Abstract
Insect pests such as termites cause damages to crops and man-made structures estimated at over $30 billion per year, imposing a global challenge for the human economy. Here, we report a strategy for compromising insect immunity that might lead to the development of nontoxic, sustainable pest control methods. Gram-negative bacteria binding proteins (GNBPs) are critical for sensing pathogenic infection and triggering effector responses. We report that termite GNBP-2 (tGNBP-2) shows β(1,3)-glucanase effector activity previously unknown in animal immunity and is a pleiotropic pattern recognition receptor and an antimicrobial effector protein. Termites incorporate this protein into the nest building material, where it functions as a nest-embedded sensor that cleaves and releases pathogenic components, priming termites for improved antimicrobial defense. By means of rational design, we present an inexpensive, nontoxic small molecule glycomimetic that blocks tGNBP-2, thus exposing termites in vivo to accelerated infection and death from specific and opportunistic pathogens. Such a molecule, introduced into building materials and agricultural methods, could protect valuable assets from insect pests.
Keywords: β(1,3)-glucanase; Gram-negative bacteria binding proteins; pattern recognition receptor; termites; social insect immunity
Insect immune systems are simple, efficient, and still enigmatic (1–3). Somatic Ig hypervariability has been observed in Anopheles and Drosophila (4, 5) and may represent ancestral versions of adaptive immunity, although its evolutionary and functional significance in this context is not clear. Insects employ other, well-characterized mechanisms. Among these are pattern recognition receptors, which recognize molecular determinants unique to different classes of pathogenic microorganisms (1–3).
Gram-negative bacteria binding proteins (GNBPs) are a class of conserved receptors (6, 7) that signal the presence of pathogens once they enter the hemocoel (8). Insect GNBPs contain regions with significant homology to bacterial β-glucanases, especially β(1,3)- and β(1,3)-(1,4)-glucanases (6, 9–11) and likely represent evolutionary descendants of enzymes originally serving homeostatic or digestive functions. Several peptidoglycan recognition proteins, members of a different receptor group in mammals and insects, were shown to be in fact active amidases that either initiate protective signaling cascades or are directly bactericidal (12). GNBPs are believed to have lost enzymatic activity and thus serve only as pattern recognition receptors (9, 10, 13).
Here, we show that a termite GNBP demonstrates β(1,3)-glucanase activity, serving a critical effector function in antimicrobial defense. By analysis of the structure–function relationships of this protein, we present a small molecule glycomimetic that is capable of blocking it, thus suppressing the insect's immune system and exposing it to attacks from specific and opportunistic pathogens. This molecule represents an inexpensive, nontoxic, and environmentally safe alternative to toxic pesticide chemicals currently in global use.
Results and Discussion
Termites Express β(1,3)-Glucanase Activity.
In a previous study (6), we reported that termite GNBPs were positively selected following a single duplication event before the divergence of Mastotermes, the most ancient lineage of the Isoptera. Adaptive evolution in termite GNBPs appears to have been driven by a coevolutionary arms race and shifts in habitat that have likely exposed termites to new groups of pathogenic microorganisms (6, 14, 15). However, our sequence analysis of GNBPs from various termite lineages showed that the critical residues involved in the β(1,3)-glucanase activity remained surprisingly intact. Interestingly, the catalytic site appears to have been maintained in the GNBPs of several other insects, and their phylogenetic distribution suggests that GNBPs cluster in discrete groups that have either maintained or lost β(1,3)-glucanase activity (Fig. 1A).
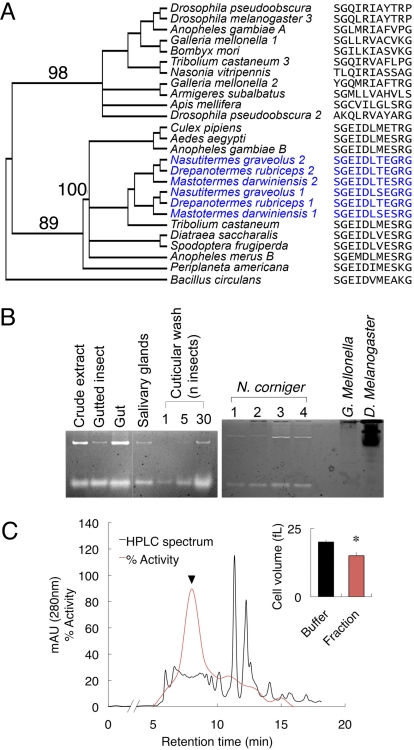
Fig. 1.
