Abstract
Background information. Primordial germ cells in developing male and female gonads are responsive to somatic cell cues that direct their sex-specific differentiation into functional gametes. The first divergence of the male and female pathways is a change in cell cycle state observed from 12.5 dpc (days post coitum) in mice. At this time XY and XX germ cells cease mitotic division and enter G1/G0 arrest and meiosis prophase I respectively. Aberrant cell cycle regulation at this time can lead to disrupted ovarian development, germ cell apoptosis, reduced fertility and/or the formation of germ cell tumours.
Results. In order to unravel the mechanisms utilized by germ cells to achieve and maintain the correct cell cycle states, we analysed the expression of a large number of cell cycle genes in purified germ cells across the crucial time of sex differentiation. Our results revealed common signalling for both XX and XY germ cell survival involving calcium signalling. A robust mechanism for apoptosis and checkpoint control was observed in XY germ cells, characterized by p53 and Atm (ataxia telangiectasia mutated) expression. Additionally, a member of the retinoblastoma family and p21 were identified, linking these factors to XY germ cell G1/G0 arrest. Lastly, in XX germ cells we observed a down-regulation of genes involved in both G1- and G2-phases of the cell cycle consistent with their entry into meiosis.
Conclusion. The present study has provided a detailed analysis of cell cycle gene expression during fetal germ cell development and identified candidate factors warranting further investigation in order to understand cases of aberrant cell cycle control in these specialized cells.
Keywords: cell cycle array, fetal ovary, fetal testis, germ cell, G1/G0 arrest, meiosis
Abbreviations: AP, alkaline phosphatase; ATM, ataxia telangiectasia mutated; CaMKII, Ca2+/calmodulin-dependent protein kinase II; CDK, cyclin-dependent kinase; Ccnd3 etc., cyclin D3 etc.; CIS, carcinoma in situ; Cks, CDC28 protein kinase; dpc, days post coitum; dpn, days post natum; Dst, dystonin; Fst, follistatin; Gas2, growth arrest-specific-2; MAPK, mitogen-activated protein kinase; Mcm, minichromosome maintenance deficient; Mdm2, murine double minute 2; Msh2, MutS homologue 2; Mvh, mouse vasa homologue; Nek3, NIMA (never in mitosis in Aspergillus nidulans)-related kinase 3; Pcna, proliferating-cell nuclear antigen; PIN1, peptidylprolyl isomerase 1; Pkd, polycystic kidney disease; RB, retinoblastoma; Rbl, retinoblastoma-like; qPCR, quantitative real-time RT–PCR; Sesn3, Sestrin 3; Shc1, Src homology 2 domain-containing transforming protein C1; SSEA-1, stage-specific embryonic antigen 1; TGCT, testicular germ cell tumour; Tnfs5ip1, tumour necrosis factor superfamily, member 5-induced protein 1
Introduction
PGCs (primordial germ cells) are the only cells in the body that undergo meiosis. Their developmental timeline is characterized by distinct cell cycle states that lead to the production of highly differentiated oocytes and spermatozoa. From initial specification at 7.25 dpc (days post coitum) in mice (Lawson and Hage, 1994; Saitou et al., 2002), a cluster of approx. 20 germ cells proliferate mitotically as they migrate to the genital ridge by 10.5–11.5 dpc (Chiquoine, 1954; Ginsburg et al., 1990; Ohinata et al., 2005). Here, they number approx. 25000 (Tam and Snow 1981; Donovan et al., 1986) and, in response to somatic cell cues, begin differentiation along the male (XY) or female (XX) pathway. XY germ cells enter G1/G0 arrest from 12.5 dpc, signifying commitment to the male pathway (Hilscher, 1974), while XX germ cells enter the first phase of meiosis from 13.5 dpc (Speed, 1982). G1/G0 arrest is completed by 14.5 dpc and maintained by the XY germ cells until after birth when they recommence several rounds of mitotic divisions before completing meiosis at the time of puberty (Setchell and Main, 1978; Western et al., 2008). XX germ cells enter meiosis in response to retinoic acid (Bowles et al., 2006; Koubova et al., 2006) and progress through to diplotene of meiosis I by 17.0 dpc–5 dpn (days post natum) (Borum, 1961; Speed, 1982). Meiosis then proceeds further as they mature into primordial follicles, but is not fully completed until fertilization (Pedersen and Peters, 1968; Gougeon, 1996).
Throughout all stages of germ cell development these cell cycle states are subject to tight regulation from the somatic cell environment. In addition to triggering the appropriate sex-specific cell cycle states in XY and XX germ cells, the somatic cells can also induce germ cell apoptosis in response to aberrant cell cycle regulation (McLaren, 1984; Coucouvanis et al., 1993; Nakatsuji and Chuma, 2001). Apoptosis is an effective mechanism for maintaining cellular integrity (Matsui, 1998), which is of high importance as germ cells are capable of acquiring ‘stemness’ despite their differentiated nature. In situations where apoptosis fails in the testis, germ cells can form CIS (carcinoma in situ) and TGCTs (testicular germ cell tumours) (Bartkova et al., 2003; Hoei-Hansen et al., 2005), which can be lethal or contribute to reduced fertility.
Despite the importance of germ cell cell cycle control for fertility, the exact machinery and regulatory mechanisms utilized have not yet been thoroughly investigated. Current knowledge about XY germ-cell G1/G0 arrest is limited to several candidate analyses and two knockout mouse models. Particularly, p63, a member of the p53 family, is expressed in G1/G0 arrested germ cells and the p63−/− mouse model displays a significant increase in the number of germ cells by birth due to a decrease in gonocyte apoptosis (Petre-Lazar et al., 2007). PIN1 (peptidylprolyl isomerase 1) has been implicated in many aspects of the cell cycle, including progression, DNA replication and checkpoint control (Lu et al., 1996; Winkler et al., 2000), and XY germ cells in Pin1−/− mutants displayed a prolonged cell cycle rate and an inability to enter G1/G0 arrest (Atchison et al., 2003). Additionally, activation of the CDK (cyclin-dependent kinase) inhibitors p15(INK4b), p16(INK4a) and p27(Kip1), as well as dephosphorylation of the RB1 (retinoblastoma 1) protein, has been identified during XY germ cell arrest (Western et al., 2008). Even though it has been hypothesized that germ cells employ a unique method of cell cycle control, these factors are all typical of somatic cell cell cycle control.
Cell cycle control common to both somatic and germ cells was also recently highlighted in a study of post-migratory, undifferentiated germ cells at 11.5 dpc, using a cell cycle array (Sorrentino et al., 2007). This study revealed germ cell control of G1-phase that was comparable with somatic cells, and included expression of Ccnd3 (cyclin D3), Rb1 and CDK inhibitors. Furthermore, it was evident that this population of germ cells was responsive to growth factor signalling and utilized cell cycle-regulated CDK inhibitor activity control in a similar way to the somatic cells analysed (Sorrentino et al., 2007).
