Abstract
The telomeres of most eukaryotes are characterized by guanine-rich repeats synthesized by the reverse transcriptase telomerase. Complete loss of telomerase is tolerated for several generations in most species, but modestly reduced telomerase levels in human beings are implicated in bone marrow failure, pulmonary fibrosis and a spectrum of other diseases including cancer. Differences in telomerase deficiency phenotypes between species most likely reflect a tumour suppressor function of telomeres in long-lived mammals that does not exist as such in short-lived organisms. Another puzzle provided by current observations is that family members with the same genetic defect, haplo-insufficiency for one of the telomerase genes, can present with widely different diseases. Here, the crucial role of telomeres and telomerase in human (stem cell) biology is discussed from a Darwinian perspective. It is proposed that the variable phenotype and penetrance of heritable human telomerase deficiencies result from additional environmental, genetic and stochastic factors or combinations thereof.
Keywords: cancer, dyskeratosis, mutations, telomerase
Introduction
Several recent studies have highlighted the tremendous importance of telomeres in clinical medicine (Yamaguchi et al, 2005; Armanios et al, 2007; Calado et al, 2009). These advances provide an opportunity to revisit some of the concepts and data that provide a link between telomeres, the turnover of various (stem) cells and the diverse pathology that is now linked to telomere dysfunction. This aim of this essay is not to provide a comprehensive review of the many papers that have implicated telomeres in human disease. Instead, I hope to provide some insight into this broad and complex topic by reviewing ideas and data from selected publications in very diverse areas.
The chicken or the egg?
The Russian evolutionary biologist Theodosius Dobzhansky, wrote ‘Nothing in Biology Makes Sense Except in the Light of Evolution' (Dobzhansky, 1973).1 The essential truth in this statement is in my view exemplified by a remarkable difference in telomere function between human beings and most other eukaryotes. This difference is illustrated by the consequences of complete and partial telomerase deficiencies. Strikingly, complete loss of telomerase is tolerated for several generations in yeast, worms, plants and mice (Table I). In each of these species, the loss of telomerase results in a measurable and predictable decline in average telomere length with each subsequent generation. After four or more generations without major changes in growth or development, telomeres become too short to protect chromosome ends and problems start to occur. Chromosome ends without sufficient telomere repeats typically are prone to fusion and the resulting dicentric chromosomes compromise the ability of cells to continue cell division2. Chromosome fusions and other molecular changes in cells of late generation telomerase-null mutant animals or plants result in a plethora of phenotypes. Most typical are a failure to reproduce, variable growth defects, accelerated ‘ageing' in some tissues and failed or delayed responses to tissue injury. In stark contrast to these consequences in late generation telomerase-null animals or plants are the often severe and life-threatening clinical phenotypes observed in patients with a modest, two-fold reduction in telomerase levels (e.g. resulting from haplo-insufficiency for one of the two essential telomerase genes) (Table I). What could be the explanation for these phenotypic differences?
Table 1.
Phenotype of telomerase reverse transcriptase gene mutations
| Species | Homozygous null (−/−) | Heterozygous (−/+) | References |
|---|---|---|---|
| Budding yeast | Viable for up to many (>10) generations | No overt phenotype | Reviewed in Lundblad (2002) |
| Fission yeast | Delayed growth defect | No overt phenotype | Haering et al (2000) |
| Caenorhabditis elegans | Viable for up to 6 generations | No overt phenotype | Cheung et al (2006) |
| Arabidopsis | Viable for up to 10 generations | No overt phenotype | Fitzgerald et al (1999) |
| Mouse | Viable for up to 6 generations | No overt phenotype | Liu et al (2000) |
| Human | Not known (lethal?) | Various disorders including Dyskeratosis Congenita, aplastic anemia and pulmonary fibrosis. Cancer predisposition | Vulliamy et al (2001), Fogarty et al (2003), Yamaguchi et al (2005), Armanios et al (2007), Calado et al (2009) |
In evolutionary terms, the cells of the germline are the most important cells: only the gametes produced by the cells of the germline have the potential to cross generations, whereas somatic cells are inevitably lost with each generation (Figure 1). The disposable soma theory provides a useful paradigm to think about reproduction, somatic cells and ageing (Kirkwood and Holliday, 1979). Cells of the germline and soma are of course highly integrated. All somatic cells and tissues, including our brain, reflect an evolutionary strategy to propagate germline DNA. Most people do not (like to) see themselves as the mortal carriers of precious, highly selected DNA contained in cells of the germline. However, the notion that our soma serves to propagate germline DNA and is ‘disposable' in the bigger scheme of things is useful in considering possible differences in telomere biology and DNA repair between cells, tissues and species.
Figure 1.
What came first, the chicken or the egg? Chickens (and hens) can be regarded as the mortal carriers of immortal germline DNA. Natural selection results in somatic (stem) cells that do not devote more energy on DNA repair and tissue maintenance than is required for the reproductive strategy and corresponding lifespan of a particular species. According to the ‘disposable soma' theory, ageing is not actively programmed, but results from the accumulation of damage after reproduction (Kirkwood and Holliday, 1979). Long-lived species that reproduce after many years need more efficient DNA repair and better protection against tumour development than comparable species that reproduce within weeks or months. Somatic cells from long-lived species show loss of telomeric DNA with each division. Telomere loss is not readily observed in cells from most short-lived species. Most likely loss of telomeric DNA represents a tumour suppressor mechanism that does not exist as such in somatic cells from short-lived species.
