Abstract
Organelle movement is essential for proper function of living cells. In plants, these movements generally depend on actin filaments, but the underlying mechanism is unknown. Here, in Arabidopsis, we identify associations of short actin filaments along the chloroplast periphery on the plasma membrane side associated with chloroplast photorelocation and anchoring to the plasma membrane. We have termed these chloroplast-actin filaments (cp-actin filaments). Cp-actin filaments emerge from the chloroplast edge and exhibit rapid turnover. The presence of cp-actin filaments depends on an actin-binding protein, chloroplast unusual positioning1 (CHUP1), localized on the chloroplast envelope. chup1 mutant lacked cp-actin filaments but showed normal cytoplasmic actin filaments. When irradiated with blue light to induce chloroplast movement, cp-actin filaments relocalize to the leading edge of chloroplasts before and during photorelocation and are regulated by 2 phototropins, phot1 and phot2. Our findings suggest that plants evolved a unique actin-based mechanism for organelle movement.
Keywords: actin filament, chloroplast photorelocation, chloroplast unusual positioning1 (CHUP1), organelle movement, phototropin
Organelle movement is ubiquitous and essential for basic cellular functions in eukaryotes, including animals, fungi, and plants. Many systems of organelle movement are based on the actin cytoskeleton. Two mechanisms of actin-based organelle movement have been identified, mainly in animals and in yeast (1). One depends on myosin, which binds organelle cargos in its tail domain and transports them by sliding on actin cables (2). The other depends on the Arp2/3 complex, which can nucleate actin filaments and form complex filament arrays. Arp2/3 complex-dependent actin polymerization at the organelle edge (that is, actin “comet tail” formation) generates the motive force to push the organelle (3).
Various organelle movements in plants have been shown to depend on actin filaments by inhibitor studies (4). Among plant organelle movements, chloroplast movement is the best-characterized response, and is therefore a good experimental system for studying the mechanisms of organelle movement (5). Chloroplasts relocate in response to external stimuli, particularly light. Weak light induces a chloroplast accumulation response so that light is captured efficiently for photosynthesis. Strong light induces an avoidance response to evade photodamage (5, 6). Through molecular genetic analysis using Arabidopsis thaliana, it was demonstrated that 2 phototropin blue light receptors (phot1 and phot2) redundantly mediated the accumulation response, and phot2 alone regulated the avoidance response (7–9). However, the mechanism of light regulation of chloroplast movement remains to be determined.
Except in very rare cases, such as a moss species, most land plant species use actin filaments exclusively (rather than microtubules) for chloroplast movement (5). Anti-actin drugs (but not anti-microtubule drugs) inhibit chloroplast movement in various green plant species (5). Interaction of chloroplasts with actin filaments was observed by actin labeling in various plant species such as a fern Adiantum capillus-veneris (10) and A. thaliana (11) and in vitro cosedimentation assays using isolated spinach chloroplasts (12).
Through molecular genetic analysis of an A. thaliana mutant impaired in chloroplast photorelocation and positioning, chloroplast unusual positioning1 (CHUP1) was identified as a possible factor linking chloroplasts to actin filaments (6, 13). The CHUP1 gene encodes a chloroplast outer membrane protein with an actin-binding motif shown to bind not only F-actin but also G-actin or profilin in vitro (13–15). However, the pattern and dynamics of chup1 cytoplasmic actin cables were not grossly different from those observed in WT (13). Therefore, the mechanism by which an actin-based system drives chloroplast movements remains obscure.
In the present study, we performed detailed analyses of actin dynamics during chloroplast photorelocation by live cell imaging of Arabidopsis plants in which actin was visualized by GFP fluorescence labeling techniques using a custom-made microscope equipped with a microbeam irradiation unit to induce precise chloroplast movement.
Results
Chloroplasts Associate with Short Actin Filaments (Cp-Actin Filaments) and Their Biased Relocalization Is Coupled to Light-Directed Chloroplast Movements.
