Abstract
Evaluation of glycosaminoglycan (GAG) concentration in articular cartilage is of particular interest to the study of degenerative joint diseases such as osteoarthritis (OA). Noninvasive imaging techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) have demonstrated the potential to assess biochemical markers of cartilage integrity such as GAG content; however, many imaging techniques are available and the optimization of particular techniques in the diagnosis of joint disease remains an active area of research. In order to highlight the differences between these various approaches, this work compares MRI (T1, T2, and T1ρ) and contrast-enhanced CT in human articular cartilage, in both the presence and absence of gadolinium-based contrast agent. Pre- and postcontrast T2 values were found to be similar on a regional level and correlated with each other. As expected, T1 values were shortened significantly on both a global and a spatial basis in the presence of gadolinium (Gd); similar results were found for T1ρ. T2 values were found to correlate mildly with postcontrast T1, T1(Gd), and with precontrast T1ρ values. In addition, contrast-enhanced CT values correlated with both precontrast T1ρ and T1(Gd) more strongly than with precontrast T2. Finally, T1(Gd) and precontrast T1ρ were found to be moderately correlated with CT data. However, T1(Gd) and precontrast T1ρ were found to be almost completely uncorrelated. Together, these results indicate that T1ρ, T2, and contrast-enhanced techniques may provide complementary information about the molecular environment in cartilage during the evolution of OA.
Introduction
Osteoarthritis (OA) is a chronic degenerative disease characterized primarily by the loss of articular cartilage. Loss of articular cartilage may lead to inflammation, pain, and associated pathology such as the growth of new vasculature, osteophyte development, and joint space narrowing. Traditionally, OA has been diagnosed by these secondary indicators of cartilage loss via radiographic examination[1], in which a planar x-ray is used to assess the presence or absence of osteophytes and the width of the joint space; determination of pathology is based on indirect measures of surrounding anatomical structures[2]. Although this is an effective approach, radiographs tend to be limited to the detection of OA only at later stages of disease progression because they lack the ability to directly image soft tissues[3].
In addition, radiographs are relatively insensitive to biochemical changes, which are essential for early diagnosis and treatment of many pathologies. For example, in OA the early stages of cartilage degeneration are often marked by the loss of the proteoglycan components of the cartilage matrix[4]. Unlike collagen, which is uncharged, proteoglycans exhibit a net negative charge in solution. This fixed charge density attracts sodium and other small positive ions. These small ions in turn pull additional water into the cartilage matrix via osmosis and create a positive pressure within the tissue that helps articular cartilage to resist compressive loading forces encountered during normal activities such as walking and running. Disruption of proteoglycans may lead initially to swelling via increased osmotic pressure followed by the eventual loss of cartilage volume which accompanies the corresponding loss of particulates composed of degraded proteoglycan. As such, proteoglycan depletion and the concomitant degradation of the cartilage matrix has been hypothesized as one of the initiating events of the pathologic process leading to OA[5, 6].
This awareness of biochemical correlates of pathology has spurred an increasing interest in employing the capabilities of MRI imaging (MRI) in the assessment of biochemical changes in the hopes of early diagnosis and treatment of diseases such as OA. MRI has all of the distinct advantages conferred from being a noninvasive assessment technique; moreover, it can assess cartilage morphology directly and has shown promise for the detection of soft tissue changes. For example, lesions found in T2-weighted images and T2 maps have been correlated with degradations of cartilage matrix (i.e., fibrillation, clefts)[7], T1ρ relaxation times with proteoglycan degradation[8], and T1 values using delayed gadolinium enhanced MR imaging of cartilage (dGEMRIC) with proteoglycan content of cartilage[9, 10]. MRI has also been used to study the sodium content of cartilage directly by using new techniques to image sodium ions[11–13]. Each of these techniques offers a unique glimpse of the pathological processes encountered during the development of OA. T2 imaging studies are of interest to OA because T2 is sensitive to tissue hydration. Early degradative changes in the extracellular matrix (ECM) affect tissue hydration not only by increasing the overall water content via osmosis but also by increasing the mobility of water. T2 has been found to be inversely correlated with both cartilage volume and thickness[14, 15], and focal areas of increased T2 levels have been found to correspond to cartilage lesions upon arthroscopic evaluation[16, 17]. In addition, early osteoarthritic changes in T2 have been linked to changes in collagen content[18–20]. However, T2 is not correlated with proteoglycan loss in the literature.