In an analogous approach, we used a cell cycle array to identify the cell cycle machinery of XX and XY germ cells as they differentiate and enter their respective sex-specific cell cycle states prior to birth. In the present study, we discuss the known functions of the genes identified in our screen in relation to fetal germ cell cell cycle control and draw comparisons from postnatal development. Our results highlighted a common calcium-signalling pathway in both XX and XY germ cells, and identified extensive regulation of apoptosis within the XY germ cell population. Additionally we saw that G1/G0 arrest involved up-regulation of a RB family member and p21. Although our array did not detect meiosis-specific genes, we were able to identify the suppression of several mitotic genes in the XX germ cell population. Our results provide a comprehensive molecular description of cell cycle regulation of fetal germ cells as they undertake their sex-specific differentiation during development.
Results
Germ cell purity and 12.5 versus 14.5 dpc XX and XY germ cell analysis
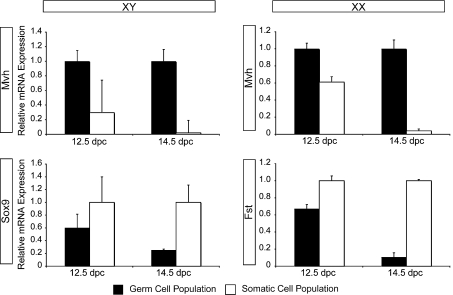
In order to profile changes in cell cycle gene expression during sex differentiation, we isolated XY and XX germ cells from 12.5 and 14.5 dpc gonads using an antibody-based magnetic purification system. We optimized this purification technique and identified SSEA-1 (stage-specific embryonic antigen 1) as the most effective at 12.5 dpc, and E-cadherin for 14.5 dpc populations (data not shown). Using these antibodies we achieved a different level of purity for each time point and sex. Based on the expression level of germ cell- and somatic cell-specific genes (Figure 1), along with visual assessment of AP (alkaline phosphatase) staining of the purified populations (ratio of AP-positive cells to AP-negative cells; results not shown), we determined the approximate purity as follows: 12.5 dpc XY >60%, 12.5 dpc XX >65%, 14.5 dpc XY >75% and 14.5 dpc XX >90%.
Figure 1. Analysis of XY and XX isolated germ cell population purities.
12.5 and 14.5 dpc germ cell populations were assessed for gene expression of the germ cell marker Mvh, and the somatic cell markers Sox9 (XY) and Fst (XX). qPCR analysis from purified populations normalized to 18S RNA. n=3, error bars represent S.E.M. Highest expression of Mvh was detected in all germ cell populations. Expression of Sox9 was highest in the XY somatic cell populations, with greatest specificity at 14.5 dpc. Expression of Fst was also highest in the XX somatic cell populations, with greatest specificity at 14.5 dpc.
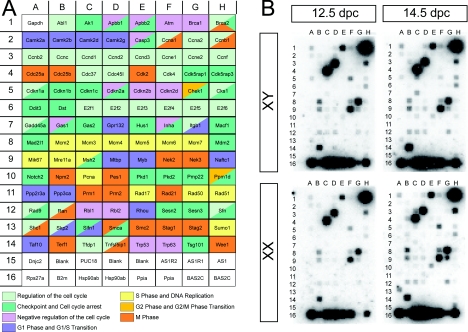
Using cDNA generated from the isolated germ cell populations, we profiled the expression level of 112 cell cycle-related genes for each time point (Figure 2A). Membrane hybridization represented three individual germ cell isolations for each sex and time point (Figure 2B). Using these populations we were able to make four comparisons: XX versus XY germ cells at 12.5 and 14.5 dpc and 12.5 versus 14.5 dpc for XX and XY germ cells. Because microarray analysis is generally known to underestimate fold changes in gene expression (Dallas et al., 2005), we focused on fold differences greater than 1.3. This threshold has also been used in other microarray studies (McHale et al., 2009). Using these criteria, gene expression changes across the germ cell populations revealed both up- and down-regulation of genes across all phases of the cell cycle (Table 1). From each comparison we next validated a selection of genes using qPCR [quantitative real-time RT–PCR (reverse transcription–PCR)] (Figure 3). Six out of nine genes analysed displayed a greater fold change than that detected by the array (Table 2), most likely reflecting the sensitivity of qPCR analysis relative to the microarray analysis (Dallas et al., 2005).
Figure 2. Gene identities and germ cell-hybridized membranes of the cell cycle array.
(A) Gene identities as spotted on to a SuperArray Cell Cycle Array (OMM-020). Genes have been colour-coded into their respective phases of the cell cycle in which they function: regulation of the cell cycle, checkpoint and cell cycle arrest, negative regulation of the cell cycle, G1-phase and G1/S transition, S-phase and DNA replication, G2-phase and G2/M transition and M- phase. (B) Hybridized membranes from three separate collections of XX and XY germ cell populations at 12.5 and 14.5 dpc showing the exposure used to generate fold expression changes between samples.
Table 1. Fold induction of cell cycle genes expressed in 12.5 and 14.5 dpc XX and XY germ cell populations.
