Abstract
Prostate cancer is one of the most common neoplasias in men. The tumor suppressor Par-4 is an important negative regulator of the canonical NF-κB pathway and is highly expressed in prostate. Here we show that Par-4 expression is lost in a high percentage of human prostate carcinomas, and this occurs in association with phosphatase and tensin homolog deleted from chromosome 10 (PTEN) loss. Par-4 null mice, similar to PTEN-heterozygous mice, only develop benign prostate lesions, but, importantly, concomitant Par-4 ablation and PTEN-heterozygosity lead to invasive prostate carcinoma in mice. This strong tumorigenic cooperation is anticipated in the preneoplastic prostate epithelium by an additive increase in Akt activation and a synergistic stimulation of NF-κB. These results establish the cooperation between Par-4 and PTEN as relevant for the development of prostate cancer and implicate the NF-κB pathway as a critical event in prostate tumorigenesis.
Keywords: AKT, aPKC, IL-6, inflammation, prostate cancer
Prostate cancer is one of the most common malignancies and the second leading cause of cancer death in males (1). The disease is complex in its development and response to therapy, and it cannot be predicted when or whether an indolent prostate tumor will become clinically aggressive. Significant limitations in current treatment methods warrant an intense focus on this type of cancer. Moreover, the development of targeted antitumor therapies will require a better understanding of the signaling cascades involved in the initiation and progression of prostate cancer.
Par-4 is a gene highly expressed in the prostate that was initially identified in an in vitro differential screen for proapoptotic genes in human prostate carcinoma cell lines (2). The Par-4 gene maps to chromosome 12q21, a region frequently deleted in certain malignancies, and encodes a protein (38 kDa) containing a leucine-zipper domain in the carboxy-terminal region, which interacts with a variety of proteins (3), including the atypical protein kinases (aPKCs), PKCζ and PKCλ/ι (4). Par-4 has been proposed to impair cell survival through the inhibition of the aPKCs and the consequent down-modulation of NF-κB and its prosurvival transcriptional targets (5–7). We have previously shown that the genetic inactivation of Par-4 in mice leads to reduced lifespan and spontaneous tumorigenesis (6). Particularly relevant to this study, Par-4-null mice develop spontaneous benign neoplasias in hormone-dependent tissues, including prostate (6). In addition, we have also shown that Par-4 is downregulated in ≈40% of human endometrial carcinomas and human lung adenocarcinomas (8, 9). Moreover, loss of Par-4 dramatically increases Ras-induced lung carcinoma formation in association with enhanced NF-κB and Akt activity (9). The latter results unveiled an unanticipated role for Par-4 as an indirect inhibitor of Akt, both in vitro and in vivo, through down-modulation of PKCζ (9). Together, these observations identify Par-4 as a tumor suppressor in the NF-κB and Akt pathways in lung cancer (9).
The phosphatase and tensin homolog deleted from chromosome 10 (PTEN) tumor suppressor is a central regulator of human prostate carcinogenesis (10). PTEN alterations have been extensively implicated in human prostate cancer; PTEN deletions and mutations occur on at least 1 allele in up to 30% of primary cancers, and homozygous PTEN inactivation is frequently associated with metastatic prostate tissues (11, 12). In addition, loss of PTEN expression correlates with higher Gleason scores in human prostate cancer (13). PTEN encodes a lipid phosphatase that is a negative regulator of the PI-3K/Akt pathway (14) and, consequently, loss of PTEN function results in aberrant activation of the Akt pathway in prostate cells (14–16). In keeping with this, genetic ablation of Akt1 is sufficient to suppress tumor development in PTEN+/− mice (17). This relates to an emerging paradigm in cancer biology in which signaling activation is enhanced by the concomitant reduction of tumor suppressors acting in the same pathway, thus promoting tumor progression. For example, the tumor suppressor promyelocytic leukemia cooperates with PTEN inside the nucleus to inhibit Akt (18). In addition, PTEN loss synergizes with defects in a number of negative regulators of proliferation, such as Nkx3.1, p27, or p18INK4c to promote the progression of benign prostate tumors to invasive carcinoma (19–21). Consistent with this, transgenic expression of activated Akt in the murine prostate induces prostatic intraepithelial neoplasia (PIN) (22). However, Akt activation is not sufficient to drive this relatively benign form of neoplasia to more aggressive cancer phenotypes (22). This result suggests a 2-hit model for prostate tumor development involving the cooperation of complementary networks of tumor suppressors.
In this regard, signaling cascades other than Akt that are involved in the regulation of cell growth and survival could come into play during tumor progression. An important pathway is the NF-κB cascade, which appears to play a central role in carcinogenesis (23), although its implication in prostate cancer still needs to be better understood. Because Par-4 is a negative regulator of NF-κB (3, 7), and Par-4 loss leads to benign prostate neoplasias, we hypothesized that Par-4 deficiency in conjunction with the loss of an Akt inhibitor like PTEN could be instrumental in prostate cancer progression. Here we have investigated the cooperation between Par-4 and PTEN in prostate tumorigenesis and report that PTEN heterozygosity synergizes with Par-4 loss to promote the progression to prostate carcinoma. We also show that there is a concomitant loss of Par-4 and PTEN in human prostate carcinomas, suggesting the existence of a pathologically relevant biochemical and functional cooperation between these 2 tumor suppressors impinging the Akt and NF-κB pathways.