T1ρ describes longitudinal relaxation in the rotating frame. Because T1ρ measurements can probe very low frequency interactions, they are especially suited to probing spin-lattice energy exchange between water and large molecules such as those which comprise the ECM in articular cartilage. Disruption of this matrix, specifically disruption of the proteoglycan content of the matrix, leads to an increase in water molecule motion and hence to an increase in measured T1ρ[8, 21, 22]. In addition, proteoglycans are known to be major players responsible for the physical resilience of articular cartilage, and their loss has been correlated with a loss of mechanical integrity within articular tissue. Moreover, because proteoglycan loss is thought to precede the development of symptomatic OA, there is an interest in T1ρ as a noninvasive method for early assessment of disease development[23].
In contrast, T1 dGEMRIC studies have shown that proteoglycan content may be more directly inferred from the presence or absence of contrast agent accumulation within cartilage[6, 9, 10, 24]. In dGEMRIC studies, charged contrast agent such as gadopentate (Gd-DTPA2-) is injected intravenously into a patient and allowed to diffuse into articular cartilage over at least two hours[25]. Because the gadopentate molecule exhibits a net negative charge, it is repulsed by the similarly negative fixed charge density due to proteoglycans within the cartilage matrix[26]. Damage to the matrix and the corresponding loss of proteoglycan component may be inferred from imaging data indicating an increased concentration of contrast agent within focal areas of articular cartilage. dGEMRIC methods are appealing for assessment of biological changes in OA due to this sensitivity to proteoglycan content.
In addition, contrast-enhanced computed tomography (CT) has also been proposed as an alternative to dGEMRIC[27]. In a similar manner to dGEMRIC imaging, CT employs a charged contrast agent and sodium imaging uses the sodium ions to identify focal areas of proteoglycan loss. However, because detection of contrast agent is accomplished directly via measurements of increased x-ray attenuation, these methods require increased amounts of contrast agent to be injected into the patient prior to imaging and hence an increased risk of complications related to contrast administration. Despite this limitation, CT methods are attractive because the imaging procedure is quick and can produce images with high resolution and isotropic voxel dimensions. In the wake of the development of new imaging methods for assessment of cartilage biochemistry, there is a need for a careful, systematic comparison of the proposed imaging parameters to assess the ability of each to assess pathology and progression of OA. Moreover, the relationships between each of these parameters have not been completely characterized; as such it is unknown which of the various imaging methods available might be complementary and hence could provide a better overall picture of OA pathology. The purpose of this study is to compare MRI (T1, T2, and T1ρ) and contrast-enhanced CT in human articular cartilage, in both the presence and absence of gadolinium-based contrast agent.
Methods
Specimen Preparation
Sixteen human osteochondral specimens were used in this study; four were obtained from OA patients undergoing total knee arthroplasty surgeries and twelve were harvested from cadavers obtained from the National Disease Research Institute (NDRI). Specimens were stored at −80°C until use; when needed, specimens were allowed to equilibrate overnight in an isotonic saline bath at 4°C. Baseline MRI data were obtained for T1, T2, and T1ρ. Specimens were then soaked overnight at 4°C in an isotonic saline solution containing 1mM gadolinium (Magnevist, Berlex, NJ) to approximate conditions present in the joint during the course of a dGEMRIC study. The following day, MRI data were again acquired for T1, T2, and T1ρ. Following contrast-enhanced MRI imaging, specimens were again soaked overnight in solution containing 250 mM gadolinium in preparation for contrast-enhanced computed tomography (CT) studies.