| Gene symbol | Gene description | GenBank® accession number | XY 14.5 versus 12.5 | XX 14.5 versus 12.5 | 12.5 XY versus XX | 12.5 XX versus XY | 14.5 XY versus XX | 14.5 XX versus XY |
|---|---|---|---|---|---|---|---|---|
| Mcm6 | Minichromosome maintenance deficient 6 | NM_118567 | 3.72 | 1.69 | ||||
| Camk2g | Ca2+/calmodulin-dependent protein kinase II γ | NM_178597 | 3.52 | 1.92 | 1.4 | 1.31 | ||
| Tnfsf5ip1 | Tumour necrosis factor superfamily, member 5-induced protein 1 | NM_134138 | 2.79 | 1.4 | 1.59 | |||
| Nek3 | NIMA (never in mitosis in Aspergillus nidulans)-related kinase 3 | NM_011848 | 2.24 | 1.88 | ||||
| Ccne2 | Cyclin E2 | NM_009830 | 1.99 | 1.68 | ||||
| Sfn | Stratifin | NM_018754 | 1.85 | |||||
| Cdk5rap1 | CDK5 regulatory subunit associated protein 1 | NM_025876 | 1.84 | |||||
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A (p21) | NM_007669 | 1.82 | 1.29 | 1.34 | |||
| Mdm2 | Transformed mouse 3T3 cell double minute 2 | NM_010786 | 1.78 | 2.38 | ||||
| Nfatc1 | Nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 | NM_016791 | 1.7 | 1.95 | ||||
| Cdk4 | Cyclin-dependent kinase 4 | NM_009870 | 1.64 | 1.62 | ||||
| Rbl2 | Retinoblastoma-like 2 | NM_011250 | 1.61 | 1.3 | ||||
| Cdk2 | Cyclin-dependent kinase 2 | NM_016756 | 1.58 | 1.79 | ||||
| Cdk5rap3 | CDK5 regulatory subunit associated protein 3 | NM_030248 | 1.57 | |||||
| Atm | Ataxia telangiectasia mutated homologue | NM_007499 | 1.56 | 1.63 | ||||
| Msh2 | MutS homologue 2 | NM_008628 | 1.51 | 2 | ||||
| Camk2a | Ca2+/calmodulin-dependent protein kinase II α | NM_077407 | 1.5 | 1.74 | 1.46 | |||
| Camk2b | Ca2+/calmodulin-dependent protein kinase II β | NM_007595 | 1.5 | 1.35 | 1.4 | 1.55 | ||
| Camk2d | Ca2+/calmodulin-dependent protein kinase II δ | NM_023813 | 1.49 | 1.39 | ||||
| Mcm4 | Minichromosome maintenance deficient 4 homologue | NM_008565 | 1.49 | 1.74 | ||||
| Shc1 | Src homology 2 domain-containing transforming protein C1 | NM_011368 | 1.46 | –14.11 | 25.33 | |||
| Trp53 | Transformation related protein 53 | NM_011640 | 1.45 | 1.44 | ||||
| Ccna1 | Cycin A1 | NM_007628 | 1.39 | |||||
| Pkd2 | Polycystic kidney disease 2 | NM_008861 | 1.37 | 1.54 | ||||
| Pcna | Proliferating cell nuclear antigen | NM_011045 | 1.34 | 1.4 | ||||
| Trp63 | Transformation related protein 63 | NM_011641 | 1.32 | 1.36 | ||||
| Ran | RAN, member RAS oncogene family | NM_009391 | 1.27 | 1.39 | ||||
| Pkd1 | Polycystic kidney disease 1 | NM_013630 | 1.25 | –2.92 | 3.96 | |||
| Mtbp | Mdm2, transformed 3T3 cell double minute p53-binding protein | NM_134092 | 1.05 | 1.44 | ||||
| Rbl1 | Retinoblastoma-like 1 | NM_011249 | 1.04 | –1.62 | 1.75 | |||
| Sesn3 | Sestrin 3 | NM_030261 | –1.21 | –2.67 | 2.6 | |||
| Cks1b | CDC28 protein kinase 1b | NM_016904 | –1.53 | 1.32 | ||||
| E2f6 | E2F transcription factor 6 | NM_033270 | –1.63 | –1.61 | 1.3 | |||
| Gas2 | Growth arrest-specific 2 | NM_008087 | –2.6 | 1.96 | ||||
| Cdc25b | Cell division cycle 25 homologue B | NM_023117 | –2.43 | 3.85 | ||||
| Dst | Dystonin | NM_134448 | –1.32 | 2.42 | ||||
| Mre11a | Meiotic recombination 11 homologue A | NM_018567 | 4.5 | |||||
| Ppp23a | Protein phosphatase 2 (formerly 2A), regulatory subunit B, α | XM_001005535 | 1.53 | 2.13 | ||||
| Rad9 | RAD9 homologue | NM_011237 | 1.32 | |||||
| Ccnd1 | Cyclin D1 | NM_007631 | 5.61 | |||||
| Prm2 | Protamine 2 | NM_008933 | 2.13 | |||||
| Gas1 | Growth arrest-specific 1 | NM_008086 | 2.03 | |||||
| Wee1 | Wee 1 homologue | NM_009516 | 1.95 | |||||
| Mcm4 | Minichromosome maintenance deficient 4 homologue | NM_008563 | 1.74 | |||||
| Npm2 | Nucleophosmin/nucleoplasmin 2 | NM_007892 | 1.74 | |||||
| E2f5 | E2F transcription factor 5 | NM_007595 | 1.7 |
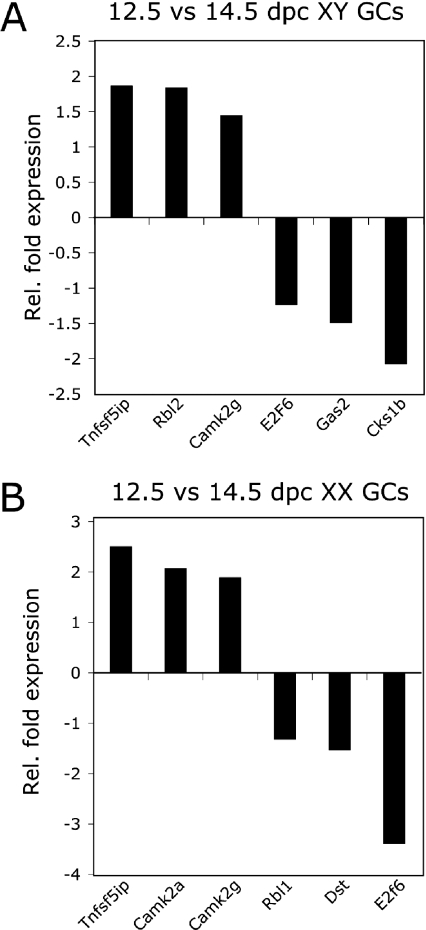
Figure 3. Validation of significantly up- and down-regulated transcripts identified from 12.5 versus 14.5 dpc germ cell populations.
qPCR analysis from purified populations normalized to 18S RNA, n=3. (A) Increased fold expression of Tnfs5ip, Rbl2 and Camk2g and decreased fold expression of E2f6, Gas2 and Cks1b in XY 14.5 dpc populations relative to XY 12.5 dpc populations. (B) Increased fold expression of Tnfs5ip, Camk2a and Camk2g and decreased fold expression of Rbl1, Dst and E2f6 in XX 14.5 dpc populations relative to XX 12.5 dpc populations. Gc, germ cell; Rel., relative.