Termites exhibit an unusual β(1,3)-glucanase activity. (A) Distance tree of insect GNBPs rooted with B. circulans β(1,3)-glucanase. Numbers depict bootstrap values for basal lineages. Glucanase motif sequences are adjacent to species name. Termite species appear in blue. Numbers following species name indicate GNBP-1, -2, etc. (B) β(1,3)-Glucanase activity in termite tissues and cuticular washes, assayed on Carboxymethyl Curdlan Remazol Brilliant Blue gels (lanes: 1, soldier; 2, large worker; 3, large worker; 4, 10 workers; 5–7, 1, 5, and 30 large workers, respectively) (Left). Comparison of activity in extracts from termites (lanes: 1, Nasutitermes corniger collected from Florida; 2–4, N. corniger collected from different colonies in Panama), Drosophila melanogaster and Galleria mellonella (Right). (C) Activity profile of fractionated termites separated by HPLC. (Inset) Cytotoxic effect of the peak active fraction (marked with an arrowhead) from termites on Metarhizium anisopliae conidia measured by flow cytometry as the effect on cell volume in femtoliters (*, P < 0.05 vs. buffer).
Measurement of β(1,3)-glucanase activity in Nasutitermes corniger, performed by electrophoresis on a chromogenic substrate gel, revealed significant activity in body tissues and secretions including salivary glands and cuticular washes (Fig. 1B). Other termite species (Zootermopsis augusticollis, Cryptotermes secundus, and Rubriceps flavipes) also showed robust β(1,3)-glucanase activity. This activity was demonstrated by all castes. Other insects, such as Galleria mellonella and Drosophila melanogaster (Fig. 1B), had no activity. Still, this activity is not limited to termites as suggested by our sequence analysis and as recently reported for several pest species (16–18).
The fungal entomopathogen Metarhizium anisopliae is a natural termite pathogen and is currently being developed for the biological control of termites and other insect pests (19). M. anisopliae conidia treated with β(1,3)-glucanases purified from either Helix pomatia or Bacillus subtilis collapsed due to turgor pressure loss and leakage of intracellular components. Similarly, conidia treated with a termite protein size-exclusion fraction coinciding with peak β(1,3)-glucanase activity likewise collapsed, and their cell volume decreased by 25% (Fig. 1C, cell volume reduction shown in Inset).
Termite GNBP-2 Is an Active β(1,3)-Glucanase Induced by Pathogenic Patterns.
To associate a termite GNBP with the observed activity, antibodies were raised against termite GNBP-1 and -2 (tGNBP-1 and tGNBP-2, respectively). Native, tGNBP-2 was successfully isolated and purified by immunoaffinity chromatography from both termite extract and nests built from various materials (Fig. 2A) and exhibited robust β(1,3)-glucanase activity (Fig. 2B). No equivalent proteins were precipitated from soil or wood. tGNBP-2 was detected in termite HPLC fractions displaying peak β(1,3)-glucanase activity (Fig. S1) and was found to be expressed on the surfaces of two distinct hemocyte populations, granular hemocytes (Electronic Volumelo/Side Scatterhi) and large, less dense hemocytes (Electronic Volumehi/Side Scatterlo, presumably plasmatocytes) (Fig. 2C). The hydrophobic tail of tGNBP-2 is highly homologous to the tail of Drosophila GNBP (20) and therefore likely serves as a GPI anchor. Existence in both soluble and membrane-associated forms is common among pattern receptors including mammalian CD14 (21) and various insect proteins (22).
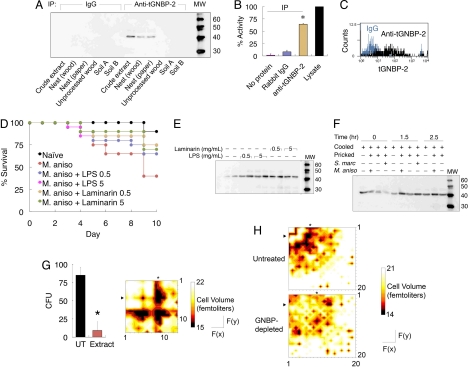
Fig. 2.
tGNBP-2 is an effector β(1,3)-glucanase induced by pathogenic patterns. (A) Immunoprecipitation of tGNBP-2 from termite extracts, nests, unprocessed wood, and soils A and B sampled from different locations. (B) β(1,3)-Glucanase activity of termite immunoprecipitate isolated by rabbit IgG or anti-tGNBP-2 (*, P < 0.05 vs. no protein and rabbit IgG). (C) Expression of tGNBP-2 on hemocyte surface, measured by flow cytometry. (D) Survival of termites infected with Metarhizium anisopliae (M. aniso) following immunization with LPS or laminarin at either 0.5 or 5 mg/mL along a course of 10 days (mean of 2 groups, n = 12/group; all immunized termite groups were P < 0.05 vs. M. aniso only, and P > 0.05 vs. naïve termites with the exception of 0.5 mg/mL LPS). (E) tGNBP-2 expression after exposure of termites to either LPS or laminarin at either 0.5 or 5 mg/mL (left and right lanes in each sample represent two independent repeats). (F) Expression of tGNBP-2 after termite infection with M. anisopliae (M. aniso) or Serratia marcescens (S. marc). Cooled only (cooled) or cooled and pricked (pricked) termites are shown as controls. (G) Effect of crude termite extract on conidial growth (in CFUs) on potato-dextrose agar plates (*, P < 0.05 vs. untreated conidia) (Left). Cytotoxicity map of fractionated termites on M. anisopliae conidia measured by cell volume in femtoliters (Right). F(x),F(y) represent fractions 1–13 on either axis. The tGNBP-2+ fraction is marked by an arrowhead; asterisks mark putative antimicrobial peptides. (H) Loss of cytotoxicity after depletion of tGNBP-2 by antibody precipitation. F(x),F(y) represent fractions 1–20 on either axis.