Using transgenic Arabidopsis plants expressing the GFP-mouse talin fusion protein (13) (and also tdTomato-fimbrin fusion protein), we analyzed actin dynamics in vivo in leaf petiole or mesophyll cells. Under intermittent GFP excitation (≈7.5 × 103 μmol m−2s−1 for 1 s) at 3-s intervals while chloroplasts were stationary, the fluorescence images revealed the presence of short actin filaments around the chloroplast peripheral region (referred to herein as chloroplast-actin filaments, abbreviated “cp-actin filaments”) (Fig. 1A Inset, and Movie S1 and Movie S2) as well as cytoplasmic actin cables. Cp-actin filaments demonstrated a complex pattern of dynamics, appearing and disappearing very rapidly in a nonsynchronous manner.
Fig. 1.
Biased cp-actin filament relocalization during chloroplast photorelocation movement. (A) Chloroplast avoidance response was induced by a high-intensity blue microbeam (377 μmol m−2 s−1) (10 μm in width). Arrowheads represent biased cp-actin filaments that were easily seen. (Inset) Nonbiased cp-actin filaments before chloroplast movement. (B and C) Magnified images of dynamics of cp-actin filaments in 2 chloroplasts indicated with arrows in A. Red circles, the position of moving chloroplasts; MB, the microbeam-irradiated area. (D) Movements of 4 chloroplasts during an avoidance response. Two control chloroplasts outside the irradiated area are indicated by cyan and yellow. Two chloroplasts in the irradiated area are indicated by blue and magenta. (Left Inset) shows the chloroplast positions at 5-min intervals after irradiation. Main image shows velocities. (Right Inset) shows the difference of GFP-talin fluorescence between the front and rear halves of the chloroplasts. (E) Reorganization of cp-actin filaments (indicated in black) during avoidance movement induced by continuous blue GFP excitation light irradiation. Numbers, time in seconds after irradiation. Blue and pink circles, the positions where GFP fluorescence intensities were recorded in F. (F) Change in GFP fluorescence intensity at the front and rear of the chloroplast in E. (G) Correlation between biased localization of cp-actin filaments and chloroplast speed during avoidance movements.
We used a custom-made microscope to observe GFP fluorescence images simultaneously during chloroplast movement induced by microbeam irradiation of specific parts of the cell (Fig. 1 A–C and Movie S3). Before irradiation, the velocities of chloroplast movement were approximately zero. When irradiated continuously with a microbeam of high-intensity blue light (377 μmol m−2 s−1), chloroplasts in or near the irradiated area (i.e., numbers 1 and 2 in Fig. 1D, Left Inset) moved transiently at velocities up to 1 μm/min out of the irradiated zone (Fig. 1D), but chloroplasts outside of the irradiated area did not move (i.e., numbers 3 and 4 in Fig. 1D, Left Inset; they were ≈30 μm or farther away from the beam.). An avoidance response was also induced when blue GFP excitation light was given continuously (Fig. 1E and Movie S4 and Movie S5). Cp-actin filaments transiently disappeared immediately after the high-intensity blue light irradiation [Fig. 1 A–C and E (30 and 39 s)] but gradually reappeared at the leading edge of the chloroplasts [Fig. 1E (63 and 72 s) and F, and Movies S3–S5]. After a full accumulation of cp-actin filaments in the front region of chloroplast (Fig. 1E, 90 s), we observed the chloroplast envelope extending toward the direction of chloroplast movement under both transmission and electron microscopy, although only when the avoidance response was induced (Fig. S1). When the chloroplasts moved out of the beam and subsequently stopped, the cp-actin filaments became nonbiased and redistributed around the entire chloroplast periphery, similar to what was seen before microbeam irradiation (Fig. 1 B and C). Chloroplasts located farther away from the microbeam (such as numbers 3 and 4 in Fig. 1D, Left Inset) neither changed their motility (Fig. 1D) nor showed biased cp-actin filament distribution (Fig. 1D, Right Inset). The appearance of biased cp-actin filaments was also evident for chloroplasts that accumulated toward a low-intensity blue microbeam (3.8 μmol m−2 s−1) (Fig. S2).