At the single cell level, many signalling and cell cycle checkpoint pathways are integrated to allow cells to make life or death decisions (Cook et al, 2009). Many of the molecules involved in such decisions are highly conserved between species. However, constant selective pressure has also resulted in very specific differences between cells and between species. No doubt, many aspects of the pathways involved in cell signalling and cell cycle checkpoints and their connectivity remain to be uncovered and better understood within specific cells. Differences in such pathways within cells of a given species need to be understood better before the added complexity of species-specific differences can be fruitfully dissected. However, in the context of growth control and malignant transformation, some differences between species have already been described. For example, DNA damage foci originating from short, dysfunctional telomeres (d'Adda di Fagagna et al, 2003) increase in number with accumulated cell divisions and with age in human and primate cells, but not in murine somatic cells and tissues (Sedivy, 2007). These and other observations (Hahn et al, 1999; Smogorzewska and de Lange, 2002) support the idea that loss of telomeric DNA prevents tumour growth and contributes to ageing in human beings and primates, but probably does not function in this way in laboratory mice (with long telomeres), plants, worms, flies and yeast. Interestingly, telomere loss can be made to also limit cell proliferation and tissue renewal in mice (Hao et al, 2005; Deng et al, 2008). In these studies, cell proliferation appears to be limited primarily by telomere loss and only indirectly by telomerase levels.
Differences in DNA repair between cells and species?
Consider two species with a greatly different lifespan. The comparable somatic (stem) cells from such species are expected to have different requirements in terms of the type and efficiency of various DNA repair pathways to ensure that germline DNA is successfully propagated across generations. According to the ‘disposable soma' hypothesis, ageing results primarily from failure to maintain genome integrity in somatic cells after reproduction (Kirkwood and Holliday, 1979). The key concept here is that organisms with cells that do spend more energy on DNA repair and other cellular ‘maintenance' functions than is strictly required for production of viable offspring are likely to be at a disadvantage relative to those that do not. Thus, differences in lifespan and reproductive strategy between species will be reflected in differences between the type and efficiency of various DNA repair pathways. The notion that the efficiency of DNA repair is subject to natural selection is not as popular as the more commonly emphasized ideas that evolution proceeds by selection of variants within a species that carry favourable DNA mutations or the simpler notion that ageing is a consequence of DNA damage (Schumacher et al, 2008). The emphasis on DNA repair rather than DNA damage in the context of ageing has practical implications: a focus on increasing the efficiency of DNA repair is not the same as a focus on reducing DNA damage. Indeed, limited exposure to DNA damage could stimulate repair and be more effective at increasing lifespan (Kaiser, 2003) than attempts to limit DNA damage altogether (e.g. with ‘anti-oxidants'). This line of reasoning is supported by the known benefit of (moderate) exercise and low-dose irradiation on the lifespan in various organisms (Calabrese and Baldwin, 2003; Radak et al, 2008).
No doubt, many differences in the type and efficiency of DNA repair pathways in specific cells within tissues and comparable (stem) cells between species remain to be uncovered. However, some notable differences have already been described. For example, DNA repair changes dramatically during neuronal differentiation (Nouspikel and Hanawalt, 2000) and the repair of double strand (ds) DNA breaks through molecules such as Ku70/80 and DNA-PK in the non-homologous end joining (NHEJ) pathway is much more efficient in human than in murine cells (Banuelos et al, 2008 and references therein). Differences in NHEJ between mice and man could reflect the fact that human (stem) cells reside for much longer periods in a dormant G0 or G1 state of the cell cycle compared with their murine counterparts. Repair of ds DNA breaks through homologous recombination is less efficient in G0 or G1 (Aylon et al, 2004) and better NEJH repair of DNA (ds) breaks in human cells may reflect the slower turnover of human cells (Wilson et al, 2008). The main point here is that the efficiency and the type of DNA repair in somatic as well as germline cells have been and are subject to natural selection. As a result, the efficiency of DNA repair in stem cells from species that reproduce rapidly is expected to be less than that in stem cells from species that take years to reproduce.3
Highly effective DNA repair required in somatic stem cells from long-lived species creates a novel risk: abnormal somatic stem cells, though perhaps less likely to arise, are more likely to propagate their abnormal genomes and survive. Such risks are superimposed on the inherent risk of living longer: more time for mutations to accumulate. It seems plausible that the additional risks to stem cells in long-lived species have favoured the evolution of telomere attrition as tumour suppression mechanism (reviewed in Aubert and Lansdorp, 2008). The lack of a similar telomere-related counting mechanism in short-lived species and model organisms provides an explanation for the different consequences of telomerase deficiency in human beings and model organisms (Table I). Unfortunately, the tumour suppressor function of telomeres in human cells poses a major hurdle in studies of human telomere biology. For example, suitable murine models for human carcinomas, the most common type of human malignancies, are only obtained after telomere function is severely compromised (Artandi et al, 2000). The difference in telomere biology between mice and man has also hampered the development of suitable murine models for other human diseases including Werner's syndrome (Chang et al, 2004).
