Abstract
Natural killer (NK) cells are lymphocytes of the innate immune system able to recognize and kill tumors lacking self-MHC class I molecules. This “missing-self” recognition is mediated by the lack of engagement of MHC class I-specific inhibitory NK cell receptors that include the killer cell Ig-like receptors (KIR) in humans and Ly49 molecules in mice. A promising immunotherapeutic strategy against MHC class I+ cancer cells is to block NK cell inhibitory receptors using monoclonal antibodies (mAb). However, interactions between MHC class I molecules and their inhibitory receptors are also required for the acquisition of NK cell functional competence, a process referred as to “education.” In addition, inhibitory receptors are involved in self-tolerance on educated NK cells. Here, we developed a preclinical mouse model in which all NK cells are educated by a single transgenic inhibitory receptor, human KIR2DL3, through the engagement with its HLA-Cw3 ligand. This approach revealed that NK cells could be reprogrammed to control the development of mouse syngenic tumors in vivo. Moreover, in vivo anti-KIR mAb treatment induced the killing of HLA+ target cells without breaking self-tolerance. Finally, the long-term infusion of anti-KIR mAb neither abolished NK cell education nor tumor cell recognition. Therefore, these results strongly support the use of inhibitory receptor blockade in cancer patients.
Keywords: anti-tumor therapy, innate immunity, pre-clinical model, tolerance
Natural killer (NK) cells are lymphocytes of the innate immune system, initially identified by their capacity to kill tumors. They are also involved in antimicrobial responses and act as regulatory cells during inflammation (1). NK cell effector functions include direct cytotoxicity, as well as cytokine and chemokine productions (e.g., IFN-γ). NK cell activation is regulated by an array of stimulatory and inhibitory cell surface receptors that sense potential target cells. Inhibitory receptors include several killer cell Ig-like receptors (KIR) in humans, Ly49 molecules in mice, and CD94/NKG2A heterodimers in both species, which recognize respectively classical and nonclassical major histocompatibility complex (MHC) class I molecules, constitutively expressed by most nucleated cells (2–5). MHC class I-specific inhibitory receptors and their ligands (H-2 in mice and HLA in humans) are highly polymorphic molecules encoded by multigenic, multiallelic families of genes that are inherited independently. NK cells have thus to discriminate self in a context where self-molecules differs from individuals to individuals. Like T lymphocytes, NK cells are educated to self versus altered-self discrimination, but the molecular strategies involved in this education are different. T cell education involves the stimulatory T cell receptor whereas NK cell education is mediated through the engagement of the MHC class I-specific inhibitory receptors (4, 6–10). This education, also termed “licensing,” leads to the maturation of a NK cell functional repertoire (i.e., the ensemble of stimulation toward which NK cells are reactive), which is adapted to self-MHC class I environment (4, 9–11). Consequently, NK cells in MHC class I-deficient hosts are hyporesponsive to stimulatory receptor stimulation and thereby tolerant to self. In physiological conditions, 2 types of self-tolerant NK cells coexist: functionally competent NK cells, whose effector responses are inhibited by the recognition of self MHC class I molecules, and hyporesponsive NK cells that cannot detect self-MHC class I (9, 10). NK cell education does not result in an on/off switch, but rather in a quantitative tuning of NK cell responsiveness: The more inhibitory receptors recognizing self-MHC class I are expressed, the more NK cells are responsive to cells lacking self-MHC class I (11–15).
The molecular mechanisms underlying the MHC-dependent NK cell education are still unknown, but have been shown in mice to require a functional immunoreceptor tyrosine-based inhibitory motif in the intracytoplasmic tail of Ly49 inhibitory receptors (6).
Several studies have suggested that the manipulation of NK cell missing-self recognition may have important clinical benefit in leukemic patients (16–19). In particular, retrospective studies of KIR-HLA mismatched stem cell transplantation in acute myeloid leukemia patients showed that the lack of KIR engagement on donor NK cells by patient MHC class I molecules was associated with a significant reduced risk for leukemia relapse (20, 21). The manipulation of NK cell alloreactivity in these settings implies haploidentical hematopoietic transplantations, which are associated with considerable adverse effects, including graft versus host disease mediated by allogenic T cells. A safer strategy would be to block NK cell inhibitory receptors in an autologous setting (17). Such a strategy is currently tested in phase I clinical trials with a fully human mAb (1–7F9). This mAb recognizes KIR2D inhibitory receptors and blocks their interaction with the human MHC class I molecules HLA-C, leading to NK cell-mediated lysis of leukemic cells (38). However, one of the main concerns for using this therapeutic approach in humans is the risk of generating a strong reactivity against normal self-tissues. Here, we report the generation of transgenic mice expressing a single inhibitory KIR in the context of its HLA ligand and in absence of endogenous mouse MHC class I molecules. These mice were used to manipulate NK cell education and to serve as a preclinical model for testing the effects of anti-KIR mAb treatment in vivo.