The presence of active tGNBP-2 in nest material indicates that termites incorporate but also maintain the levels of this protein in the nest structure. This observation points to a possible mechanism wherein tGNBP-2 functions as a nest-embedded sensor that cleaves and releases pathogenic components, which then prime termites for improved antimicrobial immunity. Indeed, termites exposed to either Gram-negative bacteria-derived LPS or an algae-derived β(1,3)-d-glucan show improved resistance to M. anisopliae infection (Fig. 2D). Analysis of tGNBP-2 expression revealed significant up-regulation following exposure to either molecular component (Fig. 2E). Moreover, infection of termites with either Serratia marcescens or M. anisopliae also induced the expression of tGNBP-2 as early as 1.5 h postinfection, whereas control groups for stress caused by cooling and pin-pricking remained close to baseline levels (Fig. 2F). This finding is in agreement with a previously reported Drosophila GNBP (20) and shows that tGNBP-2 is pattern specific rather than stress induced. The use of tGNBP-2 as an external defense system is likely to have been instrumental in the evolution of complex societies with elaborate nest architectures. Moreover, evolution of social behaviors, such as the increased mutual grooming observed following exposure to fungal pathogens (23), may have been directed by the protective function of salivary tGNBP-2.
tGNBP-2 Is an Antimicrobial Effector Protein.
Two important questions were: (a) Is the β(1,3)-glucanase activity of tGNBP-2 an effector activity, and (b) does this activity critically contribute to the total effector capacity of the termite immune system, or is it redundant to additional mechanisms? Crude termite extract, representing the total effector capacity, is cytotoxic to Metarhizium conidia (Fig. 2G Left). To observe this at a higher resolution, the extract was fractionated into either 13 or 20 size fractions crossed in 13 × 13 or 20 × 20 matrices, respectively, and the cytotoxicity of every fraction combination toward Metarhizium was evaluated by flow cytometric analysis of conidia cell volume.
Combining tGNBP-2+ fractions with fractions corresponding to ≈5,000 Da molecules (Fig. 2G Right) resulted in significant synergistic cytotoxicity compared with that exerted by tGNBP-2 alone. This synergistic effect was abolished by depletion of tGNBP-2 (Fig. 2H). One possible explanation for this intriguing finding is that tGNBP-2 cooperates with small antimicrobial peptides such as termicin and spinigerin (24–26) by compromising cell wall integrity and enhancing peptide penetration into the cell, a mechanism used by plant β(1,3)-glucanases (27). These antimicrobial peptides are constitutively expressed at high levels in termites, and mass spectrometric analysis suggested the presence of these peptides by size (Fig. S2). Termicins are also secreted by salivary glands (26). This combinatorial mapping demonstrates that tGNBP-2 is a critical component of the termite antimicrobial potential.
Structure–Function Analysis of the Pattern Recognition and Enzymatic Activity of tGNBP-2.
To examine whether tGNBP-2 represents a functional pattern recognition receptor, we quantified the interactions between this protein and a range of intact termite pathogens. Isolated tGNBP-2 exhibited binding to both Gram-negative bacteria and fungi, with significantly higher affinity to bacteria than to fungi (Fig. 3A).
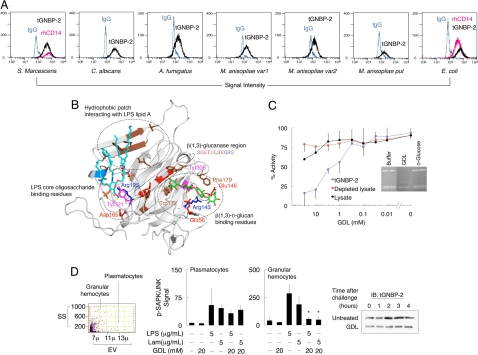
Fig. 3.