Imaging Acquisition
MRI data were acquired on a 3T GE Excite Signa system (General Electric, Milwaukee, WI) using a quadrature transmit/receive wrist coil (Clinical MR Solutions, Brookfield, WI). The protocol included four sequences: sagittal three-dimensional water excitation high-resolution spoiled gradient-recalled (SPGR) imaging (TR/TE = 15/6.7 ms, flip angle = 12, FOV = 7 cm, matrix = 256 × 256, slice thickness = 1 mm, bandwidth = 31.25 kHz, number of excitation [NEX] = 1), T1 mapping using a series of fast spin-echo inversion recovery sequences (TR/TE= 10/4.56 ms, FOV = 7 cm, matrix = 256 × 256, slice thickness = 1 mm, TI = 50, 130, 200, 400, 800, 2100 ms respectively), followed by T1ρ and T2 mapping sequences. A sagittal 3D T1ρ-weighted imaging sequence developed previously in our lab was applied in this study[28]. Briefly, the sequence is composed of two parts: magnetization preparation based on spin-lock techniques for the imparting of T1ρ contrast, and a elliptical-centered segmented 3D SPGR acquisition immediately after T1ρ preparation during transient signal evolution. A RF cycling technique was applied to eliminate the adverse impact of longitudinal relaxation on quantitative accuracy. A variable flip angle train was designed to provide a flat signal response to eliminate the filtering effect in k-space caused by transient signal evolution. The duration of the spin-lock pulse was defined as time of spin-lock (TSL), and the strength of the spin-lock pulse was defined as spin-lock frequency (FSL). The number of α pulses after each T1ρ magnetization preparation was defined as views per segment (VPS). There was a relatively long delay (time of recovery, Trec) between each magnetization preparation to allow enough and equal recovery of the magnetization before each T1ρ preparation. The imaging parameters were: TR/TE = 9.3/3.7 ms; FOV = 14 cm, matrix = 256 × 128, slice thickness = 1 mm, BW = 31.25 kHz, VPS = 64, Trec = 1.5 s, TSL = 0, 10, 40, 80 ms, FSL = 500 Hz. The T2 quantification sequence is based on T2 preparation pulses contained an MLEV train of nonselective composite 90x180y90x refocusing pulses [29, 30], followed by a SPGR sequence as for T1ρ mapping (TE = 4.1, 14.5, 25, 45.9 ms).
CT images were obtained on a Scanco Medical XtremeCT, with an x-ray tube voltage of 60 kVp and current of 900 µA. Images were reconstructed isotropically at 41µm using software provided with the system.
Image post-processing
Pre- and postcontrast T1ρ and T2 maps were generated by fitting the data on a pixel-by-pixel basis to the equation S(TSL) ~ exp(-TSL/T1ρ) and S(TE) ~ exp(-TE/T2) respectively. T1 maps were generated with a pixel-by-pixel 3-parameter fitting program. R1 and R1(Gd) maps were calculated for each sample where R1 = 1/T1 and R1(Gd) = 1/T1(Gd) for each voxel and where (Gd) indicates a measurement made in the presence of gadolinium; ΔR1 was calculated as the difference between R1(Gd) and R1. Resulting images underwent rigid registration using the VTK CISG toolkit. To facilitate a direct comparison, after registration, computed tomography data were downsampled to match the resolution present in the MRI images. Cartilage was segmented semiautomatically using the first echo T1ρ images via a Bezier spline-based MATLAB program developed in-house. The generated 3D contours were overlaid to aligned T1, T2, and T1ρ maps, and also with CT data for statistical analysis.
Statistical Analysis
Median values for each image slice were computed and used to calculate Spearman rank correlations between imaging parameters. T1, T2, and T1ρ values before and after the addition of contrast were compared using a paired t-test.