Results
Par-4 Deficiency Alters the Prostate Epithelium and Leads to Neoplasia Through PKCζ.
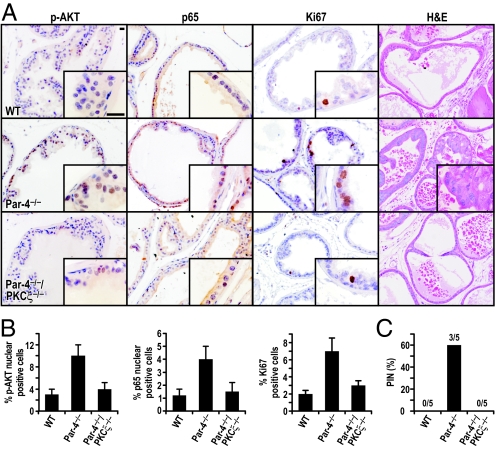
Recent results from our laboratory unveiled an important role for Par-4 in the control of NF-κB and Akt phosphorylation through PKCζ in lung tissue and in cell culture (9). Therefore, it would be of great interest to determine whether NF-κB and Akt are activated in Par-4-deficient preneoplastic prostates and whether that activation depends on PKCζ. It is also important to define the contribution of PKCζ to the induction of prostate neoplasia in Par-4−/− mice. To address these important questions, we analyzed the signaling pathways altered in preneoplastic prostates from Par-4−/− mice and also from Par-4−/−/PKCζ−/− double-knockout mice. Interestingly, we found a reproducible increase in the activation of Akt in the prostate epithelial cells of Par-4−/− mice that is reverted in the Par-4−/−/PKCζ−/− prostates (Fig. 1 A and B). These results are in keeping with our recently published data demonstrating a role for the Par-4/PKCζ module in the control of Akt in vivo (9), and extend its function to the prostate. Consistent with the proposed implication of Par-4 in NF-κB regulation, analysis of a set of well-established NF-κB-dependent transcripts revealed a reproducible increase in the levels of these mRNAs in Par-4−/− prostates as compared to WT controls [supporting information (SI) Table S1]. Of special relevance is IL-6, which has been shown to be an important inflammatory cytokine that plays a central role in various types of cancer (24). Consistent with this, RelA (p65) staining revealed increased NF-κB activation in Par-4-deficient preneoplastic prostates but not in the Par-4−/−/PKCζ−/− prostates see (Fig. 1 A and B). Collectively, these results establish the Par-4/PKCζ complex as a bona fide regulator of the NF-κB and Akt pathways in prostate.
Fig. 1.
Loss of PKCζ reverts the Par-4−/− phenotype in the prostate. Mice (4-week-old) of the indicated genotype were treated for 4 weeks with s.c. pellets containing testosterone (25 mg) and estradiol (2.5 mg). (A) Tissue sections were stained with anti-phospho-Akt(S473), anti-p65 (Rel A), or anti-Ki67 antibody, or H&E. Representative sections of analyzed prostate tissue after treatment are shown. n = 5 mice per genotype. (Scale bar, 50 μm.) (B) Quantification of cells showing positive nuclear staining for phospho-Akt, p65 or Ki-67. Results are the mean ± SD of counts from 10 different fields per mouse of a total of 5 mice in each condition. (C) Quantification of incidence of hormonally induced PIN lesions.
We next determined whether the loss of PKCζ could have an impact on the proliferation of Par-4-deficient preneoplastic prostate epithelial cells. Analysis of Ki67 expression, an indicator of proliferation, revealed an increase in the proliferative index of the prostatic epithelium in Par-4−/− as compared to WT controls. Importantly, this increase was dramatically reduced in the Par-4−/−/PKCζ−/− prostates (see Fig. 1 A and B), which indicates that PKCζ channels signals downstream of Par-4 that are relevant to cell proliferation.
Based on the above findings, we next tested the hypothesis that PKCζ inactivation would prevent PIN induction by Par-4 deficiency. In this experiment, we treated WT, Par-4−/−, and Par-4−/−/PKCζ−/− mice (4 weeks old) for 4 weeks with a testosterone-estradiol mixture, provided continuously through s.c. pellets, as described previously (6), which is carcinogenic for mice with genetic alterations in tumor suppressors. After treatment, we determined the appearance of PIN in the prostates of these mice. Consistent with our previous observations (6), the hormonal treatment promoted the induction of PIN in Par-4−/− mice, but not in WT mice. Interestingly, the phenotype of the Par-4−/−/PKCζ−/− mice was, in this respect, the same as the WT mice, showing no PIN induction (Fig. 1C). Collectively, these results genetically demonstrate that PKCζ is essential for the hyperproliferation and development of prostate neoplasia triggered by Par-4 deficiency.