Results and Discussion
In Vivo Coexpression of KIR2DL3 and HLA-Cw3 Leads to Mouse NK Cell Education.
Mouse inhibitory receptors are not uniformly expressed on NK cells leading to a heterogeneous population of NK cells that are not equally educated by self-MHC class I (7, 11). In a C57BL/6 (B6) background, 10%–15% of NK cells express no inhibitory receptors for self-MHC class I (7), whereas subsets of NK cells express 1, 2, or 3 inhibitory receptors for self-MHC class I (i.e., Ly49C, Ly49I, or NKG2A). This variegated expression leads to a high diversity of functional competence among NK cell subsets. Indeed, the more Ly49 receptors compatible with MHC molecules of a given mouse strain are expressed on NK cells, the more they are responsive (11, 15). To overcome this complexity, we generated a genetic model in which all NK cells are equal regarding the MHC class I inhibitory signal they receive during education. In H2-Kb, H2-Db double knock out (KO) mice (KbDbKO) on a B6 background, NK cells are hyporesponsive because of the lack of engagement of their inhibitory receptors (6, 7). These KbDbKO mice were crossed to transgenic mice for a human KIR, KIR2DL3 (TgKIR), and to transgenic mice expressing the HLA-Cw3 molecule, one of the KIR2DL3 ligands (TgHLA). Consistent with previous results obtained in TgKIR/HLA mice (22), all NK cells in double transgenic double KO mice (KbDbKO-TgKIR/HLA) homogenously express KIR2DL3. Moreover, HLA-Cw3 is ubiquitously expressed and parallels that of H-2 molecules in WT mice (23). Thus, in KbDbKO-TgKIR/HLA mice, all NK cells express only 1 inhibitory receptor specific for human MHC class I, from which they should receive the same educating signals.
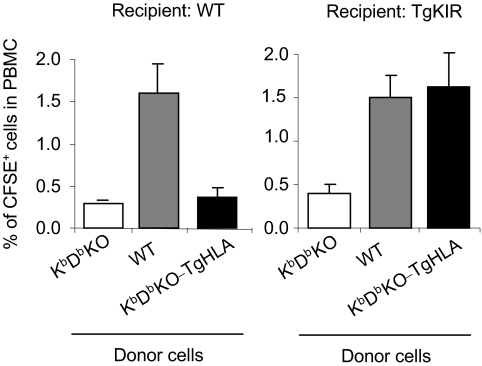
Our previous studies on TgKIR mice indicated that KIR2DL3 acts as an inhibitory receptor in mouse cells (22, 24). To confirm these results in different settings, we performed in vivo transfer experiments of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled cells. Splenocytes from KbDbKO, WT, and KbDbKO-TgHLA mice were adoptively transferred to WT or TgKIR recipients. The ability of NK cells to reject mouse cells lacking mouse or human MHC class I molecules was assessed in spleen and blood of recipient mice 40 h after injection (Fig. 1 and supporting information (SI) Fig. S1). These experiments showed that the rejection of KbDbKO cells by TgKIR mice is reversed by the transgenic expression of HLA-Cw3 on injected cells. In contrast, both KbDbKO and KbDbKO-TgHLA splenocytes were rejected in WT animals showing that HLA molecules are not recognized by mouse inhibitory receptors. Therefore, the engagement of transgenic KIR is sufficient to inhibit mouse NK cell activating pathways similarly to MHC class I specific mouse inhibitory receptors, validating this genetic model for further investigations on NK cell education.
Fig. 1.
Engagement of KIR2DL3 by its ligand HLA-Cw3 protects target cells from lysis in vivo. WT, KbDbKO, and KbDbKO-TgHLA were stained with the fluorescent dye CFSE, mixed at an equal ratio, and transferred into WT or TgKIR recipients. Mice were killed 40 h after injection, and the percentage of CFSE-labeled cells in peripheral blood cells was assessed by flow cytometry. 3 mice per group (number of experiments = 4).