GDL blocks pattern recognition and signaling by tGNBP-2. (A) Binding of tGNBP-2 to pathogenic bacteria and fungi (Serratia marcescens, Candida albicans, Aspergillus fumigatus, Metarhizium anisopliae variants 1 and 2, putative M. anisopliae isolated from a dead insect, and Escherichia coli) compared with recombinant human CD14 (rhCD14). IgG, isotype controls; tGNBP-2, tGNBP-2 signal; rhCD14, recombinant human CD14 signal. (B) A computational model of tGNBP-2 (gray) with side chains interacting with β(1,3)-d-glucan and eritoran (an LPS analog) colored as follows: red, acidic; brown, hydropohobic; blue, basic; light blue, Asn/Gln; magenta, Tyr. β(1,3)-d-glucan C atoms are green; eritoran C atoms are cyan. In both, O atoms are red, P atoms orange, and N atoms blue. (C) Dose–response inhibition of β(1,3)-glucanase activity of tGNBP-2, termite lysate, or tGNBP-2-depleted lysate by GDL. (Inset) Zymogram of termite extract with GDL or a d-glucose control. (D) Inhibition of SAPK/JNK signaling in hemocytes by suppression of tGNBP-2 measured by flow cytometry (G, granulocytes; M, monocytes; GDL, d-δ-gluconolactone; n = 2,000) (Left). GDL (20 mM) blocks induction of tGNBP-2 by laminarin challenge in termite cells, measured by Western blot (Right).
The structural basis of this dual function was studied using a homology-based model where β(1,3)-d-glucan and eritoran (an LPS analog) are docked into tGNBP-2. This model suggests that β(1,3)-d-glucans are bound and catalyzed at the intact glucanase region, whereas a distinct hydrophobic patch upstream to it binds LPS (Fig. 3B). Homologous patches have been shown to bind LPS in other insects (28). The model shows that the β(1,3)-d-glucan is potentially held in place by 6 residues, 5 of which are chemically similar in termite GNBP-2 proteins and in Bacillus circulans β(1,3)-glucanase but vary among other insect GNBPs (Table S1), providing a structural insight into the enzymatic activity of tGNBP-2. Interestingly, we found that binding of LPS and catalysis of β(1,3)-d-glucans do not cross-interfere with each other, showing that they are distinct both structurally and functionally (Fig. S3).
Rational Design of a Glycomimetic tGNBP-2 Blocker.
These findings implicated the β(1,3)-glucanase activity of tGNBP-2 as a critical component in termite antimicrobial defense. We therefore sought a way to block this protein that will target only its unusual β(1,3)-glucanase activity and leave its other parts intact. This requirement along with our aim of designing a pest control strategy highlighted a small molecule approach. We hypothesized that a glycomimetic derivative of the pathogenic patterns recognized by tGNBP-2 would be both a structurally rational and a synthetically feasible blocker.
The combination of a pocket, formed by the 6 residues immobilizing the glycan, and the active core that flanks this pocket determines that a β(1,3) glycosidic linkage must be the reducing-end determinant of the polysaccharide chain and presented to the active core. Therefore, a glucan chain (n ≥ 1) with a terminal modification was hypothesized to occupy the receptor and hold it an inactive state.
To validate this concept, we started with the simplest chain, a single glucose molecule, whereas a modification of the reducing end would provide a determinant probably sufficient to inactivate the receptor. We therefore selected an existing molecule that completely satisfied these requirements, the glucose derivative d-δ-gluconolactone (GDL) (29–31).
GDL efficiently blocked the activity of tGNBP-2 but left other β-glucanases intact (Fig. 3C), indicating good specificity for the purpose of this study. In response to a β(1,3)-d-glucan challenge, termite hemocytes responded by stress-activated protein kinase (SAPK)/JNK phosphorylation (32). GDL inhibited this activation response and the subsequent induction of tGNBP-2 (Fig. 3D). Interestingly, however, of the two hemocyte populations expressing tGNBP-2, only granular cells were blocked by GDL, suggesting that plasmatocytes have additional glucan-recognizing mechanisms and might have distinct roles in antimicrobial immunity.
GDL Suppresses Termite Antimicrobial Immunity In Vivo.
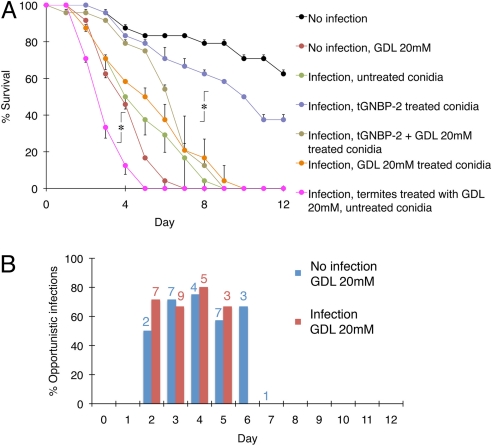
Finally, we aimed at demonstrating the effects of tGNBP-2 blockade on termite survival. Termites died rapidly following infection with M. anisopliae. When conidia were treated with isolated tGNBP-2 before infection, termite survival was remarkably similar to that of uninfected groups, indicating that conidia were inactivated by tGNBP-2. However, GDL restored susceptibility to infection. Termites treated with GDL for 24 h before infection showed accelerated mortality (Fig. 4A). GDL had no direct toxic effect on conidia themselves, as any inhibitory effect of fungal glucanases is likely irrelevant before germination. Interestingly, GDL treatment also caused accelerated mortality even in the absence of active infection. This result is explained by postmortem analysis of dead termites from both GDL treatment groups. The analysis showed that termites were killed by M. anisopliae as well as by additional opportunistic pathogens, namely, Gram-negative bacteria, such as Serratia and Pseudomonas, and fungi (Fig. 4B, Fig. S4, and Fig. S5). Notably, these observations are unlikely to have resulted from inhibited feeding, because termite gut symbionts provide multiple pathways for polysaccharide utilization (33), many of which are not blocked by GDL (34). The different survival kinetics in the in vivo experiments (Figs. 2D and 4A) are due to survivability variations among different Nasutitermes colonies used in this study. We are currently investigating potential factors affecting this variability.