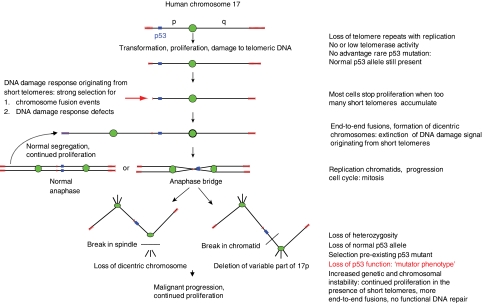
If one accepts that telomere loss indeed evolved to limit the growth of pre-malignant cells in human beings, it should be noted that this mechanism has two serious flaws (Figure 2). First, the mechanism is subject to failure when two chromosome ends with insufficient telomere repeats fuse with each other. Such fusion events (e.g. between sister chromatids or between different chromosome ends) will extinguish the DNA damage signals that originated from the short telomeres. The removal of this checkpoint is predicted to allow cells to enter mitosis. Dicentric chromosomes formed by end-to-end fusion are likely to break on mitosis and initiate cycles of chromosome bridge/breakage/fusion (de Lange, 1995). This type of genome instability greatly facilitates deletion and amplification of genes and, as a result, the malignant evolution of (pre-) malignant cells. Second, by limiting the growth of (pre-) malignant cells, the DNA damage response triggered by short telomeres (d'Adda di Fagagna et al, 2003) will become subject to strong negative selective pressure. Such selection favours cells with defective DNA damage responses. Indeed, this seems a plausible mechanism, whereby most human tumours acquire defective DNA damage responses (e.g. over half of all human cancers have mutations in the p53 gene (Vousden and Lane, 2007)). As DNA damage responses are upstream in many different DNA repair pathways, cells selected on the basis of their inability to respond appropriately to short telomeres will display defects in several different DNA repair pathways. Indeed, it seems plausible that genome instability triggered by unstable chromosome ends in cells with defective DNA damage response result in the ‘mutator cell' phenotype that is characteristic of many tumour cells (Loeb, 2001). That chromosomal instability can be initiated by disruption of telomere function and that microsatellite instability and other genomic alterations often seen in cancer cells could arise from defective DNA damage responses indirectly selected by telomere shortening does not seem to be widely accepted (Michor et al, 2005). These ideas nevertheless seem plausible and worthy of further investigation. Apart from the difficulty of finding suitable model organisms, such studies are complicated by the transient period of rampant genome instability driven by telomere dysfunction. Sooner or later, most abnormal cells typically stabilize their chromosome ends (and at that stage typically highly abnormal genomes) by upregulation of telomerase activity (Kim et al, 1994). The frequent amplification of the telomerase reverse transcriptase gene in human lung cancer suggests that break–fusion–bridge cycles involving chromosome 5p is frequently involved in the upregulation of telomerase activity in those cells (Weir et al, 2007; Kang et al, 2008).
Figure 2.
Telomere loss: an imperfect tumour suppressor mechanism. Loss of telomeric DNA after replication or damage to telomeric DNA limits the proliferation of abnormal (stem) cells. The flaws (red arrow) in the telomere-related tumour suppressor mechanism are illustrated here in a hypothetical scenario involving the short (p) arm of human chromosome 17. Chromosome 17p was chosen for illustration because it has a short track of telomere repeats in a majority of normal individuals (Martens et al, 1998; Britt-Compton et al, 2006) and because abnormalities involving the p53 gene (located on 17p13.1) are present in a majority of human cancers. Critically short telomeres are presumed to activate a DNA damage response similar to DNA double strand breaks. This DNA damage response (presumably mediated through ATM and p53) will result in growth arrest or apoptosis in all cells in most instances. However, selection on the basis of intact DNA damage responses will favour rare cells with (1) defective DNA damage responses or (2) cells in which the short telomeres are fused (eliminating the DNA damage signal). This can lead to loss of p53, genome instability and a ‘mutator phenotype' as illustrated. Eventually, cells with high telomerase levels are selected to stabilize typically highly rearranged tumour genomes. Alternatively, some tumour cells may bypass the ‘telomere checkpoint' altogether by upregulation of telomerase activity earlier in tumour development.
How many stem cell divisions?
If telomere attrition evolved to limit the number of somatic cell divisions in long-lived species, one might ask how many times stem cells in tissues actually divide. Unfortunately, and perhaps surprisingly, no clear answer to this important question is currently available. Haematopoietic stem cells (HSCs) are probably the most extensively studied stem cell population. From studies of both human and murine HSCs, it has become clear that HSCs represent a very heterogeneous population of cells that do not self-renew in the absolute sense of the word (Lansdorp, 1997). The functional properties of purified ‘candidate' stem cell populations change markedly during development in both man (Lansdorp et al, 1993) and mouse (Bowie et al, 2006; Kim et al, 2007). Such functional changes correspond to a rather abrupt change in HSC turnover early in life, which is reflected in a rapid decline in the rate of telomere attrition after 3–4 years in human beings (Rufer et al, 1999) and 1–2 years in baboons (Baerlocher et al, 2007). The turnover of murine HSCs also drops quite abruptly, but at 4–6 weeks after birth (Bowie et al, 2007; Kim et al, 2007). The majority of transplantable HSCs in adult mice are derived from a very small pool of quiescent cells that divide less than 10 times over a lifetime (Wilson et al, 2008). The picture that is emerging is that HSCs are dividing very infrequently in adults and only switch from dormancy to self-renewal following transplantation or other stress conditions. Very limited turnover of adult stem cells is also compatible with the minimal loss of telomere length observed in granulocytes from normal human individuals between 20 and 60 years of age (Lansdorp, 2008) and the sequential recruitment of quiescent progenitors that was observed to sustain cell production in human long-term bone marrow cultures (Lansdorp and Dragowska, 1993).
On the basis of telomere length data from different mammalian species, it has been proposed that HSCs divide less than 200 times over a lifetime and that this number is evolutionarily conserved (Shepherd et al, 2007). If this number is correct, it suggests that very few cell divisions in the haematopoietic system are ‘wasted'. The total number of blood cells required for life-long blood cell production in man can be calculated to be in the order of 4 × 1016 cells (∼1012 cells/day × 365 days × 100 years). In theory, only 55 divisions of a single cell could satisfy this need (255=4 × 1016). With inclusion of additional cell divisions required to compensate for inevitable cell death and loss through differentiation, estimates of less than 200 cell divisions over a lifetime seem reasonable.