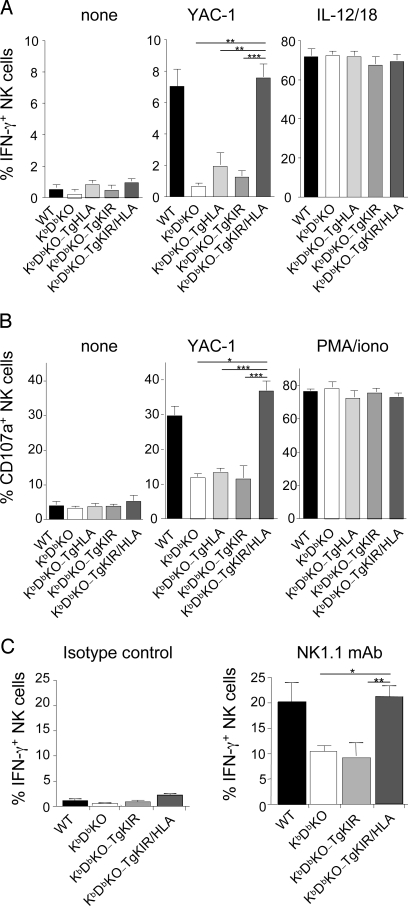
A result of education resides in the increase of NK cell ex vivo responsiveness (IFN-γ production or granule-dependent cytotoxicity) toward tumor targets or after activating receptor stimulation (6–8). To assess the ability of a human KIR to promote NK cell education in vivo in the presence of its ligand, we compared NK cell responses in WT, KbDbKO, and KbDbKO-TgKIR/HLA mice. KbDbKO-TgKIR and KbDbKO-TgHLA mice were used as controls. Splenic NK cell functions were tested ex vivo using cross-linking of the NK1.1 activating receptor, the tumor cells YAC-1, interleukin (IL)-12 and IL-18 or phorbol 12-myristate 13-acetate (PMA), and ionomycin (Fig. 2). As expected, KbDbKO NK cells were hyporesponsive to stimulation with the tumor YAC-1 (Fig. 2 A and B) as judged by both IFN-γ production and degranulation (i.e., CD107a cell surface exposure, a surrogate marker of NK cell cytotoxicity). The single transgenic expression of human KIR or HLA did not modify the responsiveness of mouse KbDbKO NK cells (Fig. 2 A and B). In contrast, when both molecules were coexpressed in the same animals, NK cell reactivity was comparable with that of WT NK cells. Mouse NK cells could thus be genetically reprogrammed for activation by tumor cells using a human inhibitory receptor and its HLA ligand.
Fig. 2.
In vivo induction of NK cell education by KIR2DL3 and HLA-Cw3 interactions. Splenocytes from WT, KbDbKO, KbDbKO-TgHLA, KbDbKO-TgKIR, and KbDbKO-TgKIR/HLA were isolated and stimulated 4 h in vitro by the tumor cell YAC-1, a mix of IL-12 and IL-18, a mix of PMA and ionomycin, anti-NK1.1 mAb-, or isotype control-coated plates. NK cell degranulation (CD107a cell surface exposure) (B) and intracellular IFN-γ production (A and C) were measured by multiparameter FACS analysis on NK1.1+CD3− cells (A and B) or NKp46+CD3− cells (C). The results (mean ± SEM) of 9 experiments including WT mice (n = 7), KbDb mice (n = 3), KbDb-TgHLA mice (n = 5), KbDb-TgKIR mice (n = 7), and KbDb-TgKIR/HLA mice (n = 10) are represented. Statistical analysis was performed using a nonparametric Mann–Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NK1.1 (Nkrp1c) is an activating receptor expressed by all mature NK cells in B6 mice (3). Signaling through this receptor leads to INF-γ production and CD107a cell surface exposure in WT NK cells, but is impaired in uneducated NK cells (6, 7, 11). This cell-free system allows the assessment of the intrinsic responsiveness of the NK cells without possible interference with unknown inhibitory ligand potentially expressed on target cells. The surface density of NK1.1, detected by flow cytometry, was the same in KbDbKO-TgKIR/HLA, KbDbKO-TgKIR, KbDbKO-TgHLA, WT, and KbDbKO mice. As expected, NK cells from KbDbKO and KbDbKO-TgKIR mice were hyporesponsive to NK1.1 triggering (Fig. 2C). In contrast, NK cell IFN-γ production after NK1.1 stimulation was restored in KbDbKO-TgKIR/HLA animals (Fig. 2C). As KIR2DL3 directly interacts with HLA-Cw3 (25), these results showed that KIR-HLA interaction leads to NK cell education in vivo. KIR-HLA education modulated some, but not all, activating pathways. Indeed, NK cells were equally responsive to cytokine (IL-12 and IL-18) or PMA/ionomycin stimulation in all of the strains of mice tested (Fig. 2 A and B). These last results are consistent with our current knowledge of mouse NK cell education (6, 7), as KbDbKO and WT NK cells are also equally responsive to these stimulations (Fig. 2B). Of note, the frequency of reactive NK cells was not significantly increased in KbDbKO-TgKIR/HLA compared with WT mice despite the fact that they uniformly express an inhibitory receptor for self and should theoretically be all educated by HLA-Cw3. These results suggest that other signals regulate NK cell responsiveness. Along this line, cytokines like IL-15 or IL-18 involved in NK cell priming (26–28) may also act as limiting factors for NK cell reactivity in KbDbKO-TgKIR/HLA mice, as they do in WT animals.
Reprogramming of NK Missing-Self Recognition.