Fig. 4.
GDL suppresses termite antimicrobial defense. (A) Effect of gain or loss of tGNBP-2 function on termites infected with Metarhizium anisopliae along the course of 12 days (GDL, d-δ-gluconolactone; mean of 2 groups, n = 12/group; *, P < 0.05 tGNBP-2 treated conidia vs. other groups, P < 0.05 GDL treated termites with untreated conidia vs. other groups, n.s. no significant difference vs. no infection). (B) Postmortem analysis of dead termites from GDL treatment groups representing percentage of termites confirmed to have been infected by microbial pathogens. Numbers above bars represent daily death count.
Conclusion
The strategy presented here is an alternative to toxic pesticides given that it is natural, nontoxic, and biodegradable. GDL and similar glycomimetics could potentially be engineered toward a field formulation with minimal adverse effects on surrounding ecosystems, for example, by using nanoparticles to immobilize them. Our strategy may not be limited to termites, because the glucanase activity of tGNBP-2 appears to be shared by several pest species (16–18) as suggested by our sequence and structural analysis. A final interesting note, because GDL is a product of a biosynthetic pathway, plants could conceivably be engineered to produce it in high amounts and at specific compartments, which would bolster their immunity to pest attacks. Glycomimetic synthesis in this context could be optimized as to not interfere with future industrial utilization of plant carbohydrates as a non-fossil-fuel source.
Materials and Methods
Study Species.
N. corniger nests were collected in April of 2006 from Gamboa in the Republic of Panama. The termites in their carton nests were maintained in plastic boxes at 28 °C and 75% humidity and provided with birch wood and water ad libitum.
Bioinformatic Analysis.
Insect GNBPs were identified with BLAST search using Nasutitermes GNBPs (DQ058898–058922). Representative GNBPs were aligned with ClustalX (35). The phylogenetic tree was constructed by distance analysis with PAUP* version 4.0b10 (36). The tree was rooted with protein sequence from B. circulans β(1,3)-glucanase (AAC60453). One-thousand replicates were used to calculate bootstrap values, and branches were collapsed with a 50% consensus rule.
Termite Extracts, Tissue Samples, Cuticular Washes, and Hemocytes.
N. corniger workers, large workers, or soldiers (whole termites or isolated tissues) were surface sterilized (5% hypochlorite followed by sterile water) and homogenized in acetate buffer (0.2 M, pH 5.0, 4 μL per termite) with Biomasher columns (pore size 80–145 μm; Cartagen) according to the manufacturer's instructions. Cuticular washes were prepared in 0.1% Tween 80 (10 μL per worker). Wash samples were concentrated with a P10 Microcon filter (Millipore). Hemocytes were isolated from 10 chilled N. corniger workers (1 μL per termite) as described (37).
Flow Cytometry.
Hemocyte staining was performed essentially as described elsewhere (38). The antibodies used were: primary, anti-tGNBP-2 (5 μg/mL) or rabbit IgG (5 μg/mL; Sigma); secondary antibody, anti-rabbit PE (1:400; Invitrogen). Intracellular flow cytometry for protein phosphorylation was performed as described (38). The antibodies used were: primary, anti-phospho SAPK/JNK (Fig. S6, 1:100; Cell Signaling); secondary, anti-rabbit fluorescein (1:400; Invitrogen). Flow cytometry was performed on a Beckman-Coulter Cell Lab Quanta SC MPL flow cytometer.
β(1,3)-Glucanase Assays.
β(1,3)-Glucanase activity was measured by a gel electrophoresis assay (39) and by a flow cytometric method (Fig. S7). Briefly, samples were run on Carboxymethyl Curdlan Remazol Brilliant Blue (Loewe Biochemica) gel, and clearing zones representing foci of enzymatic activity were photographed. For flow cytometry, laminarin (Sigma) was labeled with rhodamine green-X succinyl ester (Invitrogen) as described (40) and adsorbed on 3.0-μm-diameter polystyrene microspheres (Polysciences) by a 3-h incubation in carbonate/bicarbonate buffer (50 mM, pH 9.6) at 37 °C. Microspheres were then washed and reconstituted in sodium acetate (0.1 M, pH 5.5). Samples were mixed with 1 μL of the microsphere suspension, incubated for 15–30 min at 37 °C, and analyzed. Commercially available purified β(1,3)-glucanase from H. pomatia or B. subtilis was used at 0.1 milliunits/mL as a positive control, and results were expressed as percentage activity of this control.