Table 2. Comparison of gene fold changes detected in the microarray analysis and qPCR.
| XY germ cells | XX germ cells | |||
|---|---|---|---|---|
| Gene | Array | qPCR | Array | qPCR |
| Rbl2 | 1.61 | 1.84 | ||
| Gas2 | –2.60 | –1.49 | ||
| Cks1b | –1.530 | –2.07 | ||
| Tnfsf5ip | 2.79 | 1.86 | 1.40 | 2.51 |
| Camk2g | 3.52 | 1.45 | 1.92 | 1.89 |
| E2f6 | –2.16 | –1.60 | –1.61 | –1.66 |
| Camk2a | 1.74 | 2.07 | ||
| Rbl1 | –1.62 | –1.33 | ||
| Dst | –1.32 | –3.48 | ||
Comparison of XX versus XY germ cells at 12.5 dpc identified very few genes with a fold difference greater than 1.3 between these populations, with only five and six genes up-regulated in the XX and XY germ cell populations respectively. This cell cycle expression profile is consistent with actively cycling germ cells in both sexes. Conversely, comparison of XX versus XY populations at 14.5 dpc highlighted a large number of genes more highly expressed in the XX germ cells (34 genes), as opposed to the XY germ cell population, which had only two genes up-regulated at this time. This result is indicative of a comparison between a population that is actively cycling (XX germ cells) and a population that has ceased cell division (XY germ cells), supporting the suitability of our isolation technique for this study.
Cell cycle analysis of 12.5 versus 14.5 dpc XY germ cells
Germ cells in the XY gonad have been reported to enter G1/G0 arrest from 12.5 dpc onwards, and this arrest is complete for most of the germ cells by 14.5 dpc (Western et al., 2008). Consistent with this timeline, our analysis of 14.5 versus 12.5 dpc XY germ cell populations revealed that 26 genes were up-regulated and three genes were down-regulated at 14.5 dpc and showed enrichment in the G1-phase of the cell cycle (Table 1). Specifically, those genes up-regulated that were involved in G1/G0 arrest included the classic cell cycle-arrest genes Rbl2 (retinoblastoma-like 2), CDK inhibitor p21 (Cdkn1a) and Pkd2 (polycystic kidney disease-2). Genes involved in the G1-phase of the cell cycle included the CaMKII (Ca2+/calmodulin-dependent protein kinase II) subunits Camk2a, -2b, -2d and -2g in addition to Nfatc1 (nuclear factor of activated T-cells-1). The G1-phase cyclins included Ccna1 and Ccne2 and CDKs Cdk4 and Cdk2. Eight up-regulated genes involved in checkpoint control were p53, p63, ATM (ataxia telangiectasia mutated), Mdm2 (murine double minute 2), encoding CDK5 subunits Cdk5rap1 and Cdk5rap3, Msh2 (MutS homologue 2) and Shc1 (Src homology 2 domain-containing transforming protein C1).
Three genes involved in the S-phase of the cell cycle in the 14.5 dpc XY germ cell population included minichromosome maintenance genes Mcm4 (minichromosome maintenance deficient 4), Mcm6 and Pcna (proliferating-cell nuclear antigen). We identified only three genes involved in the G2/M-phase of the cell cycle, namely Nek3 [NIMA (never in mitosis in Aspergillus nidulans)-related kinase 3], Sfn (Stratifin) and Tnfs5ip (tumour necrosis factor superfamily, member 5-induced protein 1) (Clast3). From our array we observed three genes that were down-regulated from 12.5 to 14.5 dpc in XY germ cells and these were involved in checkpoint control: CDK1 [Cks1 (CDC28 protein kinase 1)], Gas2 (growth arrest-specific-2) and the transcription factor E2f6 (Figure 4A).
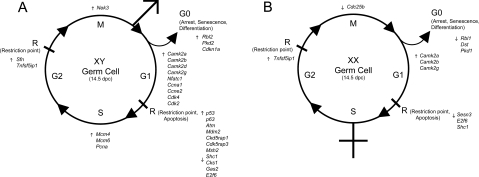
Figure 4. Functional gene groupings of significantly up- and down-regulated transcripts in XY and XX germ cell populations at 14.5 dpc relative to 12.5 dpc populations.
(A) A total of 13 genes involved in G1/G0 were up-regulated in XY 14.5 dpc populations. Seven genes up-regulated and three genes down-regulated were involved in restriction points and apoptosis. Three, two and one genes that are involved in S-phase, G2- and M-phase respectively were also up-regulated. (B) Three genes were up-regulated and three genes down-regulated that are involved in G1/G0 in the 14.5 dpc XX germ cell populations. Three genes involved in restriction points and apoptosis were down-regulated and one gene each involved in G2- and M-phase were up- and down-regulated respectively.
Cell cycle analysis of 12.5 versus 14.5 dpc XX germ cells
In contrast with XY germ cells in the testis, XX germ cells continue mitotic division after colonizing the ovary and enter meiosis from 13.5 dpc (Speed, 1982). Subsequent meiotic entry is less synchronous than that of XY germ cell G1/G0 arrest and XX germ cell entry into prophase I of meiosis is not completed until 17.0 dpc–5 dpn (Borum, 1961; Speed, 1982). As this array did not contain any meiosis-specific gene probes, we only observed four up-regulated and seven down-regulated genes in the XX cell population (Table 1). Up-regulated genes in the XX germ cells involved in G1-phase included three CaMKII subunits Camk2a, -2b and -2g. Tnfsf5ip, involved in G2/M-phase, was also up-regulated. Down-regulation of genes involved in G0 arrest included another member of the RB family Rbl1 in addition to Dst (dystonin) and Pkd1. Those involved in checkpoint control and apoptosis in the XX germ cell population included Shc1, E2f6 and Sesn3 (Sestrin 3). Lastly, the G2/M-phase gene Cdc25b was also down-regulated in our analysis (Figure 4B).
Discussion
Germ cell sex differentiation, triggered by the somatic cell environment, heralds the beginning of a multitude of cell cycle changes that accommodate meiosis in these cells and lead to the differentiation of highly specialized gametes (oocytes or spermatozoa) (McLaren, 1988). Tight control of cell cycle progression is required at each stage to prevent aberrant proliferation that results in germ cell tumours or apoptosis that contribute to infertility (Hoei-Hansen et al., 2005). In order to gain insight into the cell cycle regulation that controls the earliest sex-specific differentiation during development, we employed a cDNA microarray to profile 112 cell cycle-related genes across XX and XY germ cells at 12.5 and 14.5 dpc.
XY germ cell G1-phase
XY germ cells are reported to enter G1/G0 arrest from 12.5 dpc in the developing testis and maintain this state until after birth (McLaren and Buehr, 1990; Western et al., 2008). From our array we identified multiple subunits (Camk2a, -2b, -2d and -2g) of CaMKII (Sheng et al., 1991; Matthews et al., 1994; Fukunaga and Miyamoto, 1999; Cai et al., 2008;); however, as these subunits were also detected in the XX germ cell population, we suggest that calcium signalling has a survival role in these environments, as has been suggested previously (Yano et al., 1998; Bok et al., 2007), rather than signalling sex-specific responses for these cells. Interestingly, however, MAPK (mitogen-activated protein kinase) p38, which can activate calmodulin kinases and regulate proliferation, differentiation and cell survival, is activated specifically within the G1/G0 arrested germ cell population (K. Ewen, personal communication). These findings suggest that the calcium–MAPK pathway may be an active signalling mechanism used by the XY gonocytes.