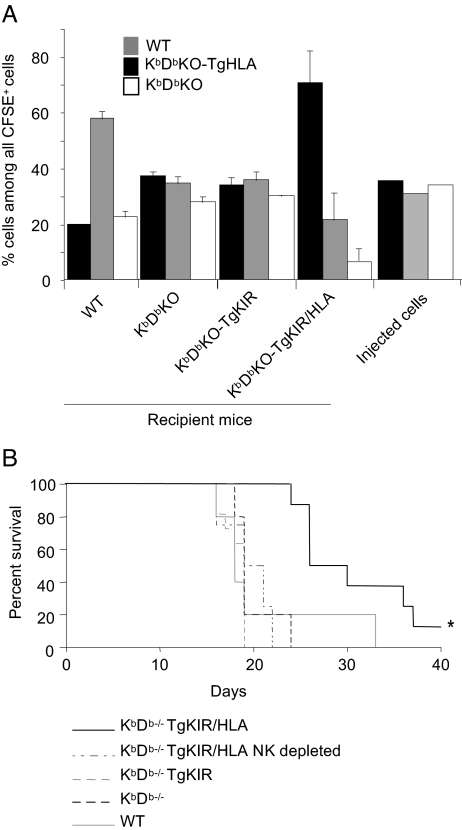
NK cell reprogramming was then tested in vivo. Upon transfer, healthy hematopoietic cells lacking self-MHC class I ligands are known to be rejected by NK cells (29, 30). We asked whether the education of mouse NK cells by KIR-HLA interaction was sufficient to confer them the ability to recognize and reject HLA− healthy cells. To address this point, we measured the in vivo rejection of HLA− splenocytes in KbDbKO-TgKIR/HLA mice. A mix of CFSE-labeled WT, KbDbKO, and KbDbKO-TgHLA splenocytes (Fig. S2) was adoptively transferred into KbDbKO and KbDbKO-TgKIR/HLA recipient mice. WT and KbDbKO-TgKIR recipients were used as positive and negative control, respectively. In vivo cytotoxicity was assessed 20–40 h after transfer in spleen (Fig. 3A). As expected, WT mice rejected both KbDbKO and KbDbKO-TgHLA cells, confirming that human HLA-Cw3 was not able to engage mouse inhibitory receptors. Uneducated KbDbKO NK cells as well as NK cells from KbDbKO-TgKIR spared the 3 subsets equally, indicating that the mere expression of an inhibitory receptor in absence of ligand interaction does not induce NK cell education. However, not only KbDbKO cells but also WT splenocytes were eliminated in the KbDbKO-TgKIR/HLA model, whereas HLA+ cells were recognized as self (Fig. 3A). The expression of KIR and HLA was thus sufficient to increase the NK cell repertoire to missing-HLA recognition, resulting in the killing of WT cells. Of note, we observed a slight trend toward a weaker elimination of WT cells than that of KbDbKO cells, suggesting that mouse Ly49 and CD94/NKG2A inhibitory receptors that can recognize mouse MHC class I molecules on WT CFSE-labeled cells might still be functional for the negative regulation of NK cell effector function, despite their lack of role in NK cell education in KbDbKO-TgKIR/HLA mice.
Fig. 3.
Reprogramming of missing-self recognition in vivo. (A) WT, KbDbKO, and KbDbKO-TgHLA splenocytes were stained with the fluorescent dye CFSE, mixed at an equal ratio, and transferred into WT, KbDbKO, KbDbKO-TgKIR, or KbDbKO-TgKIR/HLA recipients. Mice were killed 20–40 h after injection, and the percentage of each cell type among CFSE-labeled cells was assessed in blood lymphocytes of the recipients by flow cytometry (see Fig. S2 for details). A representative experiment (3 mice per group) of 3 is shown. Of note, in these experiments, cell counts of each of the 3 CFSE+ subsets recovered in spleen of KbDbKO and KbDbKO-TgKIR recipients was in the same range as the counts of CFSE+ autologous cells found in WT or in KbDbKO-TgKIR/HLA recipients, indicating that all of the 3 subsets were spared in these models where NK cells are hyporesponsive. (B) RMA tumor cells (104 cells) were transferred into WT, KbDbKO, KbDbKO-TgKIR, or KbDbKO-TgKIR/HLA mice or NK-cell-depleted KbDbKO-TgKIR/HLA mice at day 0. Mice survival was monitored for 40 days (cumulative results of 2 experiments are shown, n = 5 to 11 mice per group). *, P < 0.05 (Logrank test).