Loss of Par-4 Expression in Human Prostate Carcinomas.
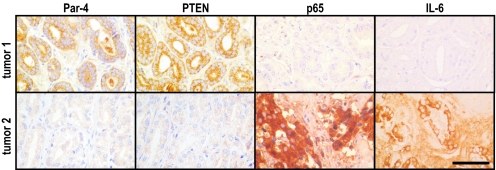
To determine the relevance of Par-4 as a prostate tumor suppressor, human prostate carcinomas were analyzed for Par-4 expression and promoter methylation. Immunohistochemical (IHC) analysis revealed that prostate carcinomas (n = 41) could be classified between Par-4-negative/low (59%) and Par-4-positive (41%) (Fig. S1A and Tables S2 and S3). Similar to what we described earlier for endometrial cancer (8), there was a significant association between Par-4 promoter methylation and lack of Par-4 expression (see Fig. S1 and Tables S2 and S3). Small cores from these tumors were assembled in a tissue microarray and subsequently analyzed by IHC for Par-4 and PTEN. Interestingly, a significant direct correlation between Par-4 and PTEN protein levels was observed (Table 1 and Fig. 2). The majority of Par-4-positive tumors were also positive for PTEN, and conversely, those tumors with negative/low PTEN also had negative/low Par-4 (see Table 1). This concomitant loss of Par-4 and PTEN suggests the existence of cooperation between these 2 tumor suppressors. PTEN inactivation has previously been found to be associated with higher Gleason scores and to more aggressive prostate cancer. Similarly, we observed a correlation between Par-4 loss and high Gleason scores (see Table 1). Together, these results place Par-4 loss as a relevant step in prostate tumor progression, like PTEN deficiency, and reveal that Par-4 inactivation is at least in part usually achieved by aberrant de novo methylation of its promoter.
Table 1.
Par-4 and PTEN association in human prostate carcinomas
| Par-4 |
||||
|---|---|---|---|---|
| Negative n (%) | Intermediate n (%) | High n (%) | P-value (Chi2) | |
| PTEN (n = 37) | ||||
| Negative | 4 (80) | 3 (21.4) | 2 (11.1) | |
| Intermediate | 1 (20) | 7 (50) | 7 (38.9) | |
| High | 0 (0) | 4 (28.6) | 9 (50) | 0.021 |
| Nuclear p65 (n = 37) | ||||
| Negative | 1 (20) | 5 (35.7) | 13 (72.2) | |
| Intermediate | 2 (40) | 7 (50) | 5 (27.8) | |
| High | 2 (40) | 2 (14.3) | 0 (0) | 0.037 |
| IL-6 (n = 37) | ||||
| Negative | 1 (20) | 11 (78.6) | 15 (83.3) | |
| Intermediate | 3 (60) | 1 (7.1) | 2 (11.1) | |
| High | 1 (20) | 2 (14.3) | 1 (5.6) | 0.038 |
| Gleason score (n = 37) | ||||
| 2–6 | 1 (20) | 5 (35.7) | 12 (66.7) | |
| 7–10 | 4 (80) | 9 (64.3) | 6 (33.3) | 0.092 |
Fig. 2.
Association between Par-4 and PTEN expression in human prostate cancer. Immunostaining for Par-4, PTEN, p65, and IL-6 in human prostate cancer samples. Representative examples of a tumor positive for both Par-4 and PTEN (tumor 1; Upper) and of a tumor negative for both Par-4 and PTEN (tumor 2; Lower). Par-4 and PTEN levels are inversely correlated with 2 parameters of NF-κB activation: nuclear p65 and IL-6. (Scale bar, 200 μm.)
Simultaneous Deficiency in Par-4 and PTEN Promote Prostate Adenocarcinoma.