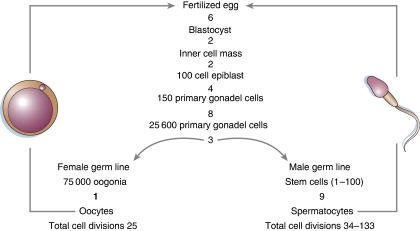
The estimate of the number of divisions in HSCs is at odds with the thousands of divisions proposed, for example stem cells in the gut (Potten et al, 2002). The notion that stem cells in the intestine of mice divide much more than those in the bone marrow is also at odds with data from mice that lack telomerase (Blasco et al, 1997; Liu et al, 2000) and turnover estimates of tissues, including blood and intestine, derived from 14C dating (Spalding et al, 2005). Mice that lack telomerase show a loss of around 5 kb per generation (Blasco et al, 1997). Assuming a loss of 100 bp of telomeric DNA per cell division, subsequent generations of telomerase-null mice are calculated to be separated by ∼50 divisions. This estimate is in close agreement with independent estimates of 34–133 cell divisions in the male and 25 in the female germline (Figure 3, adopted from Drost and Lee, 1995). If intestinal stem cells were indeed to divide thousands of times, one would expect a severe gut phenotype in the first generation of TERC KO mice unless intestinal stem cells could somehow avoid telomere loss or maintain telomeres in the absence of telomerase.4 More likely, intestinal cells, such as Lgr5+ cells, that divide every 24 h (Barker et al, 2007) include a small subset of Lgr5+ stem cells and a large proportion of Lgr5+ progenitor cells similar to CD34 cells in human bone marrow.5 Only a very small fraction (<1%) of BM CD34+ cells are probably true HSCs (Terstappen et al, 1991; Novelli et al, 1998), and only a minor fraction of sorted single Lgr5+ cells is capable of initiating crypt formation in vitro (Sato et al, 2009).
Figure 3.
Estimated number of cell divisions in the female and male murine germline from one generation to the next (adapted from Drost and Lee, 1995). The number of cell divisions is given below the indicated developmental stages. Thus, to go from a fertilized egg to a blastocyst takes an estimated 6 divisions; 8 more divisions are needed to produce 150 primary gonadal cells; the latter go through 11 more divisions before cells commit to either the female germline (1 more cell division before mature oocytes is produced) or the male germline (in which the estimated number of divisions from stem cell to spermatocyte is 9). Production of spermatocytes in males continues throughout adult life. Estimates of the number of divisions at the level of spermatogonial stem cells are hampered by uncertainties that are similar to those discussed for haematopoietic and intestinal stem cells. For the (young) mice without telomerase in laboratory settings, it is estimated that at most 50 divisions separate one generation from the next.
Telomerase deficiencies and other telomere disorders
Uncertainties about the number of stem cells in various tissues and their turnover are important in the discussion of human telomerase deficiency disorders. It seems likely that most, if not all, phenotypic consequences of human telomerase deficiency result from compromised telomere function that limits the proliferation of (stem) cells. If this is correct, the phenotypic manifestations of telomerase disorders could in fact instruct us about the turnover of stem cells in various tissues. This reasoning explains in part why telomeres and ‘telomerase disorders' are of interest to a growing number of ‘stem cell' researchers.
The first and most well-studied ‘telomere disorder' is Dyskeratosis Congenita (DC). This is a rare disorder and fewer than a thousand patients have been described. Patients with DC typically present with three distinctive clinical characteristics: skin pigmentation abnormalities, nail dystrophy and abnormalities of the oral mucosa known as leukoplakia (Walne and Dokal, 2008; Savage and Alter, 2009). The most common fatal complications of DC are related to bone marrow failure, pulmonary fibrosis and cancer (Walne and Dokal, 2008; Savage and Alter, 2009). The most frequent cancers are head and neck cancer (squamous cell carcinoma), cancer of the tongue and acute myeloid leukaemia (Alter et al, 2009). In addition, a variety of other clinical abnormalities have been described in DC patients (reviewed in Kirwan and Dokal, 2008). Hepatic cirrhosis has been described in DC and severe liver disease is not uncommon after HSC transplantation for marrow failure in patients with DC (Calado and Young, 2008). Several genes have now been implicated in DC; most notably, all three genes encoding components of the minimal (Cohen et al, 2007) telomerase enzyme complex (Walne and Dokal, 2008; Savage and Alter, 2009). Mutations were initially discovered in DKC1, located on the X chromosome (Heiss et al, 1998). A link with telomerase was first suggested when the nucleolar protein encoded by DKC1, dyskerin, was found to stabilize telomerase RNA and maintain telomere length through telomerase (Mitchell et al, 1999). Mutations in hTERC and hTERT themselves were later discovered in autosomal dominant DC (Vulliamy et al, 2001; Fogarty et al, 2003; Armanios et al, 2005). In total, ∼50% of patients with DC have a mutation in one of the three genes of the telomerase complex (Aubert and Lansdorp, 2008). It seems likely that mutations in other ‘telomere maintenance' genes contribute to the remaining ∼50% of cases. This notion is supported by the recent discoveries of mutations in genes of DC patients such as TINF2, NHP2 and NOP10 (Walne et al, 2007; Savage et al, 2008; Vulliamy et al, 2008).