NK cells are known to recognize and kill tumors in vivo. Rauscher murine leukemia virus antigen (RMA) cells are T cell lymphomas from a B6 origin. A MHC class I-deficient variant of this cell line, RMA-S, is a well-characterized NK cell target (31). RMA cells thus express stimulatory ligands, but their NK-mediated lysis is inhibited by the engagement of inhibitory receptors by MHC class I molecules. To test whether KIR-HLA-educated NK cells would be able to control mouse tumor growth, a lethal dose of RMA cells was injected in KbDbKO-TgKIR/HLA, KbDbKO-TgKIR, KbDbKO, or WT mice. Monitoring the survival of the transferred animals, we observed an increased lifespan of ≈50% in KbDbKO-TgKIR/HLA mice when compared with KbDbKO-TgKIR controls (Fig. 3B). The survival curve of WT mice after RMA transfer almost paralleled that of KbDbKO or KbDbKO-TgKIR mice, showing that even if NK cells of WT mice are fully responsive (Fig. 2), MHC class I molecules (H-2b) expressed by RMA cells impaired their killing by NK cells. Despite the expression of H-2b-specific inhibitory receptors on a fraction of NK cells from KbDbKO-TgKIR/HLA, these NK cells were efficient in the antitumor response delaying the onset of the disease. This delay was abolished by pretreatment of KbDbKO-TgKIR/HLA with anti-NK1.1 antibodies, which deplete NK cells (Fig. 3B). The simplest interpretation of these data is that NK cells in KbDbKO-TgKIR/HLA mice were educated to missing-HLA recognition. Particularly, the KIR+Ly49C/I− NKG2A− NK cell subset should be responsible for this effect. We cannot formally exclude that these NK cells have acquired an additional stimulatory receptor specific for RMA. However, we show that educating signals mediated by KIR-HLA interactions conferred to NK cells the capacity to control the growth of a syngenic tumor expressing mouse MHC class I but lacking HLA.
Altogether, the results presented in Figs. 2 and 3 show that mouse MHC class I is not necessary for NK cell education, granting that other inhibitory receptor–ligand interactions are at work. Previous works have shown that human NK cell responsiveness was correlated with the expression of inhibitory receptor for self-MHC class I (8, 12, 13). We show here in a mouse model that in vivo engagement of an inhibitory KIR by its HLA ligand is sufficient to reprogram uneducated mouse NK cells into competent effector cells. Moreover, the expression of this inhibitory receptor confers to NK cells a new repertoire of recognition resulting in missing-HLA recognition. Two main models of NK cell education have been proposed (9, 10). According to the first one, called the “stimulatory” or “arming” model, NK cells are initially hyporesponsive and become educated when their Ly49 receptors engage self-MHC class I delivering possibly stimulatory signals. The second model, called the “inhibitory” or “disarming” model, proposes that NK cells are responsive by default and that upon persistent stimulation by unknown stimulatory receptor(s), the modulation of activation signals by inhibitory receptors is required to avoid hyporesponsiveness. We cannot discriminate between these 2 hypotheses. Nevertheless, our results unambiguously show that a human inhibitory KIR can branch to the mouse signaling pathways involved in NK cell education.
In Vivo Elimination of HLA+ Cells Upon Anti-KIR mAb Infusion.
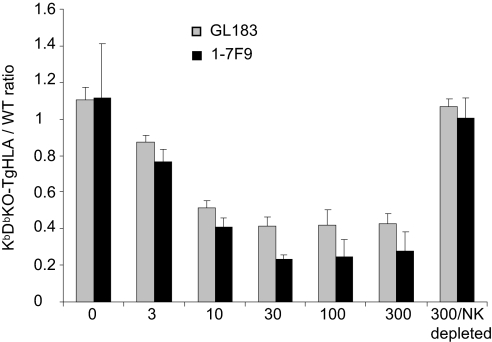
A fully human IgG4 anti-KIR2D mAb, 1–7F9, was generated with the aim of being used in anticancer therapy (38). Because the affinity of the well-characterized mouse IgG1 anti-KIR2DL3 mAb, GL183 (32), is comparable with that of 1–7F9 and the 2 mAb compete with each other on the KIR2DL3 molecule, we tested both mAbs in parallel. To assess the capacity of this antibody to promote HLA-Cw3+ target cell elimination in vivo, we performed adoptive transfers of a mix (1:1 ratio) of KbDbKO-TgHLA and WT splenocytes in TgKIR mice. To avoid the recognition of HLA-Cw3 by T and B cells, the experiments were performed using TgKIR mice bred onto a Rag-deficient background (RagKO-TgKIR). In this model, all NK cells expressed KIR2DL3 but were educated via mouse MHC class I specific receptors. The ratio between KbDbKO-TgHLA and WT donor splenocytes was detected by flow cytometry in recipients' spleen and blood 20 h after transfer (Fig. 4 and Fig. S3). Rag-TgKIR mice rejected KbDbKO cells, but were tolerant to KbDbKO-TgHLA and WT cells. In contrast, when mice were pretreated for 24 h with increasing doses of GL183 mAb, KbDbKO-TgHLA cells were eliminated in vivo (Fig. 4). NK cells mediated this effect, as it was abolished when NK cells were depleted with an anti-NK1.1 mAb (Fig. 4). In this assay, the blocking effect of 1–7F9 was equivalent to that of GL183 (Fig. 4). Thus, anti-KIR mAb infusion triggers missing-HLA recognition by educated NK cells in vivo.
Fig. 4.