Results
Sample image data are in shown in Figure 1. At the right and the left of the figure are grayscale CT and SPGR images for a single sagittal slice of imaging data. In addition, color overlays for T1(Gd), precontrast T1ρ, precontrast T2, and CT image data for corresponding regions of interest (ROIs) are shown. Interestingly, a focal area (arrow) of elevated T1(Gd) values appears to correspond to a similar location of decreased precontrast T1ρ values and also to an area of decreased CT values, which suggests qualitatively that spatial correlations between these parameters may be present.
Figure 1.
Sample image data showing SPGR, T1(Gd), T2, T1ρ, and CT values for a single specimen. SPGR and CT images are shown at the left and right. Colormaps for T1(Gd), T2, T1ρ, and CT data are shown overlaid on the SPGR grayscale image in the center.
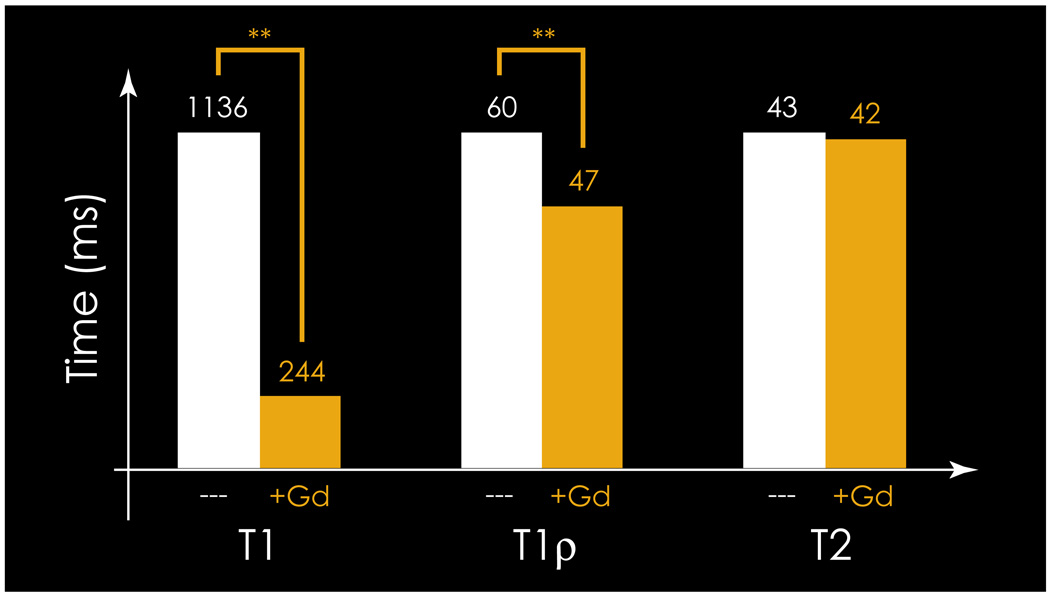
Figure 2 shows the effect of 1 mM gadolinium contrast on T1, T1ρ, and T2. Addition of 1mM contrast was found to shorten T1 and T1ρ values significantly, but was not found to affect T2 values in articular cartilage. As shown in the figure, T1 values were found to decrease significantly from a median value of 1136 ms (precontrast) to a median value of 244 ms (postcontrast) upon equilibration with 1 mM gadolinium. T1ρ values were also found to decrease significantly from a median of 60 ms (precontrast) to 47 ms (postcontrast) upon addition of gadolinium. As expected, T2 values were relatively unaffected by the addition of gadolinium (median 43 vs 42 ms).
Figure 2.
Mean T1(Gd), T2, and T1ρ, in the presence and absence of 1mM Gd. ** indicates p<0.005.