Some of the fluorescence intensity reduction may be due to photobleaching. However, photobleaching is not the main cause for fluorescence reduction in the chloroplast under the high-intensity blue light. First, fluorescence reduction was transiently induced under continuous irradiation with high-intensity blue light, but the fluorescence increased shortly thereafter showing biased distribution (that is the generation of cp-actin filaments) (Fig. 1 and Movie S3 and Movie S4). If the reduced fluorescence is due to photobleaching, fluorescence recovery should not occur during continuous irradiation. Second, there was little, if any, reduction in fluorescence intensity in phot2 or phot1phot2 mutant plants (see Fig. 3 B and C), indicating that the reduced fluorescence intensity in response to high-intensity blue light is a physiologically relevant response.
Fig. 3.
Reorganization of cp-actin filaments during chloroplast photomovement in phot1, phot2, and phot1phot2 mutants. (A–F) Chloroplast movement was induced by continuous irradiation with a microbeam (MB; 10 μm in width). Cp-actin filament behaviors under high-intensity blue light (377 μmol m−2 s−1) were observed every 5 min (A–C). Those under low-intensity blue light (3.8 μmol m−2 s−1) were observed every 10 min (D–F). Red circles indicate moving chloroplasts. Blue circles indicate lack of transient disappearance of cp-actin filaments in phot2 mutant under high-intensity blue light. (G–J) Disappearance of cp-actin filaments after 5 min of high-intensity blue microbeam irradiation (30 μm in width) (G) and increase after 10 min of low-intensity blue microbeam irradiation (the same width) (H) occurred in the WT but not in the phot1phot2 double mutant. Motility of chloroplasts located inside the microbeam area was determined as the integrated distance traveled for 60 min under high-intensity (I) or low-intensity (J) blue light. Data of G–J are presented as means and SEs derived from 7 independent experiments.
Although the cytoplasmic actin cables may modulate chloroplast movement, their role in chloroplast movement appears to be minor. Light regulation of cytoplasmic actin cables was examined in WT and phot1phot2 and chup1 mutants to study the involvement of cytoplasmic actin cables in chloroplast movement (Fig. S3). No changes in label intensity were detected before and during microbeam irradiation (both inside and outside of the microbeam). Even when cytoplasmic actin cables were attached to moving chloroplasts, there was no observed correlation with the direction and/or timing of the chloroplast movement (Fig. 1 and Movie S4). More importantly, phot1phot2 and chup1 mutants that lacked chloroplast photorelocation (9, 13) demonstrated cytoplasmic actin cable dynamics similar to those of WT plants (see Figs. 2G and 3 C and F).
Fig. 2.
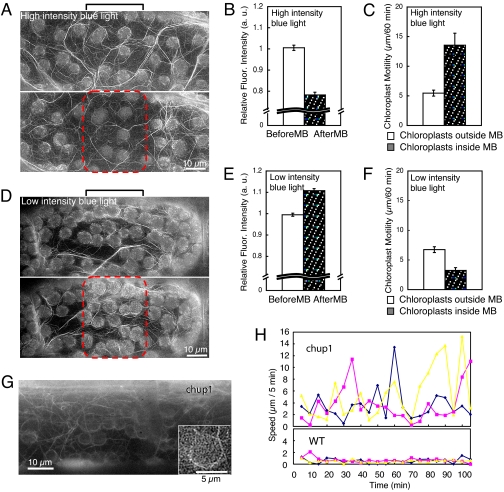
Cp-actin filament-dependent chloroplast motility. (A) Cp-actin filaments disappearance under high-intensity blue light before (Upper) and 5 min after (Lower) microbeam irradiation. (D) Cp-actin filaments increase under low-intensity blue light before (Upper) and 10 min after (Lower) microbeam irradiation. Microbeam, 30 μm in width as indicated by brackets and red dotted lines. (B and E) Changes in the relative amount of cp-actin filaments (based on fluorescence intensity) before and after blue light irradiation. (C and F) Motility of chloroplasts located inside and outside the microbeam. The integrated distance traveled for 60 min under high- or low-intensity light was determined. Data in B, C, E, and F indicate a mean and SEs of 9 chloroplasts derived from 3 independent experiments, respectively. (G) The absence of cp-actin filaments in the chup1 mutant. (Inset) High magnification of a chloroplast without cp-actin filments. (H) Chloroplast motility along cytoplasmic streaming is high in chup1 mutants (Movie S9) compared with WT. Each colored curve represents an independent chloroplast.