Mutations in hTERT and hTERC have also been found in diseases other than DC, including other bone marrow failure syndromes such as aplastic anaemia (AA) (Yamaguchi et al, 2003, 2005; Marrone et al, 2004; Ly et al, 2005; Vulliamy et al, 2005) and myelodysplastic syndrome (MDS) (Yamaguchi et al, 2003), and in diseases not typically associated with blood disorders such as idiopathic pulmonary fibrosis (IPF) (Armanios et al, 2007). In AA, 3 of 200 patients were found to have mutations in hTERC (Yamaguchi et al, 2003) and an additional 3.5% (7/200) had mutations in hTERT (Yamaguchi et al, 2005). In IPF, 1.4% (1/73) of patients were identified with hTERC mutations, whereas 6.8% (5/73) of patients with familial IPF had mutations in hTERT (Armanios et al, 2007).
Families with mutations in telomerase genes typically display disease ‘anticipation', meaning that the disease typically presents at an earlier age in each subsequent generation (Vulliamy et al, 2004; Armanios et al, 2005). The most likely explanation for disease anticipation in telomerase disorders is that limiting amounts of telomerase activity in the germline result in gametes with shorter telomeres and, consequently, offspring with shorter telomeres. Shorter telomeres in stem cells at birth reduce the number of stem cell divisions before short telomeres trigger a DNA damage response and, eventually, senescence or cell death. It is implied here that all disease manifestations of telomerase disorders are likely to result from compromised cell and tissue renewal. From the spectrum of phenotypes of patients with known telomerase deficiencies, one can deduce that the stem cells of the oral mucosa, the skin (especially in the head and neck area), the nails, the lung, the bone marrow and the liver rely on telomerase to sustain proliferation the most or have the highest turnover of all the stem cells in the human body.
Why specific tissues seem to be affected more than others in individual patients is incompletely understood. It seems possible that environmental factors contribute to disease manifestations, for example smoking in patients with pulmonary fibrosis or alcohol consumption in patients presenting with hepatic cirrhosis. In such patients, a reduced stem cell reserve could lead to symptoms by increasing the demand on the turnover of remaining tissue-specific stem cells by environmental toxins. Among environmental ‘toxins', factors induced by viral or other infections, such as interferon alpha (Essers et al, 2009), or factors stimulated by, for example, sustained psychological stress (Epel et al, 2004) should probably be included. Indeed, such factors are likely to accelerate tissue turnover and thereby ageing even in individuals without specific ‘telomere maintenance' disorders. Other known or unknown ‘extrinsic' factors, including specific diseases, could also affect tissue-specific stem cell numbers. The role of telomere attrition in cardiovascular disease is of particular interest. Telomere length was found to be much shorter in endothelial cells from the aorta compared with those from pulmonary arteries of the same organ donor, and it was suggested that this could reflect differences in hemodynamic stress to the vessel wall (Chang and Harley, 1995). Striking cytogenetic abnormalities have also been described to accumulate in aorta endothelial cells with age (Aviv et al, 2001). Despite uncertainties about the replicative or regenerative potential of endothelial cells, it seems plausible that this capacity will not exceed that of other somatic (stem) cells. Extrinsic factors, such as smoking, diabetes, high blood pressure, diet, etc., could result in loss of endothelial cells or function and trigger cardiovascular disease in the absence of additional genetic factors that compromise the replicative potential of cells such as heritable telomerase deficiencies (Farhat et al, 2008; Wong et al, 2009).
Reduced telomerase levels as well as short telomeres are expected to compromise the number as well as the replicative potential of stem cells that remain in specific tissues after extrinsic or intrinsic damage from the environment. Alternatively, disease could be triggered by additional genetic factors. For example, cell-specific turnover could be increased by a specific genetic defect or a polymorphic trait that increases the likelihood of cell death in response to injury. Presumably, such genetic co-factors can be distinguished from environmental co-factors by careful studies of both environmental exposure as well as the genotype of a given patient relative to family members that are not affected or that display other disease manifestations.
Finally, it seems possible that disease manifestations of human telomerase disorders are the result of stochastic telomere loss events at individual telomeres during development. Telomere repeats are lost not only as a result of the ‘end-replication problem', but also as a result of sporadic ‘truncation' events that could have several different causes (Lansdorp, 2005). Recently, it was found that within the haematopoietic system the most primitive haematopoietic cells have fewer abruptly shortened telomeres compared with more differentiated cells (Hills et al, 2009). The level of telomerase could be important when more than a few sporadic telomere loss events occur or accumulate in a cell at any given time (Aubert and Lansdorp, 2008). In cells with overall shorter telomeres and lower telomerase levels to start with, cell death events could occur more frequently during normal development. Both the number and the replicative potential of remaining tissue-specific stem cells could be compromised stochastically depending on when cell death occurred during development and whether remaining cells had to go through extra divisions to make up for the loss of cells. The distinction between environmental, genetic and stochastic reasons for the variable clinical presentation of patients with telomerase disorders is a major research challenge. This challenge is augmented further by the distinct possibility that the specific disease manifestation in an individual patient results from a combination of these and other factors.