We also identified the G1-phase cyclin Ccna1 in XY germ cells. Interestingly, Ccna1 is regarded as a germ cell-specific cyclin with expression almost entirely restricted to the spermatogonia of the adult testis (Sweeney et al., 1996) and Ccna1−/− male mice are sterile (Wolgemuth et al., 2004). The CDKs Ckd2 and Cdk4 were also identified. Of these, Ckd2 is required for the initiation of meiosis and male and female Ckd2−/− animals are infertile (Barbacid et al., 2005).
XY germ cell G1/G0 arrest
We have implicated several established cell cycle-arrest genes in XY germ cell G1/G0 arrest. Particularly, the RB family, to which Rbl2 belongs, regulates cell growth, differentiation and apoptosis, and is expressed in a cell cycle-dependent manner throughout spermatogenesis (Yan et al., 2001; Toppari et al., 2003). Although Rb1 itself was not included on this array, RB1 is translated and activated during XY G1/G0 arrest (Western et al., 2008), suggesting an important role for this family in XY germ cell biology. Additionally, the CDK inhibitor p21 was up-regulated. p21 inhibits cell cycle progression by interacting with cyclin–CDK complexes, which induce apoptosis in adult testes in a p53-dependent manner (el-Deiry et al., 1994; Moreno et al., 2001). The p21−/− model displays a somatic and germ cell proliferation defect in the adult testis (Deng et al., 1995); however, it is unclear whether this is a result of overproliferation or reduced apoptosis. The up-regulation of p21 during embryogenesis has also been detected by Western et al. (2008) and further experiments are under way to determine whether p21 is also responsible for germ cell apoptosis or arrest at this stage.
XY germ cell apoptosis and checkpoint control mechanisms
Our analysis revealed a robust control mechanism for apoptosis and checkpoint monitoring in the XY 14.5 dpc germ cell population. In particular, p53, p63 and ATM presumably act to reinforce germline integrity by apoptosis, of which p53 is the master regulator controlling DNA-damage and checkpoint failure-induced apoptosis in many cell types (reviewed by Meulmeester and Jochemsen, 2008). p53 is regulated by PI3K (phosphoinositide 3-kinase)/Akt (also called protein kinase B) (reviewed by Kimura et al., 2008) and TGF-β (transforming growth factor-β) signalling (reviewed by Sasai et al., 2008), the latter of which has been shown to play a role in germ cell survival in the embryonic testis (Memon et al., 2008). ATM is required in the pre-meiotic gonocyte stem cell population of the adult testis (Elson et al., 1996; Takubo et al., 2008); however, our analysis is the first report of Atm expression in XY fetal germ cells. Mdm2 and Msh2, involved in p53-mediated apoptosis (Mostert et al., 2000) and replication error correction (Velasco et al., 2004) respectively were also up-regulated in XY germ cells. Consistent with an important role in maintaining germline integrity, mutations of both Mdm2 and Msh2 have been implicated in germ cell tumours (Velasco et al., 2004). Our identification of genes involved in postnatal tumours gives further support to the notion that fetal germ cell cell cycle control is crucial for their transformation into CIS and TGCTs (Bartkova et al., 2000; Hoei-Hansen et al., 2005).
XY germ cell progression past G1-phase
Whereas most of the genes identified in our array function within G1 and G1/G0 arrest, there was also an up-regulation of a small number of genes involved in the progression past G1-phase in which XY germ cells reside. It has been suggested by Moreno et al. (2001) that the G1/G0 arrest of the germ cells is not a complete block in G0, but rather a slowing of G1-phase due to the observation that XY germ cells continue to increase in size during arrest and display high radiosensitivity, symptomatic of cycling cells (Moreno et al., 2001). Our analyses lend some support to this notion at the level of transcription. Indeed, three genes involved in S-phase were expressed in the 14.5 dpc XY germ cell population. Minichromosome maintenance genes Mcm4, Mcm6 and Pcna gene products are involved in DNA replication (Swiech et al., 2007) and mismatch repair (Stone et al., 2008) respectively. Additionally, Tnfs5ip (Clast3) and Nek3, involved in later progression of the cell cycle (Tanaka and Nigg, 1999; Bahar et al., 2002), were also identified in our array.
Suppression of meiosis in XY germ cells
In contrast with XX germ cells, XY gonocytes do not enter meiosis until puberty (Setchell and Main, 1978). Appropriately, we observed the down-regulation of several genes involved in checkpoint control and suppression of germ cell-specific genes in XY germ cells. These included Cks1, which encodes a CDK that modulates cell cycle progression through the G1/S checkpoint, which is required for progression of meiosis in the adult testis. Accordingly, Cks1−/− mutants display arrested spermatocytes in metaphase I of meiosis (Donovan and Reed, 2003), suggesting that fetal germ cells suppress this transcript that is required for the later stages of meiosis. Lastly, E2f6, which encodes a unique E2F member that is known to suppress XY germ cell-specific genes (Pohlers et al., 2005), was also appropriately down-regulated in XY germ cells.
Down-regulation of mitotic genes in XX germ cells
XX germ cells begin non-synchronous entry into prophase I of meiosis from 13.5 dpc onwards (McLaren, 2000). Therefore, using the mitotic cell cycle array employed in the present study, we did not detect any genes associated with meiosis; however, the down-regulation of several mitotic genes was identified. In particular, another member of the RB family, Rbl1, was down-regulated in XX germ cells, reinforcing the sex-specific expression of this family. Suppression of apoptotic genes was also apparent, with down-regulation of Dst and Pkd1 (van Adelsberg, 1999; Bilyy et al., 2005), as well as Sesn3, which is responsive to p53 activation during apoptosis induction (Kopnin et al., 2007). Suppression of apoptotic genes at this time point fits with reported oocyte apoptosis that occurs at 13.5 dpc, and again from 15.5 to 17.5 dpc in the ovary (Coucouvanis et al., 1993). Cdc25b, also down-regulated in XX germ cells, encodes a phosphatase required to activate CDKs for cell cycle progression. In the adult ovary, this phosphatase has been shown to be essential for CDK1 activation during the resumption of meiosis past prophase I (Lincoln et al., 2002; Solc et al., 2008).