Similarly, visualization of microtubules using a GFP-α tubulin line revealed that microtubule organization did not correlate with chloroplast distribution or photorelocation (Movie S6). Furthermore, depolymerization of microtubules with 5 μM oryzalin had no effect on chloroplast movement, indicating that microtubules are not involved in chloroplast movement. Taken together, these findings suggest that chloroplast movement is correlated with cp-actin filaments and suggest that biased cp-actin filaments may be a prerequisite for generating the motive force required for subsequent chloroplast movement.
When the differences in GFP fluorescence intensities between the front and rear halves of chloroplasts were plotted against the speeds of chloroplast movement, a close correlation between the 2 was evident for both avoidance (Fig. 1G) and accumulation responses (Fig. S2). The greater the blue light intensity, the greater the difference observed in GFP fluorescence intensities and the greater the speed of chloroplast movement (Fig. 1G).
Importantly, when we analyzed chloroplast photorelocation and behaviors of cp-actin filaments in Arp2/3 complex-deficient mutants (16), these mutants showed WT responses (Movie S7), indicating that the Arp2/3 complex is not involved in cp-actin filament polymerization and chloroplast movement.
A Relationship Between Nonbiased Cp-Actin Filaments and Chloroplast Anchoring.
When a wider microbeam (30 μm in width) of high-intensity blue light was continuously applied, chloroplasts inside the beam area remained there for extended periods of time (typically more than 1 h, Movie S8). The amount of cp-actin filaments was reduced during this period (Fig. 2 A and B), and chloroplast movement in random directions increased (Fig. 2C and Fig. S4A), suggesting that they detached from the plasma membrane. In contrast, under low-intensity blue light, cp-actin filaments increased in number (Fig. 2 D and E), and random motility was reduced (Fig. 2F and Fig. S4B). No apparent changes in cytoplasmic actin cables were observed under these conditions. These results indicate a strong correlation between cp-actin filament abundance and chloroplast motility.
CHUP1 was identified through molecular genetic analysis of chup1, a mutant with impaired chloroplast movement and positioning (6, 13). In the chup1 mutant, chloroplasts aggregated in clusters and did not show light-directed movement in response to microbeam irradiation (Movie S9). Cp-actin filaments were not detected in the chup1 mutant, although cytoplasmic actin cables appeared to be normal (Fig. 2G) (13). Chloroplast motility in the chup1 mutant was highly variable among cells, but chloroplasts moved rapidly via cytoplasmic streaming in many cells (Fig. 2H, Fig. S5, and Movie S9), a behavior not observed in WT cells (Fig. 2H). These findings reinforce the hypothesis that the function of cp-actin filaments is to connect chloroplasts to the plasma membrane. These findings suggest that CHUP1 may possibly function in cp-actin filament regulation of the chloroplast.
Phototropins Mediate Directional Photomovement and Anchoring of Chloroplasts by Regulating Cp-Actin Filaments.
Two phototropin blue light receptors (phot1 and phot2) redundantly mediate the accumulation response, and phot2 alone regulates the avoidance response (7–9). The phot1 mutant cells exhibited both accumulation and avoidance movement, and cp-actin filament dynamics were similar to WT (Fig. 3 A and D). phot2 mutant plants lacked avoidance movement but demonstrated an accumulation response under high-intensity light (Fig. 3B) and low-intensity light (Fig. 3E) with biased cp-actin filaments. Notably, the cp-actin filaments did not disappear under high-intensity light in the phot2 mutant (Fig. 3B, blue circles). Therefore, phot2 mediates transient cp-actin filament disappearance in response to strong blue light. Neither photorelocation movements nor motility changes were induced in the phot1phot2 double mutant (Fig. 3C, F, I, J, and Fig. S5). Likewise, no initial loss in cp-actin filaments nor biased cp-actin filaments were observed in the microbeam experiments in the phot1phot2 double mutant (Fig. 3 C, F–H, and Fig. S5). These results further support the role of cp-actin filament reorganization in chloroplast movement and show that phototropin function provides a directional cue for cp-actin reorganization.