Telomere defects and cancer predisposition
Telomerase is upregulated in a majority of human cancers (Kim et al, 1994; Cao et al, 2008). This upregulation is believed to be required to allow malignant cells to divide after genetic rearrangements enabled by telomere dysfunction. In view of the telomerase requirement in (late stage) cancer growth, the recent finding that heritable defects appear to predispose for the development of acute myeloid leukaemia seems perhaps counterintuitive (Calado et al, 2009). However, patients with DC or bone marrow failure resulting from mutations in telomerase genes often develop leukaemia or other types of cancer (Alter et al, 2009). Leukaemia and other malignancies are also well-known complications of high-dose chemotherapy regimens and MDS. Most likely, the loss of stem cells by telomere dysfunction or treatment creates an environment in which abnormal cells can flourish. This could reflect persistent feedback signals stimulating stem cell proliferation from the microenvironment. With many stem cells present, such stimulation is expected to occur only transiently, whereas with few or no normal stem cells responding, such stimulatory signals could persist and stimulate the growth of abnormal cells (e.g. cells with defective DNA damage responses). The malignant progression of such abnormal cells could be further facilitated by mutations induced by earlier treatment. In other words, normal stem cell numbers could indirectly suppress malignant transformation of cells, whereas loss of stem cells could predispose for malignant transformation. The difficulties related to the identification of stem cells in vivo that were discussed above greatly complicate studies on the role of the number of stem cells in tumour development. However, it seems possible that a decline in the number of tissue-specific stem cells could contribute to the exponential increase in tumour development with age. Such a relationship is suggested by the paradoxical relationship between hypo- and hyperproliferative disorders. For example, Calado et al, reported in a recent paper that heritable hypomorphic mutations in the telomerase reverse transcriptase gene predispose for acute myeloid leukaemia (Calado et al, 2009). In this study of AML patients (n=594), a common variant of the telomerase reverse transcriptase gene hTERT, A1062T, was present three times more frequently in patients than in controls (n=1110, P=0.0009). The mutant telomerase allele was found to decrease the enzymatic activity of telomerase by ∼50% in the TRAP telomerase assay (Calado et al, 2009). However, the A1062T hTERT variant did not appear to compromise telomerase activity in another study (Alder et al, 2008), and the precise functional consequences of A1062T expression in human (stem) cells remain to be clarified. As in most patients only one TERT allele was affected, telomerase activity in the stem cells of these patients is expected to be around 75% of normal levels. How reduced telomerase levels in these patients relate to the development of AML is not clear. One possibility is that telomeres were on average shorter at birth and that shorter telomeres limited normal stem cell proliferation with selection of abnormal cells as discussed above. Second, it is possible that full telomerase levels are critical in HSCs to maintain telomere function of critically short telomeres. Even a slight reduction in telomerase levels could increase the probability of cell death after, for example, oxidative damage to telomeric DNA in stem cells. Telomeres in the most primitive haematopoietic cells were found to have the lowest frequency of abruptly shortened telomeres in the haematopoietic system (Hills et al, 2009) and oxidative damage is a major cause of telomere attrition (von Zglinicki, 2002).
Two recent studies have suggested that risk of developing lung cancer is associated with genetic markers (SNP's) that map to the TERT locus (McKay et al, 2008; Wang et al, 2008). These results were confirmed in a large SNP study, which also looked at a variety of other tumours (Rafnar et al, 2009). In this large study of over 30 000 cancer cases and 45 000 controls, a significant association of SNP rs401681[C] within the TERT locus on chromosome 5p15.33 was found with basal cell carcinoma, lung cancer and cancer of the urinary bladder, prostate and cervix. Interestingly, the same SNP seems to confer protection against cutaneous melanoma, and the cancers that are associated with this SNP all have a strong environmental component to their risk. Although the precise role of genetic variation within the TERT locus remains to be elucidated, it is tempting to speculate that this SNP is linked to hypomorphic TERT alleles similar to the A1062T variant that was found to be more frequently in patients with AML than normal controls.
Concluding remarks
Recent studies have put telomerase and telomere research square at the centre of cancer research. Not only is amplification of the hTERT gene one of the most common genetic abnormalities in lung adenocarcinoma, one of the most common human cancers (Weir et al, 2007; Kang et al, 2008), it now is becoming clear that SNP's within the TERT locus are among the most reproducible risk factors for the development of different types of cancer (McKay et al, 2008; Wang et al, 2008; Rafnar et al, 2009). The finding that hypomorphic TERT mutations increase the risk for the development of acute myeloid leukaemia (Calado et al, 2009), chronic lymphocytic leukaemia (Hills and Lansdorp, NYAS in press) and probably other cancers, together with the increased risk for leukaemia development associated with bone marrow failure in AA, MDS and DC, strongly implicates stem cell failure and reduced stem cell numbers as risk factors for tumour development. More direct measures of stem cell numbers in vivo are needed to examine the relationship between stem cell numbers and tumour development in patients with defective telomere maintenance as well as in normal individuals as a function of age. Measurements of the average telomere length as well as the length at individual chromosome ends in specific cells and tissues are expected to provide valuable information about the involvement of telomeres in normal ageing and tumour biology.
Acknowledgments
Work in my laboratory is supported by grants from the Canadian Institutes of Health Research (MOP38075 and GMH79042) and the National Cancer Institute of Canada (with support from the Terry Fox Run). I thank Gerry Krystal and anonymous reviewers for critically reading the paper.
American Biology Teacher, volume 35, pages 125–129.
The consequences of fused and broken chromosomes in cells differ greatly between species. For example, multiple anaphase bridges were observed in late generation telomerase-null Arabidopsis plants. This level of genomic instability is not tolerated by mammalian cells (see Riha et al, 2001).
In this context, it is perhaps of interest to consider that the presence of many error-prone DNA polymerases in various genomes could reflect an evolutionary advantage of cells capable of generating many mutations in times of stress; e.g. see Tippin et al, 2004.
Of note, a gut phenotype was observed in the first generation of CAST/EiJ mice with short telomeres lacking telomerase (Hao et al, 2005).
How this notion can be reconciled with the elegant lacZ lineage tracing experiments by Barker et al is not clear. Differences in Cre-induced recombination between cell types and/or variable detection of lacZ in slowly versus rapidly dividing cells could possibly contribute to the failure to detect differences in the frequency of lacZ positive crypts at variable time points after Lgr5-induced Cre expression.