Conclusions
Our cell cycle gene expression analysis has highlighted several important cell cycle mechanisms utilized by XY and XX germ cells during the crucial time of sex differentiation in the developing gonads. In particularly, apoptosis regulation is apparent for both populations, along with calcium/calmodulin involvement, presumably for cell survival. The present study has provided a solid basis for the future investigation of both well- and little-known cell cycle genes in the regulation of sex-specific differentiation of fetal germ cells. Investigation into the expression of some of these newly identified genes in cases of TGCTs may provide further insight into the regulation of these highly specialized cells in cases of aberrant cell cycle control. Particular focus should surround the regulation of the RB family, p21, p53 and ATM in XY germ cells. Finally, regulation of Dst and Pkd1 in addition to Cdc25b in the XX germ cells may shed light on the regulation of meiosis triggered by retinoic acid and other unidentified somatic factors.
Experimental
MiniMACS germ cell isolation
Germ cells were isolated from CD1 mouse embryo gonads dissected in ice-cold PBS at 12.5 and 14.5 dpc with mesonephros removed. Gonads were rinsed twice in Gibco cell dissociation buffer (13151-014; Invitrogen) and incubated with agitation in the dissociation buffer for 5 min at 37°C before dissociation using a 21G needle. Dissociated cells were diluted to 1.5 ml with MACS buffer (PBS containing 2 mM EDTA and 0.5% BSA) and centrifuged at 600 g at 4°C for 10 min. Pelleted cells were resuspended in 1:50 dilution of mouse anti-human SSEA-1 antibody (12.5 dpc; Santa Cruz Biotechnology) or rat anti-mouse E-cadherin antibody (14.5 dpc; Santa Cruz Biotechnology) in MACS buffer and incubated with agitation at 4°C for 45 min. Cells were then washed twice with MACS buffer by centrifugation and incubated in 1:5 dilution of rabbit anti-mouse IgG magnetic beads (SSEA-1;130-047-102; Miltenyi Biotec) or goat anti-rat IgG magnetic beads (E-cadherin; 130-048-501; Miltenyi Biotec) in MACS buffer with agitation at 4°C for 15 min. Cells were then washed twice with MACS buffer by centrifugation, resuspended in 1.5 ml of MACS buffer and applied to a presoaked MiniMACS column (130-042-201; Miltenyi Biotec) on a magnetic backing. Somatic cells were eluted with 3 ml of MACS buffer and the column was then removed from the magnetic backing and germ cells were eluted in 3 ml of MACS buffer. Purified germ cells were then either centrifuged at 16200 g for 1 min for immediate RNA isolation, or pelleted at 600 g for 10 min before staining for AP. Purity of the germ cell population was determined using both AP staining and qPCR analysis for expression of the germ cell marker Mvh (mouse vasa homologue) (Saitou et al., 2002) and somatic cell markers Sox9 [Sry-related HMG (high-mobility group) box protein 9] (XY) (Wilhelm et al., 2005) and Fst (follistatin) (XX) (Vainio et al., 1999).
cDNA array
Total RNA was isolated from purified germ cells using the ArrayGrade™ total RNA isolation kit (GA-013; SuperArray) as per the manufacturer's instructions. RNA quality was determined by spectroscopy at 260 and 280 nm using a Nanodrop (Nanodrop Technologies). Total RNA (1 μg) was amplified and labelled with the biotin TrueLabeling-AMP™ 2.0 system (GA-030; SuperArray) as per the manufacturer's instructions using Biotin-16-UTP (11388908910; Roche Applied Science). The commercial GEArray membranes (Oligo GEArray DNA Microarray: Mouse Cell Cycle Q Series; OMM-020; Superarray; www.SABiosciences.com/ArrayList.php) were prehybridized for 8 h at 60°C and hybridized with cRNA target samples overnight at 60 °C. Membranes were then washed with 2× SSC (1× SSC is 0.15 M NaCl/0.015 M sodium citrate) and 1% SDS and then in 0.1× SSC and 0.5% SDS at 60°C for exactly 15 min. Chemiluminescent detection was performed using AP-conjugated streptavidin and CDP-Star chemiluminescent substrate. Membranes were then exposed to an X-ray film (Fuji Photo Film).
cDNA array analysis
A Hewlett–Packard ScanJet scanner was used to scan the signal for each array membrane. The signal intensity for each probe was analysed using the GEArray Analysis Suite. The total background for each membrane was subtracted from the total average value of each tetraspot for each cDNA sample. This value was normalized to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) signal control on each membrane. Transcript levels were then compared between arrays to identify the genes up- and down-regulated in each sample.
qPCR
Specific genes identified as being up- or down-regulated were measured on at least three independent cDNA samples using qPCR. Then 12.5 and 14.5 dpc XX and XY germ cells were isolated as described above and total RNA from each collection was isolated using a Micro RNA kit (Qiagen) as per the manufacturer's instructions including the optional DNaseI step. cDNA was synthesized from 1 μg of RNA by reverse transcription (Superscript III; Invitrogen) using random primers (Promega) according to the manufacturer's instructions. The ABIPrism-7000 Sequence Detector System was used to analyse relative cDNA levels. Primers were designed for each mRNA using the Universal Probe library tool (https://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp) (see Supplementary Table S1 at http://www.biolcell.org/boc/101/boc1010587add.htm). All qPCR experiments were performed in triplicate and repeated on three separate biological germ cell collections representing four pooled litters and results are represented as means±S.E.M. Briefly, samples were analysed in 25 μl reactions containing 1 μl of cDNA prepared as described above, SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, U.S.A.) and 1 μl of each forward and reverse primer (3.75 μM). Cycling conditions used an initial 10 min step at 95°C followed by 40 cycles of 95°C for 15 s and 60°C for 1 min in a two-step thermal cycle. Dissociation curves were analysed for each primer set to verify the amplification of a single product and cDNA samples were normalized against 18S rRNA using the 2▵▵−Ct method (Ct is threshold cycle value).
Online data
Funding
This work was supported by the Australian Research Council; and the National Health and Medical Research Council of Australia. C.S. is supported by a University of Queensland Postgraduate Research Scholarship. D.W. is funded by the National Health and Medical Research Council of Australia. P.K. is a Federation Research Fellow of the Australian Research Council.