Cp-Actin Filaments Localize at the Interface Between the Chloroplast and the Plasma Membrane and Are Generated from the Edge of Chloroplasts.
To study the precise localization and possible polymerization dynamics of cp-actin filaments, we developed a protoplast system in which both accumulation and avoidance responses could be induced (Movie S10). Three-dimensional confocal microscopy analyses of GFP-talin fluorescence revealed that cp-actin filaments are only localized at the interface between the chloroplast and the plasma membrane, irrespective of biased or nonbiased distribution (Fig. 4A, Movie S11, and Movie S12).
Fig. 4.
Localization and polymerization dynamics of cp-actin filaments. (A) Three dimensional reconstruction of a chloroplast (shown with red chlorophyll autofluorescence) using photographs taken every 0.1 μm in the z axis by confocal microscopy and cp-actin filaments (shown with green fluorescence of GFP-talin). Distribution of cp-actin filaments at the plasma membrane side of chloroplasts (which is obvious from chloroplast shape) both in nonbiased and biased cp-actin filaments (Movie S10 and Movie S11, respectively). (B) Disappearance of cp-actin filaments under a high-intensity laser beam (1 mW) for GFP excitation (Movies S13). (C) Disappearance followed by reappearance of cp-actin filaments under a low-intensity laser beam (0.4 mW) (Movie S14). (D) Cp-actin filament images taken every 3 s (b1 and b2) or 10 s (c1) in rectangles B and C (see Movie S14) (E) Kymographs representing cp-actin disappearance (made of 120 photographs taken every 0.5 s on the white lines in b1 and b2 of B) and reappearance (made of 180 photographs taken every 1 s on the white lines in c1 and c2 of C) at the chloroplast edge. Arrows show chloroplast edges. The outside of the chloroplast is on the left. (F) Repeated appearance of cp-actin filaments at the same points of the chloroplast edge (shown with arrowheads). A protoplast was repeatedly observed by fluorescence microscopy for 30 s followed by 1–2-min incubation in the dark. Photographs were taken at the beginning (1, 3, 5, and 7) and ending (2, 4, 6, and 8) of the observation period. A 30-s observation caused cp-actin filament disappearance and a 1–2 min dark treatment allowed the recovery.
For precise analyses of behavior of cp-actin filaments using movies, photographs of a whole or part of a protoplast were obtained every 0.2 to ≈0.5 s under continuous irradiation with a laser-scanning beam for GFP image acquisition (1 mW by Diode 488–100; Carl Zeiss). If part of a protoplast was irradiated with the laser beam, the chloroplasts next to the beam-irradiated area showed a biased distribution of cp-actin filaments at the side of the chloroplast opposite to the beam (Fig. 4B and Movie S13). Cp-actin filaments were clearly observed at the beginning of microscopic examination (Fig. 4B) but gradually disappeared within 1 min (Movie S13). Cp-actin filaments shortened during this process toward the chloroplast periphery and finally disappeared at the chloroplast edge (Fig. 4 B and D-b1, and D-b2). Cp-actin filaments initially disappeared when the intensity of the laser beam was reduced (0.4 mW), but they reappeared after 30 s to 1 min at the same positions (Fig. 4 C and D-c1 and Movie S14). Appearances of cp-actin filaments and disappearances with respect to the chloroplast edge were clearly observed in the kymographs (Fig. 4E). The appearances and disappearances were repeatedly observed within a few minutes at the same positions (Fig. 4F, arrowheads) when protoplasts were treated with dark and strong light cycles (Fig. 4F). This finding indicates that cp-actin assembly sites exist on the chloroplast edge and have a rather long half-life (Fig. 4 C and F). Cp-actin filaments disappears (or shortens) centrifugally (Fig. 4D), suggesting that cp-actin filaments may have a polarity with respect to the edge of the chloroplast.