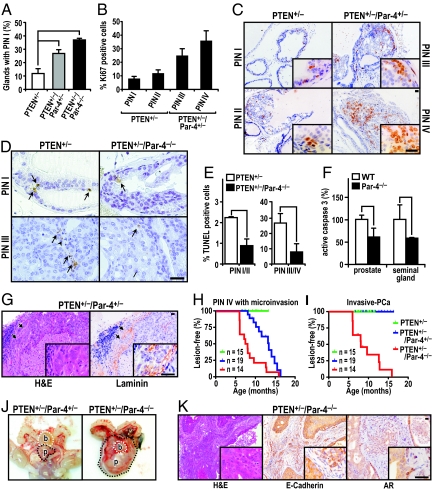
As the loss of Par-4 is associated with PTEN deficiency in human prostate cancer (see Table 1) and increased Akt activity in preneoplastic murine prostates (see Fig. 1), we reasoned that complete deletion of Par-4 in the context of PTEN heterozygosis should lead to more aggressive prostate lesions. To address this question, we crossed Par-4−/− mice with PTEN+/− mice to generate PTEN+/−/Par-4+/− and PTEN+/−Par-4−/− mice. Total or partial ablation of Par-4 in mice in a PTEN-heterozygous genetic background did not significantly affect their overall survival (Fig. S2A), which was mainly determined by the development of multicentric lymphoproliferative disease. This suggests that Par-4 gene dosage does not impinge on the latency or mortality associated with PTEN-driven lymphoproliferative disease. Importantly, however, the loss of Par-4 does cooperate with PTEN-heterozygosity in prostate cancer. Thus, whereas the majority of prostate glands in Par-4−/− mice were normal, with only a few glands becoming mildly hyperplastic in mice older than 12 months of age (data not shown), Par-4 deficiency in a PTEN+/− background had an impact on cancer initiation, increasing the incidence of low-grade PIN (PIN I) lesions in a manner dependent on the Par-4 gene dosage (Fig. 3A). Interestingly, Par-4 also cooperates with PTEN heterozygosity in the progression to high-grade lesions. Thus, whereas at 6 months of age the prostates of PTEN+/− mice were free of high-grade PIN (PIN III and PIN IV) (see representative lesions in Fig. S2B), the prostates of PTEN+/−/Par-4+/− mice developed high-grade PIN at 100% penetrance (not shown). Consistently, the proliferative index of the prostate epithelium, measured as the percentage of nuclei positive for Ki67, correlated with PIN progression (Fig. 3 B and C). Of note, the absence of Par-4 in a PTEN+/− background led to enhanced survival of neoplastic cells (Fig. 3 D and E). These are important observations because, whereas the oncogenic cooperation between PTEN deficiency and other tumor suppressors, such as Nkx3.1, p18, p27, p53 or Tsc2, has been assigned mainly to an increase in proliferation (20, 25), Par-4 deficiency is unique in the sense that it involves an increase in both survival and proliferation. In keeping with this, Par-4 deletion decreased castration-induced apoptosis in the prostates and seminal gland of Par-4-deficient mice, further demonstrating the important role of Par-4 in the control of prostate cell survival (Fig. 3F).
Fig. 3.
Loss of Par-4 cooperates with PTEN+/− to promote invasive prostate carcinoma. (A) Par-4 status affects prostate cancer initiation and low grade PIN (PIN I) is increased depending on Par-4 gene dosage. n = 5 mice per genotype. *, P < 0.05; **, P < 0.01. (B and C) The proliferative index (%Ki67 positive) correlates with PIN grade. Representative Ki67 staining of prostates from PTEN+/− and PTEN+/−/Par-4+/− mice at 6 months of age is shown. (D and E) Apoptosis was measured in PINs by TUNEL in mice of the indicated genotype (n = 3 per genotype). Representative TUNEL stainings are shown (arrows in D). At least 15 PINs of each group (PIN I/II or PIN III/IV) were measured for each genotype at 8 months of age. Values correspond to the number of apoptotic cells per lesion. *, P < 0.05. (F) Apoptosis, measured as active caspase 3-positive areas, in prostates of mice of the indicated genotypes (n = 4 per genotype) 3 days after castration. *, P < 0.05; **, P < 0.01. (G) Representative H&E staining of microinvasive carcinoma associated with high-grade PIN in the dorsolateral area of a 12-month-old PTEN+/−/Par-4+/− mouse (Left). Laminin immunostain demonstrates penetration of small nests (arrowheads) through the basement membrane into the surrounding stroma. n = 5 mice per genotype (Right). (H and I) Kaplan-Meier curves of incidence of advanced prostate intraepithelial neoplasia (PIN IV) with microinvasion (H) or of invasive prostate carcinoma (I). A clear correlation between the progression of prostate tumorigenesis and loss of 1 or 2 Par-4 alleles in combination with PTEN heterozygosity was observed. (J) Representative examples of macroscopic appearances of age-matched PTEN+/−/Par-4+/− and PTEN+/−/Par-4−/− mouse organs at 12 months of age are shown. Significant enlargement of prostate (p) was seen; (b) denotes the bladder. (K) Representative examples of invasive carcinoma in the dorsolateral area of a 6-month-old PTEN+/−/Par-4−/− mouse. Staining methods include H&E, E-cadherin, and androgen receptor (AR). n = 5 mice per genotype. (Scale bar = 50 μm.)