References
- Atchison F.W., Capel B., Means A.R. PIN1 regulates the timing of mammalian primordial germ cell proliferation. Development. 2003;130:3579–3586. doi: 10.1242/dev.00584. [DOI] [PubMed] [Google Scholar]
- Bahar R., J O.W., Kawamura K., Seimiya M., Wang Y., Hatano M., Okada S., Tokuhisa T., Watanabe T., Tagawa M. Growth retardation, polyploidy, and multinucleation induced by Clast3, a novel cell cycle-regulated protein. J. Biol. Chem. 2002;277:40012–40019. doi: 10.1074/jbc.M205345200. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Ortega S., Sotillo R., Odajima J., Martin A., Santamaria D., Dubus P., Malumbres M. Cell cycle and cancer: genetic analysis of the role of cyclin-dependent kinases. Cold Spring Harb. Symp. Quant. Biol. 2005;70:233–240. doi: 10.1101/sqb.2005.70.005. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Thullberg M., Rajpert-De Meyts E., Skakkebaek N.E., Bartek J. Cell cycle regulators in testicular cancer: loss of p18INK4C marks progression from CIS to invasive germ cell tumours. Int. J. Cancer. 2000;85:370–375. doi: 10.1002/(sici)1097-0215(20000201)85:3<370::aid-ijc13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Lukas C., Sorensen C.S., Meyts E.R., Skakkebaek N.E., Lukas J., Bartek J. Deregulation of the RB pathway in human testicular germ cell tumours. J. Pathol. 2003;200:149–156. doi: 10.1002/path.1353. [DOI] [PubMed] [Google Scholar]
- Bilyy R., Kit Y., Hellman U., Tryndyak V., Kaminskyy V., Stoika R. In vivo expression and characteristics of novel alpha-D-mannose-rich glycoprotein markers of apoptotic cells. Cell Biol. Int. 2005;29:920–928. doi: 10.1016/j.cellbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Bok J., Wang Q., Huang J., Green S.H. CaMKII and CaMKIV mediate distinct prosurvival signaling pathways in response to depolarization in neurons. Mol. Cell Neurosci. 2007;36:13–26. doi: 10.1016/j.mcn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp. Cell Res. 1961;24:495–507. doi: 10.1016/0014-4827(61)90449-9. [DOI] [PubMed] [Google Scholar]
- Bowles J., Knight D., Smith C., Wilhelm D., Richman J., Mamiya S., Yashiro K., Chawengsaksophak K., Wilson M.J., Rossant J., et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Cai H., Liu D., Garcia J.G. CaM kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc. Res. 2008;77:30–34. doi: 10.1093/cvr/cvm010. [DOI] [PubMed] [Google Scholar]
- Chiquoine A.D. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat. Rec. 1954;118:135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E.C., Sherwood S.W., Carswell-Crumpton C., Spack E.G., Jones P.P. Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp. Cell Res. 1993;209:238–247. doi: 10.1006/excr.1993.1307. [DOI] [PubMed] [Google Scholar]
- Dallas P.B., Gottardo N.G., Firth M.J., Beesley A.H., Hoffmann K., Terry P.A., Freitas J.R., Boag J.M., Cummings A.J., Kees U.R. Gene expression levels assessed by oligonucleotide microarray analysis and quantitative real-time RT–PCR – how well do they correlate? BMC Genomics. 2005;6:59. doi: 10.1186/1471-2164-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J.W., Elledge S.J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Donovan P.J., Reed S.I. Germline exclusion of Cks1 in the mouse reveals a metaphase I role for Cks proteins in male and female meiosis. Cell Cycle. 2003;2:275–276. [PubMed] [Google Scholar]
- Donovan P.J., Stott D., Cairns L.A., Heasman J., Wylie C.C. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell. 1986;44:831–838. doi: 10.1016/0092-8674(86)90005-x. [DOI] [PubMed] [Google Scholar]
- el-Deiry W.S., Harper J.W., O'Connor P.M., Velculescu V.E., Canman C.E., Jackman J., Pietenpol J.A., Burrell M., Hill D.E., Wang Y., et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- Elson A., Wang Y., Daugherty C.J., Morton C.C., Zhou F., Campos-Torres J., Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K., Miyamoto E. Current studies on a working model of CaM kinase II in hippocampal long-term potentiation and memory. Japan. J. Pharmacol. 1999;79:7–15. doi: 10.1254/jjp.79.7. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Snow M.H., McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Hilscher W. [Kinetics of prespermatogenesis and spermatogenesis] Verh. Anat. Ges. 1974;68:39–62. [PubMed] [Google Scholar]
- Hoei-Hansen C.E., Rajpert-De Meyts E., Daugaard G., Skakkebaek N.E. Carcinoma in situ testis, the progenitor of testicular germ cell tumours: a clinical review. Ann. Oncol. 2005;16:863–868. doi: 10.1093/annonc/mdi175. [DOI] [PubMed] [Google Scholar]
- Kimura T., Tomooka M., Yamano N., Murayama K., Matoba S., Umehara H., Kanai Y., Nakano T. AKT signaling promotes derivation of embryonic germ cells from primordial germ cells. Development. 2008;135:869–879. doi: 10.1242/dev.013474. [DOI] [PubMed] [Google Scholar]
- Kopnin P.B., Agapova L.S., Kopnin B.P., Chumakov P.M. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J., Menke D.B., Zhou Q., Capel B., Griswold M.D., Page D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K.A., Hage W.J. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found. Symp. 1994;182:68–84. doi: 10.1002/9780470514573.ch5. discussion 84–91. [DOI] [PubMed] [Google Scholar]
- Lincoln A.J., Wickramasinghe D., Stein P., Schultz R.M., Palko M.E., De Miguel M.P., Tessarollo L., Donovan P.J. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat. Genet. 2002;30:446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- Lu K.P., Hanes S.D., Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- Matsui Y. Developmental fates of the mouse germ cell line. Int. J. Dev. Biol. 1998;42:1037–1042. [PubMed] [Google Scholar]
- Matthews R.P., Guthrie C.R., Wailes L.M., Zhao X., Means A.R., McKnight G.S. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol. Cell. Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale C.M., Zhang L., Lan Q., Li G., Hubbard A.E., Forrest M.S., Vermeulen R., Chen J., Shen M., Rappaport S.M., et al. Changes in the peripheral blood transcriptome associated with occupational benzene exposure identified by cross-comparison on two microarray platforms. Genomics. 2009;93:343–349. doi: 10.1016/j.ygeno.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp. Soc. Exp. Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- McLaren A. Somatic and germ-cell sex in mammals. Philos. Trans. R. Soc. London Ser. B. 1988;322:3–9. doi: 10.1098/rstb.1988.0109. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol. Cell. Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- McLaren A., Buehr M. Development of mouse germ cells in cultures of fetal gonads. Cell Differ. Dev. 1990;31:185–195. doi: 10.1016/0922-3371(90)90131-f. [DOI] [PubMed] [Google Scholar]