Table 1 displays the correlations between MRI and CT imaging parameters. As shown, precontrast T2 values were found to correlate equally mildly with precontrast T1ρ, T1(Gd), and ΔR1 values (Spearman r= 0.24, −0.19, and 0.25, respectively, and p < 0.05 for each). In addition, precontrast T1ρ values were found to correlate very well (r = 0.68) with precontrast T1 and less well with other parameters. ΔR1 values were found to correlate extremely well (r = −0.96) with T1(Gd), although they were only mildly correlated with the other MRI imaging measures. Interestingly, T1(Gd) and precontrast T1ρ imaging data were uncorrelated in this study. As expected, precontrast T2 values were found to be uncorrelated with computed tomography data. In addition, T1(Gd) and precontrast T1ρ values were found to be mildly correlated (r = 0.34 and −0.41, respectively, p < 0.05) with computed tomography. Finally, ΔR1 was found to be moderately correlated with computed tomography (r = 0.48, p<0.05).
Table 1.
Spearman rank correlations between imaging parameters.
| T2 | T1 | T1(Gd) | Delta R1 | CT Attenuation | |
|---|---|---|---|---|---|
| T1ρ | 0.24* | 0.68** | 0.01 | 0.14 | 0.34** |
| T2 | 0.41** | −0.19 | 0.25* | 0.02 | |
| T1 | 0.14 | 0.06 | 0.18 | ||
| T1(Gd) | −0.96** | −0.41** | |||
| Delta R1 | 0.48** | ||||
indicates p < 0.05.
indicates p < 0.005.
Discussion
MRI imaging has become prominent in the assessment of OA due to its ability to visualize soft tissues. In particular, the development of early OA has been associated with biochemically-driven changes such as cartilage swelling and macromolecular destruction, especially destruction of collagen and proteoglycan[31]. As a result, the dGEMRIC technique for cartilage imaging has received much attention for its intuitive mechanistic appeal relating to the measurement of fixed charge density and hence its ability to characterize spatial variations of proteoglycans in cartilage. In addition, T1ρ imaging has been shown to be sensitive to the macromolecular content of articular cartilage and as such has the potential to detect changes in proteoglycan content as well. Moreover, elevated osmotic pressure associated with inflammation and macromolecular destruction has generated much interest in T2 characterization of OA due to the sensitivity of T2 to fluid[18, 19].
In this study precontrast T2 values correlated equally well on a regional basis with T1(Gd), ΔR1, and precontrast T1ρ. However, the correlation was quite mild in all cases, which is likely based on the influence of local fluid accumulation. Although both precontrast T2 and T1ρ are influenced by water motility, this result might be expected partially on the basis that T1ρ is sensitive to large molecular weight proteoglycans, whose degradation may not necessarily correlate on a spatial basis with fluid accumulation. In addition, the low effect of fluid on T1(Gd) and ΔR1 can be explained via Donnan equilibrium calculations in which gadolinium accumulation is predominately determined by the presence or absence of charged glycosaminoglycan within the cartilage matrix[26]. These calculations suggest that the equilibrium accumulation of contrast in articular cartilage is not expected to be influenced by fluid due to identical effects of hydration on fixed and mobile charge density, even though the rate of influx of contrast may be affected by the hydration of the ECM.
In addition, it was found that precontrast T1ρ correlated quite well with precontrast T1, but was only mildly correlated with other MRI measures. Because spin-locking theoretically minimizes T2 decay effects, a higher precontrast correlation between T1ρ and T1 than between T1ρ and T2 is consistent with effective spin-locking technique. Furthermore, it suggests that increasing spin locking frequency would increase the differences between correlations with T1 and T2. Biochemically, the difference in correlations may be explained in part by the fact that both T1 and T1ρ precontrast relaxation rates are influenced heavily by the molecular content of the cartilage matrix while influences on T2 are through a more indirect mechanism[9, 23, 32]. This influence may not be specific to a particular macromolecular constituent. For example, although proteoglycans have received much interest as macromolecules whose loss may be indicative of OA progression, it is reasonable to assume that precontrast T1ρ changes seen in OA are at least in part affected by other macromolecular constituents of the ECM such as collagen because the proteoglycan content of cartilage is on the order of 3–6% by (wet) weight. However, in early OA, it is thought that proteoglycans are lost preferentially throughout the matrix; hence precontrast T1ρ may be specific to their loss at the onset of disease. In addition, although precontrast T1ρ may be affected by collagen, the spin-locking technique suppresses dipolar interaction and therefore dependence of precontrast T1ρ on collagen; thus precontrast T1ρ may be more sensitive to PG[33, 34]. Precontrast T1ρ may be dominated by PG due to its increased sensitivity to molecules in the proteoglycan weight range as compared to those in the collagen weight range. Precontrast T1ρ relaxation rate (1/ T1ρ) has also been shown to decrease linearly with decreasing PG content in ex vivo bovine patellae[21] and has been proposed as a more specific indicator of PG content than precontrast T2 relaxation in trypsinized cartilage[8]. Finally, precontrast T1ρ possesses a higher dynamic range than precontrast T2, which may indicate an ability to discriminate between disease and health states with increased accuracy.