Interestingly, the high-grade PIN lesions progressed to microinvasive carcinoma in the PTEN+/−/Par-4+/− double heterozygotes (Fig. 3 G and H), as evidenced by disruption of the basal membrane of the epithelium identified by laminin staining and the presence of small nests of cells invading the surrounding stroma (see Fig. 3G). More importantly, the synergy in the progression to prostate carcinoma depends on Par-4 in a gene-dosage-dependent manner, as the loss of both Par-4 alleles in the context of PTEN+/− promoted fully invasive carcinoma with an onset at 6 months of age, high penetrance, and dramatically enlarged prostates (Fig. 3 I and J). Invasive prostate carcinomas recapitulated the aggressive features of human prostate cancer, such as invasion, foci of highly anaplastic cells, vascular emboli, and a solid pattern of growth (Fig. S2C). Moreover, high-grade PIN lesions were observed in all 3 lobes (anterior, ventral, and dorsolateral), while invasive prostate carcinoma occurred predominantly in the dorsolateral prostate. To define the origin of carcinomas in the double-mutant prostates, we performed IHC analyses of various markers. As shown in Fig. 3K, PTEN+/−Par-4−/− epithelial cancer cells were positive for E-cadherin staining and expressed high levels of androgen receptor, a hallmark of secretory epithelium, but were negative for the neuroendocrine cell marker synaptophysin (Fig. S2D). These results indicate the epithelial origin of the invasive carcinoma developed as a result of the loss of Par-4 in the context of PTEN heterozygosity. Together, these observations indicate that Par4-deficiency has a profound impact on prostate tumorigenesis in association with PTEN-deficiency, affecting the number, size, progression, and severity of lesions from benign intraepithelial neoplasias to aggressive carcinomas in a manner dependent on Par-4 gene dosage.
Akt and NF-κB Activation in PTEN+/−/Par-4−/− Prostate Epithelium.
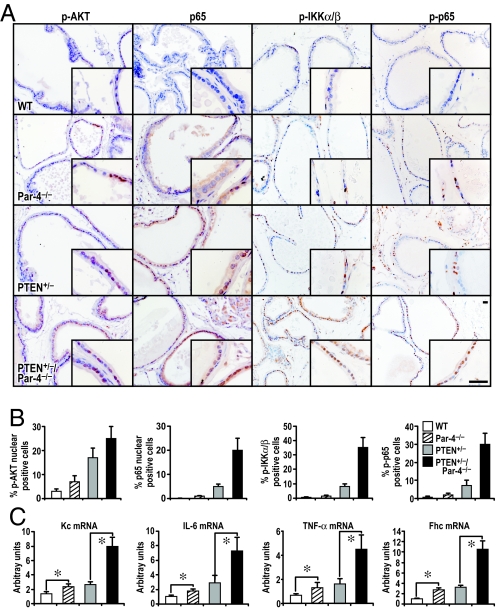
Because both Par-4 and PTEN are negative regulators of Akt [(9, 10), see also Fig. 1], we predicted that the inactivation of Par-4 in a PTEN+/− background would lead to an even greater increase in Akt activity, as compared to that induced in the single-mutant prostates. To address this possibility, we determined Akt activity in preneoplastic prostates from mice of different genotypes by IHC with anti-pAkt antibody. Results in Fig. 4 A and B demonstrate that the total loss of Par-4 in a PTEN heterozygous background results in more nuclear pAkt staining than in WT or single-mutant prostates. However, compared to the single mutants, the increase in pAkt in the PTEN+/−/Par4−/− double mutants is only additive and, therefore, difficult to reconcile with the observed dramatic effect on tumor onset and progression.
Fig. 4.
Par-4 and PTEN deficiencies cooperate in Akt and NF-κB activation in preneoplastic prostate. (A) Representative sections of preneoplastic prostate glands from 6-month-old mice of the indicated genotypes are shown. Tissue sections were stained with anti-phospho-Akt(S473) anti-p65 (Rel A), anti-phospho-IKKα/β (S176/180), or anti-phospho-p65 (S276) antibody. (Scale bar, 50 μm.) (B) Quantification of cells showing positive staining for each antibody. Results are the mean ± SD of 10 different fields per mouse of a total of 5 mice in each condition. (C) Quantification by qRT-PCR of mRNA levels of the following NF-κB targets: Kc, IL-6, TNF-α, and Fhc. Results are shown as mean ± SD. *, P < 0.05.
In this regard, it is well established that Par-4 negatively controls NF-κB (7). Thus, we sought to determine whether the simultaneous mutation of Par-4 and PTEN would lead to a synergistic activation of NF-κB in preneoplastic prostates. To address this question, we stained these prostates with an anti-p65 (RelA) antibody and scored for nuclear translocation of p65 (RelA), an established marker of NF-κB activation. While Par-4 or PTEN insufficiency separately gave rise to a significant, although modest, activation of NF-κB, this parameter was dramatically activated in the double-mutant preneoplastic prostates, suggesting a synergistic effect of the 2 mutations for NF-κB activation (see Fig. 4 A and B). When other parameters of this pathway were assessed, such as IKK activation, measured as phospho-IKK levels, or the phosphorylation of p65, a similar synergistic response was observed (see Fig. 4 A and B and Fig. S3). This was consistent with a synergistic increase in mRNA levels of NF-κB target genes, such as Kc, IL-6, TNF-α, and Fhc in the double mutants as determined by qRT-PCR (Fig. 4C), and is in agreement with the enhanced IL-6 levels observed in preneoplastic prostate of the double mutant mice as compared to the single mutants (see Fig. S3). This suggests that the synergistic activation of NF-κB in the doubly mutant prostate epithelium is the critical event for tumor progression in this system.