1–7F9 and GL183 anti-KIR mAb induce the in vivo elimination of HLA+ cells in RagKO-TgKIR mice. WT and KbDbKO-TgHLA splenocytes were labeled with CFSE, mixed at an equal ratio, and transferred into RagKO-TgKIR recipients which were pretreated or not with increasing doses of the anti-KIR mAbs 1–7F9 or GL183 (from 3 to 300 μg per mouse). For a group of mice treated with the higher dose of antibodies (300 μg per mouse), NK cells were depleted with anti-NK1.1 mAbs 1 day before cell transfer. Recipient mice were killed 20 h after injection, and the percentage of each cell type among lymphocyte spleen cells of the recipients was assessed by flow cytometry. The mean ± SD of the ratio between KbDbKO-TgHLA and WT CFSE+ cells is shown (3 mice per group). Each titration experiment (1 for GL183 antibody and 2 for 1–7F9 antibody) included a total of 21 mice.
In vivo blocking of KIR with mAb does not break self-tolerance. Previous studies in the mouse have shown that infusion of anti-Ly49C/I blocking mAbs could be efficient against MHC class I+ syngenic tumors without major deleterious effect for the recipient mice (33–35). However, in these experiments, a F(ab′)2 fragment with short half-life and low affinity was used, and in WT B6 mice almost half of Ly49C/I+ NK cells also express the inhibitory receptor CD94/NKG2A that could be engaged by Qa-1, reducing the risk of self-reactivity. In humans, the size of KIR2D+ NK cell subsets is highly variable among individuals (4). In patients expressing several KIR2D and their ligands, the use of high-affinity, long half-life, anti-KIR2D mAbs could target the activation of a high number of NK cells and thus significantly increase the risk of autoimmunity. To evaluate the toxicity of anti-KIR mAb infusion, we used the KbDbKO-TgKIR/HLA model in which NK cells are monoclonal regarding their MHC class I-induced education and in which the blocking anti-KIR mAb bind to self-specific inhibitory receptors expressed on 100% of NK cells. We only used the mouse GL183 mAb and not the human 1–7F9 mAb in these long-term settings because the mouse immune system reacted against the human mAb, avoiding a complete saturation of KIR2DL3. KbDbKO-TgKIR/HLA mice were treated every 4 days with 400 μg of the anti-KIR2DL3 mAb GL183 during 3 weeks. GL183 was tolerated by the mouse immune system and allowed a complete KIR2DL3 saturation during the course of the treatment (Fig. S4). The blood hematopoietic compartment was analyzed at 4 time points (days 0, 7, 14, and 21) after the first injection. Mice treated with the vehicle only were analyzed in parallel. NK cell frequency in spleen and blood was not significantly modified, and, phenotypically, NK1.1, NKp46, and KLRG1 expression levels at NK cell surface were not significantly affected by mAb treatment. Multiple parameters were measured, including leukocyte, erythrocyte, and platelet counts, hemoglobinemia, hematocrit, mean corpuscular volume, red cell distribution width, and mean platelet volume (Table S1). Among leucocytes, the frequency of lymphocytes, monocytes, neutrophils, eosinophils, and basophils was also assessed (Table S2). Using these readouts, no adverse hematological effect was observed even after 21 days of KIR2DL3 saturation. In addition, mice did not present any sign of disease, and their weight remained stable during antibody infusion (Table S1). Finally, spleen cell counts did not vary significantly at any time during the treatment. Similar results were obtained when KbDbKO-TgKIR mice were treated with GL183 mAb. Thus, the blocking of inhibitory receptor in vivo using saturating concentration of mAbs did not induce detectable signs of autoimmune disorder.
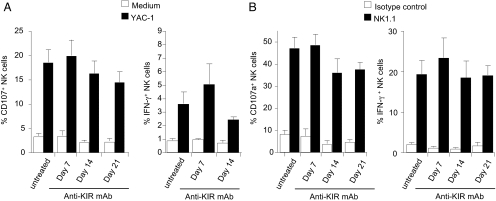
Long-Term Treatment with Anti-KIR mAb Does Not Abolish NK Cell Education.
Because KIR blocking in vivo did not induce detectable hematopoietic toxicity in KbDbKO-TgKIR/HLA model, NK cell tolerance was ensured. This result suggested either a modulation in NK cell education or that NK cells were not toxic enough to lead to clinical autoimmune symptoms in vivo, even when blocked through anti-inhibitory receptor. Answering these questions is critical for the use of blocking inhibitory receptors in clinical settings, because a potential negative effect of anti-KIR mAb on NK cell education could lead to a complete inhibition of NK cell functions, abolishing their antitumor effects. To address these issues, we tested whether the continuous KIR blocking over a long period (21 days) resulted in a reduction of NK cell reactivity against tumors. In KbDbKO-TgKIR/HLA mice the responsiveness (IFN-γ production and CD107a surface exposure) toward the tumor YAC-1 remained stable over the course of mAb treatment (Fig. 5A). Similar results were observed when NK cells were stimulated by NK1.1 cross-linking using mAb-coated plates (Fig. 5B). These data thus showed that despite the long-term blocking of the interaction between KIR and HLA, NK cell responsiveness was not drastically impaired. Anti-KIR mAb treatment in vivo thus did not abolish NK cell education.
