphorylation activity associated with Arabidopsis and human cryptochrome-1. European Journal of Biochemistry. 2003;270(14):2921–2928. doi: 10.1046/j.1432-1033.2003.03691.x. [DOI] [PubMed] [Google Scholar]
- 137.Cho H-Y, Tseng T-S, Kaiserli E, Sullivan S, Christie JM, Briggs WR. Physiological roles of the light, oxygen, or voltage domains of phototropin 1 and phototropin 2 in Arabidopsis . Plant Physiology. 2007;143(1):517–529. doi: 10.1104/pp.106.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nagai A, Suzuki K, Ward E, et al. Overexpression of plant histidinol dehydrogenase using a baculovirus expression vector system. Archives of Biochemistry and Biophysics. 1992;295(2):235–239. doi: 10.1016/0003-9861(92)90512-u. [DOI] [PubMed] [Google Scholar]
- 139.Stapleton A, Beetham JK, Pinot F, et al. Cloning and expression of soluble epoxide hydrolase from potato. The Plant Journal. 1994;6(2):251–258. doi: 10.1046/j.1365-313x.1994.6020251.x. [DOI] [PubMed] [Google Scholar]
- 140.Tada S, Hatano M, Nakayama Y, et al. Insect cell expression of recombinant imidazoleglycerolphosphate dehydratase of Arabidopsis and wheat and inhibition by triazole herbicides. Plant Physiology. 1995;109(1):153–159. doi: 10.1104/pp.109.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schledz M, Al-Babili S, von Lintig J, et al. Phytoene synthase from Narcissus pseudonarcissus: functional expression, galactolipid requirement, topological distribution in chromoplasts and induction during flowering. The Plant Journal. 1996;10(5):781–792. doi: 10.1046/j.1365-313x.1996.10050781.x. [DOI] [PubMed] [Google Scholar]
- 142.Al-Babili S, von Lintig J, Haubruck H, Beyer P. A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. The Plant Journal. 1996;9(5):601–612. doi: 10.1046/j.1365-313x.1996.9050601.x. [DOI] [PubMed] [Google Scholar]
- 143.Allina SM, Pri-Hadash A, Theilmann DA, Ellis BE, Douglas CJ. 4-coumarate:coenzyme a ligase in hybrid poplar. Properties of native enzymes, cDNA cloning, and analysis of recombinant enzymes. Plant Physiology. 1998;116(2):743–754. doi: 10.1104/pp.116.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pratelli R, Lacombe B, Torregrosa L, et al. A grapevine gene encoding a guard cell K+ channel displays developmental regulation in the grapevine berry. Plant Physiology. 2002;128(2):564–577. doi: 10.1104/pp.010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Philippar K, Büchsenschütz K, Abshagen M, et al. The K+ channel KZM1 mediates potassium uptake into the phloem and guard cells of the C4 grass Zea mays . The Journal of Biological Chemistry. 2003;278(19):16973–16981. doi: 10.1074/jbc.M212720200. [DOI] [PubMed] [Google Scholar]
- 146.Philippar K, Fuchs I, Lüthen H, et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hartje S, Zimmermann S, Klonus D, Mueller-Roeber B. Functional characterisation of LKT1, a K+ uptake channel from tomato root hairs, and comparison with the closely related potato inwardly rectifying K+ channel SKT1 after expression in Xenopus oocytes. Planta. 2000;210(5):723–731. doi: 10.1007/s004250050673. [DOI] [PubMed] [Google Scholar]
- 148.Xicluna J, Lacombe B, Dreyer I, et al. Increased functional diversity of plant K+ channels by preferential heteromerization of the Shaker-like subunits AKT2 and KAT2. The Journal of Biological Chemistry. 2007;282(1):486–494. doi: 10.1074/jbc.M607607200. [DOI] [PubMed] [Google Scholar]
- 149.Sottocornola B, Visconti S, Orsi S, et al. The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. The Journal of Biological Chemistry. 2006;281(47):35735–35741. doi: 10.1074/jbc.M603361200. [DOI] [PubMed] [Google Scholar]