To confirm the relevance of NF-κB activation in the cooperation of Par-4 and PTEN mutations in human prostate cancer, we studied in the same human tissue microarray samples used above whether the expression levels of Par-4 and PTEN correlate with activated NF-κB, as determined by nuclear p65 (Rel A) and IL-6 IHC staining. Importantly, we found that decreased levels of PTEN and Par-4 were significantly associated with an increase in both nuclear p65 and IL-6 expression, as well as with disease progression (see Fig. 2 and Table 1). These results demonstrate that the simultaneous inactivation of both tumor suppressors lead to increased activation of the NF-κB pathway in human prostate cancer tumors.
Cell Autonomous Cooperation Between Par-4 and PTEN Deficiencies.
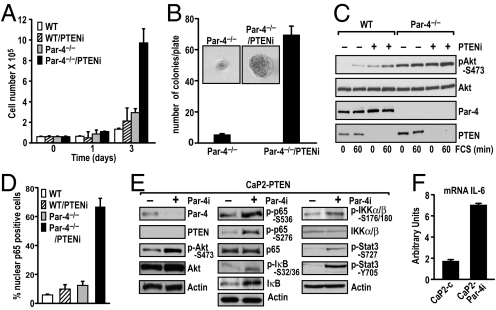
We next investigated whether the synergistic effect of simultaneous PTEN and Par-4 deficiencies detected in prostate tumorigenesis is a cell-autonomous phenomenon that can be generalized to other cell types. We first determined the proliferative properties of Par-4-deficient immortalized embryonic fibroblasts (EFs) in which PTEN levels were knocked down by lentiviral shRNA (PTENi). The data in Fig. 5A demonstrate that cell proliferation was not greatly affected by the loss of Par-4 alone or by the down-regulation of PTEN. However, the simultaneous inactivation of both proteins dramatically enhanced cell proliferation, indicating a cell-autonomous and synergistic interaction between the 2 proteins. Results in Fig. 5B reinforce this notion, as Par-4-null/PTENi cells were able to form colonies in soft agar, whereas Par-4-deficient (see Fig. 5B) or PTENi (not shown) cells could not. The data in Fig. 5C show that PTEN depletion was efficient in the RNAi-infected cells. Fig. 5C also shows that Par-4 deficiency or PTEN knockdown increased pAkt levels in EFs, as expected. In addition, pAkt levels in the doubly deficient cells revealed an additive increase in Akt activity. Of great relevance from the point of view of our in vivo data shown in Fig. 4, the nuclear translocation of p65 (Rel A) in the double Par-4−/−/PTENi EFs synergistically increased as compared to that in Par-4−/− or PTENi cells (Fig. 5D). Taken together, these results demonstrate that the simultaneous inactivation of Par-4 and PTEN in vivo or in vitro leads to synergistically increased NF-κB levels in vivo in a cell-autonomous manner.
Fig. 5.
Synergistic proliferation, transformation, and NF-κB activation in cell cultures. (A) WT and Par-4−/− EFs were retrovirally infected with control shRNAi or an shRNAi specific for PTEN (PTENi), and growth curves were determined at different times. (B) Colony formation in soft agar from Par-4−/− EFs infected with control shRNAi or PTEN shRNAi (PTENi). The total number of colonies per plate was scored by counting and represented as mean ± SD of 6 plates from 2 independent experiments; representative picture showing the colony size difference (Inset). (C) Akt activation in response to serum (FCS) was increased in Par-4−/− EFs treated with PTEN shRNAi. EF extracts were analyzed with the different antibodies (as labeled) by immunoblotting. (D) NF-κB activation analyzed by immunofluorescence staining with anti-p65 (Rel A). The percentage of p65 nuclear-positive cells was determined by counting 10 fields for each experimental condition. Results are the mean ± SD. (E) Knockdown of Par-4 (Par-4i) was achieved by lentiviral infection in the prostatic epithelial cell line, PTEN-CaP2, and cell extracts were analyzed by immunoblotting with the different antibodies (as labeled). (F) qRT-PCR analysis of IL-6 mRNA levels in PTEN-CaP2 cells infected with control or Par-4 lentivirus (Par-4i). Results are shown as mean ± SD.
To further reinforce the above concept, we used the murine PTEN-null CaP2 prostate epithelial cell line (26). The knockdown of Par-4 by shRNA (Par-4i) lentiviral infection in these cells led to a detectable increase in pAkt as compared to control cells (Fig. 5E, Left), and more interestingly, to a robust activation of NF-κB (determined as phosphorylation of p65, IκB, and IKK) in the Par-4 knockdown cells as compared to the control cell line (see Fig. 5E, Middle and Right). Consistent with this, levels of IL-6 mRNA, a bona fide NF-κB-dependent gene, were also significantly activated in Par-4 knockdown cells (Fig. 5F). Interestingly, this enhanced expression of IL-6 in CaP2-Par-4i cells correlated with a higher level of Stat3 phosphorylation in these samples (see Fig. 5E, Right), in keeping with the notion that the IL-6 produced under these conditions is biologically active. These results suggest that the cooperation between Par-4 and PTEN is a cell-autonomous effect and that the activation of Akt and NF-κB are important contributors to their mechanism of action.