Antifungal Assays.
M. anisopliae conidia were incubated with single insect extracts (or the equivalent of a single insect from extract prepared from 8–10 pooled workers) in sodium acetate (50 mM, pH 5.0) containing 0.025% Tween 80 and 50 μg of ampicillin (40 μL of incubation mix included ≈100 conidia and crude extract corresponding with one termite). This mix was incubated for 18–24 h at 25 °C, then plated onto potato dextrose agar 100 × 15 mm plates and supplemented with 50 μg/mL ampicillin. CFUs were counted following a 4-day incubation at 25 °C. Femtoliter changes in conidial cell volume were directly measured by flow cytometry.
Size-Exclusion HPLC.
Extract from 30 termites diluted 1:1 in sodium acetate (0.1 M, pH 5.5) was injected into a YMC Pack Diol 200 column (300 × 8 mm ID, particle size 5 μm, pore size 20 nm; YMC) and separated on an Agilent 1100 Series instrument at 1 mL/min.
Immunoprecipitation and Western Blots.
Anti-tGNBP-2 antibodies were raised in rabbits and purified by standard methods (GenScript). Antibodies were covalently linked to 1.0-μm-diameter Dynabeads MyOne carboxylic-acid-functionalized magnetic beads (Invitrogen) according to the manufacturer's instructions. Fresh termite extract was diluted 1:1 with immunoprecipitation buffer (25 mM Tris·HCl, pH 7.6, 150 mM NaCl, 1% vol/vol Nonidet P-40, and 1% vol/vol protease inhibitor mixture) and incubated with beads for at least 1 h at room temperature. Beads were washed with immunoprecipitation buffer and eluted with a neutral pH buffer (Pierce). Eluate was desalted and concentrated using a centrifugal column (Vivaspin 10K; Sartorius) and reconstituted in sodium acetate (0.1 M, pH 5.5). SDS/PAGE and Western blots were performed and developed by standard procedures; loading amounts were validated by bicinchoninic acid assay. Anti-tGNBP-2 was used at 2.5 μg/mL followed by anti-rabbit HRP (Cell Signaling) at 1:1,000. Soil samples A and B were collected from two different locations in Boston (Reticulitermes sp. inhabiting these locations exhibited positive β(1,3)-glucanase activity).
Homology-Based Structural Modeling of tGNBP-2.
The template structure of an endo-β(1,3)-glucanase from alkaliphilic Nocardiopsis strain F96 (PDB ID: 2HYK) was chosen by the SWISS-MODEL auto-homology-modeling Web portal to construct a structural model for tGNBP-2. The binding of β(1,3)-glucan was investigated by docking a representative β(1,3)-glucan containing a tetrasaccharide obtained from its cocrystal structure with a Bacillus macerans endo-β(1,3)-glucanase (PDB ID: 1U0A) to the active site containing the consensus β(1,3)-glucanase sequence. The binding coordinates of eritoran (an LPS analog) were obtained from its cocrystal structure with Toll-like receptor 4/MD2 complex (PDB ID: 2Z65).
Combinatorial Cytotoxicity Mapping.
Samples of M. anisopliae conidia in sodium acetate (0.1 M, pH 5.5) were incubated in 384-well plates with samples from HPLC fractions (see Size-Exclusion HPLC) in different combinations (F1 + F1, F1 + F2, F1 + F3, …, F2 + F2, F2 + F3, …, F3 + F3, …; 10 μL of each fraction) overnight at 25 °C and analyzed by flow cytometry. tGNBP-2 was depleted from 250 μL of each fraction as described in Immunoprecipitation and Western Blots.
In Vitro Binding Assays.
Isolated tGNBP-2 or rhCD14 (R&D Systems) were linked to 3.0-μm-diameter polystyrene beads (Polysciences) by a 3-h incubation in boric acid (0.1 M, pH 8.5), followed by 3 washes in cold PBS containing 0.1% vol/vol rabbit serum. Formaldehyde-inactivated pathogenic strains were labeled with FITC as previously described (41). Beads were incubated with labeled pathogens in buffer at 37 °C for 30 min and analyzed by flow cytometry.
In Vivo Pin-Prick Infection Assay.
Ten workers were cooled on ice and pricked with a sterilized insect pin immersed in a solution of either M. anisopliae or S. marcescens. Termites were then released at room temperature for specific time intervals of 1.5 and 2.5 h. The experiment was terminated by extracting the termites and freezing the extract for analysis.
In Vivo Survival and Exposure Assays.