More recently, the use of contrast agents has proven useful in the evaluation of articular cartilage[24, 35–37]. Specifically, small, mobile, charged contrast agents can be used to probe the density of charged macromolecules in the ECM such as proteoglycans. Proteoglycan loss is thought to precede development of OA and thus the ability to probe this property of cartilage may provide a method of early diagnosis of underlying pathology. Interestingly, in this study T1(Gd) was found to be uncorrelated to precontrast T1ρ although it has been documented that both measures are sensitive to proteoglycan loss in articular cartilage. The low precontrast correlation between T2 and T1ρ suggests that hydration may play a role in the discrepancy, although it is doubtful that this will account for the entire effect seen here. Although it was not assessed in this study, it is likely that both collagen and proteoglycan were degraded in many of the samples leading to complex changes in precontrast T1ρ. In addition, it is likely that destruction of proteoglycan is a heterogeneous process in which the loss of fixed charge density does not correlate exactly with the loss of macromolecular mass. The exact ratio of charge to mass lost will depend on the specific degradative enzymatic pathway which predominates, and this is quite likely to vary from region to region within the joint. Such a heterogeneous loss of charge relative to mass (or vice versa) could certainly explain the results presented here.
Employing methodology similar to that of dGEMRIC imaging, contrast-enhanced computed tomography techniques have also been studied as a potential method for imaging proteoglycans in cartilage; as expected, CT attenuation values in this study were found to correlate on both a regional and a spatial basis with T1(Gd). Interestingly, however, the correlation was moderate. One possible explanation for this is degradation of the cartilage sample during the overnight soak in contrast solution. In this study, we attempted to minimize any potential degradation by using low temperatures (4°C) in order to inhibit enzyme activity and by carefully adjusting the total solute concentration to be isotonic to blood in order to inhibit osmotic swelling or shrinkage. These precautions could be further augmented by blocking the bone chemically, although this was not done here.
This suggests that the quantitation of contrast accumulation is biased differently in the two techniques, which is reasonable given that the physical mechanisms of x-ray absorption and nuclear magnetic energy transfer are widely different. Contrast-enhanced computed tomography has a potential advantage in that it detects the presence of contrast agent by directly sensing its electron density instead of relying indirectly on its effect on tissue relaxation times. In addition, relaxation times are affected by such effects as inhomogeneities in the magnetic field which make absolute quantitation of relaxation times challenging. At the present time, however, concerns related to radiation exposure and administration of increased contrast agent dose will likely require some technological advancement before the use of computed tomography becomes widespread in human studies of OA.
Together, these results indicate that T1ρ, T2, and contrast-enhanced techniques may provide complementary information about the molecular environment in cartilage during the evolution of OA. In particular we found the low correlation between precontrast T1ρ and dGEMRIC to be quite surprising, suggesting that the mechanistic picture of proteoglycan loss early in OA may not be complete. In addition, we note only a moderate correlation between contrast-enhanced computed tomography and the dGEMRIC index, which suggests that T1(Gd) may be influenced by factors in addition to contrast agent accumulation. Finally, we report that precontrast T2 values were essentially uncorrelated with the other imaging measures studied here, indicating that T2 may provide additional, complementary information beyond what can be obtained with T1(Gd), T1ρ, or contrast-enhanced computed tomography. We expect that further study will expand our understanding of these relationships.