Functional Relevance of the NF-κB Pathway to the Mechanism of Action of Par-4 and PTEN Mutations.
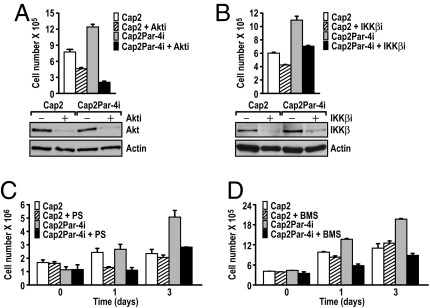
To demonstrate the functional contribution of activation of the Akt and NF-κB pathways in vivo, we tested whether the knockdown of Akt or IKKβ was sufficient to block the enhanced cell proliferation observed in the CaP2-Par-4i cells. Results in Fig. 6 A and B show that both pathways contribute to the proliferative advantage of CaP2-Par-4i cells, as siRNAs for Akt and IKK significantly inhibit this effect. To further test the functional role of the NF-κB activation in this system, we used 2 well-characterized highly specific small-molecule IKK pharmacological inhibitors, PS1145 and BMS-345541 (27, 28). Interestingly, treatment of CaP2-Par-4i cells with these molecules dramatically blocked cell growth in these cells (Fig. 6 C and D), consistent with the IKKβ sRNAi experiment. This suggests that activation of both pathways is responsible for the cooperative effect of Par-4 and PTEN inactivation in cell cultures in vitro, and it most likely accounts for the in vivo phenotype of the double mutant prostates.
Fig. 6.
Functional contribution of the NF-κB pathway in Par-4 and PTEN cooperation. (A and B) CaP2 or CaP2-Par-4i cells were treated with a control siRNA or with siRNAs specific for Akt (A) or IKKβ (B) and cell viability was determined by Trypan Blue exclusion 72 h after transfection. Cell extracts were analyzed in parallel by Western blot with antibodies for Akt, IKKβ, and actin (Lower). Results are the mean ± SD of triplicates. (C and D) Effect of treatment of CaP2 or CaP2-Par-4i cells with 2 inhibitors of the NF-κB pathway: PS1145 (10 μM) or BMS-345541(1 μM).
Discussion
The role of Par-4 in Akt regulation, and the fact that it is highly expressed in prostate (2, 9), opened the possibility that Par-4 could be a player in prostate cancer, as the Akt pathway has been shown to be relevant in this type of neoplasia in both mice and humans (29). Interestingly, we report here a frequent loss of Par-4 in human prostate cancers, often associated with aberrant promoter methylation, a situation reminiscent of what we have reported before in human endometrial cancer (8). Moreover, we observed a correlation between Par-4 and PTEN loss, as well as between Par-4 deficiency and high Gleason score. Taken together, this suggests that loss of the 2 tumor suppressors has a strong cooperative effect on human prostate carcinogenesis. This observation led us to reason that ablation of Par-4 in the context of PTEN heterozygosity should lead to aggressive forms of prostate cancer. The data we show here demonstrate that, in fact, this is the case, because the inactivation of Par-4 in the context of PTEN heterozygosity resulted in invasive adenocarcinoma that correlates with the additive activation of Akt and the synergistic stimulation of NF-κB in preneoplastic prostates. These 2 signaling cascades, NF-κB and Akt, have been mostly implicated in cell survival and, accordingly, we observed in castration experiments and TUNEL analysis that Par-4 deficiency has an important effect on the survival of prostate epithelial cells. This appears to be a proprietary characteristic of Par- 4 deficiency because it has not been reported for other tumor suppressors in combination with PTEN deficiency (20, 21, 25).
Notably, previous studies have demonstrated that both pathways, Akt and NF-κB, are deregulated in prostate cancer. In this regard, the activity of NF-κB is higher in androgen-independent cell lines and xenografts, as well as in metastatic prostate cancer compared with localized disease (30). Furthermore, increased NF-κB activation correlates with poor prognosis and predicts relapse (31). On the other hand, deregulated expression and mutations of PTEN occur with high frequency in prostate cancer, leading to aberrant activation of Akt and its downstream targets (29). However, not all of the phenotypes associated with PTEN loss can be fully explained by the activation of Akt (32), and it is increasingly apparent that PTEN possesses functions that are independent of its ability to suppress PI3K (32). Therefore, it is possible that PTEN deficiency in the context of cooperation with the inactivation of other tumor suppressors may set in place new molecular mechanisms to promote prostate tumorigenesis and progression. Our data demonstrate the existence of such a unique mechanism involving the synergistic induction of NF-κB because of the simultaneous inactivation of Par-4 and PTEN. Moreover, this effect is cell-autonomous and can be recapitulated in cultures of EFs and epithelial prostate cancer cells. Of note, blockade of the NF-κB pathway with an IKKβ sRNAi or with small-molecule inhibitors reverted the proliferative advantage of PTEN- and Par-4-deficient cells. More importantly, and consistent with these results, our IHC analysis of a set of human prostate cancer tumors showed that the loss of PTEN and Par-4 expression correlated with increased activation of the NF-κB pathway. An interesting aspect of these studies is that inactivation of Akt in these cell cultures also impaired cell proliferation in the Par-4/PTEN doubly inactivated cells, even though its activation in this context was not synergistic. These results could be interpreted to mean that Akt is necessary, but might not be sufficient, to drive the fully invasive prostate cancer phenotype, which would require the synergistic activation of NF-κB. Consistent with this notion are previously published observations that the transgenic expression of activated Akt in prostate is not sufficient to drive the invasive phenotype (22). Data from Baldwin's laboratory suggest the existence of a link between Akt and NF-κB in PTEN-deficient prostate cancer cells (33). Future studies should address the potential role of Akt as a permissive step in the synergistic activation of NF-κB during prostate cancer progression from a benign phenotype to more invasive stages.