- 150.Leng Q, Mercier RW, Hua B-G, Fromm H, Berkowitz GA. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiology. 2002;128(2):400–410. doi: 10.1104/pp.010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hua B-G, Mercier RW, Leng Q, Berkowitz GA. Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiology. 2003;132(3):1353–1361. doi: 10.1104/pp.103.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vincill ED, Szczyglowski K, Roberts DM. GmN70 and LjN70. Anion transporters of the symbiosome membrane of nodules with a transport preference for nitrate. Plant Physiology. 2005;137(4):1435–1444. doi: 10.1104/pp.104.051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Piñeros MA, Cançado GMA, Kochian LV. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: functional and structural implications. Plant Physiology. 2008;147(4):2131–2146. doi: 10.1104/pp.108.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meyer A, Eskandari S, Grallath S, Rentsch D. AtGAT1, a high affinity transporter for γ-aminobutyric acid in Arabidopsis thaliana . The Journal of Biological Chemistry. 2006;281(11):7197–7204. doi: 10.1074/jbc.M510766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chaumont F, Barrieu F, Jung R, Chrispeels MJ. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiology. 2000;122(4):1025–1034. doi: 10.1104/pp.122.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dordas C, Chrispeels MJ, Brown PH. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiology. 2000;124(3):1349–1361. doi: 10.1104/pp.124.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Werner M, Uehlein N, Proksch P, Kaldenhoff R. Characterization of two tomato aquaporins and expression during the incompatible interaction of tomato with the plant parasite Cuscuta reflexa . Planta. 2001;213(4):550–555. doi: 10.1007/s004250100533. [DOI] [PubMed] [Google Scholar]
- 158.Sakr S, Alves G, Morillon R, et al. Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiology. 2003;133(2):630–641. doi: 10.1104/pp.103.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu L-H, Ludewig U, Gassert B, Frommer WB, von Wirén N. Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis . Plant Physiology. 2003;133(3):1220–1228. doi: 10.1104/pp.103.027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Loqué D, Ludewig U, Yuan L, von Wirén N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiology. 2005;137(2):671–680. doi: 10.1104/pp.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O. Novel regulation of aquaporins during osmotic stress. Plant Physiology. 2004;135(4):2318–2329. doi: 10.1104/pp.104.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei W, Alexandersson E, Golldack D, Miller AJ, Kjellbom PO, Fricke W. HvPIP1;6, a barley (Hordeum vulgare L.) plasma membrane water channel particularly expressed in growing compared with non-growing leaf tissues. Plant and Cell Physiology. 2007;48(8):1132–1147. doi: 10.1093/pcp/pcm083. [DOI] [PubMed] [Google Scholar]
- 163.Lin W, Peng Y, Li G, et al. Isolation and functional characterization of PgTIP1, a hormone-autotrophic cells-specific tonoplast aquaporin in ginseng. Journal of Experimental Botany. 2007;58(5):947–956. doi: 10.1093/jxb/erl255. [DOI] [PubMed] [Google Scholar]
- 164.Choi W-G, Roberts DM. Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. The Journal of Biological Chemistry. 2007;282(33):24209–24218. doi: 10.1074/jbc.M700982200. [DOI] [PubMed] [Google Scholar]
- 165.Mahdieh M, Mostajeran A, Horie T, Katsuhara M. Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant and Cell Physiology. 2008;49(5):801–813. doi: 10.1093/pcp/pcn054. [DOI] [PubMed] [Google Scholar]
- 166.Shitan N, Bazin I, Dan K, et al. Involvement of CjMDR1, a plant multidrugresistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica . Proceedings of the National Academy of Sciences of the United States of America. 2003;100(2):751–756. doi: 10.1073/pnas.0134257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gustavsson S, Lebrun A-S, Nordén K, Chaumont F, Johanson U. A novel plant major intrinsic protein in Physcomitrella patens most similar to bacterial glycerol channels. Plant Physiology. 2005;139(1):287–295. doi: 10.1104/pp.105.063198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Becker D, Geiger D, Dunkel M, et al. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15621–15626. doi: 10.1073/pnas.0401502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Durrett TP, Gassmann W, Rogers EE. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology. 2007;144(1):197–205. doi: 10.1104/pp.107.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bassham DC, Raikhel NV. Plant cells are not just green yeast. Plant Physiology. 2000;122(4):999–1001. doi: 10.1104/pp.122.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]