Fig. 5.
Anti-KIR mAb treatment does not significantly impair NK cell function. KbDbKO-TgKIR/HLA mice were treated every 4 days by 400 μg of anti-KIR mAb GL183 starting at day 0. NK cell functions were assessed in spleen cells at days 7, 14, and 21. Splenocytes were stimulated 4 h in vitro with or without the tumor cell YAC-1 (A) or with anti-NK1.1 mAb-coated plates or isotype control-coated plates (B). NK cell degranulation (CD107a cell surface exposure) and intracellular IFN-γ production were measured by multiparameter FACS analysis on NK1.1+CD3− cells (A) or NKp46+CD3− cells (B). The results indicate mean ± SEM of 2 experiments (n = 4 to 6 mice per group).
It is somewhat surprising that after a 3-week-long blockade of KIR, NK cells retained responsiveness, because the KIR-HLA interaction is the only one that can theorically educate NK cells in these mice. A recent paper has shown an involvement of cis-interactions (i.e., engagement of inhibitory Ly49A and H-2Dd on the same cell) in NK cell education (36). This process might also apply to KIR-HLA interactions. In this case, the mAb may not be as efficient in inhibiting cis-interactions than trans-interactions, explaining why education is maintained upon antibody infusion in our model. Another possible explanation would be that education takes place in a compartment that is poorly accessible to mAb. Further studies will be needed to address this question.
In addition, the stimulatory signals provided to NK cells by activating ligands expressed on normal cells are likely to be qualitatively and/or quantitatively different as compared with those provided upon tumor encountering (or antibody-mediated receptor stimulation). These differences may account for the different outcomes regarding self-tolerance and reactivity to YAC-1 tumor cells observed in our experiments when inhibitory receptors were blocked. It is also possible that the isolation of normal splenocytes in vitro and their subsequent transfer in recipient mice (Fig. 1 and Fig. 3A) induced at their surface the up-regulation of stimulatory ligands that were not present at the steady state. Rejection is often partial in these models, and a fraction of cells may be altered during the in vitro processing, rendering them more sensitive to NK cells. Irrespective of these possibilities, it remains that anti-KIR treatment did not break self-tolerance and preserved NK cell responsiveness to tumors emphasizing the interest of this immunotherapy strategy. Along this line, the KIR repertoire in human is polyclonal and includes also stimulatory receptors (KIR-S). 1–7F9 mAb recognizes inhibitory KIR2DL1/L2/L3 but also KIR2DS1/S2 stimulatory molecules (38). We could not address the effect of the antibody binding to KIR-S receptors by using the KbDb-TgKIR/HLA model, but we have shown that KIR-2DS triggering on NK cells from human PBMC did not induce any significant activation (38). However, the effect of the antibody on KIR-2DS-expressing patients in vivo remains to be addressed.
In conclusion, using a genetic model of NK cell education, we showed that mouse NK cells could be educated with a human inhibitory receptor only in the context of engagement with its HLA ligand. This education “across species” was sufficient to reprogram NK cell cytotoxicity toward mouse tumors in vitro and in vivo. Moreover, the infusion of blocking anti-KIR mAbs did not break tolerance to self-hematopoietic cells and did not induce a detectable autoimmunity. Finally, the long-term blocking of inhibitory receptors by anti-KIR mAbs in vivo did not reverse their responsiveness toward tumors, at least in vitro. Further studies will be needed to test whether the in vivo elimination of tumors upon treatment with blocking antibodies is conserved to maintain clinical benefits and to evaluate the best protocols of mAb infusion (doses, kinetics of administration). However, these results strongly support the blockade of inhibitory NK cell receptors in cancer patients.
Materials and Methods
CFSE Assay for in Vivo Rejection of Target Cells.
This method for quantitative measurements of in vivo killing has been adapted from a previous study (37). Briefly, populations of spleen cells expressing or not a missing-self phenotype were labeled with 0.5 or 3 μM CFSE (Invitrogen) and mixed at 1:1 or at 1:1:1 ratios. In some experiments, an additional staining was performed using an anti-H-2Kb/Db mAb (28.8.6, mIgG2a, BD PharMingen) to discriminate 2 populations stained with 0.5 μM CFSE (Fig. S2). The ratio between the different populations before coinjection was determined by FACS analysis and compared with the ratio in the blood or spleen at various time points after inoculation, allowing a quantitative measurement of the rejection of donor cells in the recipients. Donors and recipients were sex-matched.
Antibody Treatments in Vivo.