Conidia (≈3 × 107/mL) were centrifuged at 12,000 × g for 1 min and then suspended in 600 μL of 0.1% Tween 80 by vortexing. Two groups of 12 large workers were exposed to filter paper (Whatman 5) moistened with these suspensions in 35 × 15 mm Petri dishes for 20 h. The filter paper was replaced with sterile water-moistened filter paper, and dead termites were removed after a daily census of survivorship. tGNBP-2 treatment was performed with conidia suspended in a sample of the immunoprecipitated protein overnight at 25 °C, followed by extensive washing in PBS containing 0.1% Tween 80. Dead termites were removed daily, surface sterilized with 70% ethanol, and incubated in sterile plates at room temperature for 4 days for postmortem analysis. For exposure to laminarin or LPS, filter papers were moistened with 300 μL of 0.5 or 5 mg/mL solutions of either carbohydrate. Termites were kept on moist filter paper for 24 h before exposure with conidia (107/mL). Controls included termites that were not exposed to sugars, conidia, or both (0.1% Tween 80).
Statistical Analysis and Significance of Survival Data.
Data were analyzed by Mann–Whitney U test and Wilcoxon signed-rank tests with Bonferroni corrections for multiple testing. Termite survival data were analyzed by Cox regression analysis. Data are presented as means ± SD.
Supplementary Material
Acknowledgments.
We thank the administrators of the Smithsonian Tropical Research Institute (Republic of Panama) for use of their facilities and obtaining permits for the collection of termite colonies; Prof. R. H. Crozier for helpful comments on this paper; and all of the members of the R.B.R. and R.S. groups for discussions and technical assistance. This research was supported by National Science Foundation Career Development Grant DEB-0447316 (to R.B.R.) and National Institutes of Health Grants GM57073 (to R.S.). I.B. is a Novartis Postodoctoral Fellow of the Life Sciences Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904063106/DCSupplemental.
References
- 1.Bischoff V, et al. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:1175–1180. doi: 10.1038/ni1123. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4:e229. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson FL, et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- 6.Bulmer MS, Crozier RH. Variation in positive selection in termite GNBPs and Relish. Mol Biol Evol. 2006;23:317–326. doi: 10.1093/molbev/msj037. [DOI] [PubMed] [Google Scholar]
- 7.Jiggins FM, Kim KW. Contrasting evolutionary patterns in Drosophila immune receptors. J Mol Evol. 2006;63:769–780. doi: 10.1007/s00239-006-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimopoulos G, Richman A, Muller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keitel T, Simon O, Borriss R, Heinemann U. Molecular and active-site structure of a Bacillus 1,3–1,4-β-glucanase. Proc Natl Acad Sci USA. 1993;90:5287–5291. doi: 10.1073/pnas.90.11.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warr E, Das S, Dong Y, Dimopoulos G. The Gram-negative bacteria-binding protein gene family: Its role in the innate immune system of Anopheles gambiae and in anti-Plasmodium defence. Insect Mol Biol. 2008;17:39–51. doi: 10.1111/j.1365-2583.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown GD, Gordon S. Immune recognition of fungal β-glucans. Cell Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 12.Royet J, Dziarski R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 13.Planas A, Juncosa M, Lloberas J, Querol E. Essential catalytic role of Glu134 in endo-β-1,3–1,4-d-glucan 4-glucanohydrolase from B. licheniformis as determined by site-directed mutagenesis. FEBS Lett. 1992;308:141–145. doi: 10.1016/0014-5793(92)81262-k. [DOI] [PubMed] [Google Scholar]
- 14.Miller LR. Systematics of the Australian Nasutiterminae with reference to evolution within the Termitidae (Isoptera) Canberra, Australia: Austalian National University; 1997. PhD thesis. [Google Scholar]
- 15.Rosengaus RB, Moustakas JE, Calleri DV, Traniello JF. Nesting ecology and cuticular microbial loads in dampwood (Zootermopsis angusticollis) and drywood termites (Incisitermes minor, I. schwarzi, Cryptotermes cavifrons) J Insect Sci. 2003;3:31. doi: 10.1093/jis/3.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balasubramani V, Sayyed AH, Crickmore N. Genetic characterization of resistance to deltamethrin in Plutella xylostella (Lepidoptera: Plutellidae) from India. J Econ Entomol. 2008;101:1911–1918. doi: 10.1603/0022-0493-101.6.1911. [DOI] [PubMed] [Google Scholar]
- 17.Genta FA, Terra WR, Ferreira C. Action pattern, specificity, lytic activities, and physiological role of five digestive β-glucanases isolated from Periplaneta americana. Insect Biochem Mol Biol. 2003;33:1085–1097. doi: 10.1016/s0965-1748(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 18.Pauchet Y, Freitak D, Heidel-Fischer HM, Heckel DG, Vogel H. Immunity or digestion: Glucanase activity in a glucan-binding protein family from lepidoptera. J Biol Chem. 2009;284:2214–2224. doi: 10.1074/jbc.M806204200. [DOI] [PubMed] [Google Scholar]