Discussion
Myosin-dependent transport along cytoplasmic actin cables and Arp2/3 complex-dependent comet-tailed movement are the prevailing mechanisms for actin-based organelle movement in animals and yeast (1). Like animals and yeast, plants have multiple myosins (17) and a full complement of Arp2/3 complex components (18). Recent comprehensive analyses of myosin mutant lines suggest that myosins are not involved in chloroplast photorelocation movement (19, 20), and we showed that mutants defective in Arp2/3 genes retained normal chloroplast photorelocation movement. Although redundant functions of multiple myosin isoforms and other actin-based mechanism cannot be excluded, the cp-actin filament-mediated chloroplast movement in plant cells reported here may represent an actin-based movement machinery that is distinct from those identified to date in other organelles. Note that the components of chloroplast movement identified are all plant-specific (5), and we showed here that the photoreceptor phototropin and an actin-binding protein CHUP1 played an pivotal role in mediating cp-actin filament regulation during chloroplast photorelocation movement.
Weak light induces chloroplast accumulation response, so that chloroplasts move toward and accumulate in the irradiated area. In chloroplasts outside the irradiated area, cp-actin filament reorganization was induced, and biased cp-actin filaments were formed on at the front of accumulating chloroplasts toward the irradiated area (Fig. S2). Thus, long-distance directional signals caused cp-actin filament reorganization on chloroplasts outside of the irradiated area. The biased cp-actin filaments induced by weak blue light for the accumulation response were formed in both the phot1 and phot2 mutants, but not in the phot1phot2 double mutant, indicating that both phot1 and phot2 can produce the directional signal necessary for cp-actin filament reorganizations during the accumulation response (Fig. 3). In response to strong light irradiation in WT plants, cp-actin filaments on irradiated chloroplasts disappeared, and chloroplasts demonstrated increased motility in random directions (Figs. 1 and 2 A–C). This disappearance was transient, but it continued until immediately before the chloroplasts began to avoid from the strong light. The speed of chloroplast movement during the avoidance response depended on light intensity and phot2 abundance (21). In this study, we also showed that the differences in the amount of cp-actin filaments at the front and the rear of chloroplasts were also dependent on light intensity. This relationship between the bias of cp-actin filaments and the rate of chloroplast movement provides strong support for a model in which blue light intensity determines the speed of directional chloroplast movement by regulating the difference in the amount of cp-actin filaments between the front and the rear of the chloroplasts.
Our results also shed light on an important role of cp-actin filaments in the stationary phase as well as during the movement of chloroplasts. Weak blue light induced an increase in cp-actin filaments on the entire periphery of chloroplasts in the irradiated area and this increase depended on both phot1 and phot2 (Fig. 3). This increase in cp-actin filaments under low light was associated with the decrease of chloroplast motility, and this decrease did not occur in phot1phot2 (Fig. 3). Moreover, in a chup1 mutant that lacks cp-actin filaments, chloroplasts were aggregated and moved rapidly via cytoplasmic streaming (Fig. 2 G and H). Finally, confocal microscopic analyses showed that cp-actin filaments only polymerized and localized at the interface between the chloroplast and the plasma membrane (Fig. 4). All these results strongly suggest a function of cp-actin filaments on chloroplast anchoring to the plasma membrane. Note that CHUP1 was localized on the outer envelope of the chloroplast and bound not only to F-actin but also to G-actin or profilin in vitro (13–15), suggesting that CHUP1 may possibly function in cp-actin filament regulation at the chloroplast envelope. Further analyses of cp-actin filaments will be required to understand how cp-actin filaments generate the motive force for chloroplast movement.
Materials and Methods
Plant Material and Growth.
Arabidopsis plants were grown in plastic dishes or pots under light/dark regimes at 23 °C. Light was provided by three 20-W white fluorescent tubes (FL20SW; Toshiba Lighting & Technology). The GFP-talin-expressing WT line (Col gl1) was the same as described in ref. 13. The GFP-talin-expressing lines were obtained in mutant backgrounds by genetic crossing. A tdTomato-fimbrin-expressing line was generated by transforming a construct containing tdTomato fused to the N-terminal region of an actin binding domain 2 of Arabidopsis fimbrin 1 (22). A GFP-α tubulin expression line was used to visualize microtubules in the WT background (Col gl1) (23). Inner cells of the adult leaf petiole containing chloroplasts were used for microbeam irradiation. Specimens were mounted in 0.22 M mannitol or silicone oil (KF96; Shin-Etsu Chemical). Arp2/3 mutants were provided by ABRC (SALK_003448 for ARP2 and dis1-1 for ARP3:16).