- Memon M.A., Anway M.D., Covert T.R., Uzumcu M., Skinner M.K. Transforming growth factor beta (TGFbeta1, TGFbeta2 and TGFbeta3) null-mutant phenotypes in embryonic gonadal development. Mol. Cell. Endocrinol. 2008;294:70–80. doi: 10.1016/j.mce.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulmeester E., Jochemsen A.G. p53: a guide to apoptosis. Curr. Cancer Drug Targets. 2008;8:87–97. doi: 10.2174/156800908783769337. [DOI] [PubMed] [Google Scholar]
- Moreno S.G., Dutrillaux B., Coffigny H. Status of p53, p21, mdm2, pRb proteins, and DNA methylation in gonocytes of control and gamma-irradiated rats during testicular development. Biol. Reprod. 2001;64:1422–1431. doi: 10.1095/biolreprod64.5.1422. [DOI] [PubMed] [Google Scholar]
- Mostert M., Rosenberg C., Stoop H., Schuyer M., Timmer A., Oosterhuis W., Looijenga L. Comparative genomic and in situ hybridization of germ cell tumors of the infantile testis. Lab. Invest. 2000;80:1055–1064. doi: 10.1038/labinvest.3780110. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N., Chuma S. Differentiation of mouse primordial germ cells into female or male germ cells. Int. J. Dev. Biol. 2001;45:541–548. [PubMed] [Google Scholar]
- Ohinata Y., Payer B., O'Carroll D., Ancelin K., Ono Y., Sano M., Barton S.C., Obukhanych T., Nussenzweig M., Tarakhovsky A., et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Pedersen T., Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Petre-Lazar B., Livera G., Moreno S.G., Trautmann E., Duquenne C., Hanoux V., Habert R., Coffigny H. The role of p63 in germ cell apoptosis in the developing testis. J. Cell. Physiol. 2007;210:87–98. doi: 10.1002/jcp.20829. [DOI] [PubMed] [Google Scholar]
- Pohlers M., Truss M., Frede U., Scholz A., Strehle M., Kuban R.J., Hoffmann B., Morkel M., Birchmeier C., Hagemeier C. A role for E2F6 in the restriction of male-germ-cell-specific gene expression. Curr. Biol. 2005;15:1051–1057. doi: 10.1016/j.cub.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Saitou M., Barton S.C., Surani M.A. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- Sasai N., Yakura R., Kamiya D., Nakazawa Y., Sasai Y. Ectodermal factor restricts mesoderm differentiation by inhibiting p53. Cell. 2008;133:878–890. doi: 10.1016/j.cell.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Setchell B.P., Main S.J. Drugs and the blood–testis barrier. Environ. Health Perspect. 1978;24:61–64. doi: 10.1289/ehp.782461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Thompson M.A., Greenberg M.E. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Solc P., Saskova A., Baran V., Kubelka M., Schultz R.M., Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev. Biol. 2008;317:260–269. doi: 10.1016/j.ydbio.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino E., Nazzicone V., Farini D., Campagnolo L., De Felici M. Comparative transcript profiles of cell cycle-related genes in mouse primordial germ cells, embryonic stem cells and embryonic germ cells. Gene Expression Patterns. 2007;7:714–721. doi: 10.1016/j.modgep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Speed R.M. Meiosis in the foetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma. 1982;85:427–437. doi: 10.1007/BF00330366. [DOI] [PubMed] [Google Scholar]
- Stone J.E., Ozbirn R.G., Petes T.D., Jinks-Robertson S. Role of proliferating cell nuclear antigen interactions in the mismatch repair-dependent processing of mitotic and meiotic recombination intermediates in yeast. Genetics. 2008;178:1221–1236. doi: 10.1534/genetics.107.085415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C., Murphy M., Kubelka M., Ravnik S.E., Hawkins C.F., Wolgemuth D.J., Carrington M. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- Swiech L., Kisiel K., Czolowska R., Zientarski M., Borsuk E. Accumulation and dynamics of proteins of the MCM family during mouse oogenesis and the first embryonic cell cycle. Int. J. Dev. Biol. 2007;51:283–295. doi: 10.1387/ijdb.062239ls. [DOI] [PubMed] [Google Scholar]
- Takubo K., Ohmura M., Azuma M., Nagamatsu G., Yamada W., Arai F., Hirao A., Suda T. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Tam P.P., Snow M.H. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J. Embryol. Exp. Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- Tanaka K., Nigg E.A. Cloning and characterization of the murine Nek3 protein kinase, a novel member of the NIMA family of putative cell cycle regulators. J. Biol. Chem. 1999;274:13491–13497. doi: 10.1074/jbc.274.19.13491. [DOI] [PubMed] [Google Scholar]
- Toppari J., Suominenf J.S., Yan W. The role of retinoblastoma protein family in the control of germ cell proliferation, differentiation and survival. APMIS. 2003;111:245–251. doi: 10.1034/j.1600-0463.2003.11101281.x. discussion 251. [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkila M., Kispert A., Chin N., McMahon A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- van Adelsberg J.S. The role of the polycystins in kidney development. Pediatr. Nephrol. 1999;13:454–459. doi: 10.1007/s004670050639. [DOI] [PubMed] [Google Scholar]
- Velasco A., Riquelme E., Schultz M., Wistuba I.I., Villarroel L., Pizarro J., Berlin A., Ittmann M., Koh M.S., Leach F.S. Mismatch repair gene expression and genetic instability in testicular germ cell tumor. Cancer Biol. Ther. 2004;3:977–982. doi: 10.4161/cbt.3.10.1135. [DOI] [PubMed] [Google Scholar]
- Western P.S., Miles D.C., van den Bergen J.A., Burton M., Sinclair A.H. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008;26:339–347. doi: 10.1634/stemcells.2007-0622. [DOI] [PubMed] [Google Scholar]
- Wilhelm D., Martinson F., Bradford S., Wilson M.J., Combes A.N., Beverdam A., Bowles J., Mizusaki H., Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev. Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Winkler K.E., Swenson K.I., Kornbluth S., Means A.R. Requirement of the prolyl isomerase Pin1 for the replication checkpoint. Science. 2000;287:1644–1647. doi: 10.1126/science.287.5458.1644. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D.J., Lele K.M., Jobanputra V., Salazar G. The A-type cyclins and the meiotic cell cycle in mammalian male germ cells. Int. J. Androl. 2004;27:192–199. doi: 10.1111/j.1365-2605.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- Yan W., Kero J., Suominen J., Toppari J. Differential expression and regulation of the retinoblastoma family of proteins during testicular development and spermatogenesis: roles in the control of germ cell proliferation, differentiation and apoptosis. Oncogene. 2001;20:1343–1356. doi: 10.1038/sj.onc.1204254. [DOI] [PubMed] [Google Scholar]
- Yano S., Tokumitsu H., Soderling T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.