- 19.Lomer CJ, Bateman RP, Johnson DL, Langewald J, Thomas M. Biological control of locusts and grasshoppers. Annu Rev Entomol. 2001;46:667–702. doi: 10.1146/annurev.ento.46.1.667. [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, et al. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and β-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J Biol Chem. 2000;275:32721–32727. doi: 10.1074/jbc.M003934200. [DOI] [PubMed] [Google Scholar]
- 21.Labeta MO, Landmann R, Obrecht JP, Obrist R. Human B cells express membrane-bound and soluble forms of the CD14 myeloid antigen. Mol Immunol. 1991;28:115–122. doi: 10.1016/0161-5890(91)90094-z. [DOI] [PubMed] [Google Scholar]
- 22.Metheniti A, et al. Evidence for a LPS-binding protein in medfly hemocyte surface: mediation in LPS internalization but not in LPS signaling. Arch Insect Biochem Physiol. 2003;54:25–36. doi: 10.1002/arch.10096. [DOI] [PubMed] [Google Scholar]
- 23.Traniello JF, Rosengaus RB, Savoie K. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc Natl Acad Sci USA. 2002;99:6838–6842. doi: 10.1073/pnas.102176599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulmer MS, Crozier RH. Duplication and diversifying selection among termite antifungal peptides. Mol Biol Evol. 2004;21:2256–2264. doi: 10.1093/molbev/msh236. [DOI] [PubMed] [Google Scholar]
- 25.Da Silva P, et al. Solution structure of termicin, an antimicrobial peptide from the termite Pseudacanthotermes spiniger. Protein Sci. 2003;12:438–446. doi: 10.1110/ps.0228303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberty M, et al. Insect immunity. Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J Biol Chem. 2001;276:4085–4092. doi: 10.1074/jbc.M002998200. [DOI] [PubMed] [Google Scholar]
- 27.Park SW, Lawrence CB, Linden JC, Vivanco JM. Isolation and characterization of a novel ribosome-inactivating protein from root cultures of pokeweed and its mechanism of secretion from roots. Plant Physiol. 2002;130:164–178. doi: 10.1104/pp.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilej M, et al. Distinct carbohydrate recognition domains of an invertebrate defense molecule recognize Gram-negative and Gram-positive bacteria. J Biol Chem. 2001;276:45840–45847. doi: 10.1074/jbc.M107220200. [DOI] [PubMed] [Google Scholar]
- 29.Cianciotto N, Rappuoli R, Groman N. Detection of homology to the beta bacteriophage integration site in a wide variety of Corynebacterium spp. J Bacteriol. 1986;168:103–108. doi: 10.1128/jb.168.1.103-108.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copa-Patino JL, Reyes F, Perez-Leblic MI. Purification and properties of a 1,3-β-glucanase from Penicillium oxalicum autolysates. FEMS Microbiol Lett. 1989;53:285–291. doi: 10.1016/0378-1097(89)90232-2. [DOI] [PubMed] [Google Scholar]
- 31.Pitson SM, Seviour RJ, McDougall BM, Stone BA, Sadek M. Purification and characterization of an extracellular (1 → 6)-β-glucanase from the filamentous fungus Acremonium persicinum. Biochem J. 1996;316:841–846. doi: 10.1042/bj3160841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sluss HK, et al. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 33.Warnecke F, et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- 34.de A Ximenes F, de Paula Silveira FQ, Filho EX. Production of β-xylosidase activity by Trichoderma harzianum strains. Curr Microbiol. 1996;33:71–77. doi: 10.1007/s002849900077. [DOI] [PubMed] [Google Scholar]
- 35.Higgins DG, Thompson JD, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- 36.Wilgenbusch JC, Swofford D. Inferring evolutionary trees with PAUP*. Curr Protoc Bioinformatics. 2003 doi: 10.1002/0471250953.bi0604s00. Chapter 6:Unit 6.4. [DOI] [PubMed] [Google Scholar]
- 37.Rosengaus RB, Cornelisse T, Guschanski K, Traniello JF. Inducible immune proteins in the dampwood termite Zootermopsis angusticollis. Naturwissenschaften. 2007;94:25–33. doi: 10.1007/s00114-006-0151-9. [DOI] [PubMed] [Google Scholar]
- 38.Irish JM, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Kalix S, Buchenauer H. Direct detection of β-1,3-glucanase in plant extracts by polyacrylamide gel electrophoresis. Electrophoresis. 1995;16:1016–1018. doi: 10.1002/elps.11501601170. [DOI] [PubMed] [Google Scholar]
- 40.Meunier F, Wilkinson KJ. Nonperturbing fluorescent labeling of polysaccharides. Biomacromolecules. 2002;3:857–864. doi: 10.1021/bm0255241. [DOI] [PubMed] [Google Scholar]
- 41.Hartshorn KL, White MR, Crouch EC. Contributions of the N- and C-terminal domains of surfactant protein d to the binding, aggregation, and phagocytic uptake of bacteria. Infect Immun. 2002;70:6129–6139. doi: 10.1128/IAI.70.11.6129-6139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.