Microbeam Irradiation and Fluorescence Microscopy.
We developed a microscope system based on an Axiovert 200M inverted microscope (Carl Zeiss) that permits simultaneous microbeam irradiation and epifluorescence observation of cells. The equipment contains 2 optical systems: one for ordinal epifluorescence microscopy and the other for microbeam irradiation with the stimulus light. The system permits time-lapse observation of GFP fluorescence at defined intervals in epifluorescence mode, as well as continuous microbeam irradiation of cells with defined wavelengths and intensities (excluding the period of fluorescence image acquisition). Switching between the 2 modes was performed by turning a mirror that connected the 2 optical systems. GFP and chlorophyll autofluorescence were captured with Carl Zeiss XF 116–2 and FS17 filter units (band-pass filter BP515–565 was replaced with long-pass filter LP520), respectively, using a Planapochromat ×63 objective (NA 1.4; Carl Zeiss). The blue light stimulus for microbeam irradiation was obtained by filtering the light from a 100-W halogen lamp through an interference filter (peak wavelength, 451 nm; half band width, 32 nm; Optical Coatings). Microbeam irradiation was performed under a background of red light illumination. Red light was obtained by inserting an interference filter (peak wavelength, 662.2 nm; half band width, 5 nm; Optical Coatings) in the transmission microscopy light path. Because the blue excitation band for GFP overlaps with the effective wavelength of chloroplast photomovement, the excitation level was reduced as much as possible (estimated to be ≈7.5 × 103 μmol m−2s−1). GFP image acquisition intervals were also set so as not to interfere with photomovement. With a 5-min interval between fluorescence image acquisitions, the chloroplast avoidance movement was induced by 37.7–377 μmol m−2s−1 (10–100 Wm−2) blue light. The accumulation response was induced by 3.8 μmol m−2s−1 (1 Wm−2) blue light with a 10-min interval between fluorescence image acquisitions. Transmission and fluorescence images were captured with a cooled CCD camera (CoolSNAP HQ; Nippon Roper) controlled by MetaMorph software (Nihon; Molecular Devices). The images obtained were processed and analyzed with Object Image ver. 2.09 and ImageJ ver. 1.33.
Electron Microscopy.
Leaves were fixed in 2.5% glutaraldehyde, postfixed in 1% OsO4, dehydrated in acetone, and embedded in Spurr's resin. Thin sections were stained with 4% uranyl acetate and 0.4% lead citrate and examined with a transmission electron microscope (model H-7600; Hitachi).
Confocal Microscopic Analyses of Cp-Actin Dynamics in Mesophyll Protoplasts.
Mesophyll cell protoplasts were prepared from rosette leaves of 4-week-old WT plants treated with digestion medium (pH 5.7) containing 1% (wt/vol) cellulase R-10 (Yakult Honsya), 0.1% pectolyase Y-23 (Kyowa Chemical Products), 0.2% BSA, 20 mM KCl, 0.4 M mannitol, 10 mM CaCl2, and 20 mM Mes. Samples were digested by shaking (15 rpm) at 28 °C for 10 min and an additional 3 times (5 min each) with fresh digestion medium. Isolated protoplasts were washed 3 times with White's mineral salt solution (24) and incubated overnight at 4 °C before use. For microscopic observation, protoplasts were attached to poly-L-lysine-coated coverslips and mounted in White's mineral salt solution. Fig. 4A used a custom-made high-speed 3-dimensional confocal microscope (see ref. 25 for details), Fig. 4 B–E used a confocal microscope (LSM5 LIVE; Carl Zeiss), and Fig. 4F used a fluorescence microscope (Imager Z1; Carl Zeiss).
Supplementary Material
Acknowledgments.