Acknowledgement
The research was supported by NIH K25 AR053633 and RO1 AR46905.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman R, Fries J, Bloch D. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30:11. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 2.Rogers J, Watt, Dieppe P. A comparison of the visual and radiographic detection of bony changes at the knee joint. BMJ. 1990;300:367–368. doi: 10.1136/bmj.300.6721.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan WP, et al. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. Ajr Am J Roentgenol. 1991;157(4):799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- 4.Dijkgraaf LC, et al. The structure biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53(10):1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 5.Bashir A, et al. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd DTPA 2- -enhanced MR imaging. Radiology. 1997;2052:551–558. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- 6.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 7.Mlynarik V, et al. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging. 1999;10(4):497–502. doi: 10.1002/(sici)1522-2586(199910)10:4<497::aid-jmri1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Regatte RR, et al. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9(12):1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 9.Bashir A, et al. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41(5):857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Nieminen MT, et al. Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J Biomech. 2004;37(3):321–328. doi: 10.1016/s0021-9290(03)00291-4. [DOI] [PubMed] [Google Scholar]
- 11.Wheaton AJ, et al. Sodium magnetic resonance imaging of proteoglycan depletion in an in vivo model of osteoarthritis. Acad Radiol. 2004;11(1):21–28. doi: 10.1016/s1076-6332(03)00574-9. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro EM, et al. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47(2):284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy R, et al. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39(5):697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- 14.Blumenkrantz G, et al. ACR. San Antonio, TX: 2004. Cartilage T2 as a Marker of Progression of Osteoarthritis. [Google Scholar]
- 15.Dunn TC, et al. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick L, et al. Severity of articular cartilage abnormality in patients with osteoarthritis: evaluation with fast spin-echo MR vs arthroscopy. AJR. 1994;162:99–103. doi: 10.2214/ajr.162.1.8273700. [DOI] [PubMed] [Google Scholar]
- 17.Peterfy CG. Imaging of the disease process. Curr Opin Rheumatol. 2002;14(5):590–596. doi: 10.1097/00002281-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Menezes NM, et al. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 19.Mosher TJ, et al. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177(3):665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 20.Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: A microscopic MRI (microMRI) study. Magn Reson Med. 2002;48(3):460–469. doi: 10.1002/mrm.10216. [DOI] [PubMed] [Google Scholar]
- 21.Duvvuri U, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 22.Regatte RR, et al. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229(1):269–274. doi: 10.1148/radiol.2291021041. [DOI] [PubMed] [Google Scholar]
- 23.Li X, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein D, et al. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11:465–478. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 25.Burstein D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10(5):365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cockman MD, et al. Quantitative imaging of proteoglycan in cartilage using a gadolinium probe and microCT. Osteoarthritis Cartilage. 2006;14(3):210–214. doi: 10.1016/j.joca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Li X, et al. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittain JH, et al. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 30.Foltz W, et al. Optimized spiral imaging for measurement of myocardial T2 relaxation. Magn Reson Med. 2003;49(6):1089–1097. doi: 10.1002/mrm.10467. [DOI] [PubMed] [Google Scholar]
- 31.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 32.Li X, et al. In Vivo 3T Spiral Imaging Based Multi-slice T1ρ Mapping of Knee Cartilage in Osteoarthritis. Mag. Res. Med. 2005;54(4):929–936. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 33.Makela HI, et al. Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun. 2001;289(4):813–818. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 34.Duvvuri U, et al. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci U S A. 2001;98(22):12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurkijarvi JE, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med. 2004;52(1):41–46. doi: 10.1002/mrm.20104. [DOI] [PubMed] [Google Scholar]
- 36.Williams A, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. AJR Am J Roentgenol. 2004;182(1):167–172. doi: 10.2214/ajr.182.1.1820167. [DOI] [PubMed] [Google Scholar]
- 37.Williams A, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52(11):3528–3535. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]