Purified anti-KIR mAbs 1–7F9 (human IgG4) and GL183 (mouse IgG1) were injected i.v. at the indicated doses and time points. GL183 antibody recognizes KIR2DS2/L3/L2 molecules (32), and 1–7F9 is a fully human monoclonal antibody that recognizes KIR2DS1/S2/L1/L2/L3. Receptor saturation was tested 2 h after mAb injection in the peripheral blood and then when mice were killed for analysis at days 7, 14, or 21. For NK1.1 depletions, 100 μg of the PK136 (mIgG2a) antibody was given i.v. 24 h before the experiment.
SI.
See SI Materials and Methods for more details.
Supplementary Material
Acknowledgments.
We thank C. Beziers-Lafosse for graphic arts and F. Lemonnier and G. Hammerling for their gift of KdDb KO and TgHLA-Cw3 mice, respectively. The laboratory of E.V. and S.U. is supported by European Union FP6, LSHB-CT-2004-503319-Allostem, Ligue Nationale contre le Cancer (“Equipe labellisée La Ligue”), Agence Nationale de la Recherche, Institut National du Cancer, Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Ministère de l'Enseignement Supérieur et de la Recherche. E.V. is a scholar of the Institut Universitaire de France.
Footnotes
Conflict of interest statement: C.S., P.A., C.B., M.B., and N.F. are employees of Innate-Pharma. E.V. is a cofounder and shareholder of Innate-Pharma. F.R. is an employee, cofounder, and shareholder of Innate-Pharma. N.V. is an employee of Novo-Nordisk.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901653106/DCSupplemental.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Moretta L, Moretta A. Unravelling natural killer cell function: Triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 4.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL. NK Cell Recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity. 2006;24:249–257. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 11.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 12.Cooley S, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, et al. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 14.Johansson S, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201:1145–1155. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: The rheostat model. J Immunol. 2009;182:4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velardi A, Ruggeri L, Moretta A, Moretta L. NK cells: A lesson from mismatched hematopoietic transplantation. Trends Immunol. 2002;23:438–442. doi: 10.1016/s1471-4906(02)02284-6. [DOI] [PubMed] [Google Scholar]
- 17.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 18.Miller JS, et al. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;110:578–586. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JS, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 20.Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri L, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: Challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cambiaggi A, et al. Natural killer cell acceptance of H-2 mismatch bone marrow grafts in transgenic mice expressing HLA-Cw3 specific killer cell inhibitory receptor. Proc Natl Acad Sci USA. 1997;94:8088–8092. doi: 10.1073/pnas.94.15.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dill O, Kievits F, Koch S, Ivanyi P, Hämmerling GJ. Immunological function of HLA-C antigens in HLA-Cw3 transgenic mice. Proc Natl Acad Sci USA. 1988;85:5664–5668. doi: 10.1073/pnas.85.15.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ugolini S, et al. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat Immunol. 2001;2:430–435. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 25.Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature. 2000;405:537–543. doi: 10.1038/35014520. [DOI] [PubMed] [Google Scholar]
- 26.Chaix J, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schleicher U, et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bix M, et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature Article. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 30.Hoglund P, Glas R, Ohlen C, Ljunggren HG, Karre K. Alteration of the natural killer repertoire in H-2 transgenic mice: Specificity of rapid lymphoma cell clearance determined by the H-2 phenotype of the target. J Exp Med. 1991;174:327–334. doi: 10.1084/jem.174.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 32.Ciccone E, et al. Involvement of HLA class I alleles in NK cell specific funtion: Expression of HLA-Cw3 confers selective protection from lysis by alloreactive NK clones displaying a defined specificity (specificity 2) J Exp Med. 1992;176:963–971. doi: 10.1084/jem.176.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh CY, et al. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3137. doi: 10.1182/blood.v97.10.3132. [DOI] [PubMed] [Google Scholar]
- 34.Koh CY, Ortaldo JR, Blazar BR, Bennett M, Murphy WJ. NK-cell purging of leukemia: Superior antitumor effects of NK cells H2 allogeneic to the tumor and augmentation with inhibitory receptor blockade. Blood. 2003;102:4067–4075. doi: 10.1182/blood-2003-04-1367. [DOI] [PubMed] [Google Scholar]
- 35.Koh CY, et al. NK inhibitory-receptor blockade for purging of leukemia: Effects on hematopoietic reconstitution. Biol Blood Marrow Transplant. 2002;8:17–25. doi: 10.1053/bbmt.2002.v8.pm11846352. [DOI] [PubMed] [Google Scholar]
- 36.Chalifour A, et al. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Oberg L, et al. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo - role of KARAP/DAP12-dependent and -independent pathways. Eur J Immunol. 2004;34:1646–1653. doi: 10.1002/eji.200424913. [DOI] [PubMed] [Google Scholar]
- 38.Romagné F, et al. Pre-clinical characterization of 1-7F9, a novel human anti-KIR therapeutic antibody that augments NK-mediated killing of tumor cells. Blood. 2009 doi: 10.1182/blood-2009-02-206532. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.