We thank R.Y. Tsien (University of California, San Diego) and T. Hashimoto (Nara Institute of Science and Technology, Ikoma, Japan) for tdTomato construct and GFP-TUA6 line, respectively; Y. Sato for his help in a new microscope system construction; M. Kondo for electron-microscopy; M. Kimura (University of Tsukuba, Tsukuba, Japan) for a tdTomato-fimbrin line; and J.M. Christie and J. Silverthorn for critical reading of the manuscript. We also thank the Arabidopsis Biological Resource Center for seed stocks. This work was supported by a Grant-in-Aid for Scientific Research (17570042, 19039027, and 19570045 to A.K.; 13139203, 13304061, and 16107002 to M.W.; 20870030 to N.S.) and SORST, Japan Science and Technology Agency (T.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906250106/DCSupplemental.
References
- 1.Fehrenbacher KL, Bulldog IR, Pon LA. Taking the A-train: Actin-based force generators and organelle targeting. Trends Cell Biol. 2003;13:472–477. doi: 10.1016/s0962-8924(03)00174-0. [DOI] [PubMed] [Google Scholar]
- 2.Sellers JR. Myosins: A diverse superfamily. Biochim Biophys Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 3.Goley ED, Welch MD. The ARP2/3 complex: An actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 4.Wada M, Suetsugu N. Plant organelle positioning. Curr Opin Plant Biol. 2004;7:626–631. doi: 10.1016/j.pbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Suetsugu N, Wada M. Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol Chem. 2007;388:927–935. doi: 10.1515/BC.2007.118. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara M, et al. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- 7.Kagawa T, et al. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 8.Jarillo JA, et al. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, et al. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadota A, Wada M. Photoinduction of formation circular structures by microfilaments on chloroplasts during intracellular orientation in protonemal cells of the fern Adiantum capillus-veneris. Protoplasma. 1992;167:97–107. [Google Scholar]
- 11.Kandasamy MK, Meagher RB. Actin-organelle interaction: Association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskel. 1999;44:110–118. doi: 10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Kumatani T, et al. Possible association of actin filaments with chloroplasts of spinach mesophyll cells in vivo and in vitro. Protoplasma. 2006;229:45–52. doi: 10.1007/s00709-006-0189-8. [DOI] [PubMed] [Google Scholar]
- 13.Oikawa K, et al. Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell. 2003;15:2805–2815. doi: 10.1105/tpc.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oikawa K, et al. Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol. 2008;148:829–842. doi: 10.1104/pp.108.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt von Braun S, Schleiff E. The chloroplast outer membrane protein CHUP1 interacts with actin and profilin. Planta. 2008;227:1151–1159. doi: 10.1007/s00425-007-0688-7. [DOI] [PubMed] [Google Scholar]
- 16.Mathur J, Mathur N, Kernebeck B, Hülskamp M. Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell. 2003;15:1632–1645. doi: 10.1105/tpc.011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy ASN, Day IS. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2001;2:1–18. doi: 10.1186/gb-2001-2-7-research0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeks MJ, Hussey PJ. ARP2/3 and SCAR: Plants move to the fore. Nat Rev Mol Cell Biol. 2005;6:954–964. doi: 10.1038/nrm1765. [DOI] [PubMed] [Google Scholar]
- 19.Aviser D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV. Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. 2008;146:1098–1108. doi: 10.1104/pp.107.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peremyslov VV, Prokhnevsky AI, Aviser D, Dolja VV. Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol. 2008;146:1109–1116. doi: 10.1104/pp.107.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagawa T, Wada M. Velocity of chloroplast avoidance movement is fluence rate dependent. Photochem Photobiol Sci. 2004;3:592–595. doi: 10.1039/b316285k. [DOI] [PubMed] [Google Scholar]
- 22.Sheahan MB, Rose RJ, McCurdy DW. Organelle inheritance in plant cell division: The actin cytoskeleton is required for unbiased inheritance of chloroplasts, mitochondria and endoplasmic reticulum in dividing protoplasts. Plant J. 2004;37:379–390. doi: 10.1046/j.1365-313x.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- 23.Ueda K, Matsuyama T, Hashimoto T. Visualization of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma. 1999;206:201–206. [Google Scholar]
- 24.Kagawa T, Wada M. Light-induced nuclear positioning in prothallial cells of Adiantum capillus-veneris. Protoplasma. 1993;177:82–85. [Google Scholar]
- 25.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.