In summary, our data establish a unique paradigm whereby Par-4 and PTEN mutations show accelerated tumor progression through the cooperation of the loss of these tumor suppressors in prostate carcinogenesis by the activation of the Akt and NF-κB cascades. Therefore, the use of NF-κB inhibitors alone or in combination with PI3K/Akt targeted molecules might be a promising therapeutic strategy in prostate cancers where both tumor suppressors have been inactivated.
Materials and Methods
Mice.
Par-4−/−, PKCζ−/−, and PTEN+/− mice were described previously (7, 34, 35). All mice were born and kept under pathogen-free conditions. Animal handling and experimental procedures conform to institutional guidelines (University of Cincinnati Institutional Animal Care and Use Committee, and Guidelines for Humane Endpoints for Animals Used in Biomedical Research at the Spanish National Cancer Research Center). All genotyping was done by PCR.
Histological Analysis.
Prostates were dissected and fixed in 10% neutral buffered formalin for 24 h, dehydrated, and embedded in paraffin. Sections (5 μm) were cut and stained with H&E. An extended section of Materials and Methods is provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Maryellen Daston for editing this manuscript, Glenn Doerman for preparing the figures, and Lyndsey Cheuvront for technical assistance. This work was funded in part by the University of Cincinnati-Consejo Superior de Investigaciones Cientificas Research Collaborative Agreement and by National Institutes of Health Grant R01-AI072581 (to J.M.). Research at the laboratory of M.S. is funded by the Spanish National Cancer Research Center and by grants from the Spanish Ministry of Education, the European Union (INTACT and PROTEOMAGE), and the “Marcelino Botin” Foundation. C.S. was supported by a contract from Instituto de Salud Carlos III/FIS and Fundacion Progreso y Salud, Consejeria de Salud Junta de Andalucia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813055106/DCSupplemental.
References
- 1.Lee JT, et al. Targeting prostate cancer based on signal transduction and cell cycle pathways. Cell Cycle. 2008;7:1745–1762. doi: 10.4161/cc.7.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sells SF, et al. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 3.Moscat J, Diaz-Meco MT. Par-4 keeps the atypical PKCs at bay. Cell Cycle. 2003;2:71–72. [PubMed] [Google Scholar]
- 4.Diaz-Meco MT, et al. The product of Par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 5.Lafuente MJ, et al. Regulation of mature T lymphocyte proliferation and differentiation by Par-4. EMBO J. 2003;22:4689–4698. doi: 10.1093/emboj/cdg460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Cao I, et al. Tumour-suppression activity of the proapoptotic regulator Par4. EMBO Rep. 2005;6:577–583. doi: 10.1038/sj.embor.7400421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Cao I, et al. Genetic inactivation of Par4 results in hyperactivation of NF-kB and impairment of JNK and p38. EMBO Rep. 2003;4:307–312. doi: 10.1038/sj.embor.embor769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno-Bueno G, et al. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67:1927–1934. doi: 10.1158/0008-5472.CAN-06-2687. [DOI] [PubMed] [Google Scholar]
- 9.Joshi J, et al. Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis. EMBO J. 2008;27:2181–2193. doi: 10.1038/emboj.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- 12.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 13.McMenamin ME, et al. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 14.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 15.Sun H, et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ML, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trotman LC, et al. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA. 2002;99:2884–2889. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai F, Pei XH, Pandolfi PP, Xiong Y. p18 Ink4c and Pten constrain a positive regulatory loop between cell growth and cell cycle control. Mol Cell Biol. 2006;26:4564–4576. doi: 10.1128/MCB.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 22.Majumder PK, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci USA. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 24.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Kim MJ, et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA. 2002;99:2884–2889. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao J, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 27.Yemelyanov A, et al. Effects of IKK inhibitor PS1145 on NF-kappaB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene. 2006;25:387–398. doi: 10.1038/sj.onc.1209066. [DOI] [PubMed] [Google Scholar]
- 28.Burke JR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 29.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 30.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 31.Lessard L, et al. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res. 2006;12:5741–5745. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- 32.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 33.Dan HC, et al. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leitges M, et al. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 35.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.