The author declares a financial interest in Repeat Diagnostics Inc., a company specializing in leucocyte telomere length measurements using flow FISH.
References
- Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA III, Lansdorp PM, Loyd JE, Armanios MY (2008) Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Rosenberg PS (2009) Cancer in dyskeratosis congenita. Blood (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW (2005) Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA 102: 15960–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA III, Lansdorp PM, Greider CW, Loyd JE (2007) Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326 [DOI] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406: 641–645 [DOI] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88: 557–579 [DOI] [PubMed] [Google Scholar]
- Aviv H, Khan MY, Skurnick J, Okuda K, Kimura M, Gardner J, Priolo L, Aviv A (2001) Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis 159: 281–287 [DOI] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, Kupiec M (2004) The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J 23: 4868–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerlocher GM, Rice K, Vulto I, Lansdorp PM (2007) Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell 6: 121–123 [DOI] [PubMed] [Google Scholar]
- Banuelos CA, Banath JP, MacPhail SH, Zhao J, Eaves CA, O'Connor MD, Lansdorp PM, Olive PL (2008) Mouse but not human embryonic stem cells are deficient in rejoining of ionizing radiation-induced DNA double-strand breaks. DNA Repair (Amst) 7: 1471–1483 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34 [DOI] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ (2007) Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci USA 104: 5878–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ (2006) Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest 116: 2808–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt-Compton B, Rowson J, Locke M, Mackenzie I, Kipling D, Baird DM (2006) Structural stability and chromosome-specific telomere length is governed by cis-acting determinants in humans. Hum Mol Genet 15: 725–733 [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA (2003) Toxicology rethinks its central belief. Nature 421: 691–692 [DOI] [PubMed] [Google Scholar]
- Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, Lansdorp PM, Hogge D, Chanock SJ, Estey EH, Falcao RP, Young NS (2009) Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci USA 106: 1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Young NS (2008) Telomere maintenance and human bone marrow failure. Blood 111: 4446–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bryan TM, Reddel RR (2008) Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci 99: 1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Harley CB (1995) Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA 92: 11190–11194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA (2004) Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet 36: 877–882 [DOI] [PubMed] [Google Scholar]
- Cheung I, Schertzer M, Rose A, Lansdorp PM (2006) High incidence of rapid telomere loss in telomerase-deficient Caenorhabditis elegans. Nucleic Acids Res 34: 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR (2007) Protein composition of catalytically active human telomerase from immortal cells. Science 315: 1850–1853 [DOI] [PubMed] [Google Scholar]
- Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG (2009) Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458: 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- de Lange T (1995) Telomere dynamics and genome instability in human cancer. In Telomeres, Blackburn EH, Greider CW (eds), pp 265–293. Plainview, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Deng Y, Chan SS, Chang S (2008) Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8: 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T (1973) Am Biol Teach 35: 125–129 [Google Scholar]
- Drost JB, Lee WR (1995) Biological basis of germline mutation: comparisons of spontaneous germline mutation rates among drosophila, mouse, and human. Environ Mol Mutagen 25 (Suppl 26): 48–64 [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101: 17312–17315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A (2009) IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458: 904–908 [DOI] [PubMed] [Google Scholar]
- Farhat N, Thorin-Trescases N, Voghel G, Villeneuve L, Mamarbachi M, Perrault LP, Carrier M, Thorin E (2008) Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can J Physiol Pharmacol 86: 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald MS, Riha K, Gao F, Ren S, McKnight TD, Shippen DE (1999) Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc Natl Acad Sci USA 96: 14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, Read EJ, Lansdorp PM, Young NS (2003) Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet 362: 1628–1630 [DOI] [PubMed] [Google Scholar]
- Haering CH, Nakamura TM, Baumann P, Cech TR (2000) Analysis of telomerase catalytic subunit mutants in vivo and in vitro in Schizosaccharomycespombe. Proc Natl Acad Sci USA 97: 6367–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA (1999) Creation of human tumour cells with defined genetic elements. Nature 400: 464–468 [DOI] [PubMed] [Google Scholar]
- Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW (2005) Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell 123: 1121–1131 [DOI] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I (1998) X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet 19: 32–38 [DOI] [PubMed] [Google Scholar]
- Hills M, Lucke K, Chavez EA, Eaves CJ, Lansdorp PM (2009) Probing the mitotic history and developmental stage of hematopoietic cells using single telomere length analysis (STELA). Blood 113: 5765–5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J (2003) Hormesis. A healthful dab of radiation? Science 302: 378. [DOI] [PubMed] [Google Scholar]
- Kang JU, Koo SH, Kwon KC, Park JW, Kim JM (2008) Gain at chromosomal region 5p15.33, containing TERT, is the most frequent genetic event in early stages of non-small cell lung cancer. Cancer Genet Cytogenet 182: 1–11 [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ (2007) Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130: 470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266: 2011–2015 [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Holliday R (1979) The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci 205: 531–546 [DOI] [PubMed] [Google Scholar]
- Kirwan M, Dokal I (2008) Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet 73: 103–112 [DOI] [PubMed] [Google Scholar]
- Lansdorp PM (1997) Self-renewal of stem cells. Biol Blood Marrow Transplant 3: 171–178 [PubMed] [Google Scholar]
- Lansdorp PM (2005) Major cutbacks at chromosome ends. Trends Biochem Sci 30: 388–395 [DOI] [PubMed] [Google Scholar]
- Lansdorp PM (2008) Telomeres, stem cells and hematology. Blood 111: 1759–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp PM, Dragowska W (1993) Maintenance of hematopoiesis in serum-free bone marrow cultures involves sequential recruitment of quiescent progenitors. Exp Hematol 21: 1321–1327 [PubMed] [Google Scholar]
- Lansdorp PM, Dragowska W, Mayani H (1993) Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med 178: 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Snow BE, Hande MP, Yeung D, Erdmann NJ, Wakeham A, Itie A, Siderovski DP, Lansdorp PM, Robinson MO, Harrington L (2000) The telomerase reverse transcriptase is limiting and necessary for telomerase function in vivo. Curr Biol 10: 1459–1462 [DOI] [PubMed] [Google Scholar]
- Loeb LA (2001) A mutator phenotype in cancer. Cancer Res 61: 3230–3239 [PubMed] [Google Scholar]
- Lundblad V (2002) Telomere maintenance without telomerase. Oncogene 21: 522–531 [DOI] [PubMed] [Google Scholar]
- Ly H, Calado RT, Allard P, Baerlocher GM, Lansdorp PM, Young NS, Parslow TG (2005) Functional characterization of telomerase RNA variants found in patients with hematologic disorders. Blood 105: 2332–2339 [DOI] [PubMed] [Google Scholar]
- Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ (2004) Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood 104: 3936–3942 [DOI] [PubMed] [Google Scholar]
- Martens UM, Zijlmans JM, Poon SS, Dragowska W, Yui J, Chavez EA, Ward RK, Lansdorp PM (1998) Short telomeres on human chromosome 17p. Nat Genet 18: 76–80 [DOI] [PubMed] [Google Scholar]
- McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, McLaughlin J, Shepherd F, Montpetit A, Narod S et al. (2008) Lung cancer susceptibility locus at 5p15.33. Nat Genet 40: 1404–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michor F, Iwasa Y, Vogelstein B, Lengauer C, Nowak MA (2005) Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol 15: 43–49 [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K (1999) A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402: 551–555 [DOI] [PubMed] [Google Scholar]
- Nouspikel T, Hanawalt PC (2000) Terminally differentiated human neurons repair transcribed genes but display attenuated global DNA repair and modulation of repair gene expression. Mol Cell Biol 20: 1562–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli EM, Ramirez M, Civin CI (1998) Biology of CD34+CD38− cells in lymphohematopoiesis. Leuk Lymphoma 31: 285–293 [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115 (Part 11): 2381–2388 [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S (2008) Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 44: 153–159 [DOI] [PubMed] [Google Scholar]
- Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, Jakobsdottir M, Helgadottir H, Thorlacius S, Aben KK, Blondal T, Thorgeirsson TE, Thorleifsson G, Kristjansson K, Thorisdottir K, Ragnarsson R, Sigurgeirsson B, Skuladottir H, Gudbjartsson T, Isaksson HJ et al. (2009) Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet 41: 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha K, McKnight TD, Griffing LR, Shippen DE (2001) Living with genome instability: plant responses to telomere dysfunction. Science 291: 1797–1800 [DOI] [PubMed] [Google Scholar]
- Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM (1999) Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Savage SA, Alter BP (2009) Dyskeratosis congenita. Hematol Oncol Clin North Am 23: 215–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP (2008) TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet 82: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher B, Garinis GA, Hoeijmakers JH (2008) Age to survive: DNA damage and aging. Trends Genet 24: 77–85 [DOI] [PubMed] [Google Scholar]
- Sedivy JM (2007) Telomeres limit cancer growth by inducing senescence: long-sought in vivo evidence obtained. Cancer Cell 11: 389–391 [DOI] [PubMed] [Google Scholar]
- Shepherd BE, Kiem HP, Lansdorp PM, Dunbar CE, Aubert G, LaRochelle A, Seggewiss R, Guttorp P, Abkowitz JL (2007) Hematopoietic stem-cell behavior in nonhuman primates. Blood 110: 1806–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T (2002) Different telomere damage signaling pathways in human and mouse cells. EMBO J 21: 4338–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J (2005) Retrospective birth dating of cells in humans. Cell 122: 133–143 [DOI] [PubMed] [Google Scholar]
- Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR (1991) Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38− progenitor cells. Blood 77: 1218–1227 [PubMed] [Google Scholar]
- Tippin B, Pham P, Goodman MF (2004) Error-prone replication for better or worse. Trends Microbiol 12: 288–295 [DOI] [PubMed] [Google Scholar]
- von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344 [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283 [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I (2008) Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci USA 105: 8073–8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I (2001) The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413: 432–435 [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I (2004) Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet 36: 447–449 [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I (2005) Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis 34: 257–263 [DOI] [PubMed] [Google Scholar]
- Walne AJ, Dokal I (2008) Dyskeratosis Congenita: a historical perspective. Mech Ageing Dev 129: 48–59 [DOI] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I (2007) Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet 16: 1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Broderick P, Webb E, Wu X, Vijayakrishnan J, Matakidou A, Qureshi M, Dong Q, Gu X, Chen WV, Spitz MR, Eisen T, Amos CI, Houlston RS (2008) Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet 40: 1407–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, Shah K, Sato M, Thomas RK, Barletta JA, Borecki IB, Broderick S, Chang AC, Chiang DY, Chirieac LR, Cho J et al. (2007) Characterizing the cancer genome in lung adenocarcinoma. Nature 450: 893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129 [DOI] [PubMed] [Google Scholar]
- Wong LS, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P (2009) Telomere biology in cardiovascular disease: the TERC−/− mouse as a model for heart failure and ageing. Cardiovasc Res 81: 244–252 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS (2003) Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood 102: 916–918 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS (2005) Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352: 1413–1424 [DOI] [PubMed] [Google Scholar]